Abstract
Background: Markers of protein-energy wasting (PEW) and inflammation are common in chronic kidney disease (CKD) and are among the strongest predictors of mortality in dialysis patients.
Objective: We hypothesized that markers of PEW and inflammation show similar associations in patients with non-dialysis-dependent CKD (NDD-CKD).
Design: We examined the associations of serum albumin, white blood cell (WBC) count, percentage of lymphocytes in WBCs (%LYM), and a combination of all 3 with all-cause mortality and with the composite of predialysis mortality or end-stage renal disease (ESRD) by using fixed-covariate and time-dependent Cox models in 1220 men with NDD-CKD.
Results: Lower albumin and %LYM and a higher WBC count were significantly associated with outcomes. In time-dependent Cox models, compared with patients in whom none of these markers indicated PEW, those in whom 1, 2, or all 3 markers indicated the presence of PEW had multivariable-adjusted hazard ratios (95% CI) for all-cause mortality of 1.7 (1.2, 2.4), 2.4 (1.7, 3.4), and 3.6 (2.5, 5.1); the P for trend was <0.001. Similar associations were present in fixed-covariate models for all-cause mortality and in fixed-covariate and time-dependent models for the composite outcome.
Conclusions: Traditional and nontraditional markers of PEW display robust, strong, and independent associations with mortality in patients with NDD-CKD. Clinical trials are warranted to examine whether PEW-improving interventions can lead to better outcomes in these patients.
INTRODUCTION
Individuals with chronic kidney disease (CKD), including a large number with non-dialysis-dependent CKD (NDD-CKD) (1), experience extremely high mortality rates (2, 3). Among the most powerful predictors of poor outcomes in epidemiologic studies are markers of nutritional deficiency and/or inflammation, both of which have been extensively studied in patients receiving maintenance dialysis treatment and which together have been referred to as the “malnutrition-inflammation-cachexia syndrome.” (4) Because of the complex nature of the processes of malnutrition and inflammation and the consequent nomenclatural confusion, an expert panel recently recommended the adoption of the term protein-energy wasting (PEW) to uniformly denote the constellation of findings associated with nutritional deficiency and wasting syndrome (5).
Several biomarkers of PEW, such as the traditionally used serum albumin, and some nontraditional markers, such as the percentage of lymphocytes (%LYM) in peripheral white blood cells (WBCs) and the total WBC count, are routinely assessed in clinical practice, are inexpensive, and are widely available and can be used in observational studies to examine outcomes associated with PEW. Such biomarkers of PEW have been shown to be robust predictors of morbidity and mortality in dialysis patients (6–13). These biomarkers, however, have not been subjected to similar research scrutiny in patients with NDD-CKD. We hypothesized that PEW biomarkers are strong outcome predictors in NDD-CKD and examined all-cause mortality and the composite of predialysis mortality or end-stage renal disease (ESRD) associated with different concentrations of serum albumin, %LYM, WBC count, and a combination of the 3 in 1220 male US veterans with NDD-CKD at a single medical center.
SUBJECTS AND METHODS
Study population and data collection
We examined 1259 outpatients evaluated and treated for NDD-CKD at Salem Veteran Affairs Medical Center (VAMC) between 1 January 1990 and 30 June 2007 and followed them until 1 April 2008. Thirteen female patients and 6 patients whose race could not be categorized as either white or black were excluded. Twenty one patients did not have all 3 of the studied PEW markers measured and were also excluded. The final study population consisted of 1220 patients.
Baseline characteristics of the population were examined at the initial encounter in the Nephrology Clinic, including demographic and anthropometric characteristics, comorbid conditions including the Charlson comorbidity index, clinical assessment-based etiology of CKD, and laboratory results, as previously detailed (3, 14). Use of medications (angiotensin-converting enzyme inhibitors or angiotensin receptor blockers angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, statins, and activated vitamin D products) was assessed over the entire follow-up period and was used as a surrogate for quality of clinical care. The glomerular filtration rate (eGFR) was estimated by using the abbreviated Modification of Diet in Renal Disease Study equation (15) and categorized according to Kidney/Dialysis Outcome Quality Initiative (K/DOQI) staging (16). Laboratory measures, including serum albumin, WBC, and %LYM, were recorded longitudinally and were averaged over 6-mo time periods for time-dependent analyses. All of the biochemical measurements were performed in a single laboratory at the Salem VAMC.
