Abstract
The pair-rule segmentation gene even skipped (eve) is required to activate engrailed stripes and to organize odd-numbered parasegments (PSs). The protein product Eve has been shown to be an active repressor of transcription, and recent models for Eve function suggest that activation of engrailed is indirect, but these models have not been fully tested. Here we identify the forkhead domain transcription factor Sloppy-paired as the key intermediate in the initial activation of engrailed by Eve in odd-numbered parasegments. We also analyze the roles of the transcription factors Runt and Odd-skipped in this process. Detailed analysis of engrailed and pair-rule gene expression in various mutant combinations shows how eve activates engrailed by repressing these engrailed repressors, and further indicates that mutual repression among pair-rule genes plays an important role in establishing parasegment boundaries. We present a new model of pair-rule gene function that explains the response of these boundaries to the relative levels of Eve and Fushi Tarazu.
Keywords: Segmentation, Parasegment boundary, Pair-rule gene, Eve, Homeodomain, Transcriptional repressor, Genetic network
Introduction
The even skipped gene (eve) encodes a homeodomain (HD) transcription factor (Macdonald et al., 1986) required during Drosophila segmentation for activation of engrailed (en) and for proper organization of odd-numbered parasegments (Fujioka et al., 1995; Harding et al., 1986). It is activated in response to upstream gap genes in a striped pattern that is subsequently refined into narrow stripes that coincide cell-for-cell with the odd-numbered parasegment (PS) boundaries (Lawrence et al., 1987). This refinement involves auto-activation, in that early, broad stripes are needed to activate the refined, late stripe pattern (Goto et al., 1989; Harding et al., 1989). Somewhat paradoxically, transcription assays in cultured cells showed that Eve can act as a transcriptional repressor (Han and Manley, 1993; Jaynes and O’Farrell, 1988). This analysis identified an ala/pro-rich repressor domain similar in sequence composition to repressor domains in other proteins (Hanna-Rose and Hansen, 1996). Further analysis indicated that this Eve repressor domain can function in vitro by interacting with TBP (Austin and Biggin, 1995; Um et al., 1995), and that the Eve N-terminal region can negatively regulate this activity (Li and Manley, 1999). In embryos, ubiquitous expression of Eve led to rapid repression of some target genes, indicating that Eve is a direct repressor of those genes (Manoukian and Krause, 1992). Subsequently, a second repressor domain active in embryos was identified and was shown to interact with the corepressor Groucho (Gro) (Kobayashi et al., 2001). In contrast, the first repressor domain was shown to be Gro-independent (Jiménez et al., 1997). Recently, the corepressor Atrophin was identified through its ability to interact functionally with Eve through the Gro-independent repressor domain (Zhang et al., 2002).
The initially identified eve allele was a hypomorph with a pair-rule phenotype for which the gene was named (Nüsslein-Volhard and Wieschaus, 1980). However, eve function is required for the expression both odd- and even-numbered en stripes, which are activated by distinct mechanisms (DiNardo and O’Farrell, 1987; Howard and Ingham, 1986). The odd-numbered stripes require paired (prd) in addition to eve, while the even-numbered stripes require eve, fushi tarazu (ftz), and odd-paired (DiNardo and O’Farrell, 1987). How does Eve do this? Previous data suggested that eve might activate en indirectly. Early Eve stripes repress prd at a high concentration, and sloppy paired (slp), a repressor of en, at a low concentration, producing one cell row that has an activator, but not a repressor of en. These cells activate the odd-numbered en stripes (Fujioka et al., 1995). For the even-numbered en stripes, Eve represses another repressor of en, odd skipped (odd), at the anterior edges of ftz stripes to again create one cell row that has an activator, but not a repressor of en (Fujioka et al., 1995; Manoukian and Krause, 1992). In eve hypomorphic mutants, both sets of en stripes are expressed, but the spacing is abnormal. The odd-numbered PSs are narrower than the even-numbered ones and are deleted at late embryonic stages (Frasch et al., 1988), presumably because of the abnormal juxtaposition of cell types within them (Pazdera et al., 1998). Previous models invoking only the repression activity of eve do not explain, however, why odd-numbered en stripes do not expand in slp mutants until well after they are established (Cadigan et al., 1994b). It was suggested that secondary stripes of the primary pair-rule gene runt might serve a redundant function with slp to set the anterior borders of these en stripes (Fujioka et al., 1995), but this has not been tested.
In this paper, we analyze how the repressor activity of Eve, combined with repressive interactions among other pair-rule genes, allows it to carry out its complex series of functions in the subdivision of blastoderm embryos. In particular, we show that slp is the key intermediate between eve and en, so that repression of slp by eve activates the odd-numbered en stripes. We identify novel aspects of pair-rule gene interaction that lead to a more complete picture of how this group of genes resolves the broad patterns of gap genes into the narrow patterns of segment polarity genes in their proper relative positions. The resulting model can explain, among other things, how the relative concentrations of Eve and Ftz determine the subsequent widths of the odd- and even-numbered PSs.
Materials and methods
Drosophila strains and transgenic flies
The mutant strains used in this study were Df(2R)eve, eveR13, enE, Df(2L)edsZ1 (mutant for both slp1 and slp2), odd7L, and runtLB5 (Gergen and Wieschaus, 1986), all null alleles. Df(2R)eve and either Df(2L)edsZ1 or odd7L were recombined onto the same chromosome for the double mutant analysis. These mutations were balanced over marked balancer chromosomes to allow identification of mutant embryos, as indicated in the figure legends.
Embryo analysis
In situ hybridization to whole mount embryos was performed as described previously (Tautz and Pfeifle, 1989) using digoxigenin-labeled antisense mRNA and visualized by the alkaline phosphatase-NBT/CIPB reaction (Roche). For double staining, in situ hybridization was followed by antibody staining (Mullen and DiNardo, 1995) with polyclonal α-Eve (Frasch et al., 1987) at 1:10,000 dilution or with α-En monoclonal 4D9 (Developmental Studies Hybridoma Bank) at 1:10 dilution visualized using appropriate secondary antibodies and the HRP-DAB reaction (Mullen and DiNardo, 1995).
Results
Repression of slp by Eve activates en
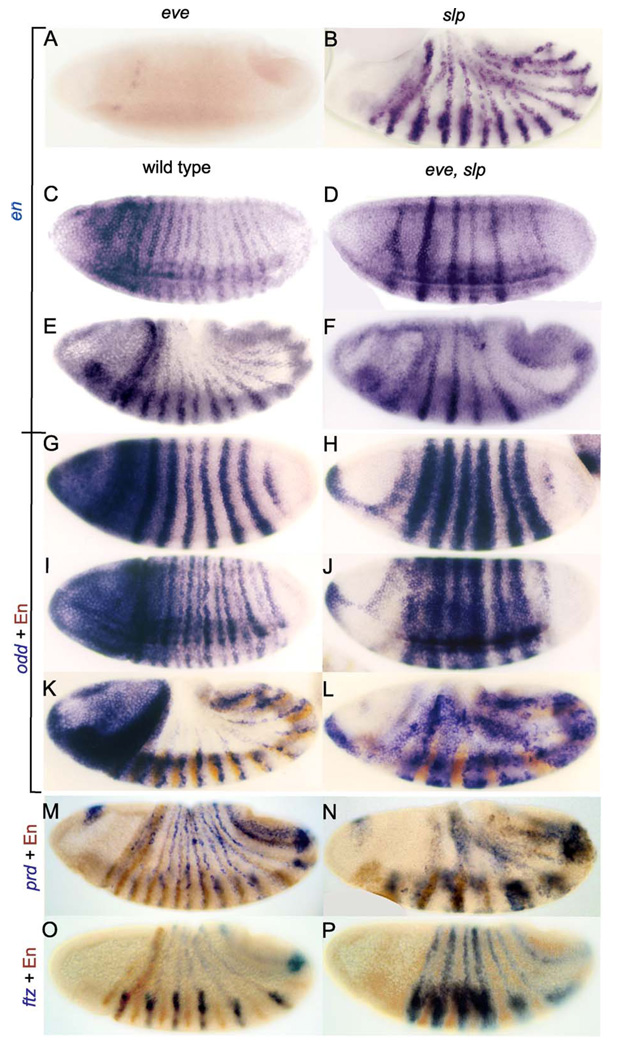
A key aspect of a previously proposed model for how eve organizes the odd-numbered PSs (Fujioka et al., 1995) was that Eve represses slp (and other repressors of en) at a low concentration, while at a higher concentration (Manoukian and Krause, 1992), it represses paired (prd, a crucial activator of en), thereby restricting en expression to a region at the edge of broad, early eve stripes. In eve mutants, both the slp and prd expression patterns expand throughout the eve domain (Baumgartner and Noll, 1990; Fujioka et al., 1995), and en is not activated. If repression of slp (and not prd) by low concentrations of eve is sufficient to activate en, then when both eve and slp are removed, en might be activated by prd in the eve domain. Activation of en in eve, slp double mutants was previously reported (Riechmann et al., 1997), but whether this occurs at the time of development when en is normally turned on or whether this is related to the ectopic activation of en that occurs at later stages in slp mutants remained unclear. We examined eve, slp double mutants and found that broad en stripes are expressed in the eve domains from the time when en expression is normally initiated (Fig. 1D, compare to Fig. 1C), showing that eve does, indeed, activate the odd-numbered en stripes through a double-negative mechanism. These abnormally broad en stripes persist to later stages (Fig. 1F and data not shown). Activation of the even-numbered en stripes also requires eve function, and it was previously shown that these en stripes require that eve repress another en repressor, odd, because they ‘‘reappear’’ in eve, odd double mutants (Coulter and Wieschaus, 1988; DiNardo and O’Farrell, 1987). In slp single mutants, en is expressed essentially normally until mid-germband extension (Fig. 1B), after which the odd-numbered en stripes begin to expand anteriorly, followed by a similar expansion of the even-numbered stripes (data not shown) (Cadigan et al., 1994b). This suggests that the positioning of the anterior borders of odd-numbered en stripes is not due solely to slp, but that another en repressor may act redundantly with slp until mid-germband extension (see below). We emphasize, however, that this putative repressor is not sufficient, in the absence of eve function, to prevent activation of en in the eve domain because en is activated there in eve, slp double mutants.
