Abstract
Individuals exposed to the Dutch Famine of 1944–45 during gestation have increased adiposity, which might be due to changes in energy intake, physical activity, or metabolic efficiency. We studied 357 persons born between January 1945 and March 1946 whose mothers experienced famine during or immediately preceding pregnancy, 298 persons born in the same 3 institutions during 1943 or 1947 (time controls), and 311 same-sex sibling controls. We obtained food frequency and physical activity data by questionnaire between 2003 and 2005 (mean age 58 y). We defined gestational exposure as exposure to a ration of <3762 kJ/d (<900 kcal/d) for at least 10 wk. For the whole study population, energy intake was 9225 ± 2650 kJ/d and physical activity was 7380 ± 4331 metabolic equivalents (MET)·min/wk. Compared with time controls, gestational famine exposure was associated with 113 kJ/d (95% CI, −272, 502) higher energy intake, 0.01 percentage point (95% CI, −0.88, 0.89) higher fat density, 688 MET·min/wk (95% CI, −1398, 23) lower physical activity, and 63 kJ/d (95% CI, −130, 259) higher predicted energy expenditure (pEE). Compared with sibling controls, gestational famine exposure was associated with 4 kJ/d (95% CI, −702, 711) higher energy intake, 2.01 percentage points (95% CI, 0.38, 3.63) higher fat density, 97 MET·min/wk) (95% CI, −1243, 1050) lower physical activity score, and 188 kJ/d (95% CI, −163, 539) higher pEE. Gender-specific associations (P < 0.05 for heterogeneity) emerged for protein density and pEE using time controls and for energy intake using sibling controls. Associations were weak, differed by choice of control, and may reflect sampling variability or methodological differences. Persistent small energy imbalances could explain the increased weight of famine-exposed individuals.
Introduction
Obesity results when energy intake exceeds expenditure over prolonged periods and even small imbalances, if they persist for long periods, can result in large absolute gains in weight (1). Several studies have reported that maternal exposure to famine in gestation is associated with increased offspring BMI in adult life (2–4). In our own study, for example, women who had experienced famine exposure during gestation were 4.3 kg heavier than women without such exposure (3).
Animal models have suggested modification of feeding behaviors and increased obesity, collectively described as couch-potato syndrome, following maternal undernutrition (5). A recent report has suggested that individuals exposed to famine early in gestation have a preference for high-fat diets compared with unexposed individuals (6). A persistent question in studies of famine exposure is whether those able to conceive during a famine, whose offspring have early-gestational famine exposure, are inherently different from other women (7) and therefore whether births to unrelated women provide an optimal reference population. Our ongoing study addresses this methodological question by including a paired sibling analysis in addition to the more commonly applied analytic strategy of comparing individuals exposed to famine during gestation to those born before or conceived after the famine (8).
In this article, we address several questions: 1) What are the differences at age 58 y in energy intake, dietary macronutrient density, and physical activity between individuals exposed to famine and those not so exposed? 2) To what extent can any differences in dietary intake be explained by differences in body size or physical activity? and 3) Does the magnitude of these differences differ by choice of reference population or by gender?
Methods
Setting.
The Dutch famine of 1944–45, which affected the western Netherlands, provides a rare opportunity to study the long-term consequences of maternal undernutrition in defined stages of gestation (9–11). Official rations, which by the end of the famine consisted almost exclusively of bread and potatoes, fell below 3762 kJ/d by November 26, 1944, and were as low as 2090 kJ/d by April 1945. The famine ceased immediately following liberation. This extraordinary period of deprivation affected fertility, weight gain during pregnancy, and infant size at birth (12–14). The reduction in fertility was greater among manual compared with the nonmanual occupational classes (9). The decline in mean birth weight of 300 g was restricted to exposure to maternal undernutrition during the 3rd trimester (14,15). Long-term consequences of gestational exposure to famine have been reported for adiposity (2–4), blood pressure (16), serum lipids (17), and coronary heart disease (18).
Population source and tracing.
We identified 3307 live-born singleton births at 3 institutions in famine-exposed cities (the midwifery training schools in Amsterdam and Rotterdam and the university hospital in Leiden) (8). We selected all 2417 births between February 1, 1945 and March 31, 1946 (infants whose mothers were exposed to the famine during or immediately preceding that pregnancy) and a sample of 890 births from 1943 and 1947 as hospital time controls (infants whose mothers did not experience famine during this pregnancy). The sample of controls included an equal number of births for each month allocated across the 3 institutions according to their size.
