Abstract
The inflammasome is a multiprotein complex involved in innate immunity. Activation of the inflammasome causes the processing and release of the cytokines interleukins 1β and 18. In primary macrophages, potassium ion flux and the membrane channel pannexin 1 have been suggested to play roles in inflammasome activation. However, the molecular mechanism(s) governing inflammasome signaling remains poorly defined, and it is undetermined whether these mechanisms apply to the central nervous system. Here we show that high extracellular potassium opens pannexin channels leading to caspase-1 activation in primary neurons and astrocytes. The effect of K+ on pannexin 1 channels was independent of membrane potential, suggesting that stimulation of inflammasome signaling was mediated by an allosteric effect. The activation of the inflammasome by K+ was inhibited by the pannexin 1 channel blocker probenecid, supporting a role of pannexin 1 in inflammasome activation. Co-immunoprecipitation of neuronal lysates indicates that pannexin 1 associates with components of the multiprotein inflammasome complex, including the P2X7 receptor and caspase-1. Moreover antibody neutralization of the adaptor protein ASC (apoptosis-associated speck-like protein containing a CARD) blocked ATP-induced cell death in oocytes co-expressing P2X7 receptor and pannexin 1. Thus, in contrast to macrophages and monocytes in which low intracellular K+ has been suggested to trigger inflammasome activation, in neural cells, high extracellular K+ activates caspase-1 probably through pannexin 1.
Pannexin 1 is a vertebrate ortholog of the invertebrate innexin gap junction proteins (1), but it does not appear to form functional gap junctions in vivo. Instead pannexin 1 acts as a membrane channel that carries ions and signaling molecules between the cytoplasm and the extracellular space (2, 3). As such, it is a candidate ATP release channel in various cell types, including erythrocytes, astrocytes, bronchial epithelial cells, and taste cells. Various functional roles have been ascribed to pannexin 1 including local vascular perfusion control and propagation of intercellular calcium waves (4–6). Recently pannexin 1 was also shown to form the large pore of the P2X7 purinergic receptor (7, 8). P2X7 plays a major role in inflammation, and its activation by extracellular ATP results in release of interleukin (IL)2-1β from macrophages, probably involving pannexin 1 as a signaling molecule (7).
IL-1β production and maturation are tightly regulated by caspase-1 incorporated into large protein complexes termed inflammasomes (9–11). The molecular composition of the inflammasome depends on the identity of the NOD-like receptor (NLR) family member serving as a scaffold protein in the complex (12). The members of the cytosolic NLR family appear to recognize conserved microbial and viral components termed pathogen-associated molecular patterns in intracellular compartments (13). The bipartite adaptor protein apoptosis-associated speck-like protein containing a CARD (ASC) bridges the interaction between NLR proteins and inflammatory caspases and plays a central role in the assembly of inflammasomes and the activation of caspase-1 in response to a broad range of pathogen-associated molecular patterns and intracellular pathogens (14). In addition, the inflammasome can be activated by danger-associated molecular patterns, molecules endogenous to the organism that signal stress or injury, including extracellular ATP acting at ionotropic P2X7 receptors, fibronectin, or monosodium urate crystals (15, 16). Moreover it has been suggested that a rapid K+ efflux through ATP-activated P2X7 receptors induces inflammasome assembly (17–20).
Despite the recent advances in the understanding of accessory proteins required for full activation of caspase-1, little is known about the signaling pathways that trigger inflammasome activation, particularly in the central nervous system (CNS). Recently we reported that spinal cord neurons contain the NLRP1/NALP1 inflammasome consisting of NLRP1, ASC, caspase-1, caspase-11, and the X-linked inhibitor of apoptosis protein (XIAP) and that spinal cord injury induces rapid activation of the inflammasome, causing processing and secretion of IL-1β and IL-18. Moreover antibody neutralization of ASC reduces caspase-1 activation and IL-1 cytokine processing, leading to significant tissue sparing and functional improvement (21). In this study, we focused on signaling events coupling pannexin 1 and P2X7 receptors to rapid caspase-1 activation in primary neurons and astrocytes. We provide compelling evidence that high extracellular K+ opens the pannexin 1 channel and activates inflammasomes in neurons and astrocytes, but not THP-1 cells, thus leading to caspase-1 activation. This signaling pathway in neurons is mediated through protein interactions between pannexin 1 and inflammasome proteins. We also provide evidence that ATP acting on P2X7 induces rapid cell death and that antibody neutralization of ASC blocks ATP-induced cell death. Thus, contrary to the widely accepted view in macrophages and monocytes that low intracellular K+ triggers inflammasome activation, high extracellular K+ surrounding cells such as neurons and astrocytes opens pannexin 1 channels and induces processing of caspase-1.
