Abstract
Background: Zinc plays an important role in antioxidant defense and the maintenance of cellular DNA integrity. However, no experimental human studies have been performed to examine the role of zinc status on DNA damage.
Objective: We evaluated the effects of dietary zinc depletion and repletion on DNA strand breaks, oxidative stress, and antioxidant defenses in healthy men.
Design: Nine healthy men with reported mean daily zinc intakes >11 mg/d were recruited. Subjects completed 3 consecutive dietary periods: baseline (days 1 to 13; 11 mg Zn/d), zinc depletion (days 14 to 55; 0.6 mg Zn/d for 1 wk and 4 mg Zn/d for 5 wk), and zinc repletion (days 56 to 83; 11 mg Zn/d for 4 wk with 20 mg supplemental Zn for first 7 d). Blood samples were collected on days 1, 13, 35, 55, and 83. DNA damage in peripheral blood cells, plasma oxidative stress, and antioxidant defense biomarkers were assessed.
Results: Dietary zinc depletion (6 wk) was associated with increased DNA strand breaks in peripheral blood cells (day 13 compared with day 55; P < 0.05), changes that were ameliorated by zinc repletion (day 55 compared with day 83; P < 0.05). Plasma zinc concentrations were negatively correlated with DNA strand breaks (r = −0.60, P = 0.006) during the zinc-depletion period. Plasma α- and γ-tocopherol concentrations, plasma total antioxidant capacity, and erythrocyte superoxide dismutase activity did not change significantly, and plasma F2-isoprostanes were unaffected by dietary period.
Conclusions: Changes in dietary zinc intake affected DNA single-strand breaks. Zinc appears to be a critical factor for maintaining DNA integrity in humans.
INTRODUCTION
Worldwide, zinc deficiency is an important public health problem that affects ≈2 billion people who do not ingest adequate amounts of zinc (1). Human zinc deficiency was first described in Iran in the early 1960s by Prasad et al (2, 3). The lack of validated biomarkers for assessing individual zinc status precludes our ability to clearly identify those with impaired zinc status. Indeed, the estimated prevalence of zinc deficiency in Americans, based on National Health and Nutrition Examination Survey (NHANES) data, suggests that ≥12% of Americans do not consume the Estimated Average Requirement (EAR) for zinc. Thus, a considerable proportion of the US population could be at risk of marginal zinc deficiency (4). The current indicators for zinc deficiency, such as plasma or hair zinc concentrations, have poor sensitivity and specificity and do not change with marginal zinc deficiency (5). Thus, the identification of sensitive biomarkers of zinc status is a critical issue in the field.
Zinc is the most abundant trace intracellular element and is required for many biological functions, including reproduction, immune function, and defense against free radicals (6, 7). Zinc is a component of >1000 transcription factors, including DNA-binding proteins with zinc fingers, and is required in >300 zinc-containing metalloenzymes. The consequences of zinc deficiency in adults have been understudied despite the recognition of symptoms of zinc deficiency for decades. Moreover, a considerable body of evidence suggests that zinc deficiency may increase the risk of some chronic diseases, including cancer (8, 9). This link may be attributed to the role of zinc in antioxidant defense and DNA damage repair. Zinc-depleted cells and experimental animals have greater susceptibility to oxidative stress and elevated concentrations of oxidatively modified proteins, lipids, and DNA damage (10–18). Because zinc also plays an essential role in DNA damage repair (19), increases in DNA damage with zinc deficiency may be due to perturbations in oxidative stress as well as impaired DNA repair functions. Overall, zinc deficiency may both increase oxidative stress and impair DNA integrity, thereby increasing the risk of cancer. In vitro and animal studies have clearly shown increased DNA damage and oxidative stress with zinc deficiency (14–17, 20–22). However, the extent to which zinc depletion regulates oxidative stress and DNA integrity in controlled human studies has yet to be comprehensively explored. Thus, the objectives of our study were to examine oxidative stress responses and DNA integrity by using a marginal zinc-depletion and -repletion protocol in healthy men. We hypothesized that marginal dietary zinc restriction would impair DNA integrity and increase oxidative stress in humans and that these deleterious effects would be reversed by zinc repletion. Collectively, the findings from this study may help identify new biomarkers for marginal zinc deficiency in humans and provide the justification for their use in future human trials.
SUBJECTS AND METHODS
Subjects
Healthy men aged 19–50 y were recruited (beginning on 28 January 2004) from the Davis and San Francisco areas, as reported previously (23). Men with a fasting plasma zinc concentration >74 μg/dL and a reported usual dietary zinc intake ≥11 mg/d—the Recommended Dietary Allowance for men—were considered to be zinc adequate and were invited to participate in the study. Average dietary zinc intakes on five 24-h dietary recalls were assessed before entry into the study. Exclusion criteria included the following: vegetarian diet, cigarette smoking, chronic use of alcohol or prescription drugs, use of illicit drugs, regular consumption of zinc supplements, or consumption of any zinc supplements during the 4 wk before entrance in the study.
All subjects gave written informed consent to participate in the study. The study protocol was reviewed and approved by the Institutional Review Boards at the University of California (Davis), the Children's Hospital and Research Center of Oakland, the Committee on Human Research at the University of California (San Francisco), and Oregon State University. The study was conducted at 2 study sites, the Ragle Human Nutrition Research Center at the University of California Davis and the General Clinical Research Center (GCRC) at the San Francisco General Hospital, University of California, San Francisco. Three subjects resided at the GCRC in San Francisco for the study duration. The other 6 subjects followed a free-living protocol, whereby they consumed either all weekday meals at the Ragle Human Nutrition Research Center or weekday lunch meals at the GCRC (breakfast and dinner were provided for home consumption). All weekend meals were provided for consumption at home.
