Abstract
We measured concentration-detection (i.e., psychometric) odor functions for the homologous ketones propanone (acetone), 2-pentanone, 2-heptanone, and 2-nonanone. Under a forced-choice procedure, stimuli were presented via an 8-channel air-dilution olfactometer that allowed natural sampling of the odorant and whose output was quantified by gas chromatography. Subjects (17 to 22 per compound) comprised young adults from both genders, all normosmics and nonsmokers. A sigmoid (logistic) equation tightly fitted group and individual functions. The odor detection threshold (ODT) was the concentration detectable at halfway (P=0.5) between chance (P=0.0) and perfect (P=1.0) detection. Odor sensitivity increased (i.e., thresholds decreased) from acetone to heptanone, remaining constant for nonanone. This relative trend was also observed in previous work and in odor thresholds compilations, but the absolute ODTs obtained here were consistently at the lower end of those reported before. Interindividual variability of ODTs was about one order of magnitude. These odor functions measured behaviorally in humans were obtained at vapor concentrations 1,000 times lower than functions measured via activation, with similar 2-ketones, of receptor neurons converging into individual olfactory glomeruli of mice, visualized with calcium sensitive dyes. Odorant concentrations presented as vapors (as in behavioral studies) and those presented as liquids (as in cellular/tissue studies) can be rendered equivalent via liquid-vapor partition coefficients and, then, compared in relative olfactory potency. These comparisons can reveal how sensitivity is progressively shaped across levels of the neural pathway.
Keywords: Concentration-detection odor functions, Homologous 2-ketones, Olfactory structure-activity relationships, Odor detection thresholds
Introduction
The study of odorant sensitivity in preparations in vitro, although necessary for elucidating the details of the stimulus-receptor interaction, may not always reflect the observed sensitivity in vivo of the behaving organism [38]. In this regard, the coherent psychophysical measurement of human olfactory sensitivity to a wide variety of volatile organic compounds (VOCs) possesses special basic and applied importance. The outcome can be used to guide the development of quantitative structure-activity relationships (QSARs) able to describe and predict odor thresholds in humans [1, 2, 4]. One convenient and orderly strategy to probe into the odor potency of the vast number of VOCs is to study homologous series of chemicals [11]. Here, we explore the olfactory detectability of selected members of the aliphatic 2-ketone series.
At the molecular level, the human olfactory receptors hOR 52D1 and hOR 1G1 have been shown to be activated by the ketones 2- and 3-nonanone, 2-decanone, and acetophenone, among a diverse set of odorants of various chemical functionalities [33]. The human olfactory receptor hOR 17-210, despite being identified as a pseudogene, has been shown to be activated by a mixture of ketones (acetophenone, camphor, beta-ionone) and by a six-odorant mixture that included the ketones camphor, acetophenone, and 2-heptanone [24, 29]. The mouse receptor mOR 912-93 was activated by aliphatic 2- and 3- ketones, and showed high sensitivity to 2-heptanone, although the human ortholog was inactive [18]. Orthologs of OR 912-93 from pig and four primate species, but not those from orangutan and human [19], also responded to aliphatic ketones. An investigation suggested that the binding of ketones to the mouse mOR 912-13 is dominated by a hydrogen bond between the carbonyl group of the ketones and a Ser105 in the receptor [20]. The human ortholog has a Gly at the position Ser105, which would make the binding much weaker rendering the ketones ineffective.
At the behavioral level, our previous work showed that human olfactory sensitivity to homologous aliphatic ketones increased with carbon chain length from 2-propanone (acetone) to 2-heptanone, but failed to increase further for 2-nonanone [10]. In this former study, the outcome consisted of odor detection thresholds (ODTs) measured under a rather conservative criterion. Vapors were delivered from squeeze bottles and quantified by gas chromatography. A similar increase in odor sensitivity (measured as ODTs) with increasing carbon chain length of aliphatic 2-ketones was observed behaviorally in squirrel monkeys and pigtail macaques [27]. In studies of human occupational health relevance, odor thresholds for the short carbon-chain ketone acetone were higher (i.e., sensitivity was lower) than for the longer ketone methyl isobutyl ketone [16, 42]. In a study on anesthetized mice, the investigators used calcium-sensitive dyes to track receptor neuron input to individual glomeruli in the olfactory bulb and measured concentration-response functions to 2-butanone and 2-hexanone among other odorants [41]. Stimuli were presented in the vapor phase and concentrations were calculated from vapor pressures in the literature. For all glomeruli tested, sensitivity to 2-hexanone was higher than for 2-butanone, such that functions for the longer-chain ketone were displaced towards lower concentrations compared to the shorter-chain ketone, but, within each glomerulus, slopes for the two ketones were similar.
In the present study, rather than measuring ODTs according to a fixed criterion, the outcome entailed defining, both at the group and at the individual level, the full concentration-detection (i.e., psychometric) function for each of four homologous ketones: propanone (acetone), 2-pentanone, 2-heptanone, and 2-nonanone. The thorough procedure employed followed that used recently to test homologous n-alcohols and acetate esters [13, 15].
Materials and Methods
An institutional review board at the University of California, San Diego, approved the protocol for all experiments described here. All participants provided written informed consent.
Stimuli
The following vapors (purity in parenthesis, FCC: Food Chemical Codex quality) were tested: propanone, i.e., acetone (99+%, FCC), 2-pentanone (98+%, FCC), 2-heptanone (98+%, FCC), and 2-nonanone (99+%, FCC). The stimuli were selected as representative of the homologous aliphatic 2-ketone series.
Subjects
We recruited a group of 39 subjects (18 female), average age (±SD): 24 (±4) years, ranging from 18 to 35 years. All were nonsmokers and performed in the normosmic range on a clinical olfactory test [8]. Not all participants were available to be tested with all four chemicals. Nevertheless, a common group of six subjects (4 female) were available for testing with all four ketones. Table 1 presents the characteristics of this common group and of the subgroup tested with each stimulus.
