Abstract
Background: Food reinforcement, ie, motivation to obtain food, is associated with energy intake and obesity. Finding ways to decrease the reinforcing value of unhealthy foods may help with adherence to diets and maintenance of weight loss. Our previous study in nonobese adults showed that daily consumption of the same snack food (food consumed apart from meals) for 14 d significantly decreased its reinforcing value.
Objectives: The aims of this study were to replicate and extend these findings to obese individuals and to examine the effects of different portion sizes of snack foods on food reinforcement.
Design: Food reinforcement and liking were tested in 31 obese and 27 nonobese women at baseline and after 2 wk of daily consumption of 0, 100, or 300 kcal/d of the same snack food.
Results: We found a significant interaction of phase, portion size, and body mass index on the pattern of operant responding for food. Obese women had a significant increase in food reinforcement after consuming the 300-kcal portion of food for 2 wk, whereas nonobese women had the opposite response. No significant differences were found on the reinforcing value with the 0- and 100-kcal portion-size conditions. Women in the 300-kcal group (obese and nonobese) reported a significant decrease in snack food liking from baseline to after daily intake.
Conclusions: These findings suggest that obese and nonobese women respond differently to the daily intake of a snack food and that this may not be a viable mechanism for reducing food reinforcement in obese women. This trial was registered at www.clinicaltrials.gov as NCT00837694.
See corresponding editorial on page 251.
INTRODUCTION
As obesity rates increase in the United States and other developed countries, it is imperative to develop novel and effective treatment strategies. The first-line treatment of overweight and obesity is energy restriction and/or increased physical activity (1). Research suggests that <10% of individuals who attempt to lose weight by making lifestyle changes will maintain weight loss after 3–5 y (2–4). One potential reason for this low success rate is that traditional diets combine energy restriction with restriction of highly liked, highly preferred snack foods. This may increase the motivation to eat restricted foods and, eventually, lead to disinhibited eating and poor weight-loss outcomes (5–7).
Food is a strong reinforcer that can be used to shape animal and human behavior (8). Studies show that food reinforcement is an empirical index of motivation to eat. The reinforcing value of food is related to energy intake, with higher food reinforcement associated with increased energy intake both in the laboratory (9, 10) and in free-living situations (10, 11). Food reinforcement also differs as a function of weight status, with obese individuals having higher levels of food reinforcement than their nonobese peers (9, 10). The reinforcing efficacy of a stimulus depends on several factors, including recent experience with the stimulus, deprivation from the stimulus, and availability of alternative reinforcers (12). These ideas are supported by studies on food reinforcement showing that food deprivation increases food reinforcement and recent consumption of a food decreases its reinforcing value (5, 7).
Some specific predictions follow from reinforcement theory about the effects of snack food variety and intake on food reinforcement. For example, restricting preferred snack foods in the diet may lead to increased reinforcing value and eventual relapse into poor eating habits. By contrast, eating a highly preferred snack food on a regular basis may lead to reinforcer satiation or monotony which, in turn, may decrease food reinforcement and help to maintain dietary adherence. In a previous study, we showed that daily intake of a 300 kcal portion of a snack food for 2 wk significantly reduced both food reinforcement and self-reported food liking in nonobese men and women (13). However, obese individuals, in general, consume a greater variety and larger portions of snack foods (14). Therefore, consuming a portion of the same snack food daily for 2 wk may not have an effect on food reinforcement if obese individuals are consuming a variety of other snack foods as well. In addition, because obese individuals find food more reinforcing than their lean peers (10, 15), it is possible that obese individuals are relatively more resistant to monotony and, therefore, food reinforcement will remain unchanged after daily snack food intake. Conversely, because studies with a variety of stimuli, including food, have shown increased responding after repeated exposure (a process known as sensitization), it is also possible that food reinforcement will increase in obese individuals after repeated consumption of the same snack food. The purpose of the current study was to compare the effects of daily snack food intake, in the absence of changes in total energy intake, in nonobese and obese women using several snack food portion sizes. We hypothesized that obese women would maintain higher levels of food reinforcement than nonobese women after daily snack food consumption and that the effects would depend on portion size.
SUBJECTS AND METHODS
Participants
Participants were obese [body mass index (BMI; in kg/m2) ≥30; n = 27] or nonobese (BMI < 30; n = 31) women between the ages of 18 and 50 y recruited from flyers posted on the University at Buffalo campus. Additional exclusionary criteria included the following: smoking, self-reported current dieting, indications of dietary restraint (see Screening procedures), a liking of <5 on a 7-point scale for all potential study foods, concurrent scores of >27 on the Binge Eating Scale (16) and a binge-eating disorder indication on the Questionnaire of Eating and Weight Patterns (17), any medications that might affect appetite (eg, methylphenidate), and any digestive, endocrine, or nervous system disorder that may limit eating.
Screening procedures
Potential participants were first screened by phone to collect demographic and basic medical information (eg, height, weight, and current medications used or illnesses). To reduce the variability that may be introduced by participants with extreme levels of dietary restraint, 14 questions modified from the restraint scale of the Three-Factor Eating Questionnaire (TFEQ; 18) were asked to get a general indication of dietary restraint. Participants were not eligible if they answered yes to >5 of the 14 questions. Once determined to be eligible, potential participants were given a list of 19 high-energy-density foods and rated each one on a 7-point scale for liking (1 = “do not like at all” and 7 = “like very much”), number of times consumed each week, perceived difficulty restricting for 2 wk and difficulty consuming daily for 2 wk (1 = “extremely easy” and 7 = “extremely difficult”). The list of foods included potato chips, Doritos (Frito Lay; Dallas, TX), tortilla chips, pretzels, popcorn, ice cream, M&Ms (Mars Inc, Chicago, IL), Twix (Mars Inc), Butterfinger (Nestle, Glendale, CA), other candy bars, Oreos (Nabisco, East Hanover, NJ), Chips Ahoy (Nabisco), other cookies, muffins, donuts, cupcakes, cake, brownies, and French fries. In addition, participants were asked if there were other snack foods that they enjoy eating that were not mentioned on this list; however, we were always able to find a food on our list that could be used for each participant. The target food to be used for testing was selected based on the liking score and the number of times consumed per week. All target foods were liked to at least a 5 on the 7-point scale and were consumed 1–4 times/wk. If several items met the criteria, one item was selected based on ease of portioning and packaging.
