Abstract
Background: The effect of breastfeeding on growth in HIV-exposed infants is not well described.
Objective: The objective was to evaluate the effect of early breastfeeding cessation on growth.
Design: In a trial conducted in Lusaka, Zambia, HIV-infected mothers were randomly assigned to exclusive breastfeeding for 4 mo followed by rapid weaning to replacement foods or exclusive breastfeeding for 6 mo followed by introduction of complementary foods and continued breastfeeding for a duration of the mother's choice. Weight-for-age z score (WAZ), length-for-age z score (LAZ), and weight-for-length z score (WLZ) and the self-reported breastfeeding practices of 593 HIV-uninfected singletons were analyzed. Generalized estimating equations were used to adjust for confounders.
Results: WAZ scores declined precipitously between 4.5 and 15 mo. The decline was slower in the breastfed infants. At 9, 12, and 15 mo, mean WAZs were, respectively, −0.74, −0.92, and −1.06 in infants who were reportedly breastfed and were −1.07, −1.20, and −1.31 in the weaned infants (P = 0.003, 0.007, and 0.02, respectively). No differences were observed past 15 mo. Breastfeeding practice was not associated with LAZ, which declined from −0.98 to −2.24 from 1 to 24 mo. After adjustment for birth weight, maternal viral load, body mass index, education, season, and marital and socioeconomic status, not breastfeeding was associated with a 0.28 decline in WAZ between 4.5 and 15 mo (P < 0.0001). During the rainy season, not breastfeeding was associated with a larger WAZ decline (0.33) than during the dry season (0.22; P for interaction = 0.02).
Conclusions: Early growth is compromised in uninfected children born to HIV-infected Zambian mothers. Continued breastfeeding partially mitigates this effect through 15 mo. Nutritional interventions to complement breastfeeding after 6 mo are urgently needed. This trial was registered at clinicaltrials.gov as NCT00310726.
INTRODUCTION
Exclusive breastfeeding for 6 mo is an important strategy for reducing postpartum mother-to-child transmission of HIV in resource-poor settings when conditions do not permit safe use of breast-milk replacements (1–4). Unfortunately, there is little evidence to guide breastfeeding practices for infants born to HIV-infected women beyond this time period (5). For the general (uninfected) population, continued breastfeeding is encouraged for ≥2 y (6–9), but the guidelines for HIV-infected women have tended to be vague. Typically, decisions about when to stop breastfeeding are left to the mother and rest on the family's ability to provide milk, animal-source, and other complementary foods and on many social, cultural, and family influences (10).
In addition to minimizing postnatal transmission of HIV, decision making for feeding infants born to HIV-infected mothers must take into account the risk of childhood infections, malnutrition, and mortality associated with not breastfeeding. The benefits of breastfeeding regarding reductions in gastrointestinal infections, respiratory infections, middle ear infections, and improvements in neurodevelopment and overall survival are well established (11–16). In settings with poor sanitation and hygiene, breastfeeding during the second year of life appears to promote growth (17, 18). In light of the high rates of diarrhea, respiratory infections, and growth faltering reported in both HIV-infected and uninfected children born to HIV-infected mothers, extended partial breastfeeding until age 2 y might be especially important (19–23).
Several studies in resource-poor areas report reductions in mortality of ≤60% in association with breastfeeding in uninfected infants born to HIV-infected mothers (24–27). In addition, we recently reported that reductions in HIV transmission achieved with early weaning were offset by increases in mortality and, as a result, early weaning had no net benefit on HIV-free survival (5).
Prior research has identified several factors that are important to growth in children born to HIV-infected mothers, including the child's HIV status (20, 22) and the mother's schooling, CD4 count, and socioeconomic status (SES) (18, 28). Rates of diarrhea and respiratory infections are also important, independent of HIV status (28). None of these studies, however, assessed the effect of timing of stopping breastfeeding on growth.
As interventions that decrease HIV transmission through breastfeeding, such as exclusive breastfeeding for the first 6 mo of life (3) and prolonged prophylaxis with antiretrovirals during the breastfeeding period (29, 30) are introduced into clinical practice, determining how to optimize the growth of infants born to HIV-infected women has become an important area of clinical and public health research. This study aims to evaluate the effect of timing of breastfeeding cessation on child growth among uninfected infants born to HIV-infected mothers in Zambia.
SUBJECTS AND METHODS
Child anthropometric measures were collected as part of the Zambia Exclusive Breastfeeding Study (ZEBS), a randomized trial described in detail elsewhere (clinicaltrials.gov; no. NCT00310726) (4, 5, 31), that evaluated the effect of short-duration exclusive breastfeeding on infant mortality and vertical transmission of HIV. Briefly, HIV-positive women attending prenatal care services from May 2001 to September 2004 at 2 sites in Lusaka, Zambia, were recruited. Women were given single-dose nevirapine (sdNVP) and were counseled about the risks and benefits of infant feeding options. Women who intended to breastfeed were eligible, and all those enrolled were encouraged to exclusively breastfeed until 4 mo. When their child reached 1 mo of age, they were randomly assigned to either a counseling program that encouraged abrupt cessation of breastfeeding at 4 mo (intervention group) or to a program that encouraged continued exclusive breastfeeding to 6 mo with gradual introduction of complementary foods (control group). The duration of breastfeeding after 4 mo in the control group was determined by personal choice.
All children in the intervention group were provided with commercially available modified cow milk infant formula and a fortified replacement cereal for 3 mo after early breastfeeding cessation. The replacement cereal, which provided 74 kcal/100 mL, was a product developed and tested in Zambia by the US Department of Agriculture and is based on the local staple maize meal and fortified with milk powder, sugar, oil, and micronutrients (see Supplemental Table 1 under “Supplemental data” in the online issue for ingredient details). Mothers were encouraged to offer replacement cereal or formula 6–8 times/d. Education about preparation, feeding practices, and hygiene was provided. Growth monitoring was done every 2 wk between 4 and 6 mo and then monthly from 6 mo as a safety net; any infant in either group with failure to thrive after weaning was provided with either the fortified study replacement cereal or with high-energy protein supplements furnished by the World Food Program, Zambia. The study was approved by the Institutional Review Boards of the investigators' institutions. Written informed consent was obtained from all participants.
On enrollment, weight, height, sociodemographic information, medical and obstetric history, and blood for hemoglobin, CD4 T cell counts, and HIV-1 RNA quantity (Roche Amplicor 1.5; Roche, Branchburg, NJ) were obtained. Nine hundred fifty-eight mothers whose newborn was alive and being breastfed and were still willing to participate in the study 1 mo postpartum were randomly assigned. Mothers and infants were followed up at clinic visits at least once a month for the first 6 mo and every 3 mo thereafter until 24 mo postpartum. Child feeding practice was assessed at each visit by maternal self-report by using a detailed questionnaire, and the exact age at which breastfeeding cessation occurred was determined. Child length and weight were measured at each study visit. Blood samples were collected at each visit to determine the child's HIV status by HIV DNA polymerase chain reaction. Information on maternal or child mortality was obtained from hospital records or family members.