Table 1.
Characteristic of subject groups.
| Subject groups | Number of subjects | Average Age (±SD) (years) | Age range (years) | Number of males | Number of females |
|---|---|---|---|---|---|
| Acetone | 17 | 24 (±5) | 18–35 | 8 | 9 |
| 2-Pentanone | 22 | 25 (±4) | 20–35 | 11 | 11 |
| 2-Heptanone | 18 | 27 (±5) | 19–35 | 9 | 9 |
| 2-Nonanone | 19 | 24 (±4) | 19–35 | 9 | 10 |
| Common subjects | 6 | 28 (±4) | 22–35 | 2 | 4 |
Apparatus and Procedure
Odorants were delivered by dynamic olfactometry employing an 8-station vapor delivery device (VDD-8). In a session, up to 6 subjects were simultaneously tested with one chemical during the course of the day (6 to 7 hs.). At the end of the session (day) each subject had provided 35 trials per concentration of the chemical and, thus, had completed testing with that chemical. The instrument and procedure have been recently described [9, 13, 14]. In summary, the salient features of the VDD-8 include [34]: 1) The stimulus source is the neat chemical, thus, no solvent needs to be used. 2) All odorant lines (made of stainless steel, glass, and Teflon) see the same concentration since dilution is achieved at the very end of the line, in a glass cone where the subject samples (sniffs). 3) Each station consists of a three-alternative (three sampling cones) forced-choice procedure against carbon-filtered air. 4) To minimize adaptation, we use an ascending concentration approach (2-fold steps between stations) where participants move in order from station 8 (the lowest concentration) to station 1 (the highest concentration). In addition, subjects are exposed just seconds at a time: 5 sec of actual stimulus out of 15 sec total exposure on any station (due to the two air blanks), with an additional 15 sec interval before moving to the next station. After finishing with the last station, a 5–10 min resting period under clean air is enforced before the process is repeated. 5) The unrestricted and simple interface between subjects and device provides an environmentally realistic exposure [22, 23] that meets the stimulus demands of natural odor sampling (sniffing) behavior [25, 26]. Gas chromatography (flame ionization detector, FID) is used for chemical-analytical quantification and control of the vapor stimulus line during actual testing. 6) To help keep an odorless background, the room containing the VDD-8, where subjects are tested, is ventilated at 330 L/sec, approximately 17 ach (air changes per hour) and no air is recirculated into the room (all entering air is fresh). In addition, local air extraction is in place directly above the outlet of the cones [9]. 7) Up to 8 subjects can be tested simultaneously during the course of the day, providing for high testing efficiency and a large amount of individual data. During testing, subjects were supervised by at least one, and sometimes two, experimenters. They made sure that each participant followed instructions and focused exclusively on his/her scoresheet. 8) The final outcome can be expressed not only as an odor detection threshold (ODT) value but also as a concentration-detection (i.e., psychometric) function that can be analyzed both at the individual and at the group level.
In addition to choosing the cone smelling different from the other two in each station, participants had to assign a confidence rating to their decision. The rating scale ranged from “1”, i.e., not confident at all, just guessing, to “5”, i.e., extremely confident.
Gas chromatography served to quantify the vapors delivered by the VDD-8, via creation of a calibration curve for mass for each ketone [12]. The concentration of the vapor stimulus line was measured before starting and one to two times per hour during every testing session (day). The average coefficient of variation of these vapor concentrations across testing sessions (days) equaled 28% for acetone, 4.4% for 2-pentanone, 16% for 2-heptanone, and 15% for 2-nonanone. The range of concentrations, in seven binary steps, tested for each ketone was as follows: For acetone, 29 to 3,653 ppb by volume; for 2-pentanone, 6.9 to 889 ppb; for 2-heptanone, 0.19 to 25 ppb; and for 2-nonanone, 0.26 to 33 ppb.
Data analysis
The results are presented as plots of detection probability, i.e., detectability (P), and confidence rating as a function of vapor concentration (log ppb by volume). Detectability, corrected for chance, ranged from a value of P = 0.0 (i.e., chance detection) to a value of P = 1.0 (i.e., perfect detection), according to:
| Equation (1) |
where P = detection probability corrected for chance, m = number of choices per trial (here, three), and p(c) = proportion correct (i.e., number of correct trials/total number of trials) [28].
Concentration-detection, that is, psychometric, functions were modeled, both at the group and at the individual level, by a sigmoid (logistic) equation of the form:
| Equation (2) |
where P = detection probability (0 ≤ P ≤ 1), Pmax = 1.0, x = vapor concentration (in log ppb by volume), and C and D are constants. C is the value of x when P=0.5, that is, when detection probability is half way between chance (P=0.0) and perfect (P=1.0) detection. This value was taken as the odor detection threshold (ODT). In turn, the constant D defines the steepness of the function. Statistical significance was established by analysis of variance (ANOVA) (SuperANOVA v.1.11, Abacus Concepts, Inc., Berkeley, CA).
Results
Figure 1, left, presents the group average psychometric function for each ketone. The odor potency of the ketones increased with carbon chain length up to heptanone, as reflected by the progressive shift to the left (towards lower concentrations) of the respective psychometric functions. Heptanone and nonanone showed mostly overlapping functions. Logically, as odor potency increased, ODTs decreased (Table 2). Figure 1, right, shows plots of group average confidence rating as a function of vapor concentration for the ketones. As expected, the increase in confidence ratings closely paralleled the increase in detectability (Figure 1, left) for every stimulus. Table 2, upper section, quantifies the group function for each ketone in terms of the values of C (±standard error, SE) and D (±SE) from equation (2), R2 as a measure of goodness of fit, and the antilog of C which is the odor detection threshold (ODT) in concentration units (ppb). The lower section of Table 2 presents analogous data but for the subgroup of 6 subjects tested in common across all ketones. The similarity between both sets of data provides support to the comparability across odorants within the study.
