Abstract
Reconstruction of flexor tendons often results in adhesions that compromise joint flexion. Little is known about the factors involved in the formation of flexor tendon graft adhesions. In this study, we developed and characterized a novel mouse model of flexor digitorum longus (FDL) tendon reconstruction with live autografts or reconstituted freeze-dried allografts. Grafted tendons were evaluated at multiple time points up to 84 days post-reconstruction. To assess the flexion range of the metatarsophalangeal joint, we developed a quantitative outcome measure proportional to the resistance to tendon gliding due to adhesions, which we termed the Gliding Coefficient. At 14 days post grafting, the Gliding Coefficient was 29- and 26-fold greater than normal FDL tendon for both autografts and allografts, respectively (p<0.001), and subsequently doubled for 28-day autografts. Interestingly, there were no significant differences in maximum tensile force or stiffness between live autograft and freeze-dried allograft repairs over time. Histologically, autograft healing was characterized by extensive remodeling and exuberant scarring around both the ends and the body of the graft, whereas allograft scarring was abundant only near the graft-host junctions. Gene expression of GDF-5 and VEGF were significantly increased in 28 day autografts compared to allografts and to normal tendons. These results suggest that the biomechanical advantages for tendon reconstruction using live autografts over devitalized allografts are minimal. This mouse model can be useful in elucidating the molecular mechanisms in tendon repair and can aid in preliminary screening of molecular treatments of flexor tendon adhesions.
Keywords: Flexor Tendon, Allograft, Autograft, Adhesion, Biomechanics
INTRODUCTION
Repair of injuries to flexor tendons is complicated by fibrotic adhesions that compromise tendon gliding and limit the range of joint flexion.1 Adhesions are especially exacerbated in injuries involving flexor digitorum profundus (FDP) and flexor digitorum superficialis (FDS) tendons in Bunnell’s “no man’s land” or zone II of the hand, which to date remain unsolved clinical problems.2, 3 As an alternative to primary repair, which still represents the standard of care for these injuries,3 surgeons often use a live tendon autograft especially when primary repair has been neglected or delayed because of infection, or in revision surgery when primary repair had failed.4
Unfortunately, flexor tendon grafting procedures also experience post-operative adhesions that limit joint flexion or cause joint contracture. The biological mechanisms of flexor tendon graft repair and adhesion formation are still poorly understood, despite being studied for decades. Adhesions following live autograft reconstruction are thought to arise through intrinsic fibrosis (as a result of suturing or surgical manipulation of the live tendon graft leading to tenocyte necrosis) or through extrinsic fibrosis (whenever the tendon sheath is disrupted leading to synovial and inflammatory cellular influx),1, 5 among other factors.6–8 On the other hand, flexor tendon reconstruction with allograft tissue has been scarcely reported in the clinical literature, and has been limited to two-stage reconstruction procedures.9 Few animal studies compared the mechanisms of healing of flexor tendon autografts and allografts. In a canine model of flexor tendon reconstruction, freeze-dried allografts have been reported to be tolerated well by the host and to allow flexor tendon function similar to autografts.10 Others reported that acellular allografts induce minimal adhesion formation in bovine flexor tendons.11, 12 Despite these reports, the biological and biomechanical differences in flexor tendon autograft and allograft healing remain less well studied compared to primary repair, which has been extensively studied.
Based on the scarce evidence from the literature, we hypothesized that an acellular tendon allograft heals without the intrinsic fibrotic adhesions that are normally observed in a live autograft, which experiences excessive scarring. To test this hypothesis and to investigate differences in flexor tendon autograft and allograft repair, we developed a novel mouse model in which we repair a gap defect in the flexor digitorum longus (FDL) tendon of the hind limb with either a live autograft or an acellular, freeze-dried allograft and provide adequate immobilization to induce robust adhesion formation. In this study, we quantitatively examine the autograft and allograft gliding function and biomechanical strength. In addition, using histology and real-time RT-PCR, we examine aspects of cellular and molecular events involved in graft repair and subsequent adhesion formation.
MATERIALS AND METHODS
Processing of Freeze-Dried Allografts
FDL tendon allografts were harvested from donor C57BL/6 mice using aseptic technique and were snap frozen in liquid nitrogen before being placed in a freeze-drying chamber (FreeZone 2.5 Liter Benchtop Freeze Dry System, Labconco Corporation, Kansas City, Missouri). The tendons were lyophilized for 12 hours, after which they were stored at −80°C until the day of surgery. Before grafting, the allografts were reconstituted with sterile saline for 30 minutes.
Surgical Procedures
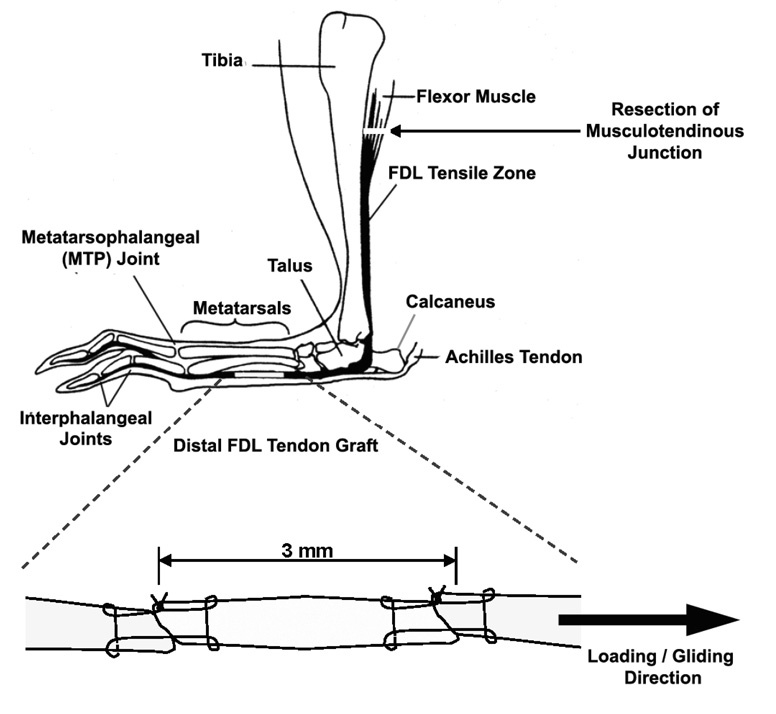
Animal studies were approved by the University of Rochester Committee for Animal Resources. Eight-week old female C57BL/6 mice were randomized into two experimental groups; live autografts and devitalized allografts. The mice were anesthetized with ketamine (60 mg/kg body weight) and Xylazine (4mg/kg body weight) via an intra-peritoneal injection. Surgeries were preformed using aseptic technique under a 2X micro dissection magnifying lens. Briefly, a longitudinal plantar incision was made on the left hind foot. The distal FDL tendon of the mouse was isolated and transected on the plantar surface of the metatarsal bones. A 3 mm freeze-dried tendon allograft that has been reconstituted in saline or a freshly harvested live autograft was sutured between the ends of host tendon using an 8–0 nylon suture in a horizontal mattress suture pattern (similar to a modified Kessler technique) (Figure 1). The tendon was then transected at the proximal musculotendinous junction to temporarily immobilize the flexor mechanism to protect against disruption of the tendon graft early during the repair period and to eliminate early tendon gliding to induce adhesion formation. The skin was closed with 4–0 silk suture. To eliminate favoring the non-operated limb, the live autografts were harvested from the right limbs. Animals receiving the freeze-dried allografts also had the right FDL tendon transected similar to the autograft donor limb. Mice were sacrificed at 0, 14, 28, 42, 63 and 84 days post surgery (n=9–12 animals per group per time point) for assessment of the metatarsophalangeal (MTP) joint flexion and biomechanical evaluation. Additional mice were sacrificed at 14 and 28 days for histology (n=3 per group per time point) and for assessment of gene expression by real-time RT-PCR (n=3 per group per time point).
Figure 1.