This analysis included 593 HIV-uninfected singleton children who were alive and in follow-up at 150 d and whose mothers were still breastfeeding at 120 d. Children who stopped breastfeeding before 120 d were excluded because all study participants were counseled to breastfeed to 4 mo; only a few stopped before this time, and an evaluation of breastfeeding cessation before 120 d was not among the study aims. Children who died or were lost to follow-up before 150 d were also excluded to minimize attrition in the 4–5-mo interval when mothers in the intervention group were instructed to wean. In addition, 150 d was chosen to give sufficient opportunity for mothers in the intervention group who planned on adhering to the study intervention to stop breastfeeding at 4 mo. Breastfeeding practice was assessed in the analyses both as randomized (intent-to-treat) and by actual breastfeeding practice at each visit. Categorical characteristics were compared by using a chi-square test or Fisher's exact test, and continuous variables were analyzed by using a t test. Weight-for-age z score (WAZ), length-for-age z score (LAZ), and weight-for-length z score (WLZ) were calculated by using the 2006 World Health Organization growth standards (32). Mean WAZ, LAZ, and WLZ were assessed at each time. The proportion of children experiencing moderate-to-severe malnutrition, defined as a WAZ < −2, was assessed cross-sectionally at each visit and cumulatively by using the Kaplan-Meier method to account for censoring by loss to follow-up and by excluding subjects who died before experiencing the event because they violated the assumption that censored subjects are still at risk of the event. To assess temporality and investigate whether child growth determines the subsequent breastfeeding practice, the Kaplan-Meier method was used to assess breastfeeding duration for low-birth-weight (<2500 g) relative to normal-birth-weight children (≥2500 g) and malnourished (WAZ < −2) relative to nonmalnourished (WAZ > −2) children at the 3-mo visit.
The effects of maternal, child, and sociodemographic characteristics on growth were assessed by using a generalized estimating equation (GEE) regression analysis with WAZ as the outcome and child age controlled for to account for the developmental age effects that we observed. An autoregressive correlation structure was used to account for repeated measurements on the same subject over time. Marital status and cohabitation with the infant's father were combined into a single variable because of the similarities in the measurements. To account for possible seasonal effects, 2 seasons were defined according to average monthly rainfall patterns for the 31-y period from 1970 to 2000 (33). The rainy season was defined as October to April (when the mean monthly rainfall was 119.8 mm), and the remainder of the year was defined as the dry season (when the mean monthly rainfall was 0.76 mm).
Individual GEE models, controlled for child age, were fit during the period of interest for all variables that were distributed significantly differently at baseline according to breastfeeding status at 150 d or factors that were shown to affect growth. Variables that were significant (P < 0.05) in the simple model were included in a multivariable GEE model. An additive SES score increased by 1 for each of the following was created: electricity, a refrigerator, stove or hotplate, or tap water in their home. Scores of 0 to 2 were categorized as “low SES” and scores of 3 to 4 were categorized as “high SES.” Those factors that remained significant were included in the final model and plausible or biologically relevant interactions with the main effect were tested. Further analysis was performed to evaluate the relation between growth and mortality by adding to the multivariable regression model a variable that designates child death and distinguishes between the last visit at which the WAZ was recorded and all prior visits. A similar model was used to evaluate whether there was any association between child growth and being lost to follow-up (LTF). Statistical analyses were performed by using SAS version 9.2 (SAS Institute Inc, Cary, NC).
RESULTS
Study population
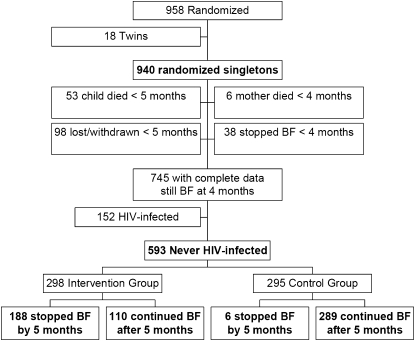
Of 958 mother-child pairs randomly assigned in the ZEBS, 593 singleton births were included in this analysis. The flow of participants and the inception of the study cohort are shown in Figure 1. The baseline characteristics of the mothers at enrollment and the infants at birth are presented in Table 1 according to the reported breastfeeding practice at 150 d. As expected, women who stopped breastfeeding their child before 150 d postpartum were significantly more likely to have been randomly assigned to the intervention group. The median age at the time that breastfeeding was stopped was 4 mo for children weaned before 5 and 15 mo for those who continued breastfeeding. Women who stopped breastfeeding early were significantly older, and there were small differences in CD4 count and viral load. Duration of breastfeeding was not associated with initiation of antiretroviral therapy prenatally or within 24 mo of the child's birth, socioeconomic factors (eg, having electricity, a refrigerator, indoor tap water, or a stove or hotplate in the home), or location or mode of delivery.
FIGURE 1.
Flow chart detailing the number of randomized subjects excluded from the analysis, the reason for their exclusion, and the distribution of included subjects by randomization group and actual breastfeeding (BF) status at 5 mo.
TABLE 1.
Baseline characteristics of 593 HIV-exposed uninfected singleton infants by breastfeeding status at 150 d regardless of randomization group
| Characteristic | Stopped breastfeeding1 (n = 194) | Continued breastfeeding1 (n = 399) | P value2 |
| Randomization group [n (%)] | |||
| Intervention | 188 (96.9) | 110 (27.6) | |
| Control | 6 (3.1) | 289 (72.4) | <0.0001 |
| Duration of breastfeeding (mo)3 | 4.2 (0.2) | 15.4 (8.2) | |
| Age (y) | 27.1 ± 5.54 | 26.2 ± 5.2 | 0.04 |
| Female sex [n (%)] | 92 (47.4) | 186 (46.6) | 0.85 |
| Maternal CD4 count (cells/mm3)3 | 348 (285) | 381 (262) | |
| <200 cells/mm3 [n (%)] | 45 (23.2) | 56 (14.1) | |
| 200–349 cells/mm3 [n (%)] | 55 (28.4) | 113 (28.5) | |
| ≥350 cells/mm3 [n (%)] | 94 (48.5) | 228 (57.4) | 0.02 |
| Maternal plasma viral load (copies/mL)3 | 34,438 (115,375) | 24,984 (63,062) | |
| <10,000 copies/mL [n (%)] | 57 (29.4) | 126 (31.7) | |
| 10,000–99,999 copies/mL [n (%)] | 84 (43.3) | 203 (51) | |
| ≥100,000 copies/mL [n (%)] | 53 (27.3) | 69 (17.3) | 0.02 |
| Birth weight (g) | 3025.1 ± 498.2 | 3064.4 ± 424 | 0.35 |
| <2500 g [n (%)] | 18 (9.4) | 30 (7.7) | |
| ≥2500 g [n (%)] | 174 (90.6) | 359 (92.3) | 0.5 |
| Hemoglobin <10 g/dL) [n (%)] | 54 (28) | 90 (22.8) | 0.17 |
| Antiretroviral therapy initiated prenatally or within 24 mo of child's birth [n (%)] | 24 (12.4) | 47 (11.8) | 0.84 |
| Maternal BMI <18.5 kg/m2 at 1 mo postpartum [n (%)] | 29 (15.5) | 43 (11.8) | 0.21 |
| Marital status | |||
| Married or living with father | 172 (88.7) | 354 (88.7) | |
| Single, not living with father | 12 (6.2) | 29 (7.3) | |
| Widowed/divorced/separated, not living with father | 10 (5.2) | 16 (4) | 0.74 |
| Education <8 y [n (%)] | 100 (51.6) | 223 (55.9) | 0.32 |
| Domestic water source [n (%)] | |||
| Tap within dwelling | 17 (8.8) | 21 (5.3) | |
| Outdoor/community tap or other | 177 (91.2) | 378 (94.7) | 0.1 |
| Electricity in the home [n (%)] | 86 (44.3) | 150 (37.6) | 0.12 |
| Refrigerator in the home [n (%)] | 29 (15) | 52 (13) | 0.51 |
| Cooking facilities [n (%)] | |||
| Stove or hot plate | 76 (39.2) | 134 (33.6) | |
| Charcoal or wood | 118 (60.8) | 264 (66.2) | 0.33 |
| Socioeconomic status [n (%)]5 | |||
| Low | 162 (83.5) | 340 (85.2) | |
| High | 32 (16.5) | 59 (14.8) | 0.59 |
| ≥1 Previous child died [n (%)]6 | 71 (42.5) | 141 (40.6) | 0.69 |
| Disclosed HIV status to partner [n (%)] | 97 (50) | 207 (51.9) | 0.86 |
| Reported no food at home on ≥1 d in past month [n (%)] | 47 (24.2) | 86 (21.6) | 0.25 |
| Full-time paid job [n (%)] | 18 (9.3) | 25 (6.3) | 0.18 |
| Place of birth [n (%)] | |||
| Home | 14 (7.2) | 41 (10.3) | |
| Clinic, hospital, other | 180 (92.8) | 358 (89.7) | 0.23 |
Based on maternal report of actual breastfeeding practice at 150 d.
