SUMMARY
Development of a multicellular organism requires precise coordination of cell division and cell type determination. The selector homeoprotein Even skipped (Eve) plays a very specific role in determining cell identity in the Drosophila embryo, both during segmentation and in neuronal development. However, studies of gene expression in eve mutant embryos suggest that eve regulates the embryonic expression of the vast majority of genes. We present here genetic interaction and phenotypic analysis showing that eve functions in the trol pathway to regulate the onset of neuroblast division in the larval CNS. Surprisingly, Eve is not detected in the regulated neuroblasts, and culture experiments reveal that Eve is required in the body, not the CNS. Furthermore, the effect of an eve mutation can be rescued both in vivo and in culture by the hormone ecdysone. These results suggest that eve is required to produce a trans-acting factor that stimulates cell division in the larval brain.
Keywords: eve, trol, Ecdysone, Neuroblast, Proliferation, Drosophila
INTRODUCTION
Proper spatiotemporal regulation of cell cycle progression is essential for the successful development of multicellular organisms. Many evolutionarily conserved cell cycle regulators have been identified in both vertebrate and invertebrate systems. Although their functions in cell cycle progression have been extensively analyzed, relatively little is known about how the cell cycle is regulated during development, especially in vivo. Several studies in Drosophila melanogaster provide us with a glimpse into the developmental regulation of the cell cycle. The coordination of embryonic cell division in mitotic domains foreshadows cell fate domains, as revealed by fate mapping studies (Foe, 1989). The timing of cell division coincides with the patterning and differentiation of imaginal discs (reviewed by Edgar and Lehner, 1996). Alterations of the cell cycle period or the number of cycles can change the expression patterns of genes that determine neuronal identity (Cui and Doe, 1995; Weigmann and Lehner, 1995). Thus, regulation of the cell cycle is closely related to the regulation of pattern formation and to differentiation during development.
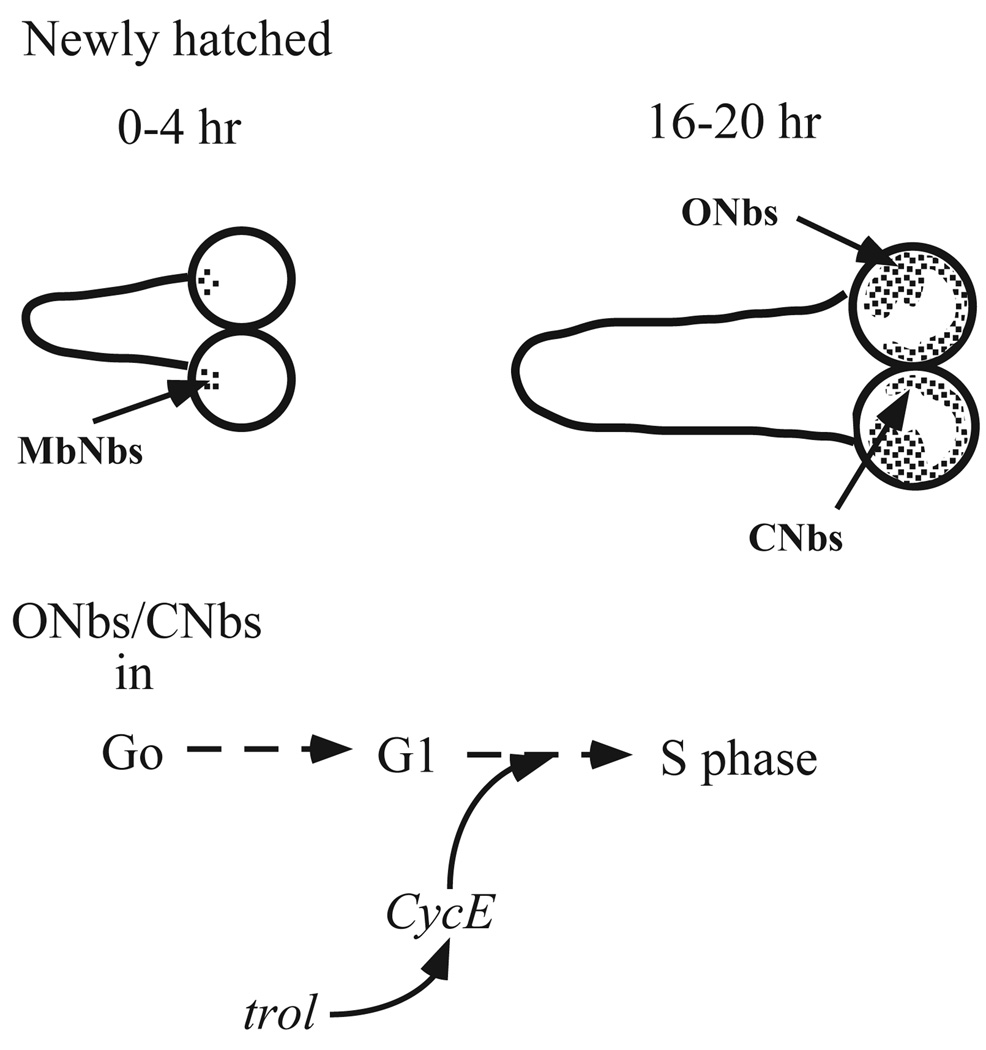
The central nervous system (CNS) of the fruit fly, Drosophila melanogaster, is an excellent system in which to study genetic control of the cell cycle in the context of development. The CNS contains several populations of neuronal precursor cells called neuroblasts with characteristic profiles of cell cycle progression during development (Hofbauer and Campos-Ortega, 1990; Ito and Hotta, 1991; Truman and Bate, 1988; White and Kankel, 1978). The adult CNS is formed by periods of neurogenesis during embryonic and larval stages. Larval phase neurogenesis occurs in a stereotyped spatial and temporal pattern as mitotically quiescent larval neuroblasts reactivate cell cycle progression (Fig. 1). Both observations of quiescent neuroblasts soon after hatching (Truman and Bate, 1988) and classical mammalian studies (Pardee, 1989), suggest that neuroblasts may initially be arrested in G0, and activate division by proceeding into G1 and then into S phase. The regulated neuroblasts are divided into sub-groups, depending on the developmental fate of their progeny and their kinetics of proliferation. Optic lobe neuroblasts stop cell division at embryonic cell cycle 17 (Campos-Ortega and Hartenstein, 1985). They remain quiescent until late first instar when they reactivate cell division and continue to divide until the pupal stage (White and Kankel, 1978). The central brain neuroblasts start cell division by mid-embryogenesis and become quiescent in late embryogenesis. They reenter the cell cycle at late first instar (Campos-Ortega and Hartenstein, 1985). Only the four mushroom body neuroblasts and one lateral neuroblast located at the ventrolateral side of each hemisphere begin cell division in embryogenesis and continue to divide through larval life (Ito and Hotta, 1991).
Fig. 1.
Neuroblast division in the larval CNS. (A) Mushroom body neuroblasts (MbNbs) divide at 0–4 hours post hatching (ph). (B) Central brain (CNbs) and optic lobe (Onbs) neuroblasts divide by 16–20 hours ph. (C) trol is postulated to stimulate cell cycle progression in CNbs and Onbs by increasing expression of the cell cycle regulator Cyclin E (CycE).
Several genes have been identified that affect neuroblast proliferation (Datta and Kankel, 1992; Ebens et al., 1993; Lipshitz and Kankel, 1985; Prokop and Technau, 1994), including anachronism (ana), terribly reduced optic lobes (trol) and even skipped (eve). trol was originally identified in a genetic screen for abnormal larval brain morphology that was due to defective patterns of neuroblast proliferation in the larval brain (Datta and Kankel, 1992). Mutations in trol cause a dramatic decrease in the reactivation of proliferation from mitotic quiescence (Datta, 1995). Recent studies suggest that trol may regulate this reactivation of neuroblast proliferation by stimulating the G1/S transition through upregulation of Cyclin E (CycE) expression (Caldwell and Datta, 1998). Several studies on trol and ana have led to the hypothesis that trol is required to overcome the repression of neuroblast cell division imposed by ana (Datta, 1995; Datta and Kankel, 1992; Ebens et al., 1993). eve, a homeodomain-containing transcriptional repressor, was identified in a screen for enhancers of the hypomorphic allele trolb22 (Park et al., 1998). Mutations in eve enhanced both the trolb22 proliferation phenotype and the associated lethality, indicating that eve may regulate transcription of cell cycle genes in the trol pathway.
eve plays a key role in many cell fate decisions in the developing embryo, ranging from segmentation to neuronal identity. While the role of eve in the determination of specific neuronal identity in the embryonic CNS appears to be part of a cell-autonomous cascade of transcription factors, its function during earlier embryonic segmentation is mediated, in part, by regulation of the localized signaling factors Hedgehog and Wingless (reviewed by Akam, 1987). Other studies have suggested that eve plays a direct role in controlling transcription of several genes, including Adh and ry (Liang and Biggin, 1998). The contrasting views of eve as a specific developmental regulator versus eve as a general transcriptional factor have yet to be resolved.