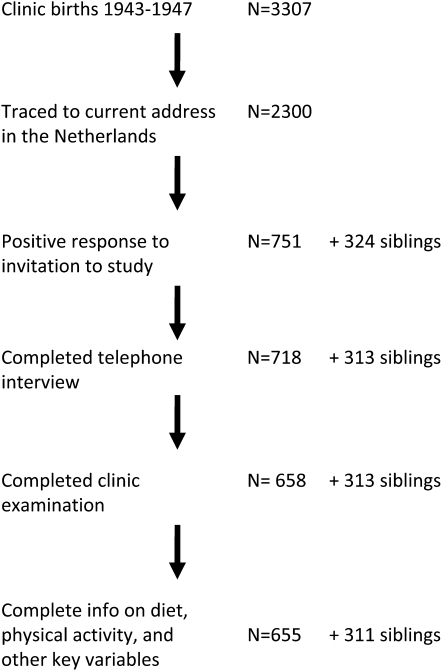
Names and addresses at birth for these 3307 infants were provided to the Population Register in the municipality of birth with a request for tracing to their current address (Fig. 1). A total of 308 (9%) were reported to have died in the Netherlands and 275 (8%) to have emigrated. The Population Register in Rotterdam declined to trace 130 individuals born out of wedlock and a current address could not be located for 294 subjects (9%). Address information was obtained for 2300 individuals (70% of the birth series). The proportion of individuals identified as deceased was highest among births in 1943 (10.4%) and lowest among births in 1947 (6.0%). Other reasons for a failure to locate a current address did not differ by year of birth or period of exposure to famine. Participants traced to a current address and those who had died, emigrated, or had not been located did not differ in birth weight or length, placental weight, maternal age at delivery, or birth order (8).
FIGURE 1 .
Flow chart of study tracing, contact, recruitment, and data availability for analysis.
A letter of invitation signed by the current director of the institution in which they were born was sent to these 2300 individuals, together with a brochure describing the study and a response card. We mailed 1 reminder letter to nonresponders. Initially, our study design called for the recruitment of same-sex sibling pairs only (one sibling per identified hospital birth) and the lack of an available sibling was a reason for ineligibility. We received some reply to 58% of the initial letters and to 44% of the reminder letters; 347 individuals (20% of 1767 respondents) expressed willingness to participate together with a sibling. None of the siblings was included in the hospital birth series. Among the 1415 who responded but declined, 951 (67%) reported not having a same-sex sibling available for study. To increase the number of study subjects, we recontacted these 951 individuals, 381 of whom expressed willingness to participate. A higher positive response to our letters from women (36%) compared with men (29%) was consistent across all exposure categories.
We conducted telephone interviews followed by a clinical examination at the Leiden University Medical Center. The standardized telephone interview (n = 1031 completed; 718 from the birth series and 313 siblings) required ∼1 h. The clinical examination took ∼4 h (n = 971 completed; 658 from the birth series and 313 siblings). All study protocols were approved by the Human Subjects Committees of Columbia University, Emory University, and Leiden University Medical Center. Study participants provided oral consent at the start of the telephone interview and written informed consent at the start of the clinical examination. All data collection was performed between 2003 and 2005.
Among the 2300 persons who were invited to join the study, those interviewed and those who were not did not differ in mean birth weight or length, placental weight, maternal age at delivery, or birth order (8). Response to our invitation, however, was lower for those born in 1947 (25%) compared with all others (35%). Eleven percent of those who were interviewed lived within 5 km of the examination site compared with 10% of those who were not interviewed, and 34% of those interviewed lived >45 km from the examination site compared with 29% of those who were not interviewed.
Diet and physical activity assessment.
The food frequency instrument is an updated version of a FFQ developed to assess dietary habits in an elderly Dutch population (19). This instrument ascertains consumption over the past 12 mo, using household measures and standardized serving sizes. Special attention is given to type of product (e.g. fat content), brand names, and seasonal food consumption. The questionnaire was mailed to the respondent following the telephone interview for completion at home and the completed instrument was reviewed by a study nurse when the participant arrived for the clinical assessment. We used the Dutch food composition database of 2001 (20) to derive daily energy, protein, fat, and carbohydrate intakes and expressed these as a percentage of total energy intakes.
We measured physical activity using the Short Questionnaire to Assess Health-Enhancing Physical Activity (21). This instrument evaluates habitual activity within the domains of occupation, leisure time, household, and transportation. It also determines whether individuals meet the health-based guideline of accumulating ≥30 min of moderately intense physical activity [≥4 metabolic equivalents (MET)7] on most, preferably all, days of the week. The instrument was completed on paper by the participant during the clinical visit. We used total activity, expressed in MET·min/wk, to represent physical activity. We computed the predicted energy expenditure (pEE) based on age, sex, height, weight, and physical activity using equations developed by the Institute of Medicine (22). Differences between reported energy intake and the pEE can be considered to be a marker of the bias inherent in the questionnaire methodology or as a measure of systematic imbalances in energy. Because biases inherent in the questionnaire are unlikely to be differential by famine exposure, residual between-group differences in this measure may represent exposure-related differences in energy balance.
Exposure to famine.
As described elsewhere (8), we used the date of last menstrual period (LMP) as noted in the hospital records to define the start of gestation unless it was missing or implausible (12%). In those cases, we inferred the LMP date from annotations on the birth record (the notation “term birth” had been added whenever applicable) or estimated gestational age based on birth weight and date of birth, assuming that the infant was of average gender-, parity-, and birth weight-specific gestation based on local growth curves (23). For each infant, the most consistent and plausible estimate of gestation was selected and used together with date of birth to infer the LMP date.