Study design
To obtain altered zinc status, the subjects completed a period of marginal dietary zinc depletion followed by a period of dietary zinc repletion (Figure 1), per protocols previously used by Ruz et al (24) with slight modification. The study was divided into 3 diet periods. During diet period (DP) 1 (baseline period, days 1–13), an adequate-zinc diet (11 mg Zn/d) was given for 13 d to ensure adequate zinc status. During DP 2 (zinc-depletion period, days 14–55), the subjects were given a liquid diet containing 0.6 mg Zn/d for 7 d followed by a low-zinc diet (4 mg Zn/d) for another 35 d. Phytate (1.3 g/d; Sigma, St Louis, MO) was added to the diet for the first 21 d of DP 2 to inhibit zinc absorption. Finally, during DP 3 (zinc-repletion period, days 56–83), the subjects consumed a zinc-adequate (11 mg Zn/d) diet for 28 d, with supplemental zinc (20 mg/d Zn as zinc gluconate) administered for the first 7 d. The subjects consumed a daily multiple vitamin supplement (100% of the Recommended Dietary Allowance) without minerals (Long's Pharmacy, Oakland, CA) throughout the study.
FIGURE 1.
Design of the dietary intervention study. The study was divided into 3 dietary periods (DPs). DP1 was from day 1 to day 13 (baseline), during which the subjects consumed a zinc-adequate diet (11 mg Zn/d) to ensure zinc-adequate status. DP2 was from day 14 to day 55 (zinc-depletion diet), during which the subjects consumed a liquid diet (0.6 mg Zn/d) for 7 d followed by a low-zinc diet (4 mg Zn/d) for another 35 d; phytate (1.3 g/d) was added to the diet from day 14 to day 34. DP3 was from day 56 to day 83 (zinc repletion), during which the subjects consumed a zinc-adequate diet (11 mg Zn/d) and were provided supplemental zinc (20 mg Zn/d as zinc gluconate; ZnG) for the first 7 d. Adapted from reference 23.
Diets
The adequate-zinc (11 mg Zn/d) and low-zinc (4 mg Zn/d) diets were initially calculated to provide 2500 kcal (55% of energy from carbohydrate, 15% from protein, and 30% from fat). The actual amounts of energy that were offered were based on the Harris-Benedict equation for estimating basal energy expenditure (25), multiplied by an activity factor of 1.5. Energy intakes were adjusted during the course of the study to maintain a constant body weight by providing energy shakes devoid of zinc that were composed of nondairy creamer, egg albumin, and flavor drink mix (Kool-Aid; Kraft Foods, Northfield, IL) in 200-kcal increments. Average total energy intakes ranged from 2461 to 3100 kcal (mean ± SD: 2838 ± 221 kcal).
Adequate-zinc diets contained beef as their main source of zinc and protein. Low-zinc diets contained chicken as their main source of protein. Both diets were matched for the amount of protein from animal sources and had a phytate-to-zinc ratio of 10. The macronutrient contents of study diets were estimated by using Food Processor Pro software (Food Pro SQL, version 9.6; ESHA, Salem, OR). Meals were served 3 times daily at 0830, 1200, and 1730. Salt and pepper seasoning packets were provided for addition to the meals ad libitum, and tap water was consumed ad libitum by subjects. Methylcellulose (2–4 g; Sigma Chemical) was added to a daily beverage during DPs 2 and 3 to ensure regular fecal flow, except during absorption study days.
A 4-d cycle of the adequate-zinc diet and a 5-day cycle of the low-zinc diet were developed and reported previously (23). The diets were prepared by the dietetic staff of the metabolic kitchen at the University of California San Francisco GCRC of San Francisco General Hospital and the Ragle Human Nutrition Research Center at the University of California Davis.
Sample collection
Whole blood samples were collected into heparinized tubes by venipuncture of the fasting subjects on days 1, 13, 35, 55, and 83. An aliquot of whole blood was used for comet DNA damage analysis. Remaining whole blood was centrifuged at 1000 × g; plasma was removed by aspiration and frozen at −80° for further analysis. Erythrocytes were washed 3 times with cold saline solution and frozen at −80° for further analysis.
Zinc and phytate analysis
Dietary zinc concentrations were measured with the use of inductively coupled plasma optical emission spectrophotometry (Varian Vista Pro, Palo Alto, CA), and phytate concentrations in the study diets and test meals were measured by HPLC with the use of a Dionex Liquid Chromatograph System (Dionex Corp, Sunnyvale, CA) at the US Department of Agriculture, Agricultural Research Service at Cornell University, Ithaca, NY. Plasma and urine were divided into aliquots and stored at –20°C until analyzed for zinc with inductively coupled plasma optical emission spectrophotometry (Varian Vista Pro).
DNA damage analysis
DNA single-strand breaks in peripheral blood cells were determined by alkali single-cell gel electrophoresis (comet assay) as described by Singh et al (26). Briefly, 25 μL fresh whole blood was mixed with 1 mL blood storage buffer [20 mmol/L EDTA, 10% dimethyl sulfoxide (DMSO), Hanks Balanced Salt Solution (HBSS)], and centrifuged at 1000 rpm for 2 min to isolate blood cells. Blood cell pellets were mixed with 200 μL 0.5% low-melting-point agarose and applied onto microscope slides (Trevigen, Gaithersburg, MD). Slides were stored in lysis buffer (Trevigen, Gaithersburg, MD) and shipped to Oregon State University within 3 d. At Oregon State University, cells embedded in the slides were lysed in comet assay lysis buffer containing 10% DMSO for 1 h. After lysis, DNA was allowed to unwind in alkali buffer (0.3 mol NaOH/L and 1 mmol EDTA/L) for 20 min. Samples then underwent electrophoresis for 20 min at 300 mA and 25 V. After the electrophoresis, neutralization buffer (0.4 mol Tris/L, pH 7.5) was dropped onto slides to neutralize excess alkali buffer. The neutralized slides were immersed in cold 100% methanol for 5 min, immersed in 100% ethanol for 5 min, and the air-dried for storage. Immediately before being measured, nuclear material was stained by 20 μL circle ethidium bromide (20 μg/mL). Comet measurements were made by image analysis with a Nikon E400 fluorescent microscope (Nikon Instruments Inc, Melville, NY) and Comet Assay III software (Perceptive Instruments, Haverhill, United Kingdom). Images of 100 randomly selected nuclei (50 nuclei from each of 2 replicate slides) were blindly analyzed from each sample. The comet measurements of tail moment were recorded and used to indicate DNA damage in human peripheral blood cells. Interindividual variation was minimized by using each individual as his own control and by using a paired-analysis approach. We normalized tail moment from other time points to those from day 13 (baseline), and statistical analysis was performed by using fold-change data for each individual relative to their own baseline.