Another factor implicated in the developmental coordination of cell division is the hormone ecdysone. Ecdysone plays a role in the initiation of imaginal histoblast division and the proliferation of post-embryonic neuroblasts in Manduca and Drosophila (Champlin and Truman, 1998). Ecdysone is also required for the activation of mitotically quiescent neuroblasts in explanted Drosophila larval CNS, but addition of ecdysone does not rescue the proliferation phenotype of trol mutant CNS in culture (Datta, 1999).
We present evidence that eve is required for production of a trans-acting signal that regulates activation of neuroblast proliferation and can be mimicked by ecdysone. We demonstrate that loss of eve function produces increased lethality and cell cycle arrest that is consistent with eve function in the trol pathway, and that eve function requires an intact C-terminal domain, in addition to the homeodomain and a repression domain. Importantly, both Eve distribution within the larval CNS and neuroblast division in a heterogenetic explant/extract system show that eve expression is not required in the regulated neuroblasts, or even in the larval CNS, to stimulate cell division, but instead is required in some other tissue(s). Furthermore, addition of ecdysone either in vitro or in vivo rescues the defective neuroblast proliferation caused by a mutation in eve. These studies reveal that heterozygous mutations in eve in one part of the larva can affect the generation of a signal that impacts cellular events in a separate organ of the developing fruit fly.
MATERIALS AND METHODS
Genetic strains and transgenes
Flies were grown in standard medium at 25°C. Markers and balancer chromosomes are described in Lindsley and Zimm (Lindsley and Zimm, 1992). trolb22 and trolsd have been previously described (Datta, 1995; Datta and Kankel, 1992; Shannon et al., 1972). The trol4 and trol8 alleles were isolated from independent mutageneses (S. D., M. C. C., M. M. R., C. R., Y. P. and S. M., unpublished). trolb22, trol4, trol8 and trolsd mutant animals were obtained from y trolb22 stock and from y trolx w/Binsn stocks. y trolb22; hs-CycE flies were constructed in our laboratory from a y trolb22 and a hs-CycE stock described previously (Caldwell and Datta, 1998). Hemizygous y trolx w/Y; eve3/+ larvae were obtained by mating of y trolx w/Binsn virgin female flies to eve3/CyO y+ male flies. Elliott Goldstein has generously provided eve mutations eve58-11, eve14-10, eve20–35 and eve10-5. The eve5/CyO, hb-lacZ stock was used to distinguish homozygous eve5 from heterozygous embryos.
The parental eve transgene, P[eve+], capable of fully rescuing eve null mutants, was described previously (Fujioka et al., 1999). Derivatives of this construct (Kobayashi, et al., 2001) contain the following alterations in the LFKPY motif near the C terminus of the protein-coding region (see Table 3): EGNΔLFK, a STOP codon inserted just before LFKPY, which removes the C-terminal 10 amino acids; EGNHA, the Hairy family Groucho interaction motif WRPW in place of FKPY; EGNPA in place of LFKPA; EGNAY in place of LFKAY.
Table 3.
Effect of LFKPY amino acid sequence on trolb22; eve3 /+ lethality
| Male |
|||
|---|---|---|---|
| Parents, (male × female) |
eve3, P/+ | CyO/+ | Ratio* |
| EGNDΔLFK (A-2) × trolb22 | 240 | 119 | 2.1± 0.3 (n=3) |
| EGNDΔLFK (A-2) × CS | 343 | 282 | 1.2±0.06 (n=2) |
| EGNHA (C-2) × trolb22 | 404 | 176 | 2.7±0.61 (n=3) |
| EGNHA (C-2) × CS | 539 | 407 | 1.4±0.14 (n=2) |
| EGNHA (F-3) × trolb22 | 223 | 139 | 1.7±0.23 (n=3) |
| EGNHA (F-3) × CS | 462 | 392 | 1.2±0.04 (n=2) |
| eve3/+; P/+ | CyO/+; P/+ | Ratio‡ | |
| P[eve+] × trolb22 | 253 | 31 | 0.12±0.02 (n=3) |
| P[eve+] × CS | 85 | 83 | 0.98 |
| EGNPA (F-2) × trolb22 | 312 | 180 | 0.53±0.08 (n=3) |
| EGNPA (F-2) × CS | 513 | 417 | 0.82±0.04 (n=2) |
| EGNAY (D-1) × trolb22 | 270 | 98 | 0.30±0.07(n=4) |
| EGNAY (D-1) × CS | 293 | 249 | 0.84±0.08 (n=2) |
| EGNAY (E-1) × trolb22 | 279 | 91 | 0.31±0.03 (n=3) |
| EGNAY (E-1) × CS | 354 | 368 | 1.1±0.1 (n=2) |
Ratio, (number of eve3, P/+ flies)/(number of CyO/+ flies).
Ratio, (number of CyO/+; P/+ flies)/(number of eve3/+; P/+flies).
Contents inside parentheses of each genotype refer to independent transgenic lines.
trol lethality screening
Virgin homozygous y trolb22 females were mated to males carrying different eve mutations. The progeny of each population were counted, and the ratio of y trolb22/Y; evex/+ to y trolb22/Y; CyO/+ was calculated. The closer the ratio is to 0, the stronger the enhancement of trolb22 lethality. Because heterozygosity at eve alone could impact viability, males from the same genotype were also crossed to Canton S females as a control. Virgin homozygous y trolb22; hs-CycE females were mated to males from eve3/CyO, y+ to ask if ectopic CycE expression can rescue the enhanced lethality of hemizygous y trolb22/ Y; eve3/+ animals.
BrdU incorporation and neuroblast counting
5′-bromodeoxyuridine (BrdU) incorporation was analyzed as previously described (Datta, 1995; Park et al., 1998). The average number of BrdU-labeled neuroblasts in sibling controls was calculated and used for normalization of neuroblast proliferation levels between experiments. Control normalized proliferation ranged from 0.8 to 1.3 in almost all genotypes. The number of labeled neuroblasts in each mutant population was also normalized by the average of sibling controls to determine if mutations in specific genes result in proliferation defects. If mutation(s) cause a proliferation phenotype, the population distribution shifts to lower values. At least three independent crosses were analyzed for each genotype.
Embryo analysis and cuticle preparation
In situ hybridization was performed as described (Tautz and Pfeifle, 1989) using digoxigenin-labeled antisense engrailed probe visualized via the alkaline phosphatase reaction with NBT/CIBP. In situ hybridization was followed by staining with anti-Eve antiserum (provided by M. Frasch). The antibody was visualized via the horse radish peroxidase reaction with DAB, as described (Mullen and DiNardo, 1995). For cuticle preparations, after devitellinization, embryos were mounted in a 1:1 mixture of Hoyer’s reagent and 30% lactic acid, then cleared by incubation at 55°C.
Sequence analysis, immunohistochemistry and Western analysis of eve5
PCR was done as previously described (Park et al., 1998). PCR products of mutated eve5 DNA were isolated and cut with EcoRI and SpeI. The resulting PCR fragments were cloned into the pBluescript vector and sequenced on both strands.
Expression of Eve proteins in larval brains and embryos was monitored by immunohistochemical analysis with mouse monoclonal 2B8 antibody (provided by N. Patel) and guinea pig anti-Eve polyclonal antibody (provided by D. Kosman). Secondary antibodies were peroxidase-conjugated goat anti-mouse IgG (Jackson ImmunoResearch, 115-035-003) or Alexa™ 488-conjugated goat anti-mouse IgG (Molecular Probe, A-11001). Homozygous eve5 embryos were identified by the lack of staining with rabbit anti-β galactosidase antibody (Chemicon, AB986) and peroxidase-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch, 111-035-003). Western analysis of Eve proteins in embryos was done as follows: 2–4 hour old embryos were dechorionated, protease inhibitor cocktail (Roche, 1-836-153) and 2× Laemmli sample buffer were added to 1× strength, and the sample was homogenized. Whole homogenate was centrifuged for 30 seconds at 14,000 g and the supernatant analyzed. Eve was detected either by guinea pig anti-Eve polyclonal antibody or by mouse monoclonal 3C10 antibody (DSHB).
Ecdysone feeding
1 mg/ml 20-hydroxyecdysone (Sigma) in BrdU-containing medium (Caldwell and Datta, 1998; Datta, 1995; White and Kankel, 1978) was used for ecdysone feeding and BrdU labeling from 16–20 hours posthatching. Plain media was used from 0–16 hours post hatching. For ecdysone feedings from 0–16 hours post hatching, 20 µl of 1 mg/ml 20-hydroxyecdysone in 10% isopropanol were mixed with 1 mg of dry yeast. Newly hatched larvae were placed in the middle of the yeast paste to maximize probability of feeding.
Preparation of larval extract and explant culture
Larval extracts from stocks of different genotypes were prepared from first instar larvae as previously described (Datta, 1999). Extracts were heat-treated after preparation and stored at –70°C. Explant culture analysis of different genotypes was as described (Datta, 1999).
Statistical analysis
Standard error of the mean was calculated. The significance of differences between the mean response indices of two different populations was evaluated using the Student’s t-test.