We characterized exposure to famine during gestation by determining the gestational ages (in weeks after the LMP) during which the mother was exposed to an official ration of <3762 kJ/d, namely between November 26, 1944, and May 12, 1945. We considered the mother exposed in gestational wk 1–10, 11–20, 21–30, or 31 to delivery if these gestational time windows were entirely included in this period. Thus, pregnancies with the LMP between November 26, 1944, and March, 4, 1945 were exposed in wk 1–10; between September 18, 1944 and December 24, 1944 in wk 11–20; between July 10, 1944, and October 15, 1944 in wk 21–30; and between May 2, 1944 and August 24, 1944 in wk 31 through delivery. By these definitions, a participant could have been exposed to famine during at most 2 adjacent 10-wk periods. Individuals exposed in at least 1 of the 10-wk periods were considered to have had any prenatal famine exposure, whereas all others were considered unexposed. None of the siblings had any gestational famine exposure.
Statistical methods.
We computed means and distributions as appropriate and used ANOVA and chi-square approaches to assess differences in sample characteristics among groups. We assessed the differences in intakes of energy and macronutrients and in physical activity scores attributable to exposure to famine using linear regression models. In models focusing on any exposure to famine, the single variable “exposure to famine during any 10-wk period of gestation” was entered. In models focusing on exposure in defined periods, all 4 10-wk periods were entered as a set into a model that already included covariates, and the overall significance of the set was tested using a 4-degree-of-freedom Wald chi-square test. We controlled for city of birth of the hospital birth series, age at assessment, and gender in all models. Models for macronutrients were adjusted for total energy intake. To address potential differences in energy requirements due to differences in physical activity and body size, we then controlled for these factors by adding terms for height, BMI, and physical activity score. For the model for physical activity, we added terms for height and BMI only. In practice, these adjustments did not alter our estimates substantively (data not shown) and only the base models are presented.
We examined additive interactions with gender. Where there was evidence of heterogeneity by gender (as defined by a P-value < 0.05 for the interaction term), we conducted stratified analysis.
To address the question of differences by type of control, we conducted 2 sets of analyses. The first was limited to the birth series, which includes all exposed birth and time controls but no sibling controls. The second focused on the sibling pairs. For the analysis limited to sibling pairs, we calculated the within-pair differences for each of the relevant measures and used these in our models. Finally, we combined the 2 populations of controls into a single reference group; in these models, we controlled for clustering at the family level using mixed models, implemented using the xtreg command in STATA.
Data are reported as means ± SD and regression estimates (95% CI). Significance was declared at P < 0.05. All statistical analyses were conducted with STATA software version 8.0 (Stata Inc.).
Results
We obtained data for analysis from 966 individuals, representing 357 hospital births with famine exposure, 298 hospital births without famine exposure, and 311 siblings (2 of whom completed the study even though their hospital-born sibling did not). The siblings were somewhat younger than the hospital birth series, but for a range of demographic and phenotypic characteristics, the 3 groups did not differ (Table 1). The differences in birth weight and in BMI have been reported previously (3,15).
TABLE 1.
Selected characteristics in 2003–2005 of Dutch individuals born in 1 of 3 hospitals in 1943–1947 and their siblings1
| Hospital controls2 | Sibling controls | Famine exposed | P-value3 | |
|---|---|---|---|---|
| Sample size, n | 298 | 311 | 357 | |
| Male, % | 46.6 | 42.4 | 45.4 | 0.56 |
| Birth weight, g | 3457 ± 506 | N/A4 | 3295 ± 504 | <0.001 |
| Age at assessment, y | 58.6 ± 1.56 | 57.2 ± 6.26 | 58.7 ± 0.42 | <0.001 |
| Completed secondary school, % | 72.8 | 68.5 | 65.3 | 0.12 |
| Current smoker, % | 24.2 | 22.5 | 25.5 | 0.67 |
| Regular alcohol consumption, % | 81.5 | 79.7 | 84.0 | 0.35 |
| Height, cm | 171.5 ± 9.0 | 171.8 ± 8.9 | 170.8 ± 8.9 | 0.35 |
| BMI, kg/m2 | 27.3 ± 4.3 | 27.0 ± 4.2 | 28.5 ± 5.0 | <0.001 |
Values are mean ± SD or percent.
Individuals born in the same institutions as exposed but not exposed to famine during gestation.
By ANOVA or chi-square test, as appropriate.
Not available for siblings.
Reported energy intake was 9225 ± 2650 kJ/d, with fat providing 35.7 ± 5.9% of energy and carbohydrate providing 42.3 ± 7.0%. The physical activity score was 7380 ± 4381 MET·min/wk and pEE was 10,028 ± 1701 kJ/d. The hospital controls and the exposed did not differ in terms of energy or macronutrient intake (Table 2). The sibling controls had lower absolute protein intakes than did the hospital births (t test; P < 0.05) but did not differ in macronutrient densities. Individuals exposed in the first half of gestation (wk 1–10 and 11–20) had somewhat higher reported absolute intakes of energy, fat, and protein and lower reported absolute intakes of carbohydrate than did the controls. The difference between the pEE and the reported energy intake was consistent across time controls (811 ± 2525 kJ/d), sibling controls (895 ± 2658 kJ/d), and exposed (719 ± 2629 kJ/d) participants.
