Abstract
Cervical carcinoma is clinically staged according to the International Federation of Gynecology and Obstetrics system; however, this staging system is frequently inaccurate, particularly with advancing stage. Imaging modalities are often used in guiding therapeutic decisions for advanced cervical cancer. However, despite technologic radiographic advances, imaging results correlate variably with the histopathology of surgical specimens. The transperitoneal laparoscopic lymphadenectomy approach offers less morbidity than the traditional laparotomy approach to surgical staging, and the retroperitoneal laparoscopic approach has been demonstrated to decrease the risk of bowel injury and reduce abdominal adhesion formation, and prior abdominal surgery does not appear to be a factor. Further prospective clinical trials are necessary to better define the role of retroperitoneal laparoscopic surgery in the management of gynecologic malignancies.
Key words: Cervical cancer, surgical staging; Transperitoneal para-aortic lymphadenectomy; Extraperitoneal para-aortic lymphadenectomy
Despite the declining death rate of cervical carcinoma, the American Cancer Society estimated almost 4000 deaths and more than 11,000 new diagnoses in 2008.1 Cervical carcinoma is clinically staged according to the International Federation of Gynecology and Obstetrics (FIGO) system; however, this staging system is frequently inaccurate, particularly with advancing stage. Clinical staging correlates poorly with the true extent of disease. Inaccuracies in staging occur in as many as 25% of patients categorized as FIGO stages I and II and in up to 65% to 90% in FIGO stage III.2 Cervical carcinoma metastasizes predominantly by the lymphatic system in an orderly fashion: initially to the pelvic lymph nodes then to the para-aortic lymph nodes. Previous studies have demonstrated a strong correlation between the incidence of nodal metastasis with tumor volume and clinical stage.3
Imaging modalities such as computed tomography (CT) and magnetic resonance imaging (MRI) are often used in guiding therapeutic decisions for advanced cervical cancer. However, despite these technologic radiographic advances, imaging results correlate variably with the histopathology of surgical specimens.4 In particular, CT and MRI are poor at detecting small volume disease in patients’ para-aortic metastases.5 Currently, positron emission tomography (PET) imaging is being evaluated as a method for detecting cervical cancer metastases prior to initiating therapy and for post-treatment surveillance to assess for recurrence. In early studies, PET scan has shown superior sensitivity (52%–85.7%) and specificity (94.4%) compared with CT and MRI in the detection of para-aortic node metastases.5
Knowing the extent of disease in patients with cervical cancer is important as it helps guide treatment. Lymphadenectomy offers the opportunity of learning whether there is involvement of the lymph nodes, not only in the pelvis, but also along the chain of lymph nodes around the aorta. Initially, lymph nodes were sampled through a traditional transperitoneal laparotomy; however, due to high complication rates, a retroperitoneal approach was developed. Dargent and colleagues6 demonstrated the feasibility and benefits of the retroperitoneal approach. The advancement of endoscopic equipment and surgical techniques in minimally invasive surgery over the past decade has decreased the morbidity associated with laparotomy. In multiple studies, staging by laparoscopy compared with laparotomy has resulted in less blood loss, shorter hospital stay, less postoperative adhesion formation, and equivalent assessment of lymph node status.7
Surgical Outcomes
Querleu and coworkers8 first described laparoscopic transperitoneal lymphadenectomy for the management of cervical cancer in 1991. Laparoscopy has many advantages over laparotomy, such as less pain, smaller incisions, quicker recovery time, decreased blood loss, shorter hospital stay, and faster return of bowel function. The transperitoneal approach allows for a thorough inspection of the abdominal cavity.8 Hertel and colleagues9 evaluated the utility of laparoscopy for staging patients with advanced cervical cancer. Specifically, the authors compared laparoscopic findings with results of MRI and CT and monitored the influence of laparoscopy to determine the treatment plan. The study enrolled 109 consecutive patients with FIGO stage Ib2 disease or higher for laparoscopic staging. Of the 101 patients undergoing para-aortic lymphadenectomy, 21 (19.3%) had positive paraaortic nodes. Intraoperative complications occurred in 3.7% (4/109) of patients, which was comparable to other studies. Their study demonstrated that compared with transperitoneal laparotomy, laparoscopic lymphadenectomy resulted in less postoperative adhesion formation. However, the study did not include a comparison group of patients that underwent laparotomy. Additionally, the authors found that treatment plans were altered in 22% of patients after laparoscopy and concluded that laparoscopic staging is of high accuracy and may help to optimize subsequent management plans.
