Abstract
Background: Glutathione plays various protective roles in the human body. Vitamin B-6 as pyridoxal-5′-phosphate (PLP) is required as the coenzyme in the formation of glutathione precursors. Despite this obligatory role of PLP, previous studies from this laboratory showed that vitamin B-6 deficiency caused elevated glutathione concentrations in rat liver and human plasma.
Objective: Our aim was to determine the effect of marginal vitamin B-6 deficiency (plasma PLP 20–30 nmol/L) on the rate of red blood cell (RBC) glutathione synthesis.
Design: We measured plasma and RBC glutathione concentrations and the fractional and absolute synthesis rates of RBC glutathione using the stable-isotope-labeled glutathione precursor [1,2-13C2]glycine in 13 healthy volunteers aged 21–39 y.
Results: Dietary vitamin B-6 restriction did not significantly affect the glutathione concentration in plasma (6.9 ± 1.9 compared with 6.7 ± 1.1 μmol/L) or RBCs (2068 ± 50 compared with 2117 ± 48 μmol/L). For RBC glutathione, the mean fractional synthesis rates were 54 ± 5%/d and 43 ± 4%/d (P = 0.10), and the absolute synthesis rates were 1116 ± 100 and 916 ± 92 μmol · L−1 · d−1 (P = 0.14) before and after vitamin B-6 restriction, respectively.
Conclusions: Marginal vitamin B-6 deficiency tended to decrease mean RBC glutathione synthesis with no effect on RBC glutathione concentration, but the responses varied widely among individuals. Because the cysteine concentration in plasma and RBC did not change during vitamin B-6 restriction, we conclude that the effects of marginal vitamin B-6 deficiency on glutathione synthesis are not caused by altered precursor concentrations.
INTRODUCTION
Glutathione is a vital factor in metabolic protective functions, including the reduction of hydroperoxides, the quenching of free radicals, and the detoxification of xenobiotics (1, 2). Glutathione synthesis from glutamate, cysteine, and glycine is tightly regulated, with its determinants including the activity of γ-glutamylcysteine synthetase and the intracellular availability of cysteine (2, 3). Cysteine can be synthesized from methionine via the transsulfuration pathway. Both enzymes of the transsulfuration pathway—cystathionine β-synthase and cystathionine γ-lyase—require vitamin B-6 in the form of pyridoxal-5′-phosphate (PLP) as coenzyme. PLP also serves as a coenzyme in the formation of glycine from serine through serine hydroxymethyltransferase.
Marginal vitamin B-6 deficiency, defined as a plasma PLP concentration between 20 and 30 nmol/L (4), occurs frequently (5) and is associated with coronary artery disease (6–8), stroke (9), and an elevated risk of Alzheimer disease (10). The mechanisms responsible for linkages between vitamin B-6 status and chronic disease have not yet been established.
A previous study from this laboratory showed that hepatic glutathione was inversely related to the dietary pyridoxine concentration over a range from fully adequate (2 mg/kg diet) through moderate (0.5 mg/kg diet) and pronounced deficiency (0.1 mg/kg) in rats (11). Plasma glutathione increased significantly during dietary vitamin B-6 restriction in healthy young men and women (12). The mechanism by which reduced vitamin B-6 status affects mammalian glutathione metabolism remains unclear. One may speculate that increases in tissue or blood glutathione might be induced by an inflammatory state (13) and oxidative stress (14–16) related to vitamin B-6 deficiency. Oxidative conditions potentially promote transsulfuration flux by activating cystathionine β-synthase (17, 18) and glutathione synthesis via activation of γ-glutamylcysteine ligase and glutathione synthetase (18). On the basis of the observations of our previous protocols and the reported connection between vitamin B-6 deficiency and oxidative stress, we hypothesize that glutathione synthesis increases after vitamin B-6 restriction in humans despite the essential role of PLP in the production of glutathione precursors.
Red blood cell (RBC) glutathione synthesis rates have been determined in humans with the use of stable-isotope-labeled glutathione precursors, such as glycine (19–23) or cysteine (24–27). RBCs are a major source of glutathione (28). As previously reported (29), we designed a steady state stable-isotope tracer infusion protocol to assess the effect of marginal vitamin B-6 deficiency on whole-body glycine kinetics in healthy men and women. This protocol used [1,2-13C2]glycine before and after dietary vitamin B-6 restriction. Our aim was to quantify the fractional synthesis rate (FSR) and the absolute synthesis rate (ASR) of RBC glutathione and to determine their dependence on vitamin B-6 status in vivo. We also report plasma and RBC concentrations of glutathione and related amino acids before and after dietary vitamin B-6 restriction in this group of 13 healthy men and women.
SUBJECTS AND METHODS
Subjects
Volunteers underwent a physical examination and were screened by standard clinical measures of hematological, hepatic, renal, and thyroid function. Medical history, dietary habits, and demographic data were assessed by a questionnaire. Of 37 recruited healthy male and nonpregnant female volunteers, 23 met the following inclusion criteria: age between 20–40 y, no history of gastrointestinal surgery, abnormal kidney or thyroid function, or any other chronic disease; no smoking or chronic drug use or alcoholism; no vitamin, amino acid, or protein supplementation; no chronic consumption of a high-protein diet; and a body mass index (in kg/m2) <28. All selected subjects were in adequate nutritional status for serum folate (>7 nmol/L), serum vitamin B-12 (>200 pmol/L), plasma PLP (>30 nmol/L), and plasma total homocysteine (<12 μmol/L).
Before the study, 6 of the 23 subjects that passed screening withdrew because of personal reasons or scheduling problems. Three subjects were withdrawn during intervention because of changes in health status and one withdrew for personal reasons. All subjects gave written informed consent. The University of Florida Institutional Review Board and the General Clinical Research Center (GCRC) Scientific Advisory Committee reviewed and approved this protocol.