TABLE 2.
Dietary intake and energy expenditure among a sample of 966 Dutch adults assessed in 2003–2005 by exposure to the Dutch Famine during gestation1
| No famine exposure
|
Famine exposed
|
||||||
|---|---|---|---|---|---|---|---|
| Hospital controls | Sibling controls | Any exposure | Wk 1–10 | Wk 11–20 | Wk 21–30 | Wk 31 to delivery | |
| Sample size, n | 298 | 311 | 357 | 74 | 127 | 144 | 132 |
| Energy intake, kJ/d | 9246 ± 2709 | 9041 ± 2633 | 9367 ± 2608 | 9718 ± 2558 | 9601 ± 2993 | 9472 ± 2847 | 9246 ± 2361 |
| Fat, g/d | 89.4 ± 34.7 | 86.1 ± 32.3 | 90.5 ± 32.7 | 94.0 ± 33.3 | 94.9 ± 37.8* | 92.2 ± 35.6 | 87.5 ± 28.9 |
| Fat, % of energy | 35.8 ± 6.5 | 35.3 ± 5.6 | 35.9 ± 5.6 | 35.9 ± 5.7 | 36.6 ± 5.7 | 36.0 ± 5.9 | 35.3 ± 5.4 |
| Protein, g/d | 95.8 ± 26.8a | 90.9 ± 25.1ab | 96.3 ± 27.6b | 100.1 ± 28.8* | 100.4 ± 32.7* | 97.6 ± 28.4 | 93.9 ± 24.1 |
| Protein, % of energy | 17.6 ± 2.9 | 17.1 ± 2.9 | 17.4 ± 3.0 | 17.4 ± 3.3 | 17.7 ± 3.1 | 17.5 ± 2.9 | 17.2 ± 2.9 |
| Carbohydrate, g/d | 231.9 ± 76.1 | 232.2 ± 73.9 | 233.8 ± 73.5 | 242.7 ± 72.4 | 236.0 ± 80.4 | 235.6 ± 76.4 | 233.3 ± 70.2 |
| Carbohydrate, % of energy | 42.2 ± 7.6 | 43.0 ± 6.5a | 41.8 ± 6.8a | 41.8 ± 6.8 | 41.2 ± 7.3* | 41.8 ± 6.5 | 42.1 ± 6.6 |
| Alcohol, % of energy | 4.4 ± 5.0 | 4.6 ± 5.0 | 4.9 ± 5.8 | 4.8 ± 6.1 | 4.5 ± 5.5 | 4.6 ± 5.2 | 5.4 ± 6.0 |
| Physical activity score, MET·min/wk | 7863 ± 4922 | 7167 ± 3715 | 7164 ± 4281 | 6733 ± 3865 | 7362 ± 3911 | 7373 ± 4573 | 6993 ± 4745 |
| pEE,2kJ/d | 10,057 ± 1831 | 9936 ± 1672 | 10,086 ± 1626 | 10,040 ± 1622 | 10,216 ± 1668 | 10,283 ± 1647 | 9994 ± 1576 |
Data are mean ± SD. *Different from combined unexposed groups, P < 0.05 (t test). Within a row, means with superscripts with a common letter differ, P < 0.10 (Bonferroni-corrected ANOVA).
Energy expenditure as predicted from sex, age, height, weight, and physical activity using equation from (22).
Table 3 presents the results for the models that compared 357 famine exposed individuals to 298 unexposed time controls. Famine exposure (considered as any exposure or as a set of 4 10-wk periods) was not associated with energy intake or macronutrient density. Compared with time controls, gestational famine exposure was associated with 113 kJ/d (95% CI, −272, 502) higher energy intake and 0.01 percentage point (95% CI, −0.88, 0.89) higher fat density, 688 MET·min/wk (95% CI, −1398, 23) lower physical activity, and 63 kJ/d (95% CI, −130, 259) higher pEE. There was heterogeneity by sex (P < 0.05) for the estimates for protein but not for the other macronutrients. In sex-stratified analyses, protein intakes were 0.33 percentage points higher (95% CI, −0.28, 0.95) for exposed men and 0.69 percentage points lower (95% CI, −1.30, 0.08) for exposed women compared with unexposed men and women, respectively. The physical activity score was 688 MET·min/wk (95% CI, −1398, 22) lower among famine-exposed individuals than among time controls. For pEE, there was heterogeneity by gender; among males, pEE was 213 kJ/d (95% CI, −510, 80) lower among those with famine exposure and among women, pEE was 301 kJ/d (95% CI, 63, 556; P < 0.05) higher, with the estimate for exposure in wk 21–30 (330 kJ/d; 95% CI, 4, 652) reaching significance. The results were not substantively altered by adjustment for height, BMI, or physical activity score (data not shown).
TABLE 3.