EXPERIMENTAL PROCEDURES
Antibodies
Rabbit anti-Rattus norvegicus ASC and NLRP1 antisera were prepared by Bethyl Laboratories as described previously (21). Chicken anti-pannexin 1 (4515) has been characterized (4), and antibody affinity-purified on a matrix with the cognate peptide (prepared by Aves Labs Inc.) was used. Other antibodies were purchased from commercial sources and included anti-NLRP1 (Abcam), anti-IL-1β (Cell Signaling Technology Inc.), anti-caspase-1 (Upstate); anti-caspase-1 (Santa Cruz Biotechnology, Inc.), anti-caspase-11 (Alexis Biochemicals), anti-caspase-11 (Santa Cruz Biotechnology, Inc.), anti-XIAP (BD Transduction Laboratories), anti-caspase-3 (Santa Cruz Biotechnology, Inc.), and anti-P2X7 receptor (Calbiochem).
Cell Culture and Treatment
Neuronal cultures were prepared from embryonic day 16–17 rat cortices as described previously (22, 23). Cortical tissue was disrupted into a cell suspension by gentle trituration and seeded in 60-mm dishes at a density of 2 × 106 cells/dish. Neurons were grown on poly-l-lysine-coated tissue culture dishes in N5 medium that contained 5% serum fraction (24). Neurons were maintained for 12 days, and the neuronal nature of the majority of cells (95%) was confirmed electrophysiologically and immunohistochemically (22). Primary astrocytes were obtained from neonatal rat cerebral cortices as described previously (22). The cells were maintained for 3 weeks in Dulbecco's modified Eagle's medium supplemented with 10% horse serum. At least 99% of the cell populations were astrocytes as determined by staining with cell-specific markers (22). The human monocytic cell line THP-1 (American Type Culture Collection) was maintained in RPMI 1640 medium supplemented with 0.05 mm 2-mecaptoethanol and 10% fetal bovine serum. Cells were pretreated with 1 mm probenecid (Alfa Aesar) for 10 min. The medium was removed and replaced with medium containing probenecid and 130 mm KCl for 30 min, 1 h, and 2 h, whereas controls received probenecid alone. Cells were washed once in ice-cold PBS and lysed as described previously (25) and prepared for immunoblot analysis.
Pannexin 1 Knockdown
Pannexin 1 was knocked down using short hairpin (sh)RNA inserted into a retroviral silencing plasmid (pRS) purchased from Origene. One microgram of Panx1 shRNA expression plasmids containing puromycin resistance was transfected into the human 1321N1 astrocytoma cells plated in 35-mm dishes using Lipofectamine 2000 reagent. After overnight incubation, transfection reagents were removed, and cells were transferred to 100-mm dishes containing selection medium (Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 1 μg/ml puromycin). After 2–3 weeks in selection medium, clones were tested for appropriate Panx1 knockdown (18) using Western blot analysis. The selected Panx1-KD 1321N1 cells were maintained in selection medium in a humidified chamber (100% humidity, 95% air, 5% CO2, 37 °C).
Immunoblotting
Primary cell cultures were lysed in lysis buffer (20 mm Tris, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 1% Triton X-100, 2.5 mm pyrophosphate, 1 mm β-glycerophosphate) with protease inhibitor mixture (Sigma-Aldrich). For immunoblot analysis of oocytes, each lane contained extracts of three oocytes that were pooled and lysed in 50 μl of oocyte Ringer's solution (OR2) by repeated passage through the end of a 1-ml disposable pipette tip. Cells were spun at 12,000 × g for 3 min, and samples were taken from the supernatant, avoiding both the pellet and the lipids on the surface. Laemmli sample buffer was added, and proteins were resolved on 10–20% Tris-HCl Criterion precast gels (Bio-Rad), transferred to polyvinylidene difluoride membranes (Millipore), placed in blocking buffer (PBS, 0.1% Tween 20, 0.4% I-Block (Applied Biosystems)), and then incubated for 1 h with primary antibodies followed by appropriate secondary horseradish peroxidase (HRP)-linked antibodies (Cell Signaling Technology Inc.). Visualization of signal was enhanced by chemiluminescence using a Phototope-HRP detection kit (Cell Signaling Technology Inc.). To control for protein loading, immunoblots were stripped with Restore Western blot stripping buffer (26) and blotted for β-tubulin using monoclonal anti-β-tubulin antibody (1:5000, BD Biosciences Pharmingen). Quantification of band density was performed using UN-SCAN-IT gelTM digitizing software (Silk Scientific, Inc.), and data were normalized to β-tubulin.