Statistical analyses
Missing data points for comorbidity index (1% missing), body mass index (BMI; 18% missing), serum phosphorus (5% missing), blood cholesterol (7% missing), hemoglobin (2% missing), and 24-h urinary protein (8% missing) were substituted by using multiple imputations. Smoking (5% missing) was analyzed by creating a dummy variable corresponding to missing smoking status. Etiologies of CKD with frequencies of <10% (including 5% glomerulonephritis, 9% chronic tubulointerstitial nephritis, and 2% hereditary diseases) were grouped together in a single category. Serum albumin, WBC, and %LYM were analyzed both as continuous variables and after categorizing patients according to the presence or absence of PEW defined as a level below or above the median value for each variable (albumin ≤ 3.7 g/dL, %LYM ≤ 22%, and WBC > 7500/mm3). The combined effect of the 3 variables was examined by categorizing patients into 4 mutually exclusive groups according to the absence of all 3 or the presence of 1, 2, or all 3 markers of PEW.
The starting time for survival analyses was the date of the first visit in the Nephrology Clinic at Salem VAMC. The main outcome measure was all-cause mortality, which was ascertained from VA electronic records (Computerized Patient Record System). The secondary outcome measure was the composite of predialysis mortality or ESRD; the latter was defined as the initiation of maintenance dialysis therapy and was ascertained from local hospital records, including Medicare Form 2728. Patients were considered lost to follow-up if no contact was documented with them for >6 mo, and they were censored at the date of the last documented contact. Associations between baseline concentrations of the PEW markers and the above outcomes were evaluated in fixed-covariate Cox models, with adjustment for potential confounders.
Time-dependent Cox models were used to capture the effect of temporal changes in PEW markers and other potential confounders. Multivariable models were constructed to adjust for potential confounders (age, race, smoking status, comorbidities, etiology of CKD, blood pressure, BMI, estimated GFR, calcium, phosphorus, bicarbonate, hemoglobin, and 24-h urinary protein). Because of collinearity between PEW markers, regression models examining associations for each individual marker excluded the other 2 markers. The nonlinearity of the associations was examined by using restricted cubic splines and by including polynomial terms. Attributable fractions (the percentage of the outcomes attributed to the exposure in exposed patients, compared with nonexposed patients) and population attributable fractions (the percentage of the outcomes attributed to the exposure in the overall population) were estimated for each categorical outcome variable. Exposure was defined as albumin ≤ 3.7 g/dL, %LYM ≤ 22%, WBC > 7500/mm3, or the presence of 1, 2, or 3 PEW markers. Attributable fractions were calculated based on incidence rate differences, and population attributable fractions were estimated from Poisson regressions (STATA “aflogit”), with CIs based on asymptotic approximations (17).
Interactions were examined in subgroup analyses and by including interaction terms for variables that showed baseline imbalance between the studied categories or that were known to be potential confounders by having been associated with both PEW independently from the biomarkers examined in this study and with clinical outcomes. Sensitivity analyses were performed by using only nonimputed values of independent variables and/or restricting analyses to a more contemporary cohort of patients enrolled after 1 January 2001. P values <0.05 were considered significant. Statistical analyses were performed by using STATA statistical software version 10 (StataCorp, College Station, TX). The study protocol was approved by the Research and Development Committee at the Salem VAMC.
RESULTS
The mean (±SD) age of the cohort was 68 ± 11 y; 24% were black, and the mean eGFR was 37 ± 17 mL · min−1 · 1.73 m−2. Most patients had CKD stages 3 (56%) and 4 (30%), with fewer patients having CKD stage 1 (1%), 2 (7%), and 5 (5%). Seven hundred thirty-five patients (60%) were enrolled after 1 January 2001. A total of 616 patients died (mortality rate: 125/1000 patient-years; 95% CI: 116, 136), and 264 reached ESRD (ESRD rate: 63/1000 patient-years; 95% CI: 56, 71) during a median follow-up of 3.3 y. Four hundred forty-four patients died before ESRD. Thirty-three patients (2.7%) were lost to follow-up; their characteristics were not significantly different (data not shown).
Baseline characteristics of patients across increments of the combination of 3 PEW markers are shown in Table 1. Patients with a higher number of PEW markers were more likely to be white, to have a higher incidence of cardiovascular disease and diabetic nephropathy, and to have a higher comorbidity index, serum phosphorus concentration, and 24-h urinary protein concentration; were less likely to have hypertensive nephrosclerosis; and had a lower diastolic blood pressure, eGFR, serum calcium concentration, and blood hemoglobin concentration. Medication use was not significantly different between the 4 groups, except for statin use, which was slightly less common in the groups with higher numbers of PEW markers.
TABLE 1.