Figure 1.
Eve activates en by repressing slp. Expression of en, absent in eve mutants, is restored in odd-numbered PSs in eve, slp double mutants. Wild-type embryos (C–P, left side) or embryos null mutant for eve (A), slp (B), or both eve and slp (C–P, right side) were stained (blue) by in situ hybridization to RNA from either en(A–F), odd (G–L), prd (M, N), or ftz (O, P); those in C–L were simultaneously stained for lacZ RNA from the hb-lacZ transgene on the balancer chromosome to identify the mutants (wild-type embryos have light blue staining throughout the head region). Those in G–P were then antibody-stained (brown) for En protein. Note that en expressionis absent inthe trunk region of eve mutants (A) butis restored (in an abnormal pattern) when slp function is simultaneously removed (D, F). Note also that odd expression is expanded in the double mutants (H, J), that odd and En expression are essentially complementary in these mutants (L), and that the En expression overlaps with that of prd (N), but not ftz (P), indicating that it is in odd-numbered PSs.
We also examined odd expression in eve, slp double mutants and found that odd stripes are extensively broadened, failing to retract from the posterior of the ftz domains as they normally do (Fig 1H and J, compare to Fig 1G and I) as well as from the anterior of the ftz domains due to the absence of eve (this is where even-numbered en stripes are normally activated). This lack of retraction from the posterior is due to the absence of slp (see below). As en expression becomes strong, it becomes clear that in eve, slp double mutants, en and odd are expressed in mutually exclusive patterns that together fill the trunk region of the embryo (Fig. 1L). We also examined prd expression and found that broad prd stripes are expressed during early gastrulation and germband extension (Fig. 1N and data not shown), relative to the much narrower stripes in the wild type (Fig. 1M), similar to the expanded prd expression seen in eve single mutants (Baumgartner and Noll, 1990). The broad en stripes are expressed within these prd stripes, suggesting that they are regulated similarly to the normal odd-numbered en stripes, which are activated by prd and repressed by slp (as well as by other en repressors, see below). That these en stripes are expressed within presumptive odd-numbered PSs is confirmed by double staining with ftz (Fig. 1P). Comparing the ftz pattern to that in the wild type (Fig. 1O), it is clear that ftz stripes fail to narrow properly in eve, slp double mutants, similar to the effect of slp mutants (Cadigan et al., 1994a).
The roles of runt and odd in restricting the odd-numbered en stripes
According to the above results, slp would appear to be involved in setting the anterior border of each odd-numbered en stripe. However, as stated above, these en stripes do not expand anteriorly in slp mutants when they are first expressed, although they do so later. This suggests that en repressors other than slp also participate in setting these borders, which become the odd-numbered PS boundaries. At the time of germband extension, secondary runt stripes are expressed just anterior to the odd-numbered en stripes. In fact, previous studies suggested that Runt is a direct repressor of odd-numbered en stripes (Manoukian and Krause, 1993; Tracey et al., 2000; Tsai and Gergen, 1994). If the presence of Runt is part of the reason for the delay of en expansion in slp mutants, then in slp, runt double mutants, en stripes should expand earlier than they do in slp mutants alone. To test this prediction, we first analyzed runt null mutants (LB5) more thoroughly than has been done previously with respect to their patterns of expression of slp and odd. This was necessary because of the complex effects of runt on the expression of other pair-rule genes. In runt mutants, hairy expression is expanded, resulting in relatively narrow and weak ftz expression (in the even-numbered PSs) (Carroll and Scott, 1986). The early odd pattern is very similar to that of ftz, and it is probably similarly regulated by hairy (Jiménez et al., 1996). Also, early, broad eve stripes persist longer than normal (in the odd-numbered PSs) (Frasch and Levine, 1987; Ingham and Gergen, 1988) due to the role of runt in repressing these stripes (Manoukian and Krause, 1993; Tsai and Gergen, 1994). These effects have secondary consequences for both slp and odd expression. The situation is further complicated by the fact that the effects of runt vary in different parts of the embryo, particularly in the even-numbered PSs, so that ftz stripes 1, 4, and 5 remain relatively broad, while others are reduced (Lawrence and Johnston, 1989). The same is true for the primary odd stripes (Fig. 2B, compare to A) that are almost complementary to Eve stripes at this stage (and essentially coincident with ftz stripes). In wild-type embryos, the primary odd stripes narrow from the anterior due to repression by Eve, as well as from the posterior, and secondary odd stripes appear in the middle portion of each eve stripe (Fig. 2C,E) (Coulter et al., 1990). In contrast, in runt null mutants, the primary odd stripes disappear essentially completely (Fig. 2D,F), while the secondary odd stripes are broader than normal, again with variation among the different stripes: they are initially close to normal within eve stripes 1 and 5, but become much broader than normal within the other eve stripes (Fig. 2F). These stripe-specific differences are presumably the result of the ‘‘gap gene-like’’ effects of runt (Tsai and Gergen, 1994), which cause, among other things, early eve stripe 5 to be weaker than normal.
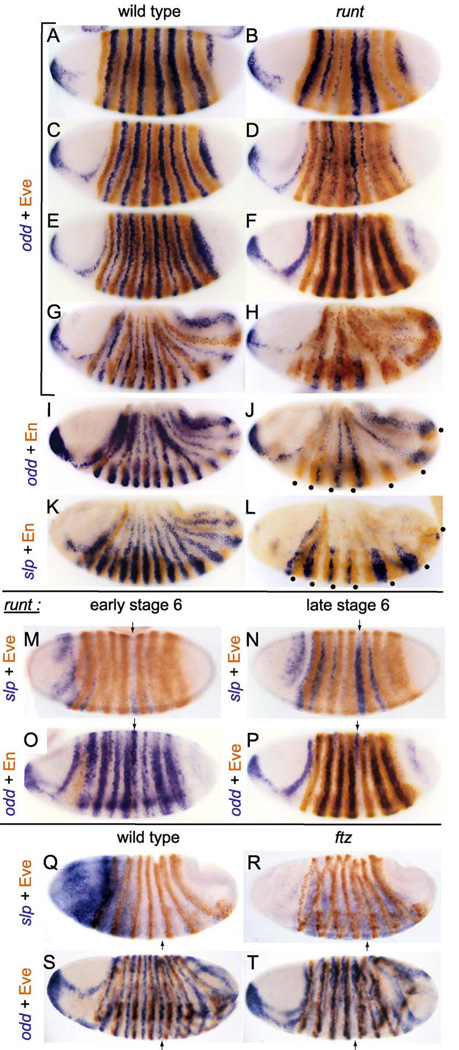
Figure 2.
Pair-rule and en expression patterns in runt null mutants, and maintenance of odd expression by ftz. Wild-type embryos (A–K, left side, and Q, S) or embryos null mutant for runt (all others except R and T, which are null for ftz) were stained (blue) by in situ hybridization to RNA for either odd (A–J, O,P, S, T) or slp (K–N, Q, R; those in Q and R were simultaneously stained for lacZ RNA from the hb-lacZ transgene on the balancer chromosome to identify the mutants, as in Fig. 1), and then antibody-stained (brown) for either Eve (A–H, M, N, P–T) or En (I–L, O). Note the stripe-specific effects of runt on odd expression at the blastoderm stage (B), with the 1st, 4th, and 5th stripes being the most normal (corresponding to PSs 2, 8, and 10). The subsequent evolution of these incipient PSs diverges from that of PSs 4, 6, and 12, in that they remain of approximately normal width (seen as gaps between Eve stripes in F, where odd expression is “replaced” by that of slp, as shown in M–P), while 4, 6, and 12 are abnormally narrow. Note also that in runt mutants, abnormally wide secondary odd stripes come up within Eve stripes 2–4, 6, and 7 (seen in F; these are in PSs 3, 5, 7, 11, and 13). By stage 8 (G–L), odd RNA is seen clearly only in odd-numbered PSs in runt mutants, while slp is expressedin even-numbered PSs: the positions of the originally strong, wide stripes of odd are now those of the wide stripes of slp, that is, PSs 2, 8,and 10. The odd-numberedEn stripes are marked with dots in J and L (stripes 1–2and 9–10 are fused), while ftz domain 4is marked with arrows in M–T. Note that in runt mutants (M–P) and in ftz mutants (R, T) odd expression is fading ventrally as slp is activated, and that in ftz mutants, slp is expressed throughout the ftz domains, while odd is lost there (although still present dorsally in T, odd is also lost there by stage 9, well before it fades in the wild type; not shown).