Dietary treatment
All meals were prepared by the Bionutrition Unit of the GCRC. The subjects consumed nutritionally adequate meals with a standardized composition for 2 d to minimize dietary variation immediately before the first infusion. Subjects began consuming a vitamin B-6–restricted diet (0.32–0.38 mg vitamin B-6/d) on the day after the first infusion and continued this regimen for 28 consecutive days (29–31). During this period, the subjects consumed breakfast at the GCRC, were given a take-out lunch and snack to eat at their convenience, and returned to the GCRC to consume their evening meal. We compensated for any vitamin and mineral inadequacies of the study diets (other than vitamin B-6) by administering custom supplements daily to the subjects. Compliance with the dietary regimen was monitored by weekly measurements of plasma PLP as described below. After the second infusion day, an over-the-counter multivitamin-multimineral supplement was provided to the subjects to facilitate restoration of normal vitamin B-6 status.
Isotope infusion protocol
The infusion protocol was previously described in detail (29, 32). [1,2-13C2]Glycine, l-[5,5,5-2H3]leucine and sodium [13C]bicarbonate were purchased from Cambridge Isotopes Laboratories (Andover, MA). The parenteral solutions of these compounds were prepared in isotonic saline, filter sterilized, and analyzed to ensure the lack of pyrogenicity and microbial contamination.
Subjects were admitted to the GCRC on the evening before the infusion protocol and consumed no food or drinks, except water, between 2100 and the first blood draw. On the morning of the infusion, a catheter was inserted in the antecubital vein of each arm: one for the tracer infusion and one for blood collection. Fasting blood samples were collected 2 h before infusion (at ≈0700) for the measurement of plasma PLP, serum folate, vitamin B-12, and plasma total homocysteine concentrations. Infusions were initiated at ≈0900 with a 5-min, ≈20 mL priming dose that delivered 9.26 μmol [1,2-13C2]glycine/kg, 1.87 μmol [5,5,5-2H3]leucine/kg, and 2.15 μmol NaH13CO3/kg. The 9-h constant infusion followed immediately thereafter at a rate of ≈20 mL/h to provide 9.26 μmol [1,2-13C2]glycine/kg and 1.87 μmol [5,5,5-2H3]leucine/kg.
Blood samples were taken at 0, 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 7.5, and 9 h of the infusion. The samples were placed immediately on ice and centrifuged within 15 min after the blood draw (1500 × g, 4°C, 10 min). Plasma was stored in microcentrifuge tubes at −80°C. After removal of the buffy coat, RBCs were washed twice with phosphate-buffered saline (pH 7.4), lysed in distilled water in a 1:1 dilution, frozen in liquid nitrogen, and stored at −80°C until analyzed.
The subjects received a nutritive formula hourly starting 2 h before the infusion to maintain a fed state (33). This formula provided a balanced pattern of amino acids at a rate based on requirements of 0.8 g protein · kg−1 · d−1, which equals an hourly protein dose of ≈0.03 g/kg with 5.23 and 5.44 kJ · kg−1 · d−1 for women and men, respectively. The formula further provided an adequate energy intake according to the requirements of 126 and 130 kJ · kg−1 · d−1 for women and men, respectively.
Sample analyses
Screening measurements
Serum folate and vitamin B-12 were analyzed with the use of a chemiluminescence-based assay (Elecsys; Roche Diagnostics, Indianapolis, IN). Plasma total homocysteine concentrations were measured as the ammonium 7-fluorobenzo-2-oxa-1,3-diazole-4-sulfonate derivative by reversed-phase HPLC with fluorescence detection (34).
Plasma and RBC concentrations of amino acids and PLP
Plasma and RBC glutathione, cysteine, and cysteinyl-glycine concentrations were measured as described for plasma total homocysteine screening by reversed-phase HPLC with fluorescence detection (34). Plasma and RBC PLP concentrations were measured as the semicarbazone derivative by reversed-phase HPLC with fluorescence detection (35, 36). Hemoglobin from lysed RBCs was measured with the use of a commercial sprectrophotometric procedure (HemoCue Hb 201+; HemoCue AB, Ängelholm, Sweden).
Gas chromatography–mass spectrometry determination of amino acid isotopic enrichment and concentration
RBC amino acids were isolated, derivatized, and analyzed by gas chromatography–mass spectrometry (GC-MS) in electron capture negative ionization mode as previously described (37). The relative abundance of specific ions was determined by selected-ion monitoring at the following m/z ratios: glycine (293–295) and leucine (349–352). Isotopic enrichments are expressed as molar ratios (mol% excess) of labeled to unlabeled isotopomers after correction for the natural abundance of stable isotopes, essentially as performed by Storch et al (33).
Liquid chromatography–mass spectrometry analysis of RBC glutathione isotopic enrichment
Whole-blood glutathione was isolated and derivatized from a modified method developed by Guan et al (38). Aliquots of whole blood (150 μL) were transferred to 1.5-mL conical microcentrifuge tubes, and the tubes were frozen at −80°C, thawed, and vortex mixed. This procedure was repeated 3 times to lyse the RBCs in the whole-blood aliquot. A 300-μL aliquot of 10 mmol/L Ellman’s solution [39.6 mg 5,5′-dithiobis-(2-nitrobenzoic acid); Sigma-Aldrich, St Louis, MO) in 10 mL of 100 mmol ammonium bicarbonate buffer solution/L, adjusted to pH = 7.5] was added to each microcentrifuge vial; the vials were vortex mixed, allowed to sit at room temperature for 15 min, and vortex mixed again. A 150-μL aliquot of 200 g sulfosalicylic acid solution/L was added, and each microcentrifuge vial was vortex mixed again. The vials were centrifuged (10,000 × g) for 15 min, and the supernatant fluid was transferred to a second microcentrifuge vial and centrifuged again at (10,000 × g) for 15 min. The supernatant fluid of each vial was then carefully transferred to 250-μL polypropylene autosampler vials and stored at −80°C until analyzed.