Derived by using a chi-square test or Fisher's exact test for cell counts <5.
Values are medians; interquartile ranges in parentheses.
Mean ± SD (all such values).
An additive variable combining domestic water source, electricity in the home, refrigerator in the home, and cooking facility. The score was increased by 1 if the participant had electricity, a refrigerator, stove or hot plate, or tap water in her home. Scores of 0 to 2 were categorized as “low socioeconomic status,” and scores of 3 to 4 were categorized as “high socioeconomic status.”
Of women who had at least one live birth.
Breastfeeding practice and anthropometric indicators
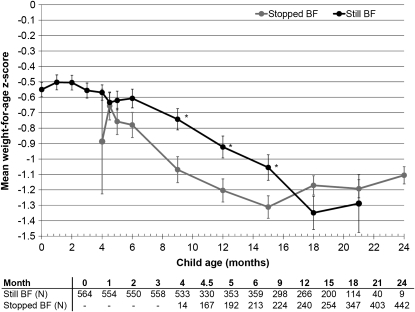
The mean baseline WAZ was −0.55 (95% CI: −0.642, −0.458) overall when all infants were still breastfeeding. WAZ declined precipitously between 4.5 and 15 mo of age in all children, but the decline was lessened among the breastfed (Figure 2 and Table 2). There was a slight recovery of mean WAZ after 15 mo among those who stopped breastfeeding and after 18 mo among those who continued to breastfeed. There was no difference in WAZs between children still being breastfed or not at 18 or 21 mo. In addition, there were no significant differences in mean WAZ between children who were randomly assigned to the intervention or control group presumably because of dilution by actual behaviors because all means were in the expected direction (Table 2).
FIGURE 2.
Mean (±SE) weight-for-age z score among 593 uninfected children born to HIV-infected mothers at each follow-up visit by actual breastfeeding (BF) practice at the time of each visit. Significant differences between groups at a single visit using the t test are indicated with asterisks (P = 0.003, 0.007, and 0.02 at 9, 12, and 15 mo, respectively). Sample sizes are presented below the graph, and the mean is plotted only when the sample size in groups exceeded 10.
TABLE 2.
Mean weight-for-age z score (WAZ) of 593 uninfected children born to HIV-infected mothers by breastfeeding status at the time of each visit and by random assignment, including the percentage of subjects moderately to severely malnourished (WAZ < −2) by breastfeeding status at the time of visit
| As practiced |
As randomized |
|||||
| Stopped breastfeeding |
Continued breastfeeding |
Intervention group |
Control group |
|||
| Age | WAZ1 | WAZ < −2 | WAZ1 | WAZ < −2 | WAZ1 | WAZ1 |
| % | % | |||||
| 0 mo | — | — | −0.55 ± 0.05 (564) | 7.8 | −0.57 ± 0.07 (284) | −0.53 ± 0.06 (280) |
| 1 mo | — | — | −0.50 ± 0.05 (554) | 8.7 | −0.47 ± 0.07 (278) | −0.53 ± 0.07 (276) |
| 2 mo | — | — | −0.50 ± 0.05 (550) | 8.0 | −0.50 ± 0.07 (279) | −0.51 ± 0.07 (271) |
| 3 mo | — | — | −0.56 ± 0.05 (558) | 10.4 | −0.58 ± 0.07 (282) | −0.53 ± 0.07 (276) |
| 4 mo | −0.89 ± 0.34 (14) | 14.3 | −0.57 ± 0.05 (533) | 9.4 | −0.61 ± 0.07 (274) | −0.55 ± 0.07 (273) |
| 4.5 mo | −0.66 ± 0.09 (167) | 11.4 | −0.63 ± 0.06 (330) | 10.9 | −0.65 ± 0.07 (248) | −0.63 ± 0.07 (249) |
| 5 mo | −0.76 ± 0.09 (192) | 10.4 | −0.62 ± 0.06 (353) | 10.8 | −0.68 ± 0.07 (267) | −0.66 ± 0.07 (278) |
| 6 mo | −0.78 ± 0.08 (213) | 13.1 | −0.61 ± 0.06 (359) | 10.6 | −0.72 ± 0.07 (287) | −0.62 ± 0.07 (285) |
| 9 mo | −1.07 ± 0.08 (224) | 24.1 | −0.74 ± 0.07 (298)2 | 15.4 | −0.92 ± 0.08 (267) | −0.84 ± 0.08 (255) |
| 12 mo | −1.20 ± 0.07 (240) | 24.6 | −0.92 ± 0.07 (266)2 | 15.8 | −1.11 ± 0.07 (261) | −1.00 ± 0.08 (245) |
| 15 mo | −1.31 ± 0.07 (254) | 27.6 | −1.06 ± 0.08 (200)2 | 21.5 | −1.22 ± 0.07 (239) | −1.17 ± 0.08 (215) |
| 18 mo | −1.17 ± 0.06 (347) | 21.9 | −1.35 ± 0.11 (114) | 34.2 | −1.19 ± 0.08 (237) | −1.24 ± 0.08 (224) |
| 21 mo | −1.19 ± 0.06 (403) | 23.1 | −1.29 ± 0.19 (40) | 27.5 | −1.19 ± 0.07 (224) | −1.21 ± 0.09 (219) |
| 24 mo | −1.11 ± 0.06 (442) | 20.1 | −1.31 ± 0.45 (9) | 33.3 | −1.05 ± 0.08 (229) | −1.17 ± 0.08 (222) |
All values are means ± SEs; n in parentheses.
