Abstract
Adipose tissue (AT) inflammation promotes insulin resistance (IR) and other obesity complications. AT inflammation and IR are associated with oxidative stress, adipocyte death, and the scavenging of dead adipocytes by proinflammatory CD11c+ AT macrophages (ATMΦ). We tested the hypothesis that supplementation of an obesitogenic (high-fat) diet with whole blueberry (BB) powder protects against AT inflammation and IR. Male C57Bl/6j mice were maintained for 8 wk on 1 of 3 diets: low-fat (10% of energy) diet (LFD), high-fat (60% of energy) diet (HFD) or the HFD containing 4% (wt:wt) whole BB powder (1:1 Vaccinium ashei and V. corymbosum) (HFD+B). BB supplementation (2.7% of total energy) did not affect HFD-associated alterations in energy intake, metabolic rate, body weight, or adiposity. We observed an emerging pattern of gene expression in AT of HFD mice indicating a shift toward global upregulation of inflammatory genes (tumor necrosis factor-α, interleukin-6, monocyte chemoattractant protein 1, inducible nitric oxide synthase), increased M1-polarized ATMΦ (CD11c+), and increased oxidative stress (reduced glutathione peroxidase 3). This shift was attenuated or nonexistent in HFD+B-fed mice. Furthermore, mice fed the HFD+B were protected from IR and hyperglycemia coincident with reductions in adipocyte death. Salutary effects of BB on adipocyte physiology and ATMΦ gene expression may reflect the ability of BB anthocyanins to alter mitogen-activated protein kinase and nuclear factor-κB stress signaling pathways, which regulate cell fate and inflammatory genes. These results suggest that cytoprotective and antiinflammatory actions of dietary BB can provide metabolic benefits to combat obesity-associated pathology.
Introduction
The accumulation of bone marrow-derived inflammatory macrophages (MΦ)7 in adipose tissue (AT) is causally implicated in the pathogenesis of insulin resistance (IR) and other obesity complications (1,2). In obese mice, these recruited ATMΦ can be distinguished from resident ATMΦ by the absence of the cell surface marker macrophage galactose N-acetyl-galactosamine specific lectin 1 (MGL1), upregulation of the dendritic cell marker CD11c, and polarization toward a classical proinflammatory (M1) phenotype (3–5). This phenotype is characterized by upregulated expression of tumor necrosis factor-α (TNFα), inducible nitric oxide synthase (iNOS), and other proinflammatory mediators that promote IR (2). Notably, ablation of CD11c+ cells or genetic abrogation of MΦ inflammatory signaling protects obese mice from IR (6,7). Macrophage infiltration and resulting AT inflammation are mechanistically linked to adipocyte death, which increases in obese mice and humans (4,5,8,9). Dead adipocytes are foci of CD11c+ ATMΦ recruitment, aggregation in crown-like structures (CLS), scavenging activity, and proinflammatory gene expression (4,5,8). Obesity-associated adipocyte death is thought to reflect 1 or more cytotoxic stresses that are elevated in the AT of obese mice and humans, in particular oxidative and endoplasmic reticulum (ER) stress (10,11).
Dietary strategies for alleviating the metabolic complications of obesity are being pursued as alternatives to pharmaceutical interventions [e.g. (12)]. The association of obesity with AT stress, adipocyte death, MΦ recruitment, and inflammatory gene expression suggested that edible berries might provide an effective alternative or supplementary intervention to attenuate obesity- associated inflammation and IR. Berries such as blueberry (BB) (Vaccinium sp) are enriched in anthocyanins, polyphenolics recognized for their ability to provide and activate cellular antioxidant protection, inhibit inflammatory gene expression, and consequently protect against oxidant-induced and inflammatory cell damage and cytotoxicity (13–15). BB-supplemented diets and preparations are also reported to inhibit MΦ infiltration (16) and to attenuate bacterial translocation to extraintestinal sites (17), a phenomenon implicated in the inflammatory and metabolic pathology associated with diets high in fat (18). Considered together, these observations suggested that BB could attenuate or delay the adipocyte death, ATMΦ recruitment, and proinflammatory gene expression that promote obesity-associated IR.
Experimental procedures
Mice and diets.
Male C57BL/6 mice were obtained from Jackson Laboratories at 5 wk of age and housed individually at the Jean Mayer USDA-Human Nutrition Research Center on Aging as previously described (5). Ethical treatment of animals was assured by the Tufts University Institutional Animal Care and Use Committee. After several days of acclimation, mice were assigned to cohorts (n = 8 mice/cohort) that received 1 of 3 pelleted diets for 8 wk (Research Diets). These were a low-fat diet (LFD) containing 10% of energy from fat (no. D12450B), a high-fat diet (HFD) containing 60% of energy from fat (no. D12492), and a modified HFD supplemented with 4% (wt:wt) freeze-dried whole BB powder (HFD+B) (Table 1). The powder was in aluminum cans under nitrogen and was provided by the U.S. Highbush Blueberry Council. It consisted of a 1:1 blend of Vaccinium ashei (Tifblue) and Vaccinium corymbosum (Rubel). Energy from sucrose and total carbohydrates were adjusted in the HFD to be equivalent to energy from sucrose and total carbohydrates in the HFD+B. BB powder (14.5 MJ/kg) contributed 2.7% of the total energy in the HFD+B diet. Diets were irradiated, packed under inert gas in individual 2.5-kg foil bags, and stored at −20°C until use. Fresh diet was provided at least twice per week to minimize oxidization of the fats and deterioration of the anthocyanins.
TABLE 1.
