Abstract
Dietary fish oils, rich in (n-3) PUFA, including eicosapentaenoic acid and docosahexaenoic acid, have been shown to have antiinflammatory properties. Although the antiinflammatory properties of fish oil may be beneficial during a chronic inflammatory illness, the same antiinflammatory properties can suppress the inflammatory responses necessary to combat acute viral infection. Given that (n-3) fatty acid-rich fish oil supplementation is on the rise and with the increasing threat of an influenza pandemic, we tested the effect of fish oil feeding for 2 wk on the immune response to influenza virus infection. Male C57BL/6 mice fed either a menhaden fish oil/corn oil diet (4 g fish oil:1 g corn oil, wt:wt at 5 g/100 g diet) or a control corn oil diet were infected with influenza A/PuertoRico/8/34 and analyzed for lung pathology and immune function. Although fish oil-fed mice had lower lung inflammation compared with controls, fish oil feeding also resulted in a 40% higher mortality rate, a 70% higher lung viral load at d 7 post infection, and a prolonged recovery period following infection. Although splenic natural killer (NK) cell activity was suppressed in fish oil-fed mice, lung NK activity was not affected. Additionally, lungs of infected fish oil-fed mice had significantly fewer CD8+ T cells and decreased mRNA expression of macrophage inflammatory protein-1-α, tumor necrosis factor-α, and interleukin-6. These results suggest that the antiinflammatory properties of fish oil feeding can alter the immune response to influenza infection, resulting in increased morbidity and mortality.
Introduction
Dietary long-chain PUFA derived from fish oil have been shown to have beneficial effects on chronic inflammatory and autoimmune disorders (1,2) and long-chain PUFA such as eicosapentaenoic acid [20:5(n-3)] and docosahexaenoic acid [22:6(n-3)] appear to be most beneficial (3,4). A number of studies report that the immunosuppressive effects of PUFA are a result of decreased cytokine production and from reductions in T cell proliferation, activation, and signaling (5–7). Studies of rodents fed fish oil-enriched diets have shown a reduction in natural killer (NK)4 cell activity (8), decreased lymphocyte proliferation (9,10), and decreased antigen presentation functions (9,11–13). In addition, decreases in ex vivo production of tumor necrosis factor-α (TNFα), interleukin (IL)-1, IL-2, IL-6, and interferon (IFN)-γ have also been reported (10,14–18).
Whereas the antiinflammatory properties of PUFA may be beneficial for some chronic inflammatory illnesses, these same antiinflammatory properties may be detrimental for response to an infection when an intact immune system is needed to eradicate an invading pathogen. For example, diets supplemented with fish oils have been shown to lower host resistance to Mycobacterium tuberculosis (19), to reduce the survival of mice against infection with Listeria monocytogenes (20,21), and to decrease resistance of mice infected with Salmonella typhimurium (22). Similarly, fish oil feeding can diminish host defense against influenza virus due to delays in viral clearance (23).
Despite the availability of vaccines and antiviral agents, influenza virus continues to be a major cause of morbidity and mortality worldwide (24,25). Influenza virus infects cells of the respiratory system, resulting in an acute and diffuse inflammation of the bronchoalveolar tract (26). Following infection, a coordinated immune response consisting of both innate and adaptive mechanisms results in an accumulation of immune cells and secretion of immunomodulatory proteins designed to limit viral spread. In the mouse model of influenza virus infection, NK cells, neutrophils, and T lymphocytes increase in the lung postinfection (p.i.) and contribute to host protection (27). The secretion of both inflammatory and antiviral cytokines helps to eliminate the virus and reduce further spread. Whereas this inflammatory response is necessary for viral clearance, it also contributes to lung pathology (27). Although there are many studies documenting the antiinflammatory properties of fish oil, there are few studies that have examined the effects of fish oil on viral infection (28).
Because of the known health benefits, fish oil supplementation is on the rise. A survey conducted among health care professionals in the US reported an increase in fish oil supplementation from 24 to 30% in 2006 to 2007 (29). Additionally, in 2007, 37.4% of the U.S. population reported using fish oil or fish oil supplements for health reasons (30). With the anticipated increase of fish consumption and the increase in the usage of fish oil supplements, there is a growing concern that the beneficial antiinflammatory properties of (n-3) fatty acids may have adverse effects when inflammation is necessary to combat infection (1,28,31). This study was undertaken to investigate effects of fish oil feeding on the immune response to influenza virus infection in mice.
Materials and Methods
Animals and diet.
Six-week-old male C57BL/6J mice were purchased from Jackson Laboratories. All mice were housed at the University of North Carolina Animal Facility, which is fully accredited by the American Association for Accreditation of Laboratory Animal Care. Animals were maintained under protocols approved by the Institutional Animal Care and Use Committee. All mice were housed under a 12-h-light/-dark schedule with free access to food and water. Mice consumed ad libitum a control semipurified diet containing 5% corn oil (D07092502, Research Diets) or a fish oil-corn oil diet at physiological levels (1% corn oil + 4% fish oil, D07092503, Research Diets) for 2 wk (Table 1).
TABLE 1.