Laboratory environment
The laboratory used for these experiments was specifically constructed for eating experiments. It is equipped with an air-delivery system that circulates new air through each room ≈10 times/h. The laboratory rooms are also equipped with HEPA air purifiers containing a CPZ (carbon, permanganate, zeolite) filter to remove airborne odorants.
Procedures
All laboratory procedures were conducted in accordance with National Institutes of Health guidelines for the use of human subjects and with the approval of the University at Buffalo Social and Behavioral Sciences Institutional Review Board. Participants visited the laboratory on 3 separate occasions, each separated by 2 wk. Experimental sessions were run during a typical lunch period (1100–1400), and participants were ≥3 h postprandial. If a participant reported eating in the past 3 h, their appointment was rescheduled. Participants were told to consume their usual breakfast and were provided with a 150-kcal preload (Kellogg Smart Start Bar; 18% fat, 17% protein, and 65% carbohydrate; Kellogg Co, Battle Creek, MI) to minimize the effects of hunger on food reinforcement (19). During the first session, participants read and signed informed consent documents, completed a demographics questionnaire, and completed 3 dietary habits questionnaires (described below). The consent form stated that the purpose of the study was to “see if eating a single, highly liked snack food every day can alter its reinforcing value.” Participants were also interviewed about their food and beverage intakes the previous day by using a 5-step, multipass interview style (20). Participants were then trained to record all food and beverage intakes as well as physical activity in a habit book every day for the 4-wk duration of the experiment. In addition, participants completed three 24-h dietary recalls over the phone each week. These procedures are described in more detail below.
Food reinforcement task
The reinforcing value of food was determined by measuring the number of responses the participants made for food or food alternatives on progressive variable ratio (VR) schedules of reinforcement (±5%). The experimental environment included 2 computer stations, a table designated for reading, and a table designated for eating. At one station was a computer on which participants could earn points for food. The other station had a different computer on which subjects could work for time to spend reading Time and Newsweek magazines. Although our primary interest was in the participant's responses for food, we provided the opportunity to work for a nonfood-related activity to reduce the likelihood that subjects would engage in responding just for the sake of responding. The food used for this task was the target food described above. The portion of food used as the reinforcer was 80–100 kcal (Table 1).
TABLE 1.
Descriptive characteristics of the study participants as a function of weight status1
| Descriptive characteristics | Obese (n = 31) | Nonobese (n = 27) |
| Age (y) | 36.1 ± 1.823 | 27.6 ± 1.8 |
| BMI (kg/m2) | 37.4 ± 0.93 | 23.2 ± 0.6 |
| Three-Factor Eating Questionnaire, restraint | 9.3 ± 0.9 | 11.1 ± 1.0 |
| Three-Factor Eating Questionnaire, disinhibition | 9.9 ± 0.63 | 7.9 ± 0.7 |
| Three-Factor Eating Questionnaire, hunger | 7.1 ± 0.6 | 6.5 ± 0.7 |
| Binge Eating Scale | 17.3 ± 1.33 | 7.4 ± 1.4 |
| Race [n (%)] | ||
| White | 19 (61.3) | 24 (88.9) |
| African American | 9 (29.0)3 | 0 (0) |
| Asian | 1 (3.2) | 1 (3.7) |
| Other or mixed race | 1 (3.2) | 2 (7.4) |
| Income [n (%)] | ||
| ≤$9999 | 8 (25.8) | 16 (59.3) |
| $10,000–$29,999 | 13 (41.9) | 2 (7.4) |
| ≥$30,000 | 10 (32.3) | 9 (33.3) |
| Education [n (%)] | ||
| High school | 15 (48.4) | 14 (51.9) |
| Completed college | 16 (51.6) | 13 (48.1) |
| Current student | 11 (35.5) | 20 (74.1) |
Potential differences in baseline characteristics were compared by ANOVA with weight status (obese or nonobese) as the between-subjects factor. Categorical variables such as race, income, and education were compared by using chi-square tests.
Mean ± SD (all such values).
Significantly different from the nonobese participants, P < 0.05.
Participants were instructed on the use of the computer-generated task to earn points toward the food or time spent reading. The task was similar to a slot machine, with 3 boxes containing different shapes that were different colors and arranged in different orientations. When the left button on the mouse was pressed, the shapes rotated and changed color. When all of the shapes matched, the participant earned one point. After 5 points were earned, the subject received either a portion of their preferred snack food (brought into the room by the experimenter) or 2 min of reading time. The schedules of reinforcement were progressive VR schedules with response requirements of VR 4, 8, 16, 32, 64, 128, 256, 512, 1024, etc, for each point. In other words, participants were able to earn 1 point after approximately 4 responses on the first trial, and a portion of food after 20 responses (5 points), with the response requirements doubling after each portion of food was earned. The computers recorded the participants' points earned throughout the session as well as the rate of responding. Subjects were instructed to perform one activity at a time (ie, play the computer, eat, or read) and that the session would end when they no longer wished to earn points for access to food or time to spend reading. This design, based on previous studies using this methodology (9, 13), was meant to ensure that the participant felt free to work for as little or as much food as desired. The participant could communicate with the experimenter through an intercom system. A pitcher of filtered water was left on the table where the food was presented along with a cup. Participants were told they could drink water ad libitum. After providing the instructions, the experimenter left the room and the participant began the task.
Experimental groups and daily snack food intake procedures
The final 2 wk of the experiment was the daily intake phase. After completion of the food reinforcement task, participants were randomly assigned to 1 of 3 snack food conditions. Participants in the 100- and 300-kcal groups were provided with 14 portions of their target food and told to consume one each day until they returned to the laboratory for the final testing session. Participants were not given any additional instructions about how and when to consume the food, so some participants may have chosen to eat smaller portions throughout the day, whereas others consumed the food in a single sitting. In addition, participants were instructed to consume their normal diet and incorporate the foods provided as part of their daily snack food intake. Our goal was to alter snack food monotony without changing total energy intake. Participants in the 0-kcal group were not provided with any snack food nor were they given any instructions about snack food consumption, other than to try and keep food and beverage consumption the same as it had been for the baseline phase of the experiment. All participants were given a phone number to call every day stating that they recorded in their habit book for that day (all participants) and that they consumed their target food (100- and 300-kcal groups only). These daily telephone calls served as our primary measure of study compliance. As an incentive to comply with telephone call instructions, the participants were given $1.00 for each phone call and a $5.00 bonus if all phone calls were completed during the 2-wk period. They were also given $2.00 for each 24-h dietary recall completed and an $8.00 bonus if all 6 dietary recalls were completed within the 2-wk period. After the daily intake phase, the participant returned to the laboratory and completed the food reinforcement task for the target food.