The derivatized glutathione samples were measured for 13C tracer content by LC-MS by using a Thermo-Finnigan Surveyor LC coupled with a Thermo-Finnigan (San Jose, CA) LCQ Deca XP Plus ion trap mass spectrometer, configured in positive electrospray ionization (ESI) mode. The ESI spray voltage was set at 4.5 kV; sheath gas (N2) was kept at a setting of 40, and the capillary temperature was maintained at 225°C. The LC column used was an Atlantis dC18, 1 × 150-mm, 3-μm bead column (Waters Corp, Milford, MA) kept at 40°C. The LC was run isocratically at 87% water, 12.5% acetonitrile, and 0.5% glacial acetic acid. The total run time was 11 min, with a mobile phase flow of 55 μL/min and a typical retention time for derivatized glutathione of ≈7 min. The LC effluent was diverted away from the ESI source for the initial 3 min of each LC run to keep any unreacted Ellman’s solution and residual sulfosalicylic acid from entering the ESI-LC-MS source. All mass spectrometry data were collected in “zoom scan” mode at ≈1 scan/s covering the isotopic envelope for the [M+H]+ ion of the derivatized glutathione (m/z = 504.5–508.5) to maximize mass resolution between isotopomers. After data acquisition, the intensity of each mass was averaged for the glutathione peak by using the Thermo-Finnigan Xcalibur Qual browser software. The peak areas for the [M+H]+ ion for unlabeled glutathione (m/z = 505) and [13C2]glutathione (m/z = 507) were then exported to an Excel worksheet, and the area ratios were converted to enrichment as mole% excess 13C2-glutathione with the use of standard equations.
Kinetic principles and analysis
As reported previously (29, 32), the combined use of [1,2-13C2]glycine and [2H3]leucine permits determination of the kinetics of whole-body glycine turnover, the conversion of glycine to serine, the rate of glycine decarboxylation, and its role as a source of 1-carbon units in 1-carbon metabolism. [2H3]Leucine was included to evaluate any nutritional effects on protein turnover as indicated by leucine flux (31, 39). The plateau enrichment (Ep) for all infused amino acid tracers was calculated as the mean of the plasma isotopic enrichments for the ≈1.5-h to 9-h time points for the infused [13C2]glycine and [2H3]leucine tracers. Steady state kinetics of amino acid tracers were calculated by using standard equations (40) as described earlier (29, 32). The flux of leucine (QLeu) in the plasma pool was calculated as
where ILeu is the [2H3]leucine infusion rate, ELeu is the enrichment of the [2H3]leucine tracer, and EpLeu is the plateau enrichment of [2H3]leucine in plasma.
Glycine flux (QGly) was calculated as
where IGly is the [13C2]glycine infusion rate, EGly is the enrichment of the [13C2]glycine tracer, and EpGly is the plateau enrichment of plasma [13C2]glycine corrected for intracellular overestimation (41, 42).
The FSR represents the fraction of the total pool that is synthesized per unit of time. The direct incorporation of the infused [1,2-13C2]glycine tracer (glycine M+2) in glutathione synthesis was determined and, thus, the synthesis of glutathione M+2. The FSR of RBC glutathione was approximated by using the initial rate of glutathione M+2 enrichment (slope of linear increase among the time points 4, 6, 7.5, and 9 h after infusion) and the estimated plateau enrichment of RBC glycine (EpRBC glycine).
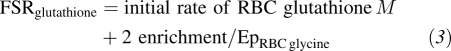 |
The RBC glycine enrichment at plateau (EpRBC glycine) was determined by fitting enrichment data to single exponential curves defined by the equation
where E is the enrichment at time t (h), Ef is the enrichment at infinity (ie, plateau enrichment), and k is the rate constant (h−1) from the fitted curve (43). Data were fit to this single exponential regression equation by using the “exponential rise to maximum” function of SigmaPlot 2002 (SPSS version 8.02; SPSS, Rochester, MN).
The ASR was derived from the FSRglutathione and the RBC total glutathione pool:
Statistical analysis
In all tracer measurements, isotopic enrichment was expressed as the ratio of labeled to nonlabeled isotopomers after correction for the natural abundance of stable isotopes. All data are presented as means ± SEMs. A paired t test was used to assess differences between normal vitamin B-6 status and marginal vitamin B-6 deficiency. Correlations between static and dynamic parameters of glutathione synthesis were determined with the use of the Spearman’s rank correlation coefficient. Linear regression was used to determine the influence of static and dynamic variables on the rate of glutathione synthesis before and after vitamin B-6 restriction and on the change in the ASR and FSR of RBC glutathione. Tertiles of RBC glutathione FSR were evaluated by using one-factor ANOVA, with multiple comparisons performed by using the Holm-Sidak method. Data were analyzed by using Microsoft Office Excel 2007 (Microsoft Corporation, Redmond, WA), SigmaStat 3.0, and SPSS 16.0 (SPSS Inc, Rochester, MN).
RESULTS
Dietary vitamin B-6 restriction significantly reduced the plasma PLP concentration (Table 1) by 55 ± 3%. All 13 subjects had reached the range of plasma PLP associated with marginal vitamin B-6 deficiency after 22 ± 2 d of consuming the vitamin B-6–restricted diet. Concurrent with the decrease in plasma PLP concentration, vitamin B-6 restriction significantly reduced the RBC PLP concentration (Table 1) by 18 ± 2%. Marginal vitamin B-6 deficiency did not alter the plasma and RBC concentrations of glutathione, cysteine, and cysteinyl-glycine. As reported previously (29), these subjects showed no change in plasma serine, methionine, or total homocysteine concentrations, whereas their plasma glycine and cystathionine concentrations significantly increased because of vitamin B-6 restriction.
TABLE 1.