Composition of experimental diets1
| Control1
|
Fish oil2
|
|||
|---|---|---|---|---|
| Ingredient | g/kg | kJ/kg | g/kg | kJ/kg |
| Casein | 200 | 3347 | 200 | 3347 |
| dl-Methionine | 3 | 50 | 3 | 50 |
| Corn starch | 219 | 3665 | 219 | 3665 |
| Sucrose | 420 | 7029 | 420 | 7029 |
| Cellulose | 60 | 0 | 60 | 0 |
| Corn oil | 50 | 2259 | 10 | 376 |
| Fish oil | 0 | 0 | 40 | 1506 |
| t-BHQ | 1.008 | 0 | 1 | 0 |
| Mineral mix S10001 | 35 | 0 | 35 | 0 |
| Vitamin mix V10001 | 10 | 167 | 10 | 167 |
| Choline bitartrate | 2 | 0 | 2 | 0 |
| d,l-α Tocopheryl acetate2 | 0 | 0 | 0.405 | 0 |
Formulated and supplied from Research Diets, Inc. AIN-78A rodent diet with modifications. Control corn oil diet no. D07092502, fish oil diet no. D07092503 containing menhaden oil with 2.1% archidonic acid, 14.2% eicosapentaenoic acid, and 12.2% docosahexaenoic acid.
Vitamin E additionally added to the fish oil diet.
Virus and infection.
The mouse-adapted strain of influenza A/Puerto Rico/8/34 (American Type Culture Collection) was propagated in the allantoic fluid of 10-d-old fertilized hen's eggs and the viral titer was determined by hemagglutination assay (32). Following 2 wk of dietary treatment, mice were anesthetized with an intraperitoneal injection of a ketamine (0.6 mg/kg)/xylazine (0.35 mg/kg) solution and infected intranasally with 0.05 mL of 2 hemagglutinating units of A/Puerto Rico/8/34 virus diluted in PBS. Previous studies from our laboratory determined that this dose of virus is sufficient to effectively elicit an immune response with normal mortality in mice (32). Mice were weighed daily following infection and percent weight loss compared with starting weight at time of infection was calculated.
Pathology.
The left lobe of the lung was removed at d 0 (uninfected mice) 3, 7, 10, 15, and 21 p.i. and perfused with 4% paraformaldehyde, paraffin embedded, cut in 6-μm sections, and stained with hematoxylin and eosin. Pathology grading was performed semiquantitatively according to the relative degree of inflammatory infiltration as previously described (33).
Quantification of lung virus titer.
As previously described, lung viral titers were determined by a modified tissue culture infectious dose 50 (TCID50) using hemagglutination as an endpoint (32). Briefly, supernates from lung homogenates were serially diluted and used to infect Madin-Darby canine kidney cells. Virus titers were determined based on the presence or absence of hemagglutination of human O RBC and TCID50 was determined by the method of Reed and Muench (34).
Enumeration of NK cell populations.
Lungs and spleens were removed at d 0 and 3 p.i. Lungs were incubated in a collagenase solution (1500 units/lung) for 1 h. Lung and spleen were processed into single-cell suspensions using a stomacher (Seward) and strained through a 40-μm nylon filter. Cell numbers between different samples were equalized to 5 × 105 cells/sample and stained with fluorescein anti-DX5 isothiocyanate (FITC) and anti-CD3 phycoerythrin (PE) (BD Pharmingen). Fluorescence was measured using a FACSCalibur flow cytometer (Becton Dickinson) equipped with a 488-nm argon laser and a 647-nm diode laser. The lymphocyte population was gated and NK cells were identified as CD3–DX5+ within the gate.
Determination of NK cell cytotoxicity.
Total lung and spleen cells were analyzed using a standard chromium-51 release assay in triplicate following a previously published method (32).
Percent specific lysis was calculated by the following equation:
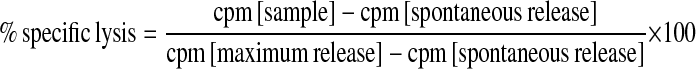 |
Quantification of immune cells.
To obtain bronchoalveolar lavage fluid whole lungs were lavaged 3 times with PBS at d 3, 7, 10, and 15 p.i. RBC were lysed using ACK lysis buffer (0.15 mol/L NH4Cl, 1 mmol/L KHCO3, 0.1 mmol/L Na2EDTA in double-distilled H2O, pH 7.4). Samples were washed twice in PBS/2% bovine serum and stained with fluorescent antibody for 30 min on ice, followed by 3 additional washes in PBS/2% bovine serum. At least 5 × 105 cells were stained with the following anti-mouse monoclonal antibodies: FITC anti-CD3, APC-anti-CD4 and peridinin-chlorophyll-protein anti-CD8, PE anti-CD11b and FITC anti-GR-1 (BD Biosciences). Cell populations were analyzed using a FACSCalibur flow cytometer (Becton Dickinson). T cells were identified using the follow antibodies: FITC-anti-CD3, APC-anti-CD4, and peridinin-chlorophyll-protein-anti-CD8 and neutrophils: PE anti-CD11b and FITC anti-GR-1(35).
Quantitation of lung mRNA cytokine levels.
The right lobe of the lung was removed at d 0 (uninfected), 3, 7, and 10 p.i. Total RNA was isolated using the TRIzol method and RT was conducted with a Superscript II First Strand Synthesis kit (Invitrogen). Following previously described methods (32), mRNA levels were measured for TNFα, IL-2, IL-6, IL-12, IFNα and IFNβ, regulated upon activation, normal T cell expressed and secreted, macrophage inflammatory protein-1-α (MIP-1α), and glyceraldehyde-3-phosphate dehydrogenase using quantitative real-time PCR. All data were expressed as fold-change from uninfected mice fed the same diet. Control and fish oil-fed mice did not differ in glyceraldehyde-3-phosphate dehydrogenase levels at any time point. All data were expressed as fold-change from uninfected mice fed the same diet.
Statistical analysis.