Composition of LFD, HFD, and HFD+B
| LFD
|
HFD
|
HFD+B
|
||||
|---|---|---|---|---|---|---|
| Ingredient | g/kg | kJ/kg | g/kg | kJ/kg | g/kg | kJ/kg |
| Casein | 189.6 | 3174.7 | 258.4 | 4328.2 | 255.7 | 4282.9 |
| l-Cystine | 2.8 | 47.6 | 3.9 | 64.9 | 3.8 | 64.2 |
| Corn starch | 545.0 | 9127.2 | 0.0 | 0.0 | 0.0 | 0.0 |
| Maltodextrin 10 | 118.5 | 1984.2 | 161.5 | 2705.2 | 159.8 | 2676.8 |
| Sucrose | 0.0 | 0.0 | 88.9 | 1488.9 | 63.9 | 1070.7 |
| Cellulose | 47.4 | 0.0 | 64.6 | 0.0 | 57.5 | 0.0 |
| Lard | 19.0 | 714.3 | 316.6 | 11929.8 | 313.3 | 11804.7 |
| Soybean oil | 23.7 | 892.9 | 32.3 | 1217.3 | 32.0 | 1204.6 |
| Mineral mix1 | 9.5 | 63.5 | 12.9 | 86.2 | 12.8 | 85.6 |
| Dicalcium phosphate | 12.3 | 0.0 | 16.8 | 0.0 | 16.6 | 0.0 |
| Calcium carbonate | 5.2 | 0.0 | 7.1 | 0.0 | 7.0 | 0.0 |
| Potassium citrate·1H20 | 15.6 | 0.0 | 21.3 | 0.0 | 21.1 | 0.0 |
| Vitamin mixture2 | 9.5 | 155.7 | 12.9 | 211.4 | 12.8 | 209.7 |
| Choline bitartrate | 1.9 | 0.0 | 2.6 | 0.0 | 2.6 | 0.0 |
| BB powder | 0.0 | 0.0 | 0.0 | 0.0 | 40.9 | 581.6 |
| FD&C blue dye no. 1 | 0.0 | 0.0 | 0.047 | 0.0 | 0.047 | 0.0 |
| FD&C red dye no. 40 | 0.0 | 0.0 | 0.000 | 0.0 | 0.024 | 0.0 |
| FD&C yellow dye no. 5 | 0.024 | 0.0 | 0.000 | 0.0 | 0.000 | 0.0 |
| Total | 1000 | 16160.0 | 1000 | 22032.0 | 1000 | 21980.9 |
Containing the following (g/kg mineral mix): sodium chloride, 259; magnesium oxide, 41.9; magnesium sulfate.7H2O, 257.6; chromium KSO4·12H2O, 1.925; cupric carbonate, 1.05; sodium fluoride, 0.2; potassium iodate, 0.035; ferric citrate, 21.0; manganous carbonate, 12.25; ammonium molybdate·4H2O, 0.3; sodium selenite, 0.035; zinc carbonate, 5.6; sucrose, 399.105. Sucrose in the mineral mix provided 63.5 kJ energy/kg diet.
Containing the following (g/kg vitamin mix): all-trans retinol acetate, 0.8; cholecalciferol, 1.0; dl-α-tocopheryl acetate, 10.0; menadione sodium bisulfate, 0.08; biotin (1.0%), 2.0; cyanocobalamin (0.1%), 1.0; folic acid, 0.2; nicotinic acid, 3.0; calcium pantothenate, 1.6; pyridoxine-HCl, 0.7; riboflavin, 0.6; thiamin-HCl, 0.6; sucrose, 978.42. Sucrose in the vitamin mix provided 163.9 kJ energy/kg diet.
Anthocyanin analysis of BB powder.
BB powder was dissolved in 5% acetonitrile in water containing 1% formic acid to produce a 100-mg/L extract. The anthocyanin content of the extract (Table 2) was determined by liquid chromatography tandem MS (LC-MS/MS) (Supplemental Methods) using a modification of the method of Kalt et al. (19).
TABLE 2.
Relative anthocyanin composition in BB powder incorporated into the HFD as determined by LC-MS/MS1
| g/kg dry weight | |
|---|---|
| Delphinidin-3-galactoside | 1.383 |
| Delphinidin-3-glucoside | 0.467 |
| Cyanidin-3-galactoside | 0.493 |
| Cyanidin-3-glucoside | 0.333 |
| Cyanidin-3-arabinoside | 0.673 |
| Peonidin-3-galactoside | 0.538 |
| Peonidin-3-glucoside | 13.915 |
| Peonidin-3-arabinoside | 4.378 |
| Malvidin-3-galactoside | 0.156 |
| Malvidin-3-arabinoside | 9.104 |
| Total | 31.44 |
Detailed methods for the LC-MS/MS analysis of BB anthocyanins are available as Supplemental Methods.
Metabolic variables.
Energy intake and indirect calorimetry were obtained for subsets of mice (n = 5–8/group) between study d 45 and 52 using metabolic chambers (TSE Calorimetry Systems). The TSE system simultaneously and continuously monitored food intake, oxygen consumption, and CO2 production. Data were collected for 72 h, during which time mice had free access to food and water. The first 24 h were considered an acclimation period and excluded from analyses.
IR.
Intraperitoneal insulin tolerance tests (ITT) were performed on food-deprived (6 h), nonanesthetized mice. Glucose measures were obtained from whole tail vein blood using an automated glucometer at baseline and at 30, 45, 60, and 90 min following intraperitoneal injection of human insulin (0.75 mU/kg). Glucose concentrations following insulin injection were calculated as a proportion of the value at baseline. Glucose area under the curve (AUC) was calculated using these values and analyzed by ANOVA. Plasma insulin concentrations of food-deprived mice were measured using ELISA (5).
Histology and immunohistochemistry.
Mice were killed by cervical dislocation following CO2 narcosis. Epididymal AT (eAT) and inguinal subcutaneous AT (scAT) were dissected, fixed, embedded in paraffin, and sectioned (8). Digital images were acquired with an Olympus DX51 light microscope. For each mouse, morphometric data were obtained from digitized tracings of ≥500 adipocytes from 3 or more sections cut at least 50 μm apart. Adipocyte size was calculated as described (5).
Adipocyte death.
To quantify the frequency of dead adipocytes in individual mice, high resolution images of at least 2 histological sections of eAT at least 50 μm apart were scored for CLS (5). A minimum of 600 adipocytes were counted for each mouse. Each tissue section was scored for CLS by 3 observers who were unaware of the study objectives or dietary treatments. Percent CLS [(number dead adipocytes/number total adipocytes) × 100] for each mouse was calculated as the mean of the 3 values.
Quantitative-PCR.