A schematic illustration of the live autograft or freeze-dried allograft reconstruction of the murine distal FDL tendon. The tendon is transected at the proximal musculotendinous junction to temporarily immobilize the flexor mechanism to protect against the disruption of the tendon graft and to stimulate adhesions.
Assessment of Metatarsophalangeal Joint Flexion
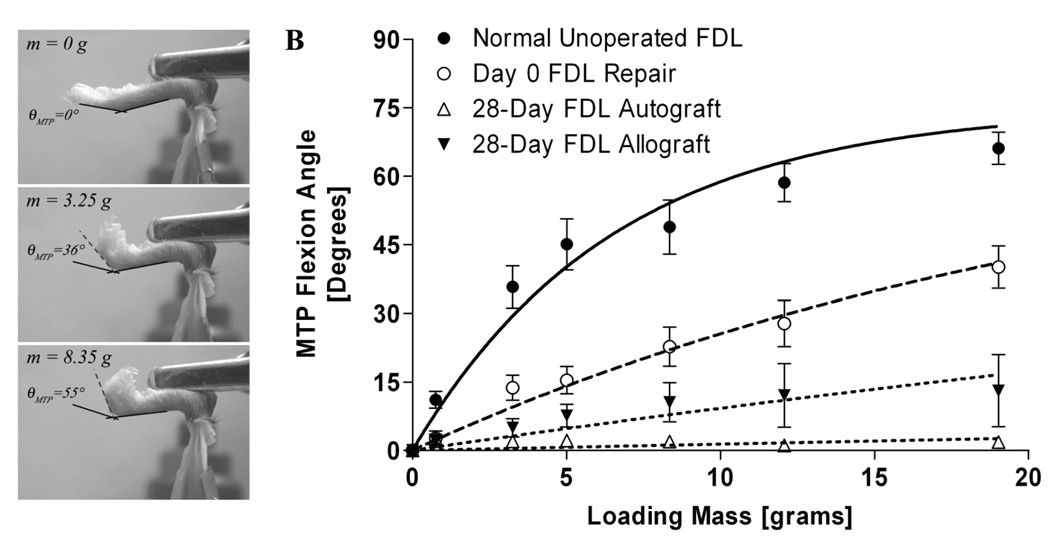
To evaluate the range of MTP joint flexion, we developed a novel assay to quantify the resistance to flexion due to grafting and adhesion formation after FDL tendon reconstruction (Figure 2). Immediately following sacrifice, the lower hind limb was disarticulated from the knee and the proximal FDL tendon along the tibia was released just proximal the tarsal tunnel without disrupting the skin at the ankle or foot. The proximal end of the tendon was then secured between two square pieces of tape using a thin layer of cyanoacrylate as previously described.13 The lower hind limb was fixed in a custom apparatus where the tibia was rigidly gripped to prevent rotation (Figure 2A). To standardize the neutral position, the toes were passively extended by the examiner and allowed to return to an unloaded position before a digital image was taken medially to determine the neutral position (zero load) of the MTP joint. The FDL tendon was incrementally loaded in the same anatomical direction as flexor muscle line of force. The loading was accomplished using dead weights (0 – 19 grams) that were statically suspended from a hook and line passing through the proximal FDL tendon/tape composite. The dead weights were suspended for 30 seconds before the digital pictures were taken to avoid creep effects. With each increment of load, a digital image was taken to quantify the MTP flexion angle relative to the neutral position. The MTP joint flexion angles were measured from the digital images by 2 independent observers (SH & JJ) using ImageJ software (http://rsb.info.nih.gov/ij/) and plotted versus the applied loads (Figure 2B). Based on the flexion curve of the normal tendon, the flexion data were fitted to a single-phase exponential association equation of the form: MTP Flexion Angle = β × [1 − exp(−m/α)] (R2=0.93±0.07, p<0.05); where m is the applied load (Prism GraphPad 3.0, GraphPad Software, Inc., San Diego, CA). The curve fit was constrained to the maximum flexion angle (β) for normal tendons that was determined to be 75° for the maximum applied load of 19 grams. The constant α governing the rate of rise of the flexion curve with increased loading was determined by nonlinear regression as a measure of the resistance to MTP joint flexion due to impaired gliding and therefore termed the Gliding Coefficient.
Figure 2.
(A) Assessment of MTP joint flexion upon FDL tendon loading. The lower hind limb of the mouse was disarticulated from the knee, and the proximal FDL tendon was isolated and loaded incrementally using dead weights in the direction of the anatomical pull starting with a neutral unloaded position. At each load a digital picture was taken. Subsequently the MTP flexion angle was measured relative to the unloaded position. (B) Representative flexion curves (flexion angles versus applied loads) of the MTP joint in normal (unoperated) and grafted FDL tendons (days 0 and 28 post grafting). Discrete data points represent measured flexion angles (mean ± SEM). Lines represent best fit curves based on modeling the data using the single-phase exponential association equation MTP Flexion Angle = β × [1 − exp(−m/α)], where m is the applied mass, β is the maximum flexion angle (75° for normal unoperated FDL tendons), and α is the Gliding Coefficient.
Biomechanical Testing
Following the adhesion test, the proximal extent of the FDL tendon at the myotendinous junction was identified then freed from surrounding tissue using blunt dissection along the length of the tendon to the tarsal tunnel. The tendon was then released at the tarsal tunnel, with dissection medially along the bone. Once the tendon was freed from the tunnel, the calcaneus was removed, freeing the proximal end of the tendon for direct gripping in the mechanical test as described by Mikic et al (2001).13 The distal tendon and graft interfaces were not disrupted or dissected since the mechanical testing involved direct gripping of the distal bones of the foot without disrupting the graft or the branching tendon insertion into the phalanges. The specimens were placed in sterile gauze soaked with saline to maintain adequate tissue hydration. The FDL tendon was then mounted on the Instron 8841 DynaMight™ axial servohydraulic testing system (Instron Corporation, Norwood, MA) using custom grips and tested following published protocols.13 The tendon was loaded in tension in displacement control at a rate of 30 mm/minute until failure. Force-displacement curves were plotted and the maximum tensile force and stiffness were determined. Failure modes were carefully observed and recorded.
Histology
Grafted limbs were harvested at 14 and 28 days post surgery (n=3 autografts and n=3 allografts per time point) by disarticulating the intact foot and tibia at the knee joint. Tissues were prepared for histology using routine techniques. Briefly, the harvested lower limb were fixed in 10% neutral buffered formalin with the tibia at 90° relative to the foot, and then decalcified in 10% EDTA at 4°C for 28 days. The decalcified tissues were then dehydrated in a gradient of alcohols and then embedded en bloc in paraffin to preserve the anatomical relationship between the grafted tendon and surrounding tissues. The line of the FDL tendon was marked on the sole of the foot using India ink with the aid of the reconstruction suture. Serial 3µm sagittal sections through the FDL tendon plane were then cut, mounted on glass slides, and stained with Orange G and Alcian Blue.
Gene Expression using Real-Time RT-PCR
RNA isolation and quantitative real-time RT-PCR were performed as briefly described.14 Grafted tendons from mice sacrificed at 14 and 28 days post-surgery (n=3 autografts and n=3 allografts per time point) and age-matched normal unoperated tendons (n=3) were harvested and immediately frozen in liquid nitrogen. Tendons from either group were pooled and minced by manual homogenization (mortar and pestle) and then flushed through a 22G needle with a syringe for further mechanical breakup of any remaining tissue. Total RNA was isolated using TRIZOL (Invitrogen Corporation, Carlsbad, CA). Single-stranded cDNA was made using a reverse transcription kit (AbGene Inc. USA, Rochester, NY) and used as a template for real-time PCR with SYBR Green PCR Master Mix (AbGene) and gene specific primers (Table 2) in a Rotor-Gene 2000 Real-Time DNA Detection System (Corbett Research, Sydney, Australia). The mean cycle threshold (Ct) values from quadruplicate measurements were used to calculate the gene expression standardized to β-actin expression as an internal control. Gene expression data were normalized and expressed as fold-increase or fold-decrease (mean ± SEM) relative the normal unoperated FDL tendon expression which was normalized to 1.