The net result in runt nulls is that, by early in gastrulation, odd is expressed within eve stripes (but only weakly in 1 and 5), but not in the ftz domains (Fig. 2H,J). In fact, this odd expression is sandwiched between pairs of late eve stripes, which are present at both the anterior and posterior edges of early eve stripes, rather than only at the anterior, as in the wild type, due to the ‘‘loss of polarity’’ that occurs in runt mutants (Carroll and Scott, 1986). This loss of polarity is reflected in ectopic late eve and en stripes in runt hypomorphs (data not shown), while in runt nulls, no ‘‘extra’’ en stripes are apparent (DiNardo and O’Farrell, 1987) (Fig. 2J). The process that ‘‘duplicates’’ odd-numbered en stripes in runt hypomorphs may be similar to that which occurs in the eve domains of runt nulls because none of the en stripes in runt nulls appear to be ftz-dependent (although a part of the en expression in ftz domain 4 may be; data not shown). Thus, in runt nulls, the ftz domains do not produce their normal en stripes, so that the en pattern appears to be more normal than in runt hypomorphs, where the presence of the ftz-dependent en stripes makes it apparent that there is an ectopic en stripe in the posterior of each eve domain (data not shown).
At the same time that odd expression is disappearing from the ftz domains in runt nulls, slp expression is induced. The timing of this slp activation is similar to that in the wild type (Grossniklaus et al., 1992), which occurs just before en activation. In the wild type, slp expression is confined to the posterior part of each PS, just anterior to each en stripe (Fig. 2K), but in runt nulls, it is expressed throughout each variably sized ftz domain (Fig. 2L). In fact, during germband extension, the patterns of odd, en, and slp appear to be mutually exclusive, and together they ‘‘fill up’’ the trunk region of the embryo (Fig. 2J,L; dots mark odd-numbered en stripes), as they do in the wild type, albeit in very different patterns. This complementarity is consistent with the observed interactions among these genes, in that en can be repressed by ectopic expression of either slp (Cadigan et al., 1994a) or odd (Saulier-Le Dréan et al., 1998), slp is repressed by ectopic odd (Saulier-Le Dréan et al., 1998), and ectopic En also represses slp (Kobayashi et al., 2003). Furthermore, the induction of slp within the ftz domains in runt nulls just precedes the loss of odd expression (Fig. 2M–P, arrows indicate ftz domain 4), suggesting that slp may be responsible for repression of odd. In the wild type, odd remains in the ftz domains anterior to each slp stripe (and posterior to each en stripe).
The complete loss of primary odd stripes in runt nulls may be a result of weaker than normal ftz expression. Even ftz stripes 1, 4, and 5 appear weaker than normal, although they remain broad (data not shown). As shown previously (Nasiadka and Krause, 1999) and in Fig. 2T, ftz is required to maintain these odd stripes. Furthermore, the activation of slp just precedes the fading of odd from the ftz domain, both in ftz mutants and in the wild type (Fig. 2Q–T, arrows indicate ftz domain 4). Thus, repression of odd by slp may restrict odd to the middle of each even-numbered PS in the wild type, where ftz expression is maintained at a high level (it has faded from the posterior half of the PS as part of the normal narrowing of ftz stripes). Thus, weakened ftz expression in runt nulls may be insufficient to maintain odd expression as slp is induced.
This proposed role of slp in the repression of primary odd stripes would predict that in slp null mutants, these odd stripes might fail to narrow from the posterior. (As stated above, narrowing from the anterior also occurs in the wild type due to repression by eve.) This is in fact the case as shown in Fig. 3B and D (compare with 3A and C; dots indicate regions of expansion). The primary odd stripes remain broader than normal, abutting the odd-numbered en stripes, which appear essentially normal at this stage. Because odd is probably sufficient to repress en (DiNardo and O’Farrell, 1987; Saulier-Le Dréan et al., 1998), this can explain why these en stripes fail to expand in slp mutants until later stages. The later expansion of en expression is roughly coincident with the fading of odd expression in this region (data not shown) and results in the repression of alternate wingless (wg) stripes (positions of dots in Fig. 3F). Later still, all the en stripes expand, and all the wg stripes are lost (Cadigan et al., 1994b).
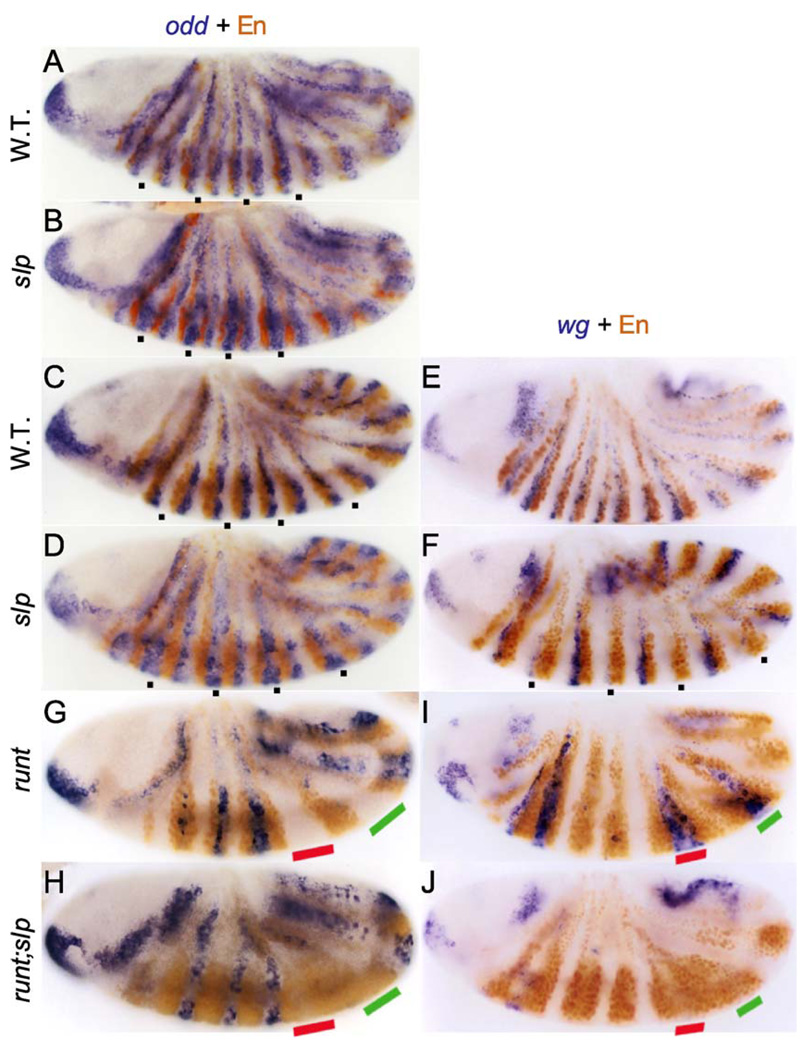
Figure 3.
Contributions of odd and runt to the odd-numbered parasegment boundaries. Wild-type embryos or those null mutant for slp (B, D, F), runt (G, I), or double mutant for runt and slp (H, J) were stained (blue) by in situ hybridization to RNA for either odd (left column) or wg (right column), followed by anti-En staining (brown). Note, in slp mutants at stages 7 and 8 (B, D), the expanded odd expression just anterior to odd-numbered En stripes (the posterior parts of PSs 2, 4, 6, and 8 are marked in A–D and F with small squares). At these stages, En expression is approximately normal; however, at stage 9 (F), odd-numbered En stripes have expanded anteriorly, and wg expression in the same regions has been lost. Note also that the regions where neither odd nor En is expressed in runt mutants (G, I) express En in runt, slp double mutants (H, J); PSs 8 and 10, where slp (Fig. 2) and wg (I) are expressed between En stripes in runt single mutants, are marked with red and green bars, respectively.