AT were dissected, snap-frozen in liquid nitrogen, and stored at −80°C. Total RNA was extracted, quantified, and analyzed by SYBR Green real-time PCR on an Applied Biosystems 7300 Real-time PCR system (5). Fold expression relative to an endogenous control gene (cyclophilin B) was calculated as 2−ΔΔCt using mice fed the LFD as the “comparer.” Primers used were: macrophage phi 4/80 (F4/80) (5′-CTTTGGCTATGGGCTTCCAGTC-3′, 5′-GCAAGGAGGACAGAGTTTATCGTG-3′), CD11c (5′-CTGGATAGCCTTTCTTCTGCTG-3′, 5′-GCACACTGTGTCCGAACTC-3′), Galectin-3 (5′-CAACAGGAGAGTCATTGTGTGTAAC-3′, 5′-TTCAACCAGGACTTGTATTTTGAAT-3′), MGL1 (5′-TCTCTGAAAGTGGATGTGGAGG-3′, 5′-CACTACCCAGGTCAAACACAATCC-3′), IL-6 (5-CCAGTTGCCTTCTTGGGACT-3′, 5′-GGTCTGTTGGGAGTGGTATCC-3′), iNOS (5′-CAGAGGACCCAGAGACAAGC-3′, 5′-TGCTGAAACATTTCCTGTGC-3′), IL-10 (5′-CCAGGGAGATCCTTTGATGA-3′, 5′-CATTCCCAGAGGAATTGCAT3′), TNFα (5′-AATGGAAGGTTGGACGAAAA-3′, 5′-GAGGCAACCTGACCACTCTC-3′), monocyte chemoattractant protein-1 (MCP1) (5′-ACTGAAGCCAGCTCTCTCTTCCTC-3,' 5′-TTCCTTCTTGGGGTCAGCACAGAC-3′), glucose-regulated protein, 78kDa (5′-GGCCAAATTTGAAGAGCTGA-3′, 5′-GCTCCTTGCCATTGAAGAAC-3′), C/EBP-homologous protein/growth arrest/DNA-damage-inducible 153 (5′-TCTTGACCCTGCGTCCCTAG-3′, 5′-TGGGCACTGACCACTCTGTTT-3′), Glutathione Peroxidase 3 (GPX3) (5′-ATGGTACCACTCATACCGCC-3′, 5′-CATCCTGCCTTCTGTCCCT-3′), Adiponectin (5′-GAATCATTATGACGGCAGCA-3′, 5′-TCATGTACACCGTGATGTGGTA-3′), Leptin (5′-TTCACACACGCAGTCGGTAT-3′, 5′- TGGTCCATCTTGGACAAACTC-3′), Arginase (5′-AGGAACTGGCTGAAGTGGTTA-3′, 5′-GATGAGAAAGGAAAGTGGCTG-3′), Ym1 (5′-AGAAGGGAGTTTCAAACCTGG-3′, 5′-GTCTTGCTCATGTGTGTAAGT-3′), Cyclophilin B (5′-ATGTGGTTTTCGGCAAAGTT-3′, 5′-TGACATCCTTCAGTGGCTTG-3′).
Statistics.
Data are expressed as means ± SEM. Data were analyzed using SYSTAT v10. ANOVA or GLM procedures were used in conjunction with protected post hoc tests (Tukey's honestly significant difference). Associations between 2 variables are reported as the Pearson correlation coefficient. Frequency data of adipocyte death were transformed as arcsin√x prior to statistical analysis. Differences were considered significant at P < 0.05.
Results
Effects of BB on HFD-induced weight gain, adiposity, and metabolic variables.
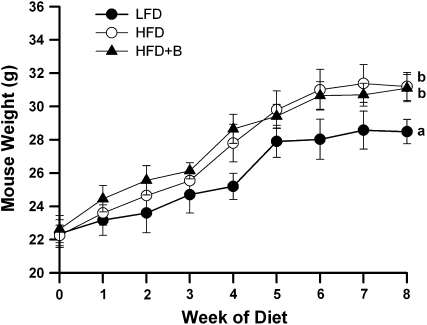
As expected, mice fed the HFD for 8 wk gained more weight (≤3 g) than mice fed the LFD (P < 0.005; Fig. 1). However, the addition of BB to the HF diet did not protect against HFD-induced weight gain, as the body weights of mice fed the HFD (31.2 ± 0.8 g) and the HFD+B (31.1 ± 0.8 g) did not differ (Fig. 1). Similar to differences in body weight, the eAT mass of mice fed the HFD or HFD+B was greater than that of mice fed the LFD (P = 0.03), but they did not differ from one another (Table 3). In addition, the adipocyte size in eAT of HFD- and HFD+B-fed mice was greater than in LFD-fed mice (P < 0.001) but did not differ from one another (Table 3). Similarly, scAT depots in HFD- and HFD+B-fed mice tended to be heavier than scAT of mice fed the LFD (P = 0.06 and 0.07, respectively) but did not differ from each other (Table 3). Collectively, these results indicate that the addition of BB to the HFD did not have protective effects on body weight, adiposity, or adipocyte hypertrophy. Consistent with these data, gene expression of adiponectin was lower in the HFD and HFD+B groups than in the LFD-fed mice (P = 0.02; data not shown) and that of leptin, which was higher than in the LFD group (P = 0.04; data not shown).
FIGURE 1 .
Body weight of mice fed the LFD, HFD, or HFD+B for 8 wk. Values are means ± SEM, n = 8. Means at wk 8 without a common letter differ, P < 0.05.
TABLE 3.
Adiposity, energy intake, substrate utilization, and indices of metabolic rate in mice fed the LFD, HFD, or HFD+B for 8 wk1
| LFD | HFD | HFD+B | |
|---|---|---|---|
| Adiposity | |||
| eAT weight, g | 0.80 ± 0.06a | 1.49 ± 0.22b | 1.35 ± 0.12b |
| scAT weight, g | 0.54 ± 0.03a | 0.86 ± 0.13b | 0.84 ± 0.09b |
| Adipocyte size, μm2 | 2,210 ± 90a | 5,150 ± 320b | 5,090 ± 670b |
| Metabolic variables | |||
| Energy intake, kJ·d−1 | 50.55 ± 4.30a | 55.99 ± 2.64b | 56.06 ± 5.65b |
| Respiratory exchange ratio, arbitrary units | 0.83 ± 0.02b | 0.72 ± 0.01a | 0.73 ± 0.01a |
| VO2, mg·kg−1·h−1 | 1459 ± 42b | 1288 ± 29a | 1334 ± 53a |
| Heat, kJ·h−1·kg−1 | 29.74 ± 0.95b | 25.57 ± 0.60a | 26.53 ± 1.07a |
Values are means ± SEM, n = 5–8. Means in a row without a common letter differ, P < 0.05.
