Abstract
Recent evidence suggests that extranuclear action of retinoid receptors is involved in mediating the pleiotropic effects of retinoids. However, whether they reside in the cytoplasm remains elusive. Here, we showed that retinoic acid receptor-γ (RARγ) was cytoplasmic in confluent cells, or when cells were released from serum depletion or treated with growth factors. In studying the regulation of RARγ subcellular localization, we observed that ectopically overexpressed RARγ was mainly cytoplasmic irrespective of serum concentration and cell density. The cytoplasmic retention of RARγ was inhibited by ligand retinoic acid (RA). In addition, coexpression of retinoid X receptor-α (RXRα) resulted in nuclear localization of RARγ through their heterodimerization. Mutagenesis studies revealed that a C-terminal fragment of RXRα potently prevents RA-induced RARγ nuclear localization and transcriptional function. Furthermore, our results showed that the cytoplasmic retention of RARγ was due to the presence of its unique N-terminal A/B domain, which was subject to regulation by p38 MAPK-mediated phosphorylation. Deletion or mutation of the N-terminal A/B domain largely impaired its cytoplasmic localization. Together, our data demonstrate that the subcellular localization of RARγ is regulated by complex interactions among ligand binding, receptor phosphorylation, and receptor dimerizations.
The pleiotropic effects of retinoids, natural and synthetic vitamin A derivatives, is mediated by two classes of nuclear receptor family, the retinoic acid receptors (RARs)3 and the retinoid X receptors (RXRs), which are encoded by three distinct genes, α, β, and γ (1, 2). In addition, different isoforms can be generated from each receptor, which differ in their N-terminal sequences, through differential promoter usage and alternative splicing (1, 2). The evolutionary conservation of these receptor subtypes and isoforms and their distinct patterns of expression during development and in the adult organism suggest that each of them has discrete functions (3, 4). RARγ, but not RARα, plays a role in predisposing murine keratinocytes to Ras-induced tumorigenesis, in retinoic acid (RA)-induced cell cycle arrest and apoptosis in keratinocytes (5), and in the regulation of the balance between hematopoietic stem cell self-renewal and differentiation (6). At the level of transcriptional regulation, overexpression of RARγl inhibits transactivation of RA target genes by other RARs (7), and several specific RARγ target genes have recently been identified in F9 cells (8).
RARs activate transcription by binding to cis-acting response elements in the promoter/enhancer region of target genes as homodimer or heterodimer with RXRs (1, 2, 9). RXRs, besides forming heterodimer with RARs in retinoid signaling, can act as heterodimerization partners for many other nuclear receptors to activate transcription of their target genes in a variety of signaling pathways (1, 2). Retinoid receptors, like other nuclear receptors, consist of three main functional domains: the non-conserved N-terminal A/B domain, the central DNA-binding domain containing two zinc finger motifs and nuclear localization signal (NLS), and the multifunctional C-terminal ligand-binding domain (LBD) containing regions for receptor dimerization, ligand binding, and the ligand-dependent transactivation function. Dimerization interfaces that largely mediate heterodimerization of RXRα with RARs have been mapped to regions in the C terminus, corresponding to helices 9 and 10 in the canonical nuclear receptor LBD structure (10, 11). The N-terminal A/B domain contains conserved serine residues, which belong to consensus phosphorylation sites for proline-dependent protein kinases such as cyclin-dependent kinases (Cdks) and the mitogen-activated protein kinases (MAPKs) (12–14), and phosphorylation of these sites can regulate RAR transactivation (15). Phosphorylation of the A/B domain of RARγ is indispensable for differentiation of F9 cells upon RA and cAMP treatment (16), whereas RARα binds to CDK-activating kinase, resulting in an enhanced CDK-activating kinase activity and cell proliferation (17).
Unlike steroid hormone receptors such as glucocorticoid receptor (GR) and androgen receptor that rapidly translocate from the cytoplasm to the nucleus upon ligand-induced activation, retinoid receptors are considered to mainly reside in the nucleus independent of the presence of ligand (18–20). However, certain rapid retinoid responses, such as activation of GTPase Rac (21), protein kinase C (22), and ERK2 (23), and phosphorylation of cAMP-response element-binding protein (24, 25), cannot be explained by classic transcriptional regulation. Thus, subcellular localization of retinoid receptors changes during development of the testes (26) and during various stages of the menstrual cycle (27). Cytoplasmic localization of retinoid receptors in some cell types has been shown to be associated with important biological processes, including growth (28), apoptosis (29), differentiation (30), and inflammation (31, 32). Different RARβ isoforms also exhibit distinct subcellular localization, with RARβ2 being primarily nuclear and RARβ4 being cytoplasmic (33). Thus, subcellular localization may represent another mechanism by which retinoid receptors mediate the pleiotropic effects of retinoids.
Cytoplasmic localization of nuclear receptors not only regulates transcription by modulating their availability in the nucleus but also plays an active role in the cross-talk with other signal transduction pathways. Nur77 migrates from the nucleus to the cytoplasm where it targets mitochondria by binding to Bcl-2 (34–36), providing a molecular basis for integration of nuclear receptor signaling to mitochondrial apoptotic machinery. RXR rapidly inhibits Rac activation and intracellular calcium release by binding to G protein Gq in human platelets that contain no nucleus (21). RXR migrates from the nucleus to the cytoplasm during nerve growth factor-induced PC12 cell differentiation (30) and targets mitochondria to induce apoptosis (29). Interaction of RARγ with cytoplasmic c-Src mediates neuritogenic differentiation (28). Altered retinoid receptor subcellular localization has been shown to be associated with cancer progression (37, 38). Thus, understanding how subcellular localization of retinoid receptors is regulated will provide additional important information regarding the mechanism of retinoid receptor action.
Here we report that RARγ often resides in the cytoplasm when cells are cultured at high density, released from serum starvation, or treated with growth factors. Using transient transfection assays, we show that the cytoplasmic accumulation of RARγ is largely dependent on its N-terminal A/B domain, which is modulated by phosphorylation of its Ser-77 and Ser-79 residues by p38 MAPK. Cytoplasmic retention of RARγ is also inhibited by ligand binding and heterodimerization with RXRα. Thus, our results demonstrate that the subcellular localization of RARγ is regulated by complex interplays among ligand binding and receptor phosphorylation and that its N-terminal A/B region plays a critical role in determining its cytoplasmic accumulation.
EXPERIMENTAL PROCEDURES
Cell Culture and Plasmid Constructs
HEK293T and HeLa cells were grown in Dulbecco's modified Eagle's medium, H460 and SW480 cells were grown in minimal essential medium, and ZR-75-1 cells were cultured in RPMI 1640 medium, supplemented with 10% fetal bovine serum. The expression vectors for RARs, RXRα, and reporter TREpal-tk-CAT were described previously (9, 29, 39, 40). Constructions of expression vectors for RXRα deletion mutants have been described previously (29). RARγ/AB and ΔN-RARγ were constructed by cloning the RARγ A/B domain (amino acids 1–89) and ΔN-RARγ (amino acids 90–454) into pCMV-Myc expression vector, respectively. The RARγ A/B domain was obtained by PCR using forward primer (5′-ccggaattcccatggccaccaataaggag-3′) and reverse primer (5′-ccgctcgagctatggcttgtagacccgagg-3′). The ΔN-RARγ fragment was generated by PCR using forward primer (5′-ccggaattccatgcttcgtgtgcaatgac-3′) and reverse primer (5′-ccgctcgagtcaggctggggacttcag-3′). Point mutants of RARγ were generated with a QuikChange site-directed mutagenesis kit (Stratagene) according to the manufacturer's recommendation.
Western Blotting
Cell lysates were boiled in SDS sample buffer, resolved by SDS-PAGE (8 or 12.5% polyacrylamide), and transferred to nitrocellulose (35, 36, 41). After transfer, the membranes were blocked in 5% milk in TBST (10 mm Tris-HCl, pH 8.0, 150 mm NaCl, and 0.05% Tween 20) for 30 min and incubated with primary antibody in 5% milk in TBST for 2 h at room temperature. The membranes were washed three times with TBST, and incubated for 1 h at room temperature in TBST containing horseradish peroxidase-linked anti-mouse or rabbit immunoglobulin (Santa Cruz Biotechnology). After washing in TBST for three times, immunoreactive products were detected by chemiluminescence with an enhanced chemiluminescence system (ECL, Amersham Biosciences).
Alkaline Phosphatase Treatment
Cells were boiled for 10 min in alkaline phosphatase lysis buffer (100 mm Tris-HCl, pH 8.0, 100 mm NaCl, 5 mm MgCl2, 2 mm phenylmethylsulfonyl fluoride, and 0.6% SDS) and incubated with alkaline phosphatase (200 units/ml, Roche Applied Science) for 4 h at 37 °C (41).