RESULTS
eve transheterozygotes show defective neuroblast proliferation
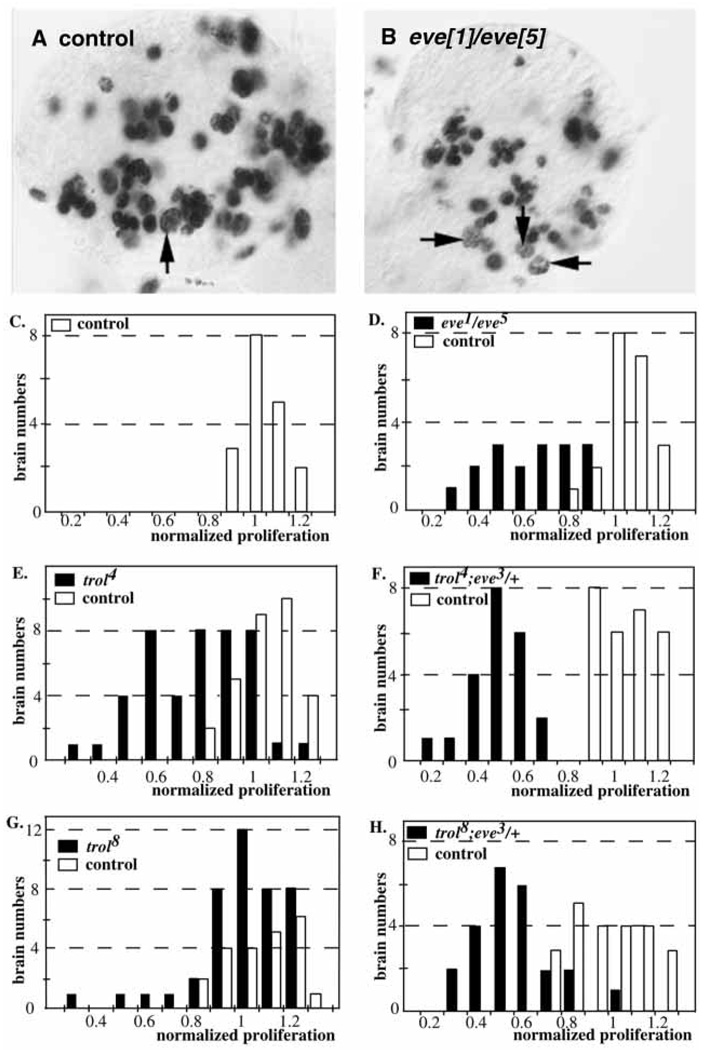
We have previously shown that eve is a dominant enhancer of trolb22, and that heterozygosity for strong eve3 or eve4 mutations does not cause defective proliferation (Park et al., 1998), suggesting that eve is in the trol pathway. If so, homozygous eve mutations might cause a proliferation phenotype in the larval CNS. Complementation analysis showed that a very small number of eve1/eve5 transheterozygous flies survived to adulthood (O’Brien et al., 1994). This enabled us to ask if eve1/eve5 larval brains exhibit defective proliferation. y w/Y; eve1/CyO, y+ flies were crossed to y w; eve5/CyO, y+ females, and eve1/eve5 mutants were selected as yellow larvae. eve1/eve5 transheterozygotes show decreased BrdU labeling, resulting in a shift of normalized BrdU incorporation from control values (ranging from 0.8 to 1.2 in sibling eve1/+ or eve5/+ animals, Fig. 2A,C) to lower values (ranging from 0.4 to 0.9 in eve1/eve5 mutants, Fig. 2B,D). Comparison of average normalized proliferation reveals a significant reduction in eve1/eve5 transheterozygotes (0.57±0.04, n=19) versus controls (1.00±0.02, n=24).
Fig. 2.
Proliferation in eve, trol and trol;eve/+ mutants. BrdU incorporation from 16–20 hours ph. Arrows indicate labeled neuroblasts. (A) Control brain lobe. (B) eve1/eve5 brain lobe. Quantitation of the number of BrdU-labeled neuroblasts in mutant and control samples. White bars indicate control samples. Black bars indicate mutant samples. (C) Control brains. (D) eve1/eve5. (E) trol4. (F) trol4;eve3/+. (G) trol8. (H) trol8;eve3/+.
New eve alleles define a threshold for enhancement of trolb22 lethality
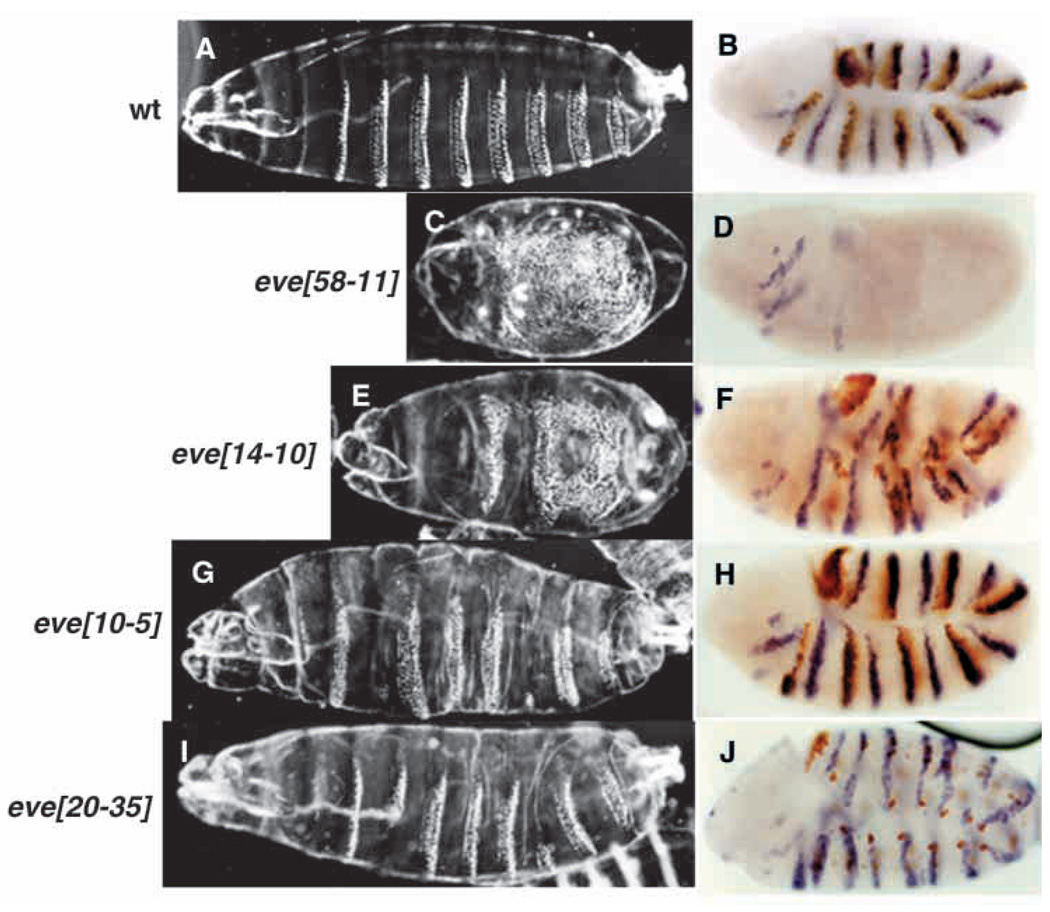
Four new eve alleles (Bour et al., 1995) were assayed for their ability to enhance the lethality of trolb22. The phenotypic strengths of the new alleles were assayed by examining both their embryonic cuticular phenotypes and the expression patterns of engrailed (en) transcripts at earlier stages (Fig. 3). eve58-11 showed a null cuticular phenotype (Fig. 3C) and a severely defective en expression pattern (Fig. 3D), consistent with the lack of Eve staining, and also strongly enhanced trolb22 lethality when heterozygous (Table 1). eve14-10 showed a hypomorphic cuticular phenotype, clearly weaker than that of eve58-11 (Fig. 3E), and the expression patterns of en and Eve showed correspondingly milder defects (Fig. 3F). Both eve10-5 and eve20–35 showed weak hypomorphic cuticular phenotypes (Fig. 3G,I), and the en and Eve patterns were closer to wild type (Fig. 3H,J). eve10-5, eve20–35 and eve14-10 did not enhance trolb22 lethality. Taken together, the embryonic phenotypes and the interactions with trolb22 suggest that enhancement of trolb22 lethality requires a major reduction in eve function, close to 50% (i.e. that produced by a null/+ eve genotype).
Fig. 3.
Embryonic phenotypes of eve alleles. Cuticular phenotypes. (A) Control. (C) eve58-11. (E) eve14-10. (G) eve10-5. (I) eve20–35. engrailed expression at stage 10. (B) Control. (D) eve58-11. (F) eve14-10. (H) eve10-5. (J) eve20–35. In all panels anterior is towards the left, ventral is downwards.
Table 1.