As expected, energy intakes in mice fed the HFD or HFD+B were greater than in LFD-fed mice (P = 0.02; Table 3). Moreover, compared with LFD mice, HFD- and HFD+B-fed mice had lower respiratory exchange ratios (P < 0.001), oxygen consumption (P = 0.03), and heat production (P = 0.01) (Table 3). Importantly, the HFD and HFD+B cohorts did not differ in any of these measures (Table 3). These data indicate that greater energy intake, altered substrate utilization favoring fat over carbohydrate, and lower metabolic rate contribute to the greater body weight and adiposity in mice fed the HFD and HFD+B compared with those fed the LFD. Together, these results suggest that BB supplementation did not significantly alter HFD-associated changes in energy intake or metabolic rate, consistent with the increases in body weight and adiposity (Fig. 1; Table 3).
BB protects against HFD-induced IR.
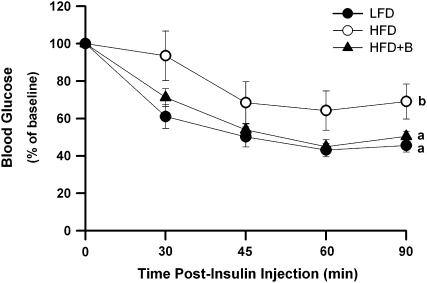
ITT were performed after 8 wk of dietary treatment to assess the effects of BB on HFD-induced peripheral IR (assessed as ITT[AUC]). IR was greater in mice fed the HFD (glucose AUC = 787.8 ± 35.2 mmol/L · 90 min) compared with those fed the LFD (glucose AUC = 513.8 ± 14.1 mmol/L · 90 min; P = 0.005; Fig. 2), consistent with prior studies from our laboratory (5). Notably, mice fed the HFD+B were less insulin resistant than mice fed the HFD (glucose AUC = 549.6 ± 19.9 mmol/L · 90 min; P = 0.03; Fig. 2). In fact, the ITT[AUC] did not differ between the LFD and HFD+B cohorts (Fig. 2). Consistent with the observed improvement in insulin sensitivity, the blood glucose concentrations tended (P = 0.07) to be lower in food-deprived HFD+B mice (7.34 ± 0.58 mmol/L) compared with the HFD mice (8.57 ± 0.51 mmol/L) but not to the LFD mice (5.80 ± 0.35 mmol/L). Together, these results demonstrate that mice fed the HFD+B for 8 wk were less insulin resistant and had a mild improvement in glucose homeostasis compared with mice fed the HFD. However, plasma insulin concentrations in food-deprived mice fed the HFD (148.0 ± 37.0 pmol/L) or the HFD+B (148.1 ± 45.0 pmol/L) were ∼100% greater (P = 0.04 and 0.04, respectively) than those in LFD-fed mice (70.6 ± 13.7 pmol/L). Thus, the HFD+B did not ameliorate the modest hyperinsulinemia in mice fed the HFD.
FIGURE 2 .
ITT in mice fed the LFD, HFD, or HFD+B for 8 wk. Data represent the clearance of blood glucose in response to an insulin bolus in food-deprived mice. Glucose values at 30, 45, 60, and 90 min after intraperitoneal insulin injection were expressed for each mouse as a proportion of the value at baseline (100%). Values are means ± SEM, n = 8. ITT [AUC] for lines without a common letter differ, P < 0.05.
BB protects against HFD-induced adipocyte death.
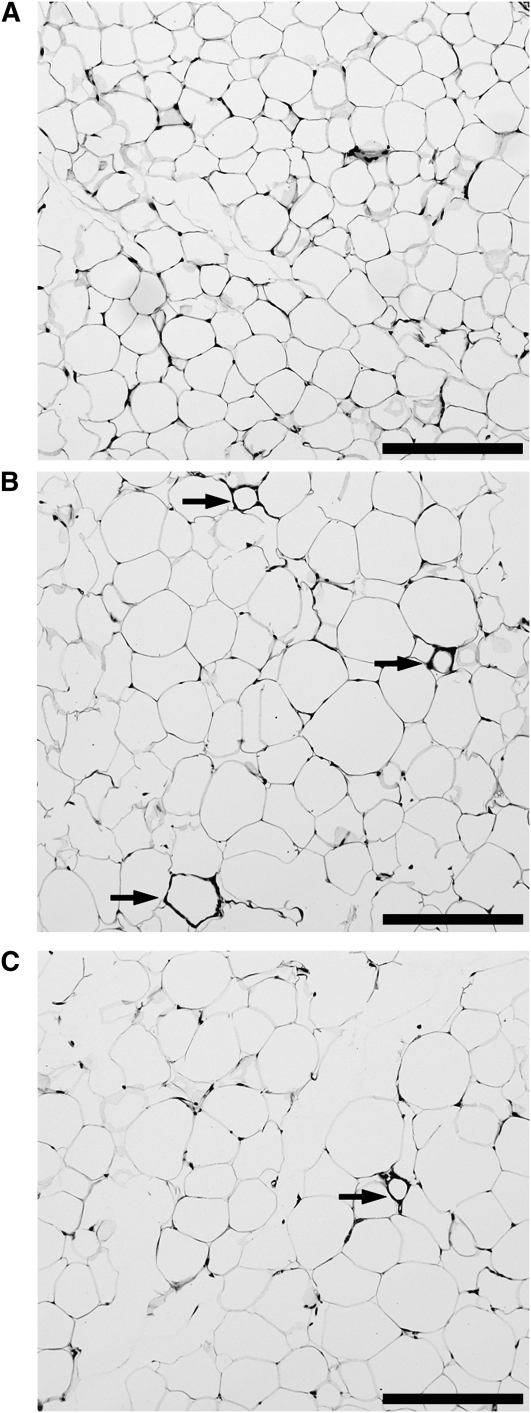
The frequency of dead adipocytes was 5-fold greater in mice fed the HFD (0.64% ± 0.08) than in mice fed the LFD (0.10% ± 0.05) (P < 0.001; Fig. 3A,B). Supplementation of the HFD with BB was associated with a ∼50% lower frequency of dead adipocytes (0.33% ± 0.03) compared with the HFD (P = 0.02; Fig. 3B,C), coincident with lower IR (Fig. 2). The frequency of dead adipocytes tended to be correlated with IR (ITT[AUC]) when data from mice fed all 3 diets were included (r = 0.46; P = 0.06). Together, these results confirm the positive association of obesity-induced IR and adipocyte death (5) and demonstrate that the beneficial effects of BB on whole body IR are coincident with BB-mediated attenuation of adipocyte death.