Figure 1.
Group concentration-detection (i.e., psychometric) odor functions (left) and confidence ratings as a function of concentration (right) for the four ketones. Psychometric functions were modeled by the sigmoid equation (2). For acetone, each point represents the average of 595 trials made by 17 subjects; for 2-pentanone, 770 trials by 22 subjects; for 2-heptanone, 630 trials by 18 subjects; and for 2-nonanone, 665 trials by 19 subjects. Bars indicate standard errors (SE).
Table 2.
Upper section. Values describing the group psychometric function (n = number of subjects) for each ketone, including odor detection threshold (ODT), the constants C and D, with their respective standard error (SE), and the square of the correlation coefficient (R2). Lower section. Same as above but for the group of six common subjects tested with all ketones.
| All subjects | |||||||
|---|---|---|---|---|---|---|---|
| n | ODT (ppb) | C (log ppb) | SE (C) | D | SE (D) | R2 | |
| Acetone | 17 | 832 | 2.92 | ±0.024 | 0.27 | ±0.022 | 0.993 |
| 2-Pentanone | 22 | 100 | 2.00 | ±0.013 | 0.23 | ±0.012 | 0.998 |
| 2-Heptanone | 18 | 4.8 | 0.68 | ±0.024 | 0.27 | ±0.022 | 0.993 |
| 2-Nonanone | 19 | 5.5 | 0.74 | ±0.018 | 0.29 | ±0.017 | 0.996 |
| Common Subjects | |||||||
| n | ODT (ppb) | C (log ppb) | SE (C) | D | SE (D) | R2 | |
| Acetone | 6 | 794 | 2.90 | ±0.041 | 0.26 | ±0.037 | 0.979 |
| 2-Pentanone | 6 | 100 | 2.00 | ±0.017 | 0.18 | ±0.015 | 0.996 |
| 2-Heptanone | 6 | 4.5 | 0.65 | ±0.031 | 0.21 | ±0.027 | 0.988 |
| 2-Nonanone | 6 | 6.6 | 0.82 | ±0.023 | 0.21 | ±0.020 | 0.993 |
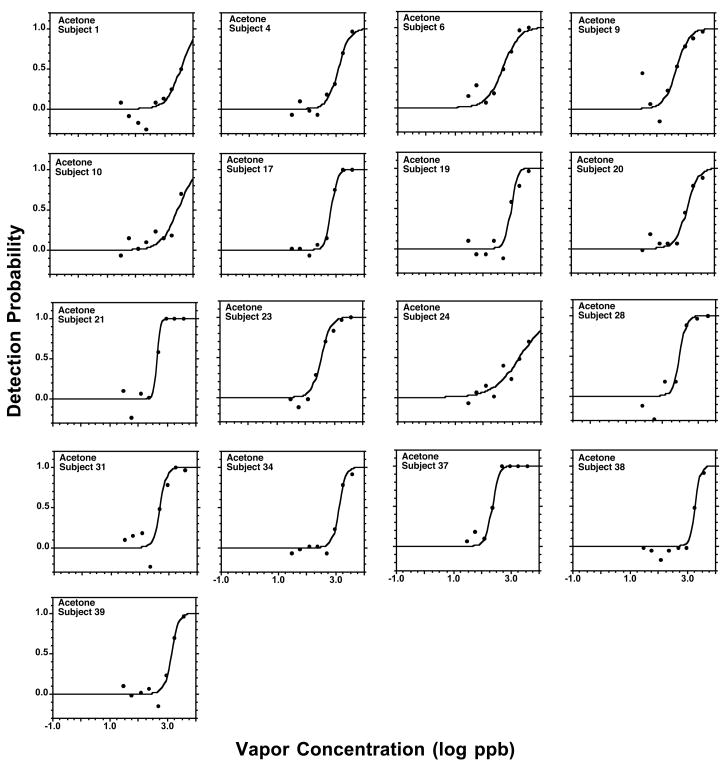
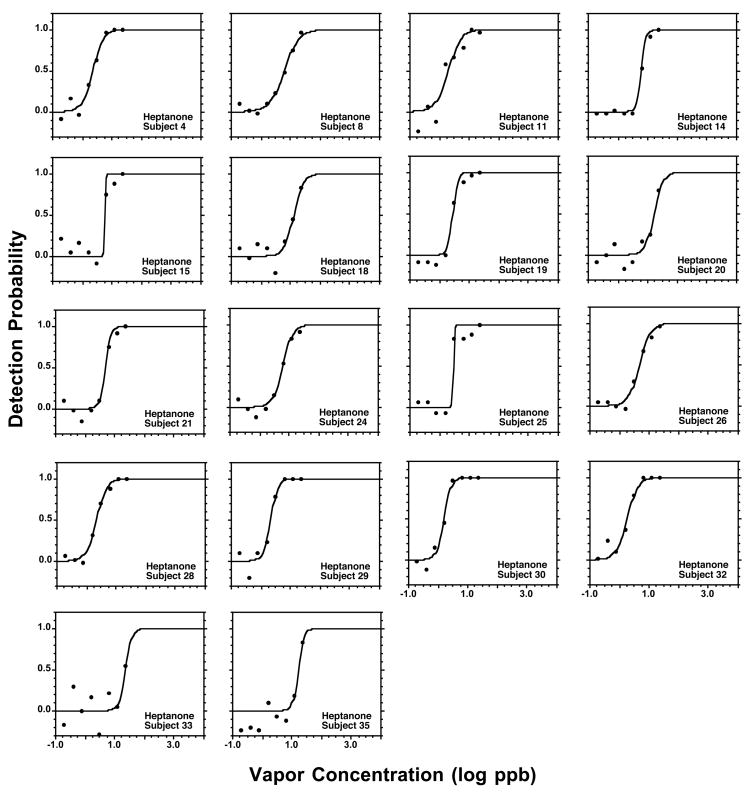
Figures 2 to 5 present individual psychometric functions for the ketones. Each subject was assigned a unique number, so the performance of participants tested on more than one ketone can be followed across chemicals. In turn, Table 3 provides a quantification of each individual function in terms of the constants C and D, and of R2. The outcome shows that the sigmoid equation (2) provides a very adequate fit to the experimental data, both at the group and individual level. Out of the 76 individual functions across all ketones (Figures 2 to 5) in only one case a participant (Subject 7, nonanone) did not achieve, even at the highest concentration tested, a detectability of P=0.5. Still, the participant was quite close, achieving at the highest concentration a detectability of P=0.4 in a context of a continuous increase in detection across the 3 highest concentrations.