Oxidative stress and antioxidant status
Plasma F2-isoprostanes were measured as an index of lipid peroxidation and indicator of oxidative stress in vivo (27). The sum of various F2-isoprostanes with the appropriate mass-to-charge ratio and fragmentation characteristics and arachidonic acid (AA) were measured in plasma, as described previously (28) with minor modification. Briefly, 500 μL plasma was subjected to alkaline hydrolysis, acidified, extracted with ethyl acetate–hexane, and dried under nitrogen gas. The concentrated extract was separated by HPLC-tandem mass spectrometry (HPLC system consisting of 2 LC-10ADvp pumps, a DGU-14A degasser, a SIL-HTC autosampler/system controller, and a CTO-10Avp column oven; Shimadzu, Columbia, MD). The prostaglandin F (PGF) analytes and AA were detected by selected reaction monitoring on a triple-quadrupole mass spectrometer operated in negative mode (API 3000; Applied Biosystems/MDS Sciex, Foster City, CA). Monitored in the PGF experiment were 15-series PGFs (m/z 353.2–193.1), 5-series PGFs (m/z 353.2–115.0), and 8-iso-PGF2α-d4 internal standard (m/z 357.2–197.1). Standard curves were constructed by using 8 concentrations of the analytes 8-iso-PGF2α and PGF2α (100–4000 pg/mL). In the AA experiment, both AA and AA-d8 internal standard (m/z 311.2–239.1) were monitored. Standard curves were calculated from 8 concentrations of AA (100–4000 ng/mL). Quantitation was performed by using Analyst 1.4.1 software (Applied Biosystems/MDS Sciex). Interindividual variation for each subject was minimized by using each participant as his own control, and the change in the isoprostane values relative to day 13 (baseline) was calculated and analyzed with appropriate statistical analysis, as described below.
To assess plasma total antioxidant capacity, the ferritin reducing ability of plasma (FRAP) was measured as described previously (29). Briefly, diluted plasma samples (1:4) were mixed on a 96-well plate with 300 μL freshly prepared FRAP reagent [25 mL sodium acetate buffer (300 mmol/L), 2.5 mL 2,4,6-tripyridyl-1,3,5-triazine (TPTZ, 10 mmol/L), and 2.5 mL FeCl3 (20 mmol/L)]. Samples were incubated for 15 min at 37°C before being read at 593 nm on a microplate reader (Spectramax 190; Molecular Devices, Sunnyvale, CA). FRAP values were calculated by using trolox as standards.
Plasma α-tocopherol and γ-tocopherol were extracted and measured by HPLC-electrochemical detection as described previously (30, 31). Concentrations of α-tocopherol standards were determined spectrophotometrically by using ε292 nmEtOH = 3270 M−1 • cm−1 and of γ-tocopherol by using ε298 nmEtOH = 3810 M−1 • cm−1 (30).
Superoxide dismutase (SOD) activity was measured by using the xanthine oxidase-cytochrome c method according to McCord and Fridovich (32) and L'Abbé and Fischer (33). The hemoglobin concentration in cell lysate was measured by using Drabkin's method (34). Briefly, the reaction was initiated by adding xanthine oxidase (Calbiochem, CA) to the reaction mix containing 20 mmol sodium carbonate buffer/L (pH 10.0), 0.1 mmol EDTA/L, 50 μmol xanthine/L (Sigma), and 10 μmol cytochrome c/L (Sigma) in a final volume of 200 μL. The rate of increase in absorbance at 408 nm was monitored with a plate reader (Molecular Devices). One SOD activity unit was defined as the quantity of SOD required to produce 50% inhibition of the reduction rate of cytochrome c under the experimental conditions.
Sample size and statistical analyses
On the basis of the actual sample size of 9 men (2 subjects missing day 13 comet slides) and the observed variation in tail moment, we were able to detect a within-subject difference in tail moment of 0.51 by dietary zinc period, with 80% power and a 5% chance of type I error.
Statistical analysis was performed with the use of PRISM (version 4.0; GraphPad Software, San Diego, CA). Comparisons between time points were made by repeated-measures analysis of variance with Bonferroni's multiple comparison post hoc test when appropriate. Multiple regressions were conducted to assess the association of change between plasma zinc and DNA strand breaks with subjects as a factor. Data were considered statistically significant at P < 0.05. All data are reported as mean ± SEM unless otherwise indicated.