FIGURE 3 .
Frequency of dead adipocytes in mice fed the LFD (A), HFD (B), or HFD+B (C) for 8 wk. Representative hematoxylin- and eosin-stained histological sections of eAT from mice are shown. Dead adipocytes are indicated by CLS of ATMΦ surrounding remnant lipid droplets (arrows). Scale bar = 200 μm.
BB attenuates the HFD-associated change in gene hallmarks of recruited (M1) compared with resident (M2) ATMΦ.
CD11c and MGL1 gene expression provide useful measures of the numbers of recruited (M1-polarized) ATMΦ (CD11c+/MGL1−) and resident (M2-polarized) ATMΦ (CD11c−/MGL1+) (3,20). Employing real-time PCR, we determined gene expression levels of markers of total eAT monocytes/MΦ (galectin-3), differentiated MΦ (F4/80), recruited ATMΦ (CD11c), and resident ATMΦ (MGL1), respectively. The HFD was associated with greater galectin-3 (P = 0.04) and CD11c gene expression (P = 0.02; Table 4). These results are consistent with HFD-induced recruitment of monocytes (galectin-3+) into eAT and their subsequent maturation into M1-polarized (CD11c+) ATMΦ (F4/80+). Because CD11c+ ATMΦ preferentially localize to dead adipocytes (4), these results are also in agreement with the greater frequency of dead adipocytes in the eAT of HFD-fed mice (Fig. 3) (5,8).
TABLE 4.
Gene expression (real-time PCR) in whole eAT for gene markers of ATMΦ and ATMΦ polarization, inflammatory mediators, and oxidative stress in mice fed the LFD, HFD, or HFD+B for 8 wk1
| LFD | HFD | HFD+B | |
|---|---|---|---|
| ATMΦ markers | |||
| Galectin 3 | 1.00 ± 0.10a | 1.78 ± 0.28b | 1.52 ± 0.24b |
| F4/80 | 1.00 ± 0.17 | 1.34 ± 0.19 | 1.13 ± 0.13 |
| CD11c | 1.00 ± 0.26a | 3.66 ± 1.10b | 2.55 ± 0.66b |
| MGL1 | 1.00 ± 0.12 | 0.91 ± 0.08 | 1.15 ± 0.09 |
| Ym1 | 1.00 ± 0.16 | 1.21 ± 0.28 | 1.42 ± 0.23 |
| CD11c / MGL1 | 1.00 ± 0.17a | 6.17 ± 1.50b | 1.42 ± 0.47a |
| Inflammatory mediators | |||
| TNFα | 1.00 ± 0.14a | 2.60 ± 0.41b | 1.19 ± 0.24a |
| MCP-1 | 1.00 ± 0.26a | 4.32 ± 1.06b | 2.95 ± 0.88b |
| IL-6 | 1.00 ± 0.12 | 1.35 ± 0.19 | 1.11 ± 0.14 |
| iNOS | 1.00 ± 0.17 | 1.55 ± 0.24 | 1.03 ± 0.17 |
| IL-10 | 1.00 ± 0.21a | 2.29 ± 0.40b | 1.46 ± 0.29a |
| Oxidative stress | |||
| GPx3 | 1.00 ± 0.42a,b | 0.62 ± 0.16a | 1.24 ± 0.31b |
Values are means ± SEM, n = 8. Means in a row with superscripts without a common letter differ, P < 0.05.
In contrast, expression of MGL1 and Ym1, hallmarks of resident (M2-polarized) ATMΦ (3,21), were not significantly altered by the HFD (Table 4). Thus, the ratio of CD11c gene expression:MGL1 gene expression was greater in mice fed the HFD than in those fed the LFD (P = 0.02; Table 4). Remarkably, this index of the relative numbers of M1 and M2 ATMΦ was lower in mice fed HFD+B than in mice fed the HFD (P = 0.05) and did not differ from mice fed the LFD (Table 4). Similar results were obtained when gene expression of Ym1 was considered (data not shown). Together, these results suggest that BB selectively affects the recruitment and/or maturation of M1-polarized (CD11c+/MGL1−) ATMΦ in mice made obese by HFD.
BB protects against HFD-induced ATMΦ inflammatory gene expression in eAT.
TNFα and iNOS, which are produced predominantly by M1-polarized ATMΦ, and IL-6 and MCP-1, which are produced by ATMΦ and other AT cells, have each been implicated as disrupters of insulin signaling and/or promoters of obesity-associated IR in vivo (2). Consistent with the role of CD11c+ ATMΦ in obesity-associated AT inflammation, CD11c gene expression was correlated with gene expression of TNFα (r = 0.73; P < 0.001), iNOS (r = 0.54; P = 0.009), interleukin (IL)-6 (r = 0.53; P = 0.02), and MCP-1 (r = 0.49; P = 0.04; Table 4) when data from mice from all 3 diets were included. Compared with LFD-fed mice, feeding the HFD resulted in greater expression of TNFα (P = 0.005) and MCP-1 (P = 0.05; Table 4). Notably, supplementing the HFD with BB was associated with reduced expression of TNFα (P = 0.01; Table 4). Considered together, these results indicate that BB can reduce mRNA levels of ATMΦ-derived proinflammatory genes that promote obesity-associated IR.
IL-10 is an immunosuppressive cytokine that limits the inflammatory response to pathogens and injury, thereby preventing or minimizing tissue damage. IL-10 is upregulated by resident M2-polarized ATMΦ during the course of HF diet-induced AT inflammation, ostensibly as a counter-regulatory mechanism (3,5,21). In the present study, IL-10 gene expression in eAT was greater in mice fed the HFD than in those fed the LFD (P = 0.003; Table 4). Surprisingly, BB supplementation of the HFD was associated with a 50% lower IL-10 gene expression compared with HFD alone (P = 0.02; Table 4), coincident with attenuated ATMΦ expression of proinflammatory genes (Table 4). These results suggest that the biological action(s) of BB result in downregulation of HFD-induced inflammatory gene expression in both M1-polarized and M2-polarized ATMΦ.