Figure 2.
Individual psychometric odor functions for acetone. Each point represents the outcome of 35 trials made by that participant.
Figure 5.
Analogous to Figure 2 but for 2-nonanone.
Table 3.
Quantification of each individual psychometric odor function shown in Figures 2 to 5 in terms of the values of C (i.e., the ODT in log ppb), D, and R2, as fitted by equation (2).
| Acetone (n=17) | Pentanone (n=22) | Heptanone (n=18) | Nonanone (n=19) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | C (log ppb) | D | R2 | Subject | C (log ppb) | D | R2 | Subject | C (log ppb) | D | R2 | Subject | C (log ppb) | D | R2 |
| 1 | 3.55 | 0.25 | 0.72 | 3 | 2.59 | 0.11 | 0.84 | 4 | 0.36 | 0.18 | 0.97 | 2 | 1.06 | 0.25 | 0.93 |
| 4 | 3.09 | 0.19 | 0.97 | 4 | 1.95 | 0.09 | 0.96 | 8 | 0.80 | 0.25 | 0.98 | 4 | 1.01 | 0.16 | 0.90 |
| 6 | 2.68 | 0.28 | 0.91 | 5 | 2.29 | 0.15 | 0.93 | 11 | 0.30 | 0.21 | 0.89 | 7 | 1.61 | 0.27 | 0.66 |
| 9 | 2.67 | 0.22 | 0.78 | 8 | 1.91 | 0.14 | 0.98 | 14 | 0.78 | 0.08 | 0.99 | 8 | 0.87 | 0.30 | 0.95 |
| 10 | 3.45 | 0.28 | 0.75 | 11 | 1.64 | 0.20 | 0.98 | 15 | 0.77 | 0.02 | 0.92 | 12 | 0.53 | 0.34 | 0.84 |
| 17 | 2.85 | 0.11 | 0.99 | 14 | 2.18 | 0.37 | 0.85 | 18 | 1.12 | 0.17 | 0.86 | 13 | 0.31 | 0.11 | 0.95 |
| 19 | 2.96 | 0.11 | 0.93 | 16 | 2.21 | 0.10 | 0.95 | 19 | 0.45 | 0.08 | 0.98 | 16 | 0.24 | 0.15 | 0.99 |
| 20 | 3.03 | 0.19 | 0.94 | 17 | 1.98 | 0.15 | 0.98 | 20 | 1.22 | 0.15 | 0.88 | 19 | 0.48 | 0.25 | 0.89 |
| 21 | 2.64 | 0.06 | 0.96 | 19 | 1.88 | 0.15 | 0.97 | 21 | 0.69 | 0.10 | 0.97 | 20 | 0.79 | 0.22 | 0.97 |
| 23 | 2.55 | 0.17 | 0.98 | 20 | 2.23 | 0.06 | 0.97 | 24 | 0.79 | 0.17 | 0.97 | 21 | 0.74 | 0.23 | 0.95 |
| 24 | 3.26 | 0.47 | 0.84 | 21 | 2.17 | 0.21 | 0.86 | 25 | 0.47 | 0.02 | 0.96 | 22 | 0.51 | 0.10 | 0.86 |
| 28 | 2.49 | 0.10 | 0.94 | 22 | 1.58 | 0.14 | 0.96 | 26 | 0.68 | 0.19 | 0.98 | 23 | 0.61 | 0.19 | 0.96 |
| 31 | 2.70 | 0.12 | 0.88 | 23 | 1.69 | 0.14 | 0.98 | 28 | 0.35 | 0.17 | 0.99 | 24 | 1.09 | 0.12 | 0.97 |
| 34 | 3.11 | 0.12 | 0.98 | 24 | 2.02 | 0.11 | 0.99 | 29 | 0.33 | 0.12 | 0.97 | 27 | 1.07 | 0.24 | 0.94 |
| 37 | 2.35 | 0.12 | 0.97 | 27 | 2.13 | 0.17 | 0.98 | 30 | 0.20 | 0.12 | 0.99 | 28 | 0.73 | 0.09 | 0.99 |
| 38 | 3.28 | 0.09 | 0.96 | 28 | 1.65 | 0.11 | 0.98 | 32 | 0.27 | 0.18 | 0.96 | 31 | 0.77 | 0.23 | 0.92 |
| 39 | 3.15 | 0.13 | 0.96 | 30 | 1.98 | 0.11 | 0.98 | 33 | 1.37 | 0.11 | 0.45 | 34 | 0.50 | 0.20 | 0.98 |
| 31 | 2.11 | 0.09 | 0.96 | 35 | 1.24 | 0.09 | 0.79 | 36 | 1.04 | 0.30 | 0.85 | ||||
| 32 | 2.05 | 0.27 | 0.96 | 37 | 0.43 | 0.17 | 0.89 | ||||||||
| 33 | 2.42 | 0.24 | 0.87 | ||||||||||||
| 34 | 1.66 | 0.12 | 0.97 | ||||||||||||
| 37 | 1.85 | 0.10 | 0.95 | ||||||||||||
| Average | 2.93 | 0.18 | 2.01 | 0.15 | 0.68 | 0.13 | 0.76 | 0.21 | |||||||
| S.E. | 0.08 | 0.02 | 0.06 | 0.02 | 0.09 | 0.01 | 0.08 | 0.02 | |||||||
The outcome of a two-way ANOVA for the factors gender and ketone on the individual values of C (i.e., the ODT in log ppb) revealed a significant effect for ketone {F(3,68) = 191, p<0.001} but not for gender or the gender x ketone interaction. Post-hoc tests showed that the value of C differed significantly between all pairs of ketones (P<0.05) except heptanone vs. nonanone. The results provide statistical support to the data shown in Figure 1 and Tables 2 and 3, where ODTs across ketones decrease with carbon chain length form acetone to heptanone but remain similar for heptanone and nonanone. Despite the fact that the average of individual values of D (i.e., the steepness of the function) varied little across ketones (see Table 3), an ANOVA on these values for the factors gender and ketone showed a significant effect for ketone {F(3,68) = 3.055, p = 0.034} but not for gender or the interaction. In this case, post-hoc tests revealed that only the D values for nonanone and heptanone were significantly different (p = 0.05, Bonferroni/Dunn, all means).