Debriefing and subject payment
On completion of both phases, the participants had their height and weight measured, were told the purpose of the study, and were given a debriefing form highlighting the theoretical rationale behind these experiments as well as background literature. They were compensated in the form of a check based on adherence to the protocol. The maximum amount of money that could have been earned was $91.00.
Dietary recalls and habit books
To determine usual energy intake, 24-h dietary recalls were conducted at the beginning of each testing session as well as 3 times (2 weekdays and 1 weekend day) each week over the telephone (20, 21). The experimenter guided the subject through the recall process using a 5-step, multipass interview style (20). Briefly, the first pass included asking the participant to make a quick, uninterrupted list of all foods and beverages consumed. The second pass was a review of the quick list. For the third pass, the experimenter returned to the beginning of the list and asked for the times that foods and beverages were consumed and for portion sizes. The fourth pass was a review of the detailed information. During the final pass, the participant was asked to recall any other foods that they may have forgotten, such as foods that were eaten in small amounts. During laboratory visits, measuring cups and spoons, rulers, and pictures of portions of food were provided to help the participants estimate portion sizes. The total number of calories consumed was calculated for the recall based on manufacturers' labels and from the Food Works database (http://www.nutritionco.com/FoodWorks.htm; The Nutrition Company, 2008).
We also provided the participants with weekly habit books in which to record all food and beverage intake and physical activity for the 4 wk of the study to assess typical snack food consumption. There were 2 books for the baseline period and 2 books for the period after daily intake. Habit books were cross-checked with 24-h dietary recalls, and discrepancies were probed by the experimenter. In addition, habit books were used to calculate snack food variety. Briefly, unhealthy snack foods were divided into the following categories: French fries, chips, crackers, popcorn/pretzels, cake/pie/brownies, ice cream, cookies, candy, and other. We included an “other” category because there were some foods that did not fit into the above categories (eg, honey roasted nuts, pudding, and flavored gelatin). The number of snack food categories from which foods were consumed each day and the total number of snack food portions consumed each day were recorded by a trained staff member and cross-checked by another trained staff member to use as a measure of snack variety. These measures included both self-selected snack foods and the snack foods that we provided. Interrater reliability was 95.1% for snack food categories and 93.1% for snack food portions.
We chose to use both habit books and telephone dietary recalls for 2 reasons. First, per our past experience, self-recording of dietary intake data are often incomplete. The telephone recalls allowed us to collect some data on food intake in case the habit book recording was not reliable. Second, we have found in the past that regular telephone contact with participants increases compliance and reduces attrition.
Demographic and anthropometric characteristics
A general demographics questionnaire was used to assess education status, annual income, race, and ethnicity. Height (cm) and weight (lb) were measured by using a Digi-Kit digital stadiometer (North Bend, WA) and a Tanita digital weight scale (Arlington Heights, IL) and used to calculate BMI. Individuals were considered obese if their BMI was ≥30 and nonobese if their BMI was <30 (22).
Eating questionnaires
At the end of the first session, participants completed 3 eating questionnaires: the TFEQ (18), the Questionnaire of Eating and Weight Patterns (QEWP; 17), and the Binge Eating Scale (BES; 16). The TFEQ has 3 subscales that assess dietary restraint, hunger, and disinhibition. The QEWP and BES were used to rule out binge-eating disorder.
Food liking, hunger, and fullness
Before and after the food-reinforcement task, participants rated how hungry and how full they felt on 100-mm visual analog scales. The scales were anchored by “not hungry/full at all” and “extremely hungry/full.” They were also asked to rate how much they liked the target food on a 100-mm visual analog scale anchored by “not like at all” and “like very much.”
Analytic plan
Potential differences in baseline characteristics were compared by using a 2-factor analysis of variance (ANOVA) with weight status (obese or nonobese) and portion-size group (0, 100, or 300 kcal) as the between-subjects factors. Categorical variables, such as race, income, and education, were compared by using chi-square tests. Participant compliance, based on the total amount of money earned, was compared by using a 2-factor ANOVA with weight-status and experimental group as the between-subjects factors. The experiment was divided into 2 phases. The first 2 wk were considered the baseline phase, during which no food was provided and we collected food and beverage intake data to establish typical consumption patterns. The second 2 wk were referred to as the daily intake phase, during which we continued to collect food and beverage intake data, but snack food portions were provided for daily consumption to the 100-kcal and 300-kcal groups. Between-session changes (from baseline to the after daily intake phase) in body weight, energy presented during the experimental session, energy consumed (as assessed by 24-h dietary recalls), and snack food variety were compared by using a mixed analysis of covariance (ANCOVA) with weight status and portion-size group as the between-subjects factors, phase as the within-subjects factor, and age, BES score, minority status, and TFEQ-disinhibition score as covariates. Within- (before and after session) and between- (baseline and after daily intake) session changes in hunger and food liking were compared by using a 4-factor mixed ANCOVA with weight status and portion-size group as the between-subjects factors, before/after and phase (baseline to after daily intake) as the within-subjects factors, and age, BES score, minority status, and TFEQ-disinhibition score as covariates. Post hoc comparisons were made by using linear contrasts, and Bonferroni corrections were applied to control for multiple comparisons. To determine whether daily consumption of the snack food decreased liking, planned comparisons of before-session food liking with phase and portion-size group as between-subjects factors were also conducted.