Effects of BB on HFD-induced oxidative stress in eAT.
Obesity promotes oxidative and ER stress in AT and these stresses are mechanistically linked to AT inflammation and the metabolic complications of obesity (10,11,22). To assess the effect of BB supplementation on oxidative stress in AT, we quantified gene expression of GPx3, a sensitive index of oxidative stress in AT (22). GPx3 gene expression was greater in mice fed the HFD+B compared with those fed the HFD (P = 0.05) and tended (P = 0.11) to be greater than in mice fed the LFD (Table 4). These data suggest that protection from HFD-induced adipocyte death (Fig. 3) and AT inflammation (Table 4) is coincident with attenuated oxidative stress in AT of mice fed the HFD+B diet. Surprisingly, neither HFD nor HFD+B had any effect on adipose ER stress, assessed as gene expression of glucose-regulated protein 78 and C/EBP-homologous protein [(23); data not shown]. These results suggest that oxidative stress precedes ER stress during the development of obesity and AT inflammation in this model.
Discussion
The genus Vaccinium (e.g. BB, bilberry, cranberry) has been used traditionally as a source of folk remedies for established diabetic symptoms, primarily as leaf or stem infusions or decoctions (24). Here, we demonstrate that supplementing a HFD with Vaccinium berry powder inhibits the early inflammatory events in AT that promote obesity-associated IR. Consistent with these observations, mice fed the HFD+B diet for 8 wk were substantially protected from IR based on significant improvements in the ITT [AUC] compared with those fed HFD. The ITT measures whole-body glucose disposal in response to an insulin bolus and reflects both increased skeletal muscle glucose uptake and decreased liver glucose production. BB also improved HFD-induced hyperglycemia in food-deprived mice, although this improvement was modest and was not coincident with attenuated hyperinsulinemia. Because plasma glucose concentrations largely reflect hepatic glucose output (which is normally suppressed by insulin), these observations suggest that supplementing the HFD with BB did not reverse HFD-induced hepatic IR. Hyperinsulinemic-euglycemic clamp studies will be required to verify this conclusion. Overall, however, these results demonstrate that dietary BB can protect against whole-body IR and can improve glycemia in a model of obesity-induced IR.
A prior study of BB effects on diet-induced obesity and obesity complications reported no protection from IR in mice fed a BB-supplemented HFD for 12 wk (25). However, these results were confounded by significantly greater food intake and adiposity in mice fed the BB-supplemented HFD. Similarly in our studies, mice fed the HFD+B for 12 wk were ∼3 g heavier and 30% more obese than HFD mice (P = 0.03; data not shown), reflecting higher energy intake and somewhat reduced energy expenditure after wk 8 (data not shown). Intriguingly, however, despite the greater adiposity of HFD+B mice, ITT[AUC] values did not differ between HFD and HFD+B mice (P = 0.92; data not shown), suggesting that BB supplementation may have continued to provide metabolic benefit after 8 wk.
Peripheral IR is thought to be caused by increased circulating levels of FFA and lipids (2,26). One mechanism contributing to elevated circulating FFA in obesity is adipocyte lipolysis (11,26). Inflammatory mediators upregulated in AT in obesity (e.g. TNFα, iNOS, IL-6) exacerbate lipolysis by antagonizing the antilipolytic effects of insulin on adipocytes and/or by directly stimulating lipolysis (11,26). Accordingly, the insulin-sensitizing effects of BB in the present study were associated with attenuated AT inflammation, manifest as lower frequency of dead adipocytes and lower gene expression indices of ATMΦ infiltration and inflammatory activation. Studies from our laboratory and others indicate that adipocyte death and ATMΦ infiltration are mechanistically intertwined in the pathogenesis of AT inflammation. Specifically, the infiltration, accumulation, and proinflammatory activation of M1-polarized ATMΦ in obesity are functionally linked to the selective recruitment of CD11c+ ATMΦ to scavenge dead adipocytes and their remnant lipid (4,5,8). Thus, a decrease in the proportion of ATMΦ expressing CD11c+ and coincident reductions in inflammatory cytokines in eAT of mice fed the HFD+B diet is consistent with the lower frequency of dead adipocytes in these mice.
It is noteworthy that whereas HFD+B was associated with frequencies of adipocyte death and levels of CD11c gene expression intermediate between those of mice fed the LFD and HFD, AT gene expression of TNFα did not differ between mice fed the HFD+B compared with those fed the LFD. This observation suggests that, in addition to reducing adipocyte death and ATMΦ infiltration, BB blocks HFD-induced AT inflammation by inhibiting inflammatory gene expression in ATMΦ (and presumably other AT cells). Prior studies of BB protective actions in contexts other than obesity (15,16,27) suggest that one potential mechanism of this inflammatory gene downregulation is through inhibition of stress-induced mitogen-activated protein kinase and nuclear factor κB signaling pathways, which modulate inflammatory gene expression in MΦ (28). Future studies employing alternative sampling methods (e.g. flow cytometry) will be required to determine the extent to which the salutary effect of BB on HFD-induced ATMΦ inflammation reflects reduced ATMΦ infiltration or, alternatively, reduced ATMΦ inflammatory gene expression. In addition, our data do not exclude the possibility that the antiinflammatory effects of the HFD+B also reflect the previously reported actions of BB to inhibit bacterial translocation to extraintestinal sites (17). Extraintestinal bacterial translocation and the resulting low-grade endotoxemia are associated with chronic HFD regimes and are implicated in the development of AT inflammation and IR (18). Irrespective of the mechanism(s) of action, we suggest that the ability of BB to inhibit inflammatory gene expression in AT plays a critical role in preventing obesity-associated IR in the present study. However, a limitation of our study is the absence of ELISA data confirming that BB-associated patterns of gene expression are manifest as functionally significant alterations in expressed inflammatory proteins.