Concentration of plasma and red blood cell (RBC) pyridoxal-5′-phosphate (PLP), glutathione, total cysteine, and cysteinylglycine before and after dietary vitamin B-6 restriction1
| At normal vitamin B-6 status | At marginal vitamin B-6 deficiency | |
| Plasma PLP (nmol/L) | 55 ± 4 | 23 ± 12 |
| RBC PLP (pmol/g hemoglobin) | 429 ± 19 | 352 ± 142 |
| Plasma glutathione (μmol/L) | 6.9 ± 1.9 | 6.7 ± 1.1 |
| RBC glutathione (μmol/L packed red cells) | 2068 ± 50 | 2117 ± 48 |
| Plasma total cysteine (μmol/L) | 237 ± 33 | 237 ± 29 |
| RBC total cysteine (nmol/g hemoglobin) | 141 ± 8 | 144 ± 8 |
| Plasma cysteinylglycine (μmol/L) | 31.2 ± 1.4 | 29.7 ± 1.3 |
| RBC cysteinylglycine (nmol/g hemoglobin) | 193 ± 15 | 200 ± 18 |
All values are means ± SEMs; n = 13.
Significantly different from normal vitamin B-6 status, P < 0.0001 (paired t test).
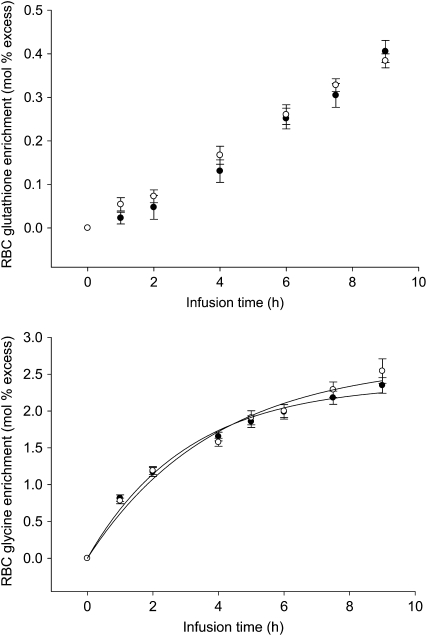
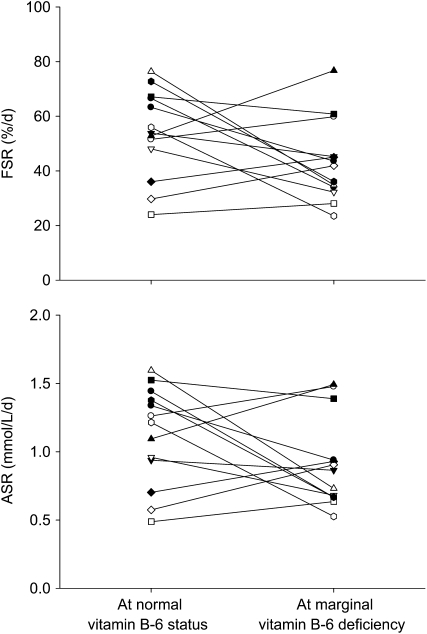
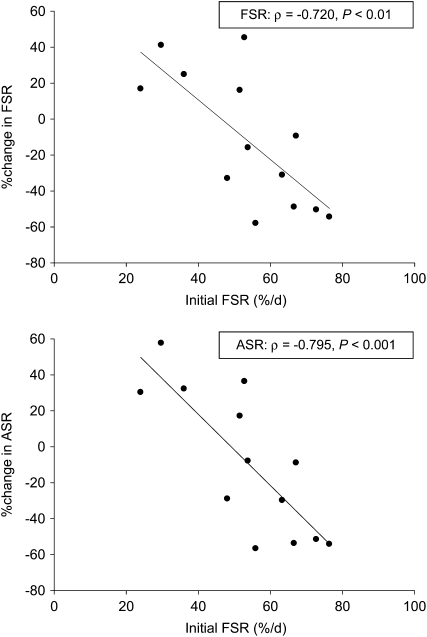
The progressive isotopic enrichment of RBC glutathione and its precursor RBC glycine in this protocol are presented in Figure 1. The means for FSR and ASR of RBC glutathione (Table 2) were slightly lower in marginal vitamin B-6 deficiency; however, this did not reach statistical significance (P = 0.10 and P = 0.14, respectively). The individual FSR of RBC glutathione responded to vitamin B-6 restriction, ranging from a 58% decrease to a 45% increase (Figure 2A). The individual ASRs of RBC glutathione ranged from a 57% decrease to a 58% increase (Figure 2B). To further examine the heterogeneity of this response, we ranked FSR values according to the extent and direction of absolute change due to vitamin B-6 restriction and divided them into tertiles (Table 3). ASR and FSR of RBC glutathione at normal vitamin B-6 status significantly correlated with the extent of change in ASR and FSR observed due to vitamin B-6 restriction, respectively (ASR: ρ = −0.698, P < 0.01; FSR: ρ = −0.692, P < 0.01). The change in RBC PLP concentration in response to vitamin B-6 restriction was significantly related to the change in RBC cysteine concentration (ρ = −0.748, P < 0.01). Linear regression showed that the change in ASR and FSR of RBC glutathione in response to vitamin B-6 restriction were both negatively dependent on the FSR at normal vitamin B-6 status (Figure 3).
FIGURE 1.
Mean (±SEM) red blood cell (RBC) glutathione and glycine enrichments before (•) and after (○) vitamin B-6 restriction.
TABLE 2.
Fractional synthesis rate (FSR) and absolute synthesis rate (ASR) of red blood cell (RBC) glutathione and whole-body glycine and leucine flux before and after vitamin B-6 restriction1
| Normal vitamin B-6 status | Marginal vitamin B-6 deficiency | |
| FSR of RBC glutathione (%/d) | 53.7 ± 4.5 | 43.2 ± 4.1 |
| ASR of RBC glutathione (μmol · L packed red cells−1 · d−1) | 1116 ± 100 | 916 ± 92 |
| Whole-body glycine flux (μmol · kg−1 · h−1) | 448 ± 20 | 446 ± 16 |
| Whole-body leucine flux (μmol · kg−1 · h−1) | 106 ± 4 | 106 ± 7 |
All values are means ± SEMs; n = 13.