Dominant enhancement of trolb22 lethality by new eve alleles
| Male |
Female |
|||||
|---|---|---|---|---|---|---|
| Genotype of cross (male × female) |
evex/+ | CyO/+ | Ratio* | evex/+ | CyO/+ | Ratio* |
| eve58-11/CyO × trolb22 | 0 | 74 | 0.00 | 28 | 357 | 0.08 |
| eve58-11/CyO × CS | 66 | 81 | 0.81 | 84 | 92 | 0.91 |
| eve14-10/CyO × trolb22 | 283 | 295 | 0.96 | 977 | 958 | 1.02 |
| eve14-10/CyO × CS | 253 | 238 | 1.06 | 272 | 294 | 0.93 |
| eve10-5/CyO × trolb22 | 118 | 119 | 0.99 | 347 | 325 | 1.07 |
| eve10-5/CyO × CS | 90 | 89 | 1.0 | 106 | 114 | 0.93 |
| eve20–35/CyO × trolb22 | 67 | 73 | 0.92 | 310 | 318 | 0.91 |
| eve20–35/CyO × CS | 94 | 99 | 0.95 | 77 | 110 | 0.70 |
Ratio, (number of evex/+ flies)/(number of CyO/+ flies).
eve3 enhances the proliferation phenotype of two trol alleles
We tested two trol alleles of intermediate strength, trol4 and trol8, for their ability to be enhanced by mutations in eve. trol4 and trol8 are independent lethal alleles, and both trol4/trolb22 and trol8/trolb22 are viable (S. D., M. C. C., M. M. R., C. R., Y. P. and S. M., unpublished). Analysis of larval neuroblast proliferation in y trol4 w/Y brains revealed a bimodal distribution. BrdU labeling was within control values in 43% of the samples (19 out of 44, ranging from 0.78 to 1.2), and was decreased in 57% of the samples (25 out of 44, ranging from 0.29 to 0.76, Fig. 2E). In contrast, heterozygosity of eve3 in a trol4 background caused defective proliferation in 100% of the samples (total of 22 brains, Fig. 2F). Hemizygous y trol8 w/Y animals had a much weaker proliferation phenotype than did trol4 mutants. Only 23% (6/26) exhibited defective proliferation, with ratios ranging from 0.28 to 0.76 (Fig. 2G). The remaining samples (20/26) showed proliferation consistent with wildtype levels. Introduction of a heterozygous eve3 mutation in a trol8 background increased the number of brains with defective proliferation to 88% (21/24) (Fig. 2H). Thus, like trolb22, both intermediate trol alleles are significantly enhanced by eve3.
Ectopic expression of cyclin E can rescue the enhanced neuroblast proliferation phenotype
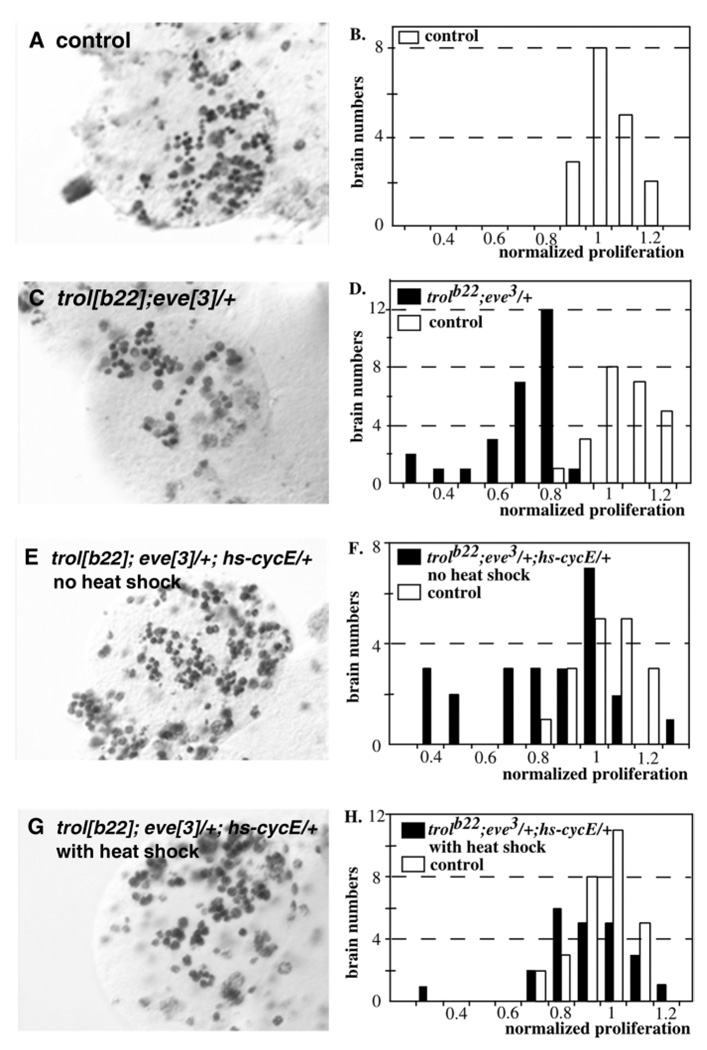
Ectopic expression of cyclin E from a hs-CycE transgene consistently rescues the defective neuroblast proliferation in trolsd first instar larval CNSs (Caldwell and Datta, 1998). To determine if expression of CycE would also rescue the increased neuroblast arrest of y trolb22/Y; eve3/+ animals, we examined the effect of expression from a hs-CycE transgene. In y trolb22/Y; eve3/+ animals, weak over expression of CycE (no heat shock) partially rescued the neuroblast phenotype. Without heat induction only 35% (7/20) of y trolb22/Y; eve3/+ animals (Fig. 4E,F) carrying the hs-CycE transgene showed defective proliferation compared to 96% (26/27) of y trolb22/Y; eve3/+ animals without the transgene (Fig. 4C,D). Furthermore, strong induction of CycE expression with a 30 minute heat shock rescued the neuroblast phenotype almost completely (96%, 22/23; Fig. 4G,H). Interestingly, weak induction of CycE did not rescue the enhanced lethality of y trolb22/Y; eve3/+ animals.
Fig. 4.
Rescue of neuroblast phenotypes by expression of Cyclin E. BrdU incorporation in mutant and control brain lobes from 16–20 hours ph. (A) Control. (C) trolb22;eve3/+. (E) trolb22;eve3/+;hs-CycE/+, no heat shock. (G) trolb22;eve3/+;hs-CycE/+, with heat shock. Quantitation of the number of BrdU-labeled neuroblasts. (B) Control. (D) trolb22;eve3/+. (F) trolb22;eve3/+;hs-CycE/+, no heat shock. (H) trolb22;eve3/+;hs-CycE/+, with heat shock.
eve protein is not expressed in larval brain neuroblasts
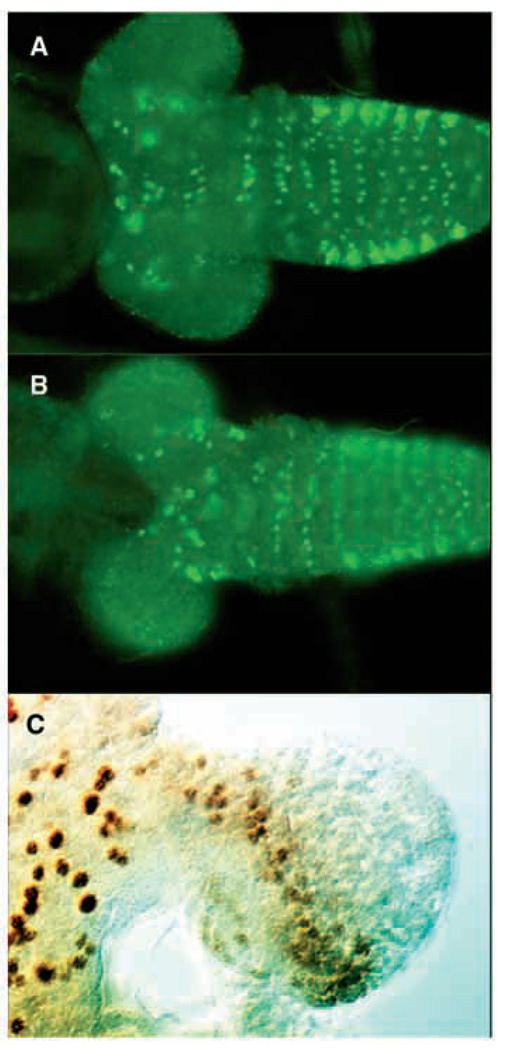
We examined the distribution of eve protein in the brains of wild-type early, mid and late first instar larvae using immunohistochemistry. The distribution of Eve protein within the CNS did not change appreciably during first instar. Eve protein is expressed in the CNS mainly in the thoracic region (Fig. 5A,B). eve-expressing cells appear to be mostly ganglion mother cells and neurons as characterized by their smaller size in comparison with thoracic neuroblasts and their more internal position on the ventral side of the thoracic ganglion. A few ganglion mother cells and neurons in the central brain region expressed Eve protein, but no cells in the optic lobe proliferation center stained positively for Eve (Fig. 5A, B). Cells in the brain lobes do stain for engrailed protein (Fig. 5C), indicating that penetration of the tissue is not an issue. Thus, Eve does not appear to be expressed in larval neuroblasts close to the time when their proliferation is affected by eve and trol mutations.