As noted above, secondary runt stripes are present in the same portions of even-numbered PSs where slp is expressed in the wild type and where odd remains in slp mutants. Having characterized runt null mutants, we were in a position to test the effects of removing both runt and slp function. The most normal even-numbered PSs in runt nulls, at least in terms of their width, are PSs 4 and 5, where the broadest slp stripes are expressed (Fig. 2L; marked with red and green bars, respectively, in Fig 3G–J). As shown in Fig. 3I, wg is also expressed in this region (coincident with slp expression). In runt, slp double mutants, these regions express en (as do the other, narrower even-numbered parasegmental domains; Fig. 3H,J; compare with Fig. 3G,I). This result is consistent with the idea that both runt and slp contribute to setting the anterior boundaries of the odd-numbered PSs by repressing late eve and en. However, the observed expansion of odd in slp mutants suggests that runt may not be directly required for this function and that slp may be primarily responsible for setting this boundary in wild-type embryos. In the absence of slp, odd expands into the region, keeping en off until later (described above), while in runt nulls, odd is lost in this region. In runt, slp double mutants, odd is not present in the ftz domains, so that en can be activated.
Repression among the repressors of en contributes to segmentation
As shown above, in runt mutants, the trunk region is subdivided by the expression patterns of three genes (Fig. 2), en (essentially coincident with late eve expression), odd, and slp. Furthermore, in runt, slp double mutants (Fig. 3H), the embryo is subdivided by odd and en expression (which coincides with late eve), while in eve, slp double mutants, the trunk region is also subdivided by odd and en, but in a very different pattern (Fig. 1L). In eve null mutants, en expression is completely missing in the trunk region (Fig. 1A), while odd is present only in the ftz domains (Fig. 4A), and slp is expressed throughout the eve domains (Fujioka et al., 1995), so that again, en, odd, and slp subdivide the embryo. The cross-repressive interactions between slp and odd, and between late eve and slp, can account for the lack of overlap of their resolved patterns. Once established, mutual repression between en and slp (Alexandre and Vincent, 2003; Cadigan et al., 1994b; Kobayashi et al., 2003) can reinforce the odd-numbered PS boundary set up at the late eve– slp interface.
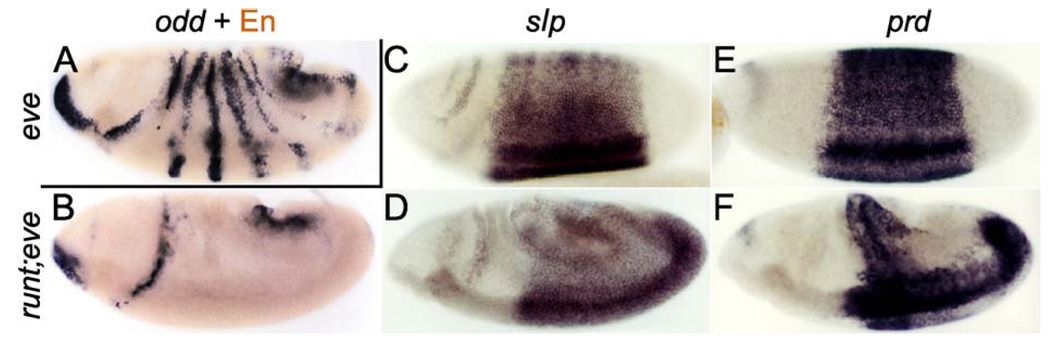
Figure 4.
The role of eve, runt, and odd in limiting slp and prd expression. Embryos either null mutant for eve (A) or double mutant for runt and eve (B–F) were stained (black) by in situ hybridization to RNA for either odd (A, B), slp (C, D), or prd (E, F), followed by either anti-En staining (A, B; brown) or anti-Eve staining (C–F; the embryos shown did not stain, indicating that they are mutant for eve). Note that odd expression, which is present in eve nulls (A), is absent from the trunk region in runt, eve double mutants (B), and that these same double mutants express both slp and prd throughout the trunk (C–F; prd has faded from most dorsal and lateral regions by late stage 8, seen in F).
If the observed phenotypes are in fact due to the interactions described above, and if these interactions are sufficient to account for pair-rule gene cross-regulation, we can make several predictions of dramatic phenotypes that should occur in runt, eve double mutants. First, there should be no odd expression in the trunk region during gastrulation. This is indeed found to be the case (Fig. 4B). Second, we would expect slp to be expressed throughout the trunk region due to the absence of both eve and odd. This prediction is also borne out (Fig. 4C,D). We would also expect all periodicity in the pattern of prd expression to be lost in eve, slp double mutants. We found that this is indeed the case (Fig. 4E,F). Thus, repressive interactions between slp and odd, the repressors of en (and late eve), as well as repression of slp and odd by eve, appear to play an important role in organizing the pair-rule and en expression patterns. These interactions, while they can lead to complex effects, may be functionally rather simple in that eve, odd, and slp repress each others expression, while en (regulated similarly to late eve) is repressed by odd and slp (and possibly runt), and activated by ftz and prd. The upstream interactions that subdivide the embryo into two-segment-wide swaths of repeating pattern in the wild type can be thought of as setting up a repeating pattern of odd, slp, and late eve stripes (including the ‘‘minor’’ eve stripes in the anterior of the ftz domains, which are expressed at the same time as late eve stripes, overlapping the even-numbered en stripes). The segment polarity genes are then activated in a similar repeating pattern. The odd-numbered en stripes essentially follow the late eve pattern, replacing it at later stages. In fact, it has been shown that in the absence of en, late eve expression persists (Harding et al., 1986), consistent with a direct role for en in turning off eve. The wg pattern is a subset of the slp pattern (Grossniklaus et al., 1992). After the wg and en patterns are set up, they are maintained by positive feedback involving the secreted proteins Wg and Hedgehog, while slp and en continue to have roles in the maintenance of these patterns (Cadigan et al., 1994b; Fujioka et al., 2002).
Discussion
Repression of slp and odd by Eve to activate en
Previous genetic studies suggested that the activation of en by eve might be indirect because en stripes are restored in the ftz domains (even-numbered PSs) when both eve and the en repressor odd are simultaneously removed (DiNardo and O’Farrell, 1987), and en stripes are also restored when both eve and the en repressor slp are removed (Riechmann et al., 1997). The latter observation is consistent with a previous model of how eve organizes odd-numbered PSs by repressing different target genes at different concentrations (Fujioka et al., 1995), a model also consistent with the rescue of viable adult flies by expression of only the early, broad stripes of eve (in an eve null background), and the complete rescue of segmentation (in eve null mutants) by a chimeric repressor containing only the eve HD and repressor domains from En (Fujioka et al., 2002). We confirmed this model by showing that the en stripes expressed in eve, slp double mutants are present within odd-numbered parasegmental primordia (Fig. 1P), and that they are activated as early in development as are normal en stripes (Fig. 1D). The latter point also indicates that slp has a primary role in the initial activation of en stripes by eve, rather than acting only later to restrict en stripes, a possibility that was suggested by the fact that en stripes only expand at later stages of germband extension in slp mutants (Cadigan et al., 1994b). Two possible explanations for the delay in en expansion in slp mutants emerged from our studies. One is provided by the fact that odd stripes abut the anterior edges of odd-numbered en stripes in slp mutants due to the lack of retraction of these stripes (Fig. 3B,D). Thus, slp represses odd, and in the absence of slp, expanded odd expression may prevent en stripes from expanding until later stages, when odd expression fades. Another possibility is discussed below.
The complex role of runt in segmentation
A previous model of eve function suggested that runt acts redundantly with slp as a repressor of en to help set the anterior margins of odd-numbered PSs (Fujioka et al., 1995). Eve represses both slp and secondary runt stripes, both of which expand into the eve domains in eve mutants (Fujioka et al., 1995), and ectopic expression of either runt or slp is sufficient to repress en (Cadigan et al., 1994a; Manoukian and Krause, 1993). We tested this model by examining runt, slp double mutants. The interpretation of expression patterns in these mutants is complicated by the fact that runt is a primary pair-rule gene that drastically affects the patterns of expression of the other pair-rule genes. In runt null mutants, the other primary pair-rule genes hairy and eve are both overexpressed, with hairy stripes becoming variably wider, and eve stripes remaining broad well into germband extension, when they are normally restricted to the anterior edges of the odd-numbered PSs (Ingham and Gergen, 1988). Due possibly to indirect effects, ftz, odd, and slp all have drastically affected expression patterns as well. We catalogued these effects of removing runt function, and then looked at how they were altered by simultaneously removing slp function.
The straightforward prediction of the model that runt and slp act redundantly to set the anterior margins of odd-numbered en stripes is that these stripes will expand anteriorly in runt, slp double mutants. Such an anterior expansion means that they would extend into the ftz domains. In runt single mutants, the ftz domains are abnormally narrow, with the exception of ftz domains 1, 4, and 5, which are close to normal width (Lawrence and Johnston, 1989) (Fig. 2J,L; ftz domain 7 is broad, but the effects there appear to be different, and we have not dealt with them here). Consistent with the model, in runt, slp double mutants, en is expressed throughout the ftz domains (Fig. 3H,J).
Functional redundancy between runt and slp?