Co-immunoprecipitation Assays
For Co-IP assay (29, 36), HEK293T cells grown in 60-mm dishes were transfected with indicated expression vectors for 24 h and treated with ligands for 1 h. After washing three times with cold PBS, cells were lysed in 1 ml of P-RIPA buffer (1% Nonidet P-40, 0.5% deoxycholic acid, 0.1% SDS, 10 mm EDTA in PBS) containing protease inhibitors (Sigma). Lysate was precleared by incubating with normal mouse IgG and protein A/G-Sepharose (Santa Cruz Biotechnology) for 2 h at 4 °C. Precleared lysate was then incubated with 1 μg of anti-Myc monoclonal antibody (9E10, Santa Cruz Biotechnology) overnight at 4 °C. Immunocomplexes were then precipitated with 40 μl of protein A/G-Sepharose. After extensive washing with P-RIPA buffer, beads were boiled in 40 μl of loading buffer and analyzed by Western blotting.
Immunofluorescence Microscopy
Cells seeded on cover slips in 24-well plates overnight were transfected with appropriate expression vectors for 24 h and treated with ligands for 1 h. The cells were fixed in 4% paraformaldehyde in PBS for 10 min, washed twice with PBS, and then permeabilized with 0.1% Triton X-100 in PBS for 10 min (29, 35, 36). Fixed cells were preincubated for 30 min in PBS containing 5% bovine serum albumin at room temperature. Cells were stained with primary antibody (anti-Myc monoclonal antibody, 1:400 dilution) for 1 h at room temperature followed by incubation with secondary antibody conjugated with Alexa Fluor568 (Invitrogen) or Cy5 (1:1000 dilution). For staining endogenous RARγ, cells grown on coverslips were stained with rabbit polyclonal anti-RARγ antibody (M-454, Santa Cruz Biotechnology) as described above. 4,6-Diamidino-2-phenylindole (0.1 μg/ml) was added to the secondary antibody mixture to visualize nuclei. Fluorescence images were collected and analyzed using an inverted fluorescence microscope or MRC-1024 MP laser-scanning confocal microscope (Bio-Rad).
Reporter Assay
HeLa cells were seeded at 5 × 104 cells/well in 24-well plates. Cells were transfected with 50 ng of TREpal-tk-CAT plasmid, 20 ng of β-galactosidase expression vector (pCH 110, Amersham Biosciences), 50 ng of expression vectors for receptors using Lipofectamine 2000 (Invitrogen). Cells were treated for 20 h with RAR or RXR ligands. CAT activity was normalized with β-galactosidase activity for transfection efficiency (9, 39, 42).
RESULTS
Subcellular Localization RARγ Is Regulated by Growth Conditions
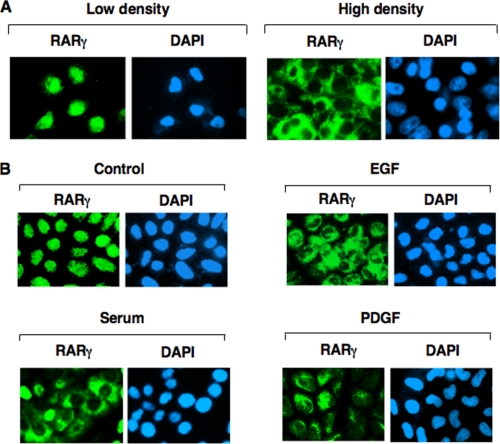
In studying whether retinoid receptors exhibited differential subcellular localization in response to cellular stimulation, we observed that RARγ exhibited strikingly different intracellular localization patterns in H460 lung cancer cells depending on culture conditions. In non-confluent cells, immunostaining with anti-RARγ antibody revealed that typically >95% of cells had RARγ nuclear staining. In contrast, RARγ was predominantly (>70%) cytoplasmic in confluent cells (Fig. 1A). The subcellular localization of RARγ was also regulated by serum concentration. When subconfluent H460 cells were cultured in serum-free medium, RARγ was predominantly nuclear. However, cytoplasmic localization of RARγ was observed when cells were released from 24 h of serum starvation (Fig. 1B). Furthermore, treatment of H460 cells cultured in serum-free medium with various growth factors, such as epidermal growth factor or platelet-derived growth factor, also resulted in increased RARγ cytoplasmic staining (Fig. 1B). Such effects of cell density and growth factors were not observed when RARα was studied (data not shown). Thus, RARγ frequently resides in the cytoplasm, depending on cell density, serum concentration, and the presence of growth factors.
FIGURE 1.
Regulation of intracellular localization of RARγ by cell density, serum concentration, and growth factors. A, effect of cell density on RARγ subcellular localization. H460 cells were seeded at low density (0.2 × 106 cells per 100-mm plate) or high density (7.0 × 106 cells per 100-mm plate) and 12 h later, cells were immunostained using anti-RARγ antibody (Santa Cruz Biotechnology, M-454). Nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI). More than 95% of low density cells showed RARγ nuclear staining, whereas ∼70% of confluent cells displayed RARγ cytoplasmic staining. B, effect of serum and growth factors on RARγ subcellular localization. H460 cells were seeded at 1.0 × 106 cells per 100-mm plate in complete medium overnight and then changed to serum-free medium for 24 h. Cells were then treated with either serum (10%), epidermal growth factor (100 ng/ml), or platelet-derived growth factor (10 ng/ml) for 6 h and immunostained using anti-RARγ antibody. About 90% subconfluent serum-deprived cells showed RARγ nuclear staining, whereas treatment with serum, epidermal growth factor, and platelet-derived growth factor resulted in 70%, 60%, and 50% of cells displaying RARγ cytoplasmic staining, respectively.
Transfected RARγ Predominantly Resides in the Cytoplasm
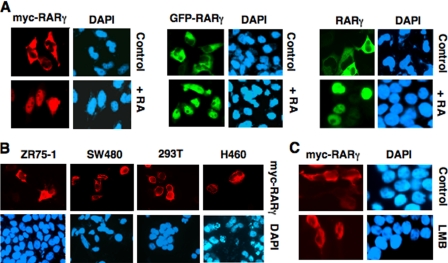
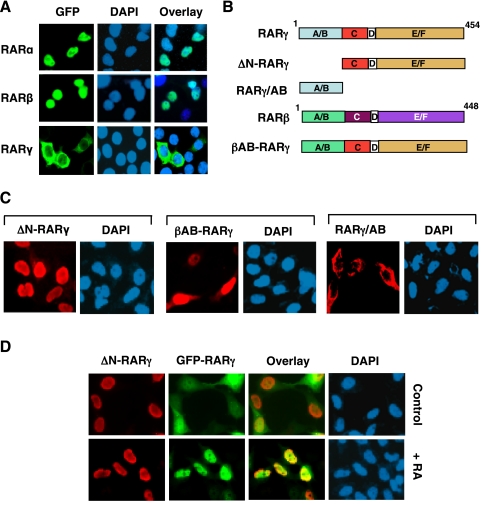
To study the regulation of subcellular localization of RARγ, we transfected Myc-RARγ (RARγ1 tagged with the Myc epitope) expression vector into HeLa cells. Immunostaining of transfected cells with anti-Myc antibody showed that ectopically expressed Myc-RARγ was mainly cytoplasmic (Fig. 2A). Cytoplasmic localization of Myc-RARγ was independent on cell density (data not shown), which was in contrast to the endogenous RARγ whose subcellular localization depended on growth conditions (Fig. 1). To study whether cell density-independent cytoplasmic localization of Myc-RARγ was due to Myc epitope tagged, RARγ fused with green fluorescent protein (GFP) was transfected into HeLa cells. Fluorescence microscopy analysis showed that GFP-RARγ also resided in the cytoplasm. Furthermore, untagged RARγ displayed similar cytoplasmic localization when examined by anti-RARγ antibody (Fig. 2A). Cytoplasmic localization of Myc-RARγ, GFP-RARγ, and RARγ was potently inhibited by ligand binding as treatment of cells with RA resulted in their exclusive nuclear localization. To determine whether the cytoplasmic localization of ectopically expressed RARγ could be observed in other cell types, we transfected Myc-RARγ into several cell lines, including ZR-75-1 breast cancer, SW480 colon cancer, H460 lung cancer, and HEK293T embryonic kidney cells (Fig. 2B). Similar to that observed in HeLa cells, Myc-RARγ was mainly cytoplasmic in these cells, demonstrating that cytoplasmic localization of overexpressed RARγ is not cell type-specific. As nuclear receptors are known to shuttle between the cytoplasm and the nucleus and their subcellular localization is often determined by a balance between activities of nuclear export sequence (NES) and NLS (43), we determined whether cytoplasmic retention of RARγ might depend on its sustained nuclear export. Myc-RARγ-transfected cells were treated with leptomycin B (LMB), which is known to inhibit classic Crm-1-dependent nuclear export (44). Fig. 2C shows that treatment of cells with LMB had no apparent effect on cytoplasmic accumulation of Myc-RARγ, suggesting that retention of transfected RARγ in the cytoplasm was not dependent on its continuous nuclear export.