Discussion
Group average data
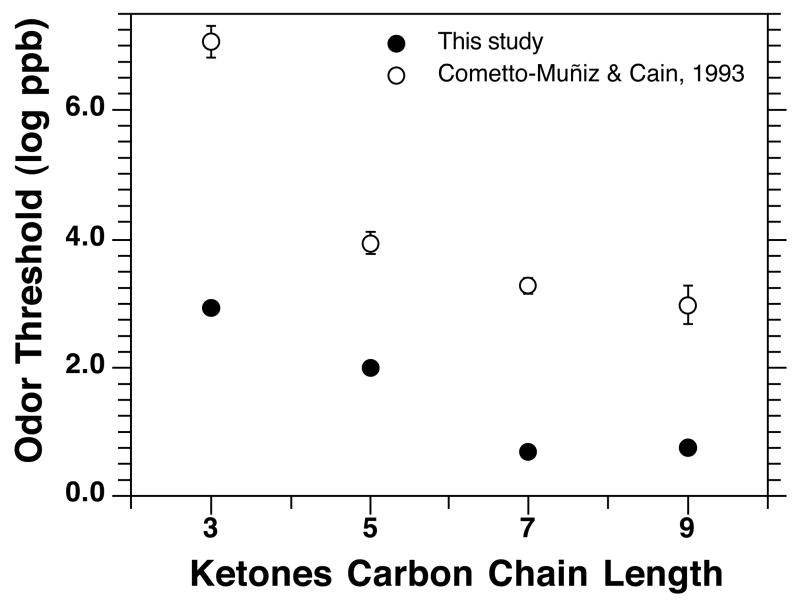
In terms of ODTs, Figure 6 compares the values obtained here with those of our previous investigation. In both cases, thresholds decline across homologous ketones, approaching a plateau at the level of 2-heptanone. Nevertheless, the present thresholds are lower by 4 orders of magnitude for acetone, and between 2 and 2.5 orders of magnitude for the other three ketones. A similar comparative outcome between former and present ODTs has been observed and discussed for homologous n-alcohols and acetate esters [13, 15]. We conclude that the previous delivery system and methodology captured well the relative odor potency of homologs within each series but produced artificially high thresholds. As shown here and in the recent studies cited, the improvements implemented in the generation, delivery, control, and reliability of the vapor stimulus, added to the measurement of full psychometric functions, have resulted in odor thresholds that better reflect human absolute odor sensitivity. Figure 7 illustrates how the present ODTs for the ketones fall among the lowest values from those listed, for the same odorants, in two comprehensive compilations of odor detection thresholds in air [17, 39].
Figure 6.
Group average odor detection thresholds (ODTs) reported now (filled circles) and previously [10] (empty circles) for homologous 2-ketones. Bars, covered by the symbol in the case of the present data, indicate standard error (SE).
Figure 7.
Showing how the group average ODTs obtained here (crosses) for the 2-ketones compare with those compiled by van Gemert [39] (filled symbols) and those compiled and standardized by Devos et al. [17] (empty symbols) from the literature. (For clarity, ODTs from the various studies in the compilations are spread out along the x-axis.)
Individual data
Individual psychometric functions, as those for the group, were also fitted with high correlation coefficients by equation (2) (Table 3 and Figures 2 to 5). Among the 6 subjects tested in common across all ketones, subjects 28 and 19 were consistently the most sensitive, subjects 21 and 4 were medium sensitive, and subjects 20 and 24 were consistently the less sensitive (Table 3). These outcomes encourage the comparison of interindividual olfactory sensitivity among all participants (17≤n≤22) tested with each ketone. The ratio of the least sensitive to the most sensitive subject in terms of ODTs (expressed in ppb) equaled 16 for acetone, 10 for pentanone, 15 for heptanone, and 24 for nonanone, i.e., at or slightly above one order of magnitude. In terms of the interquartile range of individual ODTs expressed as constant C, i.e., log ppb, the interindividual variability equaled 0.48 for acetone, 0.30 for pentanone, 0.44 for heptanone, and 0.50 for nonanone.
The above picture of interindividual variability in odor thresholds agrees well with recent data from homologous n-alcohols and acetates measured with the same apparatus, i.e., the VDD-8, and similar methodology [13, 15]. The variability measured here is within the range observed in some previous studies [32] but much smaller than that reported in other investigations [6, 21, 31, 43]. To avoid producing artificially high estimates of interindividual variability in the present study, we have taken particular care to address three critical factors noted in previous research: 1) an accurate analytical quantification and control of the vapor concentrations tested [7]; 2) a natural and sufficient availability of the odorant(s) delivered [22, 23, 25, 26]; and 3) a large enough amount of data per person to secure a representative individual performance [36, 37].