Due to the crucial functions of runt as a primary pair-rule gene, in runt mutants, the ftz domains are incompletely organized. Rather than slp replacing odd in the posterior half of each even-numbered PS, and odd persisting just anterior to slp, as in the wild type, slp completely replaces odd (Fig. 2). Similarly, in runt, slp double mutants, odd is lost throughout the ftz domains (Fig. 3H). This differs from slp single mutants, where odd persists in the posterior part of the ftz domains and may therefore be responsible for preventing expansion of en until mid-germband extension, when odd expression fades. Thus, the expansion of en in runt, slp double mutants is consistent either with secondary runt stripes providing a redundant function with slp in setting the anterior borders of odd-numbered en stripes, or with odd delimiting these borders when slp is mutated. A third possibility is that both are true, that is, runt and slp may each be required for this function in the wild type, while in the absence of slp, expanded odd expression substitutes for slp, until it fades during germband extension. A way to distinguish between these possibilities might be to examine en expression in odd, slp double (null) mutants to determine whether secondary runt expression is sufficient to prevent en expansion. Unfortunately, these are not available because odd and slp are very close together on the 2nd chromosome.
Either runt, slp, or odd appears to be sufficient to repress the odd-numbered en stripes when ectopically expressed (Cadigan et al., 1994a; Manoukian and Krause, 1993; Saulier-Le Dréan et al., 1998), supporting the possibility of redundancy. However, in eve nulls, both the slp and secondary runt expression patterns expand throughout the eve domains (Fujioka et al., 1995), and in eve, slp double mutants, low level runt expression also occurs throughout the eve domain (data not shown). Despite this, in the absence of slp, en is activated (Fig. 1), perhaps because secondary runt expression is not activated soon enough (it normally becomes detectable just as en is activated) and is relatively weak. This suggests a stringent requirement for slp. Thus, runt may serve only an auxiliary role at this stage of segmentation.
A remaining question is why odd is not present in the ftz domains of runt, slp double mutants (Fig. 3H). The explanation presented here for the loss of odd from the ftz domains of runt nulls is that slp represses it (Fig. 2), so why is odd not maintained there in runt, slp double mutants? As odd is known to be very sensitive to repression by Eve (Manoukian and Krause, 1992), this may be explained by the fact that late eve stripes expand into this region (data not shown), which presumably occurs because slp and runt are absent while prd is present (Fujioka et al., 1995). In wild-type embryos, prd fades from the middle of each ftz domain before en is activated, but as in runt nulls (Baumgartner and Noll, 1990), this does not occur in runt, slp mutants (data not shown). This is consistent with the fact that odd is not there, because prd persists throughout the ftz domain in odd mutants (Baumgartner and Noll, 1990). Apparently, the combination of the persistence of early eve (due to the absence of runt) and the reduced level of ftz (due to expanded hairy expression, which can also be ascribed to the absence of runt), in the absence of slp, results in the activation of late eve and the repression of odd throughout the ftz domain. Thus, in runt, slp mutants, odd is not maintained at a sufficient level to prevent late eve expression, and Eve therefore represses odd. However, the regulation of prd in the ftz domain is complex and may also involve odd-paired (Baumgartner and Noll, 1990; DiNardo and O’Farrell, 1987) or naked cuticle (Mullen and DiNardo, 1995; Zeng et al., 2000). These genes are known to be involved in regulating odd and en in this region (DiNardo and O’Farrell, 1987; Mullen and DiNardo, 1995), but we have not examined their roles.
A model of pair-rule gene interactions
Key observations emerging from these studies are the repressive interactions between slp and odd, which help to explain the phenotype of runt mutants, and the identification of slp as a primary intermediary in the activation of en by eve. Several other important interactions were also revealed by these studies, and are described above and in Fig. 5A and Table 1. When combined with the previously characterized interactions among pair-rule genes, they can account for such complex effects as those seen in runt mutants. They also allow us to understand the process of segmentation more succinctly as a series of interactions that subdivide the trunk region into repeating patterns of en (coincident with late eve stripes), odd, and slp. These patterns come to be mutually exclusive due at least in part to corepressive interactions among the three genes (see Fig. 5), and serve as a template for the expression of other segment polarity genes, such as wg (which is activated within each slp stripe, adjacent to en). The experiments of Fig. 4 constitute a test of the model. The model shows how most periodicity is lost in the ftz domains of runt null mutants, as well as accounting for the loss of periodicity in the eve domains of eve nulls. In neither case is all periodicity lost because hairy, odd, and ftz are still expressed in striped patterns. However, the model predicts that all periodicity in the odd, slp, and prd patterns will be lost in runt, eve double mutants, and this prediction is borne out: both slp and prd are expressed throughout the trunk region in the double mutants, and odd expression is completely lost there. This suggests that the model does, indeed, account for the key interactions between spatially localized factors that act to establish the periodic pattern in Drosophila embryos.
Figure 5.
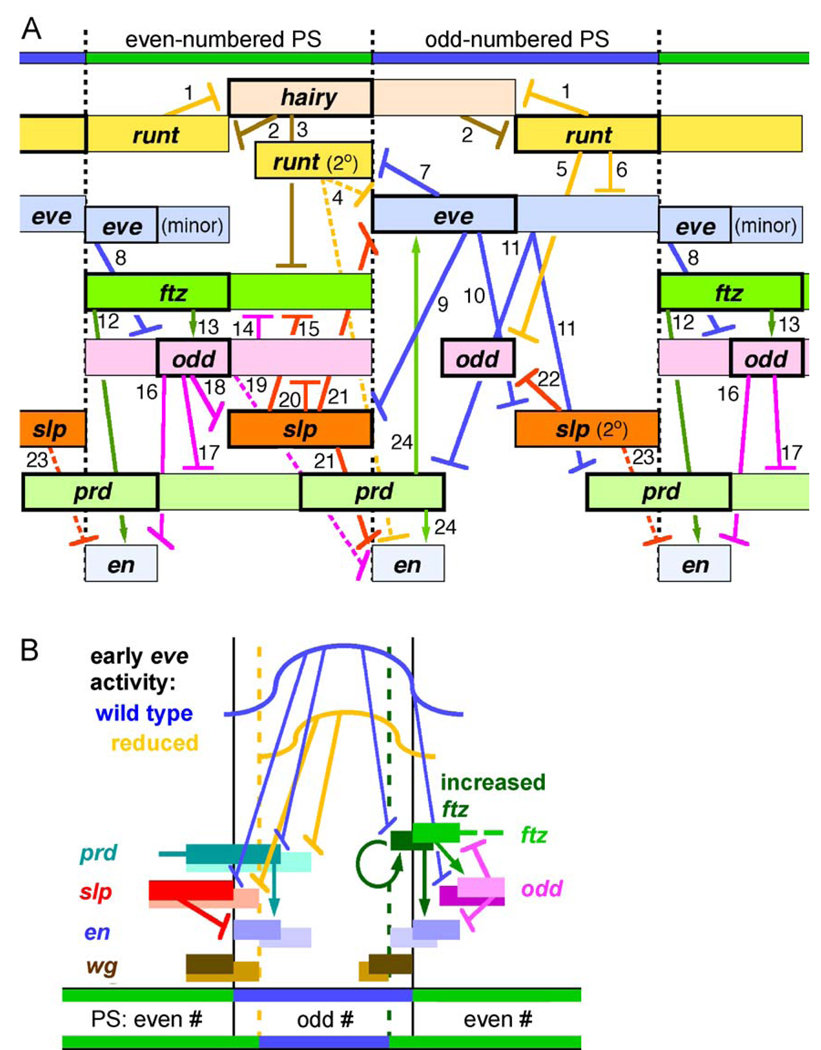
A model of pair-rule gene interactions that position en stripes. Regulatory interactions are represented by arrows (activation) or T-shapes (repression). Anterior is to the left. (A) The anterior and posterior extents of initial expression patterns are represented by colored boxes. Refined patterns that are either activated later or result from subsequent interactions among pair-rule genes are shown either as a heavily outlined box or (in the cases of runt and eve) as a box at a slightly lower position in the diagram (for eve, these “minor” stripes remain weak relative to the thick-outlined late eve stripes). The initial patterns of the primary pair-rule genes hairy, runt, and eve are largely determined by the concentrations of the gap proteins, and ftz also has a strong input from gap proteins. Mutually repressive interactions between hairy and runt further contribute to the formation of their complementary patterns, which are diagrammed here. Regulatory interactions that point downward in the diagram contribute to the initial patterns of expression of downstream genes, while those that point upward generally produce a subsequent refinement of the initial pattern or regulate a part of the pattern that appears later. The experimental justification for each interaction is listed in Table 1, with the exception of number 10, which is as follows: in runt nulls, where eve expression persists abnormally, odd comes on in broad stripes in the eve domain in place of slp (Fig. 2); coupled with the fact that in eve nulls, slp is expressed throughout the eve domain (Fujioka et al., 1995), this suggests that late eve expression is normally responsible for setting the anterior border of secondary slp expression; this function may be taken over by odd as odd represses eve (see 6b, Table 1), and odd may also help to limit the posterior border of odd-numbered en stripes at this stage (Saulier-Le Dréan et al., 1998). Interaction number 4 is shown as a dotted line because, as described more fully in the text, odd expression is lost from the ftz domains in runt nulls as well as in runt, slp double mutants, so that odd may be primarily responsible for keeping en and late eve from expanding anteriorly in slp mutants, while slp may be solely responsible for setting this border in the wild type. Implicit in a number of these regulatory relationships is the fact that an effect of one gene on another may change during the course of refinement of pair-rule gene patterns, and similarly, that an effect in odd-numbered PSs does not imply the corresponding effect in even-numbered PSs, and vice versa. Such