Baseline characteristics of individuals stratified by the number of protein-energy wasting (PEW) markers present1
| Number of PEW markers of malnutrition/inflammation below or above median value |
|||||
| 0 (n = 212) | 1 (n = 447) | 2 (n = 401) | 3 (n = 160) | P for linear trend2 | |
| Age (y) | 68.2 ± 11.73 | 67.3 ± 10.7 | 69.6 ± 10.5 | 68.7 ± 10.4 | 0.09 |
| Race, black [n (% of total)] | 64 (30) | 125 (28) | 72 (18) | 34 (21) | 0.001 |
| DM [n (% of total)] | 118 (56) | 244 (55) | 208 (52) | 101 (63) | 0.4 |
| ASCVD [n (% of total)] | 109 (51) | 238 (53) | 251 (63) | 98 (61) | 0.003 |
| Smoking [n (% of total)] | 41 (20) | 117 (28) | 97 (25) | 45 (30) | 0.1 |
| Comorbidity index | 2.2 ± 1.6 | 2.3 ± 1.6 | 2.7 ± 1.8 | 2.8 ± 1.8 | <0.001 |
| Etiology of CKD [n (% of total)] | |||||
| Diabetes | 44 (21) | 138 (31) | 119 (30) | 57 (36) | 0.007 |
| Hypertension | 69 (33) | 119 (27) | 108 (27) | 27 (17) | 0.003 |
| Ischemic | 27 (13) | 49 (11) | 56 (14) | 20 (13) | 0.6 |
| Other | 65 (31) | 130 (29) | 115 (29) | 48 (30) | 0.8 |
| Calcitriol use [n (% of total)] | 69 (33) | 131 (29) | 132 (33) | 49 (31) | 0.9 |
| ACEI/ARB use [n (% of total)] | 165 (78) | 341 (76) | 303 (76) | 117 (73) | 0.3 |
| Statin use [n (% of total)] | 148 (70) | 296 (66) | 251 (63) | 99 (62) | 0.05 |
| BMI (kg/m2) | 29.2 ± 5.1 | 29.5 ± 5.7 | 29.0 ± 5.9 | 29.4 ± 7.7 | 0.7 |
| SBP (mm Hg) | 146 ± 25 | 151 ± 26 | 150 ± 27 | 147 ± 28 | 0.8 |
| DBP (mm Hg) | 73 ± 14 | 76 ± 15 | 74 ± 16 | 70 ± 16 | 0.04 |
| eGFR (mL · min−1 · 1.73 m−2) | 39.2 ± 16.8 | 38.2 ± 17.2 | 35.5 ± 15.6 | 35.8 ± 17.5 | 0.04 |
| Serum albumin (g/dL) | 4.0 ± 0.2 | 3.7 ± 0.5 | 3.6 ± 0.4 | 3.2 ± 0.4 | <0.001 |
| Lymphocytes in WBC (%) | 30.4 ± 6.7 | 26.0 ± 8.0 | 19.8 ± 6.7 | 15.0 ± 4.9 | <0.001 |
| Blood WBC (1000/mm3) | 5.8 ± 0.9 | 7.0 ± 1.9 | 8.3 ± 2.2 | 9.8 ± 2.1 | <0.001 |
| Serum cholesterol (mg/dL) | 192 ± 49 | 191 ± 54 | 188 ± 54 | 182 ± 53 | 0.07 |
| Serum calcium (mg/dL) | 9.3 ± 0.4 | 9.2 ± 0.6 | 9.1 ± 0.6 | 9.0 ± 0.6 | <0.001 |
| Serum phosphorus (mg/dL) | 3.7 ± 0.7 | 3.8 ± 0.8 | 3.9 ± 0.8 | 3.9 ± 0.8 | 0.008 |
| Serum bicarbonate (mEq/L) | 25.5 ± 3.3 | 25.5 ± 3.3 | 25.7 ± 3.4 | 25.8 ± 3.7 | 0.3 |
| Blood hemoglobin (g/dL) | 12.9 ± 1.8 | 12.8 ± 1.8 | 12.7 ± 1.9 | 12.0 ± 2.0 | <0.001 |
| Proteinuria (g/24 h) | 460 (377, 561)4 | 707 (659, 815) | 814 (702, 944) | 1202 (940, 1538) | <0.001 |
Markers of PEW were defined as serum albumin <3.7 g/dL, percentage of lymphocytes in white blood cell (WBC) count <22%, and WBC count >7500/mm3. DM, diabetes mellitus; CKD, chronic kidney disease; ASCVD, atherosclerotic cardiovascular disease; ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; SBP, systolic blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate.
Comparisons were made by using chi-square test for linear trend.
Mean ± SD (all such values).