Co-immunoprecipitation
To assess the protein composition and association of proteins in the inflammasome, primary neuronal cultures (2 × 106 cells) were lysed in 200 μl of lysis buffer (20 mm Tris, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 1% Triton X-100, 2.5 mm pyrophosphate, 1 mm β-glycerophosphate) with protease inhibitor mixture (Sigma-Aldrich). Approximately 200 μg of neuronal lysates were immunoprecipitated with anti-ASC or anti-pannexin 1 antibodies using TrueBlotTM anti-rabbit Ig or PrecipHen (Aves Labs Inc.) immunoprecipitation beads. Neuronal lysates were precleared by adding 50 μl of anti-rabbit TrueBlot beads to 200 μg of lysate in a microcentrifuge tube. The mixture was incubated for 1 h at 4 °C, and beads were pelleted by centrifugation at 12,000 × g for 30 s. The supernatant was recovered and immunoprecipitated with 5 μg of anti-ASC or anti-pannexin 1 and incubated at 4 °C overnight. Fifty microliters of anti-rabbit TrueBlot beads or anti-chicken PrecipHen beads were added to the mixture, incubated for 2 h, and then centrifuged at 12,000 × g for 30 s. The pelleted beads were washed five times in lysis buffer, resuspended in loading buffer, and heated at 95 °C for 3 min before analysis by immunoblotting using antibodies against ASC, NLRP1, capase-11 and caspase-1, caspase-3, XIAP, and pannexin 1.
Preparation of Oocytes
Oocytes were prepared as described previously (27). Xenopus laevis oocytes were isolated by incubating small pieces of ovary in 2 mg/ml collagenase type I (Worthington) in Ca2+-free OR2 (82.5 mm NaCl, 2.5 mm KCl, 1.0 mm MgCl2, 1.0 mm CaCl2, 1.0 mm Na2HPO4, 5.0 mm HEPES (pH 7.5) with antibiotics (10,000 units/ml penicillin and 10 mg/ml streptomycin) and stirring at one turn/s for 3 h at room temperature. After thorough washing with regular OR2, oocytes devoid of follicle cells and having a uniform pigmentation were selected and stored in OR2 at 18 °C.
cRNA and Electrophysiology
RNA for mouse pannexin 1 was prepared using the mMessage mMachine in vitro transcription kit (Ambion). Oocytes were injected with 20–40 nl of cRNA (1 μg/μl) and incubated for 18–48 h at 18 °C. RNA for human pannexin 1 and P2X7 was similarly prepared and injected. Oocytes were then incubated for 7–8 days prior to electrophysiological analysis at room temperature. Oocytes were tested using two-electrode voltage clamp (Model OC725C, Warner Instruments) under constant perfusion according to the protocols described in the figures.
ASC Antibody Neutralization
Oocytes expressing both human pannexin 1 and P2X7 receptor were tested 7–8 days after injection of RNA. Cells were clamped at −40 mV, and 5-s test pulses to −35 mV were applied. After base-line readings were taken in OR2, cells were exposed to 500 μm ATP for 4 min and then returned to OR2. After it was established that the ATP reliably resulted in cell death, remaining co-expressing cells were then injected with 60 nl of anti-ASC (diluted 1:10 in PBS) or 60 nl of control IgG (diluted 1:10 in PBS). After 1 h, the reinjected cells were exposed to 500 μm ATP and tested as before.
Dye Uptake
Parental and Panx1-KD 1321N1 human astrocytoma cells were exposed for 30 min to control solution (145 mm NaCl, 5 mm KCl, 1.4 mm CaCl2, 1.0 mm MgCl2, 10 mm HEPES, pH 7.4) and to a depolarizing solution (60 mm NaCl, 50 mm KCl, 1.4 mm CaCl2, 1.0 mm MgCl2, 10 mm HEPES, pH 7.4) containing 10 μm YoPro1. After treatment, cells were washed with control solution and then fixed with p-formaldehyde, and cover slips were mounted using Vectashield mounting medium containing 4′,6-diamidino-2-phenylindole. YoPro fluorescence intensity was measured from the whole field of view of high K+ (50 mm)-treated cells and normalized to that obtained under control (5 mm K+) conditions. Images were acquired using MetaFluor software (Universal Imaging Corp.) and a digital camera (Photometrics HQ2) attached to a Nikon inverted TE-2000E microscope equipped with a 10× objective and fluorescein isothiocyanate and 4′,6-diamidino-2-phenylindole filter sets.