Statistical analyses were performed using JMP 7 Statistical software and SAS 9.1 software. Normally distributed data were analyzed by 2-way ANOVA with diet and day p.i. as main effects. Student's t test was used for post hoc comparison between the dietary groups and Tukey's honestly significant differences was used for post hoc comparisons among the days p.i. Nonparametric data were analyzed using the Kruskal Wallis test. Counted data were analyzed using logistic regression analysis (P < 0.05) when Wald CI for odds ratios did not include 1. Survival data were analyzed using the Kaplan-Meier survival estimates. Significance that compares the survival curve estimates of the 2 diet groups was analyzed by the log rank test. Differences were considered significant at P < 0.05.
Results
Fish oil-fed mice recover more slowly from influenza virus infection.
In mice, weight loss is a marker for illness severity following influenza infection and subsequent weight gain is an indicator for recovery. Both control and fish oil-fed mice lost considerable amounts of weight during the course of infection. Control mice began to regain weight after d 10 p.i.; however, fish oil-fed mice did not begin to regain weight until d 14 p.i. Between d 11 and 18 p.i., the percent body weight change remained significantly lower in the fish oil-fed mice compared with the control mice. By d 20 p.i., the body weights did not differ between the groups (Fig. 1).
FIGURE 1 .
Percent body weight change in mice fed control or fish oil diets following influenza infection. Values are means ± SEM, d 0 n = 61 (control) or d 0 n = 71 (fish oil). *Groups differ at each time, P < 0.05.
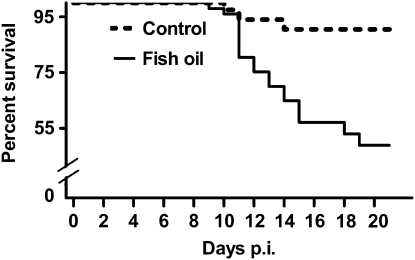
Fish oil feeding results in a higher mortality rate following influenza infection.
Increased severity of infection in the fish oil-fed mice was also reflected in the mortality rates. Beginning at d 11 p.i., fish oil-fed mice had a significantly higher mortality rate compared with control mice. At d 20 p.i., the fish oil-fed mice had a 51% mortality rate compared with a 10% mortality rate in control mice (Fig. 2).
FIGURE 2 .
Percent survival in mice fed control or fish oil diets following influenza infection. Control, n = 61 (d 0); fish oil, n = 71 (d 0). Log rank, P < 0.001.
Fish oil feeding improves lung pathology post-influenza virus infection.
Influenza virus infection results in an infiltration of inflammatory cells in the lung. Lung pathology was examined at various times p.i. Lung pathology was significantly reduced in influenza-infected fish oil-fed mice at d 7, 10, 15, and 21 p.i. compared with infected controls (Fig. 3).
FIGURE 3 .
Degree of lung pathology in mice fed control or fish oil diets following influenza infection. Values are means ± SEM, n = 3-6 per time point except d 10, n = 11 (control and fish oil). *Different from control, P < 0.05. Pathology score was as follows: 0, no inflammation; 1+, mild influx of inflammatory cells with cuffing around vessels; 2+, increased inflammation with ∼25–50% of the total lung involved; 3+, severe inflammation involving 50–75% of the lung; and 4+, almost all lung tissue contained inflammatory infiltrates.
Fish oil feeding increases virus load in the lung p.i.
Because the fish oil-fed mice had reduced inflammation in the lungs and the inflammatory response is necessary for viral control, lung viral titers were measured in the mice. On d 7 p.i., fish oil-fed mice had a viral load that was 7.1-fold higher that of controls (Fig. 4).
FIGURE 4 .
Lung influenza viral titers in mice fed control or fish oil diets during influenza infection. Lung virus titers were determined by TCID50. Values are means ± SEM, n = 4-6 per time point except d 7, n = 10 (control and fish oil). *Different from control, P < 0.05.
Fish oil feeding affects NK cell cellularity and cytotoxicity.
NK cells represent the first line of defense post-influenza infection (36). Previous studies have demonstrated reduced splenic NK activity with fish oil feeding (8). At d 3 p.i., the splenic NK population was significantly reduced in the fish oil-fed mice compared with controls (Fig. 5A) and NK (Fig. 5B). In the lungs at d 3 p.i., the fish oil-fed mice had less than one-half the total number of NK cells compared with control mice (Fig. 5C); however, lung NK activity did not differ between groups (Fig. 5D).
FIGURE 5 .
Effect of influenza infection and fish oil feeding on spleen NK cell number (A), spleen NK cell cytotoxicity (B), lung NK cell number (C), and lung NK cell cytotoxicity (D) in mice fed control or fish oil diets on d 3 after infection with influenza. Values are means ± SEM, n = 6. *Different from control, P < 0.05.
Fish oil feeding reduces neutrophils in lung of infected mice.
Recruitment of neutrophils to the site of infection is an essential early component of the immune response to influenza infection (37). In the lungs of fish oil-fed uninfected mice (d 0), the total number of neutrophils was 71% lower than in control mice (Fig. 6). Following infection, neutrophil numbers in the lung increased in both groups; however, the total neutrophil number remained significantly lower in the fish oil-fed mice.
FIGURE 6 .
Effect of fish oil feeding on neutrophil infiltration of the lung in mice fed control or fish oil diets during influenza infection. Values are means ± SEM, n = 5-6 per time point (control and fish oil). *Different from corresponding d 0, P < 0.05. #Different from corresponding control, P < 0.05.
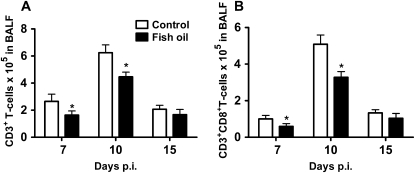
Fish oil feeding reduces CD8+ T cells in the infected lung.