RESULTS
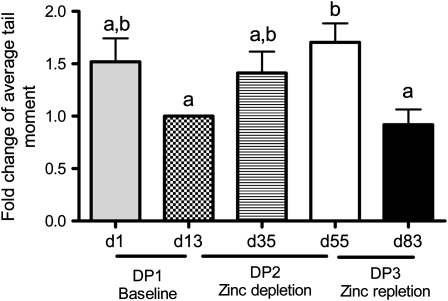
Subject characteristics and zinc status measurements throughout the study are published elsewhere (23). In summary, the mean (±SD) age of the subjects was 38 ± 8 y, and body mass index (in kg/m2) ranged from 20.8 to 25.9. None of the subjects were anemic. Mean (±SD) dietary zinc intake and plasma zinc concentrations were 12.9 ± 2.2 mg/d and 84.8 ± 8.5 μg/dL, respectively, at the start of the study. The comet assay has been used in human intervention trials to measure DNA single-strand breaks in vivo from peripheral blood cells (35). Thus, we used this technique to evaluate the effects of dietary zinc intake on DNA integrity. The number of DNA strand breaks increased during the zinc-depletion period (DP2), as indicated by increased average tail moment (Figure 2). In particular, average tail moments increased by 57% from baseline by the end of the depletion period (day 13 compared with day 55; P < 0.05), which suggests that 6 wk of low zinc intake significantly increased DNA damage in mixed peripheral blood cells. Importantly, the increases in DNA damage were reversible after zinc repletion, which suggests that the extent of DNA damage was dependent on dietary zinc status. At the end of the zinc-repletion period (DP3), average tail moments decreased by 39.9% compared with the end of the depletion period (day 83 compared with day 55; P < 0.01), and were ≈91.8% of the baseline level (day 83 compared with d13, P > 0.05), demonstrating that zinc repletion normalized DNA damage. Interestingly, we detected a 34% trend for a decrease in average tail moments during the baseline period (DP1: day 13 compared with day 1; P = 0.09), which suggests that the subjects may have had moderate DNA damage before enrollment in the study. Collectively, these data suggest that consuming a zinc-adequate balanced-nutritious diet (11 mg Zn/d) for 13 d may have helped to reduce DNA damage and thereby lowered baseline levels to below those observed initially, when the volunteers were still consuming their usual diets. Overall, these data suggest that dietary zinc status affects DNA damage in peripheral blood cells and that adequate zinc status may be essential to maintain DNA integrity in humans. Importantly, the alterations in DNA integrity occurred before significant changes in plasma zinc were detected. During the study, as reported previously, plasma and urinary zinc concentrations measured at the beginning (day 13) and at the end of the zinc-depletion period (day 55) did not differ significantly. Mean plasma zinc concentrations were 0.79 ± 0.9 and 0.79 ± 1.0 μg/mL on days 13 and 55, respectively. However, plasma zinc concentrations were 13% higher at the end of the zinc-repletion period than at the end of the zinc-depletion period (0.86 ± 1.0 μg/mL; day 83 compared with day 56; P < 0.05). Urinary zinc concentrations were not significantly different between metabolic periods (23). Although there was no significant change in plasma zinc concentrations during the zinc-depletion period, plasma zinc concentrations showed a negative correlation with measures of DNA damage (Figure 3A). Interestingly, the correlations were greater during the zinc-depletion and -repletion periods of the study, and the correlation during the baseline period was not statistically significant (Figure 3, B and C).
FIGURE 2.
Mean (±SEM) fold changes in average tail moments from baseline in dietary periods (DPs) 1, 2, and 3. Six weeks of dietary zinc depletion increased DNA single-strand breaks in human peripheral blood cells, measured by comet assay. Blood samples were collected on day 1 (beginning of the study), day 13 (baseline), day 35 (middle of zinc depletion), day 55 (end of zinc depletion), and day 83 (end of zinc repletion). Two comet slides were made for each human subject at each time point. Images of 100 randomly selected nuclei (50 nuclei from each of 2 replicate slides) were measured blindly for tail moment. n = 7. Significant differences between means were determined by repeated-measures ANOVA followed by Bonferroni's multiple comparisons test. Groups with different lowercase letters are significantly different. DNA damage gradually increased during zinc depletion; zinc repletion reversed the damage back to baseline levels.
FIGURE 3.
Mean fold changes in average tail moments from baseline in peripheral blood cells for each subject. Tail moments correlated significantly with plasma zinc concentrations during the entire study period [A; days (d) 1–83] and during the zinc-depletion and -repletion period (C; d13–d83) but not during the baseline period (B; d1–d13). Multiple regression analysis was conducted to assess the association of change between variables with subjects as a factor. DNA single-strand breaks were measured by comet assay, and plasma zinc concentrations were measured by inductively coupled plasma optical emission spectrophotometry. Blood samples were collected on day 1 (beginning of the study), day 13 (baseline), day 35 (middle of zinc depletion), day 55 (end of zinc depletion), and day 83 (end of zinc repletion). Two comet slides were made for each human subject at each time point. Images of 100 randomly selected nuclei (50 nuclei from each of 2 replicate slides) were measured blindly for tail moment. There was a significant correlation between fold changes in average tail moments in peripheral blood cells and plasma zinc concentrations for the whole study period (P = 0.014) and the zinc-depletion and -repletion period (P = 0.0083) but no significant correlation for the baseline period (P = 0.67).
We measured plasma total antioxidant capacity by FRAP assay, which was unaffected by zinc intake throughout the investigation (Table 1; P = 0.37). However, plasma concentrations of α-tocopherol and γ-tocopherol differed significantly by dietary period (α-tocopherol, P = 0.004; γ-tocopherol, P = 0.0013). Specifically, tocopherol concentrations tended to increase during the zinc-depletion period (day 55 compared with day 13: α-tocopherol increased by 14%, P = 0.30; γ-tocopherol increased by 26%, P = 0.07). During zinc repletion, γ-tocopherol concentrations decreased significantly, whereas α-tocopherol concentrations did not change (day 83 compared with day 55: α-tocopherol decreased by 8%, P > 0.05; γ-tocopherol decreased by 30%, P < 0.05).
TABLE 1.