Table 2.
Effects of the non-destructive assessment of the MTP joint flexion on the tensile biomechanical properties of 28-day FDL autografts and allografts *
| Maximum Force [N] | Stiffness [N/mm] | |||
|---|---|---|---|---|
| Autograft | Allograft | Autograft | Allograft | |
| No MTP Joint Flexion Test | 1.51 ± 0.23 | 1.43 ± 0.33 | 1.33 ± 0.16 | 1.07 ±0.18 |
| After MTP Joint Flexion Test | 2.19 ± 0.32 | 2.24 ± 0.31 | 1.62 ± 0.22 | 1.58 ± 0.20 |
Mean ± SEM; n=5 per group
Statistical Analysis
Data analysis including 2-way Analysis of Variance with Bonferroni post-hoc multiple comparisons (α=0.05) and the nonlinear regression analyses were performed using Prism GraphPad 4.0 statistical software. The Gliding Coefficient data were generated by fitting individual tendon flexion curves to the mathematical model using an algorithm in PRISM which utilizes the Marquardt method to minimize the sum of squares of errors between measured and modeled values over a range of α and β values. The goodness of fit was assessed using the R2 value and by correlating the Gliding coefficient data to the MTP joint ROM.
RESULTS
Effects of Allograft Processing by Freeze-Drying
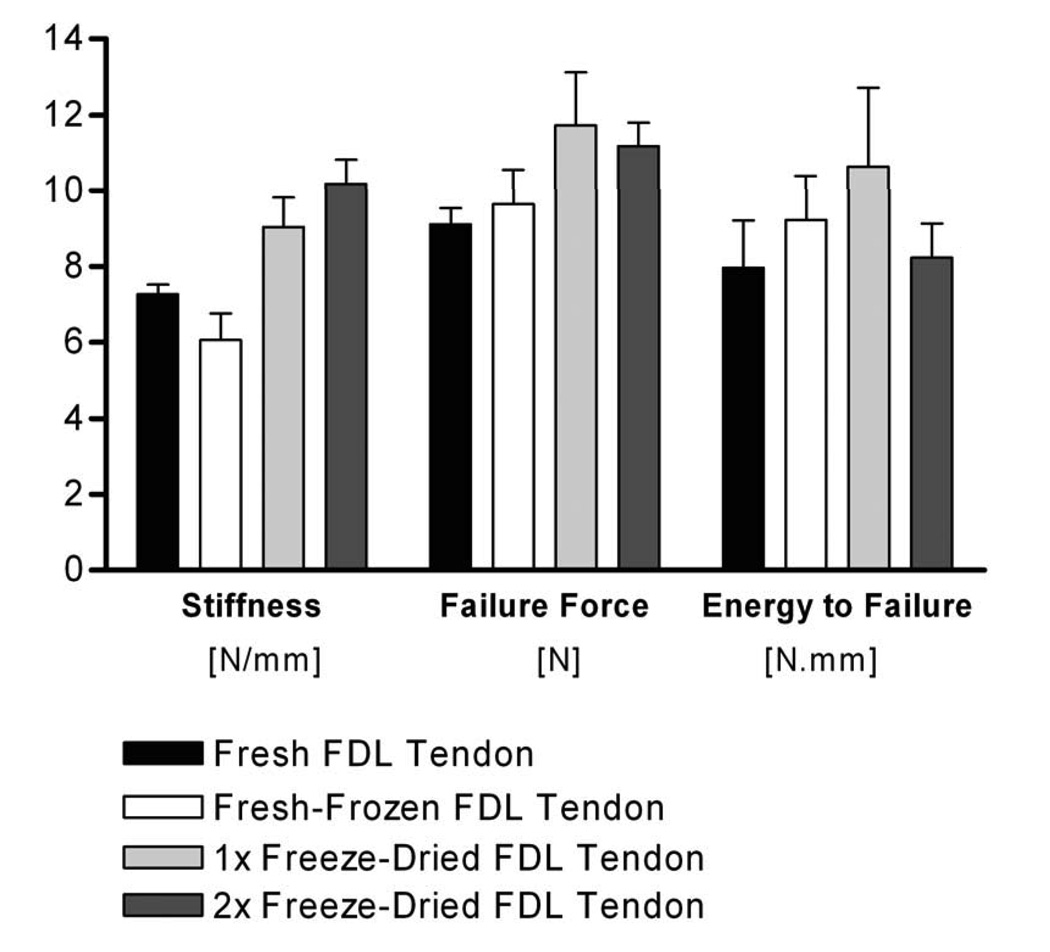
To determine the effects of freeze-drying on the mouse FDL tendon tensile biomechanical properties, FDL tendons were harvested from cadaver mice and tested biomechanically in tension either immediately without freezing, after a single −20°C freeze-thaw cycle, after freeze-drying and reconstitution in PBS once, or after freeze-drying and reconstitution in PBS twice (n=6 tendons per group). There were no significant changes in the mechanical properties (failure force, stiffness, and energy to failure) of the once or twice freeze-dried tendons compared to the fresh and fresh-frozen tendons (Figure 3). When failure modes were examined, 58% of the once or twice freeze-dried tendons failed in the mid-substance whereas 50% of the fresh frozen tendons and 83% of the fresh tendons failed in this manner. The remaining tendons failed either near the insertion to the bone distally or at the proximal tendon-muscle insertion.
Figure 3.
Effects of freeze-drying on the mouse FDL tendon tensile biomechanical properties. FDL tendons were harvested from cadaver mice and tested biomechanically either immediately without freezing (Fresh), after a single −20°C freeze-thaw cycle (Fresh-Frozen), after being freeze-dried and reconstituted in PBS once (1× Freeze-Dried), or after being freeze-dried and reconstituted in PBS twice (2× Freeze-Dried). Data presented as mean ± SEM.