Fig. 5.
Eve localization in the larval brain. Eve localization in late first instar brain. (A) Ventral view. (B) Dorsal view. (C) Engrailed localization in a late first instar brain. In panels A and B anterior is towards the left. In panel C anterior is to the top.
In an explant proliferation assay, eve function is required in the larval extract
The expression pattern of eve in the larval brain suggested that Eve protein might act in some other tissue to affect neuroblast proliferation in the brain itself. This hypothesis was tested in vitro using a brain explant culture system (Table 2) (Datta, 1999). When wild-type (CS) brains were cultured with wild-type extract, 39% (7/18) of the samples had numbers of dividing neuroblasts that were similar to those observed in vivo. When y trolb22/Y; eve3/+ CNSs were cultured with extract from y trolb22/Y; eve3/+ first instar larvae, just 7.8±1.3% of the samples (4/53 total) showed in vivo levels of neuroblast proliferation, a percentage comparable to that observed in y trolb22/Y; eve3/+ animals in vivo. Similarly, only 6.3±1.3% (4/66 total) of brains derived from y trolb22/Y; eve3/+ animals showed normal neuroblast proliferation when cultured with eve3/+ extract, consistent with a locus of action of trol within the neuroblasts. In sharp contrast, 36% (9/25) of y trolb22/Y; eve3/+ brains cultured with wild-type (CS) extract had levels of neuroblast proliferation within normal in vivo levels. These levels are close to those of wild-type brains cultured with wild-type extract (above), suggesting that eve is not functioning within the brain to affect proliferation. Strikingly, when trolb22 mutant brains (wild type for eve) were cultured in extract derived from eve3/+ first instar larvae (wild type for trol), none of the samples (0/42 total) showed normal activation of neuroblast division, showing that eve affects the extract.
Table 2.
Non-autonomous eve function is required for neuroblast proliferation
| Brain | 30% Larval extract | % of Normal proliferation* |
|---|---|---|
| CS | CS | 38.9 |
| trolb22; eve3/+ | trolb22; eve3/+ | 7.8±1.3 |
| trolb22; eve3/+ | eve3/+ | 6.3±1.4 |
| trolb22 | eve3/+ | 0±0 |
| trolb22; eve3/+ | trolb22; eve3/+ with 10 µg/ml 20E | 39.8±2.7 |
% of normal proliferation, (number of samples with normal proliferation)/(total number of samples) in independent batches of tissue explant experiments±s.e.m.
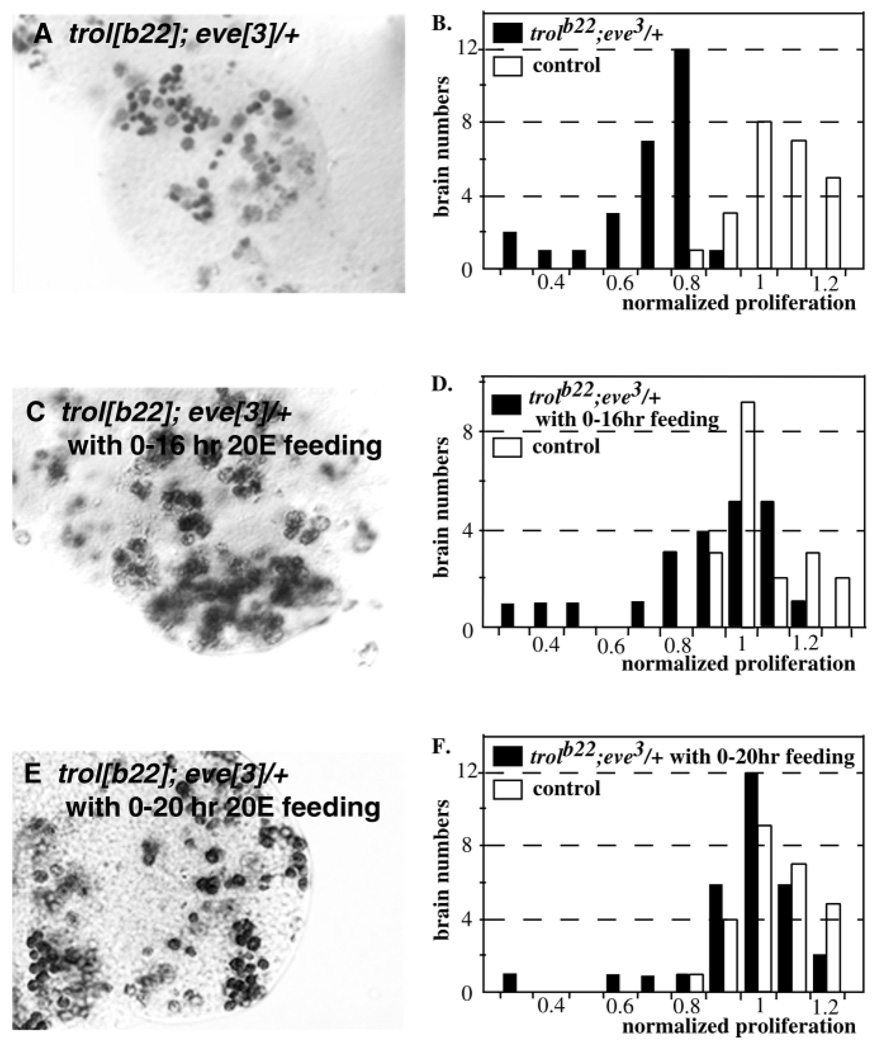
Addition of ecdysone rescues defective proliferation
Previously, we have shown that the addition of ecdysone to the medium in the brain explant culture system is required for the activation of neuroblast proliferation (Datta, 1999). This led us to ask if ecdysone would suppress the eve-mediated loss of neuroblast proliferation. When y trolb22/Y; eve3/+ brains were cultured in y trolb22/Y; eve3/+ extract, to which 10 m g/ml ecdysone had been added, 40±2.7% (27/67 total, Table 2) of the samples revealed normal activation of neuroblast division. This is strikingly similar to the results for wild-type brains cultured with wild-type extract (see above). These results were confirmed in vivo by feeding studies. When y trolb22/Y; eve3/+ animals were fed 1 mg/ml ecdysone from 0–20 hours after hatching, the number of samples showing neuroblast proliferation within the control range rose from 4% (1/27) (Fig. 6A,B) to 90% (27/30) (Fig. 6E,F). Interestingly, while 68% (15/22) of y trolb22/Y; eve3/+ animals fed ecdysone from 0–16 hours (Fig. 6C,D) showed normalized neuroblast proliferation levels that were within the control range (0.8–1.2), all of the samples (14/14) from animals fed ecdysone from 16–20 hours showed normal proliferation levels, indicating that ecdysone addition is most effective during the last four hours of the treatment period.
Fig. 6.
Rescue of neuroblast proliferation defects by ecdysone. BrdU incorporation in brain lobes of mutant and control brains from 16–20 hours ph. (A) trolb22;eve3/+. (C) trolb22;eve3/+ fed ecdysone from 0–16 hours ph. (E) trolb22;eve3/+ fed ecdysone fro 0–20 hours ph. Quantitation of the number of BrdU labeled neuroblasts. (B) trolb22;eve3/+. (D) trolb22;eve3/+ fed ecdysone from 0–16 hours ph. (F) trolb22;eve3/ fed ecdysone from 0–20 hours ph.