Changes in the pattern of operant responding for food as a function of phase (baseline compared with after daily intake) were compared by using mixed-effects regression models (MRMs) (23). MRMs can be modeled based on the pattern of the data. Because responding on progressive ratio schedules of reinforcement to assess reinforcer efficacy usually shows an increase followed by a late-session decrease in responding (24), the MRMs included both linear and quadratic components. Improvement in fit of the quadratic plus linear trends was tested by using a 2-tailed log likelihood test with 2 df. The MRM compared the pattern of operant responses for food with BMI and portion-size group as time-invariant predictors, the schedule of reinforcement and experimental phase as the time-variant predictors, and age, minority status, and scores on the BES and the disinhibition subscale of the TFEQ as covariates. Each of the covariates was also tested as a moderator of responding by interacting it with phase and schedule of reinforcement (2). Because none of the participants in any condition responded on the final schedule of reinforcement (which required 1024 responses for each point), this schedule was excluded from the analysis. Interactions from the primary MRM analysis were probed by using separate MRM analyses on each phase, weight status group, and portion-size group using the linear and quadratic trends where appropriate. All analyses were conducted using SYSTAT 11.0 (Systat Software Inc, Chicago, IL).
RESULTS
Descriptive statistics
Descriptive characteristics of the participants are shown in Table 1. Compared with the nonobese women, the obese women had significantly higher BMI values (F1,56 = 175.91, P < 0.0001), were significantly older (F1,56 = 10.48, P = 0.002), scored significantly higher on the disinhibition subscale of the TFEQ (F1,56 = 5.30, P = 0.03), scored significantly higher on the BES (F1, 56 = 6.98, P < 0.01), and had a higher percentage of minorities (χ21 = 5.12, P = 0.02). There were no differences by weight status for income, education, compliance with experimental procedures, or TFEQ restraint or hunger subscales (all P > 0.05). There were no differences in any of the participant characteristics as a function of portion-size group nor were there any interactions between weight status and portion-size group (all P > 0.05; data not shown).
Food liking and hunger
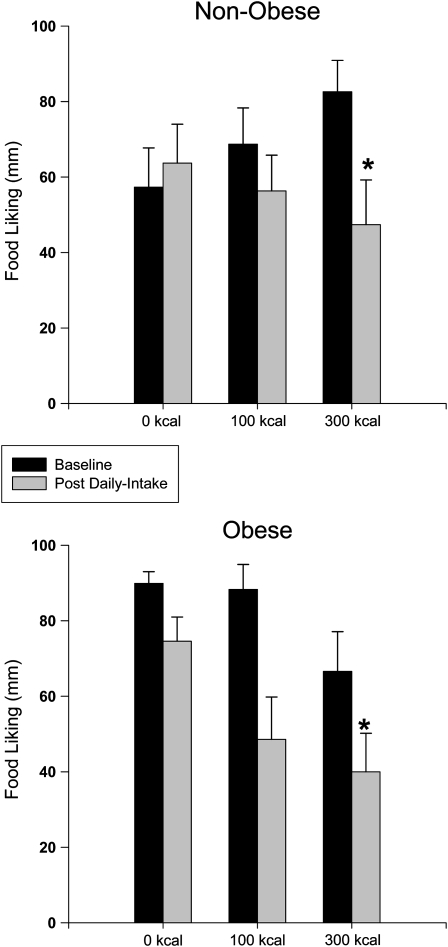
There was a main effect of before-session (39.0 ± 3.4) compared with after-session (22.4 ± 2.9) on hunger (F1, 208 = 25.08, P < 0.0001) but no effect of phase, weight status, or portion-size group and no interactions among these factors (all P > 0.05). There was a main effect of before-session compared with after-session (F1, 208 = 13.09, P < 0.0001) and phase (F1, 208 = 18.23, P < 0.0001) and an interaction of weight status and portion-size group (F2, 208 = 4.49, P = 0.012) on food liking. When before-session liking was compared across phase and portion-size and weight-status groups, there was a main effect of phase (F1, 56 = 25.58, P < 0.001) and an interaction between portion-size group and phase (F2, 52 = 4.31, P = 0.02; Figure 1). Linear contrasts showed that there was a significant decrease in preexperimental food liking from baseline to after the daily intake phase in the 300-kcal group.
FIGURE 1.
Mean (±SEM) values for self-reported food liking in nonobese (n = 31) and obese (n = 27) participants at baseline and after 2 wk of daily intake of different portions (0, 100, or 300 kcal) of the same snack food (post daily intake). Liking was reported on a 100-mm visual analog scale anchored by “do not like” at 0 mm and “like a lot” at 100 mm. Within- (before and after session) and between- (baseline and after daily intake) session changes in food liking were compared by using a 4-factor mixed ANOVA with weight-status and portion-size group as the between-subjects factors and before session/after session and phase (baseline to after daily intake) as the within-subjects factors. Post hoc comparisons were made by using linear contrasts, and Bonferroni corrections were applied to control for multiple comparisons. When before-session liking was compared across phase and portion-size and weight-status groups, there was a main effect of phase (F1, 56 = 25.58, P < 0.001) and an interaction between portion-size group and phase (F2, 52 = 4.31, P = 0.02). Significant reductions were observed in before-session snack food liking from baseline to the after daily intake phase only in the 300-kcal group. *Significantly different from baseline, P = 0.015.
Energy consumption and snack food variety from dietary recalls
There was a main effect of weight status on self-reported energy intake, assessed by 24-h dietary recalls, (F1, 52 = 7.2, P = 0.01; Table 2) with obese women reporting significantly more energy intake than nonobese women (2276 ± 114 compared with 1900 ± 93 kcal). However, when these results were corrected for body weight, the obese women reported significantly less energy intake per pound of body weight (22.95 ± 1.1 kcal/kg) than did the nonobese women (30.8 ± 1.4 kcal/kg; P < 0.0001; Table 2). There were no significant differences in self-reported energy intake across the different phases of the experiment (F2, 52 = 0.85, P = 0.36) and or in portion-size group (F2, 52 = 0.98, P = 0.38) and no significant interactions between weight status and portion-size group (F2, 52 = 1.80, P = 0.17; Table 2).
TABLE 2.