Steepness of the psychometric functions
Under equation (2), constant D quantifies the steepness of the function such that the lower the value of D, the steeper the function. D can be calculated from the group data (Table 2) or from the average of individual values of D (Table 3). In both cases, D did not vary greatly across ketones. For the group data (all subjects), D ranged between 0.23 and 0.29 with no consistent trend across chain length (Table 2, upper section). For the average of individual data, D ranged between 0.21 and 0.13, with a decreasing trend from acetone to heptanone and an upward shift for nonanone (Table 3). The group value for D is influenced not only by differences in the steepness of individual functions but also by differences in individual sensitivity, i.e., the position of the function along the concentration (x) axis, quantified by C. In contrast, the average of individual values of D is less influenced by differences in individual values of C. For this reason, group values of D across ketones (Table 2) are more uniform and higher (reflecting a shallower function) than the average of individual values of D (Table 3).
In analogy with concentration-response functions in pharmacology [5, 35], and assuming that odor detection reflects, at least in part, the ligand binding characteristics in olfaction, we have used the average of individual D values across homologous n-alcohols and acetates to suggest a mechanism of interaction between VOCs and olfactory receptors [13, 15]. For these two series, D declined (i.e., the function steepness increased) with carbon chain length up to the largest homologs tested, 1-octanol and octyl acetate, respectively. We proposed a system where the VOC interacts with a set of receptors (R) to form a VOC-receptor complex that then breaks down, allowing the VOC to be transported away:
| Equation (3) |
Then, assuming that the complex concentration reaches a steady state under a given set of conditions, the concentration will be given by equation (4), derived from the Michaelis-Menten equation [30], where k1′ in the numerator is k1 times the constant receptor concentration, k1′ = k1 · {R}.
| Equation (4) |
We then showed [13] that the smaller the dissociation constant (k2) of the VOC-olfactory receptor complex, the steeper the slope of any plot of complex concentration versus VOC concentration. This implies that k2 should be small for the higher homologs tested and large for the lower ones (ethanol and ethyl acetate). If the biophase where the VOC is released after dissociating from the receptor were more polar (less hydrophobic) than that of the receptor, polar molecules (ethanol, ethyl acetate) will be transported faster than less polar molecules (1-octanol, octyl acetate). Two potential biophases that could carry the VOC away are the bloodstream and the nasal mucus, both largely aqueous and, thus, likely to be more polar than the receptor biophase. The increase in the average steepness of individual psychometric functions with carbon chain length across alcohols, acetates, and, up to a certain extent, ketones agrees with a concomitant decrease in the value of k2 and with the above interpretation. Nevertheless, the present results with the ketones show that this trend reverses upon reaching the largest homolog tested, 2-nonanone, whose high value of D (i.e., a shallower function) is comparable to that of acetone, the smallest homolog. A possible explanation is that when odorant molecules reach a certain dimension, as with nonanone, it is quite likely that the odorant-receptor interaction is diminished and that the value of k1 or of k1′ decreases, thus modifying the steepness of the function. Changes in odorant specificity and/or potency upon exceeding a certain size along homologous 2-ketones have also been observed in molecular studies of the mouse olfactory receptor mOR912-93, which, interestingly, shows maximal specificity at carbon length seven, i.e., 2-heptanone [18].
Concentration-response functions at the cellular/tissue and behavioral levels
Using a battery of odorant vapors that included 2-butanone and 2-hexanone, an investigation measured concentration-response functions for these two ketones in the stimulation of single glomeruli from the olfactory bulb of mice [41]. There were differences in slope between glomeruli but in all cases, in line with the present findings, functions for the longer-chain ketone (2-hexanone) were displaced to the left (i.e., towards lower concentrations) compared to functions for the shorter-chain ketone (2-butanone). Vapor concentrations were not actually measured but calculated from values of vapor pressure taken from the literature, a procedure that should be done with caution due to potentially large differences among literature sources, see [12]. The functions were fitted by a form of the Hill equation, comparable to the sigmoid equation (2) used here. The concentration producing half-maximal response was in the order of 1 to 2 microM for 2-hexanone and 10 to 40 microM for 2-butanone. We did not test the 4- and 6- carbon ketones but did test the 3- and 5- carbon homologs. For comparison, the concentration producing a detectability half-way between chance and perfect detection in this study (i.e., P=0.5, the ODT) was only 4 nM for 2-pentanone and 34 nM for acetone, values about 1,000 times lower. From a physicochemical point of view, concentrations from the two studies can be directly compared since both were delivered in vapor-phase. The enhanced sensitivity of olfaction when concentration-response functions are measured at higher levels (or more complex structures) of the pathway has been recently discussed and exemplified, see [13]. In addition, perireceptor factors can also increase sensitivity. For example, concentration-response curves have also recently helped to show that odorant binding proteins in nasal mucus, apart from their suggested role as odorant transporters and scavengers, can bind to olfactory receptors to form a complex that facilitates subsequent odorant binding, and, thus, enhance the response across a wide odorant concentration range [40].
Performing systematic comparisons of olfactory concentration-response functions for the same odorants (preferably on the same species) gathered at incrementally higher levels of the sensory pathway, e.g., molecular, cellular, tissue, and behavioral, can provide important information about how olfactory sensitivity is shaped across the neural pathway. As pointed out recently [13], stimulation of olfactory preparations is often done in a liquid-phase so concentrations are measured in that phase, whereas in behavioral studies delivery of the stimulus (to air-breathing species) is done in the vapor (gas) phase and concentrations should preferentially be measured in such phase. For any given odorant, gas (Cgas) and liquid (Cliquid) concentrations are related by the partition coefficient (Kgas to liquid) between the gas and the liquid media, according to:
| Equation (5) |
Knowledge of such coefficient and of the concentration in one medium allows one to calculate the equivalent concentration in the other medium. A recent paper has gathered partition coefficients from gas to water for up to 374 VOCs [3]. Interestingly, the study also assembled coefficients for partition from gas to physiological saline, the basic medium in which most olfactory preparations “in vitro” are tested. This opens the door to begin a systematic comparison of olfactory concentration-response functions obtained at different levels of the neural pathway, providing another insight to understand how the system blends together to trigger odor detection.