Association of dietary intakes with exposure to the Dutch Famine during gestation among 655 Dutch men and women born in 3 hospitals in the Netherlands 1943–1947 and assessed in 2003–20051
| Period of famine exposure
|
||||||
|---|---|---|---|---|---|---|
| Any exposure | Wk 1–10 | Wk 11–20 | Wk 21–30 | Wk 31 to delivery | P-value2 | |
| n | 357 | 74 | 127 | 144 | 132 | |
| Energy, kJ/d | 113 (−272, 502) | 351 (−280, 982) | 259 (−263, 782) | 146 (−351, 640) | 50 (−460, 556) | 0.56 |
| Fat, % of energy | 0.01 (−0.88, 0.89) | −0.31 (−1.76, 1.14) | 0.60 (−0.61, 1.80) | 0.03 (−1.11, 1.17) | −0.71 (−1.88, 0.45) | 0.51 |
| Protein,#% of energy | −0.15 (−0.59, 0.29) | 0.01 (−0.70, 0.73) | 0.30 (−0.30, 0.90) | 0.09 (−0.48, 0.65) | −0.33 (−0.91, 0.25) | 0.50 |
| Males | 0.33 (−0.28, 0.95) | 0.25 (−0.78, 1.27) | 0.73 (−0.16, 1.63) | 0.40 (−0.43, 1.23) | −0.14 (−0.99, 0.71) | 0.19 |
| Females | −0.69 (−1.30, −0.08) | −0.16 (−1.17, 0.86) | −0.12 (−0.93, 0.69) | −0.26 (−1.03, 0.52) | −0.49 (−1.29, 0.31) | 0.72 |
| Carbohydrate, % of energy | −0.48 (−1.58, 0.63) | −0.22 (−2.03, 1.60) | −1.02 (−2.53, 0.48) | 0.15 (−1.27, 1.57) | 0.06 (−1.40, 1.52) | 0.70 |
| Physical activity score, MET·min/wk) | −688 (−1398, 23) | −1022 (−2187, 143) | −205 (−1171, 761) | −121 (−1034, 792) | −780 (−1717, 157) | 0.27 |
| pEE,#kJ/d | 63 (−130, 259) | −75 (−393, 238) | 100 (−163, 364) | 159 (−92, 405) | −96 (−351, 159) | 0.43 |
| Males | −213 (−510, 79) | −284 (−761, 196) | −79 (−497, 339) | 21 (−368, 410) | −389 (−786, 8) | 0.32 |
| Females | 301* (46, 556) | 130 (−293, 552) | 263 (−75, 606) | 330* (4, 652) | 130 (−79, 464) | 0.08 |
Estimates are linear regression coefficients for exposure in the specified period and associated 95% CI and are adjusted for familial clustering, city of birth of the hospital-born participant, age at interview, and sex. Models for fat, protein, and carbohydrate are also adjusted for total energy intake. Estimates for 10-wk periods are also adjusted for exposure in adjacent 10-wk periods. Comparison group is hospital controls (n = 298). *Different from controls, P < 0.05. #Test for heterogeneity: P < 0.05 for any exposure.
Wald test (4 degrees of freedom) for overall test of significance for set of 4 10-wk periods.
There were 309 same-gender sibling pairs (162 pairs with a famine-exposed sibling, 147 pairs in which neither sibling had famine exposure) available for analysis. Compared with sibling controls, gestational famine exposure was associated with 4 kJ/d (95% CI, −702, 711) higher energy intake, 2.01 percentage points (95% CI, 0.38, 3.63) higher fat density, 97 MET·min/wk (95% CI, −1243, 1050) lower physical activity score, and 188 kJ/d (95% CI, −163, 539) higher pEE (Table 4). Energy intake showed heterogeneity by gender (P < 0.05) and was 1053 kJ/d (95% CI, −2207, 96) lower in exposed men and 744 kJ/d (95% CI, −130, 1618) higher in exposed women compared with their unexposed siblings. Carbohydrate density was −1.92 percentage points lower (95% CI, −3.89, 0.06) in individuals exposed to famine at any point in gestation compared with their siblings and exposed and unexposed siblings did not differ in protein. There was no evidence for heterogeneity by sex for any macronutrients. The results were not substantively altered by adjustment for height, BMI, or physical activity score (data not shown).
TABLE 4.