Energy intake (raw and adjusted for body weight) in the laboratory and outside of the laboratory during the baseline and daily intake phases by weight status and portion-size group1
| Baseline |
After daily intake |
|||||
| 0 kcal | 100 kcal | 300 kcal | 0 kcal | 100 kcal | 300 kcal | |
| Nonobese women | ||||||
| Laboratory, unadjusted | 227.8 ± 43.8 | 225.9 ± 47.8 | 227.8 ± 43.8 | 168.9 ± 47.4 | 125.6 ± 38.2 | 168.9 ± 47.4 |
| Laboratory, adjusted | 2.5 ± 1.5 | 3.8 ± 0.8 | 3.4 ± 0.7 | 2.3 ± 1.0 | 2.2 ± 0.7 | 2.7 ± 0.8 |
| Home, unadjusted | 1675 ± 118 | 1895 ± 117 | 2148 ± 151 | 1598 ± 188 | 1862 ± 148 | 2240 ± 182 |
| Home, adjusted | 26.8 ± 2.3 | 32.7 ± 2.5 | 32.2 ± 2.2 | 25.5 ± 3.2 | 32.8 ± 3.6 | 33.7 ± 2.7 |
| Obese women | ||||||
| Laboratory, unadjusted | 218.9 ± 37.4 | 242.3 ± 46.4 | 212.1 ± 74.9 | 221.5 ± 24.3 | 145.9 ± 50.7 | 229.7 ± 104.1 |
| Laboratory, adjusted | 2.1 ± 0.4 | 2.4 ± 0.4 | 2.2 ± 0.8 | 2.1 ± 0.3 | 1.5 ± 0.4 | 2.3 ± 1.0 |
| Home, unadjusted | 2442 ± 126 | 2255 ± 250 | 2276 ± 244 | 2279 ± 125 | 2148 ± 227 | 2271 ± 181 |
| Home, adjusted | 22.4 ± 1.1 | 23.1 ± 2.4 | 23.4 ± 1.9 | 20.9 ± 1.0 | 22.0 ± 2.2 | 23.5 ± 1.2 |
All values are means ± SEs. Between-session changes (from baseline to after daily intake phase) in energy consumed during the experimental session (kcal consumed in the laboratory) and energy consumed as assessed by 24-h dietary recalls (kcal consumed at home) were compared by using a mixed ANOVA with weight-status and portion-size group as the between-subjects factors and phase as the within-subjects factor. The data were compared by using the raw values as well as the values adjusted for body weight (kcal/kg). Post hoc comparisons were made by using linear contrasts. There was a main effect of weight status on energy intake from 24-h dietary recalls for the uncorrected data, with obese women consuming more energy than the nonobese women. After the data were adjusted for body weight, there was still a main effect of weight status (F1,52 = 21.98, P < 0.0001), but it was in the opposite direction, with nonobese participants consuming more kcal/kg than obese individuals. There was no effect of weight status or group on laboratory energy intake (adjusted or unadjusted), but there was a significant main effect of phase (F1,52 = 4.88, P = 0.03) with a decrease in laboratory energy intake from baseline to the daily intake phase.
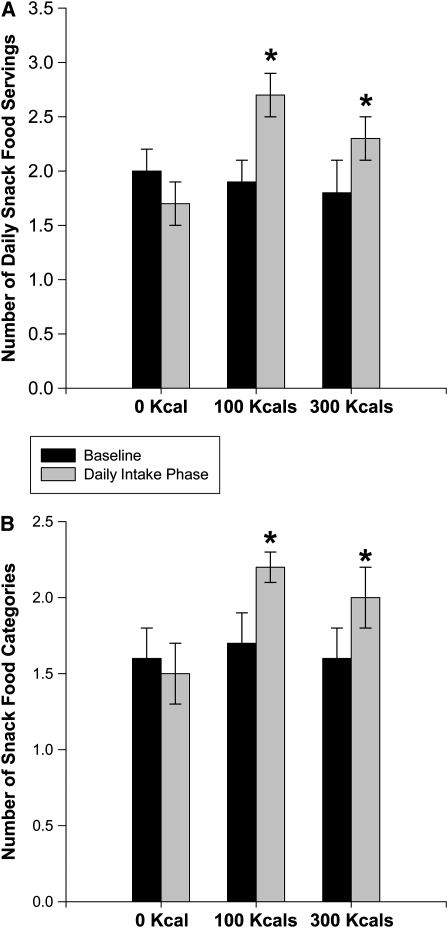
There was a significant interaction of portion-size condition and phase (baseline compared with daily intake) on both the number of categories from which snack foods were consumed (F2, 47 = 5.31, P = 0.008) and the number of snack food portions consumed (F2, 47 = 2.58, P = 0.001). Linear contrasts showed that both the number of snack food categories and number of snack food portions increased from baseline to the daily intake phase in the 100- and 300-kcal groups as compared with the 0-kcal group, with no difference as a function of weight status for either comparison (F1, 47 = 0.39, P = 0.51 and F1, 47 = 1.81, P = 0.18; Figure 2).
FIGURE 2.
Mean (±SEM) number of daily snack food servings consumed (A) and the number of different snack food categories from which daily snacks were selected (B) in women in each of the portion-size conditions (0, 100, and 300 kcal) during the baseline and daily intake phases. The data were compared by using a mixed ANOVA with experimental phase (baseline and after daily intake) as the within-subjects factor and weight-status and portion-size condition as the between-subjects factors. Post hoc comparisons were made by using linear contrasts. There was a significant interaction of portion-size condition and phase on both the number of categories from which snack foods were consumed (F2, 48 = 4.88, P = 0.01) and the number of snack food portions consumed (F2, 48 = 8.02, P = 0.001). Linear contrasts showed that both the number of snack food categories and the number of snack food portions increased from baseline to the daily intake phase in the 100- and 300-kcal groups as compared with the 0-kcal group, with no difference as a function of weight status for either comparison (F1, 48 = 0.39, P = 0.51, and F1, 48 = 1.81, P = 0.18).