FIGURE 2.
Individual fractional synthesis rate (FSR) and absolute synthesis rate (ASR) of red blood cell glutathione before and after vitamin B-6 restriction in 13 healthy men and women. Symbols represent individual subjects.
TABLE 3.
Tertiles of the absolute change in the fractional synthesis rate (FSR) of red blood cell (RBC) glutathione due to vitamin B-6 restriction1
| Absolute change in FSR of RBC glutathione | |
| %/d | |
| Upper tertile (n = 5) | 11.5 ± 3.4a |
| Middle tertile (n = 4) | −12.6 ± 3.1b |
| Lower tertile (n = 4) | −35.8 ± 2.2c |
All values are means ± SEMs. Means with different superscript letters are significantly different, P < 0.001 (one-factor ANOVA with multiple comparisons performed by using the Holm-Sidak method).
FIGURE 3.
Linear regression between the change in fractional synthesis rate (FSR) and absolute synthesis rate (ASR) of red blood cell glutathione after vitamin B-6 restriction and the initial FSR value at normal vitamin B-6 status in 13 healthy men and women.
DISCUSSION
Vitamin B-6 depletion and marginal vitamin B-6 deficiency have been shown to increase glutathione concentrations in rat liver (11, 44) and human plasma (12). To our knowledge, this was the first study to investigate the effect of vitamin B-6 status on in vivo glutathione synthesis in healthy men and women. We showed that marginal vitamin B-6 deficiency tended to decrease RBC glutathione synthesis rates without affecting the plasma and RBC total glutathione concentration. The response of RBC glutathione synthesis rate to vitamin B-6 restriction varied greatly among subjects and was shown to depend on the initial FSR value (Figure 3). Changes in RBC glutathione synthesis rates were not predicted by the concentration or extent of change in plasma and RBC cysteine and glutathione. Thus, the effect of marginal vitamin B-6 deficiency on RBC glutathione synthesis rates appeared to be unrelated to the supply of the precursor amino acid cysteine. This finding is consistent with recent results of mathematical modeling simulations that predicted the effect of mild vitamin B-6 deficiency on blood glutathione concentration to be solely related to increased oxidative stress and not to the reduced velocity of PLP-dependent enzymes involved in cysteine formation (45). The variability in responses among subjects might result from factors influencing the activity and expression of γ-glutamyl-cysteine-synthetase, eg antioxidants and hormones such as insulin and glucocorticoids (1). Because deficiencies of γ-glutamyl-cysteine-synthetase and glutathione synthetase occur very rarely and are related to severe clinical symptoms (46), we do not believe that the observed variation in the basal rate of glutathione synthesis was associated with genetic variation. Furthermore, we were unable to postulate a mechanism by which cellular PLP depletion would directly alter glutathione synthesis.
Despite the fact that marginal vitamin B-6 deficiency yielded substantial and individually varied glutathione synthesis responses, plasma and RBC glutathione concentrations remained constant in our subjects. Similar findings were reported by Lyons et al (26), who observed reduced whole-blood glutathione synthesis rates but unaltered whole-blood glutathione concentrations after a 10-d sulfur amino acid–free diet in healthy men. In addition, mathematical modeling predicted constant blood glutathione concentrations during increasing oxidative stress and decreasing cytoplasmic (largely hepatic) glutathione concentrations (45). A possible explanation for this observation is a reduced glutathione turnover (26), potentially caused by reduced glutathione transport (47). In rat heart and liver tissues, a more severe vitamin B-6 deficiency induced an increase in glutathione peroxidase activity and to a lesser extent in glutathione reductase activity (15). This shift in enzyme activity evoked a decrease in the ratio of reduced to oxidized glutathione, whereas glutathione concentrations remained unaltered (15). Although our results indicate that the effects of marginal vitamin B-6 deficiency on glutathione synthesis are independent of the cysteine supply, the underlying mechanisms responsible for the variation in response in glutathione synthesis with concurrently constant RBC glutathione concentration remain to be determined.
Cysteine formation through the transsulfuration pathway is an important source of cysteine for glutathione synthesis. The dependence of glutathione synthesis on cystathionine β-synthase was shown in an in vitro model in which the glutathione concentration decreased by half within 17.5 min after inactivation of cystathionine β-synthase (48). As shown in this study and previously (12), marginal vitamin B-6 deficiency did not alter plasma or RBC cysteine concentrations. Furthermore, the hepatic cysteine concentration was maintained in rats with moderate levels of deficiency (11). In vivo cysteine flux is maintained in human vitamin B-6 deficiency states (12). Likewise, this research group has shown that the in vitro activity of hepatic cystathionine γ-lyase is depressed even in rats with mild vitamin B-6 deficiency (11), but the hepatic cysteine concentration in rats is approximately constant over a wide range of moderate vitamin B-6 deficiency states (11). We previously proposed that elevated cystathionine, under conditions in which cystathionine is lower than the Michaelis constant for cystathionine γ-lyase, compensates at least partially for the reduction in cystathionine γ-lyase caused by PLP depletion (11, 12) to allow transsulfuration flux to be maintained in marginal vitamin B-6 deficiency. We suggest that cysteine formation through transsulfuration is resilient to marginal vitamin B-6 deficiency and, thus, cysteine production does not limit glutathione synthesis at this level of deficiency. This situation differs from more severe vitamin B-6 deficiency that is capable of reducing cysteine availability to the extent glutathione synthesis is impaired and glutathione concentration is reduced (49).