complexities can be explained by the existence of distinct regulatory elements in a gene, which drive expression of different aspects of its pattern and respond to distinct regulatory inputs. This is the case for the regulation of eve. In other cases, such as for runt, it may not be possible to dissect the cis-acting sequences into distinct elements (Klingler et al., 1996), and such complexities may be due to multiple factors acting combinatorially through common or overlapping elements. (B) Regulatory interactions can account for the well-established concentration-dependent positioning of PS boundaries by Eve and Ftz (see text for appropriate references). In the anterior half of each odd-numbered PS, the early eve stripe provides a concentration gradient of Eve protein just before and during cellularization of the blastoderm (shown as a blue curve at the top). Reduced Eve activity is represented by the yellow curve; other expression patterns are represented by colored boxes, with altered patterns that result from either decreased eve activity, manifested primarily at the left PS boundary in the diagram, or increased ftz activity, manifested primarily at the right PS boundary, represented by boxes offset below the wild-type patterns. The odd-numbered en stripe is activated by Prd and repressed by Slp, and both prd and slp are repressed by Eve. A high level of Eve is required to repress prd (Fujioka et al., 1995; Manoukian and Krause, 1992), which is activated earlier than slp in the trunk region, while a low level of Eve suffices to repress slp. The anterior border of this en stripe is positioned by the posterior edge of the slp stripe, while its posterior border is positioned by the posterior edge of prd expression, both of which are sensitive to the genetic dose of eve (Fujioka et al., 1999) and to the level of Eve’s repressor activity (Fujioka et al., 2002; Kobayashi et al., 2001). The net result of these interactions is that the positions of both the anterior and posterior borders of the odd-numbered en stripe respond to changes in the level of Eve, with lower Eve levels resulting in reduced-width odd-numbered PSs (and a boundary at the dotted yellow line), and therefore expanded even-numbered PSs. The positions of the even-numbered en stripes are particularly sensitive to the concentration of Ftz. Ftz activates these en stripes, while Odd represses them, setting the anterior and posterior borders, respectively. Higher levels of Ftz (or increased stability of Ftz) result in auto-activation over a wider region (Hiromi and Gehring, 1987; Ish-Horowicz et al., 1989), but also cause an anterior expansion of odd stripes. Eve represses odd at low concentrations, and is also capable of repressing ftz at higher concentrations (DiNardo and O’Farrell, 1987; Fujioka et al., 1995; Manoukian and Krause, 1992), while Odd causes subsequent downregulation of ftz expression. This combination of interactions allows the positions of the even-numbered en stripes to move anteriorly in response to higher Ftz activity, expanding the even-numbered PSs (with a boundary at the dotted green line) at the expense of the odd-numbered ones. Reduced levels of Ftz function or increased levels of Eve function produce the opposite effects of those described above, and mechanisms that are the converse of those described can account for these effects. The positions of the wg stripes move in conjunction with those of the en stripes. At the anterior boundaries of the odd-numbered PSs, wg is repressed by both En (Heemskerk et al., 1991) and late Eve expression (Ingham et al., 1988; Manoukian and Krause, 1992), which shares an anterior border with en (Lawrence et al., 1987). At the anterior boundaries of the even-numbered PSs, wg is repressed by ftz and en (Heemskerk et al., 1991; Ingham et al., 1988; Ish-Horowicz et al., 1989), so that in each case, wg expression is activated just anterior to that of en.
Table 1.
Summary of pair-rule interactions.
| Interaction # (Fig. 5A) | Experimental basis for interaction | Genetic background | Reference |
|---|---|---|---|
| 1 | hairy expression expands | runt− | Ingham and Gergen, 1988; Hartmann et al., 1994 |
| 2 | runt expression expands | hairy− | Ingham and Gergen, 1988 |
| runt is activated by a Hairy-ADa fusion | hs-HairyAct | Jiménez et al., 1996 | |
| 3 | ftz stripes expand, fail to narrow properly | hairy− | Ingham and Gergen, 1988 |
| ftz is activated by a Hairy-ADa fusion | hs-HairyAct | Jiménez et al., 1996 | |
| 4 | both late eve and odd-numbered en stripes expand anteriorly (they do so only later in slp single mutants; however, see legend to Fig. 5A) | runt−; slp− | this work |
| odd-numbered en stripes are very sensitive to repression by Runt | ectopic runt | Aronson et al., 1997; Manoukian and Krause, 1993 | |
| 5 | secondary odd stripes expand posteriorly (they do not in slp−) | runt− | this work (Fig. 2) |
| 6b | early eve stripes persist | runt− | Ingham and Gergen, 1988 |
| eve is rapidly repressed | hs-runt | Manoukian and Krause, 1993 | |
| eve is activated by a Runt-ADa fusion | hs-RunAct | Jiménez et al., 1996 | |
| 7 | secondary (late) runt expression expands throughout the eve domain | eve− | Fujioka et al., 1995 |
| runt is rapidly repressed | hs-eve | Manoukian and Krause, 1992 | |
| 8 | odd fails to retract from the anterior of ftz domains, preventing activation of enc | eve− | Coulter and Wieschaus, 1988; DiNardo and O'Farrell, 1987; Fujioka et al., 1995 |
| odd is rapidly repressed | hs-eve | Manoukian and Krause, 1992 | |
| 9 | slp expression expands throughout the odd-numbered parasegments, preventing activation of en | eve− | Fujioka et al., 1995 (this work, Fig. 1) |
| 10 | (see legend to Fig. 5A) | this work, Fig. 2 | |
| 11 | prd expression expands throughout the eve domain | eve− | Baumgartner and Noll, 1990; Fujioka et al., 1995 |
| prd is rapidly repressed | hs-eve | Manoukian and Krause, 1992 | |
| 12 | even-numbered en stripes are not activated | ftz− | DiNardo and O'Farrell, 1987; Howard and Ingham, 1986 |
| ectopic ftz activates en | hs-ftz | Ish-Horowicz et al., 1989 | |
| 13 | ftz is required to maintain odd expressiond (and thereby to limit slp expression, as seen in runt nulls) | ftz−(runt−) | Nasiadka and Krause, 1999 (this work, Fig. 2) |
| odd is rapidly activated | hs-ftz | Nasiadka and Krause, 1999 | |
| 14 | ftz stripes fail to narrow properlye | odd− | Mullen and DiNardo, 1995 |
| 15 | ftz stripes fail to narrow properlyf | slp− | Cadigan et al., 1994b |
| 16 | odd sets the posterior border of even-numbered en stripes | odd− | DiNardo and O'Farrell, 1987 |
| eve− rescue | Fujioka et al., 1995 | ||
| hs-eve | Manoukian and Krause, 1992 | ||
| 17 | prd persists throughout the ftz domain | odd− | Baumgartner and Noll, 1990; Saulier-Le Dréan et al., 1998 |
| 18g | slp is expressed throughout ftz domains in runt nulls due to failure of ftz to maintain odd expressionh | runt− (ftz−) | this work (Fig. 2) (Nasiadka and Krause, 1999) |
| 19,20 | odd expands, preventing odd-numbered en stripes from expanding anteriorly (until odd fades during germband extension)i | slp− | this work (Fig. 3) |
| 21 | odd-numbered en stripes “come back” when slp is removed from eve− | eve−, slp− | this work (Fig. 1) |
| ectopic slp expression represses en | hs-slp | Cadigan et al., 1994a | |
| 22 | 2° odd stripes missing in eve−, due to expansion of slp | eve− | this work (Fig. 4); (Fujioka et al., 1995) |
| 23 | even-numbered en stripes expand anteriorly late in germband extension | slp− | Cadigan et al., 1994b |
| ectopic slp expression represses en | hs-slp | Cadigan et al., 1994a | |
| 24 | prd activates both late eve and odd-numbered en stripes | eve− | Fujioka et al., 1996; DiNardo and O'Farrell, 1987 |
Activation domain.
eve is also repressed by odd (Saulier-Le Dréan et al., 1998), which may contribute to further narrowing not diagrammed in Fig. 5A (odd stripes in the odd-numbered PSs appear later than those in the even-numbered PSs); in addition, further narrowing occurs as early eve expression fades and late eve is activated by Prd, so that final late eve stripes are essentially coincident with odd-numbered en stripes.
Although shown in Fig. 5A as emanating from the later “minor stripe” aspect of eve expression in the diagram, early eve stripes are probably sufficient for this function (Fujioka et al., 1995).
This regulation is likely to be direct (Nasiadka and Krause, 1999).
This effect may be indirect (Saulier-Le Dréan et al., 1998).