Structural analysis of the eve5 allele
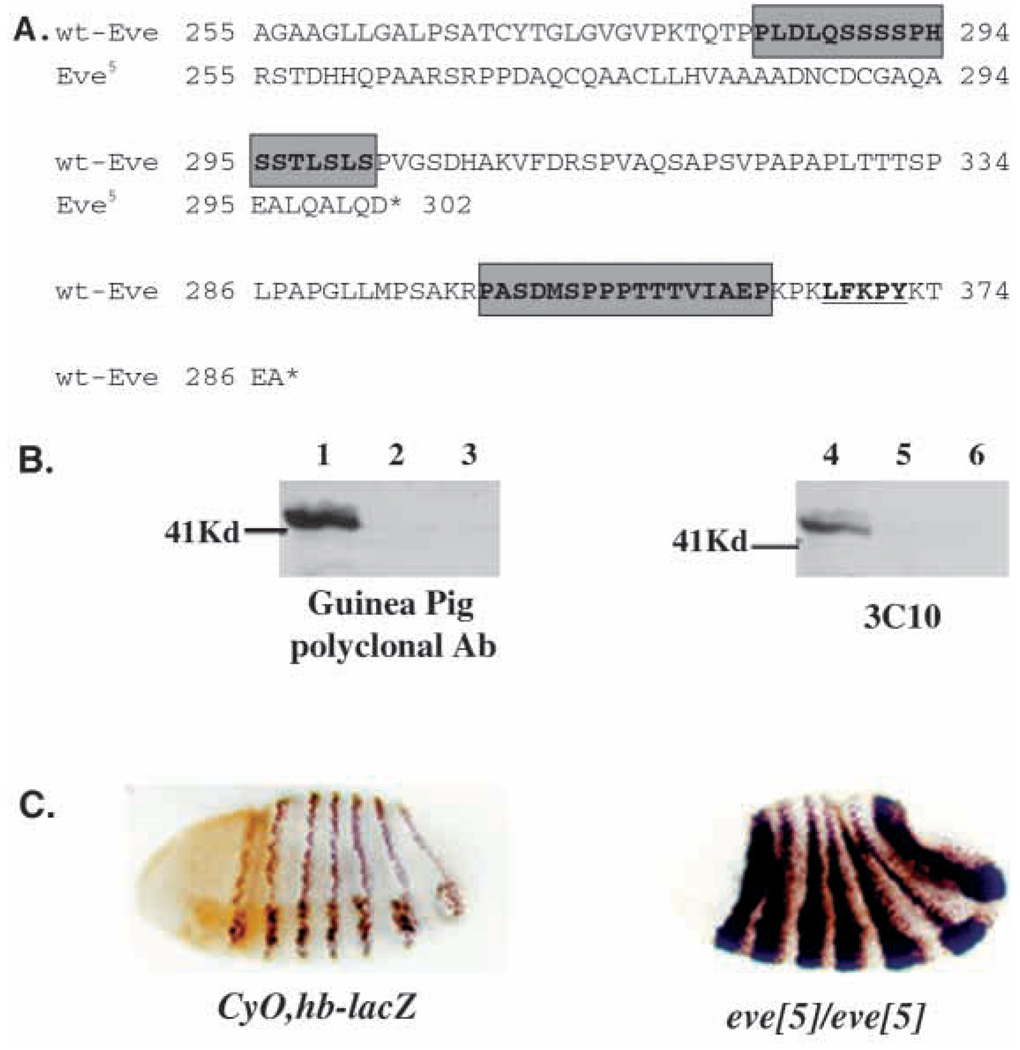
To investigate the structural requirements for Eve function we analyzed the molecular lesion in the eve5 allele. It was previously shown that the eve5 mutation removes the 3′ portion of the Eve-coding region, leaving intact the DNA-binding domain and a transcriptional repression domain (Park et al., 1998). To identify the specific sequences deleted, PCR products amplified from eve5 genomic DNA were cloned. Sequence analysis showed (Fig. 7A) that the deletion starts at the first base of codon ala-255 and ends at the second base of the pro-326 codon, resulting in deletion and frameshift mutations from amino acid 255 to amino acid 376, the C terminus of the wild-type Eve protein. The deletion eliminates PEST sequences found between amino acids 283 and 335, suggesting that the Eve5 protein may have enhanced stability. This predicted increase in stability is in agreement both with comparative immunohistochemistry on eve5 mutant and wild-type embryos, and with western analysis. Staining for Eve protein at embryonic stages 6, 8 and 10 in eve5 mutant and wild-type embryos revealed a dramatic increase in the quantity of Eve detected in the eve5 embryos at all three stages (Fig. 7C). Consistent with the lack of obvious mutations in transcriptional control regions (Park et al., 1998), the spatial pattern of Eve protein in the mutant embryos appears normal, as does the level of eve mRNA (data not shown). The putative increase in stability of the Eve5 protein is also supported by western analysis. Wild-type Eve is sufficiently unstable that it cannot be detected on western blots even in samples prepared in the presence of high levels of protease inhibitors from 2–4 hour embryos, which show maximum levels of Eve immunostaining (Fig. 7B). In contrast, the Eve5 protein can easily be detected in samples from heterozygous eve5/CyO embryos by immunoblotting both with the monoclonal antibody 3C10 and with polyclonal anti-Eve antiserum (Fig. 7B). The band identified by each of the anti-Eve antibodies corresponds to approximately 40 kDa in size, while the predicted size of the unmodified Eve5 protein is about 33 kDa, suggesting that Eve may be post-translationally modified in vivo. Eve protein expressed in cultured Drosophila cells also migrates significantly slower than expected from its molecular weight (Han and Manley, 1993).
Fig. 7.
Molecular analysis of eve5. (A) Nucleotide and predicted protein sequence comparison of eve5 with wild type. Shaded boxes indicate predicted PEST sequences. Underline indicates Groucho interacting motif. (B) Western blot of eve5/+ and control samples. Lanes 1, 2 eve5/CyO; 2, 5 eve4/CyO; 3, 6 CS. (C) Immunohistochemical detection of wild-type Eve at late stage 6 of embryogenesis (left), when wild-type Eve protein levels are decreasing and Eve5 protein at early stage 7 (panel).
Transgenes with a deletion of the Groucho-interacting domain rescue most of eve function in the trol pathway
The C-terminal frameshift mutation in the eve5 allele eliminates a Groucho-interacting motif, LFKPY, that is required for full Eve function in segmentation (Kobayashi et al., 2001). We showed previously that eve5 causes weak trolb22 lethality and a corresponding enhancement of the proliferation phenotype (Park et al., 1998). This suggests that the Groucho-interacting domain may be required for eve function as a cell cycle regulator. Therefore, we tested the ability of three independent transgenes with insertions on the second chromosome to rescue the enhancement of trol lethality by eve3 (Table 3). The EGNΔLFK transgene, which contains a small deletion of the Groucho-interacting motif, fully rescued the trolb22 lethality enhanced by heterozygosity at eve; i.e. the transgene supported viability in a trolb22; eve/+ background comparable with that of CS controls. Two independent EGNHA transgenes, containing a hairy Groucho-interacting motif (WRPW, Fisher et al., 1996) instead of the eve Groucho-interacting motif, showed rescue to an extent similar to that of the EGNΔLFK transgene. Three other independent transgenes inserted on the third chromosome, which have amino acid substitutions that disrupt the Groucho interaction in yeast two-hybrid assays and are similarly defective in segmentation function (Kobayashi et al., 2001) also showed rescue of the enhanced trolb22 lethality (Table 3). However, in the absence of an eve mutation, none of the transgenes resulted in enhancement of trolb22 lethality that was due to eve overexpression to the same extent as did a wild-type eve transgene (expressing a normal Eve protein with an intact Groucho-interacting motif) (Park et al., 1998). These results are consistent with the partial loss of eve segmentation function caused by these disruptions of the Eve-Groucho interaction, which is about 50% (Kobayashi et al., 2001), and with the fact that a reduction of close to 50% in eve function is required to enhance the trol phenotype. They also suggest that the Eve-Groucho interaction contributes to Eve function in the trol pathway (see Discussion).
DISCUSSION
eve is part of the trol pathway
Heterozygous eve mutations dramatically increase trolb22 lethality and interact synergistically with trolb22 to uncover a proliferation phenotype (Park et al., 1998). These data suggest that eve is a part of the trol pathway. If so, then a stronger reduction in Eve activity alone might also cause a proliferation phenotype. Fig. 2 clearly shows that eve1/eve5 transheterozygous animals have a neuroblast proliferation defect, consistent with the interpretation that eve is in the trol pathway.
A corollary of the hypothesis that eve is part of the trol pathway is that neuroblasts arrested in a y trolb22/Y; eve3/+ animal are in the same cell cycle phase as those arrested in trolsd mutants and can be rescued by expression of CycE, as was previously shown for trolsd (Caldwell and Datta, 1998). In fact, the proliferation defect in some y trolb22/Y; eve3/+ mutant brains is rescued by low levels of CycE expression, while higher levels result in rescue in virtually every individual (Fig. 4). The partial rescue by low levels of CycE expression indicates that the trol pathway in y trolb22/Y; eve3/+ animals is less compromised than that in trolsd mutants, which require high levels to obtain rescue, and further supports the hypothesis that trol and eve function in a common proliferation pathway.
The eve-trol interaction is not allele specific
Known mutations in both the eve homeodomain (eve1 and eve2) and a transcriptional repression domain (eve4) enhance trolb22 phenotypes, suggesting that trolb22 enhancement is due to eve function as a DNA-binding transcriptional repressor. However, eve mutations do not enhance the strongest trol allele, trolsd. This led us to ask whether trolb22 enhancement by eve was due to a partial loss of function of eve and trol or to allele-specific interactions. We tested several new eve alleles (Table 1), and found a good correspondence between the ability to enhance trol and the severity of the eve embryonic phenotype, strongly supporting the hypothesis that the trolb22-eve interaction is due to a general loss of eve function. The lack of trol enhancement by weak eve mutations also indicates that enhancement requires a reduction in Eve activity of close to 50%. However, trol enhancement is not due to a defect in segmentation. In addition to the fact that heterozygous eve mutants produce few segmentation defects, this is supported indirectly by observations on another transcriptional regulator, ftz, which was also identified as a trol enhancer (C. D. Hough and S. D., unpublished). Heterozygous ftz mutations enhance trolb22 lethality and produce a proliferation phenotype similar to that of eve mutations. A ftz transgene that restores the early pair rule expression of ftz (but not CNS expression) cannot rescue enhancement (Y. P. and S. D., unpublished), implying that segmentation defects are not the cause of trol enhancement by ftz.