Significantly different from stopped breastfeeding within a single age category, P < 0.05 (t test).
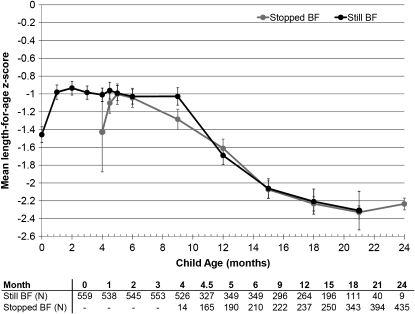
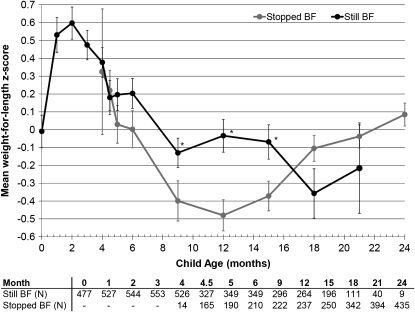
Breastfeeding practice at a particular visit was not associated with LAZ at the same visit, but LAZ also declined with child age from −0.98 at 1 mo to −2.24 at 24 mo (Figure 3). Mean WLZ exhibited a similar decline to WAZ among subjects who stopped breastfeeding between 4.5 and 12 mo with a subsequent recovery during the second year (Figure 4).
FIGURE 3.
Mean (±SE) length-for-age z score among 593 uninfected children born to HIV-infected mothers at each follow-up visit by actual breastfeeding (BF) practice at the time of each visit. No significant differences were observed (t test). Sample sizes are presented below the graph, and the mean is plotted only when the sample size in groups exceeded 10.
FIGURE 4.
Mean (±SE) weight-for-length z score among 593 uninfected children born to HIV-infected mothers at each follow-up visit by actual breastfeeding (BF) practice at the time of each visit. Significant differences between groups at a single visit are indicated by asterisks (P = 0.05, 0.0005, and 0.02 at 9, 12, and 15 mo, respectively; t test). Sample sizes are presented below the graph, and the mean is plotted only when the sample size in groups exceeded 10.
The occurrence of moderate-to-severe malnutrition, defined as a WAZ < −2, was greater at each visit among children who stopped breastfeeding from 4.5 to 15 mo. Among those subjects who had not experienced moderate-to-severe malnutrition by 4 mo, 40.5% became moderately to severely malnourished by 24 mo (data not shown).
Reverse causality
To assess whether child growth determines subsequent breastfeeding practice, the Kaplan-Meier method was used to compare age of breastfeeding cessation by birth weight and WAZ at 3 mo. The duration of breastfeeding for low-birth-weight (<2500 g) children was similar to that of normal-birth-weight children. During the first year, WAZ was not associated with breastfeeding duration; however, children who were moderately to severely malnourished (WAZ < −2) at 3 mo were more likely to continue breastfeeding during the second year (log-rank, P = 0.03). Similarly, of the 200 children still breastfeeding at 15 mo, those who were moderately to severely malnourished at that time point were more likely to continue breastfeeding during the next 3–5 mo (Wilcoxon test, P = 0.04).
Other factors associated with growth
Cross-sectional WAZs at 1, 6, 12, 18, and 24 mo based on maternal characteristics at enrollment and infant characteristics at birth are shown in Table 3. These time points were chosen for inclusion in the table as evenly spaced and clinically meaningful time points. Statistical significance was established by using a bivariate GEE model that controlled for child age. The presence of electricity, a refrigerator, tap water, or a stove or hotplate within the home was associated with significantly higher WAZs. Mothers with >8 y of education or who were married had significantly heavier children, whereas mothers who had a viral load >100,000 copies/mL or a body mass index (BMI; in kg/m2) <18.5 1 mo postpartum had significantly lighter infants. Low-birth-weight infants had significantly lower WAZs throughout the 2-y period. The maternal CD4 count had a positive effect on WAZ but was not statistically significant, whereas child sex, parity, food insecurity at baseline, maternal hemoglobin, full-time paid employment, and initiation of antiretroviral therapy had no effect on WAZ.
TABLE 3.
Mean weight-for-age z score (WAZ) of 593 uninfected children born to HIV-infected mothers by independent predictors of child growth at 1, 6, 12, 18, and 24 mo of age regardless of breastfeeding status
| 1 mo |
6 mo |
12 mo |
18 mo |
24 mo |
|||||||
| Characteristic | n | Mean WAZ | n | Mean WAZ | n | Mean WAZ | n | Mean WAZ | n | Mean WAZ | P value1 |
| Infant sex | |||||||||||
| Male | 297 | −0.53 | 303 | −0.73 | 267 | −1.10 | 247 | −1.29 | 243 | −1.16 | |
| Female | 257 | −0.47 | 269 | −0.61 | 239 | −1.01 | 214 | −1.13 | 208 | −1.05 | 0.24 |
| Maternal CD4 count | |||||||||||
| <200 cells/mm3 | 93 | −0.60 | 97 | −0.83 | 84 | −1.13 | 78 | −1.13 | 78 | −1.07 | |
| 200–349 cells/mm3 | 160 | −0.65 | 162 | −0.75 | 139 | −1.04 | 126 | −1.34 | 121 | −1.15 | |
| ≥350 cells/mm3 | 299 | −0.40 | 311 | −0.59 | 281 | −1.05 | 256 | −1.18 | 250 | −1.11 | 0.08 |
| Maternal plasma viral load | |||||||||||
| <10,000 copies/mL | 172 | −0.32 | 176 | −0.58 | 163 | −1.00 | 149 | −1.13 | 149 | −0.98 | |
| 10,000–99,999 copies/mL | 267 | −0.50 | 275 | −0.58 | 243 | −0.96 | 226 | −1.14 | 213 | −1.09 | |
| ≥100,000 copies/mL | 114 | −0.78 | 120 | −1.03 | 99 | −1.40 | 85 | −1.58 | 88 | −1.38 | 0.0007 |
| Birth weight | |||||||||||
| <2500 g | 46 | −2.19 | 46 | −1.65 | 39 | −1.71 | 36 | −2.04 | 37 | −1.79 | |
| ≥2500 g | 497 | −0.33 | 515 | −0.58 | 460 | −0.99 | 419 | −1.13 | 407 | −1.04 | <0.0001 |
| Hemoglobin | |||||||||||
| <10 g/dL | 131 | −0.55 | 137 | −0.67 | 116 | −0.96 | 111 | −1.20 | 103 | −1.05 | |
| ≥10 g/dL | 417 | −0.49 | 429 | −0.68 | 384 | −1.08 | 346 | −1.23 | 343 | −1.13 | 0.4 |
| Maternal BMI 1 mo postpartum | |||||||||||