Operant responding for food
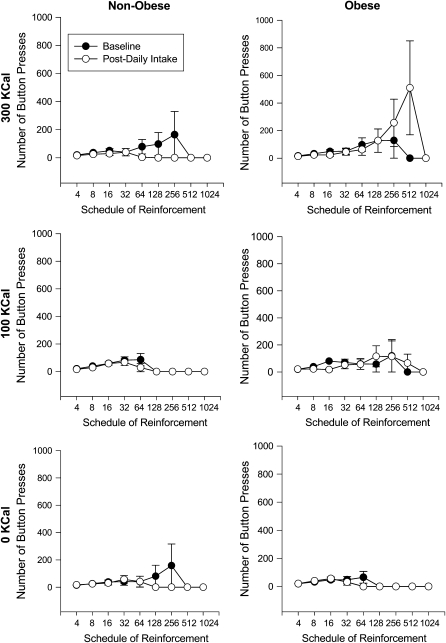
The MRM showed that there was a significant interaction of BMI, phase, and portion-size group on the pattern of responding across schedules of reinforcement for the linear (P < 0.001) and quadratic (P < 0.001) trends, with control for age, hunger, scores on the 3 subscales of the TFEQ (dietary restraint, disinhibition, and hunger), minority status, and BES score. The quadratic function improved the model fit over the linear trend (χ22 = 13.89, P < 0.001). This relation was further explored by conducting separate MRM analyses within each portion-size condition. The 300-kcal group, but not the 0-kcal or the 100-kcal group showed a significant interaction of BMI and time on operant responding for both the linear (P < 0.001) and the quadratic (P < 0.001) trends. The quadratic function improved the model fit over the linear (χ21 = 4.77, P < 0.05). None of the variables served as moderators of the changes from baseline to the daily intake phase for any of the analyses. The separate MRM analyses of the baseline and daily intake phases showed that there were no differences in the pattern of operant responding as a function of BMI (P = 0.80) or group (P = 0.66) at baseline, but after the daily intake phase there was a significant interaction of reinforcement schedule, experimental group, and BMI (P < 0.0001) and reinforcement schedule and group (P = 0.01). Analysis of each weight-status group separately showed that there was a significant interaction of experimental phase and portion-size group in the nonobese women for both the linear (P = 0.01) and the quadratic (P = 0.01) trends and for the linear trend in the obese women (P < 0.0001; Figure 3).
FIGURE 3.
Mean (±SEM) number of operant responses for food in nonobese (n = 31) and obese (n = 27) participants in the 300-, 100-, and 0-kcal groups at baseline and after daily intake across different schedules of reinforcement. Changes in the pattern of operant responding for food as a function of phase (baseline compared with after daily intake) were compared by using mixed-effects regression models (MRMs) (23). The MRMs compared the pattern of operant responses for food after the daily intake phase to responses for food at baseline, with BMI and portion-size group as time-invariant predictors, the schedule of reinforcement as the time-variant predictor, and age, hunger, and scores on the Binge Eating Scale and the 3 subscales of the Three-Factor Eating Questionnaire (dietary restraint, disinhibition, and hunger) as covariates. Each of the covariates was also tested as a moderator of responding by interacting it with phase and schedule of reinforcement. Because none of the participants in any condition responded on the 1024 schedule, this schedule was excluded from the analysis. Interactions from the primary MRM analysis were probed using separate MRM analyses for each phase, weight-status group, and portion-size group by using the linear and quadratic trends where appropriate. There was a significant group × weight status × reinforcement schedule effect (P < 0.0001). Further probing of this analysis showed that obese individuals in the 300-kcal group responded significantly more for food after the daily intake phase than at baseline (P < 0.0001), and nonobese individuals in the 300-kcal group responded less for food after the daily intake phase than at baseline (P = 0.01). There were no differences in the 0- or 100-kcal groups (all P > 0.05).
DISCUSSION
The results showed that daily consumption of a 300-kcal snack for 2 wk resulted in a bidirectional shift in food reinforcement, with nonobese women decreasing and obese women increasing food reinforcement. This occurred despite the fact that there was a significant reduction in self-reported food liking in both the nonobese and obese women in the 300-kcal portion-size group. There was no difference in food reinforcement as a function of weight status, experimental phase, or portion-size condition for either the 0-kcal or the 100-kcal group. Food liking did not change in the 0-kcal group, and only the obese women in the 100-kcal group showed a significant reduction in food liking. These results indicate that obese and nonobese women have differential changes in food reinforcement after repeated intake of the same snack food, whereas food liking decreases after repeated consumption regardless of weight status.
There are several potential explanations for the increase in food reinforcement in obese women. First, common neurobiological pathways underlie overeating and drug abuse (25, 26). Therefore, as with drug responses, there could be an inverted U-shaped curve for snack food reinforcement. It is possible that the 300-kcal portion of food was on the descending limb of the curve for nonobese participants (reinforcer satiation); thus, when they returned to the laboratory after 2 wk of daily consumption, this snack food was at a low level of reinforcement. In contrast, the 300-kcal portion could have been optimal, or at the peak of the curve, for obese women and when they returned to the laboratory they found this food very reinforcing. If this is the case, an even larger daily portion size would be required for reinforcer satiation and a decrease in food reinforcement in obese individuals in a similar manner as seen for the 300-kcal portion in the nonobese individuals (13). Similarly, the 100-kcal portion may have been too small to exert any effects on food reinforcement, which supports the hypothesis that there is an “optimal dose” or portion size. Another possibility is that daily intake of snack food led to incentive sensitization to the snack food in obese women. Incentive sensitization is an increase in the incentive motivational properties of a stimulus after repeated exposure (27–30). Research has shown increased motivated responding for food after initial exposure to food cues (31, 32). These studies were conducted within a single experimental session, and, to our knowledge, there has been no research on the incentive sensitization to food over days or weeks of exposure. In the present study, only the obese participants showed sensitization, which is consistent with incentive sensitization theory, which states that sensitization will occur after repeated stimulation in susceptible individuals under specific circumstances (28). Perhaps obesity is a risk factor for sensitization to food stimuli. If this is the case, the question becomes whether chronic overeating leads to susceptibility to sensitization to food or if susceptibility to sensitization is a risk factor for overeating and obesity.
In contrast with the effects of daily snack food on food reinforcement, obese and nonobese women reported a significant decrease in food liking after daily consumption of the 300-kcal food portion. This finding is similar to studies on monotony in which hedonic ratings of eaten and uneaten food are examined after days or weeks of consumption. Raynor and Wing (33) showed that 4 d of consumption of the same snack food under controlled laboratory conditions resulted in a significant decrease in hedonic ratings for that food, relative to individuals who consumed a different snack food on each of the 4 test days. These effects are not specific to snack foods, because meal foods consumed once a week for 10 wk (34) or every day for 5 d (35) showed a reduction in pleasantness compared with free-choice and variety control foods. Hetherington et al (36) examined monotony for chocolate and for bread and butter after daily consumption for 22 d. They found that liking of chocolate decreased, but liking of bread and butter remained stable. This suggests that some foods that are consumed frequently may be resistant to monotony. In addition, although liking of chocolate decreased significantly over time, the ad libitum consumption of chocolate increased, lending support to the hypothesis that “liking” and “wanting” are separable processes.