To determine whether fish oil feeding affects T cell numbers in the lung, total numbers of T lymphocytes (CD3+) as well as CD4+ and CD8+ populations were identified in bronchoalveolar lavage fluid following influenza challenge. Although the CD3+ T cell number peaked at d 10 p.i. in both diet groups, fish oil-fed mice had significantly fewer CD3+ T cells at d 7 and 10 p.i. (Fig. 7A). This reduction in CD3+ T lymphocytes was due to fewer CD8+ T cells (Fig. 7B), as the number of CD4+ T cells did not differ between groups at any time point (data not shown). By d 15 p.i., CD3+ lymphocyte numbers did not differ between groups.
FIGURE 7 .
Total lung cell populations of CD3+ lymphocytes (A) and CD8+ cytotoxic T cells (B) in the bronchoaveolar lavage fluid in mice at d 7, 10, and 15 after infection with influenza. Values are means ± SEM, n = 5-6 per time point (control and fish oil). *Different from control, P < 0.05.
Fish oil feeding decreases cytokine and chemokine mRNA expression in lungs of influenza-infected mice.
A coordinated production of cytokines and chemokines occurs in the lungs during an influenza virus infection (27). To determine whether fish oil feeding could influence production of these inflammatory mediators, mRNA levels were measured for various cytokines and chemokines. In infected mice, fish oil feeding resulted in significantly lower mRNA expression of MIP-1α, TNFα, and IL-6 at d 7 p.i. compared with control mice (Fig. 8). Levels of mRNA for IL-2, IL-12, IFNα, IFNβ, and regulated upon activation, normal T-cell expressed and secreted did not differ between infected diet groups at any time p.i. (data not presented).
FIGURE 8 .
Lung mRNA levels of proinflammatory cytokines in mice on d 7 after infection with influenza. Lung mRNA levels of IL-6, MIP-1α, and TNFα at d 7 after infection with influenza. Values are means ± SEM, n = 4-6 per time point (control and fish oil). *Different from control, P < 0.05.
Discussion
The favorable effects of dietary fish oils stem from the potential of (n-3) fatty acids to reduce excessive inflammation (3,38). Studies have shown beneficial antiinflammatory properties of dietary fish oils on chronic diseases such as rheumatoid and osteoarthritis, inflammatory bowel disease, cardiovascular disease, type 2 diabetes, and Alzheimer's disease (39). However, suppression of the immune system may be deleterious when inflammation is required to eliminate the invading pathogen, such as during influenza virus infection (28). Following infection with influenza, a controlled and coordinated immune response is essential for resolving the infection. The availability of (n-3) fatty acid-rich fish oil supplements and the increasing inclusion of (n-3) fatty acids in prepared foods (40) add to the importance of understanding how the immunosuppressive properties of fish oils may impact the host's ability to respond to influenza virus infection.
Mice fed a fish oil-rich diet had decreased lung pathology following influenza infection compared with controls; however, the fish oil-fed mice also had a prolonged recovery time, significantly increased viral titer and importantly, a significantly higher mortality rate. Thus, in the case of influenza virus infection, reduced lung inflammation was associated with a poor outcome p.i. To understand the direct effect of fish oil feeding on the immune response to influenza virus infection, numbers and phenotypes of cells infiltrating the lung tissue were measured. At d 3 p.i., fish oil-fed mice had fewer numbers of NK cells infiltrating their lungs compared with control mice. NK cells provide an early defense against influenza virus infection and are important in reducing viral load prior to activation of the adaptive immune response (36). NK cell trafficking to the site of infection is dependent on a variety of inflammatory mediators, including the expression of a cytokine/chemokine gradient, upregulation of adhesion molecules, and activation of G-protein–coupled receptors (41–43). Fatty acids of the (n-3) group have been shown to decrease surface expression of vascular adhesion molecules and to alter G-protein–coupled membrane receptors (38,44,45), suggesting that fish oil feeding may have interfered with the signaling required for NK cell trafficking into the lungs. However, other possibilities for reduced NK in the lung include a reduction in general numbers, resulting in fewer NK cells trafficking to the lung.
Although the NK cell number was reduced in both the spleen and lungs of fish oil-fed mice, impairment in NK activity was found only in splenic NK cells. The differences between spleen and lung NK activity may be due to exposure to cytokines. There are a number of cytokines that enhance NK cytotoxicity, including IL-12, IL-18, and IFNα and β (36,42); therefore, NK activity in the lungs of fish oil-fed mice may have increased due to exposure to the cytokine milieu at the site of infection. During influenza virus infection, production of inflammatory cytokines occurs at the site of infection and therefore the spleen is not exposed to this localized inflammatory response. Indeed, Yaqoob et al. (8) demonstrated enhanced NK activity in PUFA-treated NK cells following exposure to IFNγ.
Fish oil feeding also affected numbers of neutrophils in the lung following infection. During early virus-induced inflammatory responses, neutrophils rapidly traffic into infected airways, where they play a critical role in limiting virus replication and activating innate immunity (37,46). Neutrophil migration is controlled in part by the release of chemokines, cytokines, and leukotrienes. MIP-1α has been shown to play a critical role in each aspect of neutrophil trafficking, including rolling, stationary adhesion, and tissue recruitment in vivo (47,48). In addition, studies by Ramos et al. (48) demonstrated that ovalbumin-induced neutrophil migration in immunized mice was mediated by MIP-1α via the release of TNFα and leukotriene B4. Moreover, influenza-infected MIP-1α knockout mice exhibited reduced lung inflammation and delayed viral clearance compared with infected wild type mice (49). In our study, we showed that fish oil-fed mice lacked MIP-1α and TNFα mRNA induction. Together, these data suggest that the failure to upregulate MIP-1α and TNFα mRNA in fish oil-fed mice may have resulted in reduced neutrophil trafficking to the lungs following influenza infection.