Antioxidant status of human subjects during the zinc-depletion and -repletion periods1
| Day 1 | Day 13 | Day 35 | Day 55 | Day 83 | P2 | |
| FRAP (μmol/L) | 613.5 ± 31.3a | 606.5 ± 23.7a | 636.6 ± 30.2a | 634.8 ± 30.01a | 594.4 ± 36.8a | 0.37 |
| Plasma α-tocopherol (μmol/L) | 20.49 ± 1.61a | 23.67 ± 1.98a,b | 26.30 ± 1.73b | 27.13 ± 1.78b | 25.20 ± 1.75b | 0.004 |
| Plasma γ-tocopherol (μmol/L) | 1.55 ± 0.23a | 1.69 ± 0.18a,b | 2.10 ± 0.14b | 2.13 ± 0.22b | 1.48 ± 0.13a | 0.0013 |
| Erythrocyte SOD activity (U/mg Hb) | 99.5 ± 4.5a | 96.1 ± 2.0a,b | 89.2 ± 4.9b | 89.2 ± 3.7b | 91.3 ± 3.6b | 0.001 |
| Arachidonic acid (μg/mL) | 147.0 ± 13.8a | 141.4 ± 13.7a | 135.6 ± 12.4a | 138.0 ± 14.5a | 139.3 ± 11.7a | 0.26 |
All values are means ± SEMs; n = 9. FRAP, ferritin reducing ability of plasma; SOD, superoxide dismutase. Values within a row with different superscript letters are significantly different, P < 0.05 (Bonferroni's multiple comparisons test).
Represents the main effects of the analysis by repeated-measures ANOVA.
We also measured erythrocyte SOD activity as an additional component of the antioxidant defense system (36). CuZnSOD is the only SOD in erythrocytes, and it is an important zinc-containing antioxidant defense enzyme found in the circulation. Erythrocyte SOD activity tended to decrease after marginal zinc depletion and increase after zinc repletion, but the changes were not statistically significant (Table 1).
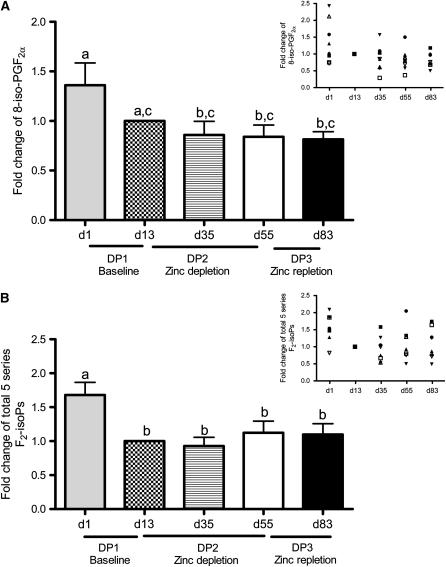
To determine whether zinc depletion affected lipid peroxidation and oxidative stress, we measured plasma F2-isoprostane concentrations. F2-isoprostanes are generated from nonenzymatic peroxidation of AA and are considered the reference standard for the measurement of lipid peroxidation and oxidative stress (27). Concentrations of F2-isoprostanes in biological fluids are highly related to the incidence of many oxidative stress–associated diseases, such as diabetes, obesity, and cardiovascular diseases (37). AA is the precursor fatty acid of F2-isoprostanes, and we hypothesized that plasma AA could be affected by changes in altered zinc status (15). Thus, we measured plasma AA concentrations simultaneously with F2-isoprostanes, but found that plasma AA concentrations did not change significantly during the study (Table 1; P = 0.26). Likewise, dietary zinc depletion did not affect plasma 8-iso-PGF2α and 5-series F2-isoprostane concentrations (Figure 4). Similar to the observations concerning DNA single-strand breaks, however, F2-isoprostanes tended to decline during the baseline period (day 1 compared with day 13). The mean (± SE) F2-isoprostane concentrations on day 13 after the baseline period were 2131 ± 835.7 pg/mL for 8-iso-PGF2α and 4323 ± 1187 pg/mL for 5 series F2-isoprostanes.
FIGURE 4.
Mean (±SEM) fold changes in plasma 8-iso-15(R)-prostaglandin2α [8-iso-15(R)-PGF2α; A] and in total 5-series F2-isoprostanes (F2-isoPs; B) during dietary periods (DPs) 1, 2, and 3 (insets: individual subject data). The values were calculated based on the sum of 8-iso-15(R)-PGF2α and 8-iso-PGF2α (pg/mL) and the percentage change relative to baseline [day (d) 13]. n = 8. Significant differences between means were determined by repeated-measures ANOVA followed by Bonferroni's multiple comparisons test. Groups with different lowercase letters are significantly different (P < 0.05).
DISCUSSION
The present intervention study showed that low dietary zinc intake resulted in an increase in DNA single-strand breaks in healthy human volunteers. Consumption of a marginally zinc-deficient diet by these same subjects had no significant effect on their plasma and urinary zinc concentrations (23), which are commonly used biomarkers for assessing zinc status. However, plasma zinc concentrations were correlated with changes in DNA strand breaks during dietary zinc repletion. It is difficult to assess an individual's zinc status because the symptoms of zinc deficiency are usually nonspecific, and homeostatic mechanisms tightly maintain tissue and circulating zinc concentrations within fairly narrow ranges. The existing biomarkers for zinc deficiency, such as plasma zinc concentrations and activities of zinc-dependent enzymes, are problematic in that they lack sensitivity and/or specificity for individual-level assessment (38). The poor sensitivity and specificity of plasma zinc hinder the progress of research on zinc deficiency and its potential health consequences. We report here, using a highly controlled human zinc feeding trial, that alterations in DNA integrity occur quickly with marginal zinc depletion. Importantly, these changes occur before measurable alterations in plasma zinc concentrations. Our data support the notion that functional consequences of zinc deficiency occur before plasma zinc concentrations decline and highlight the essential role of zinc for maintaining DNA integrity in vivo.