FIGURE 2.
Cytoplasmic retention of transfected RARγ. A, ectopically expressed RARγ resides in the cytoplasm and translocates into the nucleus upon RA treatment. HeLa cells cultured in 24-well plates were transfected with RARγ, Myc-RARγ, or GFP-RARγ expression vector (100 ng/well) as indicated and treated with vehicle (Control) or RA (0.1 μm) for 1 h. Myc-RARγ-transfected cells were immunostained using monoclonal anti-Myc antibody, and RARγ-transfected cells were stained with anti-RARγ antibody. About 85% of cells displayed cytoplasmic localization of transfected RARγ, whereas >95% of cells showed RARγ nuclear staining when treated with RA. B, cytoplasmic localization of Myc-RARγ in different cell types. Myc-RARγ (100 ng/well in 24-well plates) was transfected into the indicated cell line, and its localization was examined by immunostaining using anti-Myc antibody. More than 50% of cells showed RARγ cytoplasmic staining. C, cytoplasmic localization of RARγ does not depend on its nuclear export. HeLa cells were transfected with Myc-RARγ (100 ng/well in 24-well plates) and treated with or without LMB (2.5 ng/ml, Sigma). Subcellular localization of Myc-RARγ was examined by immunostaining.
Regulation of RARγ Subcellular Localization by RXRα
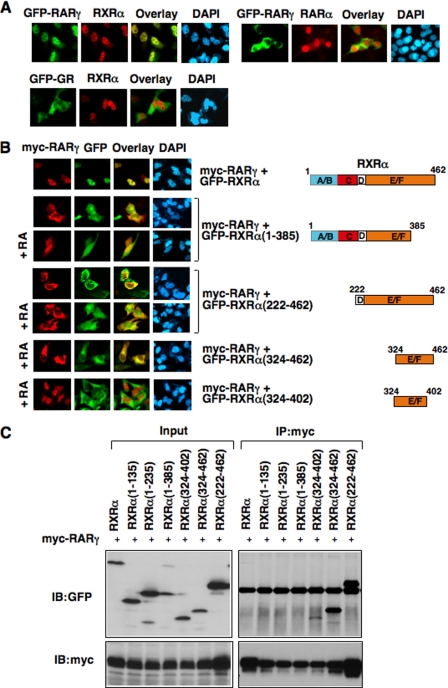
Our observations that the cytoplasmic localization of endogenous RARγ depended on culture condition while transfected RARγ was constitutively cytoplasmic suggested the presence of endogenous RARγ-binding protein(s) that acted to keep RARγ in the nucleus and were limiting. Because RXRα was known to modulate subcellular distribution of its heterodimerization partners (29, 43, 45, 46), we examined whether RXRα represented such a limiting factor. As shown in Fig. 3A, when RXRα was cotransfected with GFP-RARγ, both receptor proteins were found exclusively in the nucleus. Such a relocalization of RARγ from the cytoplasm to the nucleus by RXRα occurred in the absence of RA treatment and was specific, because the cytoplasmic localization of GR was not affected by RXRα overexpression. In addition, cotransfection of RARα had no effect on the cytoplasmic localization of GFP-RARγ. Thus, RXRα is an important regulator of the subcellular localization of RARγ, similar to the effect of RARγ ligand.
FIGURE 3.
Inhibition of RARγ cytoplasmic localization by RXRα. A, RXRα induces RARγ nuclear accumulation. The indicated receptor expression vectors (GFP-RARγ, 100 ng; GFP-GR, 100 ng); RXRα, 300 ng; RARα, 300 ng) were transfected into HeLa cells cultured in 24-well plates, and their subcellular localization was analyzed by immunostaining using anti-RXRα (for staining RXRα) or anti-RARα (for staining RARα) antibody. More than 95% of cotransfected cells showed nuclear localization of RXRα/RARγ or RXRα. B, C-terminal fragments of RXRα block ligand-induced nuclear translocation of RARγ. HeLa cells cultured in 24-well plates were cotransfected with expression vectors for Myc-RARγ (100 ng/well) and indicated RXRα fragments fused with GFP protein (300 ng/well), and cells were then treated with or without RA (0.1 μm). Cells were immunostained using anti-Myc antibody and examined by fluorescence microscopy. About 80% of cotransfected cells showed the images presented. C, interaction of RARγ with RXRα and RXRα mutants. The indicated GFP-tagged RXR expression vector (1 μg/dish) were cotransfected with Myc-RARγ (1 μg/dish) into HEK293T cells cultured in 60-mm dishes, and their interactions were analyzed by Co-IP assays using anti-Myc antibody. Blots were probed with either anti-GFP or anti-Myc antibody to determine the efficacy and specificity of interaction. Input represents 5% of lysates used for Co-IP experiments.
LBD of RXRα Specifically Blocks Nuclear Import of RARγ
To study how RXRα regulated RARγ subcellular localization, several RXRα mutants were constructed. An excess amount of expression vector encoding each of the RXRα mutants was transfected with Myc-RARγ to determine their effect on the cytoplasmic localization of Myc-RARγ. Deletion of the A/B domain from RXRα had no effect on its ability to induce RARγ nuclear import (not shown). Although cotransfection of RXRα completely retained Myc-RARγ in a ligand-independent manner, removal of the very C-terminal region (amino acids 386–462) from RXRα completely impaired its effect, as cotransfection of RXRα-(1–385) did not result in relocalization of RARγ into the nucleus in the absence of RA (Fig. 3B). Co-IP assay showed that RXRα-(1–385) could not heterodimerize with RARγ (Fig. 3C), suggesting that heterodimerization of RARγ and RXRα was required for their nuclear localization. Consistently, RXRα-(1–385) did not interfere with the effect of RA on inducing Myc-RARγ nuclear import. We also evaluated the effect of the RXRα LBD, RXRα-(222–462), which alone is cytoplasmic (29). The mutant bound strongly with RARγ in Co-IP assay (Fig. 3C). When RXRα-(222–462) and RARγ were cotransfected, both receptor proteins were almost exclusively localized in the cytoplasm. Surprisingly, RA failed to induce nuclear localization of RARγ, suggesting that RXRα-(222–462) blocked the effect of RA on inducing RARγ nuclear import. The nuclear presence of RXRα-(222–462) in the presence of RA likely reflected the formation of RXRα-(222–462) homodimer due to excess amount of the receptor mutant in the presence of RA. Because RXRα-(222–462) lacks the putative RXRα NLS (between amino acids160–165) (43), these results suggest that activation of the RXRα NLS is crucial for the nuclear localization of RXRα/RARγ heterodimer. To further determine the effect of RXRα-(222–462), two smaller fragments of the RXRα LBD were generated. RXRα(324–462), which bound strongly with RARγ (Fig. 3C), was able to inhibit the ability of RA in inducing RARγ nuclear import, whereas RXRα-(324–402), which bound weakly to RARγ, had no effect. Thus, induction of RARγ nuclear localization by RXRα requires its NLS and the C-terminal region of the RXRα can inhibit the ability of RA in inducing RARγ nuclear import.