Although transperitoneal laparoscopy decreases intra-abdominal adhesions, it does not address the difficulties encountered with mobilization and retraction of small bowel, adhesiolysis, mobilization of the sigmoid colon, or identification of the ureters. Because retroperitoneal laparotomy for lymph node assessment had previously been shown to decrease enteric complications due to less intra-abdominal adhesion formation, a retroperitoneal laparoscopic approach seemed quite feasible. One of the initial studies evaluating the feasibility of the laparoscopic retroperitoneal lymphadenectomy by Vasilev and McGonigle10 suggested that the retroperitoneal approach provided more rapid access to the node area, decreased risk of injury on entry to bowel or large vessels, and decreased risk for electrosurgical bowel injury.
Further reviews have demonstrated that by avoiding entry into the peritoneal cavity, the risk of adverse events such as postoperative ileus, intraperitoneal adhesions, and intestinal obstruction was eliminated.7 Another advantage of the retroperitoneal approach is that prior abdominal surgery-associated adhesions do not seem to impact the success of the operation.11
Occelli and colleagues12 examined animal models comparing the risk of postoperative adhesions with transperitoneal versus retroperitoneal laparoscopic lymphadenectomy. They found that the overall adhesion rates were 76% in the transperitoneal group and 43% in the retroperitoneal group. Of interest, the majority of the adhesions formed with the retroperitoneal approach localized outside of the para-aortic external radiation field.
In a retrospective study of 184 patients by Leblanc and colleagues,13 pretherapeutic laparoscopic staging was deemed safe, feasible, and reproducible. Information from pathologic examination of para-aortic lymph nodes impacted treatment planning in up to 58% of patients, sparing 75% with stages IIB-IVA disease overtreatment. More significantly, patients with minimal para-aortic nodal disease treated with extended-field radiotherapy had the same survival as patients with negative nodes treated with pelvic radiotherapy. In this study, the perceived major drawback to the retroperitoneal approach was the concern for the relatively high rate of lymphocele formation. Fourteen of the first 104 patients (13.4%), 2 of whom required a reoperation (one had a laparoscopic marsupialization of the paracolic gutters, whereas the other had an open procedure for drainage of an abscessed lymphocyst). For the remaining 77 patients, the authors performed preventative marsupialization of the left paracolic gutter. Following this preventative measure, only 3 (3.8%) of the 77 developed symptomatic lymphocysts, all of which resolved spontaneously.
Recently, Tillmans and Lowe14 published the first series examining outpatient management for laparoscopic aortic lymph node dissection. The study enrolled 18 patients with either stage IIB or IIIB cervical carcinoma. All patients underwent a preoperative CT scan (all of which were negative for aortic disease) followed by laparoscopic lymphadenectomy. Occult aortic nodal metastasis was detected in 2 patients (11%). The median blood loss was 25 cc and the median nodal count was 10, which compared favorably to the previously reported literature.6–8 The authors demonstrated that outpatient laparoscopic extraperitoneal aortic lymphadenectomy is both safe and feasible. Additionally, the authors performed a cost analysis comparing nonsurgical (CT, MRI, and PET) and surgical (outpatient laparoscopic extraperitoneal lymphadenectomy) management. The cost analysis indicated that outpatient laparoscopic extraperitoneal lymphadenectomy appears equivalent to PET scan and MRI, but more expensive than CT scan.14
Although laparoscopy has proven to be feasible, it remains a technically challenging operation. The robotic da Vinci® Surgical System (Intuitive Surgical, Inc., Sunnyvale, CA) may lend value in performing this surgery as it provides a steady 3-dimensional view, instruments with articulating tips, and less reliance on the surgeon’s movements (increasing accuracy and precision).15 Vergote and coworkers16 reported on technique and operative results of robotic retroperitoneal para-aortic lymphadenectomy in 5 patients with IIB or IIIB disease. The procedure included a transperitoneal laparoscopy through the umbilicus (to evacuate leakage of CO2 from retroperitoneal to intraperitoneal cavity). A total of 4 trocar ports were used with the patient slightly tilted to the left side. In all of the patients a retroperitoneal drain was left above the pubis symphysis and removed without difficulty the next day. There was 1 operative complication in which the right ureter was damaged, but successfully repaired robotically without sequelae. Postoperatively, there were no complications. From the authors’ preliminary experience, robotic para-aortic lymphadenectomy may offer advantages over standard laparoscopy. As with any surgical procedure, an intimate knowledge of the anatomy is an essential ingredient to success (Figure 1).