Increased adipocyte death in obesity is suggested (8) to reflect the elevated levels of cytotoxic stressors reported for obese AT (10,11,22). In the present study, higher frequencies of dead adipocytes in mice fed the HFD were coincident with lower gene expression of GPx3, an oxidative stress-sensitive gene in AT (22). Notably, feeding the HFD+B diet was not associated with lower GPx3 gene expression coincident with lower frequency of dead adipocytes. Together, these observations provide an empirical association between AT oxidative stress and obesity-associated adipocyte death. As with the inflammation-suppressive effects of BB on ATMΦ, the cytoprotective effects of BB on adipocytes are, in theory, consistent with the demonstrated ability of BB to modulate mitogen-activated protein kinase and nuclear factor κB signaling pathways (16,27,29), because these 2 pathways coordinately regulate cell fate (30). BB-enhanced adipocyte survival may also indirectly reflect the effects of BB on ATMΦ, specifically the attenuation of ATMΦ TNFα, because this inflammatory mediator promotes oxidative stress and apoptosis (31,32). Consistent with the notion that the actions of BB to prevent adipocyte apoptosis reflect the salutary effects of BB on ATMΦ are recent observations (7,33) suggesting that inflammatory CD11c+ ATMΦ participate in adipocyte execution as well as clearance [see also (8)].
Our data demonstrating comparable weight and adiposity in mice fed either the HFD or HFD+B distinguish the protective effects of whole BB extract on AT inflammation and IR in the present study from the actions of flavonoids and other polyphenolics that achieve similar protection by blocking adiposity per se (25,34). In particular, despite its presence in the diet we observed none of the reported actions (34,35) of the anthocyanin cyanidin-3 glucoside (C3G) to block HFD-induced obesity or to promote insulin sensitivity by altering retinol binding protein-4 and glucose transporter 4 gene expression (data not shown). Prior et al. (25) similarly did not detect antiobesity effects of a BB extract containing ≥2-fold higher concentrations of C3G than that (0.2% of diet) reported to robustly inhibit HF diet-induced obesity (34). However, Prior et al. (25) reported that a purified preparation of BB anthocyanins partially inhibited HF diet-induced increases in adiposity, suggesting that the “whole” BB matrix may restrict the bioavailability or bioactivity of C3G and potentially other BB polyphenolics.
We find it additionally encouraging that in humans, 2.7% of total energy from BB (as in the present study) could be achieved with 20 g/d of BB powder or 140 g/d of whole BB (based on a daily energy intake of 14.5 MJ). Thus, the metabolic benefits of BB are, in theory, attainable with feasible levels of dietary intervention. These benefits are likely to reflect at least in part the biological activities of BB anthocyanins. Although anthocyanin bioavailability is generally considered to be low (36), recent in vivo studies suggest that even low levels of anthocyanins can be highly bioavailable and well retained in tissues (19). Although our data strongly suggest that AT is an important site of BB actions to ameliorate obesity complications, it is plausible that the protective effects of BB in the present study reflect retention and direct action of BB components in non-AT, including skeletal muscle, liver, pancreas, and circulating immune cells. Studies elucidating the tissue distribution of BB anthocyanins and anthocyanin metabolites in mice fed a HFD+B will help clarify the cell targets and molecular mechanism(s) by which dietary BB protects against obesity-induced AT inflammation and IR.
Supplementary Material
Supported by the United States Highbush Blueberry Council (M.S.O.), the American Diabetes Association (M.S.O.), USDA contract 5819507707 (A.S.G.), NIH T32DK062032 (J.D.), and NIH TH32HL69772 (G.B.).
Author disclosures: J. DeFuria, G. Bennett, K. J. Strissel, J. W. Perfield, A. S. Greenberg, and M. S. Obin, no conflicts of interest. P. E. Milbury currently holds research funds from the United States Highbush Blueberry Council.
Supplemental Methods are available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: AT, adipose tissue; ATMΦ, adipose tissue macrophage; AUC, area under the curve; BB, blueberry; C3G, cyanidin-3 glucoside; CLS, crown-like structure; eAT, epididymal adipose tissue; ER, endoplasmic reticulum; GPx3, glutathione peroxidase 3; HFD, high-fat diet; HFD+B, high-fat diet plus blueberry; IL, interleukin; iNOS, inducible nitric oxide synthase; IR, insulin resistance; ITT, insulin tolerance test; LC-MS/MS, liquid chromatography tandem MS; LFD, low-fat diet; MΦ, macrophage; MCP-1, monocyte chemoattractant protein 1; MGL1, macrophage galactose N-acetyl-galactosamine specific lectin 1; scAT, subcutaneous adipose tissue; TNFα, tumor necrosis factor-α.