Methionine and cysteine contribute equally to glutathione synthesis as shown in rat hepatocytes (50). At an adequate intake of methionine, the dietary cysteine supply did not alter the RBC glutathione synthesis rate in healthy adults (51). A diet free of methionine and cysteine significantly reduced the whole-blood glutathione synthesis rate, whereas it did not affect the whole-blood glutathione concentration (26). In our study, the subjects consumed an adequate supply of protein (73 ± 4 g/d) and of methionine and cystine (1.7 ± 0.1 and 1.3 ± 0.1 mg/d) during the 28-d vitamin B-6–restricted diet. Cysteine in plasma fluctuates depending on protein and glutathione breakdown in tissues (3). Glutathione breakdown has been shown to account for 50% of cysteine flux (24). A 30% decrease in the plasma cysteine concentration after a 10-d sulfur amino acid–free diet (26) could be explained primarily by reduced protein breakdown. In our study, marginal vitamin B-6 deficiency did not change whole-body protein turnover as indicated by constant whole-body leucine flux. The observed variation in effects of vitamin B-6 restriction on the glutathione synthesis rate was, therefore, unrelated to changes in protein turnover or sulfur amino acid intake.
RBC glutathione has widely been used in the quantification of in vivo glutathione synthesis rate (19–22). In rabbits (52) and pigs (53), faster glutathione synthesis was determined in liver than in blood cells. Compared with control rats without infection, septic rats had lower glutathione synthesis rates in whole blood but higher rates in liver, spleen, large intestine, lung, muscle, and heart (54). This observation would explain our findings of slightly reduced mean RBC glutathione synthesis in the present study and an elevated liver glutathione concentration in vitamin B-6–deficient rats in our previous report (11). Also, Hsu et al (44) found increased glutathione synthesis rates in vitamin B-6–depleted rat liver and only mildly increased RBC glutathione concentrations. In addition, it should be recognized that the effects of vitamin B-6 restriction on RBC glutathione synthesis would result from reduced PLP in the liver and other tissues capable of 1-carbon metabolism and transsulfuration. The rat liver PLP concentration decreased proportionally to the dietary pyridoxine concentration, which indicated that the dietary vitamin B-6 supply affects the vitamin B-6 concentration and, therefore, the coenzyme function at the tissue level (11). Whereas the reductions in plasma and RBC PLP reflect the dietary vitamin B-6 intake, it should not be assumed that the fluxes of such PLP-dependent processes would be equally affected at various extents of vitamin B-6 deficiency.
In conclusion, we showed with this human tracer study, a rat model (11), and a mathematical model (45) that vitamin B-6 status has an effect on glutathione metabolism that varies from person to person. Our results also indicate that variations in the RBC glutathione synthesis rates and in glutathione concentrations do not occur as a result of an impaired supply of the precursor amino acid cysteine. Additional research is needed to clarify the mechanism behind the effect of low vitamin B-6 status on glutathione metabolism.
Acknowledgments
We thank all the volunteers for their participation.
The authors’ responsibilities were as follows—YL: responsible for study coordination, subject recruitment and screening, data collection and analysis, and primary preparation of the manuscript; BO: responsible for data collection and analysis of RBC glutathione enrichment; LRG and CK: responsible for study coordination and subject recruitment and screening; DEM: responsible for supervision of data collection on glutathione enrichment and for manuscript preparation; PWS: was a coinvestigator and was responsible for the experimental design, clinical oversight, and manuscript preparation; and JFG: was the principal investigator and was responsible for the experimental design, oversight of data collection, and manuscript preparation. None of the authors had a personal or financial interest in this research or a conflict of interest of any kind.
REFERENCES
- 1.Lu SC. Regulation of hepatic glutathione synthesis: current concepts and controversies. FASEB J 1999;13:1169–83 [PubMed] [Google Scholar]
- 2.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr 2004;134:489–92 [DOI] [PubMed] [Google Scholar]
- 3.Stipanuk MH. Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr 2004;24:539–77 [DOI] [PubMed] [Google Scholar]
- 4.Leklem JE. Vitamin B-6: a status report. J Nutr 1990;120(suppl 11):1503–7 [DOI] [PubMed] [Google Scholar]
- 5.Morris MS, Picciano MF, Jacques PF, Selhub J. Plasma pyridoxal 5′-phosphate in the US population: the National Health and Nutrition Examination Survey, 2003-2004. Am J Clin Nutr 2008;87:1446–54 [DOI] [PubMed] [Google Scholar]
- 6.Friso S, Girelli D, Martinelli N, et al. Low plasma vitamin B-6 concentrations and modulation of coronary artery disease risk. Am J Clin Nutr 2004;79:992–8 [DOI] [PubMed] [Google Scholar]
- 7.Lin PT, Cheng CH, Liaw YP, Lee BJ, Lee TW, Huang YC. Low pyridoxal 5′-phosphate is associated with increased risk of coronary artery disease. Nutrition 2006;22:1146–51 [DOI] [PubMed] [Google Scholar]