Assessment of Metatarsophalangeal Joint Flexion
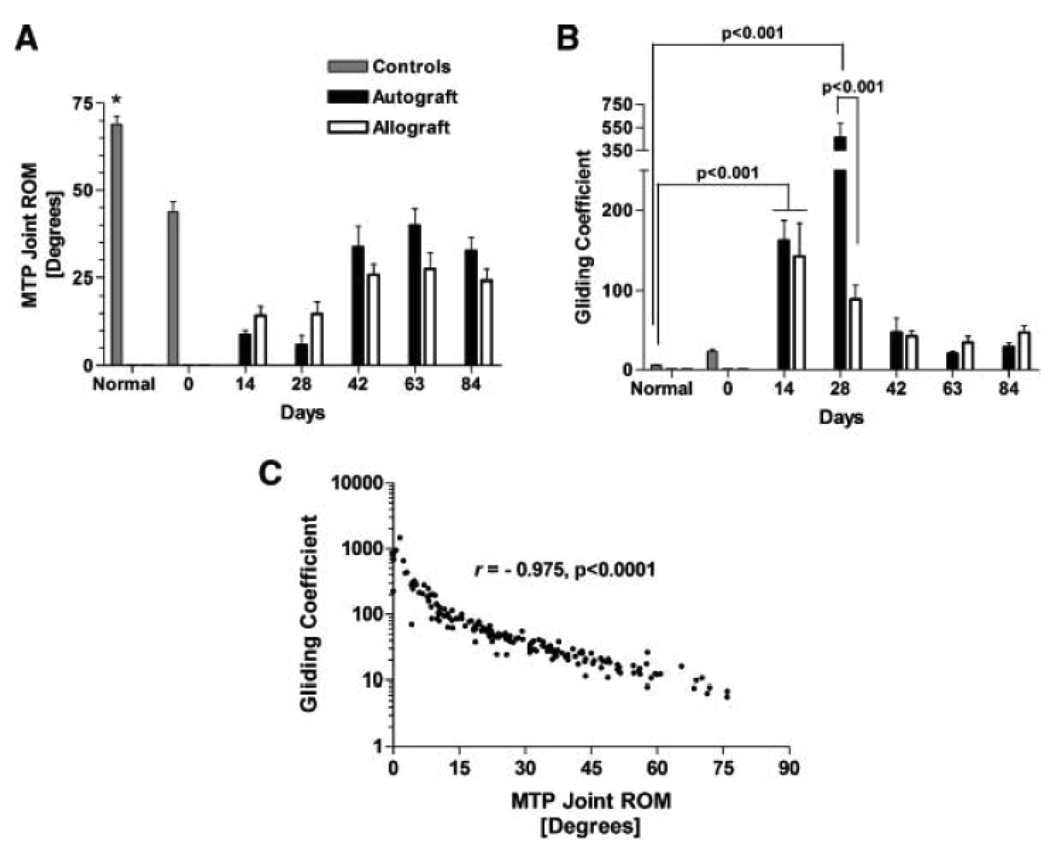
Both autografts and allografts experienced significant reductions in the range of motion (ROM) of the MTP joint (defined as the flexion angle upon the application of the maximum excursion load of 19 grams) (Figure 2B and Figure 4A). The MTP joint ROM for normal tendons was significantly greater than reconstructed tendons at all time points regardless of the graft type (p<0.001). The ROM for the14 and 28 day autografts and allografts were reduced compared to the other time points but these differences were not statistically significant. The autografts’ MTP joint ROM at 14 and 28 days were only 60% and 40% of the corresponding allografts’ ROM, respectively, although these differences were also not statistically significant. These results are consistent with the Gliding Coefficient data. At 14 days post grafting, the Gliding Coefficient was 29- and 26-fold greater than normal FDL tendon (n=8) for both autografts (n=12) and allografts (n=12), respectively (p<0.001). However, there was no significant difference between autograft and allograft Gliding Coefficients. At 28 days post grafting, the Gliding Coefficient of the autografts (n=9) was 83-fold (p<0.001) greater that normal tendon (n=8). By contrast, the Gliding Coefficient for freeze-dried allograft tendon (n=10) was increased 16-fold compared to normal tendon, and was 5-fold less than the Gliding Coefficient measured in the autograft tendons (p<0.001). By 42 days and thereafter, the Gliding Coefficients significantly decreased in both groups but remained somewhat higher than normal unoperated FDL tendons (Figure 4B). We further measured the range of MTP joint flexion (defined as the flexion angle upon the application of the maximum excursion load of 19 grams). Figure 4C shows a strong negative correlation (Spearman’s r=−0.975, p<0.0001) between the empirically determined Gliding Coefficient and the measured range of MTP joint flexion, which corroborates the validity of the Gliding Coefficient as quantitative measure of the resistance to joint flexion. Interestingly, simply transplanting a graft and then evaluating the MTP joint flexion immediately after surgery (Day 0, n=9, Figure 4B) shows that the Gliding Coefficient increases only by 2-folds compared to normal FDL tendon, which could be a result of the suture interfering with the gliding of the FDL graft, the enlargement of the graft/host junctures, or skin tightening when the incision was closed.
Figure 4.
(A) MTP joint flexion ROM and (B) Gliding Coefficients of normal unoperated FDL tendons and FDL tendon autografts and allografts at multiple time points post-grafting (mean ± SEM). Asterisk indicates significant difference between normal and operated tendons (p<0.001). (C) Correlation between the empirically determined Gliding Coefficient and the MTP range of flexion (Spearman’s r = −0.975, p<0.0001).
Biomechanical Properties of FDL Tendon Autografts and Allografts
Immediately following the assessment of the MTP joint flexion, the grafted tendons were harvested and tested biomechanically as described. We investigated whether the non-destructive assessment of the MTP joint flexion had any effects on the measured tensile biomechanical properties of specimens harvested 28 days post transplantation. The data in Table 2 demonstrated that the biomechanical properties of fresh autografts or freeze-dried allografts that were tested for MTP joint flexion were not significantly different from specimens that were not tested for MTP joint flexion assessment (p>0.05).
More importantly, there were no significant differences in maximum tensile force or stiffness between live autograft and freeze-dried allograft repairs at any time point up to 84 days post-transplantation (Table 3). While there were mild improvements over time in the tensile strength (as indicated by the maximum tensile force at failure), both autograft and allograft repairs remained less than 50% of the strength of normal FDL tendon. The stiffness for both the autograft and allograft repairs significantly increased over time reaching 75–90% of the stiffness of normal unoperated FDL tendon. On average, 87% of the autografts failed at the proximal repair site compared 74% f allografts. The remainder of the tendon grafts failed either at the distal repair site or in the graft itself with the latter being a rare incidence (∼3%).
Table 3.
Tensile biomechanical properties of autografts and allografts (Mean ± SEM) over time following FDL tendon reconstruction
| Maximum Force [N] | Stiffness [N/mm] | |||
|---|---|---|---|---|
| Normal | 9.71 ± 0.15* | 5.14 ± 0.26 # | ||
| Autograft | Allograft | Autograft | Allograft | |
| Day 0 | 0.80 ± 0.14a | 0.45 ± 0.09 a | 0.36 ± 0.08 I | 0.24 ± 0.04 I |
| Day 14 | 1.54 ± 0.14a,b | 1.67 ± 0.15 b | 1.60 ± 0.19 II | 1.46 ± 0.17 II |
| Day 28 | 1.79 ± 0.20b | 1.83 ± 0.25 b | 1.45 ± 0.13 II | 1.33 ± 0.15 II |
| Day 42 | 3.25 ± 0.26c | 2.84 ± 0.21 c | 3.50 ± 0.30 III | 3.33 ± 0.19 III |
| Day 63 | 3.73 ± 0.32c | 3.17 ± 0.26 c | 3.89 ± 0.30 III,VI | 3.90 ± 0.37 III |
| Day 84 | 3.56 ± 0.37c | 4.17 ± 0.31 d | 4.60 ± 0.43 IV | 3.83 ± 0.42 III |
p<0.001 Compared to autograft and allograft repairs at all time points
p<0.05 Compared to autograft and allograft repairs at days 0, 14, 28, and 42.