Geometric mean; 95% CI in parentheses (all such values).
Patient outcomes categorized according to the number of concomitantly present markers of PEW in fixed-covariate and time-dependent Cox models are shown in Table 2. Patients with higher numbers of PEW markers had significantly higher hazard ratios of all-cause mortality and of the composite outcome in both fixed-covariate and time-dependent models. The attributable fractions and the population attributable fractions also showed an increasing trend across the groups with increasing numbers of PEW markers (Table 2). Compared with patients with none of the PEW markers present, the concomitant presence of 1, 2, or 3 markers was associated with incrementally higher mortality in all of the studied subgroups (Figure 1). Interaction terms for diabetes mellitus and smoking were statistically significant, but the incrementally higher risk of mortality across the increasing numbers of PEW markers was uniformly present, even in these subgroups, albeit of different magnitudes (Figure 1).
TABLE 2.
Multivariable-adjusted hazard ratios (and 95% CIs), attributable fractions, and population attributable fractions for all-cause mortality and the composite outcome of predialysis all-cause mortality or end-stage renal disease associated with the number of markers of malnutrition or inflammation below or above the median value1
| Risk associated with number of PEW markers present |
|||
| 1 (n = 447) vs 0 (n = 212) | 2 (n = 401) vs 0 (n = 212) | 3 (n = 160) vs 0 (n = 212) | |
| All-cause mortality | |||
| Hazard ratio (95% CI) | |||
| Fixed-covariate | 1.3 (1.0, 1.7) | 1.7 (1.3, 2.2) | 2.1 (1.5, 2.9) |
| Time-dependent | 1.7 (1.2, 2.4) | 2.4 (1.7, 3.4) | 3.6 (2.5, 5.1) |
| Composite outcome | |||
| Hazard ratio (95% CI) | |||
| Fixed-covariate | 1.1 (0.9, 1.5) | 1.5 (1.2, 1.9) | 1.7 (1.3, 2.3) |
| Time-dependent | 1.8 (1.3, 2.5) | 2.5 (1.8, 3.4) | 3.4 (2.4, 4.7) |
| Attributable fraction | |||
| Mortality percent (95% CI) | |||
| Fixed-covariate | 22 (−1, 41) | 43 (26, 57) | 60 (45, 70) |
| Time-dependent | 39 (13, 58) | 66 (53, 76) | 80 (71, 86) |
| Composite outcome percent (95% CI) | |||
| Fixed-covariate | 26 (5, 42) | 45 (30, 58) | 59 (45, 70) |
| Time-dependent | 42 (21, 58) | 66 (54, 75) | 77 (69, 84) |
| Population attributable fraction | |||
| Mortality percent (95% CI) | |||
| Fixed-covariate | 16 (5, 25) | 27 (18, 35) | 22 (15, 39) |
| Time-dependent | 35 (15, 50) | 57 (43, 68) | 65 (54, 73) |
| Composite outcome percent (95% CI) | |||
| Fixed-covariate | 17 (8, 24) | 26 (19, 33) | 19 (13, 25) |
| Time-dependent | 40 (24, 52) | 59 (48, 68) | 64 (54, 71) |
Markers of protein-energy wasting (PEW) were defined as serum albumin <3.7 g/dL, percentage of lymphocytes in white blood cell (WBC) count <22%, and WBC count >7500/mm3. Hazard ratios were determined in fixed covariate and time-dependent Cox models using the group with no markers of PEW as referent. All Cox models were adjusted for age, race, Charlson comorbidity index, etiology of chronic kidney disease, diabetes mellitus, cardiovascular disease, smoking, systolic and diastolic blood pressure, BMI, estimated glomerular filtration rate, serum calcium, serum phosphorus, hemoglobin, bicarbonate, blood cholesterol, and 24-h urinary protein. Attributable factions were calculated based on incidence rate differences, and population attributable fractions were estimated from Poisson regressions.
FIGURE 1.
Multivariable adjusted hazard ratios (and 95% CIs) of all-cause mortality in patients with 1, 2, or 3 concomitant markers of protein-energy wasting (PEW: serum albumin <3.7 g/dL, percentage of lymphocytes in white blood cells <22%, a white blood cell count >7500/mm3) compared with patients with none of these markers in time-dependent Cox models, overall, and in selected subgroups. All models were adjusted for age, race, Charlson comorbidity index, etiology of chronic kidney disease, diabetes mellitus (DM), cardiovascular disease, smoking, systolic and diastolic blood pressure, BMI, estimated glomerular filtration rate (eGFR), serum calcium, serum phosphorus, hemoglobin (Hgb), bicarbonate, blood cholesterol, and 24-h urinary protein. Interactions with DM and smoking were statistically significant. ASCVD, atherosclerotic cardiovascular disease.