Previous studies, using in vitro and in vivo animal models, found that zinc deficiency increased DNA damage in liver or testes (17, 18, 21, 22). To our knowledge, our study is the first report of zinc-related DNA damage in peripheral blood cells of humans. Although previously published studies and the current study did not specify the differences in sensitivity to DNA damage among tissues with zinc depletion, our laboratory observed that marginal zinc deficiency increased DNA strand breaks in peripheral blood cells, without significantly increasing 8-oxo-7,8-dihydroguanine concentrations in tissues such as the liver or prostate (Y Song and E Ho, unpublished observations, 2009). Increases in DNA damage with marginal deficiency in tissues are only seen with additional exogenous stresses (Y Song, A Scrimgeour, and E Ho, unpublished observations, 2009), which suggests that the peripheral blood cells may be more sensitive to DNA damage with zinc deficiency.
Possible links between zinc deficiency and DNA damage were described previously, but the specific underlying mechanisms for these events remain unknown. Possible mechanisms include perturbations of antioxidant defenses and/or impaired DNA repair mechanisms. We have shown in previous in vitro studies that cellular zinc depletion causes oxidative stress and disruptions of DNA repair pathways (17), and further investigation into the effects of human zinc depletion on DNA repair is an important area of future research. In the current study, no changes in total antioxidant capacity were observed with marginal zinc depletion, although we did see a trend for decreased erythrocyte SOD activity. This result is consistent with one study in humans by the European Zincage project (39), which reported that erythrocyte SOD activity was inversely associated with the plasma zinc concentration.
We previously showed an increase in plasma F2-isoprostanes in zinc-deficient rats, which indicated that lipid peroxidation increased with zinc depletion (16). In marginally zinc-deficient rats we also found that hepatic vitamin E status was compromised with zinc deficiency, whereas the plasma vitamin E concentration remained unchanged (Y Song and E Ho, unpublished observations, 2009), results that are consistent with our current findings. Therefore, we postulated that during zinc depletion, liver vitamin E status is sacrificed to maintain circulating vitamin E concentrations. In marginal zinc depletion in humans, we did not detect any changes in F2-isoprostane or plasma tocopherol concentrations during zinc depletion and repletion, which may have been due to similar compensatory mechanisms, as shown in our previous rat studies. The body may be able to partially suppress lipid peroxidation caused by zinc deficiency by maintaining plasma vitamin E concentrations. Importantly, although compensatory antioxidant metabolism may help limit oxidative stress caused by low cellular zinc, this was not sufficient to prevent the DNA damage to peripheral blood cells, which suggests that oxidative stress may not be the only factor contributing to DNA damage with zinc deficiency.
One of the interesting findings from the current study is that both DNA single-strand breaks and F2-isoprostane measures tended to be elevated on entry into the study (day 1). The mean (±SE) tail moment after the baseline period was 1.008 ± 0.075, whereas it was 1.898 ± 0.260 at the time of entry to the study. Although comparison of comet values between studies is problematic because of a lack of standardization of the assay between laboratories, the values seen at the end of our baseline period (day 13) are similar to the healthy control comet moment values (≈1.0) that were reported in studies that used similar methods and analyses (40–42). Several studies have shown a positive correlation between DNA damage/comet moment and heavy smoking. Heavy smokers were found to have tail moments in their peripheral blood of 1.5 ± 0.29, whereas those in healthy controls were 1.00 ± 0.016 (41). The elevated comet measurements on day 0 in the current study suggest that, although the entry criteria were designed to exclude potentially zinc-deficient individuals, the subjects may have had suboptimal zinc or other micronutrient status at the time of enrollment into the study, which was reversed during the baseline period. The subjects were also given a multivitamin supplement (including 30 IU all-rac-α-tocopherol), so we may have also corrected other micronutrient deficiencies during the baseline period. Several other vitamins, such as vitamins E and C and folate have antioxidant and/or DNA integrity functions (43). This finding during the baseline period also highlights the potential sensitivity of DNA damage to micronutrient depletion. However, it is also important to point out that the subjects continued to take multivitamin supplements throughout the study, and correlations with plasma zinc and DNA damage were only significant during the zinc-depletion and -repletion phases. Thus, alterations in oxidative stress measures and DNA damage after the baseline period were likely to be largely attributed to zinc alterations.
The comet assay has become a widely used assay in the toxicology field because it is a quick, sensitive, and relatively inexpensive method for measuring DNA strand breaks as an index of genetic damage after exposure to genotoxic agents. A similar approach may be taken for monitoring the status of micronutrients that play a critical role in DNA integrity, such as zinc. However, results of the comet assay are not specific to zinc status, and these results may be affected by other environmental stresses, including other micronutrient deficiencies (43, 44). In addition, further standardization of the assay needs to be performed to control for interlaboratory variations. Currently, in the genotoxicity community, there is a large push to establish standardized techniques, establish reference values and internal standards, and standardize statistical analyses for the assay (45–47). Overall, the high sensitivity of measuring DNA strand breaks by comet assay may complement the poor sensitivity of plasma zinc concentrations, help to identify individuals with marginal zinc deficiency, and test the effectiveness of treatments. For example, supplementing zinc in high-risk populations, such as the elderly or vegetarians, for a few weeks may not change plasma zinc concentrations or improve any clinical symptoms; however, decreases in DNA strand breaks, as measured by the comet assay during the supplementation period, could imply a preexisting marginal zinc-deficient status and justify the continuation of supplemental zinc. Moreover, other functional assays, such as the measurement of interleukin-2 gene expression in mononuclear cells in vitro with the addition of zinc, could be a good assay for detecting human marginal zinc deficiency (48).
The prevalence of zinc deficiency in developed countries could be underestimated. Data from NHANES (2001–2002) showed that ≈12% of Americans do not consume enough zinc relative to their theoretical requirements. The current dietary intervention study shows that dietary zinc depletion increases DNA damage in humans, which suggests that marginal zinc deficiency could have significant health consequences because of zinc's essential role in maintaining DNA integrity. Moreover, the current study highlights the sensitivity of DNA integrity to marginal dietary zinc depletion compared with traditional zinc status measures and indicates that DNA single-strand breaks in peripheral blood cells could be used as a complementary biomarker for zinc deficiency in humans, which may help the progress of clinical diagnosis and zinc-deficiency research.