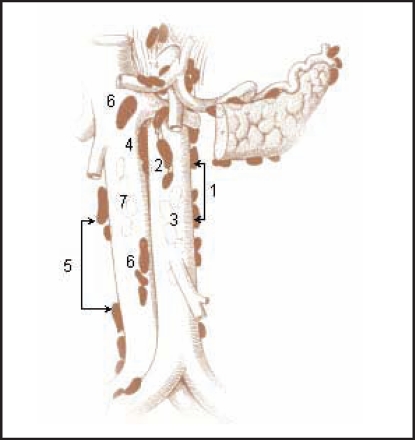
Figure 1.
Para-aortic and paracaval lymph nodes: (1) Lateral aortic; (2) preaortic; (3) postaortic; (4) intermediate lumbar; (5) lateral caval; (6) precaval; (7) postcaval. Reproduced with permission from SEER training module: abdominal lymph nodes. National Cancer Institute Web site. http://training.seer.cancer.gov/lymphoma/anatomy/chains/deep-abdominal.html. Accessed April 27, 2009.
Surgical Technique: Transperitoneal Laparoscopic Para-Aortic Lymphadenectomy
A 4-trocar technique is used. The camera port is placed in the umbilicus unless the patient has had a previous midline incision. In the latter cases, the initial trocar is placed in the left upper quadrant. Two additional trocars are placed in the right and left lower quadrants. A fourth trocar is placed in the midline suprapubically. The surgeon is positioned on the patient’s left side. The grasping forceps is in the surgeon’s left hand through the midline suprapubic port and the coagulating instrument is in the surgeon’s right hand through the left lower quadrant port. The viewing monitor is placed on the patient’s right side at the level of the right shoulder.
The procedure starts with an incision along the peritoneal surface over the lower aspect of the aorta and vena cava. Please note that the incision must be made medial to the reflection of the right ureter. The patient’s lower extremities are located on the left side of the screen and the right side of the patient is on the top of the screen. Once a fenestration is made on the peritoneal surface, the grasper is used to lift the peritoneum. The incision is extended inferiorly and superiorly while placing gentle upward traction on the peritoneal surface.
Once the peritoneum has been incised, the surgeon should identify the right psoas muscle, which can be easily found lateral to the lymph node bundle overlying the inferior vena cava. The lymph node bundle is then grasped with atraumatic grasper and the pedicles are developed, coagulated, and transected. Dissection is then continued along the entire surface of the inferior vena cava to the level of the reflection of the duodenum.
Once the lymphadenectomy over the inferior vena cava has been completed, the dissection is then performed over the surface of the aorta, inferior to the level of the inferior mesenteric artery. The dissection is extended to the level immediately below the aortic bifurcation to remove the lymph nodes over the left common iliac vein.
At this time attention is focused on the left para-aortic lymph nodes. Please note that this dissection is performed without changing either the position of the surgeon or the position of the instrumentation. The assistant should apply gentle traction on the left border of the peritoneum to expose and highlight the inferior mesenteric artery.
Dissection is then performed over the aorta superior to the inferior mesenteric artery to the level of the left renal vein. Additional lymph nodes are removed above the inferior mesenteric artery and below the left renal vein. Care must be taken not to injure the ascending lumbar veins and the hemiazygous vein as these often drain into the lower border of the left renal vein (Figure 2).
Figure 2.
Situs after finishing infrarenal paraaortic lymphadenectomy in a patient with complete lymph node debulking: (1) Vena ovarica dextra; (2) vena cava; (3) aorta; (4) arteria mesenterica inferior; (5) vena renalis sinistra. Reprinted from Gynecologic Oncology, Vol. 99, Marnitz S et al, “Is there a benefit of pretreatment laparoscopic transperitoneal surgical staging in patients with advanced cervical cancer?” pp. 536–544, Copyright 2005, with permission from Elsevier.
Surgical Technique: Extraperitoneal Laparoscopic Para-Aortic Lymphadenectomy
The patient should be positioned supine without tucking the arms.
Trendelenburg position is not necessary. The surgeon should ensure that the abdomen and the left flank are prepared. The operating surgeon should stand on the patient’s left side. An initial incision is made in the umbilicus and a laparoscope is inserted to inspect the abdominal and pelvic cavity to ensure that there is no evidence of metastatic disease.
A 10- to 12-mm incision is made in the left lower quadrant with a scalpel. This incision should be approximately 2 cm above and medial to the anterior superior iliac spine. The muscles of the anterior abdominal wall are then separated. This may be done sharply or with electrocautery. Care must be taken not to perforate the peritoneal sheath.