Figure 3.
Analogous to Figure 2 but for 2-pentanone.
Figure 4.
Analogous to Figure 2 but for 2-heptanone.
Acknowledgments
The work described in this article was funded by grant number R01 DC 002741 from the National Institute on Deafness and Other Communication Disorders (NIDCD), National Institutes of Health (NIH). Thanks are due to E. Moreno-Davis for excellent technical assistance. Thanks are also due to S. Var, J.H. Im, L. Chen and J. Nicolette for their help with some of the experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abraham MH, Gola JMR, Cometto-Muñiz JE, Cain WS. The correlation and prediction of VOC thresholds for nasal pungency, eye irritation and odour in humans. Indoor Built Environ. 2001;10:252–257. [Google Scholar]
- 2.Abraham MH, Gola JMR, Cometto-Muñiz JE, Cain WS. A model for odour thresholds. Chem Senses. 2002;27:95–104. doi: 10.1093/chemse/27.2.95. [DOI] [PubMed] [Google Scholar]
- 3.Abraham MH, Ibrahim A, Acree WE. Partition of compounds from gas to water and from gas to physiological saline at 310 K: Linear free energy relationships. Fluid Phase Equilibr. 2007;251:93–109. [Google Scholar]
- 4.Abraham MH, Sánchez-Moreno R, Cometto-Muñiz JE, Cain WS. A quantitative structure-activity analysis on the relative sensitivity of the olfactory and the nasal trigeminal chemosensory systems. Chem Senses. 2007;32:711–719. doi: 10.1093/chemse/bjm038. [DOI] [PubMed] [Google Scholar]
- 5.Brody TM. Concentration-response relationships. In: Minneman KP, Brody TM, Larner J, editors. Human Pharmacology: Molecular to Clinical. St. Louis: Mosby-Year Book; 1994. pp. 25–32. [Google Scholar]
- 6.Brown KS, Maclean CM, Robinette RR. The distribution of the sensitivity to chemical odors in man. Hum Biol. 1968;40:456–472. [PubMed] [Google Scholar]
- 7.Cain WS. Differential sensitivity for smell: “noise” at the nose. Science. 1977;195:796–798. doi: 10.1126/science.836592. [DOI] [PubMed] [Google Scholar]
- 8.Cain WS. Testing olfaction in a clinical setting. Ear Nose Throat J. 1989;68:316–328. [PubMed] [Google Scholar]
- 9.Cain WS, Schmidt R, Wolkoff P. Olfactory detection of ozone and D-limonene: reactants in indoor spaces. Indoor Air. 2007;17:337–347. doi: 10.1111/j.1600-0668.2007.00476.x. [DOI] [PubMed] [Google Scholar]
- 10.Cometto-Muñiz JE, Cain WS. Efficacy of volatile organic compounds in evoking nasal pungency and odor. Arch Environ Health. 1993;48:309–314. doi: 10.1080/00039896.1993.9936719. [DOI] [PubMed] [Google Scholar]
- 11.Cometto-Muñiz JE. Physicochemical basis for odor and irritation potency of VOCs. In: Spengler JD, Samet J, McCarthy JF, editors. Indoor Air Quality Handbook. New York: McGraw-Hill; 2001. pp. 20.1–20.21. [Google Scholar]
- 12.Cometto-Muñiz JE, Cain WS, Abraham MH. Quantification of chemical vapors in chemosensory research. Chem Senses. 2003;28:467–477. doi: 10.1093/chemse/28.6.467. [DOI] [PubMed] [Google Scholar]
- 13.Cometto-Muñiz JE, Abraham MH. Human olfactory detection of homologous n-alcohols measured via concentration-response functions. Pharmacology, Biochemistry and Behavior. 2008;89:279–291. doi: 10.1016/j.pbb.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cometto-Muñiz JE, Cain WS, Abraham MH, Gil-Lostes J. Concentration-detection functions for the odor of homologous n-acetate esters. Physiology and Behavior. 2008;95:658–667. doi: 10.1016/j.physbeh.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cometto-Muñiz JE, Cain WS, Abraham MH, Gil-Lostes J. Concentration-detection functions for the odor of homologous n-acetate esters. Physiol Behav. 2008 doi: 10.1016/j.physbeh.2008.09.021. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalton PH, Dilks DD, Banton MI. Evaluation of odor and sensory irritation thresholds for methyl isobutyl ketone in humans. Am Ind Hyg Assoc J. 2000;61:340–350. doi: 10.1080/15298660008984542. [DOI] [PubMed] [Google Scholar]
- 17.Devos M, Patte F, Rouault J, Laffort P, van Gemert LJ. Standardized Human Olfactory Thresholds. Oxford: IRL Press; 1990. [Google Scholar]
- 18.Gaillard I, Rouquier S, Pin JP, Mollard P, Richard S, Barnabe C, Demaille J, Giorgi D. A single olfactory receptor specifically binds a set of odorant molecules. Eur J Neurosci. 2002;15:409–418. doi: 10.1046/j.0953-816x.2001.01871.x. [DOI] [PubMed] [Google Scholar]
- 19.Gaillard I, Rouquier S, Chavanieu A, Mollard P, Giorgi D. Amino-acid changes acquired during evolution by olfactory receptor 912–93 modify the specificity of odorant recognition. Hum Mol Genet. 2004;13:771–780. doi: 10.1093/hmg/ddh086. [DOI] [PubMed] [Google Scholar]