This regulation is likely to be direct (Yu et al., 1999).
ftz is also required for the eve minor stripes (data not shown), which may also limit slp expression.
Ectopic odd was shown to repress slp (Saulier-Le Dréan et al., 1998), but in odd mutants, en is expressed here rather than slp (DiNardo and O'Farrell, 1987), due to its activation at higher levels of ftz (en can then repress slp).
This effect of odd is likely to be direct (Saulier-Le Dréan et al., 1998).
It has been well documented that the relative widths of odd- and even-numbered PSs are regulated by the relative levels of Eve and Ftz at the syncytial blastoderm stage (Frasch et al., 1988; Fujioka et al., 1995). However, only a partial understanding of the mechanisms involved has previously been achieved (Hughes and Krause, 2001). It has been shown that the widths of odd-numbered PSs contract when Eve function is reduced (Frasch et al., 1988; Fujioka et al., 1995), and expand when the dose of eve is increased (Fujioka et al., 1999; Hughes and Krause, 2001), or when Eve’s repressor activity is increased (Fujioka et al., 2002; Kobayashi et al., 2001). Reciprocally, when Ftz is overexpressed (Hughes and Krause, 2001), or when its level is increased by a mutation that increases its stability (Kellerman et al., 1990), the even-numbered PSs expand. In each case, expansion of one set of PSs is at the expense of the other. The primary determinant of these relative widths appears to be the positioning of the PS boundary, which coincides with the anterior borders of the en stripes. The interactions described here are sufficient to account for these effects, as illustrated in Fig. 5B, and explained in the figure legend.
In addition to the interactions shown in the model, which can account for the major effects that occur in the mutant combinations described here, a number of other interactions have been documented. Some of these are described above, but for clarity were not included in Fig. 5, while a number of others have been observed in overexpression experiments and may serve an auxiliary role in sculpting expression patterns. Further, the transitions from gap gene to pair-rule gene control of pattern formation, and that from pair-rule to segment polarity gene control, are not discrete. As a result, gap gene effects persist as primary pair-rule genes exert their effects on each other. Segment polarity gene interactions probably have a role in parallel with that of the later cross-regulatory interactions among pair-rule genes because they can begin almost as soon as their expression patterns become discernable, very soon after cellularization of the blastoderm is complete. Additionally, quite a number of genes known to be involved in early pattern formation along the anterior–posterior axis have not been included here. Most of these probably exert their effects through the genes shown in Fig. 5 (e.g., partner of paired) (Raj et al., 2000), which are usually considered to be the major players that have spatially restricted expression patterns at this stage, but some may not. Nonetheless, this model can serve as a backbone for understanding this complex system of interactions, which represent a crucial step in what is probably the best studied regulatory network guiding the development of multicellular organisms.
Acknowledgments
We thank Manfred Frasch and the Developmental Studies Hybridoma Bank for antibodies (against Eve and En, respectively), and Yukiko Emi-Sarker for excellent technical assistance. Special thanks to Henry Krause for helpful discussions and for his seminal contributions to this field over the years. Thanks also to Steve Small, Henry Krause, and anonymous reviewers for significant contributions to the final form of this report. This work was supported by NSF (0110856) and NIH (GM50231) awards to J.B.J.
References
- Alexandre C, Vincent JP. Requirements for transcriptional repression and activation by Engrailed in Drosophila embryos. Development. 2003;130:729–739. doi: 10.1242/dev.00286. [DOI] [PubMed] [Google Scholar]
- Aronson BD, Fisher AL, Blechman K, Caudy M, Gergen JP. Groucho-dependent and-independent repression activities of Runt domain proteins. Mol. Cell. Biol. 1997;17:5581–5587. doi: 10.1128/mcb.17.9.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin RJ, Biggin MD. A domain of the even-skipped protein represses transcription by preventing TFIID binding to a promoter: repression by cooperative blocking. Mol. Cell. Biol. 1995;15:4683–4693. doi: 10.1128/mcb.15.9.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner S, Noll M. Network of interactions among pair-rule genes regulating paired expression during primordial segmentation of Drosophila. Mech. Dev. 1990;33:1–18. doi: 10.1016/0925-4773(90)90130-e. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Grossniklaus U, Gehring WJ. Functional redundancy: the respective roles of the two sloppy paired genes in Drosophila segmentation. Proc. Natl. Acad. Sci. U. S. A. 1994a;91:6324–6328. doi: 10.1073/pnas.91.14.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadigan KM, Grossniklaus U, Gehring WJ. Localized expression of sloppy paired protein maintains the polarity of Drosophila parasegments. Genes Dev. 1994b;8:899–913. doi: 10.1101/gad.8.8.899. [DOI] [PubMed] [Google Scholar]
- Carroll SB, Scott MP. Zygotically active genes that affect the spatial expression of the fushi tarazu segmentation gene during early Drosophila embryogenesis. Cell. 1986;45:113–126. doi: 10.1016/0092-8674(86)90543-x. [DOI] [PubMed] [Google Scholar]
- Coulter DE, Wieschaus E. Gene activities and segmental patterning in Drosophila: analysis of odd-skipped and pair-rule double mutants. Genes Dev. 1988;2:1812–1823. doi: 10.1101/gad.2.12b.1812. [DOI] [PubMed] [Google Scholar]
- Coulter DE, Swaykus EA, Beran-Koehn MA, Goldberg D, Wieschaus E, Schedl P. Molecular analysis of odd-skipped, a zinc finger-encoding segmentation gene with a novel pair-rule expression pattern. EMBO J. 1990;9:3795–3804. doi: 10.1002/j.1460-2075.1990.tb07593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardo S, O’Farrell PH. Establishment and refinement of segmental pattern in the Drosophila embryo: spatial control of engrailed expression by pair-rule genes. Genes Dev. 1987;1:1212–1225. doi: 10.1101/gad.1.10.1212. [DOI] [PubMed] [Google Scholar]
- Frasch M, Levine M. Complementary patterns of even-skipped and fushi tarazu expression involve their differential regulation by a common set of segmentation genes in Drosophila. Genes Dev. 1987;1:981–995. doi: 10.1101/gad.1.9.981. [DOI] [PubMed] [Google Scholar]
- Frasch M, Hoey T, Rushlow C, Doyle H, Levine M. Characterization and localization of the even-skipped protein of Drosophila. EMBO J. 1987;6:749–759. doi: 10.1002/j.1460-2075.1987.tb04817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch M, Warrior R, Tugwood J, Levine M. Molecular analysis of even-skipped mutants in Drosophila development. Genes Dev. 1988;2:1824–1838. doi: 10.1101/gad.2.12b.1824. [DOI] [PubMed] [Google Scholar]
- Fujioka M, Jaynes JB, Goto T. Early even-skipped stripes act as morphogenetic gradients at the single cell level to establish engrailed expression. Development. 1995;121:4371–4382. doi: 10.1242/dev.121.12.4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka M, Miskiewicz P, Raj L, Gulledge AA, Weir M, Goto T. Drosophila Paired regulates late even-skipped expression through a composite binding site for the paired domain and the homeodomain. Development. 1996;122:2697–2707. doi: 10.1242/dev.122.9.2697. [DOI] [PubMed] [Google Scholar]
- Fujioka M, Emi-Sarker Y, Yusibova GL, Goto T, Jaynes JB. Analysis of an even-skipped rescue transgene reveals both composite and discrete neuronal and early blastoderm enhancers, and multi-stripe positioning by gap gene repressor gradients. Development. 1999;126:2527–2538. doi: 10.1242/dev.126.11.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka M, Yusibova GL, Patel NH, Brown SJ, Jaynes JB. The repressor activity of Even-skipped is highly conserved, and is sufficient to activate engrailed and to regulate both the spacing and stability of parasegment boundaries. Development. 2002;129:4411–4421. doi: 10.1242/dev.129.19.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergen JP, Wieschaus E. Dosage requirements for runt in the segmentation of Drosophila embryos. Cell. 1986;45:289–299. doi: 10.1016/0092-8674(86)90393-4. [DOI] [PubMed] [Google Scholar]
- Goto T, Macdonald P, Maniatis T. Early and late periodic patterns of even skipped expression are controlled by distinct regulatory elements that respond to different spatial cues. Cell. 1989;57:413–422. doi: 10.1016/0092-8674(89)90916-1. [DOI] [PubMed] [Google Scholar]