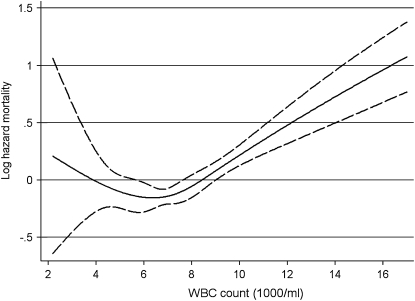
When examined individually, all 3 PEW markers showed significant associations with outcomes in time-dependent Cox models (Table 3 and Figures 2, 3, and 4Figures 2, 3, and 4). Serum albumin and %LYM also showed significant, albeit weaker, associations in fixed-covariate Cox models, whereas the WBC count was not associated with the studied outcomes in fixed-covariate models (Table 3). The association of serum albumin with mortality was linear and strictly downgoing, whereas for %LYM it was nonlinear, with a statistically significant increase in mortality observed only if %LYM was <25% (Figure 3; P < 0.001 for the quadratic term). All of the associations remained significant when examined in models using nonimputed values of independent variables and when the analyses were restricted to a more contemporary cohort of patients enrolled after 1 January 2001 (data not shown).
TABLE 3.
Multivariable-adjusted hazard ratios (and 95% CIs), attributable fractions, and population attributable fractions for all-cause mortality and the composite outcome of predialysis all-cause mortality or end-stage renal disease associated with concentrations of serum albumin, percentage of lymphocytes in the white blood cell (WBC) count, and WBC count below or above the median value1
| Serum albumin ≤3.7 (n = 547) vs >3.7 g/dL (n = 673) | Percentage of lymphocytes in WBC ≤22 (n = 587) vs >22% (n = 633) | WBC >7500 (n = 625) vs ≤7500/mL (n = 595) | |
| All-cause mortality | |||
| Hazard ratio (95% CI) | |||
| Fixed-covariate | 1.7 (1.4, 2.0) | 1.3 (1.1, 1.6) | 1.1 (0.9, 1.3) |
| Time-dependent | 2.0 (1.6, 2.4) | 1.8 (1.5, 2.1) | 1.3 (1.1, 1.5) |
| Composite outcome | |||
| Hazard ratio (95% CI) | |||
| Fixed-covariate | 1.5 (1.2, 1.7) | 1.3 (1.1, 1.5) | 1.0 (0.9, 1.2) |
| Time-dependent | 1.9 (1.6, 2.2) | 1.7 (1.4, 2.0) | 1.2 (1.0, 1.4) |
| Attributable fraction | |||
| Mortality percent (95% CI) | |||
| Fixed-covariate | 42 (31, 50) | 34 (23, 44) | 3 (−13, 17) |
| Time-dependent | 56 (48, 63) | 56 (48, 63) | 22 (9, 34) |
| Composite outcome percent (95% CI) | |||
| Fixed-covariate | 47 (38, 54) | 30 (19, 40) | 1 (−15, 15) |
| Time-dependent | 55 (48, 62) | 52 (44, 59) | 15 (1, 26) |
| Population attributable fraction | |||
| Mortality percent (95% CI) | |||
| Fixed-covariate | 11 (8, 15) | 12 (8, 16) | 4 (0, 8) |
| Time-dependent | 38 (31, 45) | 40 (33, 47) | 9 (1, 16) |
| Composite outcome percent (95% CI) | |||
| Fixed-covariate | 12 (9, 14) | 9 (6, 12) | 3 (1, 6) |
| Time-dependent | 37 (31, 43) | 37 (31, 43) | 7 (1, 13) |
Hazard ratios were determined in fixed covariate and time-dependent Cox models. All Cox models were adjusted for age, race, Charlson comorbidity index, diabetes mellitus, cardiovascular disease, smoking, systolic and diastolic blood pressure, BMI, estimated glomerular filtration rate, serum calcium, serum phosphorus, hemoglobin, bicarbonate, blood cholesterol, and 24-h urinary protein. Attributable factions were calculated based on incidence rate differences, and population attributable fractions were estimated from Poisson regressions.
FIGURE 2.
Multivariable-adjusted hazards ratios (solid line) and 95% CIs (dashed lines) of all-cause mortality associated with continuous serum albumin concentrations in a time-dependent Cox model using restricted cubic splines adjusted for age, race, Charlson comorbidity index, etiology of chronic kidney disease, diabetes mellitus, cardiovascular disease, smoking, systolic and diastolic blood pressure, BMI, estimated glomerular filtration rate, serum calcium, serum phosphorus, hemoglobin, bicarbonate, blood cholesterol, and 24-h urinary protein.