Association of intakes of energy and macronutrients and of physical activity with exposure to the Dutch Famine during gestation among 309 same-sex sibling pairs of Dutch men and women assessed in 2003–20051
| Period of famine exposure of exposed sibling
|
||||||
|---|---|---|---|---|---|---|
| Any exposure | Wk 1–10 | Wk 11–20 | Wk 21–30 | Wk 31 to delivery | P-value2 | |
| Pairs, n | 162 | 28 | 67 | 72 | 51 | |
| Energy,#kJ/d | 4 (−698, 711) | −113 (−1367, 1145) | 117 (−798, 1028) | 92 (−794, 982) | −477 (−1471, 518) | 0.88 |
| Males | −1053 (−2207, 96) | −849 (−2947, 1254) | −313 (−1940, 1317) | −380 (−1977, 1212) | −2186 (−3862, −510) | 0.10 |
| Females | 744 (−130, 1618) | 535 (−1016, 2086) | 489 (−602, 1584) | 564 (−497, 1626) | 347 (−907, 1597) | 0.60 |
| Fat, % of energy | 2.01* (0.38, 3.63) | 1.88 (−1.03, 4.79) | 1.01 (−1.11, 3.13) | 1.12 (−0.94, 3.17) | 0.25 (−2.06, 2.55) | 0.39 |
| Protein, % of energy | −0.37 (−1.16, 0.42) | 0.26 (−1.15, 1.66) | −0.79 (−1.81, 0.23) | 0.41 (−0.59, 1.40) | −0.10 (−1.21, 1.02) | 0.63 |
| Carbohydrate, % of energy | −1.92 (−3.89, 0.06) | −2.77 (−6.29, 0.76) | 0.28 (−2.28, 2.85) | −1.81 (−4.31, 0.68) | 0.14 (−2.66, 2.93) | 0.43 |
| Physical activity score, MET·min/wk | −97 (−1243, 1050) | −282 (−2328, 1765) | 149 (−1339, 1638) | 127 (−1319, 1574) | 561 (−1059, 2182) | 0.95 |
| pEE, kJ/d | 188 (−163, 539) | 159 (−464, 786) | 230 (−230, 686) | 38 (−405, 481) | 222 (−280, 719) | 0.76 |
Estimates are within-pair mean differences and associated 95% CI. Estimates for individual 10-wk periods are adjusted for within-pair differences and any additional 10-wk periods of exposure of the affected sibling. Comparison group is pairs of unaffected siblings (n = 147 pairs). *Different from controls, P < 0.05. #Test for heterogeneity by sex: P < 0.05 for any exposure.
P-value for Wald test (4 df) for overall test of significance for set of 4 10-wk periods
Models that considered both sets of controls simultaneously were consistent with the results for the time controls, with slightly narrower CI reflecting the larger sample size for both exposed and unexposed individuals available for analysis. There was significant heterogeneity by gender for protein density (data not shown).
Discussion
We examined energy and macronutrient intakes and physical activity at age 58 y in relation to gestational exposure to famine. We observed an inconsistent pattern of associations that varied depending on the choice of controls. The time controls and sibling controls differed in absolute protein intake. Using time controls born in the same hospitals as the famine-exposed participants, we observed sex-specific associations with protein intake and with pEE, whereas using siblings as controls, we observed sex-specific associations with energy intake and an increase in fat density.
There is an increasing interest in the early life and prenatal determinants of adult health. Much of this work has focused on pathophysiologic changes that might result from gestational undernutrition, including variation in organ structure (24) and epigenetic changes in the offspring DNA (25,26). An additional research direction has considered how behaviors linked to the development of disease might be altered. Vickers et al. (5) have demonstrated that rats subjected to a low-protein maternal diet exhibit a set of behaviors characterized by both hyperphagia and hypoactivity. The Dutch Famine provides a quasi-experimental setting in which this phenomenon can be investigated in humans.
Recently, Lussana et al. (6) reported that gestational famine exposure is associated with a preference for a high fat-density diet compared with time controls. We were unable to replicate these observations in our data using time controls but did find a similar pattern using sibling controls. The 2 study populations share many features: participants were born in urban Netherlands during or immediately following the famine and experienced the same post-war environments; all are currently resident in The Netherlands; the populations were the same age at examination; and the study samples are approximately the same size. Both studies used a food frequency instrument developed and validated in The Netherlands (albeit different questionnaires). There are minor differences between the 2 studies in the classification of dates of birth for those exposed and in the recruitment strategy. The major difference between the 2 studies is that Lussana et al. (6) did not recruit any sibling controls. Only in the present study can we therefore compare findings using 2 strategies for control selection. We found that the associations differed. As sibling controls share many factors, including genetic and social characteristics that are likely to influence behaviors, these could a priori be preferred as the reference population to assess potential long-term effects of pregnancy-specific exposures. Given the limited size of each study alone and, hence, the limited precision of the estimates, we think that the discordant results between the 2 studies (and also the differences within our own study depending on the choice of controls) may reflect sampling variability and that the findings from both studies need to be interpreted cautiously.
Obesity is a consequence of positive energy imbalance sustained over time. The imbalance might occur because energy intakes are elevated, expenditure is reduced, or metabolic efficiency is altered (1). Our estimates for energy intake are consistent with the modest imbalance in energy intake that would be required to result in the difference of 4.3 kg observed in the women in this cohort at age 58 y (3) and were not materially altered with adjustment for height, BMI, or physical activity, all of which are independent determinants of metabolic need and hence energy intake. These observations increase our confidence in our methodology but highlight the limitations inherent in studying relatively small effects in cohorts of limited size.
pEE is derived from a model that includes age, height, weight, and physical activity level (22). The effect of gestational exposure to famine on pEE is therefore the net result of potentially counteracting effects. Indeed, if obesity is increased and physical activity decreases, then there may be no net change in pEE. The difference between the pEE and the reported energy intake was slightly larger for both time controls and sibling controls than for the famine exposed. This difference measure represents a composite result of systematic biases in self-reported measures of diet and physical activity, as well as potential true between-group differences. We do not expect the reporting bias to vary by exposure status or control group. Famine-exposed women are heavier than those without such exposure (3) and in our present data, famine-exposed individuals reported slightly lower physical activity scores. The pattern of differences across periods of exposure to famine differs for the physical activity score (which was lowest for those exposed in wk 1–10 and 31 to delivery) and pEE (which was highest for those exposed in wk 11–30). Because these estimates are derived from small numbers of individuals, they are imprecise and again need to be interpreted cautiously.