| <18.5 kg/m2 | 72 | −0.76 | 68 | −1.09 | 60 | −1.38 | 53 | −1.51 | 52 | −1.52 | |
| ≥18.5 kg/m2 | 481 | −0.46 | 466 | −0.61 | 414 | −1.01 | 380 | −1.16 | 374 | −1.04 | 0.0007 |
| Marital status | |||||||||||
| Married or living with father | 495 | −0.48 | 505 | −0.63 | 449 | −1.00 | 413 | −1.17 | 404 | −1.07 | |
| Single, not living with father | 38 | −0.80 | 41 | −1.18 | 35 | −1.69 | 28 | −1.78 | 26 | −1.64 | |
| Widowed, divorced, or separated and not living with father | 21 | −0.56 | 26 | −0.61 | 22 | −1.17 | 20 | −1.45 | 21 | −1.24 | 0.03 |
| Education | |||||||||||
| ≤8 y | 298 | −0.61 | 310 | −0.80 | 281 | −1.17 | 251 | −1.36 | 249 | −1.22 | |
| >8 y | 256 | −0.39 | 262 | −0.53 | 225 | −0.92 | 210 | −1.04 | 202 | −0.97 | 0.003 |
| Domestic water source | |||||||||||
| Tap within dwelling | 36 | −0.09 | 36 | −0.13 | 34 | −0.62 | 33 | −0.65 | 32 | −0.59 | |
| Outdoor/community tap or other | 518 | −0.53 | 536 | −0.71 | 472 | −1.09 | 428 | −1.26 | 419 | −1.15 | 0.002 |
| Electricity in the home | |||||||||||
| Yes | 222 | −0.31 | 225 | −0.46 | 197 | −0.83 | 183 | −0.97 | 179 | −0.84 | |
| No | 332 | −0.63 | 347 | −0.81 | 309 | −1.20 | 278 | −1.38 | 272 | −1.29 | <0.0001 |
| Refrigerator in the home | |||||||||||
| Yes | 77 | −0.16 | 76 | −0.34 | 62 | −0.45 | 56 | −0.73 | 58 | −0.67 | |
| No | 476 | −0.56 | 495 | −0.72 | 443 | −1.14 | 404 | −1.28 | 392 | −1.17 | <0.0001 |
| Cooking facilities | |||||||||||
| Stove or hot plate | 197 | −0.26 | 199 | −0.46 | 175 | −0.84 | 160 | −0.96 | 158 | −0.85 | |
| Charcoal or wood | 357 | −0.64 | 372 | −0.79 | 330 | −1.17 | 300 | −1.34 | 292 | −1.24 | <0.0001 |
| Season | |||||||||||
| Dry, May–September | 221 | −0.43 | 231 | −0.67 | 215 | −0.89 | 175 | −1.10 | 188 | −1.03 | |
| Rainy, October–April | 333 | −0.55 | 341 | −0.67 | 291 | −1.18 | 286 | −1.29 | 263 | −1.17 | <0.0001 |
Derived by using the type 3 score statistic from bivariate generalized estimating equation models for 0 to 24 mo, with control for child age and repeated measures.
Adjustment for confounding
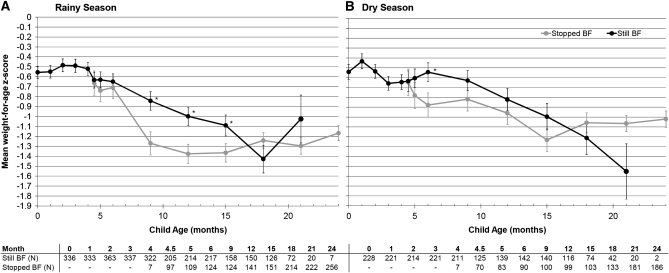
The period of WAZ decline between 4.5 and 15 mo was described by using GEE models. First, a simple model was used to assess the association of each variable with WAZ while the precipitous decline over time was controlled for by including the child's age in the model. The variables that were independently associated with WAZ are shown in Table 4. Next, each variable was added to the model including breastfeeding practice to determine whether, alone or together, these factors accounted for the association between breastfeeding and WAZ. The absence of breastfeeding was associated with a significant decrease in WAZ of 0.28 (95% CI: 0.39, 0.17) when season, birth weight, maternal viral load, BMI, marital status, education, and SES were controlled for. The benefit of breastfeeding was more pronounced in the rainy season (October–April), when it was associated with a 0.33 (95% CI: 0.21, 0.46) increase in WAZ. A smaller but still significant benefit was observed in the dry season, when breastfeeding was associated with 0.22 (95% CI: 0.1, 0.34) increase in WAZ (Figure 5; P for interaction = 0.02) after adjustment for all of the factors shown in Table 4.
TABLE 4.
Generalized estimating equation regression model that assessed the association between weight-for-age z score (WAZ) and breastfeeding practice at the time of visit for HIV-exposed children aged 4.5–15 mo
| Simple model1 |
Multivariable model2 |
|||||
| Category | Estimate | SE | P value3 | Estimate | SE | P value3 |
| Intercept | — | — | — | −0.1349 | 0.0812 | 0.0966 |
| Age, mo | −0.0595 | 0.0044 | <0.0001 | −0.0517 | 0.0045 | <0.0001 |
| Stopped breastfeeding | −0.2893 | 0.0574 | <0.0001 | −0.2821 | 0.0564 | <0.0001 |
| Rainy season, October–April | −0.1972 | 0.0250 | <0.0001 | −0.2026 | 0.0253 | <0.0001 |
| Viral load ≥100,000 copies/mL | −0.4391 | 0.1119 | <0.0001 | −0.2836 | 0.1098 | 0.0098 |
| Birth weight <2500 g | −1.0608 | 0.1594 | <0.0001 | −0.8724 | 0.1475 | <0.0001 |
| Maternal BMI <18.5 kg/m2 at 1 mo postpartum | −0.4045 | 0.1264 | 0.0014 | −0.2585 | 0.1229 | 0.0354 |
| Not married, widowed, divorced, or separated and not living with partner | −0.3404 | 0.1400 | 0.0150 | −0.2864 | 0.1442 | 0.0471 |
| Maternal education, high school or more | 0.2598 | 0.0867 | 0.0027 | 0.2233 | 0.0873 | 0.0106 |
| High socioeconomic status4 | 0.4951 | 0.1203 | <0.0001 | 0.3760 | 0.1223 | 0.0021 |
The simple model assessed the association between WAZ and each variable individually with inclusion of age to account for time.
The multivariable model assessed the association between WAZ and breastfeeding status with control for age, season, maternal viral load, birth weight, maternal BMI, marital status, education, and socioeconomic status.
Derived by using the generalized estimating equation z statistic.
An additive variable that included domestic water source, electricity in the home, refrigerator in the home, and cooking facility. The score was increased by 1 if the participant had electricity, a refrigerator, stove or hot plate, or tap water in her home. Scores of 0 to 2 were categorized as “low socioeconomic status,” and scores of 3 to 4 were categorized as “high socioeconomic status.”
FIGURE 5.