LBD of RXRα Inhibits RARγ Homodimerization and Heterodimerization
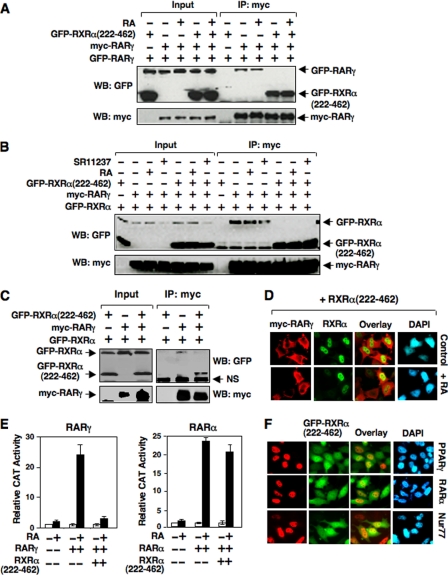
The observation that the LBD of RXRα could potently antagonize the effect of RA on regulating the subcellular localization of RARγ was interesting, because there have been several reports describing the generation of truncated RXRα fragment by proteolytic cleavage in cancer cells (47–49). To gain insight into the mechanism of RXRα-(222–462) action, we determined its effect on RARγ homodimerization and heterodimerization by Co-IP experiments. GFP-RARγ and Myc-RARγ were expressed together with or without an excess amount of GFP-RXRα-(222–462), and Myc-RARγ was immunoprecipitated using antibody against the c-Myc epitope. In the absence of GFP-RXRα-(222–462), GFP-RARγ was co-immunoprecipitated with Myc-RARγ in a RA-independent manner (Fig. 4A). When GFP-RXRα-(222–462) was cotransfected, dimerization of GFP-RARγ with Myc-RARγ was largely abolished, likely reflecting higher affinity of RARγ/RXRα-(222–462) heterodimerization than RARγ homodimerization (42). We also examined the effect of RXRα-(222–462) on RARγ/RXRα heterodimerization. GFP-RXRα, when cotransfected with Myc-RARγ, was co-immunoprecipitated by anti-Myc antibody independent of the presence of RA- and RXR-specific ligand SR11237 (Fig. 4B). However, when an excess amount of GFP-RXRα-(222–462) was cotransfected, interaction of GFP-RXRα with RARγ was inhibited (Fig. 4B). To determine the relative affinity of Myc-RARγ with RXRα and RXRα-(222–462), an equal amount of GFP-RXRα and GFP-RXRα-(222–462) was cotransfected with Myc-RARγ. Immunoprecipitation of Myc-RARγ resulted in strong co-immunoprecipitation of GFP-RXRα-(222–462) but not GFP-RXRα (Fig. 4C), consistent with our observation that RARγ interacted strongly with RXRα-(222–462) than with RXRα (Fig. 3C). Such a strong heterodimerization of RXRα-(222–462) with RARγ prompted us to examine whether it could interfere with the effect of RXRα on inducing RARγ nuclear localization. Although expression of RXRα effectively induced RARγ nuclear localization (Fig. 3A), when an excess amount of RXRα-(222–462) was cotransfected with RXRα and Myc-RARγ, Myc-RARγ was found in the cytoplasm regardless of RA treatment (Fig. 4D), suggesting an inhibitory effect of RXRα-(222–462) on RXRα-induced RARγ nuclear localization. Taken together, RXRα-(222–462) with its potent dimerization activity can modulate the dimerization property of RARγ and its ligand responsiveness.
FIGURE 4.
Effect of the LBD of RXRα, RXRα-(222–462), on RARγ dimerization and transactivation. A and B, inhibition of RARγ homodimerization (A) and RARγ/RXRα heterodimerization (B) by RXRα-(222–462). HEK293T cells cultured in 60-mm dishes were transfected with the indicated expression vectors (Myc-RARγ, 1 μg/dish; GFP-RARγ, 1 μg/dish; GFP-RXRα, 1 μg/dish; GFP-RXRα-(222–462), 3 μg/dish) and treated with RA (0.1 μm) for 1 h. Co-IP assays were conducted using anti-Myc antibody and immunoprecipitates were analyzed by Western blotting using anti-GFP antibody. C, inhibition of RARγ/RXRα heterodimerization by RXRα-(222–462). HEK293T cells cultured in 60-mm dishes were transfected with the indicated expression vectors (Myc-RARγ, 1 μg/dish; GFP-RXRα, 1 μg/dish; GFP-RXRα-(222–462), 1 μg/dish) and treated with RA (0.1 μm) for 1 h. Co-IP assays were conducted as above. NS indicates nonspecific band. D, RXRα-(222–462) prevents the effect of RXRα and RA on inducing nuclear localization of RARγ. HeLa cells cultured in 24-well plates were transfected with expression vectors for Myc-RARγ (100 ng/well), RXRα (100 ng/well), and RXRα-(222–462) (300 ng/well), and treated with RA (0.1 μm) for 1 h. Cells were stained using anti-Myc and rabbit antibody specific to N-terminal domain of RXRα followed by anti-mouse IgG-AlexaFluor 568 (Invitrogen) and anti-rabbit IgG-Cy5 (Amersham Biosciences) conjugates. The colors of Cy5 staining for full-length RXRα and GFP fluorescence were converted to green and blue, respectively, for better comparison of the localization of RARγ and full-length RXRα. E, RXRα-(222–462) inhibits the transcriptional activity of RARγ. Reporter assays were performed for RARγ or RARα as described under “Experimental Procedures.” The data shown are the means of three separate experiments. F, effect of RXRα-(222–462) on subcellular localization of RXRα heterodimerization partners. The expression vectors for peroxisome proliferator-activated receptor-γ, RARα, or Nur77 (100 ng/well) was cotransfected with GFP-RXRα-(222–462) (300 ng/well) into HeLa cells cultured in 24-well plates. The cells were immunostained using the antibodies for each nuclear receptor and analyzed by fluorescence microscopy.
LBD of RXRα Inhibits RARγ Transactivation
The fact that RXRα-(222–462) strongly antagonizes the effect of RA on inducing RARγ nuclear import prompted us to examine whether it could interfere with the effect of RA on inducing RARγ transactivation. Reporter assays using TREpal-tk-CAT reporter known to be activated by RARs (42) showed that RA strongly induced RARγ transcriptional activity. However, when RXRα-(222–462) was cotransfected, RA-induced RARγ transactivation was completely inhibited (Fig. 4E). Interestingly, activation of the TREpal-tk-CAT reporter by RARα was only slightly inhibited by RXRα-(222–462) cotransfection, demonstrating a selective inhibitory effect of RXRα-(222–462). The inhibitory effect of RXRα-(222–462) was likely due to its ability to keep RARγ but not RARα (Fig. 4F) in the cytoplasm. Interestingly, nuclear localization of several other RXRα heterodimerization partners, including peroxisome proliferator-activated receptor-γ (PPARγ) and Nur77, was not altered by cotransfection of RXRα-(222–462) (Fig. 4F). Thus, subcellular localization of RARγ may represent a regulatory mechanism for its transactivation.
N-terminal A/B Domain of RARγ Is Crucial for Its Cytoplasm Localization
Although ectopically expressed RARγ was localized in the cytoplasm, transfected RARα and RARβ were nuclear under the same conditions (Fig. 5A), suggesting that RARγ-specific sequences were responsible for its cytoplasmic retention. Among three RAR subtypes, their N-terminal A/B domains display a significant variance in sequences, whereas the sequences in their DNA-binding domain and LBD are very similar (1, 2). Therefore, we examined the role of the N-terminal A/B domain of RARγ by constructing several RARγ mutants (Fig. 5B). Deletion of the A/B domain from RARγ completely impaired its ability to reside in the cytoplasm, as the mutant (ΔN-RARγ) was exclusively nuclear (Fig. 5C). Cotransfection of ΔN-RARγ with GFP-RARγ resulted in their nuclear localization (Fig. 5D), likely due to their homodimerization (Fig. 4A). When the N-terminal A/B domain of RARγ was replaced by the corresponding domain of RARβ, the resulting chimeric protein (βAB-RARγ) (Fig. 5B) was mainly found in the nucleus. To directly test the role of the A/B domain, an expression vector encoding the A/B domain of RARγ (RARγ/AB) was transfected into HeLa cells. Interestingly, immunostaining showed that RARγ/AB was predominantly cytoplasmic. The RARγ/AB does not contain classic leucine-rich NES sequences, consistent with the inability of LMB to block RARγ cytoplasmic localization (Fig. 2C). Taken together, these results demonstrate that the N-terminal A/B domain of RARγ is responsible for its unique cytoplasmic localization.
FIGURE 5.
The N-terminal A/B domain of RARγ is crucial for cytoplasmic localization of RARγ. A, ectopically expressed RARγ, but not RARα and RARβ, is cytoplasmic. GFP-tagged RARα, -β, or -γ (100 ng/well) was transfected into HeLa cells cultured in 24-well plates, and their subcellular localization was examined by immunofluorescence microscopy. B, schematic representation of RARγ mutants. A–F domains are indicated. C, cytoplasmic localization of RARγ requires the presence of its N-terminal A/B domain. Myc-tagged ΔN-RARγ, βAB-RARγ, or RARγ/AB (100 ng/well) was transfected into HeLa cells cultured in 24-well plates, and their subcellular localization was visualized by immunostaining using anti-Myc antibody. About 95% of ΔN-RARγ-transfected cells showed nuclear staining, whereas 65% of βAB-RARγ-transfected cells and 55% of RARγ/AB-transfected cells exhibited predominant cytoplasmic receptor staining. D, ΔN-RARγ shuttles RARγ into the nucleus. GFP-RARγ (100 ng/well) and Myc-ΔN-RARγ (300 ng/well) were cotransfected into HeLa cells cultured in 24-well plates and cells were stained with anti-Myc antibody. About 80% of cotransfected cells showed nuclear localization of both proteins, whereas >95% of cotransfected cells showed their nuclear localization when treated with RA.