On d 7 and 10 p.i., CD8+ T cells were lower than in controls in the lung of influenza-infected fish oil-fed mice. Influenza-specific CD8+ T cells kill infected target cells by direct lysis and also play an essential role in influenza virus clearance and controlling morbidity (50–52). In mice that lack CD8+ T cells, infection with influenza A/PR/8/34 led to increased viral replication and mortality (53). CD4+ T cells, on the other hand, help to resolve inflammation during influenza infection; however, they are not essential for viral clearance (54,55). Our data suggest that reduction in CD8+ T cell numbers p.i. coupled with reduced numbers of neutrophils likely contributed to the increase in lung virus titer in the fish oil-fed mice.
The recruitment of T cells is dependent on cytokine-induced expression of adhesion molecules. For example, TNFα and IL-1β stimulate endothelial cells to increase expression of adhesion molecules, selectins, and intergrins (56,57). As expected with an influenza virus infection, cytokine mRNA expression of TNFα peaked at d 7 in control mice; however, this induction did not occur in fish oil-fed mice, suggesting that a lack TNFα may have contributed to the decrease in CD8+ T cell trafficking to the lung. Alternatives to trafficking include increased apoptosis and/or failure to proliferate in response to antigen stimuli (58,59). However, we examined lung tissue for increased apoptosis by TUNEL staining and found no differences in apoptosis between diet groups (data not shown).
The number of CD4+ T cells in the lung, however, was not affected in fish oil-fed influenza infected mice. Fish oil feeding may have altered pathways required for CD8+ T cell and not CD4+ T cell trafficking. For example, although both CD4+ and CD8+ T cells express CXCR3, administration of anti-CXCR3 antibody reduced CD4+ T cell infiltrate in the brain, whereas CD8+ trafficking was not affected (60). Similarly, CCR5 is also expressed on both CD4+ and CD8+ T cells, although only CD4+ T cell trafficking was affected by lack of CCR5 expression in a mouse model of hepatitis virus (61). Interestingly, mRNA for MIP-1α, the ligand for CCR5, was underexpressed in the fish oil-fed mice. Our results suggest that chemokines and perhaps their receptor expression may play a key role in the immunomodulatory effects of fish oil during influenza infection.
TNFα is produced by infected lung epithelial cells, activated macrophages, dendritic cells, neutrophils, T cells (CD8+ and CD4+), and NK cells (26,62). During viral infection, TNFα exerts antiviral activity (63), enhances the recruitment of leukocytes to the site of infection, and activates innate immune responses (64,65). IL-6 induction has pleiotropic effects, including the activation of NK cells and macrophages and stimulation of T cell differentiation during influenza infection (64,66). The potential for (n-3) fatty acids to reduce proinflammatory cytokines has been shown previously (67). For example, studies of fish oil-fed mice have demonstrated that injection with lipopolysaccharide decreased the ex vivo production of TNFα, IL-1β, and IL-6 by peritoneal macrophages and decreased TNFα, IL-1β, and IL-6 concentrations in circulation (14,17,68).
Together, these data suggest that the antiinflammatory properties of fish oil that resulted in reduced neutrophils, NK cells, and CD8+ T cells in the lung and decreased expression of mRNA for proinflammatory cytokines likely led to the increased viral titer and subsequent higher mortality rate in the fish oil-fed mice. However, the viral titers began to decrease in both fish oil-fed and control groups at a time when the former were beginning to die. This may be a case of survivor bias and those mice that ultimately died may have had a higher viral titer. We also investigated other possibilities for the high mortality rate of fish oil-fed mice, including impaired liver and/or kidney function, spread of virus to the brain, and increased lung cell apoptosis. All of these possibilities were negative (data not shown). In the future, other possibilities to explain the increased death rate of fish oil-fed mice will include an investigation to determine whether lung tissue repair mechanisms are impaired (69) or if fish oil-fed influenza-infected mice have increased and/or longer fevers potentially leading to brain damage and ultimately death (70,71).
Further mechanistic studies are needed to determine how PUFA can influence the immune response to influenza infection. For example, studies have shown that lipid raft disruptions by (n-3) fatty acids can affect signaling pathways in T lymphocytes and disrupt immunological synapse formation needed to activate T cells (6,72). However, potential adverse effects of these alterations in vivo during an influenza virus infection have not been investigated.
In this study, we utilized both a physiologically relevant concentration of dietary fish oil supplementation and a natural route of administration of a viral pathogen. Results from our study suggest that fish oil consumption has the potential to increase the severity of an influenza virus infection and perhaps other viral illnesses as well.
Acknowledgments
We thank Ray Tseng for his review of the manuscript.
Supported by an NIH-funded Clinical Nutrition Research Unit (DK056350).
Author disclosures: N. M. J. Schwerbrock, E. A. Karlsson, Q. Shi, P. A. Sheridan, and M. A. Beck, no conflicts of interest.
Abbreviations used: FITC, fluorescein isothiocyanate; IFN, interferon; IL, interleukin; MIP-1α, macrophage inflammatory protein-1-α; NK, natural killer; PE, phycoerythrin; p.i., postinfection; TCID50, tissue culture infectious dose 50; TNFα, tumor necrosis factor-α.