- 8.Robinson K, Arheart K, Refsum H, et al. Low circulating folate and vitamin B6 concentrations: risk factors for stroke, peripheral vascular disease, and coronary artery disease. European COMAC Group. Circulation 1998;97:437–43 [DOI] [PubMed] [Google Scholar]
- 9.Kelly PJ, Shih VE, Kistler JP, et al. Low vitamin B6 but not homocyst(e)ine is associated with increased risk of stroke and transient ischemic attack in the era of folic acid grain fortification. Stroke 2003;34:e51–4 [DOI] [PubMed] [Google Scholar]
- 10.Miller JW, Green R, Mungas DM, Reed BR, Jagust WJ. Homocysteine, vitamin B6, and vascular disease in AD patients. Neurology 2002;58:1471–5 [DOI] [PubMed] [Google Scholar]
- 11.Lima CP, Davis SR, Mackey AD, Scheer JB, Williamson J, Gregory JF., III Vitamin B-6 deficiency suppresses the hepatic transsulfuration pathway but increases glutathione concentration in rats fed AIN-76A or AIN-93G diets. J Nutr 2006;136:2141–7 [DOI] [PubMed] [Google Scholar]
- 12.Davis SR, Quinlivan EP, Stacpoole PW, Gregory JF., 3rd Plasma glutathione and cystathionine concentrations are elevated but cysteine flux is unchanged by dietary vitamin B-6 restriction in young men and women. J Nutr 2006;136:373–8 [DOI] [PubMed] [Google Scholar]
- 13.Friso S, Jacques PF, Wilson PW, Rosenberg IH, Selhub J. Low circulating vitamin B(6) is associated with elevation of the inflammation marker C-reactive protein independently of plasma homocysteine levels. Circulation 2001;103:2788–91 [DOI] [PubMed] [Google Scholar]
- 14.Benderitter M, Hadj-Saad F, Lhuissier M, Maupoil V, Guilland JC, Rochette L. Effects of exhaustive exercise and vitamin B6 deficiency on free radical oxidative process in male trained rats. Free Radic Biol Med 1996;21:541–9 [DOI] [PubMed] [Google Scholar]
- 15.Cabrini L, Bergami R, Fiorentini D, Marchetti M, Landi L, Tolomelli B. Vitamin B6 deficiency affects antioxidant defences in rat liver and heart. Biochem Mol Biol Int 1998;46:689–97 [DOI] [PubMed] [Google Scholar]
- 16.Taysi S. Oxidant/antioxidant status in liver tissue of vitamin B6 deficient rats. Clin Nutr 2005;24:385–9 [DOI] [PubMed] [Google Scholar]
- 17.Taoka S, Ohja S, Shan X, Kruger WD, Banerjee R. Evidence for heme-mediated redox regulation of human cystathionine beta-synthase activity. J Biol Chem 1998;273:25179–84 [DOI] [PubMed] [Google Scholar]
- 18.Vitvitsky V, Mosharov E, Tritt M, Ataullakhanov F, Banerjee R. Redox regulation of homocysteine-dependent glutathione synthesis. Redox Rep 2003;8:57–63 [DOI] [PubMed] [Google Scholar]
- 19.Jackson AA, Gibson NR, Lu Y, Jahoor F. Synthesis of erythrocyte glutathione in healthy adults consuming the safe amount of dietary protein. Am J Clin Nutr 2004;80:101–7 [DOI] [PubMed] [Google Scholar]
- 20.Jahoor F, Jackson A, Gazzard B, et al. Erythrocyte glutathione deficiency in symptom-free HIV infection is associated with decreased synthesis rate. Am J Physiol 1999;276:E205–11 [DOI] [PubMed] [Google Scholar]
- 21.Reid M, Badaloo A, Forrester T, Jahoor F. In vivo rates of erythrocyte glutathione synthesis in adults with sickle cell disease. Am J Physiol Endocrinol Metab 2006;291:E73–9 [DOI] [PubMed] [Google Scholar]
- 22.Reid M, Badaloo A, Forrester T, et al. In vivo rates of erythrocyte glutathione synthesis in children with severe protein-energy malnutrition. Am J Physiol Endocrinol Metab 2000;278:E405–12 [DOI] [PubMed] [Google Scholar]
- 23.Yu YM, Ryan CM, Fei ZW, et al. Plasma L-5-oxoproline kinetics and whole blood glutathione synthesis rates in severely burned adult humans. Am J Physiol Endocrinol Metab 2002;282:E247–58 [DOI] [PubMed] [Google Scholar]
- 24.Fukagawa NK, Ajami AM, Young VR. Plasma methionine and cysteine kinetics in response to an intravenous glutathione infusion in adult humans. Am J Physiol 1996;270:E209–14 [DOI] [PubMed] [Google Scholar]
- 25.Lyons J, Rauh-Pfeiffer A, Ming-Yu Y, et al. Cysteine metabolism and whole blood glutathione synthesis in septic pediatric patients. Crit Care Med 2001;29:870–7 [DOI] [PubMed] [Google Scholar]
- 26.Lyons J, Rauh-Pfeiffer A, Yu YM, et al. Blood glutathione synthesis rates in healthy adults receiving a sulfur amino acid-free diet. Proc Natl Acad Sci USA 2000;97:5071–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Crabben SN, Wijburg FA, Ackermans MT, Sauerwein HP. Effect of cysteine dosage on erythrocyte glutathione synthesis rate in a patient with cystathionine beta synthase deficiency. J Inherit Metab Dis (Epub ahead of print 24 January 2008) [DOI] [PubMed] [Google Scholar]
- 28.Giustarini D, Milzani A, Dalle-Donne I, Rossi R. Red blood cells as a physiological source of glutathione for extracellular fluids. Blood Cells Mol Dis 2008;40:174–9 [DOI] [PubMed] [Google Scholar]
- 29.Lamers Y, Williamson J, Ralat M, et al. Moderate dietary vitamin B-6 restriction raises plasma glycine and cystathionine concentrations while minimally affecting the rates of glycine turnover and glycine cleavage in healthy men and women. J Nutr 2009;139:452–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coburn SP, Ziegler PJ, Costill DL, et al. Response of vitamin B-6 content of muscle to changes in vitamin B-6 intake in men. Am J Clin Nutr 1991;53:1436–42 [DOI] [PubMed] [Google Scholar]