FIGURE 3.
Multivariable-adjusted hazards ratios (solid line) and 95% CIs (dashed lines) of all-cause mortality associated with continuous percentage of lymphocytes in the white blood cell (WBC) count in a time-dependent Cox model using restricted cubic splines adjusted for age, race, Charlson comorbidity index, etiology of chronic kidney disease, diabetes mellitus, cardiovascular disease, smoking, systolic and diastolic blood pressure, BMI, estimated glomerular filtration rate, serum calcium, serum phosphorus, hemoglobin, bicarbonate, blood cholesterol, and 24-h urinary protein.
FIGURE 4.
Multivariable-adjusted hazards ratios (solid line) and 95% CIs (dashed lines) of all-cause mortality associated with continuous white blood cell (WBC) count in a time-dependent Cox model using restricted cubic splines adjusted for age, race, Charlson comorbidity index, etiology of chronic kidney disease, diabetes mellitus, cardiovascular disease, smoking, systolic and diastolic blood pressure, BMI, estimated glomerular filtration rate, serum calcium, serum phosphorus, hemoglobin, bicarbonate, blood cholesterol, and 24-h urinary protein.
DISCUSSION
We examined the outcome predictability of 3 readily available biomarkers of PEW (serum albumin, %LYM, and WBC count) in 1220 men with NDD-CKD and found robust and independent associations between these biomarkers and relevant clinical endpoints. When considering a combination of the 3 factors, those with an incrementally higher number of PEW markers experienced a significantly higher mortality. On a population level, approximately two-thirds of the mortality could be attributed to the concomitant presence of the 3 studied markers of PEW. Individually, serum albumin and %LYM displayed stronger associations with mortality (with population attributable fractions of all-cause mortality of 38% and 40% for values below their median levels in time-dependent models), whereas WBC showed slightly weaker associations that were more pronounced in time-dependent models (population-attributable fraction of all-cause mortality of 9% for a WBC count >7500/mL).
Both protein-energy malnutrition and inflammation are common and often concurrent conditions in patients with CKD (4). Various indicators of these 2 conditions are associated with adverse clinical outcomes in CKD patients receiving dialysis (7, 8, 18–25). Because of the complex changes characterizing malnutrition and inflammation, which include clinical, biochemical, and nutritional factors, an expert panel recently recommended the term PEW, defined by using 4 main categories of measures that have been consistently associated with worse outcomes in dialysis patients: biochemical measures (including serum albumin), measures of body mass, measures of muscle mass, and measures of dietary intake (5). A series of additional markers has also been advocated, including peripheral blood cell counts, such as %LYM (5), with lesser amounts of data available on their importance.
Whereas the association of serum albumin (6–9), lymphocyte count, %LYM (10–12), and the WBC count (11–13) with adverse outcomes in maintenance dialysis patients is well-established, less information is available in patients with NDD-CKD. To the best of our knowledge, the association between lower albumin and adverse outcomes was examined in 3 previous studies of patients with moderately advanced CKD (26–28). Lower albumin was independently associated with higher all-cause mortality and a higher incidence of cardiovascular events in these studies. The Atherosclerosis Risk in Community (ARIC) study found a significantly higher incidence of coronary heart disease associated with higher WBC counts (27). Before our current study, no studies have examined the association of WBC count or %LYM with mortality in NDD-CKD, an no previous studies have examined the association of a combination of these markers with outcomes. Because of their relatively wide availability, these markers of PEW can be useful tools in assessing the nutritional or inflammatory status of patients with CKD and could also serve as powerful predictors of adverse outcomes in this patient population.
The pathophysiologic pathways underlying the associations between PEW and mortality are not clear; a complex mechanism of action is more likely rather than a single pathophysiologic event (4). Such mechanisms include sarcopenia-related diminished muscle function, alterations in circulating actin, and gelsolin concentrations (4, 29); lower muscle oxidative metabolism (30); impaired immune function (31); decreased sequestration of uremic toxins (32); decreased production of antiinflammatory cytokines and adiponectin (33) by lower fat stores; and atrophy of the gut lining, decreased intestinal secretions, and altered gut flora leading to a reduction in gut function and the ability to absorb nutrients (34). Even though it is unclear whether serum albumin, WBC count, or %LYM are causally involved in the chain of events leading to adverse outcomes, or if they are merely disease markers correlating with the truly causal factors, these associations put into perspective the hypothesis that actionable PEW surrogates are some of the strongest death predictors in CKD. Clinical trials will ultimately be needed to prove a true cause-effect relation between PEW and any adverse outcomes.