References
- 1.Calder PC. n-3 Polyunsaturated fatty acids and immune cell function. Adv Enzyme Regul. 1997;37:197–237. [DOI] [PubMed] [Google Scholar]
- 2.Benatti P, Peluso G, Nicolai R, Calvani M. Polyunsaturated fatty acids: biochemical, nutritional and epigenetic properties. J Am Coll Nutr. 2004;23:281–302. [DOI] [PubMed] [Google Scholar]
- 3.Calder PC. Immunoregulatory and anti-inflammatory effects of n-3 polyunsaturated fatty acids. Braz J Med Biol Res. 1998;31:467–90. [DOI] [PubMed] [Google Scholar]
- 4.Jump DB. The biochemistry of n-3 polyunsaturated fatty acids. J Biol Chem. 2002;277:8755–8. [DOI] [PubMed] [Google Scholar]
- 5.Meydani SN. Effect of (n-3) polyunsaturated fatty acids on cytokine production and their biologic function. Nutrition. 1996;12:S8–14. [PubMed] [Google Scholar]
- 6.Fan YY, McMurray DN, David N, Ly LH, Chapkin RS. Dietary (n-3) polyunsaturated fatty acids remodel mouse T-cell lipid rafts. J Nutr. 2003;133:1913–20. [DOI] [PubMed] [Google Scholar]
- 7.Stulnig TM. Immunomodulation by polyunsaturated fatty acids: mechanisms and effects. Int Arch Allergy Immunol. 2003;132:310–21. [DOI] [PubMed] [Google Scholar]
- 8.Yaqoob P, Newsholme AE, Calder P. Inhibition of natural killer cell activity by dietary lipids. Immunol Lett. 1994;41:241–7. [DOI] [PubMed] [Google Scholar]
- 9.Zhang P, Kim W, Zhou L, Wang N, Ly LN, McMurray DN, Chapkin RS. Dietary fish oil inhibits antigen-specific murine Th1 cell development by suppression of clonal expansion. J Nutr. 2006;136:2391–8. [DOI] [PubMed] [Google Scholar]
- 10.Jolly CA, Jiang YH, Chapkin RS, McMurray DN. Dietary (n-3) polyunsaturated fatty acids suppress murine lymphoproliferation, interleukin-2 secretion, and the formation of diacylglycerol and ceramide. J Nutr. 1997;127:37–43. [DOI] [PubMed] [Google Scholar]
- 11.Shaikh SR, Edidin M. Polyunsaturated fatty acids and membrane organization: elucidating mechanisms to balance immunotherapy and susceptibility to infection. Chem Phys Lipids. 2008;153:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujikawa M, Yamashita N, Yamazaki K, Sugiyama E, Suzuki H, Hamazaki T. Eicosapentaenoic acid inhibits antigen-presenting cell function of murine splenocytes. Immunology. 1992;75:330–5. [PMC free article] [PubMed] [Google Scholar]
- 13.Huang SC, Misfeldt ML, Fritsche KL. Dietary fat influences Ia antigen expression and immune cell populations in the murine peritoneum and spleen. J Nutr. 1992;122:1219–31. [DOI] [PubMed] [Google Scholar]
- 14.Yaqoob P, Calder PC. Effects of dietary lipid manipulation upon inflammatory mediator production by murine macrophages. Cell Immunol. 1995;163:120–8. [DOI] [PubMed] [Google Scholar]
- 15.Skuladottir IH, Petursdottir HD, Hardardottir I. The effects of omega-3 polyunsaturated fatty acids on TNF-α and IL-10 secretion by murine peritoneal cells in vitro. Lipids. 2007;42:699–706. [DOI] [PubMed] [Google Scholar]
- 16.Turek JJ, Li Y, Schoenlein IA, Allen KGD, Watkins BA. Modulation of macrophage cytokine production by conjugated linoleic acids is influenced by the dietary n-6:n-3 fatty acid ratio. J Nutr Biochem. 1998;9:258–66. [Google Scholar]
- 17.Renier G, Skamene E, DeSanctis J, Radzioch D. Dietary n-3 polyunsaturated fatty acids prevent the development of atherosclerotic lesions in mice. Modulation of macrophage secretory activities. Arterioscler Thromb. 1993;13:1515–24. [DOI] [PubMed] [Google Scholar]
- 18.Sanderson P, MacPherson GG, Jenkins Ch, Calder PC. Dietary fish oil diminishes the antigen presentation activity of rat dendritic cells. J Leukoc Biol. 1997;62:771–7. [DOI] [PubMed] [Google Scholar]
- 19.Paul KP, Leichsenring M, Pfisterer M, Mayatepek E, Wagner D, Domann M, Sonntag HG, Bremer HJ. Influence of n-6 and n-3 polyunsaturated fatty acids on the resistance to experimental tuberculosis. Metabolism. 1997;46:619–24. [DOI] [PubMed] [Google Scholar]
- 20.Fritsche KL, Shahbazian LM, Feng C, Berg JN. Dietary fish oil reduces survival and impairs bacterial clearance in C3H/Hen mice challenged with Listeria monocytogenes. Clin Sci (Lond). 1997;92:95–101. [DOI] [PubMed] [Google Scholar]
- 21.Puertollano MA, Puertollano E, Ruiz-Bravo A, Jimenez-Valera M, De Pablo MA, Alvarez De Cienfuegos G. Changes in the immune functions and susceptibility to Listeria monocytogenes infection in mice fed dietary lipids. Immunol Cell Biol. 2004;82:370–6. [DOI] [PubMed] [Google Scholar]
- 22.Chang HR, Dulloo AG, Vladoianu IR, Piguet PF, Arsenijevic D, Girardier L, Pechere JC. Fish oil decreases natural resistance of mice to infection with Salmonella typhimurium. Metabolism. 1992;41:1–2. [DOI] [PubMed] [Google Scholar]
- 23.Byleveld PM, Pang GT, Clancy R, Roberts DCK. Fish oil feeding delays influenza virus clearance and impairs production of interferon-γ and virus-specific immunoglobulin A in the lungs of mice. J Nutr. 1999;129:328–35. [DOI] [PubMed] [Google Scholar]
- 24.CDC. Influenza season summary, in pneumonia and influenza (P&I) mortality surveillance. 2005–06 U.S. Atlanta (GA): CDC; 2007.