(p<0.05)
(p<0.05)
Histology of FDL Tendon Autografts and Allografts
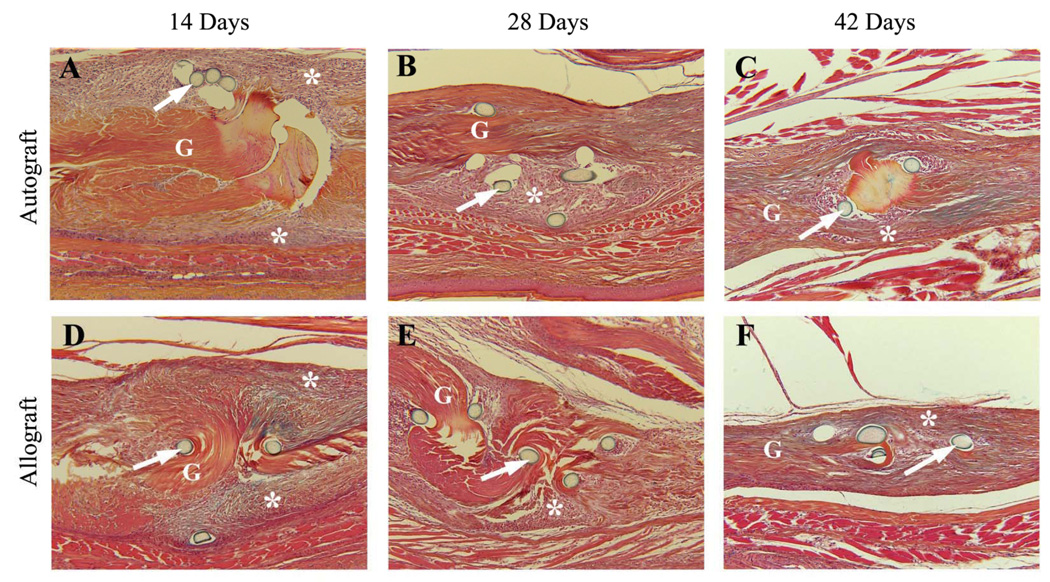
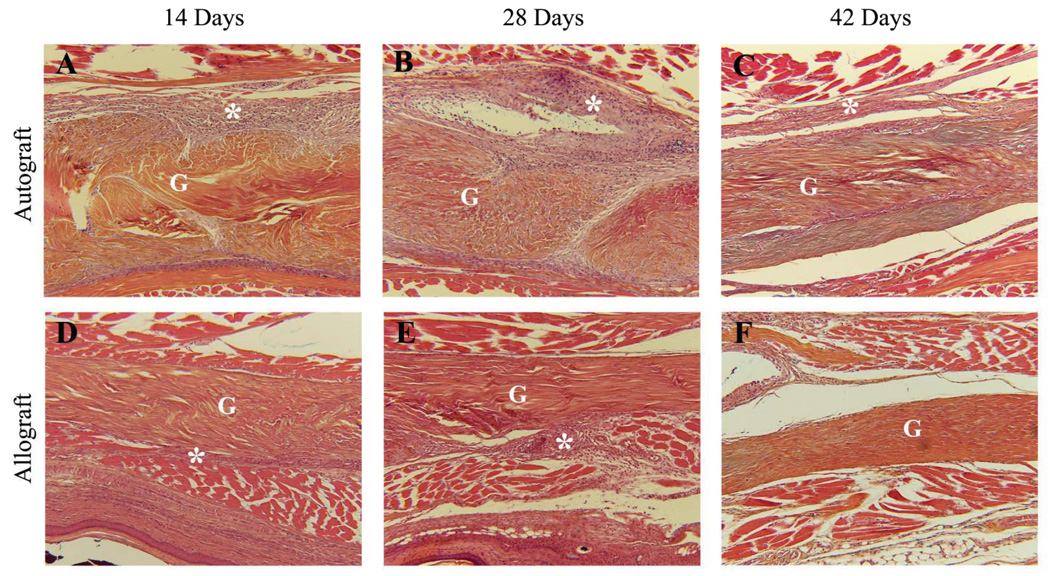
To examine the potential biological mechanisms responsible for the observed reduction in the tendon gliding function at 14 and 28 days post surgery, and the subsequent restoration of the gliding function at 42 days post grafting, tendon autografts and allografts were assessed histologically. These analyses revealed that at 14 and 28 days the host junctions of both autograft and allograft were surrounded by similar amounts of hypercellular fibrotic scar tissue, and appeared enlarged relative to the body of the graft proper (Figure 5). However, remarkable differences between autograft and allograft healing were manifested by the amount of fibrotic scar tissue surrounding the middle segments of the grafts (Figure 6). While autografts were encased by this tissue that appeared to be invading the tendon (Figure 6 A & B), the middle segment of the allografts was largely unaffected by the host and remained mostly acellular at 14 and 28 days post grafting (Figure 6 D & E). These differences were less profound by day 42 (Figure 6 C & F). These distinct modes of repair are consistent with the differences in the Gliding Coefficients between the grafts at 28 days, and suggest increased adhesions in the autografts at this time, which resolve with subsequent remodeling.
Figure 5.
Representative histologic sections of the proximal host-graft junction of the FDL tendon autografts (A–C) and allografts (D–F) at 14, 28, and 42 days post surgery. Sections were stained with Orange G/Alcian Blue (10X). Of note is that both 14-day and 28-day autograft (A & B) and allograft (D & E) ends adjacent to the suture (arrows) are surrounded by similar amounts of hyper-cellular fibrotic scar tissue (*) and appear enlarged relative to the body of the graft proper (marked as G). By day 42, the amount of scarring and the enlargement at the graft-host junction are reduced for both autografts (C) and allografts (F).
Figure 6.
Representative histologic sections of the middle segment of the FDL tendon autografts (A-C) and allografts (D–F) at 14, 28 and 42 days post surgery. Sections were stained with Orange G/Alcian Blue (10X). Of note are the remarkable differences in the amount of the hyper-cellular fibrotic scar (*) surrounding 14-day and 28-day autografts (A & B) that appears to be minimal around the acellular allografts (C & D). By 42 days the scar tissue appears to have significantly remodeled in both autografts (E) and allografts (F). Graft tissue is marked G.
Gene Expression in FDL Tendon Autografts and Allografts
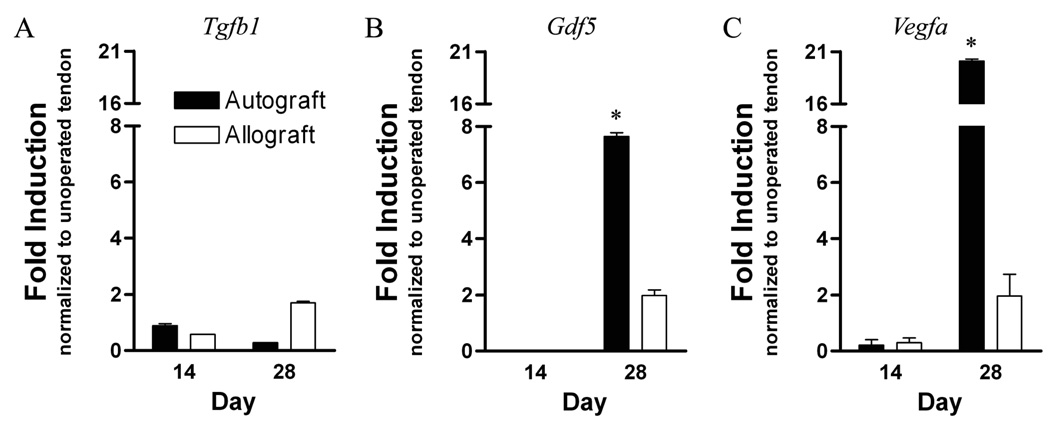
Since it is known that TGF-β1, GDF-5, and VEGF-A are potent growth factors that stimulate vascular invasion, fibrosis and tenocyte differentiation respectively, we assessed their mRNA expression levels in grafted FDL tendons at 14 and 28 days post surgery, corresponding to the maximum observed reductions in tendon gliding functions. Consistent with the robust intrinsic healing response of the live autografts, Gdf5, and Vegfa expression levels in autografts were significantly up-regulated in 28-day autografts by 7- and 20-fold respectively, compared to normal unoperated tendon (p<0.05), but the Tgfb1expression levels were not increased. In contrast, Tgfb1, Gdf5, and Vegfa expression levels were doubled in 28-day allografts compared to normal unoperated tendon controls, although these differences were not significant (Figure 7).
Figure 7.
Gene expression of (A) Tgfb1, (B) Gdf5, and (C) Vegfa in FDL tendon autografts and allografts at 14 and 28 days post grafting. Total RNA was extracted and pooled from 3 tendon grafts and processed for real-time RT-PCR. Gene expression was standardized with the internal beta-actin control and then normalized by the level of expression in normal unoperated FDL tendon. Data presented as the mean fold induction (over normal unoperated tendon) ± SEM. * p < 0.05 vs. normal unoperated tendon.
DISCUSSION
The development of comparative animal models to study the biomechanical and biological factors involved in flexor tendon adhesions is important for advancing our understanding of this debilitating problem and for designing therapeutic and rehabilitation treatment programs. A number of elegant studies in multiple human and animal models have identified passive controlled gliding motion as the most important factor in reducing the risk of adhesion formation.3, 15–18 Other studies have focused on molecular treatment of the flexor tendon injury to provide adhesion-free healing via the delivery of anti-scarring adjuvants that inhibit the effects of TGF-β and bFGF among other factors.19–23 Despite their promise, these approaches remain experimental and have yet to yield a clinical application,3 largely because our understanding of the molecular mechanisms involved in the formation of adhesions after flexor tendon injury and grafting remains incomplete.