Our study was limited by its observational nature, which allowed us to establish associations, but not causality. Our study sample was limited to male patients from a single institution; hence, our results may not apply to the larger population with NDD-CKD. Our enrollment of patients over an extended period of time carried the risk that secular trends in medical practices could have affected patient outcomes differently based on the time of enrollment. However, we examined more contemporary patients separately and found no differences in outcomes. Uneven medical care in patients belonging to different clinical subgroups could have affected our outcomes. We addressed this by assessing the use of several medical interventions that are commonly applied in patients with CKD (eg, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, statins, and activated vitamin D products) and found no significant differences in use. We adjusted our analyses for numerous potential confounding variables, but residual confounding from unmeasured variables could still have been present.
In conclusion, lower serum albumin concentrations and %LYM levels and a higher WBC count, alone or in combination, are independently associated with increased all-cause mortality in patients with moderate-to-advanced NDD-CKD. These traditional and nontraditional measures of PEW are universally available for monitoring nutritional and inflammatory status in CKD and for predicting clinical outcomes. Clinical trials are not yet available to prove that these markers of PEW are causally related to higher mortality.
Acknowledgments
The authors’ responsibilities were as follows—CPK: study conception, data acquisition, data analysis, drafting of the manuscript, and financial support; SMG: data collection and interpretation, review and approval of the final version of the manuscript; JEA: data interpretation, review and approval of the final version of the manuscript; and KK-Z: data interpretation, review and approval of the final version of the manuscript, and material support. KK-Z received grant support and/or honoraria from Novo Nordisk. None of the other authors reported a conflict of interest.
REFERENCES
- 1.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 2003;41:1–12 [DOI] [PubMed] [Google Scholar]
- 2.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 2004;164:659–63 [DOI] [PubMed] [Google Scholar]
- 3.Kovesdy CP, Trivedi BK, Anderson JE. Association of kidney function with mortality in patients with chronic kidney disease not yet on dialysis: a historical prospective cohort study. Adv Chronic Kidney Dis 2006;13:183–8 [DOI] [PubMed] [Google Scholar]
- 4.Kovesdy CP, Kalantar-Zadeh K. Why is protein-energy wasting associated with mortality in chronic kidney disease? Semin Nephrol 2009;29:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fouque D, Kalantar-Zadeh K, Kopple J, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int 2008;73:391–8 [DOI] [PubMed] [Google Scholar]
- 6.Beddhu S, Kaysen GA, Yan G, et al. Association of serum albumin and atherosclerosis in chronic hemodialysis patients. Am J Kidney Dis 2002;40:721–7 [DOI] [PubMed] [Google Scholar]
- 7.Iseki K, Kawazoe N, Fukiyama K. Serum albumin is a strong predictor of death in chronic dialysis patients. Kidney Int 1993;44:115–9 [DOI] [PubMed] [Google Scholar]
- 8.Kalantar-Zadeh K, Kilpatrick RD, Kuwae N, et al. Revisiting mortality predictability of serum albumin in the dialysis population: time dependency, longitudinal changes and population-attributable fraction. Nephrol Dial Transplant 2005;20:1880–8 [DOI] [PubMed] [Google Scholar]
- 9.Lowrie EG, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis 1990;15:458–82 [DOI] [PubMed] [Google Scholar]
- 10.Kuwae N, Kopple JD, Kalantar-Zadeh K. A low lymphocyte percentage is a predictor of mortality and hospitalization in hemodialysis patients. Clin Nephrol 2005;63:22–34 [DOI] [PubMed] [Google Scholar]
- 11.Pifer TB, McCullough KP, Port FK, et al. Mortality risk in hemodialysis patients and changes in nutritional indicators: DOPPS. Kidney Int 2002;62:2238–45 [DOI] [PubMed] [Google Scholar]
- 12.Reddan DN, Klassen PS, Szczech LA, et al. White blood cells as a novel mortality predictor in haemodialysis patients. Nephrol Dial Transplant 2003;18:1167–73 [DOI] [PubMed] [Google Scholar]