- 25.Thompson WW, Comanor L, Shay DK. Epidemiology of seasonal influenza: use of surveillance data and statistical models to estimate the burden of disease. J Infect Dis. 2006;194:S82–91. [DOI] [PubMed] [Google Scholar]
- 26.Julkunen I, Melén K, Nyqvist M, Pirhonen J, Sareneva T, Matikainen S. Inflammatory responses in influenza A virus infection. Vaccine. 2000;19:S32–7. [DOI] [PubMed] [Google Scholar]
- 27.Bruder D, Srikiatkhachorn A, Enelow RI. Cellular immunity and lung injury in respiratory virus infection. Viral Immunol. 2006;19:147–55. [DOI] [PubMed] [Google Scholar]
- 28.Anderson M, Fritsche KL. (n-3) Fatty acids and infectious disease resistance. J Nutr. 2002;132:3566–76. [DOI] [PubMed] [Google Scholar]
- 29.Kemper KJ, Gardiner P, Woods C. Changes in use of herbs and dietary supplements (HDS) among clinicians enrolled in an online curriculum. BMC Complement Altern Med. 2007;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. National Health Statistics Reports; 2008;12:2–24. [PubMed]
- 31.Mori TA, Chem CP, Beilin LJ. Omega-3 fatty acids and inflammation. Curr Atheroscler Rep. 2004;6:461–7. [DOI] [PubMed] [Google Scholar]
- 32.Smith AG, Sheridan PA, Harp JB, Beck MA. Diet-induced obese mice have increased mortality and altered immune responses when infected with influenza virus. J Nutr. 2007;137:1236–43. [DOI] [PubMed] [Google Scholar]
- 33.Beck MA, Nelson HK, Shi Q, Van Dael P, Schiffrin EJ, Blum S, Barclay D, Levander OA. Selenium deficiency increases the pathology of an influenza virus infection. FASEB J. 2001;15:1481–3. [PubMed] [Google Scholar]
- 34.Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–7. [Google Scholar]
- 35.Pauksens K, Fjaertoft G, Douhan-Håkansson L, Venge P. Neutrophil and monocyte receptor expression in uncomplicated and complicated influenza A infection with pneumonia. Scand J Infect Dis. 2008;40:326–37. [DOI] [PubMed] [Google Scholar]
- 36.French AR, Yokoyama WM. Natural killer cells and viral infections. Curr Opin Immunol. 2003;15:45–51. [DOI] [PubMed] [Google Scholar]
- 37.Tate M, Brooks A, Reading P. The role of neutrophils in the upper and lower respiratory tract during influenza virus infection of mice. Respir Res. 2008;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calder PC. Immunomodulation by omega-3 fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2007;77:327–35. [DOI] [PubMed] [Google Scholar]
- 39.Sijben JWC, Calder CP. Differential immunomodulation with long-chain n-3 PUFA in health and chronic disease. Proc Nutr Soc. 2007;66:237–59. [DOI] [PubMed] [Google Scholar]
- 40.Howe PR, Downing JA, Grenyer BF, Grigonis-Deane EM, Bryden WL. Tuna fishmeal as a source of DHA for n-3 PUFA enrichment of pork, chicken, and eggs. Lipids. 2002;37:1067–76. [DOI] [PubMed] [Google Scholar]
- 41.Robertson MJ. Role of chemokines in the biology of natural killer cells. J Leukoc Biol. 2002;71:173–83. [PubMed] [Google Scholar]
- 42.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defence: Function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. [DOI] [PubMed] [Google Scholar]
- 43.Grégoire C, Carmelo LC, Tomasello LE, Geissmann F, Vivier E, Walzer T. The trafficking of natural killer cells. Immunol Rev. 2007;220:169–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weber C, Erl W, Pietsch A, Danesch U, Weber PC. Docosahexaenoic acid selectively attenuates induction of vascular cell adhesion molecule–1 and subsequent monocytic cell adhesion to human endothelial cells stimulated by tumor necrosis factor–{alpha}. Arterioscler Thromb Vasc Biol. 1995;15:622–8. [DOI] [PubMed] [Google Scholar]
- 45.De Caterina R, Cybulsky MA, Clinton SK, Gimbrone MA, Libby P. Omega-3 fatty acids and endothelial leukocyte adhesion molecules. Prostaglandins Leukot Essent Fatty Acids. 1995;52:191–5. [DOI] [PubMed] [Google Scholar]
- 46.Hartshorn KL, Karnad A, Tauber A. Influenza A virus and the neutrophil: a model of natural immunity. J Leukoc Biol. 1990;47:176–86. [DOI] [PubMed] [Google Scholar]
- 47.Schröder JM. Chemoattractants as mediators of neutrophilic tissue recruitment. Clin Dermatol. 2008;18:245–63. [DOI] [PubMed] [Google Scholar]
- 48.Ramos CD, Fernandes KS, Canetti C, Teixeira MM, Silva JS, Cunha FQ. Neutrophil recruitment in immunized mice depends on MIP-2 inducing the sequential release of MIP-1alpha, TNF-alpha and LTB (4). Eur J Immunol. 2006;8:2025–34. [DOI] [PubMed] [Google Scholar]
- 49.Cook DN, Beck MA, Coffman TM, Kirby SL, Sheridan JF, Pragnell IB, Smithies O. Requirement of MIP-1-alpha for an inflammatory response to viral infection. Science. 1995;269:1583–5. [DOI] [PubMed] [Google Scholar]