The surgeon’s index finger is then inserted and the loose areolar tissue is separated. The surgeon should palpate immediately deep to the incision to detect the pulsation of the external iliac vessels along the psoas muscle. A balloon-tipped trocar should be inserted in this space. The balloon should be insufflated to fix the trocar to the incision site. The surgeon’s index finger is used to expose the extraperitoneal space. The balloon-tipped trocar is inserted. The extraperitoneal space is insufflated and the abdominal cavity is deflated. The laparoscope is then introduced in the extraperitoneal space, and is used to dissect the areolar tissue in the extraperitoneal space. An 11-mm trocar is introduced cephalad to the previous trocar approximately 3 to 4 cm along the midaxillary line.
The correct anatomic boundaries of the surgical field should be the left common iliac vessels medially, the psoas muscle inferiorly, the ureter superiorly, and the left renal vein as the most cephalad boundary. The ureter is seen peristalsing in the upper field of dissection. One should follow the left infundibulopelvic vessels to the left renal vein. A 5-mm trocar is placed cephalad to the previous trocar along the midaxillary line. At this point, the assistant should hold the camera and stand behind the surgeon. The surgeon should hold graspers in the left hand and the laparoscopic shear in the right hand.
Once it has been confirmed that there is no evidence of grossly positive lymph nodes, the lymphatic pad is grasped and gently separated from the aorta by blunt and sharp dissection from the psoas muscle and the sympathetic chain posteriorly and the peritoneum and ureter anterolaterally. Dissection continues close to the adventitial layer of the vessels starting from the origin of the left common iliac artery and moving up to the left renal vein. The inferior aspect of the left renal vein is identified directly or by following the left gonadal vein.
The next step is to reach the right common iliac vessels. The peritoneal sac is elevated from the left common iliac vein and then from the sacral promontory. The bifurcation of the inferior vena cava is then identified. The right common iliac vein and the right common iliac artery are freed using blunt dissection. The right common iliac artery is followed in a caudal direction down to the level of its bifurcation. The right ureter is then elevated and separated from the iliac vessels and the psoas muscle. At this time, the right lateral common iliac nodes and the presacral nodes are removed. The precaval nodes are identified and detached from the inferior vena cava. Once these steps have been accomplished, the nodal dissection is considered complete (Figure 3).
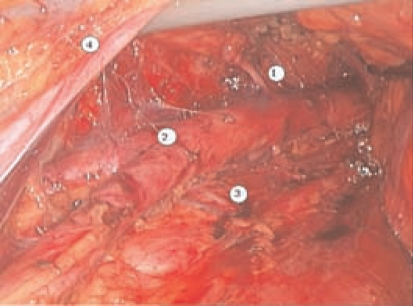
Figure 3.
Laparoscopic extraperitoneal paraaortic lymphadenectomy: (1) Inferior mesenteric artery; (2) aortic bifurcation; (3) lumbar artery and vein; (4) left ureter.
The resected lymph nodes are extracted from the extraperitoneal cavity through the 10-mm port. The operative field is evaluated for hemostasis. At this time, the extraperitoneal space is deflated, and the abdominal cavity is insufflated.
The laparoscope is once again placed through the port in the umbilicus. A 2- to 3-cm incision is made in the peritoneal sac to prevent lymphocyst formation.
Conclusion
Minimally invasive laparoscopic staging for advanced cervical cancer is viable and reproducible. Although imaging modalities are improving, the current gold standard for determining lymph node status is surgical sampling. The transperitoneal laparoscopic lymphadenectomy approach offers less morbidity than the traditional laparotomy approach. The retroperitoneal laparoscopic approach has been demonstrated to decrease the risk of bowel injury and reduce abdominal adhesion formation, and prior abdominal surgery does not appear to be a factor.7 Further prospective clinical trials are necessary to better define the role of retroperitoneal laparoscopic surgery in the management of gynecologic malignancies. Additionally, as gyneoncologists become more familiar with robotic surgery, technically challenging surgery may become less difficult.
Main Points.
Clinical staging of cervical carcinoma correlates poorly with the true extent of disease.
Lymphadenectomy can determine involvement of the lymph nodes not only in the pelvis, but also along the chain of lymph nodes around the aorta.
In multiple studies, staging by laparoscopy compared with laparotomy has resulted in less blood loss, shorter hospital stay, less postoperative adhesion formation, and equivalent assessment of lymph node status.
By avoiding entry into the peritoneal cavity, the risk of adverse events such as postoperative ileus, intraperitoneal adhesions, and intestinal obstruction was eliminated in the retroperitoneal laparoscopic lymphadenectomy.
Robotic para-aortic lymphadenectomy may offer advantages over standard laparoscopy.
Footnotes
No financial support was necessary in preparing this manuscript or acquiring data.