Studies that have concurrently examined motivation for a stimulus (wanting) and hedonic ratings of a stimulus (liking) have consistently shown that for a number of stimuli, including food (9, 37–40) and drugs (28–30), the effects of exposure on liking can be dissociated from the effects on wanting or motivation to obtain. For example, a recent study by Finlayson et al (40) showed that subjective liking and wanting for a savory food were similar when participants were hungry but diverged after consumption of a savory meal, with liking for savory foods increasing and wanting of savory foods decreasing. In the same study, when participants were hungry, liking of sweet foods was high, but wanting of sweet foods was low. However, after a savory meal was consumed, wanting and liking of sweet foods converged and were equal. This study suggests that wanting and liking are dissociable and are sensitive to different factors related to food intake. What is unique about our current findings is that all participants were exposed to the same experimental treatment and had a similar decrease in before-session food liking from baseline to after daily intake, but the decrease in food liking was associated with an increase in food reinforcement only in obese individuals in the 300-kcal portion-size group. This is consistent with other literature showing that there are not differences in baseline food liking or in changes in liking of eaten foods relative to uneaten foods (sensory specific satiation) as a function of weight status (41), but that food “wanting” or motivation to obtain food increases as a function of body weight (9, 10).
This study was not without limitations. First, we relied on self-report for daily snack food and energy intakes. It is possible that everyone did not consume all of the snacks, even though the participants in the 100-kcal and 300-kcal groups reported consuming the provided snack foods each day. This potential variability in snack food intake could have influenced our results. Second, only women were included in this study. In the past we have found that men and women respond differently to snack food administration (13); therefore, we chose to focus on women. This limits the generalization of our findings to men and eliminates the study of sex as a moderator or differential responding to daily snack food intake. Third, we did not measure ad libitum water consumption. It is possible that the volume of water consumed could have influenced motivation to work for food and/or energy intake. Finally, previous research has shown greater food reinforcement for obese than for nonobese peers (9–11). However, in the current study, no differences in food reinforcement as a function of weight status were observed at baseline; this difference only emerged after the daily intake phase. One possible reason for this is that, in the current study, we had extensive contact focused on food intake, in the form of participant daily food and beverage recording and telephone dietary recalls, before testing food reinforcement. This may have increased the participants' awareness of their food and energy intakes and influenced their responding for food. It is also possible that other methodologic or sampling differences (eg, we focused on women who were low in dietary restraint) may have accounted for the discrepancies in our results. Even if we had observed differences in food reinforcement between the obese and nonobese groups at baseline, we do not believe this would have changed our results. The primary finding was that obese women increased and nonobese women decreased their food reinforcement after the daily intake phase. If the obese women had higher food reinforcement at baseline, they may have had less room to increase responding after the daily intake phase; however, we believe that their pattern of responding would have remained the same.
In summary, we showed that obese and nonobese women shifted food reinforcement in response to 2 wk of daily intake of a 300-kcal portion of a snack food compared with daily intakes of 0 or 100 kcal, with obese women increasing food reinforcement and nonobese women decreasing food reinforcement. These effects were not attributable to differences in snack food liking or to differences in food reinforcement between the obese and nonobese women at baseline. Future studies will focus on determining whether these effects are specific to unhealthy snack foods or if we get the same response to healthy snacks as well. In addition, we will measure the stimulus specificity of these effects by determining whether the changes in food reinforcement after consuming one food will generalize to similar classes of foods. When taken together, our study suggests that daily intake of the same snack food is not a viable strategy to decrease food reinforcement in obese individuals without other manipulations of food or energy intake.
Acknowledgments
With her permission, we thank Sarah J Salvy for her comments on a previous version of this manuscript.
The authors' responsibilities were as follows—JLT: study design, supervision of data collection, data analysis, and manuscript preparation; AMB, RLB, NK, and SM: data collection, data entry, and assistance with manuscript preparation; and LHE: assistance with study design and manuscript preparation. LHE is a consultant for Kraft Foods and NuVal. None of the other authors had any potential conflicts of interest.
REFERENCES
- 1.Brownell KD. The central role of lifestyle change in long-term weight management. Clin Cornerstone 1999;2:43–51 [DOI] [PubMed] [Google Scholar]
- 2.Kraemer HC, Frank E, Kupfer DJ. Moderators of treatment outcomes: clinical, research, and policy importance. JAMA 2006;296:1286–9 [DOI] [PubMed] [Google Scholar]
- 3.Rosenbaum M, Leibel RL, Hirsch J. Obesity. N Engl J Med 1997;337:396–407 [DOI] [PubMed] [Google Scholar]
- 4.Tsai AG, Wadden TA. Systematic review: an evaluation of major commercial weight loss programs in the United States. Ann Intern Med 2005;142:56–66 [DOI] [PubMed] [Google Scholar]
- 5.Epstein LH, Truesdale R, Wojcik A, Paluch RA, Raynor HA. Effects of deprivation on hedonics and reinforcing value of food. Physiol Behav 2003;78:221–7 [DOI] [PubMed] [Google Scholar]
- 6.Polivy J, Coleman J, Herman CP. The effect of deprivation on food cravings and eating behavior in restrained and unrestrained eaters. Int J Eat Disord 2005;38:301–9 [DOI] [PubMed] [Google Scholar]