References
- 1.Surmi BK, Hasty AH. Macrophage infiltration into adipose tissue: initiation, propagation and remodeling. Future Lipidol. 2008;3:545–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shoelson S, Herreroa L, Naaza A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–80. [DOI] [PubMed] [Google Scholar]
- 3.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW II, DeFuria J, Jick Z, Greenberg AS, Obin MS. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910–8. [DOI] [PubMed] [Google Scholar]
- 6.Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–8. [DOI] [PubMed] [Google Scholar]
- 7.Solinas G, Vilcu C, Neels J, Bandyopadhyay G, Luo J-L, Naugler W, Grivennikov S, Wynshaw-Boris A, Scadeng M, et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6:386–97. [DOI] [PubMed] [Google Scholar]
- 8.Cinti S, Mitchell G , Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–55. [DOI] [PubMed] [Google Scholar]
- 9.Murano I, Barbatelli G, Parisani V, Latini C, Muzzonigro G, Castellucci M, Cinti S. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J Lipid Res. 2008;49:1562–8. [DOI] [PubMed] [Google Scholar]
- 10.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–8. [DOI] [PubMed] [Google Scholar]
- 11.Lionetti L, Mollica MP, Lombardi A, Cavaliere G, Gifuni G, Barletta A. From chronic overnutrition to insulin resistance: the role of fat-storing capacity and inflammation. Nutr Metab Cardiovasc Dis. 2009;19:146–52. [DOI] [PubMed] [Google Scholar]
- 12.Cefalu WT, Ye J, Zuberi A, Ribnicky DM, Raskin I, Liu Z, Wang ZQ, Brantley PJ, Howard L, et al. Botanicals and the metabolic syndrome. Am J Clin Nutr. 2008;87:S481–7. [DOI] [PubMed] [Google Scholar]
- 13.Williams RJ, Spencer JP, Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radic Biol Med. 2004;36:838–49. [DOI] [PubMed] [Google Scholar]
- 14.Zafra-Stone S, Yasmin T, Bagchi M, Chatterjee A, Vinson JA, Bagchi D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol Nutr Food Res. 2007;51:675–83. [DOI] [PubMed] [Google Scholar]
- 15.Lau FC, Bielinski DF, Joseph JA. Inhibitory effects of blueberry extract on the production of inflammatory mediators in lipopolysaccharide-activated BV2 microglia. J Neurosci Res. 2007;85:1010–7. [DOI] [PubMed] [Google Scholar]
- 16.Atalay M, Gordillo G, Roy S, Rovin B, Bagchi D, Bagchi M, Sen CK. Anti-angiogenic property of edible berry in a model of hemangioma. FEBS Lett. 2003;544:252–7. [DOI] [PubMed] [Google Scholar]
- 17.Osman N, Adawi D, Ahrné S, Jeppsson B, Molin G. Endotoxin- and d-galactosamine-induced liver injury improved by the administration of Lactobacillus, Bifidobacterium and blueberry. Dig Liver Dis. 2007;39:849–56. [DOI] [PubMed] [Google Scholar]
- 18.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–81. [DOI] [PubMed] [Google Scholar]
- 19.Kalt W, Blumberg JB, McDonald JE, Vinqvist-Tymchuk MR, Fillmore SAE, Graf BA, Leary JM, Milbury P. Identification of anthocyanins in the liver, eye, and brain of blueberry-fed pigs. J Agric Food Chem. 2008;56:705–12. [DOI] [PubMed] [Google Scholar]
- 20.Ito A, Suganami T, Yamauchi A, Degawa-Yamauchi M, Tanaka M, Kouyama R, Kobayashi Y, Nitta N, Yasuda K, et al. Role of CC chemokine receptor 2 in bone marrow cells in the recruitment of macrophages into obese adipose tissue. J Biol Chem. 2008;283:35715–23. [DOI] [PubMed] [Google Scholar]
- 21.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. [DOI] [PubMed] [Google Scholar]
- 22.Lee YS, Kim AY, Choi JW, Kim M, Yasue S, Son HJ, Masuzaki H, Park KS, Kim JB. Dysregulation of adipose glutathione peroxidase 3 in obesity contributes to local and systemic oxidative stress. Mol Endocrinol. 2008;22:2176–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.JI C. Dissection of endoplasmic reticulum stress signaling in alcoholic and non-alcoholic liver injury. J Gastroenterol Hepatol. 2008;23:S16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martineau LC, Couture A, Spoor D, Benhaddou-Andaloussi A, Harris C, Meddah B, Leduc C, Burt A, Vuong T, et al. Anti-diabetic properties of the Canadian lowbush blueberry Vaccinium angustifolium Ait. Phytomedicine. 2006;13:612–23. [DOI] [PubMed] [Google Scholar]
- 25.Prior RL, Wu X, Gu L, Hager TJ, Hager A, Howard LR. Whole berries versus berry anthocyanins: Interactions with dietary fat levels in the C57BL/6J mouse model of obesity. J Agric Food Chem. 2008;56:647–53. [DOI] [PubMed] [Google Scholar]
- 26.Wang S, Soni KG, Semache M, Casavant S, Fortier M, Pan L, Mitchell GA. Lipolysis and the integrated physiology of lipid energy metabolism. Mol Genet Metab. 2008;95:117–26. [DOI] [PubMed] [Google Scholar]
- 27.Williams CM, El Mohsen MA, Vauzour D, Rendeiro C, Butler LT, Ellis JA, Whiteman M, Spencer JPE. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radic Biol Med. 2008;45:295–305. [DOI] [PubMed] [Google Scholar]
- 28.Suganami T, Tanimoto-Koyama K, Nishida J, Itoh M, Yuan X, Mizuarai S, Kotani H, Yamaoka S, Miyake K, et al. Role of the Toll-like receptor 4/NF-{kappa}B pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol. 2007;27:84–91. [DOI] [PubMed] [Google Scholar]
- 29.Joseph JA, Carey A, Brewer GJ, Lau FC, Fisher DR. Dopamine and Abeta-induced stress signaling and decrements in Ca2+ buffering in primary neonatal hippocampal cells are antagonized by blueberry extract. J Alzheimers Dis. 2007;11:433–46. [DOI] [PubMed] [Google Scholar]
- 30.Herr I, Debatin K-M. Cellular stress response and apoptosis in cancer therapy. Blood. 2001;98:2603–14. [DOI] [PubMed] [Google Scholar]
- 31.Domingo P, Vidal F, Domingo JC, Veloso S, Sambeat MA, Torres F, Sirvent JJ, Vendrell J, Matias-Guiu X, et al. Tumour necrosis factor alpha in fat redistribution syndromes associated with combination antiretroviral therapy in HIV-1-infected patients: potential role in subcutaneous adipocyte apoptosis. Eur J Clin Invest. 2005;35:771–80. [DOI] [PubMed] [Google Scholar]
- 32.Mori M. Regulation of nitric oxide synthesis and apoptosis by arginase and arginine recycling. J Nutr. 2007;137:S1616–20. [DOI] [PubMed] [Google Scholar]
- 33.Patsouris D, Li P-P, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsuda T, Horio F, Uchida K, Aoki H, Osawa T. Dietary cyanidin 3-O-{beta}-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J Nutr. 2003;133:2125–30. [DOI] [PubMed] [Google Scholar]
- 35.Sasaki R, Nishimura N, Hoshino H, Isa Y, Kadowaki M, Ichi T, Tanaka A, Nishiumi S, Fukuda I, et al. Cyanidin 3-glucoside ameliorates hyperglycemia and insulin sensitivity due to downregulation of retinol binding protein 4 expression in diabetic mice. Biochem Pharmacol. 2007;74:1619–27. [DOI] [PubMed] [Google Scholar]
- 36.McGhie TK, Walton MC. The bioavailability and absorption of anthocyanins: Towards a better understanding. Mol Nutr Food Res. 2007;51:702–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.