- 13.Johnson DW, Wiggins KJ, Armstrong KA, Campbell SB, Isbel NM, Hawley CM. Elevated white cell count at commencement of peritoneal dialysis predicts overall and cardiac mortality. Kidney Int 2005;67:738–43 [DOI] [PubMed] [Google Scholar]
- 14.Kovesdy CP, Trivedi BK, Kalantar-Zadeh K, Anderson JE. Association of low blood pressure with increased mortality in patients with moderate to severe chronic kidney disease. Nephrol Dial Transplant 2006;21:1257–62 [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 1999;130:461–70 [DOI] [PubMed] [Google Scholar]
- 16.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39:S1–266 [PubMed] [Google Scholar]
- 17.Greenland S, Drescher K. Maximum likelihood estimation of the attributable fraction from logistic models. Biometrics 1993;49:865–72 [PubMed] [Google Scholar]
- 18.Chertow GM, Goldstein-Fuchs DJ, Lazarus JM, Kaysen GA. Prealbumin, mortality, and cause-specific hospitalization in hemodialysis patients. Kidney Int 2005;68:2794–800 [DOI] [PubMed] [Google Scholar]
- 19.Ikizler TA. Nutrition, inflammation and chronic kidney disease. Curr Opin Nephrol Hypertens 2008;17:162–7 [DOI] [PubMed] [Google Scholar]
- 20.Kalantar-Zadeh K, Block G, McAllister CJ, Humphreys MH, Kopple JD. Appetite and inflammation, nutrition, anemia, and clinical outcome in hemodialysis patients. Am J Clin Nutr 2004;80:299–307 [DOI] [PubMed] [Google Scholar]
- 21.Kalantar-Zadeh K, Kopple J. Malnutrition as a cause of morbidity and mortality in dialysis patients. 2nd ed. In: Kopple J, Massry S, eds Nutritional management of renal disease. Philadelphia, PA: Lipincott, Williams & Wilkins, 2004 [Google Scholar]
- 22.Kaysen GA. Inflammation nutritional state and outcome in end stage renal disease. Miner Electrolyte Metab 1999;25:242–50 [DOI] [PubMed] [Google Scholar]
- 23.Mittman N, Avram MM, Oo KK, Chattopadhyay J. Serum prealbumin predicts survival in hemodialysis and peritoneal dialysis: 10 years of prospective observation. Am J Kidney Dis 2001;38:1358–64 [DOI] [PubMed] [Google Scholar]
- 24.Rambod M, Kovesdy CP, Bross R, Kopple JD, Kalantar-Zadeh K. Association of serum prealbumin and its changes over time with clinical outcomes and survival in patients receiving hemodialysis. Am J Clin Nutr 2008;88:1485–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sreedhara R, Avram MM, Blanco M, Batish R, Avram MM, Mittman N. Prealbumin is the best nutritional predictor of survival in hemodialysis and peritoneal dialysis. Am J Kidney Dis 1996;28:937–42 [DOI] [PubMed] [Google Scholar]
- 26.Menon V, Greene T, Wang X, et al. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int 2005;68:766–72 [DOI] [PubMed] [Google Scholar]
- 27.Muntner P, He J, Astor BC, Folsom AR, Coresh J. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the atherosclerosis risk in communities study. J Am Soc Nephrol 2005;16:529–38 [DOI] [PubMed] [Google Scholar]
- 28.Weiner DE, Tighiouart H, Elsayed EF, et al. The relationship between nontraditional risk factors and outcomes in individuals with stage 3 to 4 CKD. Am J Kidney Dis 2008;51:212–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee PS, Sampath K, Karumanchi SA, et al. Plasma gelsolin, circulating actin and chronic hemodialysis mortality. J Am Soc Nephrol 2009;20:1140–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Argiles JM. Cancer-associated malnutrition. Eur J Oncol Nurs 2005;9(suppl 2):S39–50 [DOI] [PubMed] [Google Scholar]
- 31.Chinen J, Shearer WT. Secondary immunodeficiencies, including HIV infection. J Allergy Clin Immunol 2008;121:S388–92 [DOI] [PubMed] [Google Scholar]
- 32.Jandacek RJ, Anderson N, Liu M, Zheng S, Yang Q, Tso P. Effects of yo-yo diet, caloric restriction, and olestra on tissue distribution of hexachlorobenzene. Am J Physiol Gastrointest Liver Physiol 2005;288:G292–9 [DOI] [PubMed] [Google Scholar]
- 33.Mohamed-Ali V, Goodrick S, Bulmer K, Holly JM, Yudkin JS, Coppack SW. Production of soluble tumor necrosis factor receptors by human subcutaneous adipose tissue in vivo. Am J Physiol 1999;277:E971–5 [DOI] [PubMed] [Google Scholar]
- 34.Ziegler TR, Evans ME, Fernandez-Estivariz C, Jones DP. Trophic and cytoprotective nutrition for intestinal adaptation, mucosal repair, and barrier function. Annu Rev Nutr 2003;23:229–61 [DOI] [PubMed] [Google Scholar]