- 31.Davis SR, Scheer JB, Quinlivan EP, Coats BS, Stacpoole PW, Gregory JF., III Dietary vitamin B-6 restriction does not alter rates of homocysteine remethylation or synthesis in healthy young women and men. Am J Clin Nutr 2005;81:648–55 [DOI] [PubMed] [Google Scholar]
- 32.Lamers Y, Williamson J, Gilbert LR, Stacpoole PW, Gregory JF., III Glycine turnover and decarboxylation rate quantified in healthy men and women using primed, constant infusions of [1,2-13C2]glycine and [2H3]leucine. J Nutr 2007;137:2647–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Storch KJ, Wagner DA, Burke JF, Young VR. Quantitative study in vivo of methionine cycle in humans using [methyl-2H3]- and [1-13C]methionine. Am J Physiol 1988;255:E322–31 [DOI] [PubMed] [Google Scholar]
- 34.Pfeiffer CM, Huff DL, Gunter EW. Rapid and accurate HPLC assay for plasma total homocysteine and cysteine in a clinical laboratory setting. Clin Chem 1999;45:290–2 [PubMed] [Google Scholar]
- 35.Ubbink JB, Serfontein WJ, de Villiers LS. Stability of pyridoxal-5-phosphate semicarbazone: applications in plasma vitamin B6 analysis and population surveys of vitamin B6 nutritional status. J Chromatogr 1985;342:277–84 [DOI] [PubMed] [Google Scholar]
- 36.Ubbink JB, Schnell AM. High-performance liquid chromatographic assay of erythrocyte enzyme activity levels involved in vitamin B6 metabolism. J Chromatogr 1988;431:406–12 [DOI] [PubMed] [Google Scholar]
- 37.Davis SR, Stacpoole PW, Williamson J, et al. Tracer-derived total and folate-dependent homocysteine remethylation and synthesis rates in humans indicate that serine is the main one-carbon donor. Am J Physiol Endocrinol Metab 2004;286:E272–9(Published erratum appears in Am J Physiol Endocrinol Metab 2004;286:E674.) [DOI] [PubMed] [Google Scholar]
- 38.Guan X, Hoffman B, Dwivedi C, Matthees DP. A simultaneous liquid chromatography/mass spectrometric assay of glutathione, cysteine, homocysteine and their disulfides in biological samples. J Pharm Biomed Anal 2003;31:251–61 [DOI] [PubMed] [Google Scholar]
- 39.Cuskelly GJ, Stacpoole PW, Williamson J, Baumgartner TG, Gregory JF., III Deficiencies of folate and vitamin B6 exert distinct effects on homocysteine, serine, and methionine kinetics. Am J Physiol Endocrinol Metab 2001;281:E1182–90 [DOI] [PubMed] [Google Scholar]
- 40.Wolfe RR. Radioactive and stable isotope tracers in biomedicine—principles and practice of kinetic analysis. New York, NY: Wiley Liss, 1992 [Google Scholar]
- 41.Arends J, Schafer G, Schauder P, Bircher J, Bier DM. Comparison of serine and hippurate as precursor equivalents during infusion of [15N]glycine for measurement of fractional synthetic rates of apolipoprotein B of very-low-density lipoprotein. Metabolism 1995;44:1253–8 [DOI] [PubMed] [Google Scholar]
- 42.Cryer DR, Matsushima T, Marsh JB, Yudkoff M, Coates PM, Cortner JA. Direct measurement of apolipoprotein B synthesis in human very low density lipoprotein using stable isotopes and mass spectrometry. J Lipid Res 1986;27:508–16 [PubMed] [Google Scholar]
- 43.MacCoss MJ, Fukagawa NK, Matthews DE. Measurement of intracellular sulfur amino acid metabolism in humans. Am J Physiol Endocrinol Metab 2001;280:E947–55 [DOI] [PubMed] [Google Scholar]
- 44.Hsu JM, Buddemeyer E, Chow BF. Role of pyridoxine in glutathione metabolism. Biochem J 1964;90:60–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nijhout HF, Gregory JF, Fitzpatrick C, et al. A mathematical model gives insights into the effects of vitamin B-6 deficiency on 1-carbon and glutathione metabolism. J Nutr 2009;139:784–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ristoff E, Larsson A. Inborn errors in the metabolism of glutathione. Orphanet J Rare Dis 2007;2:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lunn G, Dale GL, Beutler E. Transport accounts for glutathione turnover in human erythrocytes. Blood 1979;54:238–44 [PubMed] [Google Scholar]
- 48.Mosharov E, Cranford MR, Banerjee R. The quantitatively important relationship between homocysteine metabolism and glutathione synthesis by the transsulfuration pathway and its regulation by redox changes. Biochemistry 2000;39:13005–11 [DOI] [PubMed] [Google Scholar]
- 49.Ashwood-Smith MJ, Smith AD. Rat brain glutathione and pyridoxine deficiency. Nature 1959;184(suppl 26):2028–9 [DOI] [PubMed] [Google Scholar]
- 50.Stipanuk MH, Coloso RM, Garcia RA, Banks MF. Cysteine concentration regulates cysteine metabolism to glutathione, sulfate and taurine in rat hepatocytes. J Nutr 1992;122:420–7 [DOI] [PubMed] [Google Scholar]
- 51.Courtney-Martin G, Rafii M, Wykes LJ, Ball RO, Pencharz PB. Methionine-adequate cysteine-free diet does not limit erythrocyte glutathione synthesis in young healthy adult men. J Nutr 2008;138:2172–8 [DOI] [PubMed] [Google Scholar]
- 52.Cabral CB, Bullock KH, Bischoff DJ, Tompkins RG, Yu YM, Kelleher JK. Estimating glutathione synthesis with deuterated water: a model for peptide biosynthesis. Anal Biochem 2008;379:40–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jahoor F, Wykes LJ, Reeds PJ, Henry JF, del Rosario MP, Frazer ME. Protein-deficient pigs cannot maintain reduced glutathione homeostasis when subjected to the stress of inflammation. J Nutr 1995;125:1462–72 [DOI] [PubMed] [Google Scholar]
- 54.Malmezat T, Breuille D, Capitan P, Mirand PP, Obled C. Glutathione turnover is increased during the acute phase of sepsis in rats. J Nutr 2000;130:1239–46 [DOI] [PubMed] [Google Scholar]