Acknowledgments
We gratefully acknowledge Deborah Hobbs, Alan Taylor, Davis Yu, and the Oxidative & Nitrative Stress and Cancer Chemoprotection Core laboratories at the Linus Pauling Institute and the WM Keck Collaboratory at Oregon State University for their assistance in conducting these studies.
The authors' responsibilities were as follows—YS: managed all aspects of the studies conducted at Oregon State University, including the conduct and design of all experiments, data interpretation, and initial draft of the manuscript; CSC: managed all aspects of the original clinical studies; RSB: conducted the FRAP assay; MGT: contributed to the data interpretation and manuscript revision; and KHB, JCK, and EH: contributed to the study concept, research design, data interpretation, and manuscript revision. All authors critically reviewed the manuscript. None of the authors had a personal or financial conflict of interest.
REFERENCES
- 1.International Zinc Nutrition Consultative Group Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull 2004;25(suppl):S99–203 [PubMed] [Google Scholar]
- 2.Prasad AS. Discovery of human zinc deficiency and studies in an experimental human model. Am J Clin Nutr 1991;53:403–12 [DOI] [PubMed] [Google Scholar]
- 3.Prasad AS, Halsted JA, Nadimi M. Syndrome of iron deficiency anemia, hepatosplenomegaly, hypogonadism, dwarfism and geophagia. Am J Med 1961;31:532–46 [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS), National Health and Nutrition Examination Survey Questionnaire. Hyattsville, MD. 2001–2002. Available from: http://www.cdc.gov/nchs/about/major/nhanes/nhanes01-02.htm (cited 1 May 2007)
- 5.Gibson RS, Hess SY, Hotz C, Brown KH. Indicators of zinc status at the population level: a review of the evidence. Br J Nutr 2008;99:S14–23 [DOI] [PubMed] [Google Scholar]
- 6.Powell SR. The antioxidant properties of zinc. J Nutr 2000;130:1447S–54S [DOI] [PubMed] [Google Scholar]
- 7.Bray TM, Bettger WJ. The physiological role of zinc as an antioxidant. Free Radic Biol Med 1990;8:281–91 [DOI] [PubMed] [Google Scholar]
- 8.Leone N, Courbon D, Ducimetiere P, Zureik M. Zinc, copper, and magnesium and risks for all-cause, cancer, and cardiovascular mortality. Epidemiology 2006;17:308–14 [DOI] [PubMed] [Google Scholar]
- 9.Wu T, Sempos CT, Freudenheim JL, Muti P, Smit E. Serum iron, copper and zinc concentrations and risk of cancer mortality in US adults. Ann Epidemiol 2004;14:195–201 [DOI] [PubMed] [Google Scholar]
- 10.Sakaguchi S, Iizuka Y, Furusawa S, Ishikawa M, Satoh S, Takayanagi M. Role of Zn(2+) in oxidative stress caused by endotoxin challenge. Eur J Pharmacol 2002;451:309–16 [DOI] [PubMed] [Google Scholar]
- 11.Taylor CG, Bray TM. Effect of hyperoxia on oxygen free radical defense enzymes in the lung of zinc-deficient rats. J Nutr 1991;121:460–6 [DOI] [PubMed] [Google Scholar]
- 12.Sullivan JF, Jetton MM, Hahn HK, Burch RE. Enhanced lipid peroxidation in liver microsomes of zinc-deficient rats. Am J Clin Nutr 1980;33:51–6 [DOI] [PubMed] [Google Scholar]
- 13.Yousef MI, El-Hendy HA, El-Demerdash FM, Elagamy EI. Dietary zinc deficiency induced-changes in the activity of enzymes and the levels of free radicals, lipids and protein electrophoretic behavior in growing rats. Toxicology 2002;175:223–34 [DOI] [PubMed] [Google Scholar]
- 14.Shaheen AA, el-Fattah AA. Effect of dietary zinc on lipid peroxidation, glutathione, protein thiols levels and superoxide dismutase activity in rat tissues. Int J Biochem Cell Biol 1995;27:89–95 [DOI] [PubMed] [Google Scholar]
- 15.Canali R, Vignolini F, Nobili F, Mengheri E. Reduction of oxidative stress and cytokine-induced neutrophil chemoattractant (CINC) expression by red wine polyphenols in zinc deficiency induced intestinal damage of rat. Free Radic Biol Med 2000;28:1661–70 [DOI] [PubMed] [Google Scholar]
- 16.Bruno RS, Song Y, Leonard SW, et al. Dietary zinc restriction in rats alters antioxidant status and increases plasma F2 isoprostanes. J Nutr Biochem 2007;18:509–18 [DOI] [PubMed] [Google Scholar]
- 17.Ho E, Ames BN. Low intracellular zinc induces oxidative DNA damage, disrupts p53, NFkappa B, and AP1 DNA binding, and affects DNA repair in a rat glioma cell line. Proc Natl Acad Sci USA 2002;99:16770–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho E, Courtemanche C, Ames BN. Zinc deficiency induces oxidative DNA damage and increases p53 expression in human lung fibroblasts. J Nutr 2003;133:2543–8 [DOI] [PubMed] [Google Scholar]
- 19.Ho E. Zinc deficiency, DNA damage and cancer risk. J Nutr Biochem 2004;15:572–8 [DOI] [PubMed] [Google Scholar]
- 20.Oteiza PI, Clegg MS, Keen CL. Short-term zinc deficiency affects nuclear factor-kappab nuclear binding activity in rat testes. J Nutr 2001;131:21–6 [DOI] [PubMed] [Google Scholar]
- 21.Olin KL, Shigenaga MK, Ames BN, et al. Maternal dietary zinc influences DNA strand break and 8-hydroxy-2'-deoxyguanosine levels in infant rhesus monkey liver. Proc Soc Exp Biol Med 1993;203:461–6 [DOI] [PubMed] [Google Scholar]