Mean (±SE) weight-for-age z scores of uninfected children born to HIV-infected mothers at each follow-up visit by actual breastfeeding (BF) practice at the time of each visit during the rainy (A) and dry (B) seasons. Significant differences between groups at a single visit are indicated by asterisks (dry season, 6 mo: P = 0.03; rainy season, 9, 12, and 15 mo: P = 0.004, 0.005, and 0.05 respectively; t test). The interaction between BF practice and season in the generalized estimating equation regression model that assessed the association between weight-for-age z score and BF practice at time of visit for children aged 4.5–15 mo was significant using the z statistic (P = 0.02) after age, birth weight, maternal viral load, BMI, marital status, education, and socioeconomic status were controlled for. Sample sizes are presented below each graph, and the mean is plotted only when the sample size in groups exceeded 10.
Growth and mortality
Further analysis was performed to evaluate the relation between growth and mortality. The last recorded WAZ before the death of the 51 children who died between 4.5 and 15 mo was, on average, 0.88 (95% CI: 0.54, 1.21) lower than that for children who survived (P < 0.0001), whereas the WAZs at all other visits were, on average, 0.38 (95% CI: 0.12, 0.65) lower than that for children who survived (P = 0.005) after age, breastfeeding status, season, birth weight, maternal viral load, maternal BMI, marital status, education, and SES were controlled for. The last recorded WAZ for 40 children who were LTF between 4.5 and 15 mo was not significantly different from the WAZ of children who were not LTF, and their WAZ measurements at all other visits were not significantly different from those of children who were not LTF after the same covariates were controlled for (P = 0.85 and 0.13, respectively).
DISCUSSION
Early growth was substantially compromised in this sample of HIV-uninfected children born to HIV-infected mothers in a low-resource setting when compared with international growth standards. More than 40% of the study participants experienced moderate-to-severe malnutrition by age 2 y. Continued breastfeeding partially mitigated this through 15 mo. The decline in weight gain and the increased risk of malnutrition in HIV-exposed uninfected children occurred despite provision of replacement food for ≥3 mo to children in the intervention group who stopped breastfeeding early.
A strong association between malnutrition and mortality as well as many other long-term adverse outcomes is well established in the literature (34–36). Early childhood malnutrition is associated with decreased cognitive development in the absence of remediation (37). Poor growth in early childhood has also been associated with decreased educational achievement, decreased productivity in jobs requiring manual labor, and lower birth weight in the next generation (37).
In addition to poor weight gain, we also found marked declines in linear growth as compared with the age and sex-adjusted World Health Organization norms. These trends in growth are comparable with those of urban Zambian children reported before the HIV epidemic who were breastfed to a median age of 18.3 mo, whose WAZs and LAZs declined between 6 and 23 mo of age (38). Similarly, poor patterns of linear and ponderal growth among children born to HIV-infected mothers have been reported in other studies conducted in low-resource settings. A decline in WAZs and LAZs was reported in HIV-exposed uninfected children in South Africa and the Congo, which was punctuated by a recovery in WAZ from 15 to 18 mo in South African children similar to that seen in our study and to WAZ recovery from 16 to 20 mo in Congolese children (19, 20). A precipitous decline in LAZ during the first 2 y of life was also observed in a recent cohort of 886 Tanzanian infants; however, WAZ was not reported in the study (28).
In the current study, we found that partial breastfeeding continued to be beneficial for the growth of children born to HIV-infected mothers to ≥15 mo. Ascertainment of the temporal relation between declining growth and prolonged breastfeeding is difficult. In our data, there was a weak association between continued breastfeeding after 12 mo and lower WAZ at 3 mo, similar to other reports that suggest that women prolong breastfeeding for undernourished children (39, 40). This association would tend to bias results away from showing a protective effect of breastfeeding and thus strengthens our inference that continued breastfeeding has a direct protective effect on growth outcomes. Although our data showed some recovery of WAZ after 18 mo, mean z scores did not return to levels attained during the first 6 mo, when most infants were predominantly or exclusively breastfed. Our results clearly show that breastfeeding mitigates the decline in somatic growth to 15 mo; however, the lack of continued benefit thereafter may have been due in part to “reverse causality,” because longer breastfeeding occurred among those failing to thrive at 15 mo.
Whereas our main aim was not to evaluate predictors of early childhood growth, we identified many important environmental and social factors that have implications for clinical and public health practices. We found that children of mothers who were better educated or married also had improved growth. The positive association of growth and maternal education has been reported previously in developing countries, including among children born to HIV-infected mothers (41–44). We also found that other variables related to better socioeconomic position, namely the presence of electricity, tap water, a refrigerator, or a stove or hotplate in the home were strongly associated with better child growth. The importance of socioeconomic and environmental factors on child nutrition is well established. Children from poorer households may have inadequate or inconsistent access to complementary food (food insecurity), which leads to growth faltering during the second half of infancy (18, 45, 46). Also, unsafe household water and poor hygiene likely confer an independent risk of infection and subsequent malnutrition (47–50). The rainy season appears to present a distinct hazard to growth in the young children in our study. Others have also reported a strong negative association between season and growth in similar settings in Africa (51, 52) and other low-resource settings (53–56). It appears that not breastfeeding during the rainy months poses a distinctly higher risk of malnutrition than it does during the dry months, although the risk is significant during both seasons. The observed association and interaction between season and breastfeeding is likely a small part of a larger web of associations between the physical and social environment, infectious diseases, breastfeeding, and childhood growth.
We found that indicators of advanced maternal HIV disease, including an HIV viral load >100,000 copies/mL and a low BMI, were associated with poor child growth consistent with earlier publications (57). The possible underlying causes are not known but include compromised caregiving capacity due to poor maternal health (23, 58) and possibly the negative effects of in utero milieu on infant immune function that may render them more vulnerable to infection (59). An association between worse LAZs and WLZs and a low maternal CD4 count has also been reported from a study in Tanzania (28). This study, however, did not distinguish by method of feeding. Because our study was conducted before the availability of antiretroviral medications and only the minority of women who were among the last enrolled in the study received treatment for HIV, our ability to rigorously assess the potential effect of maternal antiretroviral medications on growth was limited. Whether suppression of HIV replication and improvements in maternal health resulting from antiretroviral medications will improve postnatal growth remains to be determined.
Our study had many potentially important limitations. In this study, apart from breastfeeding practices, insufficient data were collected on other aspects of dietary intake beyond 6 mo, so we are unable to address potential nutritional explanations for the “catch up” growth that was manifest toward the end of the second year of life. Measurements of head circumference, a relevant outcome, were not ascertained and changes in unmeasured exposures such as local food availability in the study area could have affected the growth of the children in our study.
As interventions for decreasing breastfeeding-associated HIV transmission advance, the importance of improving other aspects of the health of exposed-uninfected infants becomes increasingly apparent. Future studies need to confirm our findings in other settings and should evaluate the benefits of prolonged breastfeeding, including family planning, reductions in infectious diseases, and the possible risk of HIV transmission, to help direct clinical practice and public health guidelines.