Having established that the eve-trol interaction is not eve allele specific, we proceeded to ask whether other trol mutations could also be enhanced by eve. Two independent trol alleles of intermediate strength also showed eve enhancement (Fig. 2), clearly indicating that the eve-trol interaction is not due to specific characteristics of the trolb22 allele or to background mutations in the trolb22 stock.
We compared the average reduction of normalized proliferation in different mutant populations. For the trol4 and trol8 mutations, only the population with a mutant phenotype were included in the calculation. Strong trolsd samples led to a reduction in normalized proliferation of 0.66±0.04 (n=13, Y. P. and S. D., unpublished data) of control levels, consistent with published results (Datta, 1995). eve1/eve5 transheterozygotes showed a reduction to 0.57±0.04 (n=19), while trol4/Y; eve3/+ and trol8/Y; eve3/+ brains showed a reduction to 0.57±0.03 (n=22) and 0.57±0.03 (n=21), respectively. Thus, while not all animals of each genotype had a proliferation phenotype, the magnitude of the proliferation phenotype, when present, was statistically identical in all the mutant genotypes examined. This consistent reduction of proliferation in different mutant animals suggests that a specific subpopulation of neuroblasts may require the activity of trol and eve in order to activate cell division, while the remaining neuroblasts use an alternative mechanism, which either is independent of or somehow compensates for a loss of activity in the trol pathway.
Eve protein requires several domains to control neuroblast proliferation
We had shown previously that the DNA-binding domain and a transcription repression domain in the Eve protein are likely to be required for neuroblast cell cycle activation (Park et al., 1998). Sequence analysis of the eve5 mutation suggested that the deletion of PEST sequences within the Eve5 protein (Sackerson, 1995) and the resulting protein stability, as demonstrated by both western and immunohistochemical analysis (Fig. 7B,C), might contribute to eve5 enhancement of trolb22. This possibility is consistent with our previous observation that overexpression of eve by addition of eve transgenes also enhances trolb22 lethality (Park et al., 1998). Alternatively, deletion of a domain at the C terminus of the Eve5 protein, which was recently shown to interact functionally with the corepressor Groucho (Kobayashi et al., 2001), could have diminished its activity as a cell cycle regulator. This interpretation is consistent with the partial-loss-of-function embryonic phenotype of eve5. The role of sequences at the C terminus of Eve was investigated using eve transgenes with mutant or substituted Groucho-interaction motifs.
Transgenes with truncation and amino acid substitutions in the C-terminal Groucho-interacting motif rescue lethality caused by the loss of Eve activity caused by eve3. However, these same transgenes, in the presence of two wild-type eve alleles, are not sufficient to enhance trolb22 lethality, while transgenes that express the normal Eve protein do cause enhancement. This suggests that the activity of each of the Groucho-motif mutant eve transgenes is lower than that of wild-type eve+ and is consistent with the fact that the transgenes have a reduced function in segmentation (Kobayashi et al., 2001). These data indicate that the Groucho-interaction domain, in addition to the DNA-binding domain and the Groucho-independent transcriptional repression domain, is necessary for the full function of the Eve protein in promoting neuroblast proliferation.
eve acts non-autonomously to control proliferation
All of the genetic evidence to date suggests that eve functions as a transcriptional regulator in the trol pathway. The most straightforward explanation is that eve acts within the regulated neuroblasts to promote CycE expression. Surprisingly, while Eve protein is found in the larval CNS (Fig. 5) in a pattern similar to that observed in other insect systems (Duman-Scheel and Patel, 1999), It cannot be detected in the regulated neuroblasts at any time from larval hatching to the point at which the cells have already entered S phase. This unexpected result led to the hypothesis that eve might function outside of the larval brain to provide a trans-acting signal required for neuroblast proliferation.
We have previously demonstrated that a CNS mutant for trolsd shows a defect in neuroblast proliferation even when cultured with wild-type whole-body extract, suggesting that trol is required only within the larval CNS for normal activation of neuroblast division (Datta, 1999). We used the same system to determine whether eve was required in the CNS or the body for normal proliferation to occur. As shown in Table 2, when trolb22 brains are cultured with extract derived from eve3/+ animals, a proliferation defect is observed. The magnitude of the defect is similar to that of trolb22/Y; eve3/+ brains cultured with trolb22/Y; eve3/+ extract, and comparable with the defect in trolb22/Y; eve3/+ animals in vivo. Thus, a decrease in eve activity within the body can precipitate a lack of neuroblast proliferation within a sensitized trol larval brain.
Eve-mediated enhancement of defective neuroblast proliferation can be rescued by ecdysone
Analysis of explants had previously shown that ecdysone enabled activation of neuroblast division and could substitute for larval extract (Datta, 1999). Furthermore, addition of ecdysone did not rescue the proliferation phenotype of cultured trolsd mutant brains, implying that ecdysone acts upstream of trol. Addition of ecdysone to extract from trolb22/Y; eve3/+ animals produced normal proliferation in cultured trolb22/Y; eve3/+ mutant brains (Table 2). Thus, ecdysone can overcome the lack of eve-induced activity in the extract. The ability of ecdysone to compensate for low eve expression was also seen in vivo in the rescue of proliferation defects of trolb22/Y; eve3/+ animals by ecdysone feeding (Fig. 6). Interestingly, almost complete rescue was obtained when animals were fed ecdysone from 16–20 hours posthatching, indicating that the time between ecdysone action and S phase entry is at most four hours.
eve, ecdysone and activation of imaginal neuroblast division
Mutations in eve produce specific defects in embryonic segmentation and the determination of neuronal identities in the embryonic CNS. Analyses of these phenotypes has led to the elucidation, especially for segmentation, of a complex molecular circuit that controls the expression of specific genes to set the body plan of the embryo. Yet some studies have also suggested that mutations in eve result in changes in the spatial pattern of expression of 87% of the genes in the embryo, even those such as ry, whose linkage to changes in segment identity are not obvious (Liang and Biggin, 1998). So how does a gene like eve with such apparent specificity in mutant phenotype and expression pattern affect the expression of a majority of genes in such a global fashion? Biochemical analyses indicate that Eve binds throughout the length of many genes, although how that binding might regulate gene expression is not clear (Walter et al., 1994). Another possibility is that eve is also required for the formation of an organismal-level trans-acting signal that affects the expression of other genes.
The genetic interaction between eve and trol has all the characteristics expected for two components of a common pathway: (1) the eve-trol interaction is not allele specific and the known functional domains of Eve are implicated in the interaction; (2) the strength of the interaction mirrors the strength of the eve allele in segmentation; (3) eve mutants themselves have the predicted proliferation phenotype; and (4) neuroblasts arrested in trolb22;eve/+ can be rescued by expression of CycE, as can the neuroblasts arrested in a strong trol mutant. The latter is especially revealing, as induction of CycE expression in trol mutants results in the activation of cell division ONLY in the number of neuroblasts appropriate to the developmental stage of the induction (Caldwell and Datta, 1998). That is, not all mitotically quiescent neuroblasts are arrested at the same cell cycle phase, and the extent to which CycE is a limiting factor is developmentally controlled. Therefore, as in embryonic segmentation and determination of neuronal identity, eve appears to function in a specific genetic pathway to affect the behavior of specific cells at specific times.
However, Eve is not detectable in regulated neuroblasts at any time during first instar. Furthermore, eve function is not required within the larval CNS, but is required within the larval body from which extracts are prepared. Moreover, low levels (10–20%) of extract made from eve+ (CS) animals will not support activation of neuroblast division while higher concentrations will ( Y. P. and S. D., data not shown). This concentration dependence indicates that eve does not inhibit production of a trans-acting proliferation repressor that is produced at higher levels in a eve mutant, as dilution of such a repressor would allow neuroblast division at lower rather than higher extract concentrations. These results strongly suggest that eve function is required for the production of a trans-acting factor that stimulates neuroblast division.
Is ecdysone the trans-acting factor produced in response to eve? Ecdysone can rescue eve-dependent proliferation defects both in vivo and in vitro (Fig. 6, Table 2), but not the proliferation defect of trol mutants in vitro (Datta, 1999). This suggests that ecdysone acts upstream of trol, as would be expected if it is the eve-dependent trans-acting signal, and trol acts within the receiving cells. However, while the ecdysone receptor has been detected in a few neurosecretory cells of the first instar CNS, it has not been detected in neuroblasts (Truman et al., 1994). This may indicate that only a few high-affinity receptors are required to transduce the ecdysone signal, or that ecdysone acts indirectly through the products of the neurosecretory cells. However, as Eve is not detectable in the neurosecretory cells in wild-type brain lobes (Fig. 5), it is unlikely that the added ecdysone rescues mutant animals by compensating for a loss of Eve activity in those cells. In each of these cases, eve could be acting through ecdysone production. Alternatively, ecdysone may act through a parallel pathway to that stimulated by an (unknown) eve-dependent signal. While the relationship between eve and ecdysone is not yet clear, it seems likely that eve is required for the production of an organismal-level trans-acting signal that is specifically required to stimulate larval neuroblast proliferation.