- 50.Nguyen HH, Boyaka PN, Moldoveanu Z, Novak MJ, Kiyono H, McGhee JR, Mestecky J. Influenza virus-infected epithelial cells present viral antigens to antigen-specific CD8+ cytotoxic T lymphocytes. J Virol. 1998;72:4534–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stambas J, Guillonneau C, Kedzierska K, Mintern JD, Doherty PC, La Gruta NL. Killer T cells in influenza. Pharmacol Ther. 2008;120:186–96. [DOI] [PubMed] [Google Scholar]
- 52.Topham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997;159:5197–200. [PubMed] [Google Scholar]
- 53.Bender BS, Croghan T, Zhang L, Small PA Jr. Transgenic mice lacking class I major histocompatibility complex-restricted T cells have delayed viral clearance and increased mortality after influenza virus challenge. J Exp Med. 1992;175:1143–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Allan W, Tabi Z, Cleary A, Doherty P. Cellular events in the lymph node and lung of mice with influenza. Consequences of depleting CD4+ T cells. J Immunol. 1990;144:3980–6. [PubMed] [Google Scholar]
- 55.Brown DM, Román E, Swain SL. CD4 T cell responses to influenza infection. Semin Immunol. 2004;16:171–7. [DOI] [PubMed] [Google Scholar]
- 56.Chin JE, Hatfield CA, Winterrowd GE, Brashler JR, Vonderfecht SL, Fidler SF, Griffin RL, Kolbasa KP, Krzesicki RF, et al. Airway recruitment of leukocytes in mice is dependent on alpha4-integrins and vascular cell adhesion molecule-1. Am J Physiol. 1997;272:L219–29. [DOI] [PubMed] [Google Scholar]
- 57.De Sanctis GT, Wolyniec WW, Green FHY, Qin S, Jiao A, Finn PW, Noonan T, Joetham AA, Gelfand E, et al. Reduction of allergic airway responses in P-selectin-deficient mice. J Appl Physiol. 1997;83:681–7. [DOI] [PubMed] [Google Scholar]
- 58.Sasaki T, Kanke Y, Kudoh K, Misawa Y, Shimizu J, Takita T. Effects of dietary docosahexaenoic acid on surface molecules involved in T cell proliferation. Biochim Biophys Acta. 1999;1436:519–30. [DOI] [PubMed] [Google Scholar]
- 59.Switzer KC, McMurray DN, Morris JS, Chapkin RS. (n-3) Polyunsaturated fatty acids promote activation-induced cell death in murine T lymphocytes. J Nutr. 2003;133:496–503. [DOI] [PubMed] [Google Scholar]
- 60.Stiles LN, Hosking MP, Edwards RA, Strieter RM, Lane TE. Differential roles for CXCR3 in CD4+ and CD8+ T cell trafficking following viral infection of the CNS. Eur J Immunol. 2006;36:613–22. [DOI] [PubMed] [Google Scholar]
- 61.Glass WG, Lane TE. Functional analysis of the CC chemokine receptor 5 (CCR5) on virus-specific CD8+ T cells following coronavirus infection of the central nervous system. Virology. 2003;312:407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.La Gruta NL, Kedzierska K, Stambas J, Doherty PC. A question of self-preservation: immunopathology in influenza virus infection. Immunol Cell Biol. 2007;85:85–92. [DOI] [PubMed] [Google Scholar]
- 63.Seo SH, Webster RG. Tumor necrosis factor alpha exerts powerful anti-influenza virus effects in lung epithelial cells. J Virol. 2002;76:1071–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Reeth K. Cytokines in the pathogenesis of influenza. Vet Microbiol. 2000;74:109–16. [DOI] [PubMed] [Google Scholar]
- 65.Buchweitz JP, Harkema JR, Kaminski NE. Time-dependent airway epithelial and inflammatory cell responses induced by influenza virus A/PR/8/34 in C57BL/6 Mice. Toxicol Pathol. 2007;35:424–35. [DOI] [PubMed] [Google Scholar]
- 66.Matsukura S, Kokubu F, Noda H, Tokunaga H, Adachi M. Expression of IL-6, IL-8, and RANTES on human bronchial epithelial cells, NCI-H292, induced by influenza virus A. J Allergy Clin Immunol. 1996;98:1080–7. [DOI] [PubMed] [Google Scholar]
- 67.Calder PC. Polyunsaturated fatty acids, inflammation, and immunity. Lipids. 2001;36:1007–24. [DOI] [PubMed] [Google Scholar]
- 68.Sadeghi S, Wallace FA, Calder PC. Dietary lipids modify the cytokine response to bacterial lipopolysaccharide in mice. Immunology. 1999;96:404–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cardoso CR, Souza MA, Ferro EA, Favoreto S Jr, Pena JD. Influence of topical administration of n-3 and n-6 essential and n-9 nonessential fatty acids on the healing of cutaneous wounds. Wound Repair Regen. 2004;12:235–43. [DOI] [PubMed] [Google Scholar]
- 70.Kozak W, Soszynski D, Rudolph K, Conn CA, Kluger MJ. Dietary n-3 fatty acids differentially affect sickness behavior in mice during local and systemic inflammation. Am J Physiol. 1997;272:R1298–307. [DOI] [PubMed] [Google Scholar]
- 71.Kurokawa M, Imakita M, Kumeda CA, Shiraki K. Cascade of fever production in mice infected with influenza virus. J Med Virol. 1996;50:152–8. [DOI] [PubMed] [Google Scholar]
- 72.Kim W, Fan Y-Y, Barhoumi R, Smith R, McMurray DN, Chapkin RS. n-3 polyunsaturated fatty acids suppress the localization and activation of signaling proteins at the immunological synapse in murine CD4+ T cells by affecting lipid raft formation. J Immunol. 2008;181:6236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]