Regulation of RARγ Cytoplasmic Localization by Phosphorylation
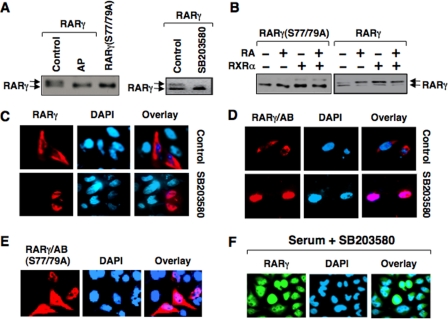
When RARγ was transfected into cells we noticed that it migrated as double bands in SDS-PAGE, and treatment of RARγ-containing lysates with alkaline phosphatase resulted in disappearance of the slow-migrating band (Fig. 6A). Thus, RARγ was phosphorylated in HeLa cells. The serine residues (Ser-77 and Ser-79 in human RARγ1) in the A/B domain of RARγ can be phosphorylated by cdk7 and p38 MAPK (12, 13). To determine whether they were responsible for RARγ phosphorylation, we constructed a RARγ mutant, in which Ser-77 and Ser-79 were mutated to alanine. The resulting mutant, RARγ(S77/79A), when transfected into HeLa cells, migrated as a single band (Fig. 6A), suggesting that Ser-77 and Ser-79 were mainly responsible for RARγ phosphorylation in the cells. Treatment of RARγ-transfected cells with SB203580, an inhibitor of the p38 MAPK known to phosphorylate Ser-77 and Ser-79 (13), resulted in disappearance of the slow migrating RARγ band (Fig. 6A), suggesting that RARγ was mainly phosphorylated by the p38 MAPK in cells, consistent with previous results (13). We also examined the effect of ligand binding and RXRα heterodimerization on RARγ phosphorylation. Again, ectopically expressed RARγ exhibited double bands on SDS-PAGE. Interestingly, the fast migrating band was reduced upon treatment of cells with RA or cotransfected with RXRα expression vector (Fig. 6B). Such an effect of RA and RXRα expression was not observed when RARγ(S77/79A) was used, suggesting that RA treatment and RXRα expression induce similar conformational change of RARγ, which renders the N-terminal phosphorylation sites of RARγ more accessible to responsible kinases.
FIGURE 6.
RARγ phosphorylation and the effect of p38 MAPK inhibitor on RARγ subcellular localization. A, transfected RARγ is phosphorylated. HEK293T cells cultured in 6-well plates were transfected with RARγ or RARγ(S77/79A) expression vector (1 μg/well) in the presence or absence of SB203580 (10 μm). Lysates prepared from RARγ-transfected cells were also treated with alkaline phosphatase (AP). Cell lysates were subject to Western blot analysis using anti-RARγ antibody. B, regulation of RARγ phosphorylation by RXRα and RA. HEK293T cells cultured in 6-well plates were transfected with RARγ or RARγ (S77/79A) expression vector (0.5 μg/well) with or without RXRα (1.5 μg/well) and treated with RA for 1 h. Cell lysates were subject to Western blot analysis using anti-RARγ antibody. C and D, inhibition of RARγ cytoplasmic accumulation by SB203580. HeLa cells cultured in 24-well plates were transfected with Myc-RARγ (C) or Myc-RARγ/AB (D) (100 ng/well), and then treated with or without SB203580 (10 μm) for 1 h. Cells were stained with anti-Myc antibody and examined by fluorescence microscopy. About 80% of transfected cells showed RARγ or RARγ/AB nuclear staining when treated with SB203580. E, mutation of phosphorylation sites in RARγ/AB impairs its cytoplasmic localization. HeLa cells cultured in 24-well plates were transfected with Myc-RARγ/AB(S77/79A) (100 ng/well). Cells were stained with anti-Myc antibody and examined by fluorescence microscopy. F, SB203580 inhibits serum-induced RARγ cytoplasmic localization. H460 cells cultured in serum-free medium for 24 h were stimulated with serum (10%) in the presence of SB203580 (10 μm) for 6 h. Cells were immunostained using anti-RARγ antibody.
To determine the role of RARγ phosphorylation in its subcellular localization, we examined the effect of SB203580 on subcellular localization of RARγ and RARγ/AB. Cells transfected with RARγ or RARγ/AB were treated with SB203580, and their subcellular localization was examined. In the absence of SB203580 treatment, RARγ and RARγ/AB were mainly cytoplasmic. In contrast, they were nuclear when cells were treated with the inhibitor (Fig. 6, C and D). When Ser-77 and Ser-79 in the A/B domain of RARγ were mutated to alanine, the resulting mutant, RARγ/ΑΒ(S77/79A), was predominantly nuclear (Fig. 6E). The observation that the A/B domain became nuclear in response to the p38 MAPK inhibitor or mutations of its phosphorylation sites was surprising because the domain does not contain a classic nuclear import signal. Thus, it is likely that nuclear localization of the A/B domain was due to its nuclear retention by binding to certain nuclear proteins under these conditions. Indeed, a previous study showed that phosphorylation of RARγ inhibited its interaction with nuclear vinexin β (50). We also examined whether SB203580 could affect the cytoplasmic localization of endogenous RARγ, and our result showed that treatment of serum-stimulated H460 lung cancer cells with SB203580 also induced nuclear localization of the endogenous RARγ (Fig. 6F). Together, these results demonstrated that phosphorylation of Ser-77 and Ser-79 is required for efficient RARγ cytoplasmic localization and that the p38 MAPK is likely involved in the phosphorylation.
Interplay between RXRα LBD and RARγ N-terminal A/B Domain
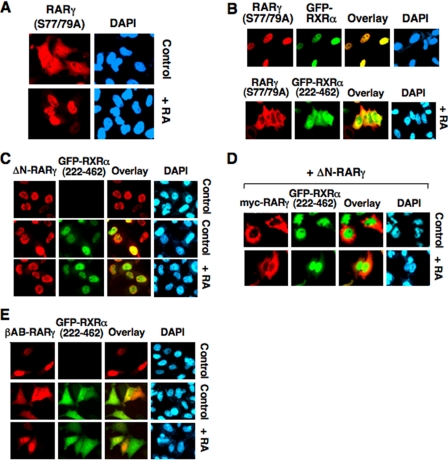
The above data demonstrated that cytoplasmic localization of RARγ depended on the presence of its N-terminal A/B domain and was inhibited by ligand binding and RXRα heterodimerization. The final localization of RARγ is likely determined by balance among these regulations. To address the contribution of each regulatory parameter, we first determined the subcellular localization of RARγ(S77/79A) and its regulation by RXRα and ligand binding. Unlike RARγ, RARγ(S77/79A) was diffusely distributed in both the cytoplasm and nucleus of cells, with predominant nuclear accumulation (Fig. 7A), likely due to lack of RARγ phosphorylation. Like RARγ, the mutant was exclusively nuclear when cells were treated with RA (Fig. 7A). Cotransfection of GFP-RXRα also led to exclusive nuclear localization of RARγ(S77/79A) (Fig. 7B). In contrast, coexpression of RXRα-(222–462) resulted in exclusive cytoplasmic localization of the RARγ mutant even in the presence of RA (Fig. 7B).
FIGURE 7.
Regulation of subcellular localization of RARγ N-terminal A/B domain mutants by RXRα and RA. A and B, regulation of subcellular localization of RARγ(S77/79A) by RXRα and RA. HeLa cells cultured in 24-well plates were transfected with RARγ(S77/79A) alone (A) or with GFP-RXRα or GFP-RXRα-(222–462) (100 ng/well) (B), and treated with RA (0.1 μm), followed by immunostaining with anti-RARγ antibody. About 95% of transfected cells showed diffused distribution of RARγ(S77/79A), which became exclusive nuclear when treated with RA, as shown in A. About 85% of cotransfected cells showed the indicated RARγ(S77/79A) staining, as shown in B. C, effect of RXRα-(222–462) on the nuclear localization of ΔN-RARγ. The Myc-ΔN-RARγ expression vector (100 ng/well) was transfected with or without GFP-RXRα-(222–462) (100 ng/well) into HeLa cells cultured in 24-well plates. Cotransfected cells were treated with RA (0.1 μm), and cells were immunostained using anti-Myc antibody and examined by fluorescence microscopy. About 95% of cotransfected cells showed the images presented. D, ΔN-RARγ differentially inhibited the cytoplasmic localization of RXRα-(222–462) and RARγ. HeLa cells cultured in 24-well plates were transfected with expression vectors for Myc-RARγ, ΔN-RARγ, and GFP-RXRα-(222–462) (100 ng/well), and treated with RA (0.1 μm) for 1 h. Cells were stained using anti-Myc antibody and examined by fluorescence microscopy. E, RXRα-(222–462) enhances cytoplasmic accumulation of βAB-RARγ. The Myc-βAB-RARγ (100 ng/well) expression vector was transfected together with GFP-RXRα-(222–462) (100 ng/well) or alone into HeLa cells cultured in 24-well plates, and cells were immunostained using anti-Myc antibody and examined by fluorescence microscopy. About 80% of cotransfected cells showed diffused distribution of βAB-RARγ and RXRα-(222–462) in the absence of RA treatment and predominant βAB-RARγ nuclear staining when treated with RA.