Acknowledgments
This work was supported by NIH grant R01 NS36737 to S. D. and NSF grant IBN9808931 to J. B. J. We thank Drs N. Patel and D. Kosman for gifts of Eve antibody; Dr E. Goldstein for eve mutant stocks; and Drs N. Patel, M. Frasch, and E. M. C. Skoulakis, as well as members of the Datta and Jaynes laboratories, for helpful discussions.
REFERENCES
- Akam M. The molecular basis for metameric pattern in the Drosophila embryo. Development. 1987;101:1–22. [PubMed] [Google Scholar]
- Bour BA, O’Brien MA, Lockwood WL, Goldstein ES, Bodmer R, Taghert PH, Abmeyer SM, Nguyen HT. Drosophila MEF2, a transcription factor that is essential for myogenesis. Genes Dev. 1995;9:730–741. doi: 10.1101/gad.9.6.730. [DOI] [PubMed] [Google Scholar]
- Caldwell M, Datta S. Expression of cyclin E or DP/E2F rescues the G1 arrest of trol mutant neuroblasts in the Drosophila larval central nervous system. Mech. Dev. 1998;79:121–130. doi: 10.1016/s0925-4773(98)00178-6. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega JA, Hartenstein V. The Embryonic Development of Drosophila melanogaster. Berlin: Springer-Verlag; 1985. [Google Scholar]
- Champlin DT, Truman JW. Ecdysteroid control of cell proliferation during optic lobe neurogenesis in the moth Manduca sexta. Development. 1998;125:269–277. doi: 10.1242/dev.125.2.269. [DOI] [PubMed] [Google Scholar]
- Cui X, Doe CQ. The role of the cell cycle and cytokinesis in regulating neuroblast sublineage gene expression in the Drosophila CNS. Development. 1995;121:3233–3243. doi: 10.1242/dev.121.10.3233. [DOI] [PubMed] [Google Scholar]
- Datta S. Control of proliferation activation in quiescent neuroblasts of the Drosophila central nervous system. Development. 1995;121:1173–1182. doi: 10.1242/dev.121.4.1173. [DOI] [PubMed] [Google Scholar]
- Datta S. Activation of neuroblast proliferation in explant culture of the Drosophila larval CNS. Brain Res. 1999;818:77–83. doi: 10.1016/s0006-8993(98)01292-x. [DOI] [PubMed] [Google Scholar]
- Datta S, Kankel DR. l(1)trol and l(1)devl, loci affecting the development of the adult central nervous system in Drosophila melanogaster. Genetics. 1992;130:523–537. doi: 10.1093/genetics/130.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman-Scheel M, Patel NH. Analysis of molecular marker expression reveals neuronal homology in distantly related arthropods. Development. 1999;126:2327–2334. doi: 10.1242/dev.126.11.2327. [DOI] [PubMed] [Google Scholar]
- Ebens AJ, Garren H, Cheyette BNR, Zipursky SL. The Drosophila anachronism locus: A glycoprotein secreted by glia inhibits neuroblast proliferation. Cell. 1993;74:15–28. doi: 10.1016/0092-8674(93)90291-w. [DOI] [PubMed] [Google Scholar]
- Edgar BA, Lehner CF. Developmental control of cell cycle regulators: A fly’s perspective. Science. 1996;274:1646–1652. doi: 10.1126/science.274.5293.1646. [DOI] [PubMed] [Google Scholar]
- Fisher AL, Ohsako S, Caudy M. The WRPW motif of the hairy-related basic helix-loop-helix repressor proteins acts as a 4-amino-acid transcription repression and protein- protein interaction domain. Mol. Cell. Biol. 1996;16:2670–2677. doi: 10.1128/mcb.16.6.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foe VE. Mitotic domains reveal early commitment of cells in Drosophila embryos. Development. 1989;107:1–22. [PubMed] [Google Scholar]
- Fujioka M, Emi-Sarker Y, Yusibova GL, Goto T, Jaynes JB. Analysis of an even-skipped rescue transgene reveals both composite and discrete neuronal and early blastoderm enhancers, and multi-stripe positioning by gap gene repressor gradients. Development. 1999;126:2527–2538. doi: 10.1242/dev.126.11.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K, Manley JL. Transcriptional repression by the Drosophila even-skipped protein: definition of a minimal repression domain. Genes Dev. 1993;7:491–503. doi: 10.1101/gad.7.3.491. [DOI] [PubMed] [Google Scholar]
- Hofbauer A, Campos-Ortega JA. Proliferation pattern and early differentiation of the optic lobes in Drosophila melanogaster. Roux’s Arch. Dev. Biol. 1990;198:264–274. doi: 10.1007/BF00377393. [DOI] [PubMed] [Google Scholar]
- Ito K, Hotta Y. Proliferation pattern of postembryonic neuroblasts in the brain of Drosophila melanogaster. Dev. Biol. 1991;149:134–148. doi: 10.1016/0012-1606(92)90270-q. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Goldstein RE, Fujioka M, Paroush Z, Jaynes JB. Groucho augments the repression of multiple Even-skipped target genes in establishing parasegment boundaries. Development. 2001;128:1805–1815. doi: 10.1242/dev.128.10.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, Biggin MD. Eve and ftz regulate a wide array of genes in blastoderm embryos: the selector homeoproteins directly or indirectly regulate most genes in Drosophila. Development. 1998;125:4471–4482. doi: 10.1242/dev.125.22.4471. [DOI] [PubMed] [Google Scholar]
- Lindsley DL, Zimm GG. The Genome of Drosophila melanogaster. San Diego, California: Academic Press; 1992. [Google Scholar]
- Lipshitz HD, Kankel DR. Specificity of gene action during central nervous system development in Drosophila melanogaster: analysis of the lethal (1) optic ganglion reduced locus. Dev. Biol. 1985;108:56–77. doi: 10.1016/0012-1606(85)90009-0. [DOI] [PubMed] [Google Scholar]
- Mullen JR, DiNardo S. Establishing parasegments in Drosophila embryos: roles of the odd-skipped and naked genes. Dev. Biol. 1995;169:295–308. doi: 10.1006/dbio.1995.1145. [DOI] [PubMed] [Google Scholar]
- O’Brien MA, Roberts MS, Taghert PH. A genetic and molecular analysis of the 46C chromosomal region surrounding the FMRFamide neuropeptide gene in Drosophila melanogaster. Genetics. 1994;137:121–137. doi: 10.1093/genetics/137.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee AB. G1 events and regulation of cell proliferation. Science. 1989;246:603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- Park Y, Fujioka M, Jaynes J, Datta S. The Drosophila homeobox gene eve enhances trol, an activator of neuroblast proliferation in the larval CNS. Dev. Genet. 1998;23:247–257. doi: 10.1002/(SICI)1520-6408(1998)23:3<247::AID-DVG9>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Prokop A, Technau GM. Normal function of the mushroom body defect gene of Drosophila is required for the regulation of the number and proliferation of neuroblasts. Dev. Biol. 1994;161:321–337. doi: 10.1006/dbio.1994.1034. [DOI] [PubMed] [Google Scholar]
- Sackerson C. Patterns of conservation and divergence at the even-skipped locus of Drosophila. Mech. Dev. 1995;51:199–215. doi: 10.1016/0925-4773(95)00365-7. [DOI] [PubMed] [Google Scholar]
- Shannon MP, Kaufman TC, Shen MW, Judd BH. Lethality patterns and morphology of selected lethal and semi-lethal mutations in the zeste-white region of Drosophila melanogaster. Genetics. 1972;72:615–638. doi: 10.1093/genetics/72.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tautz D, Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989;98:81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Truman JW, Bate M. Spatial and temporal patterns of neurogenesis in the central nervous system of Drosophila melanogaster. Dev. Biol. 1988;125:145–157. doi: 10.1016/0012-1606(88)90067-x. [DOI] [PubMed] [Google Scholar]
- Truman JW, Talbot WS, Fahrbach SE, Hogness DS. Ecdysone receptor expression in the CNS correlates with stage-specific responses to ecdysteroids during Drosophila and Manduca development. Development. 1994;120:219–234. doi: 10.1242/dev.120.1.219. [DOI] [PubMed] [Google Scholar]
- Walter J, Dever CA, Biggin MD. Two homeo domain proteins bind with similar specificity to a wide range of DNA sites in Drosophila embryos. Genes Dev. 1994;8:1678–1692. doi: 10.1101/gad.8.14.1678. [DOI] [PubMed] [Google Scholar]
- Weigmann K, Lehner CF. Cell fate specification by even-skipped expression in the Drosophila nervous system is coupled to cell cycle progression. Development. 1995;121:3713–3721. doi: 10.1242/dev.121.11.3713. [DOI] [PubMed] [Google Scholar]
- White K, Kankel DR. Patterns of cell division and cell movement in the formation of the imaginal nervous system in Drosophila melanogaster. Dev. Biol. 1978;65:296–321. doi: 10.1016/0012-1606(78)90029-5. [DOI] [PubMed] [Google Scholar]