The novel mouse model of FDL tendon grafts offers a quantitative tool to not only examine the biomechanical aspects of flexor tendon grafts, but also to potentially elucidate the molecular events involved in repair and subsequent adhesion formation via the use of transgenic mouse models of gain and loss of function. However, this model has a number of inherent limitations. The mouse model is admittedly challenging due to the small size of the FDL tendon, which requires microsurgical reconstruction under magnifying lens, however the reproducibility of the data in our study strongly supports the feasibility of this model. In addition, larger animal models (e.g. canine) that more closely resemble the size and anatomy of human flexor tendons allow for testing the effects of passive motion/loading protocols in reducing adhesions,6, 24, 26 which we were unable to reproduce in this small animal model for obvious technical reasons. Furthermore, while the mouse FDL tendon graft model does not represent a true zone II reconstruction model, we deliberately immobilized the flexor mechanism by severing the proximal FDL tendon insertion in the flexor muscle to abolish early tendon gliding. This resulted in impairment of MTP joint flexion via mechanisms that have the hallmarks of adhesions including histological evidence of fibrotic scar tissue especially around the live autografts similar to those observed in larger animals such as dogs which have been used for years in flexor tendon repair research.
To quantify the effects of adhesions on the biomechanics of the flexor mechanism in our murine FDL tendon model, we developed an innovative Gliding Coefficient as a measure of the resistance to tendon gliding and MTP joint flexion. Since we did not measure the MTP joint flexion angle by another method that would constitute a golden standard against which to assess the accuracy of the measurements, we computed the intra- and inter-observer reproducibility of the joint angle measurements and determined that the average intra- and inter-observer errors were < 1% which provides confidence about the reproducibility of the MTP joint flexion angle measurements. Furthermore, the MTP joint flexion test is non-destructive and allows for subsequent biomechanical testing of the grafts since the maximum applied excursion load of 19 grams was about 10% of the failure force of the 14 day grafts which was the earliest healing time point we tested.
As a measure of adhesions, previous studies have reported the digital range of motion upon the application of a single defined load to cause tendon excursion.21, 24 By contrast, the Gliding Coefficient is based on joint flexion data over a range of applied loads that would cause a maximum 75° flexion in a normal unoperated MTP joint. The test offers information about the joint ROM (the plateau) and the resistance to flexion with increased loading (the gliding coefficient). The Gliding Coefficient is similar to the Work of Flexion which measures the resistance to flexion over a range of applied excursions. The work of flexion test is feasible in larger animal models that allow flexion testing under displacement control without the risk of damaging the graft. However, due to the small size of the tendon and low levels of force required to effect flexion, our test was conducted under load control to ensure that we do not induce loading that would be damaging to the tendons. There are other advantages to using the Gliding Coefficient rather than reporting a single joint flexion angle. First, if the “single” flexion angle or ROM is incorrectly reported due to measurement error or due to an error in the determination of the neutral position, it would be difficult to observe this error as an outlier. Instead, by recording and plotting the flexion angle over a range of applied loads, and computing the Gliding Coefficient based on the mathematical model as a “rate” constant for joint flexion under controlled loading, we can easily identify those measurements that deviate from the model and provide erroneous estimation of the joint function. Since this is a novel measure to assess the resistance to joint flexion under load control, we examined the correlation between the GC and the maximum MTP joint flexion angle range of motion (ROM) and reported a strong negative correlation (r=−0.97) which corroborates the GC as a measure of the resistance to joint flexion sensitive to the effects of adhesions and less prone to the effects of errors inherent in measuring a single angle as the ROM.
As hypothesized, at 14 and 28 days post-grafting both live autografts and reconstituted freeze-dried allografts had significantly greater Gliding Coefficients and hence more adhesions than normal unoperated tendons or time zero repairs. Interestingly, by 42 days post-operatively and thereafter the Gliding Coefficient was not different than time zero repairs for both autografts and allografts. Histologically, the amount of fibrotic tissue surrounding the 28-day autografts and allografts is markedly reduced by 42 days. There are two possible explanations for these improvements. First, the noted improvement in joint flexion may be a result of the resumption of tendon excursion after the proximal tendon-muscle insertion had been allowed sufficient time to heal and restore the flexor mechanism. This theory is based on the anecdotal observation that the mice more actively used their operated limbs by 28 days. Previous studies have suggested that small flexor tendon excursions following injury may be sufficient for full restoration of the flexion range of motion.24 Regardless, this feature of our model is different than the clinical experience which suggests that the onset of fibrotic adhesions does not resolve spontaneously and might require meticulous tenolysis surgery.3 Second, we hypothesized that the marked increase in the expression of Gdf5 and Vegfa mRNA might be involved in the improvements in joint flexion after 28 days. Whether this increased mRNA expression translates into increased GDF5 and VEGF protein synthesis at the repair site after 28 days remains to be verified in future experiments using immunohistochemistry.
In agreement with the limited data in the literature, we found that reconstituted freeze-dried allografts did not cause increased adhesions compared to live autografts. To the contrary, 28-day live autografts experienced a significant fivefold increase in their Gliding Coefficients compared to the processed allografts. Previous studies compared the healing of flexor tendon autografts and freeze-dried allografts implanted in the paws of dogs and reported that: 1) the implanted allografts were tolerated well by the host; and 2) the implanted allografts allowed flexor tendon function similar to that allowed by autografts.10 Others reported similar observation in bovine flexor tendons suggesting that acellular allografts induced minimal adhesion formation.11, 12 It has been recognized for quite some time that even minor manipulations of a live tendon graft such as the passing of a suture through the tendon induces an “intrinsic” inflammatory stimulation of the resident cells. Since freeze-dried allografts are acellular the intrinsic inflammatory response is not expected. It is conceivable therefore that autograft transplantation may exacerbate the adhesion tissues, presumably resulting from the surgical manipulation of a live graft that might lead to inflammatory stimulation of tenocyte proliferation and migration from the live graft in addition to other intrinsic and extrinsic factors.5
A number of factors may have been responsible for the observation that murine FDL tendon allografts and autografts were similar in terms of their failure tensile properties, but remained significantly weaker than normal unoperated tendons despite modest increases over time. While both grafts initially provided a scaffold to bridge the experimental defect, the two grafts supposedly heal with different mechanisms. Live autografts likely heal via intrinsic and extrinsic mechanisms that involve the graft tenocytes as well as the influx of synovial fibroblasts, precursor cells, and inflammatory cells, respectively.3, 25 As a result, autografts underwent extensive remodeling that negatively affected the rate of accrual of biomechanical strength over time as has been reported for flexor tendon gap defects.26 By contrast, the acellular allografts can only heal by extrinsic mechanisms. Potenza et al demonstrated that extrinsic cells from the synovial capsule of the joint populated and contributed to the healing of lacerations within freeze-dried allografts implanted in canine and rabbit knee joints.27, 28 In our model, we observed modest scarring around the mostly acellular middle segment of the allograft at 14 and 28 days that remained isolated resembling a foreign body response. However at the interface with the host tendon stubs, hypercellular scarring was exuberant in bridging and remodeling the allograft-host juncture, resulting in cellular infiltration into the graft. While the allografts appeared to undergo little remodeling compared to live autografts, the accrual of biomechanical strength was still as slow as live autografts possibly due to the localization of the repair response to the graft ends. In both groups, however, the abolishment of tendon gliding and loading due to the deliberate severing of the proximal tendon-muscle insertion is likely the factor that slows return toward normal biomechanical properties. Therefore, clinical interpretations about the biomechanical equivalency of live autograft and freeze-dried allografts from this model should only be made with the limitations of the model in mind.