There is limited literature on animal models of prenatal calorie restriction and adult behavior. Vickers et al. (4) developed an animal model in which adult Wistar rats born to mothers fed a 30% calorie-restricted prenatal diet exhibited hyperphagia, hyperleptinemia, reduced voluntary locomotor activity, and larger retroperitoneal fat pads compared with adult rats born to mothers who consumed diets ad libitum during the prenatal period. In this model the effects of prenatal nutrition were amplified by postnatal hypercaloric nutrition (27). Because elevated plasma leptin levels would be expected to reduce appetite in rats, the authors suggested that prenatal calorie restriction created a state of leptin resistance (27). Later work showed that postnatal leptin treatment normalized adult appetite, voluntary locomotor activity, and fat mass among prenatally programmed rats (28). In another study, rats born to mothers fed a low-protein diet throughout gestation had a preference for a high-fat diet and an aversion to a high-carbohydrate diet, yielding higher energy intake as young adults; this effect was significantly more pronounced in female than in male rats (29). However, further work from the same group found that short-term exposure to low-protein diets at specific points in gestation was associated with lower preference for a high-fat diet among females (30). These animal data resemble prior epidemiologic data on adult body size (2–4) and the data presented here on adult diet. As in the animal model, prenatal famine exposure was associated with decreased physical activity score when the hospital controls were considered, but we could not replicate this observation in the paired-sibling analysis.
Several issues in the design and implementation of the study are worth considering. It is possible that differences in participation rates may have led to our findings. This possibility cannot be directly assessed, however. Participation rates did not differ by gender or by distance from the examination site. It is also possible that parental characteristics associated with offspring's dietary patterns differed by period of maternal exposure to famine. However, genetic differences were controlled for by including siblings born outside the famine period in the referent group and social class differences in the exposed and unexposed was minimized by using births from the same institutions. Both the food frequency and the physical activity questionnaires are imprecise instruments and the resulting measurement error is likely to have attenuated any true associations. Finally, gestational exposure to famine encompasses more than just restriction in food, with resulting reductions in intake of energy and nutrients. Maternal stress might also be a factor in the resulting offspring behavior and phenotype.
In conclusion, our study adds to the very limited data on human energy intake and expenditure after gestational famine exposure. Our findings appear to be sensitive to the choice of control population, although there is overlap of the effect estimates obtained by different control strategies due to the limited size of the study population. For the same reason, differences with the reported findings by Lussana et al. (6) need to be interpreted with caution, as these could well be due to sampling variability. It is clear, nevertheless, that even small differences in energy balance over time could have contributed to the excess of adiposity (1) in the populations observed and hence these merit detailed examination.
Supported by grants RO1 HL067914 (PI: L.H.L.) and R01 AG-028593 (PI: L.H.L.) from the NIH.
Author disclosures: A. D. Stein, A. Rundle, N. Wada, R. A. Goldbohm, and L. H. Lumey, no conflicts of interest.
Abbreviations used: LMP, last menstrual period; MET, metabolic equivalent; pEE, predicted energy expenditure.
References
- 1.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH, American Heart Association; Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. [DOI] [PubMed] [Google Scholar]
- 2.Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295:349–54. [DOI] [PubMed] [Google Scholar]
- 3.Stein AD, Kahn HS, Rundle A, Zybert PA, van der Pal-de Bruin K, Lumey LH. Anthropometric measures in middle age after exposure to famine during gestation: evidence from the Dutch famine. Am J Clin Nutr. 2007;85:869–76. [DOI] [PubMed] [Google Scholar]
- 4.Ravelli AC, van Der Meulen JH, Osmond C, Barker DJ, Bleker OP. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr. 1999;70:811–6. [DOI] [PubMed] [Google Scholar]
- 5.Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab. 2000;279:E83–7. [DOI] [PubMed] [Google Scholar]
- 6.Lussana F, Painter RC, Ocke MC, Buller HR, Bossuyt PM, Roseboom TJ. Prenatal exposure to the Dutch famine is associated with a preference for fatty foods and a more atherogenic lipid profile. Am J Clin Nutr. 2008;88:1648–52. [DOI] [PubMed] [Google Scholar]
- 7.Painter RC, Westendorp RG, de Rooij SR, Osmond C, Barker DJ, Roseboom TJ. Increased reproductive success of women after prenatal undernutrition. Hum Reprod. 2008;23:2591–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lumey LH, Stein AD, Kahn HS, van der Pal-de Bruin KM, Blauw GJ, Zybert PA, Susser ES. Cohort profile: The Dutch Hunger Winter Families Study. Int J Epidemiol. 2007;36:1196–204. [DOI] [PubMed] [Google Scholar]
- 9.Stein Z, Susser M, Saenger G, Marrolla F. Famine and human development: The Dutch Hunger Winter of 1944–1945. New York: Oxford University Press; 1975.