- 20.Hummel P, Vaidehi N, Floriano WB, Hall SE, Goddard WA., 3rd Test of the Binding Threshold Hypothesis for olfactory receptors: explanation of the differential binding of ketones to the mouse and human orthologs of olfactory receptor 912–93. Protein Sci. 2005;14:703–710. doi: 10.1110/ps.041119705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones FN. An analysis of individual differences in olfactory thresholds. Am J Psychol. 1957;70:227–232. [PubMed] [Google Scholar]
- 22.Knudsen HN, Clausen G, Fanger PO. Sensory characterization of emissions from materials. Indoor Air. 1997;7:107–115. [Google Scholar]
- 23.Knudsen HN, Valbjørn O, Nielsen PA. Determination of exposure-response relationships for emissions from building products. Indoor Air. 1998;8:264–275. [Google Scholar]
- 24.Lai PC, Bahl G, Gremigni M, Matarazzo V, Clot-Faybesse O, Ronin C, Crasto CJ. An olfactory receptor pseudogene whose function emerged in humans: a case study in the evolution of structure-function in GPCRs. J Struct Funct Genomics. 2008;9:29–40. doi: 10.1007/s10969-008-9043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laing DG. Characterisation of human behaviour during odour perception. Perception. 1982;11:221–230. doi: 10.1068/p110221. [DOI] [PubMed] [Google Scholar]
- 26.Laing DG. Natural sniffing gives optimum odour perception for humans. Perception. 1983;12:99–117. doi: 10.1068/p120099. [DOI] [PubMed] [Google Scholar]
- 27.Laska M, Miethe V, Rieck C, Weindl K. Olfactory sensitivity for aliphatic ketones in squirrel monkeys and pigtail macaques. Exp Brain Res. 2005;160:302–311. doi: 10.1007/s00221-004-2012-0. [DOI] [PubMed] [Google Scholar]
- 28.Macmillan NA, Creelman CD. Detection theory: A user’s guide. Cambridge: Cambridge University Press; 1991. [Google Scholar]
- 29.Matarazzo V, Clot-Faybesse O, Marcet B, Guiraudie-Capraz G, Atanasova B, Devauchelle G, Cerutti M, Etievant P, Ronin C. Functional characterization of two human olfactory receptors expressed in the baculovirus Sf9 insect cell system. Chem Senses. 2005;30:195–207. doi: 10.1093/chemse/bji015. [DOI] [PubMed] [Google Scholar]
- 30.Price NC, Dwek RA, Ratcliffe RG, Wormald M. Principles and Problems in Physical Chemistry for Biochemists. Oxford: Oxford University Press; 2001. [Google Scholar]
- 31.Punter PH. Measurement of human olfactory thresholds for several groups of structurally related compounds. Chem Senses. 1983;7:215–235. [Google Scholar]
- 32.Rabin MD, Cain WS. Determinants of measured olfactory sensitivity. Percept Psychophys. 1986;39:281–286. doi: 10.3758/bf03204936. [DOI] [PubMed] [Google Scholar]
- 33.Sanz G, Schlegel C, Pernollet JC, Briand L. Comparison of odorant specificity of two human olfactory receptors from different phylogenetic classes and evidence for antagonism. Chem Senses. 2005;30:69–80. doi: 10.1093/chemse/bji002. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt R, Cain WS. How to make and measure smells - practical lessons in olfactometry. Chem Senses. 2003;28:551, A597. (abstract) [Google Scholar]
- 35.Snyder R. Basic concepts of the dose-response relationship. In: Rodricks JV, Tardiff RG, editors. Assessment and Management of Chemical Risks. Washington, D.C.: American Chemical Society; 1984. pp. 37–55. [Google Scholar]
- 36.Stevens JC, Cain WS, Burke RJ. Variability of olfactory thresholds. Chem Senses. 1988;13:643–653. [Google Scholar]
- 37.Stevens JC, Dadarwala AD. Variability of olfactory threshold and its role in assessment of aging. Percept Psychophys. 1993;54:296–302. doi: 10.3758/bf03205264. [DOI] [PubMed] [Google Scholar]
- 38.Touhara K. Deorphanizing vertebrate olfactory receptors: recent advances in odorant-response assays. Neurochem Int. 2007;51:132–139. doi: 10.1016/j.neuint.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 39.van Gemert LJ. Compilations of odour threshold values in air, water and other media. Utrech: Oliemans: Punter & Partners BV; 2003. Odour Thresholds. [Google Scholar]
- 40.Vidic J, Grosclaude J, Monnerie R, Persuy MA, Badonnel K, Baly C, Caillol M, Briand L, Salesse R, Pajot-Augy E. On a chip demonstration of a functional role for Odorant Binding Protein in the preservation of olfactory receptor activity at high odorant concentration. Lab Chip. 2008;8:678–688. doi: 10.1039/b717724k. [DOI] [PubMed] [Google Scholar]
- 41.Wachowiak M, Cohen LB. Representation of odorants by receptor neuron input to the mouse olfactory bulb. Neuron. 2001;32:723–735. doi: 10.1016/s0896-6273(01)00506-2. [DOI] [PubMed] [Google Scholar]
- 42.Wysocki CJ, Dalton P, Brody MJ, Lawley HJ. Acetone odor and irritation thresholds obtained from acetone-exposed factory workers and from control (occupationally unexposed) subjects. Am Ind Hyg Assoc J. 1997;58:704–712. doi: 10.1080/15428119791012342. [DOI] [PubMed] [Google Scholar]
- 43.Yoshida M. Correlation analysis of detection threshold data for ‘standard test’ odors. Bull Fac Sci Eng Chuo Univ. 1984;27:343–353. [Google Scholar]