Admittedly, the clinical utility of freeze-dried (lyophilized) tendon allografts is debatable. There are clinical reports that suggest that freeze-dried allografts are of no significant value in the surgical management of certain indications such as chronic massive rotator cuff tears,29 and may induce intraarticular reaction when used in ACL reconstruction,30, 31 for example. However, other clinical reports indicate that freeze-dried allografts used for ligament and tendon repairs and arthroscopic reconstruction of ACL deficient knees provide satisfactory clinical results.32, 33 Animal studies including our own data indicate that implanted freeze-dried tendon/ligament allografts are similar in (biomechanical) strength to live tendon/ligament autografts.10 Furthermore, a freeze-dried animal or human tendon rehydrates easily before surgical implantation without adverse effects on their biomechanical properties as we (Figure 3) and others have reported.34
The patterns of growth factor gene expression have been previously described in flexor tendon healing,35–37 but not for autograft and allograft flexor tendon reconstruction models. In our study we evaluated the expression of Tgfb1, Gdf5, and Vegfa transcripts on days 14 and 28 post-surgery, which corresponded to the earliest time point where adhesions were observed. We found a twofold increase in these transcript levels (compared to normal tendon) in 28-day allografts. By contrast, the level of Tgfb1mRNA expression was not upregulated in the live autografts at either 14 or 28 days. It is quite possible that the upregulation in Tgfb1expression might have been an earlier event in the repair response of autografts and allografts since previous studies suggested that Tgfb1mRNA levels are nearly 3.5-fold increased in a rabbit flexor tendon healing model as early as 3 days and remain upregulated through 12 days of healing before returning to normal levels at 24 days.35 Interestingly, we observed that the level of expression of Vegfa was increased by 20-fold in the autografts at 28 days. These observations are somewhat similar to reports that demonstrated that Vegfa mRNA levels more than double at 7 and 10 days of healing following canine flexor tendon injury.36, 37 The differences in the levels and temporal kinetics of Vegfa upregulation maybe related to the relative size of the graft compared to primary healing. While local and direct GDF-5 protein delivery on collagen sponge implants has been shown to increase the tensile strength of rat Achilles tendon repair tissues,38 to the best of our knowledge the effects of this growth factor on flexor tendon adhesion formation are unknown. In our model, we observed that Gdf5 mRNA levels were sevenfold increased in 28-day autografts, which experienced the highest levels of adhesions. This increase in Gdf5 transcription was concomitant with the observed increases in VEGF expression. This observation is consistent with recent reports that suggest that GDF-5 promotes angiogenic activity of stromal cells by increasing VEGF gene expression in vitro.39 How GDF-5 and VEGF might be implicated in the observed adhesions in our murine model of flexor tendon grafts remains to be carefully evaluated.
In conclusion, we developed the first murine model of flexor tendon grafts along with an innovative outcome measure for the quantitative assessment of joint flexion function. Despite its limitations, our model has the potential to enable systematic testing of the cellular and molecular events involved in repair and adhesion formation through the utilization of transgenic mouse models in future studies. Furthermore, the model can potentially aid in rapid and inexpensive screening of novel molecular treatments of flexor tendon adhesions.
Table 1.
Primer sequences for real-time RT-PCR
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Tgfb1 | 5′-CTTTAGGAAGGACCTGGGTT-3′ | 5′-CAGGAGCGCACAATCATGTT-3′ |
| Gdf5 | 5′-TCCTTCCTGCTGAAGAAGAACA-3′ | 5′-TAAAGCTGGTGATGGTGTTGGC-3′ |
| Vegf | 5′-TTCAGAGCGGAGAAAGCATT-3′ | 5′-GAGGAGGCTCCTTCCTGC-3′ |
| Beta-actin | 5′-AGATGTGGATCAGCAAGCAG-3′ | 5′-GCGCAAGTTAGGTTTTGTCA-3′ |
ACKNOWLEDGEMENTS
Drs. Hasslund and Jacobson share first authorship as they had equal contributions to the work. The Authors would like to thank Dr. Brendan Boyce for the valuable advice and stimulating discussions, Krista Scorsone for technical assistance with the histology, and David Reynolds for technical assistance with biomechanical testing. This work was supported in part by research grants from the National Institutes of Health (PHS AR054041, AR051469, DE017096), Whitaker Foundation, the Danish Medical Research Council, the Musculoskeletal Transplant Foundation, and the Orthopaedic Research Education Foundation (OREF).
REFERENCES
- 1.Taras JS, Lamb MJ. Treatment of flexor tendon injuries: surgeons' perspective. Journal of Hand Therapy. 1999;12:141–148. doi: 10.1016/s0894-1130(99)80016-7. [DOI] [PubMed] [Google Scholar]
- 2.Bunnell S. The injured hand; principles of treatment. Ind Med Surg. 1953;22:251–254. [PubMed] [Google Scholar]
- 3.Lilly SI, Messer TM. Complications after treatment of flexor tendon injuries. J Am Acad Orthop Surg. 2006;14:387–396. doi: 10.5435/00124635-200607000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Stark HH, Anderson DR, Zemel NP, Boyes JH, Ashworth CR, Rickard TA. Bridge flexor tendon grafts. Clin Orthop Relat Res. 1989:51–59. [PubMed] [Google Scholar]
- 5.Taras JS, Kaufmann RA. Flexor Tendon Reconstruction. In: Green DP, Hotchkiss RN, Pederson WC, Wolfe SW, editors. Green's Operative Hand Surgery. Fifth ed. Philadelphia: Elsevier Churchill Livingstone; 2005. pp. 241–276. [Google Scholar]
- 6.Gelberman RH, Manske PR. Factors influencing flexor tendon adhesions. Hand Clin. 1985;1:35–42. [PubMed] [Google Scholar]
- 7.Abrahamsson SO, Gelberman RH, Amiel D, Winterton P, Harwood F. Autogenous flexor tendon grafts: fibroblast activity and matrix remodeling in dogs. J Orthop Res. 1995;13:58–66. doi: 10.1002/jor.1100130110. [DOI] [PubMed] [Google Scholar]
- 8.Seiler JG, 3rd, Chu CR, Amiel D, Woo SL, Gelberman RH. The Marshall R. Urist Young Investigator Award. Autogenous flexor tendon grafts. Biologic mechanisms for incorporation. Clin Orthop Relat Res. 1997:239–247. [PubMed] [Google Scholar]
- 9.Liu TK. Clinical use of refrigerated flexor tendon allografts to replace a silicone rubber rod. Journal of Hand Surgery - American Volume. 1983;8:881–887. doi: 10.1016/s0363-5023(83)80087-2. [DOI] [PubMed] [Google Scholar]
- 10.Webster DA, Werner FW. Mechanical and functional properties of implanted freeze-dried flexor tendons. Clinical Orthopaedics & Related Research. 1983:301–309. [PubMed] [Google Scholar]
- 11.Ramesh R, Kumar N, Sharma AK, Maiti SK, Singh GR. Acellular and glutaraldehyde-preserved tendon allografts for reconstruction of superficial digital flexor tendon in bovines. Part I–Clinical, radiological and angiographical observations. Journal of Veterinary Medicine - Series A. 2003;50:511–519. doi: 10.1111/j.1439-0442.2004.00578.x. [DOI] [PubMed] [Google Scholar]
- 12.Ramesh R, Kumar N, Sharma AK, Maiti SK, Kumar S, Charan K. Acellular and glutaraldehyde-preserved tendon allografts for reconstruction of superficial digital flexor tendon in bovines. Part II–Gross, microscopic and scanning electron microscopic observations. Journal of Veterinary Medicine - Series A. 2003;50:520–526. doi: 10.1111/j.1439-0442.2004.00579.x. [DOI] [PubMed] [Google Scholar]