Our findings indicate that continued breastfeeding through the second year of life mitigates some of the decline in nutritional status that occurs once breast milk is no longer the only or predominant source of the child's nutrition. However, because breastfeeding cannot provide for all of the child's nutritional needs at this age, it is essential that nutritional and educational interventions to complement, rather than replace, breast milk in HIV-exposed infants in low-resource settings be offered and evaluated.
Supplementary Material
Acknowledgments
We thank the Zambian families who participated in the research and all the study staff and volunteers. We also thank the following people who assisted with aspects of the study: Elwyn Chomba, Marc Bulterys, Chewe Luo, Lynne Mofenson, Ellen Piwoz, and Kevin Ryan.
The authors' responsibilities were as follows—SA, LK, DMT, and GMA: conceptualization and design; MS, CK, MM, DMT, and LK: study implementation and management; GMA: laboratory assays; SA, AF, and LK: analysis and statistics; and SA and AF: draft of the manuscript. All authors: interpretation of data and revision of manuscript for critical content. None of the authors had a personal or financial conflict of interest.
REFERENCES
- 1.Coutsoudis A, Pillay K, Spooner E, Kuhn L, Coovadia H. Influence of infant-feeding patterns on early mother-to-child transmission of HIV-1 in Durban, South Africa: a prospective cohort study. South African Vitamin A Study Group. Lancet 1999;354:471–6 [DOI] [PubMed] [Google Scholar]
- 2.Iliff PJ, Piwoz E, Tavengwa N, et al. Early exclusive breastfeeding reduces the risk of postnatal HIV-1 transmission and increases HIV-free survival. AIDS 2005;19:699–708 [DOI] [PubMed] [Google Scholar]
- 3.Coovadia HM, Rollins N, Bland R, et al. Mother-to-child transmission of HIV-1 infection during exclusive breastfeeding in the first 6 months of life: an intervention cohort study. Lancet 2007;369:1107–16 [DOI] [PubMed] [Google Scholar]
- 4.Kuhn L, Sinkala M, Kankasa C, et al. High uptake of exclusive breastfeeding and reduced early post-natal HIV transmission. PLoS One 2007;2:e1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuhn L, Aldrovandi GM, Sinkala M, et al. Effects of early, abrupt weaning on HIV-free survival of children in Zambia. N Engl J Med 2008;359:130–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization Global strategy for infant and young child feeding. Geneva, Switzerland: World Health Organization, 2003 [Google Scholar]
- 7.World Health Organization Infant and young child feeding counselling: an integrated course. Geneva, Switzerland: World Health Organization, 2006 [Google Scholar]
- 8.World Health Organization Integrated Management of Childhood Illness (IMCI). Geneva, Switzerland: Division of Child and Adolescent Health and Development, World Health Organization, 2005 [Google Scholar]
- 9.Gartner LM, Morton J, Lawrence R, et al. Breastfeeding and the use of human milk. Pediatrics 2005;115:496–506 [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization WHO HIV and infant feeding technical consultation consensus statement. Geneva, Switzerland: World Health Organization, 2006 [Google Scholar]
- 11.Howie PW, Forsyth J, Ogston S, Clark A, Florey C. Protective effect of breast feeding against infection. BMJ 1990;300:11–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.César JA, Victora C, Barros F, Santos I, Flores J. Impact of breast feeding on admission for pneumonia during postneonatal period in Brazil: nested case-control study. BMJ 1999;318:1316–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fewtrell M. The long-term benefits of having been breast-fed. Curr Paediatrics 2004;14:97–103 [Google Scholar]
- 14.Aniansson G, Alm B, Andersson B, et al. A prospective cohort study on breast-feeding and otitis media in Swedish infants. Pediatr Infect Dis J 1994;13:183–8 [DOI] [PubMed] [Google Scholar]
- 15.Anderson JW, Johnstone B, Remley D. Breast-feeding and cognitive development: a meta-analysis. Am J Clin Nutr 1999;70:525–35 [DOI] [PubMed] [Google Scholar]
- 16.Quigley MA, Kelly Y, Sacker A. Breastfeeding and hospitalization for diarrheal and respiratory infection in the United Kingdom Millennium Cohort Study. Pediatrics 2007;119:e837–42 [DOI] [PubMed] [Google Scholar]
- 17.Onyango AW, Esrey S, Kramer M. Continued breastfeeding and child growth in the second year of life: a prospective cohort study in western Kenya. Lancet 1999;354:2041–5 [DOI] [PubMed] [Google Scholar]
- 18.Fawzi WW, Herrera M, Nestel P, el Amin A, Mohamed K. A longitudinal study of prolonged breastfeeding in relation to child undernutrition. Int J Epidemiol 1998;27:255–60 [DOI] [PubMed] [Google Scholar]
- 19.Bobat R, Coovadia H, Moodley D, Coutsoudis A, Gouws E. Growth in early childhood in a cohort of children born to HIV-1-infected women from Durban, South Africa. Ann Trop Paediatr 2001;21:203–10 [DOI] [PubMed] [Google Scholar]
- 20.Bailey RC, Kamenga M, Nsuami M, Nieburg P, St Louis M. Growth of children according to maternal and child HIV, immunological and disease characteristics: a prospective cohort study in Kinshasa, Democratic Republic of Congo. Int J Epidemiol 1999;28:532–40 [DOI] [PubMed] [Google Scholar]
- 21.Villamor E, Mbise R, Spiegelman D, et al. Vitamin A supplements ameliorate the adverse effect of HIV-1, malaria, and diarrheal infections on child growth. Pediatrics 2002;109:E6. [DOI] [PubMed] [Google Scholar]
- 22.Lepage P, Msellati P, Hitimana D, et al. Growth of human immunodeficiency type 1-infected and uninfected children: a prospective cohort study in Kigali, Rwanda, 1988 to 1993. Pediatr Infect Dis J 1996;15:479–85 [DOI] [PubMed] [Google Scholar]
- 23.Thea DM, St Louis M, Atido U, et al. A prospective study of diarrhea and HIV-1 infection among 429 Zairian infants. N Engl J Med 1993;329:1696–702 [DOI] [PubMed] [Google Scholar]
- 24.Kagaayi J, Gray R, Brahmbhatt H, et al. Survival of infants born to HIV-positive mothers, by feeding modality, in Rakai, Uganda. PLoS One 2008;3:e3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thior I, Lockman S, Smeaton L, et al. Breastfeeding plus infant zidovudine prophylaxis for 6 months vs formula feeding plus infant zidovudine for 1 month to reduce mother-to-child HIV transmission in Botswana: a randomized trial: the Mashi Study. JAMA 2006;296:794–805 [DOI] [PubMed] [Google Scholar]