- 7.Raynor HA, Epstein LH. The relative-reinforcing value of food under differing levels of food deprivation and restriction. Appetite 2003;40:15–24 [DOI] [PubMed] [Google Scholar]
- 8.Thompson RH, Iwata BA. A review of reinforcement control procedures. J Appl Behav Anal 2005;38:257–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epstein LH, Temple JL, Neaderhiser BJ, Salis R, Leddy JJ. Food reinforcement, the dopamine D2 receptor genotype, and energy intake in obese and non-obese humans. Behav Neurosci 2007;121:877–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saelens BE, Epstein LH. Reinforcing value of food in obese and non-obese women. Appetite 1996;27:41–50 [DOI] [PubMed] [Google Scholar]
- 11.Epstein LH, Wright SM, Paluch RA, et al. Relation between food reinforcement and dopamine genotypes and its effect on food intake in smokers. Am J Clin Nutr 2004;80:82–8 [DOI] [PubMed] [Google Scholar]
- 12.Epstein LH, Leddy JJ, Temple JL, Faith MS. Food reinforcement and eating: a multi-level analysis. Psychol Bull 2007;133:884–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Temple JL, Chappel A, Shalik J, Volcy S, Epstein LH. Daily consumption of individual snack foods decreases their reinforcing value. Eat Behav 2008;9:267–76 [DOI] [PubMed] [Google Scholar]
- 14.Raynor HA, Jeffery RW, Phelan S, Hill JO, Wing RR. Amount of food group variety consumed in the diet and long-term weight loss maintenance. Obes Res 2005;13:883–90 [DOI] [PubMed] [Google Scholar]
- 15.Temple JL, Legierski CM, Giacomelli AM, Salvy SJ, Epstein LH. Overweight children find food more reinforcing and consume more energy than do nonoverweight children. Am J Clin Nutr 2008;87:1121–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spitzer RL, Yanovski S, Wadden T, et al. Binge eating disorder: its further validation in a multisite study. Int J Eat Disord 1993;13:137–53 [PubMed] [Google Scholar]
- 17.Gormally J, Black S, Daston S, Rardin D. The assessment of binge eating severity among obese persons. Addict Behav 1982;7:47–55 [DOI] [PubMed] [Google Scholar]
- 18.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res 1985;29:71–83 [DOI] [PubMed] [Google Scholar]
- 19.Reiss S, Havercamp S. The sensitivity theory of motivation: implications for psychopathology. Behav Res Ther 1996;34:621–32 [DOI] [PubMed] [Google Scholar]
- 20.Tran KM, Johnson RK, Soultanakis RP, Matthews DE. In-person vs telephone-administered multiple-pass 24-hour recalls in women: validation with doubly labeled water. J Am Diet Assoc 2000;100:777–83 [DOI] [PubMed] [Google Scholar]
- 21.Casey PH, Goolsby SL, Lensing SY, Perloff BP, Bogle ML. The use of telephone interview methodology to obtain 24-hour dietary recalls. J Am Diet Assoc 1999;99:1406–11 [DOI] [PubMed] [Google Scholar]
- 22.NHLBI Obesity Education Initiative Expert Panel Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. Obes Res 1998;6(suppl 2):51S–209S [PubMed] [Google Scholar]
- 23.Hedeker D, Gibbons RD. Longitudinal data analysis. Hoboken, NJ: John Wiley & Sons, 2006 [Google Scholar]
- 24.Bickel WK, Marsch LA, Carroll ME. Deconstructing relative reinforcing efficacy and situating the measures of pharmacological reinforcement with behavioral economics: a theoretical proposal. Psychopharmacology (Berl) 2000;153:44–56 [DOI] [PubMed] [Google Scholar]
- 25.Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci 2005;8:555–60 [DOI] [PubMed] [Google Scholar]
- 26.Wang GJ, Volkow ND, Logan J, et al. Brain dopamine and obesity. Lancet 2001;357:354–7 [DOI] [PubMed] [Google Scholar]
- 27.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 1993;18:247–91 [DOI] [PubMed] [Google Scholar]
- 28.Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci 2008;363:3137–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strakowski SM, Sax KW. Progressive behavioral response to repeated d-amphetamine challenge: further evidence for sensitization in humans. Biol Psychiatry 1998;44:1171–7 [DOI] [PubMed] [Google Scholar]
- 30.Strakowski SM, Sax KW, Rosenberg HL, DelBello MP, Adler CM. Human response to repeated low-dose d-amphetamine: evidence for behavioral enhancement and tolerance. Neuropsychopharmacology 2001;25:548–54 [DOI] [PubMed] [Google Scholar]
- 31.Epstein LH, Robinson JL, Temple JL, Roemmich JN, Marusewski A, Nadbrzuch R. Sensitization and habituation of motivated behavior in overweight and non-overweight children. Learn Motiv 2008;39:243–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Epstein LH, Robinson JL, Temple JL, Roemmich JN, Marusewski A. Variety influences habituation of motivated behavior for food and energy intake in children. Am J Clin Nutr 2009;89:746–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raynor HA, Wing RR. Effect of limiting snack food variety across days on hedonics and consumption. Appetite 2006;46:168–76 [DOI] [PubMed] [Google Scholar]
- 34.Zandstra EH, de Graaf C, van Trijp HC. Effects of variety and repeated in-home consumption on product acceptance. Appetite 2000;35:113–9 [DOI] [PubMed] [Google Scholar]
- 35.Meiselman HL, deGraaf C, Lesher LL. The effects of variety and monotony on food acceptance and intake at a midday meal. Physiol Behav 2000;70:119–25 [DOI] [PubMed] [Google Scholar]
- 36.Hetherington MM, Pirie LM, Nabb S. Stimulus satiation: effects of repeated exposure to foods on pleasantness and intake. Appetite 2002;38:19–28 [DOI] [PubMed] [Google Scholar]
- 37.Epstein LH, Wright SM, Paluch RA, et al. Food hedonics and reinforcement as determinants of laboratory food intake in smokers. Physiol Behav 2004;81:511–7 [DOI] [PubMed] [Google Scholar]
- 38.Finlayson G, King N, Blundell J. The role of implicit wanting in relation to explicit liking and wanting for food: implications for appetite control. Appetite 2008;50:120–7 [DOI] [PubMed] [Google Scholar]
- 39.Finlayson G, King N, Blundell JE. Liking vs. wanting food: importance for human appetite control and weight regulation. Neurosci Biobehav Rev 2007;31:987–1002 [DOI] [PubMed] [Google Scholar]
- 40.Finlayson G, King N, Blundell JE. Is it possible to dissociate ‘liking’ and ‘wanting’ for foods in humans? A novel experimental procedure. Physiol Behav 2007;90:36–42 [DOI] [PubMed] [Google Scholar]
- 41.Snoek HM, Huntjens L, Van Gemert LJ, De Graaf C, Weenen H. Sensory-specific satiety in obese and normal-weight women. Am J Clin Nutr 2004;80:823–31 [DOI] [PubMed] [Google Scholar]