- 10.Lumey LH, Ravelli AC, Wiessing LG, Koppe JG, Treffers PE, Stein ZA. The Dutch famine birth cohort study: design, validation of exposure, and selected characteristics of subjects after 43 years follow-up. Paediatr Perinat Epidemiol. 1993;7:354–67. [DOI] [PubMed] [Google Scholar]
- 11.Lumey LH, Van Poppel FW. The Dutch famine of 1944–45: mortality and morbidity in past and present generations. Soc Hist Med. 1994;7:229–46. [DOI] [PubMed] [Google Scholar]
- 12.Stein Z, Susser M. Fertility, fecundity, famine: food rations in the dutch famine 1944/5 have a causal relation to fertility, and probably to fecundity. Hum Biol. 1975;47:131–54. [PubMed] [Google Scholar]
- 13.Stein AD, Ravelli AC, Lumey LH. Famine, third-trimester pregnancy weight gain, and intrauterine growth: The Dutch Famine Birth Cohort Study. Hum Biol. 1995;67:135–50. [PubMed] [Google Scholar]
- 14.Smith C. The effect of wartime starvation in Holland upon pregnancy and its products. Am J Obstet Gynecol. 1947;53:599–608. [DOI] [PubMed] [Google Scholar]
- 15.Stein AD, Zybert PA, van de Bor M, Lumey LH. Intrauterine famine exposure and body proportions at birth: the Dutch Hunger Winter. Int J Epidemiol. 2004;33:831–6. [DOI] [PubMed] [Google Scholar]
- 16.Stein AD, Zybert PA, van der Pal-de Bruin K, Lumey LH. Exposure to famine during gestation, size at birth, and blood pressure at age 59 y: evidence from the Dutch Famine. Eur J Epidemiol. 2006;21:759–65. [DOI] [PubMed] [Google Scholar]
- 17.Roseboom TJ, van der Meulen JH, Osmond C, Barker DJ, Ravelli AC, Bleker OP. Plasma lipid profiles in adults after prenatal exposure to the Dutch famine. Am J Clin Nutr. 2000;72:1101–6. [DOI] [PubMed] [Google Scholar]
- 18.Painter RC, de Rooij SR, Bossuyt PM, Simmers TA, Osmond C, Barker DJ, Bleker OP, Roseboom TJ. Early onset of coronary artery disease after prenatal exposure to the Dutch famine. Am J Clin Nutr. 2006;84:322–7. [DOI] [PubMed] [Google Scholar]
- 19.Grootenhuis PA, Westenbrink S, Sie CM, de Neeling JN, Kok FJ, Bouter LM. A semiquantitative food frequency questionnaire for use in epidemiologic research among the elderly: validation by comparison with dietary history. J Clin Epidemiol. 1995;48:859–68. [DOI] [PubMed] [Google Scholar]
- 20.NEVO-tabel. Nederlands Voedingsstoffenbestand 2001/ Stichting Nederlands Voedingsstoffenbestand. 2001 [cited 2009 Feb 1]. Den Haag (Netherlands): Voedingscentrum. Available from: http://www.rivm.nl/nevo_en/nevo/.
- 21.Wendel-Vos GC, Schuit AJ, Saris WH, Kromhout D. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J Clin Epidemiol. 2003;56:1163–9. [DOI] [PubMed] [Google Scholar]
- 22.Institute of Medicine. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. National Academy Press: Washington, 2002. [DOI] [PubMed]
- 23.Kloosterman GJ. On intrauterine growth. Int J Gynaecol Obstet. 1970;8:895–912. [Google Scholar]
- 24.Hoet JJ, Ozanne S, Reusens B. Influences of pre- and postnatal nutritional exposures on vascular/endocrine systems in animals. Environ Health Perspect. 2000;108 Suppl 3:563–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waterland RA, Michels KB. Epigenetic epidemiology of the developmental origins hypothesis. Annu Rev Nutr. 2007;27:363–88. [DOI] [PubMed] [Google Scholar]
- 26.Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw G-J, Susser ES, Slagboom PE, Lumey LH. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci USA. 2008;105:17046–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vickers MH, Breier BH, McCarthy D, Gluckman PD. Sedentary behavior during postnatal life is determined by the prenatal environment and exacerbated by postnatal hypercaloric nutrition. Am J Physiol Regul Integr Comp Physiol. 2003;285:R271–3. [DOI] [PubMed] [Google Scholar]
- 28.Vickers MH, Ikenasio BA, Breier BH. IGF-I treatment reduces hyperphagia, obesity, and hypertension in metabolic disorders induced by fetal programming. Endocrinology. 2001;142:3964–73. [DOI] [PubMed] [Google Scholar]
- 29.Bellinger L, Lilley C, Langley-Evans SC. Prenatal exposure to a maternal low-protein diet programmes a preference for high-fat foods in the young adult rat. Br J Nutr. 2004;92:513–20. [DOI] [PubMed] [Google Scholar]
- 30.Bellinger L, Langley-Evans SC. Fetal programming of appetite by exposure to a maternal low-protein diet in the rat. Clin Sci. 2005;109:413–20. [DOI] [PubMed] [Google Scholar]