We next examined the subcellular localization of ΔN-RARγ and its regulation by RXRα-(222–462), taking the advantage of the unique property of this RXRα mutant in retaining RARγ in the cytoplasm. First we asked whether RXRα-(222–462) was able to retain ΔN-RARγ in the cytoplasm. Unlike the wild-type RARγ, ΔN-RARγ was exclusively nuclear (Fig. 7C). Coexpression of GFP-RXRα-(222–462) failed to block ΔN-RARγ from nuclear localization. Instead, RXRα-(222–462) was found in the nucleus together with ΔN-RARγ (Fig. 7C), suggesting that RXRα-(222–462) was shuttled by ΔN-RARγ into the nucleus through their heterodimerization. Interestingly, when ΔN-RARγ was coexpressed with GFP-RXRα-(222–462) and Myc-RARγ in cells, GFP-RXRα-(222–462) was still found in the nucleus, whereas the cytoplasmic localization of Myc-RARγ was only slightly affected (Fig. 7D), suggesting that ΔN-RARγ preferentially dimerized with RXRα-(222–462) over Myc-RARγ. The fact that RXRα-(222–462) was able to retain RARγ (Fig. 3B) and RARγ(S77/79A) (Fig. 7B), but not ΔN-RARγ, in the cytoplasm, further demonstrating the importance of the RARγ A/B domain in its cytoplasmic localization.
The role of the A/B domain was further illustrated by our examination of the regulation of the subcellular localization of βAB-RARγ. Although βAB-RARγ alone was predominantly nuclear (Fig. 7E), a majority of βAB-RARγ became cytoplasmic when coexpressed with RXRα-(222–462). The effect of RXRα-(222–462) however was largely abolished by RA as βAB-RARγ was nuclear when cells were treated with RA (Fig. 7E). These results suggested that A/B domain of RARβ could compromise the effect of RARγ A/B domain in retaining RARγ/RXRα-(222–462) heterodimer in the cytoplasm in the absence of RA. Together, our results demonstrate that an appropriate balance among the phosphorylation of the N-terminal A/B domain of RARγ, its ligand binding, and RXR heterodimerization determines the final localization of RARγ in cells.
DISCUSSION
Regulated protein movement between the nucleus and the cytoplasm provides a simple, reversible, and rapid means to regulate nuclear and cytoplasmic events and to coordinate interaction of signal transduction pathways. Recent studies have demonstrated the importance of rapid non-genomic action of nuclear receptors, including steroid hormone receptors and retinoid receptors. We report here that the subcellular localization of RARγ is unique among three RAR subtypes in that it often resides in the cytoplasm depending on cellular environment and growth conditions. We further demonstrate that the N-terminal A/B domain of RARγ is a major determinant of its cytoplasmic retention, which acts in coordination with phosphorylation, ligand binding, and RXRα heterodimerization to determine the final destination of RARγ protein in cells.
Cytoplasmic Localization of RARγ
Endogenous RARγ is nuclear in cells grown under normal conditions. However, when cells were cultured at high density, RARγ was predominantly cytoplasmic (Fig. 1A). Cell density-dependent intracellular localization of proteins is not unprecedented. For example, subcellular localization of von Hippel-Lindau tumor suppressor (51), the ERM family of proteins (52), aryl hydrocarbon receptor (53), and adenomatous polyposis coli (54) is controlled by cell density. RARγ was also cytoplasmic when cells cultured in serum-free medium were released from serum starvation or treated with growth factors (Fig. 1B). Thus, modulation of signal transduction pathways during growth and differentiation can alter the subcellular localization of RARγ. Consistently, a previous study showed that the subcellular localization RARγ is altered during cell growth and differentiation in the endometrium (27). In addition to being a mechanism regulating receptor transactivation, cytoplasmic localization of RARγ may be important for its non-genomic actions involved in the regulation of cell growth, apoptosis, and differentiation. For instance, RA treatment of NIH-3T3 cells resulted in accumulation of RARγ in the plasma membrane and its interaction with PI3K, an event that is required for RA-induced cell differentiation (55).
While cytoplasmic localization of many nuclear receptors have been shown to be dependent on the classic leucine-rich NES, which is CRM-1-dependent, a number of nuclear receptors can be localized in the cytoplasm through CRM-1-independent mechanism (56–59). Thus, protein-protein interaction with factors other than nuclear receptors has been suggested as an important regulatory mechanism for subcellular localization of nuclear receptors. Calreticulin mediates cytoplasmic localization of GR independently of classic leucine-rich NES (56), whereas RXRα is capable of binding to cytoplasmic G protein (21). The cytoplasmic accumulation of RARγ is unlikely due to increased nuclear export of the receptor protein because blocking Crm-1-mediated nuclear export with LMB had no effect on its cytoplasmic localization (Fig. 2C). Such an observation suggests that cytoplasmic accumulation of RARγ may be due to its retention in the cytoplasm through interaction with cytoplasmic proteins.
Inhibition of Cytoplasmic Localization of RARγ by Ligand Binding and RXRα Heterodimerization
Our studies demonstrate that ligand binding and RXRα heterodimerization represent two important activities that retain RARγ in the nucleus. Unlike other retinoid receptors, transfected RARγ is cytoplasmic but resides in the nucleus in response to RA (Fig. 2A). This is in analogues to steroid hormone receptors, such as GR and androgen receptor that translocate from the cytoplasm into nucleus upon ligand binding (18). In the context of GR, ligand binding induces a receptor conformation that dissociates GR from Hsp90, leading to its activation of NLS and nuclear accumulation. It is likely that RA binding induces a RARγ conformation that disfavors its binding to cytoplasmic proteins and/or activates its NLS. Ligand-induced RARγ nuclear translocation may lead to cotranslocation of cytoplasmic RARγ-binding proteins into the nucleus, thereby inhibiting their activities. It is noteworthy however that binding of RARγ to c-Src was RA-dependent (28) and that RA treatment is required for accumulation of RARγ in the plasma membrane in NIH-3T3 cells (55). Thus, ligand binding may act to induce RARγ cytoplasmic localization in some cell types and under certain conditions.
Our results show that RXRα induces RARγ nuclear localization through their heterodimerization (Fig. 3). Thus, the ratio of RXRα and RARγ proteins is another critical determinant of the subcellular localization of RARγ. RXRα heterodimerization likely inhibits the cytoplasmic accumulation of RARγ through its inhibition of RARγ binding to cytoplasmic proteins and/or activation of their NLS. On the other hand, our observation that RXRα-(222–462) suppressed RA-induced nuclear localization of RARγ (Fig. 3B) suggests that the RXRα NLS is required for its ability to retain RXRα/RARγ heterodimer in the nucleus. RARγ is not the first one whose subcellular localization is regulated by RXRα. Previous studies have shown that RXRα plays a dominant role in the nuclear localization of RXR/VDR heterodimer (46). However, RXRα is required for nuclear export of Nur77 through their unique heterodimerization in response to certain apoptotic stimuli (29).
Cytoplasmic localization of transfected RARγ was potently inhibited by ligand binding or RXRα heterodimerization, suggesting that interaction of RARγ with cytoplasmic proteins was not sufficient to antagonize the effect of ligand-binding or RXRα heterodimerization on inducing its nuclear translocation. However, under some conditions, such as superconfluent culture condition, endogenous RARγ was cytoplasmic (Fig. 1), presumably as an RARγ/RXRα heterodimer. Thus, interaction of RARγ with cytoplasmic proteins could be enhanced by certain cellular stimuli to overcome the effect of RXRα heterodimerization. Interestingly, recent studies have shown that RXR can also be cytoplasmic in response to stimuli that induce apoptosis (29), inflammation (31, 32), and differentiation (30). It remains to be seen whether and how cytoplasmic RXRα regulates RARγ activities under these conditions.
Our analysis of regulation of RARγ subcellular localization by RXRα revealed an unexpected function of RXRα LBD fragment, RXRα-(222–462), in the regulation of the localization and dimerization capacity of RARγ. The mutant prevented RA-induced RARγ nuclear localization (Fig. 3B), presumably through its inhibition of RARγ homodimerization and consequently the RARγ NLS activity (Fig. 4A). The mutant also inhibited RARγ/RXRα heterodimerization (Fig. 4, B and C). Of interest is that RXRα can actually be cleaved in cancer cells by cathepsin L-type protease to produce fragments of similar sizes to RXRα-(222–462) mutant (47–49). Whether proteolytic cleavage of RXRα functions as a regulatory mechanism of RARγ activities remains interesting to study.