References
- 1.American Cancer Society, authors. Cancer Facts & Figures 2008. Vol. 20. Atlanta, GA: American Cancer Society; 2008. [Accessed April 27, 2009]. Publication No. 500808. http://www.cancer.org/downloads/STT/2008CAFFfinalsecured.pdf. [Google Scholar]
- 2.Lagasse LD, Creasman WT, Shingleton HM, et al. Results and complications of operative staging in cervical cancer: experience of the Gynecologic Oncology Group. Gynecol Oncol. 1980;9:90–98. doi: 10.1016/0090-8258(80)90013-x. [DOI] [PubMed] [Google Scholar]
- 3.Nelson JH , Jr, Boyce J, Macasaet M, et al. Incidence, significance, and follow-up of para-aortic lymph node metastases in late invasive carcinoma of the cervix. Am J Obstet Gynecol. 1977;128:336–340. doi: 10.1016/0002-9378(77)90633-0. [DOI] [PubMed] [Google Scholar]
- 4.Schneider A, Hertel H. Surgical and radiographic staging in patients with cervical cancer. Curr Opin Obstet Gynecol. 2004;16:11–18. doi: 10.1097/00001703-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Heller PB, Maletano JH, Bundy BN, et al. Clinicalpathologic study of stage IIB, III, and IVA carcinoma of the cervix: extended diagnostic evaluation for paraaortic node metastasis-a Gynecologic Oncology Group study. Gynecol Oncol. 1990;38:425–430. doi: 10.1016/0090-8258(90)90085-y. [DOI] [PubMed] [Google Scholar]
- 6.Dargent D, Ansquer Y, Mathevet P. Technical development and results of left extraperitoneal laparoscopic paraaortic lymphadenectomy for cervical cancer. Gynecol Oncol. 2000;77:87–92. doi: 10.1006/gyno.1999.5585. [DOI] [PubMed] [Google Scholar]
- 7.Sonoda Y, Leblanc E, Querleu D, et al. Prospective evaluation of surgical staging of advanced cervical cancer via a laparoscopic extraperitoneal approach. Gynecol Oncol. 2003;91:326–331. doi: 10.1016/j.ygyno.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Querleu D, Dargent D, Ansquer Y, et al. Extraperitoneal endosurgical aortic and common iliac dissection in the staging of bulky or advanced cervical carcinomas. Cancer. 2000;88:1883–1891. [PubMed] [Google Scholar]
- 9.Hertel H, Köhler C, Elhawary T, et al. Laparoscopic staging compared with imaging techniques in the staging of advanced cervical cancer. Gynecol Oncol. 2002;87:46–51. doi: 10.1006/gyno.2002.6722. [DOI] [PubMed] [Google Scholar]
- 10.Vasilev SA, McGonigle KF. Extraperitoneal laparoscopic para-aortic lymph node dissection. Gynecol Oncol. 1996;61:315–320. doi: 10.1006/gyno.1996.0149. [DOI] [PubMed] [Google Scholar]
- 11.Ramirez PT, Milam MR. Laparoscopic extraperitoneal paraaortic lymphadenectomy in patients with locally advanced cervical cancer. Gynecol Oncol. 2007;104(2 suppl 1):9–12. doi: 10.1016/j.ygyno.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 12.Occelli B, Narducci F, Lanvin D, et al. De novo adhesions with extraperitoneal endosurgical para-aortic lymphadenectomy versus transperitoneal laparoscopic para-aortic lymphadenectomy: a randomized experimental study. Am J Obstet Gynecol. 2000;183:529–533. doi: 10.1067/mob.2000.105736. [DOI] [PubMed] [Google Scholar]
- 13.Leblanc E, Narducci F, Frumovitz M, et al. Therapeutic value of pretherapeutic extraperitoneal laparoscopic staging of locally advanced cervical carcinoma. Gynecol Oncol. 2007;105:304–311. doi: 10.1016/j.ygyno.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Tillmans T, Lowe MP. Safety, feasibility, and costs of outpatient laparoscopic extraperitoneal aortic nodal dissection for locally advanced cervical carcinoma. Gynecol Oncol. 2007;106:370–374. doi: 10.1016/j.ygyno.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Kho RM, Hilger WS, Hentz JG, et al. Robotic hysterectomy: technique and initial outcomes. Am J Obstet Gynecol. 2007;197:113.e1–113.e4. doi: 10.1016/j.ajog.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Vergote I, Pouseele B, Van Gorp T, et al. Robotic retroperitoneal lower para-aortic lymphadenectomy in cervical carcinoma: first report on the technique used in 5 patients. Acta Obstet Gynecol Scand. 2008;87:783–787. doi: 10.1080/00016340802146946. [DOI] [PubMed] [Google Scholar]