- 13.Mikic B, Schalet BJ, Clark RT, Gaschen V, Hunziker EB. GDF-5 deficiency in mice alters the ultrastructure, mechanical properties and composition of the Achilles tendon. J Orthop Res. 2001;19:365–371. doi: 10.1016/S0736-0266(00)90018-4. [DOI] [PubMed] [Google Scholar]
- 14.Ito H, Koefoed M, Tiyapatanaputi P, Gromov K, Goater JJ, Carmouche J, Zhang X, Rubery PT, Rabinowitz J, Samulski RJ, Nakamura T, Soballe K, O'Keefe RJ, Boyce BF, Schwarz EM. Remodeling of cortical bone allografts mediated by adherent rAAV-RANKL and VEGF gene therapy. Nat Med. 2005;11:291–297. doi: 10.1038/nm1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gelberman RH, Seiler JG, 3rd, Rosenberg AE, Heyman P, Amiel D. Intercalary flexor tendon grafts. A morphological study of intrasynovial and extrasynovial donor tendons. Scand J Plast Reconstr Surg Hand Surg. 1992;26:257–264. doi: 10.3109/02844319209015268. [DOI] [PubMed] [Google Scholar]
- 16.Dovelle S, Heeter PK, Fischer DR, Chow JA. Early controlled motion following flexor tendon graft. Am J Occup Ther. 1988;42:457–463. doi: 10.5014/ajot.42.7.457. [DOI] [PubMed] [Google Scholar]
- 17.Silfverskiold KL, May EJ, Tornvall AH. Tendon excursions after flexor tendon repair in zone. II: Results with a new controlled-motion program. J Hand Surg [Am] 1993;18:403–410. doi: 10.1016/0363-5023(93)90082-e. [DOI] [PubMed] [Google Scholar]
- 18.Silfverskiold KL, May EJ. Gap formation after flexor tendon repair in zone. II: Results with a new controlled motion programme. Scand J Plast Reconstr Surg Hand Surg. 1993;27:263–268. [PubMed] [Google Scholar]
- 19.Jorgensen HG, McLellan SD, Crossan JF, Curtis AS. Neutralisation of TGF beta or binding of VLA-4 to fibronectin prevents rat tendon adhesion following transection. Cytokine. 2005;30:195–202. doi: 10.1016/j.cyto.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 20.Chang J, Most D, Stelnicki E, Siebert JW, Longaker MT, Hui K, Lineaweaver WC. Gene expression of transforming growth factor beta-1 in rabbit zone II flexor tendon wound healing. evidence for dual mechanisms of repair. Plast Reconstr Surg. 1997;100:937–944. doi: 10.1097/00006534-199709001-00016. [DOI] [PubMed] [Google Scholar]
- 21.Chang J, Thunder R, Most D, Longaker MT, Lineaweaver WC. Studies in flexor tendon wound healing. neutralizing antibody to TGF-beta1 increases postoperative range of motion. Plast Reconstr Surg. 2000;105:148–155. doi: 10.1097/00006534-200001000-00025. [DOI] [PubMed] [Google Scholar]
- 22.Chang J, Most D, Thunder R, Mehrara B, Longaker MT, Lineaweaver WC. Molecular studies in flexor tendon wound healing. the role of basic fibroblast growth factor gene expression. J Hand Surg [Am] 1998;23:1052–1058. doi: 10.1016/S0363-5023(98)80015-4. [DOI] [PubMed] [Google Scholar]
- 23.Khan U, Kakar S, Akali A, Bentley G, McGrouther DA. Modulation of the formation of adhesions during the healing of injured tendons. J Bone Joint Surg Br. 2000;82:1054–1058. doi: 10.1302/0301-620x.82b7.9892. [DOI] [PubMed] [Google Scholar]
- 24.Silva MJ, Brodt MD, Boyer MI, Morris TS, Dinopoulos H, Amiel D, Gelberman RH. Effects of increased in vivo excursion on digital range of motion and tendon strength following flexor tendon repair. J Orthop Res. 1999;17:777–783. doi: 10.1002/jor.1100170524. [DOI] [PubMed] [Google Scholar]
- 25.Boyer MI. Flexor tendon biology. Hand Clin. 2005;21:159–166. doi: 10.1016/j.hcl.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Gelberman RH, Boyer MI, Brodt MD, Winters SC, Silva MJ. The effect of gap formation at the repair site on the strength and excursion of intrasynovial flexor tendons. An experimental study on the early stages of tendon-healing in dogs. J Bone Joint Surg Am. 1999;81:975–982. doi: 10.2106/00004623-199907000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Chow SP, Hooper G, Chan CW. The healing of freeze-dried rabbit flexor tendon in a synovial fluid environment. Hand. 1983;15:136–142. doi: 10.1016/s0072-968x(83)80002-3. [DOI] [PubMed] [Google Scholar]
- 28.Potenza AD, Herte MC. The synovial cavity as a “tissue culture in situ”–science or nonsense? J Hand Surg [Am] 1982;7:196–199. doi: 10.1016/s0363-5023(82)80088-9. [DOI] [PubMed] [Google Scholar]
- 29.Nasca RJ. The use of freeze-dried allografts in the management of global rotator cuff tears. Clinical Orthopaedics & Related Research. 1988:218–226. [PubMed] [Google Scholar]
- 30.Jackson DW, Windler GE, Simon TM. Intraarticular reaction associated with the use of freeze-dried, ethylene oxide-sterilized bone-patella tendon-bone allografts in the reconstruction of the anterior cruciate ligament. Am J Sports Med. 1990;18:1–10. doi: 10.1177/036354659001800101. discussion 10–11, [DOI] [PubMed] [Google Scholar]
- 31.Roberts TS, Drez D, Jr, McCarthy W, Paine R. Anterior cruciate ligament reconstruction using freeze-dried, ethylene oxide-sterilized, bone-patellar tendon-bone allografts. Two year results in thirty-six patients.[erratum appears in Am J Sports Med 1991 May-Jun;19(3):272] American Journal of Sports Medicine. 1991;19:35–41. doi: 10.1177/036354659101900106. [DOI] [PubMed] [Google Scholar]
- 32.Bright RW, Green WT. Freeze-dried fascia lata allografts. a review of 47 cases. J Pediatr Orthop. 1981;1:13–22. doi: 10.1097/01241398-198101010-00003. [DOI] [PubMed] [Google Scholar]
- 33.Wainer RA, Clarke TJ, Poehling GG. Arthroscopic reconstruction of the anterior cruciate ligament using allograft tendon. Arthroscopy. 1988;4:199–205. doi: 10.1016/s0749-8063(88)80027-6. [DOI] [PubMed] [Google Scholar]
- 34.Bechtold JE, Eastlund DT, Butts MK, Lagerborg DF, Kyle RF. The effects of freeze-drying and ethylene oxide sterilization on the mechanical properties of human patellar tendon. American Journal of Sports Medicine. 1994;22:562–566. doi: 10.1177/036354659402200421. [DOI] [PubMed] [Google Scholar]
- 35.Berglund M, Reno C, Hart DA, Wiig M. Patterns of mRNA expression for matrix molecules and growth factors in flexor tendon injury. differences in the regulation between tendon and tendon sheath. J Hand Surg [Am] 2006;31:1279–1287. doi: 10.1016/j.jhsa.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 36.Boyer MI, Watson JT, Lou J, Manske PR, Gelberman RH, Cai SR. Quantitative variation in vascular endothelial growth factor mRNA expression during early flexor tendon healing. an investigation in a canine model. J Orthop Res. 2001;19:869–872. doi: 10.1016/S0736-0266(01)00017-1. [DOI] [PubMed] [Google Scholar]
- 37.Bidder M, Towler DA, Gelberman RH, Boyer MI. Expression of mRNA for vascular endothelial growth factor at the repair site of healing canine flexor tendon. J Orthop Res. 2000;18:247–252. doi: 10.1002/jor.1100180212. [DOI] [PubMed] [Google Scholar]
- 38.Aspenberg P, Forslund C. Enhanced tendon healing with GDF 5 and 6. Acta Orthop Scand. 1999;70:51–54. doi: 10.3109/17453679909000958. [DOI] [PubMed] [Google Scholar]
- 39.Zeng Q, Li X, Beck G, Balian G, Shen FH. Growth and differentiation factor-5 (GDF-5) stimulates osteogenic differentiation and increases vascular endothelial growth factor (VEGF) levels in fat-derived stromal cells in vitro. Bone. 2007;40:374–381. doi: 10.1016/j.bone.2006.09.022. [DOI] [PubMed] [Google Scholar]