N-terminal A/B Domain of RARγ Is Essential for Its Cytoplasmic Localization
Our results demonstrate that the unique N-terminal A/B domain of RARγ is the major determinant of RARγ cytoplasmic accumulation. This was illustrated by our finding that deletion of the A/B domain from RARγ abolished its cytoplasmic accumulation (Fig. 5). In addition, replacement of the A/B domain of RARγ with the corresponding domain of RARβ impaired the cytoplasmic retention of RARγ. Furthermore, the A/B domain of RARγ alone was exclusively cytoplasmic (Fig. 5). The role of the A/B domain can also be shown by our observation that RXRα-(222–462) retained RARγ (Fig. 3B), but not ΔN-RARγ (Fig. 7), in the cytoplasm. A recent study demonstrates that the A/B domain of retinoid-related orphan receptors (RORs) also mediates their cytoplasmic localization through an undefined mechanism (60). How the A/B domain acts to retain RARγ in the cytoplasm remains to be determined. As discussed above, the cytoplasmic localization of RARγ was not inhibited by LMB (Fig. 2C). The fact that the A/B domain does not contain classic leucine-rich nuclear export signal again argues against a CRM1-mediated nuclear export mechanism. Thus, the A/B domain of RARγ contains proline-rich sequences capable of binding to a number of signaling or adaptor proteins (61, 62), suggesting that the A/B domain may reside in the cytoplasm through its interaction with cytoplasmic retention factors. Support of this notion comes from a recent report that the N-terminal proline-rich sequences of RARγ are required for RARγ interaction with c-Src (28).
Binding of proline-rich sequences to signaling or adaptor proteins is subjected to regulation by phosphorylation of Ser/Thr residues within or adjacent to the proline-rich sequences (61, 62). Thus, it is interesting to note that Ser-77 and Ser-79 are located in the proline-rich sequences in the A/B domain. Consistently, phosphorylation-defective RARγ/AB (Fig. 6E) and RARγ mutant (Fig. 7A) were predominantly nuclear, and inhibition of the p38 MAPK known to phosphorylate RARγ resulted in nuclear localization of RARγ and RARγ/AB mutant (Fig. 6, C, D, and F).
Although the N-terminal A/B domain of RARγ and its phosphorylation are involved in RARγ cytoplasmic localization, their activities are regulated by RARγ ligand binding and RXRα heterodimerization. RARγ was phosphorylated and yet nuclear upon RA treatment or RXRα heterodimerization (Fig. 6B). Because ligand binding and receptor heterodimerization are known to activate NLS, such effects of RA treatment and RXRα heterodimerization on RARγ nuclear localization and phosphorylation would suggest that their activation of NLS predominates over their effect on phosphorylation. Thus, the steady-state distribution of RARγ is subjected to multiple levels of regulations, including ligand binding, receptor heterodimerization, and receptor phosphorylation, which need to act in a coordinated manner to determine its final destination in cells.
The existence of three RAR subtypes and their distinct distributions during development and in the adult organisms suggest that they have distinct modes of action and are involved in the control of different biological activities (1, 2). For instance, a recent study demonstrated a non-redundant role of RARγ in mediating the hematopoietic stem cell self-renewing effects induced by RA treatment (6). However, three subtypes show extensive sequence homolog in their DNA-binding domain and LBD, suggesting that the difference in their transcriptional regulation cannot satisfactorily explain their distinct action in vivo. Thus, our illustration of the unique property of the RARγ A/B domain and the possible underlying mechanism of action suggest that different RAR subtype may have distinct modes of action, dictated by their N-terminal A/B domains.
In summary, our results demonstrate that RARγ often resides in the cytoplasm due to its unique N-terminal A/B domain and that the subcellular localization of RARγ is subject to multiple levels of regulations that act in concert to determine the final destination of RARγ protein in cells. Ligand binding and RXRα heterodimerization act to retain RARγ in the nucleus, whereas the N-terminal A/B domain confers its cytoplasmic localization, which is also subject to regulation by phosphorylation. Loss of balance among these regulatory events will result in a shift of RARγ subcellular localization. It can be envisioned that activation of certain signal transduction pathways may result in activation and/or induction of cytoplasmic RARγ-binding protein, which, together with modulation of RARγ phosphorylation will shift RARγ protein from the nucleus to the cytoplasm. Subcellular localization of RARγ likely serves as a biological switch to mediate the cross-talk between retinoid signaling and signal transduction pathways in response to various cellular stimulations. Phosphorylation-dependent subcellular retention of RARγ will provide a simple, reversible, and rapid means to control cellular responses to different environmental signals and physiological/pathophysiological conditions.
This work was supported, in whole or in part, by National Institutes of Health Grants CA87000, CA107039, GM60544, and CA109345 (to X.-k. Z.). This work was also supported by a grant from the national nuclear R & D program funded by the Korean Ministry of Sciences and Technology, the Susan G. Komen Breast Cancer Foundation, the U.S. Army Medical Research and Material Command, the California Tobacco-Related Diseases Research Program, the California Breast Cancer Research Program, and the 985 Project and 863 Program (No. 2007AA09Z404) from Xiamen University.
- RARγ
- retinoic acid receptor-γ
- RXR
- retinoid X receptor
- RA
- retinoic acid
- NLS
- nuclear localization signal
- LBD
- ligand-binding domain
- Cdk
- cyclin-dependent kinase
- MAPK
- mitogen-activated protein kinase
- GR
- glucocorticoid receptor
- CMV
- cytomegalovirus
- Co-IP
- co-immunoprecipitation
- PBS
- phosphate-buffered saline
- CAT
- chloramphenicol acetyltransferase
- GFP
- green fluorescent protein
- LMB
- leptomycin B
- NES
- nuclear export sequence.
REFERENCES
- 1.Kastner P., Mark M., Chambon P. ( 1995) Cell 83, 859– 869 [DOI] [PubMed] [Google Scholar]
- 2.Mangelsdorf D. J., Evans R. M. ( 1995) Cell 83, 841– 850 [DOI] [PubMed] [Google Scholar]
- 3.Mark M., Ghyselinck N. B., Chambon P. ( 2006) Annu. Rev. Pharmacol. Toxicol. 46, 451– 480 [DOI] [PubMed] [Google Scholar]
- 4.Taneja R., Bouillet P., Boylan J. F., Gaub M. P., Roy B., Gudas L. J., Chambon P. ( 1995) Proc. Natl. Acad. Sci. U.S.A. 92, 7854– 7858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen C. F., Goyette P., Lohnes D. ( 2004) Oncogene 23, 5350– 5359 [DOI] [PubMed] [Google Scholar]
- 6.Purton L. E., Dworkin S., Olsen G. H., Walkley C. R., Fabb S. A., Collins S. J., Chambon P. ( 2006) J. Exp. Med. 203, 1283– 1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Husmann M., Lehmann J., Hoffmann B., Hermann T., Tzukerman M., Pfahl M. ( 1991) Mol. Cell. Biol. 11, 4097– 4103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su D., Gudas L. J. ( 2008) Biochem. Pharmacol 75, 1129– 1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X. K., Pfahl M. ( 1993) Receptor 3, 183– 191 [PubMed] [Google Scholar]
- 10.Bourguet W., Vivat V., Wurtz J. M., Chambon P., Gronemeyer H., Moras D. ( 2000) Mol. Cell 5, 289– 298 [DOI] [PubMed] [Google Scholar]