- 22.Oteiza PI, Olin KL, Fraga CG, Keen CL. Zinc deficiency causes oxidative damage to proteins, lipids and DNA in rat testes. J Nutr 1995;125:823–9 [DOI] [PubMed] [Google Scholar]
- 23.Chung CS, Stookey J, Dare D, et al. Current dietary zinc intake has a greater effect on fractional zinc absorption than does longer term zinc consumption in healthy adult men. Am J Clin Nutr 2008;87:1224–9 [DOI] [PubMed] [Google Scholar]
- 24.Ruz M, Cavan KR, Bettger WJ, Thompson L, Berry M, Gibson RS. Development of a dietary model for the study of mild zinc deficiency in humans and evaluation of some biochemical and functional indices of zinc status. Am J Clin Nutr 1991;53:1295–303 [DOI] [PubMed] [Google Scholar]
- 25.Harris J, Benedict F. A biometric study of basal metabolism in man. Washington, DC: Carnegie Institute of Washington, 1919 [Google Scholar]
- 26.Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 1988;175:184–91 [DOI] [PubMed] [Google Scholar]
- 27.Musiek ES, Yin H, Milne GL, Morrow JD. Recent advances in the biochemistry and clinical relevance of the isoprostane pathway. Lipids 2005;40:987–94 [DOI] [PubMed] [Google Scholar]
- 28.Taylor AW, Bruno RS, Frei B, Traber MG. Benefits of prolonged gradient separation for high-performance liquid chromatography-tandem mass spectrometry quantitation of plasma total 15-series F-isoprostanes. Anal Biochem 2006;350:41–51 [DOI] [PubMed] [Google Scholar]
- 29.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 1996;239:70–6 [DOI] [PubMed] [Google Scholar]
- 30.Podda M, Weber C, Traber MG, Packer L. Simultaneous determination of tissue tocopherols, tocotrienols, ubiquinols, and ubiquinones. J Lipid Res 1996;37:893–901 [PubMed] [Google Scholar]
- 31.Leonard SW, Bruno RS, Paterson E, et al. 5-nitro-gamma-tocopherol increases in human plasma exposed to cigarette smoke in vitro and in vivo. Free Radic Biol Med 2003;35:1560–7 [DOI] [PubMed] [Google Scholar]
- 32.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 1969;244:6049–55 [PubMed] [Google Scholar]
- 33.L'Abbé MR, Fischer PW. Automated assay of superoxide dismutase in blood. Methods Enzymol 1990;186:232–7 [DOI] [PubMed] [Google Scholar]
- 34.Richterich R, Colombo JP, Bachmann C. Clinical chemistry: theory, practice, and interpretation. New York, NY: J Wiley, 1981 [Google Scholar]
- 35.Loft S, Moller P, Cooke MS, Rozalski R, Olinski R. Antioxidant vitamins and cancer risk: is oxidative damage to DNA a relevant biomarker? Eur J Nutr 2008;47(suppl 2):19–28 [DOI] [PubMed] [Google Scholar]
- 36.Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem 1995;64:97–112 [DOI] [PubMed] [Google Scholar]
- 37.Morrow JD. Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arterioscler Thromb Vasc Biol 2005;25:279–86 [DOI] [PubMed] [Google Scholar]
- 38.Hambidge M. Biomarkers of trace mineral intake and status. J Nutr 2003;133(suppl 3):948S–55S [DOI] [PubMed] [Google Scholar]
- 39.Mariani E, Cornacchiola V, Polidori MC, et al. Antioxidant enzyme activities in healthy old subjects: influence of age, gender and zinc status: results from the Zincage Project. Biogerontology 2006;7:391–8 [DOI] [PubMed] [Google Scholar]
- 40.Sasaki M, Dakeishi M, Hoshi S, Ishii N, Murata K. Assessment of DNA damage in Japanese nurses handling antineoplastic drugs by the comet assay. J Occup Health 2008;50:7–12 [DOI] [PubMed] [Google Scholar]
- 41.Lu Y, Morimoto K. Exposure level to cigarette tar or nicotine is associated with leukocyte DNA damage in male Japanese smokers. Mutagenesis 2008;23:451–5 [DOI] [PubMed] [Google Scholar]
- 42.Lu Y, Morimoto K, Nakayama K. Health practices and leukocyte DNA damage in Japanese hard-metal workers. Prev Med 2006;43:140–4 [DOI] [PubMed] [Google Scholar]
- 43.Ames BN, Wakimoto P. Are vitamin and mineral deficiencies a major cancer risk? Nat Rev Cancer 2002;2:694–704 [DOI] [PubMed] [Google Scholar]
- 44.Bull C, Fenech M. Genome-health nutrigenomics and nutrigenetics: nutritional requirements or 'nutriomes' for chromosomal stability and telomere maintenance at the individual level. Proc Nutr Soc 2008;67:146–56 [DOI] [PubMed] [Google Scholar]
- 45.Gallo V, Khan A, Gonzales C, et al. Validation of biomarkers for the study of environmental carcinogens: a review. Biomarkers 2008;13:505–34 [DOI] [PubMed] [Google Scholar]
- 46.Moller P. The alkaline comet assay: towards validation in biomonitoring of DNA damaging exposures. Basic Clin Pharmacol Toxicol 2006;98:336–45 [DOI] [PubMed] [Google Scholar]
- 47.Moller P. Assessment of reference values for DNA damage detected by the comet assay in human blood cell DNA. Mutat Res 2006;612:84–104 [DOI] [PubMed] [Google Scholar]
- 48.Prasad AS, Bao B, Beck FW, Sarkar FH. Correction of interleukin-2 gene expression by in vitro zinc addition to mononuclear cells from zinc-deficient human subjects: a specific test for zinc deficiency in humans. Transl Res 2006;148:325–33 [DOI] [PubMed] [Google Scholar]