- Grossniklaus U, Pearson RK, Gehring WJ. The Drosophila sloppy paired locus encodes two proteins involved in segmentation that show homology to mammalian transcription factors. Genes Dev. 1992;6:1030–1051. doi: 10.1101/gad.6.6.1030. [DOI] [PubMed] [Google Scholar]
- Han K, Manley JL. Transcriptional repression by the Drosophila even-skipped protein: definition of a minimal repression domain. Genes Dev. 1993;7:491–503. doi: 10.1101/gad.7.3.491. [DOI] [PubMed] [Google Scholar]
- Hanna-Rose W, Hansen U. Active repression mechanisms of eukaryotic transcription repressors. Trends Genet. 1996;12:229–334. doi: 10.1016/0168-9525(96)10022-6. [DOI] [PubMed] [Google Scholar]
- Harding K, Rushlow C, Doyle HJ, Hoey T, Levine M. Cross-regulatory interactions among pair-rule genes in Drosophila. Science. 1986;233:953–959. doi: 10.1126/science.3755551. [DOI] [PubMed] [Google Scholar]
- Harding K, Hoey T, Warrior R, Levine M. Autoregulatory and gap gene response elements of the even-skipped promoter of Drosophila. EMBO J. 1989;8:1205–1212. doi: 10.1002/j.1460-2075.1989.tb03493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann C, Taubert H, Jackle H, Pankratz MJ. A two-step mode of stripe formation in the Drosophila blastoderm requires interactions among primary pair rule genes. Mech. Dev. 1994;45:3–13. doi: 10.1016/0925-4773(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Heemskerk J, DiNardo S, Kostriken R, O’Farrell PH. Multiple modes of engrailed regulation in the progression towards cell fate determination. Nature. 1991;352:404–410. doi: 10.1038/352404a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiromi Y, Gehring WJ. Regulation and function of the Drosophila segmentation gene fushi tarazu. Cell. 1987;50:963–974. doi: 10.1016/0092-8674(87)90523-x. [DOI] [PubMed] [Google Scholar]
- Howard K, Ingham P. Regulatory interactions between the segmentation genes fushi tarazu, hairy, and engrailed in the Drosophila blastoderm. Cell. 1986;44:949–957. doi: 10.1016/0092-8674(86)90018-8. [DOI] [PubMed] [Google Scholar]
- Hughes SC, Krause HM. Establishment and maintenance of parasegmental compartments. Development. 2001;128:1109–1118. doi: 10.1242/dev.128.7.1109. [DOI] [PubMed] [Google Scholar]
- Ingham PW, Gergen JP. Interactions between the pair-rule genes runt, hairy, even-skipped and fushi tarazu and the establishment of periodic pattern in the Drosophila embryo. Development. 1988;104:51–60. [Google Scholar]
- Ingham PW, Baker NE, Martinez-Arias A. Regulation of segment polarity genes in the Drosophila blastoderm by fushi tarazu and even skipped. Nature. 1988;331:73–75. doi: 10.1038/331073a0. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D, Pinchin SM, Ingham PW, Gyurkovics HG. Autocatalytic ftz activation and metameric instability induced by ectopic ftz expression. Cell. 1989;57:223–232. doi: 10.1016/0092-8674(89)90960-4. [DOI] [PubMed] [Google Scholar]
- Jaynes JB, O’Farrell PH. Activation and repression of transcription by homeodomain-containing proteins that bind a common site. Nature. 1988;336:744–749. doi: 10.1038/336744a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez G, Pinchin SM, Ish-Horowicz D. In vivo interactions of the Drosophila Hairy and Runt transcriptional repressors with target promoters. EMBO J. 1996;15:7088–7098. [PMC free article] [PubMed] [Google Scholar]
- Jiménez G, Paroush Z, Ish-Horowicz D. Groucho acts as a corepressor for a subset of negative regulators, including Hairy and Engrailed. Genes Dev. 1997;11:3072–3082. doi: 10.1101/gad.11.22.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellerman KA, Mattson DM, Duncan I. Mutations affecting the stability of the fushi tarazu protein of Drosophila. Genes Dev. 1990;4:1936–1950. doi: 10.1101/gad.4.11.1936. [DOI] [PubMed] [Google Scholar]
- Klingler M, Soong J, Butler B, Gergen JP. Disperse versus compact elements for the regulation of runt stripes in Drosophila. Dev. Biol. 1996;177:73–84. doi: 10.1006/dbio.1996.0146. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Goldstein RE, Fujioka M, Paroush Z, Jaynes JB. Groucho augments the repression of multiple Even skipped target genes in establishing parasegment boundaries. Development. 2001;128:1805–1815. doi: 10.1242/dev.128.10.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Fujioka M, Tolkunova EN, Deka D, Abu-Shaar M, Mann RS, Jaynes JB. Engrailed cooperates with extradenticle and homothorax to repress target genes in Drosophila. Development. 2003;130:741–751. doi: 10.1242/dev.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence PA, Johnston P. Pattern formation of the Drosophila embryo: allocation of cells to parasegments by even-skipped and fushi tarazu. Development. 1989;105:761–767. doi: 10.1242/dev.105.4.761. [DOI] [PubMed] [Google Scholar]
- Lawrence PA, Johnston P, Macdonald P, Struhl G. Borders of parasegments in Drosophila embryos are delimited by the fushi tarazu and even-skipped genes. Nature. 1987;328:440–442. doi: 10.1038/328440a0. [DOI] [PubMed] [Google Scholar]
- Li C, Manley JL. Allosteric regulation of Even-skipped repression activity by phosphorylation. Mol. Cell. 1999;3:77–86. doi: 10.1016/s1097-2765(00)80176-8. [DOI] [PubMed] [Google Scholar]
- Macdonald PM, Ingham P, Struhl G. Isolation, structure, and expression of even-skipped: a second pair-rule gene of Drosophila containing a homeo box. Cell. 1986;47:721–734. doi: 10.1016/0092-8674(86)90515-5. [DOI] [PubMed] [Google Scholar]
- Manoukian AS, Krause HM. Concentration-dependent activities of the even-skipped protein in Drosophila embryos. Genes Dev. 1992;6:1740–1751. doi: 10.1101/gad.6.9.1740. [DOI] [PubMed] [Google Scholar]
- Manoukian AS, Krause HM. Control of segmental asymmetry in Drosophila embryos. Development. 1993;118:785–796. doi: 10.1242/dev.118.3.785. [DOI] [PubMed] [Google Scholar]
- Mullen JR, DiNardo S. Establishing parasegments in Drosophila embryos: roles of the odd-skipped and naked genes. Dev. Biol. 1995;169:295–308. doi: 10.1006/dbio.1995.1145. [DOI] [PubMed] [Google Scholar]
- Nasiadka A, Krause HM. Kinetic analysis of segmentation gene interactions in Drosophila embryos. Development. 1999;126:1515–1526. doi: 10.1242/dev.126.7.1515. [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- Pazdera TM, Janardhan P, Minden JS. Patterned epidermal cell death in wild-type and segment polarity mutant Drosophila embryos. Development. 1998;125:3427–3436. doi: 10.1242/dev.125.17.3427. [DOI] [PubMed] [Google Scholar]
- Raj L, Vivekanand P, Das TK, Badam E, Fernandes M, Finley RL, Brent R, Appel LF, Hanes SD, Weir M. Targeted localized degradation of Paired protein in Drosophila development. Curr. Biol. 2000;10:1265–1272. doi: 10.1016/s0960-9822(00)00745-4. [DOI] [PubMed] [Google Scholar]
- Riechmann V, Irion U, Wilson R, Grosskortenhaus R, Leptin M. Control of cell fates and segmentation in the Drosophila mesoderm. Development. 1997;124:2915–2922. doi: 10.1242/dev.124.15.2915. [DOI] [PubMed] [Google Scholar]
- Saulier-Le Dréan B, Nasiadka A, Dong J, Krause HM. Dynamic changes in the functions of Odd-skipped during early Drosophila embryogenesis. Development. 1998;125:4851–4861. doi: 10.1242/dev.125.23.4851. [DOI] [PubMed] [Google Scholar]
- Tautz D, Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989;98:81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Tracey WD, Jr, Ning X, Klingler M, Kramer SG, Gergen JP. Quantitative analysis of gene function in the Drosophila embryo. Genetics. 2000;154:273–284. doi: 10.1093/genetics/154.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C, Gergen JP. Gap gene properties of the pair-rule gene runt during Drosophila segmentation. Development. 1994;120:1671–1683. doi: 10.1242/dev.120.6.1671. [DOI] [PubMed] [Google Scholar]
- Um M, Li C, Manley JL. The transcriptional repressor Even-skipped interacts directly with TATA-binding protein. Mol. Cell. Biol. 1995;15:5007–5016. doi: 10.1128/mcb.15.9.5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Yussa M, Song J, Hirsch J, Pick L. A double interaction screen identifies positive and negative ftz gene regulators and Ftz-interacting proteins. Mech. Dev. 1999;83:95–105. doi: 10.1016/s0925-4773(99)00038-6. [DOI] [PubMed] [Google Scholar]
- Zeng W, Wharton KA, Jr, Mack JA, Wang K, Gadbaw M, Suyama K, Klein PS, Scott MP. Naked cuticle encodes an inducible antagonist of Wnt signalling. Nature. 2000;403:789–795. doi: 10.1038/35001615. [DOI] [PubMed] [Google Scholar]
- Zhang S, Xu L, Lee J, Xu T. Drosophila Atrophin homolog functions as a transcriptional corepressor in multiple developmental processes. Cell. 2002;108:45–56. doi: 10.1016/s0092-8674(01)00630-4. [DOI] [PubMed] [Google Scholar]