- 11.Rastinejad F., Wagner T., Zhao Q., Khorasanizadeh S. ( 2000) EMBO J. 19, 1045– 1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bastien J., Adam-Stitah S., Riedl T., Egly J. M., Chambon P., Rochette-Egly C. ( 2000) J. Biol. Chem. 275, 21896– 21904 [DOI] [PubMed] [Google Scholar]
- 13.Giannì M., Bauer A., Garattini E., Chambon P., Rochette-Egly C. ( 2002) EMBO J. 21, 3760– 3769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rochette-Egly C., Plassat J. L., Taneja R., Chambon P. ( 2000) Mol. Endocrinol. 14, 1398– 1410 [DOI] [PubMed] [Google Scholar]
- 15.Bour G., Lalevée S., Rochette-Egly C. ( 2007) Trends Cell Biol. 17, 302– 309 [DOI] [PubMed] [Google Scholar]
- 16.Taneja R., Rochette-Egly C., Plassat J. L., Penna L., Gaub M. P., Chambon P. ( 1997) EMBO J. 16, 6452– 6465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J. G., Barsky L. W., Davicioni E., Weinberg K. I., Triche T. J., Zhang X. K., Wu L. ( 2006) FASEB J. 20, 2142– 2144 [DOI] [PubMed] [Google Scholar]
- 18.Hager G. L., Lim C. S., Elbi C., Baumann C. T. ( 2000) J. Steroid Biochem. Mol. Biol. 74, 249– 254 [DOI] [PubMed] [Google Scholar]
- 19.Georget V., Lobaccaro J. M., Terouanne B., Mangeat P., Nicolas J. C., Sultan C. ( 1997) Mol. Cell. Endocrinol. 129, 17– 26 [DOI] [PubMed] [Google Scholar]
- 20.Htun H., Barsony J., Renyi I., Gould D. L., Hager G. L. ( 1996) Proc. Natl. Acad. Sci. U.S.A. 93, 4845– 4850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moraes L. A., Swales K. E., Wray J. A., Damazo A., Gibbins J. M., Warner T. D., Bishop-Bailey D. ( 2007) Blood 109, 3741– 3744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S. W., Hong J. S., Ryu S. H., Chung W. C., Yoon J. H., Koo J. S. ( 2007) Mol. Cell. Biol. 27, 6933– 6947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yen A., Roberson M. S., Varvayanis S., Lee A. T. ( 1998) Cancer Res. 58, 3163– 3172 [PubMed] [Google Scholar]
- 24.Aggarwal S., Kim S. W., Cheon K., Tabassam F. H., Yoon J. H., Koo J. S. ( 2006) Mol. Biol. Cell 17, 566– 575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cañón E., Cosgaya J. M., Scsucova S., Aranda A. ( 2004) Mol. Biol. Cell 15, 5583– 5592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dufour J. M., Kim K. H. ( 1999) Biol. Reprod 61, 1300– 1308 [DOI] [PubMed] [Google Scholar]
- 27.Fukunaka K., Saito T., Wataba K., Ashihara K., Ito E., Kudo R. ( 2001) Mol. Hum. Reprod 7, 437– 446 [DOI] [PubMed] [Google Scholar]
- 28.Dey N., De P. K., Wang M., Zhang H., Dobrota E. A., Robertson K. A., Durden D. L. ( 2007) Mol. Cell. Biol. 27, 4179– 4197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao X., Liu W., Lin F., Li H., Kolluri S. K., Lin B., Han Y. H., Dawson M. I., Zhang X. K. ( 2004) Mol. Cell. Biol. 24, 9705– 9725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katagiri Y., Takeda K., Yu Z. X., Ferrans V. J., Ozato K., Guroff G. ( 2000) Nat. Cell Biol. 2, 435– 440 [DOI] [PubMed] [Google Scholar]
- 31.Ghose R., Zimmerman T. L., Thevananther S., Karpen S. J. ( 2004) Nucl. Recept. 2, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mey J., Schrage K., Wessels I., Vollpracht-Crijns I. ( 2007) Glia 55, 152– 164 [DOI] [PubMed] [Google Scholar]
- 33.Sommer K. M., Chen L. I., Treuting P. M., Smith L. T., Swisshelm K. ( 1999) Proc. Natl. Acad. Sci. U.S.A. 96, 8651– 8656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolluri S. K., Zhu X., Zhou X., Lin B., Chen Y., Sun K., Tian X., Town J., Cao X., Lin F., Zhai D., Kitada S., Luciano F., O'Donnell E., Cao Y., He F., Lin J., Reed J. C., Satterthwait A. C., Zhang X. K. ( 2008) Cancer Cell 14, 285– 298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li H., Kolluri S. K., Gu J., Dawson M. I., Cao X., Hobbs P. D., Lin B., Chen G., Lu J., Lin F., Xie Z., Fontana J. A., Reed J. C., Zhang X. ( 2000) Science 289, 1159– 1164 [DOI] [PubMed] [Google Scholar]
- 36.Lin B., Kolluri S., Lin F., Liu W., Han Y. H., Cao X., Dawson M. I., Reed J. C., Zhang X. K. ( 2004) Cell 116, 527– 540 [DOI] [PubMed] [Google Scholar]
- 37.Chakravarti N., Lotan R., Diwan A. H., Warneke C. L., Johnson M. M., Prieto V. G. ( 2007) Clin. Cancer Res. 13, 4817– 4824 [DOI] [PubMed] [Google Scholar]
- 38.Takiyama Y., Miyokawa N., Sugawara A., Kato S., Ito K., Sato K., Oikawa K., Kobayashi H., Kimura S., Tateno M. ( 2004) J. Clin. Endocrinol. Metab. 89, 5851– 5861 [DOI] [PubMed] [Google Scholar]
- 39.Zhang X. K., Lehmann J., Hoffmann B., Dawson M. I., Cameron J., Graupner G., Hermann T., Tran P., Pfahl M. ( 1992) Nature 358, 587– 591 [DOI] [PubMed] [Google Scholar]
- 40.Zhang X. K., Salbert G., Lee M. O., Pfahl M. ( 1994) Mol. Cell. Biol. 14, 4311– 4323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han Y. H., Cao X., Lin B., Lin F., Kolluri S. K., Stebbins J., Reed J. C., Dawson M. I., Zhang X. K. ( 2006) Oncogene 25, 2974– 2986 [DOI] [PubMed] [Google Scholar]
- 42.Zhang X. K., Hoffmann B., Tran P. B., Graupner G., Pfahl M. ( 1992) Nature 355, 441– 446 [DOI] [PubMed] [Google Scholar]
- 43.Prüfer K., Barsony J. ( 2002) Mol. Endocrinol. 16, 1738– 1751 [DOI] [PubMed] [Google Scholar]
- 44.Fornerod M., Ohno M., Yoshida M., Mattaj I. W. ( 1997) Cell 90, 1051– 1060 [DOI] [PubMed] [Google Scholar]
- 45.Baumann C. T., Maruvada P., Hager G. L., Yen P. M. ( 2001) J. Biol. Chem. 276, 11237– 11245 [DOI] [PubMed] [Google Scholar]
- 46.Prüfer K., Racz A., Lin G. C., Barsony J. ( 2000) J. Biol. Chem. 275, 41114– 41123 [DOI] [PubMed] [Google Scholar]
- 47.Matsushima-Nishiwaki R., Shidoji Y., Nishiwaki S., Moriwaki H., Muto Y. ( 1996) Biochem. Biophys. Res. Commun. 225, 946– 951 [DOI] [PubMed] [Google Scholar]
- 48.Nagaya T., Murata Y., Yamaguchi S., Nomura Y., Ohmori S., Fujieda M., Katunuma N., Yen P. M., Chin W. W., Seo H. ( 1998) J. Biol. Chem. 273, 33166– 33173 [DOI] [PubMed] [Google Scholar]
- 49.Nomura Y., Nagaya T., Yamaguchi S., Katunuma N., Seo H. ( 1999) Biochem. Biophys. Res. Commun. 254, 388– 394 [DOI] [PubMed] [Google Scholar]
- 50.Bour G., Plassat J. L., Bauer A., Lalevée S., Rochette-Egly C. ( 2005) J. Biol. Chem. 280, 17027– 17037 [DOI] [PubMed] [Google Scholar]
- 51.Mohan S., Burk R. D. ( 2003) Oncogene 22, 5270– 5280 [DOI] [PubMed] [Google Scholar]
- 52.Batchelor C. L., Woodward A. M., Crouch D. H. ( 2004) Exp. Cell Res. 296, 208– 222 [DOI] [PubMed] [Google Scholar]
- 53.Ikuta T., Kobayashi Y., Kawajiri K. ( 2004) J. Biol. Chem. 279, 19209– 19216 [DOI] [PubMed] [Google Scholar]
- 54.Zhang F., White R. L., Neufeld K. L. ( 2001) Mol. Cell. Biol. 21, 8143– 8156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Masiá S., Alvarez S., de Lera A. R., Barettino D. ( 2007) Mol. Endocrinol. 21, 2391– 2402 [DOI] [PubMed] [Google Scholar]
- 56.Holaska J. M., Black B. E., Love D. C., Hanover J. A., Leszyk J., Paschal B. M. ( 2001) J. Cell Biol. 152, 127– 140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu J., DeFranco D. B. ( 2000) Mol. Endocrinol. 14, 40– 51 [DOI] [PubMed] [Google Scholar]
- 58.Tyagi R. K., Amazit L., Lescop P., Milgrom E., Guiochon-Mantel A. ( 1998) Mol. Endocrinol. 12, 1684– 1695 [DOI] [PubMed] [Google Scholar]
- 59.Glass C. K., Rosenfeld M. G. ( 2000) Genes Dev. 14, 121– 141 [PubMed] [Google Scholar]
- 60.Aschrafi A., Meindl N., Firla B., Brandes R. P., Steinhilber D. ( 2006) Biochim. Biophys. Acta 1763, 805– 814 [DOI] [PubMed] [Google Scholar]
- 61.Li S. S. ( 2005) Biochem. J. 390, 641– 653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zarrinpar A., Bhattacharyya R. P., Lim W. A. ( 2003) Sci STKE 2003, RE8. [DOI] [PubMed] [Google Scholar]