- 26.Atashili J, Kalilani L, Seksaria V, Sickbert-Bennett E. Potential impact of infant feeding recommendations on mortality and HIV-infection in children born to HIV-infected mothers in Africa: a simulation. BMC Infect Dis 2008;8:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taha TE, Kumwenda N, Hoover D, et al. The impact of breastfeeding on the health of HIV-positive mothers and their children in sub-Saharan Africa. Bull World Health Organ 2006;84:546–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Webb AL, Manji K, Fawzi W, Villamor E. Time-independent maternal and infant factors and time-dependent infant morbidities including HIV infection, contribute to infant growth faltering during the first 2 years of life. J Trop Pediatr 2009;55:83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumwenda NI, Hoover D, Mofenson L, et al. Extended antiretroviral prophylaxis to reduce breast-milk HIV-1 transmission. N Engl J Med 2008;359:119–29 [DOI] [PubMed] [Google Scholar]
- 30.Six Week Extended-Dose Nevirapine (SWEN) Study Team Bedri A, Gudetta B, et al. Extended-dose nevirapine to 6 weeks of age for infants to prevent HIV transmission via breastfeeding in Ethiopia, India, and Uganda: an analysis of three randomised controlled trials. Lancet 2008;372:300–13 [DOI] [PubMed] [Google Scholar]
- 31.Thea DM, Vwalika C, Kasonde P, et al. Issues in the design of a clinical trial with a behavioral intervention—the Zambia exclusive breast-feeding study. Control Clin Trials 2004;25:353–65 [DOI] [PubMed] [Google Scholar]
- 32.WHO Multicentre Growth Reference Study Group WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for height and body mass index-for-age: methods and development. Geneva, Switzerland: World Health Organization, 2006 [Google Scholar]
- 33.Zambia Meteorological Department. World Weather Information Service Lusaka, Zambia: World Weather Information Service; Version current 9 March 2009. Available from: http://www.worldweather.org/040/c00150.htm (cited 9 March 2009) [Google Scholar]
- 34.Caulfield LE, de Onis M, Blössner M, Black RE. Undernutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria, and measles. Am J Clin Nutr 2004;80:193–8 [DOI] [PubMed] [Google Scholar]
- 35.Pelletier DL, Frongillo E, Jr, Habicht J. Epidemiologic evidence for a potentiating effect of malnutrition on child mortality. Am J Public Health 1993;83:1130–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fawzi WW, Herrera M, Spiegelman D, el Amin A, Nestel P, Mohamed K. A prospective study of malnutrition in relation to child mortality in the Sudan. Am J Clin Nutr 1997;65:1062–9 [DOI] [PubMed] [Google Scholar]
- 37.Victora CG, Adair L, Fall C, et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet 2008;371:340–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ng'andu NH, Watts TE. Child growth and duration of breast feeding in urban Zambia. J Epidemiol Community Health 1990;44:281–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marquis GS, Habicht J, Lanata C, Black R, Rasmussen K. Association of breastfeeding and stunting in Peruvian toddlers: an example of reverse causality. Int J Epidemiol 1997;26:349–56 [DOI] [PubMed] [Google Scholar]
- 40.Simondon KB, Simondon F. Mothers prolong breastfeeding of undernourished children in rural Senegal. Int J Epidemiol 1998;27:490–4 [DOI] [PubMed] [Google Scholar]
- 41.Boyle MH, Racine Y, Georgiades K, et al. The influence of economic development level, household wealth and maternal education on child health in the developing world. Soc Sci Med 2006;63:2242–54 [DOI] [PubMed] [Google Scholar]
- 42.Villamor E, Msamanga G, Spiegelman D, et al. HIV status and sociodemographic correlates of maternal body size and wasting during pregnancy. Eur J Clin Nutr 2002;56:415–24 [DOI] [PubMed] [Google Scholar]
- 43.Albrecht S, Semrau K, Kasonde P, et al. Predictors of nonadherence to single-dose nevirapine therapy for the prevention of mother-to-child HIV transmission. J Acquir Immune Defic Syndr 2006;41:114–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van de Poel E, Hosseinpoor A, Speybroeck N, Van Ourti T, Vega J. Socioeconomic inequality in malnutrition in developing countries. Bull World Health Organ 2008;86:282–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kikafunda JK, Walker A, Collett D, Tumwine J. Risk factors for early childhood malnutrition in Uganda. Pediatrics 1998;102:E45. [DOI] [PubMed] [Google Scholar]
- 46.Van de Poel E, Hosseinpoor A, Jehu-Appiah C, Vega J, Speybroeck N. Malnutrition and the disproportional burden on the poor: the case of Ghana. Int J Equity Health 2007;6: 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oyemade A, Omokhodion F, Olawuyi J, Sridhar M, Olaseha I. Environmental and personal hygiene practices: risk factors for diarrhoea among children of Nigerian market women. J Diarrhoeal Dis Res 1998;16:241–7 [PubMed] [Google Scholar]
- 48.Muoki MA, Tumuti D, Rombo G. Nutrition and public hygiene among children under five years of age in Mukuru slums of Makadara Division, Nairobi. East Afr Med J 2008;85:386–97 [DOI] [PubMed] [Google Scholar]
- 49.D'Souza RM. Housing and environmental factors and their effects on the health of children in the slums of Karachi, Pakistan. J Biosoc Sci 1997;29:271–81 [DOI] [PubMed] [Google Scholar]
- 50.Gorter AC, Sandiford P, Pauw J, Morales P, Pérez R, Alberts H. Hygiene behaviour in rural Nicaragua in relation to diarrhoea. Int J Epidemiol 1998;27:1090–100 [DOI] [PubMed] [Google Scholar]
- 51.McGregor IA, Rahman A, Thompson B, Billewicz W, Thomson A. The growth of young children in a Gambian village. Trans R Soc Trop Med Hyg 1968;62:341–52 [DOI] [PubMed] [Google Scholar]
- 52.Maleta K, Virtanen S, Espo M, Kulmala T, Ashorn P. Seasonality of growth and the relationship between weight and height gain in children under three years of age in rural Malawi. Acta Paediatr 2003;92:491–7 [DOI] [PubMed] [Google Scholar]
- 53.Brown KH, Black R, Becker S. Seasonal changes in nutritional status and the prevalence of malnutrition in a longitudinal study of young children in rural Bangladesh. Am J Clin Nutr 1982;36:303–13 [PubMed] [Google Scholar]
- 54.Pollitt E, Arthur J. Seasonality and weight gain during the first year of life. Am J Hum Biol 1989;1:747–56 [DOI] [PubMed] [Google Scholar]
- 55.Panter-Brick C. Seasonal growth patterns in rural Nepali children. Ann Hum Biol 1997;24:1–18 [DOI] [PubMed] [Google Scholar]
- 56.Hillbruner C, Egan R. Seasonality, household food security, and nutritional status in Dinajpur, Bangladesh. Food Nutr Bull 2008;29:221–31 [DOI] [PubMed] [Google Scholar]
- 57.Kuhn L, Kasonde P, Sinkala M, et al. Does severity of HIV disease in HIV-infected mothers affect mortality and morbidity among their uninfected infants? Clin Infect Dis 2005;41:1654–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chisenga M, Kasonka L, Makasa M, et al. Factors affecting the duration of exclusive breastfeeding among HIV-infected and -uninfected women in Lusaka, Zambia. J Hum Lact 2005;21:266–75 [DOI] [PubMed] [Google Scholar]
- 59.Clerici M, Saresella M, Colombo F, et al. T-lymphocyte maturation abnormalities in uninfected newborns and children with vertical exposure to HIV. Blood 2000;96:3866–71 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.