RESULTS
High Extracellular K+ Activates Pannexin 1
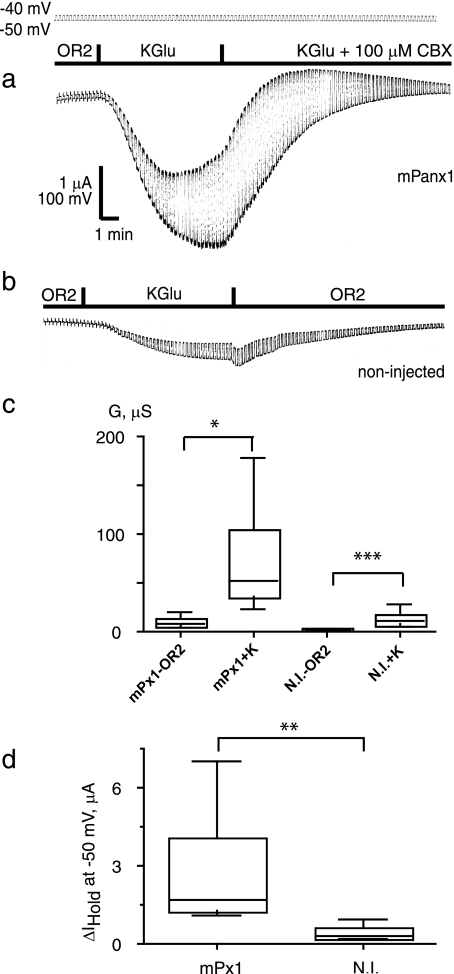
Pannexin 1 is a channel that releases ATP and other ions from the cell. Accordingly an increase of extracellular K+ concentration causes ATP release from Xenopus oocytes expressing pannexin 1 (2, 28, 29). However, electrophysiological data suggest that pannexin 1 opens only when depolarized to positive membrane potentials. To investigate this apparent discrepancy, we applied 130 mm K+ to pannexin 1 channels in voltage-clamped oocytes held at potentials at which the channel is normally closed. When high extracellular K+ was applied, currents in response to a 10-mV test pulse from a holding potential of −50 mV increased in a reproducible and reversible fashion (Fig. 1a and supplemental Fig. 1A). The K+-induced current was attenuated by carbenoxolone (Fig. 1a) and by probenecid (supplemental Fig. 2). In contrast, application of extracellular K+ to noninjected oocytes increased conductance only moderately (Fig. 1b). To quantify this increase, we measured the conductance of oocytes to this test pulse and found that 130 mm K+ increased conductance substantially in pannexin 1-expressing oocytes (Fig. 1c). Application of 130 mm K+ also increased the holding current needed to maintain cells at −50 mV compared with noninjected oocytes (Fig. 1d). The effect of K+ was dose-dependent. The lowest potassium concentration required for detectable activation of pannexin 1 currents was 20 mm (supplemental Fig. 1B). Replacement of Na+ with choline+ did not activate pannexin (not shown), indicating that the observed effects are due to K+ and not the removal of Na+. Potassium chloride was as effective as potassium gluconate to activate pannexin 1 channels (not shown).
FIGURE 1.
Activation of pannexin 1 channels by extracellular K+. Oocytes were voltage-clamped at a holding potential of −50 mV, and 10-mV depolarizing pulses at a rate of five/min were applied. Perfusion with a high K+ (130 mm) solution resulted in a large inward current and increase in membrane conductance that was sensitive to carbenoxolone (CBX) (a). High K+ induced a smaller response in uninjected oocytes (b). Box plots of changes in membrane conductance (c) and holding currents (d) induced by high K+ in oocytes expressing mouse pannexin 1 (mPx1) and in noninjected oocytes (N.I.) are shown. The box plots are used conventionally (smallest observation, lower quartile, median, upper quartile, and largest observation). Two-tailed t tests (paired (c) and unpaired (d)) yielded significance levels of <0.05 (*), 0.01 (**), or 0.001 (***) as shown. n = 8 (Panx1) and 10 (noninjected). KGlu, potassium gluconate; G, conductance; μS, microsiemens.
FIGURE 2.
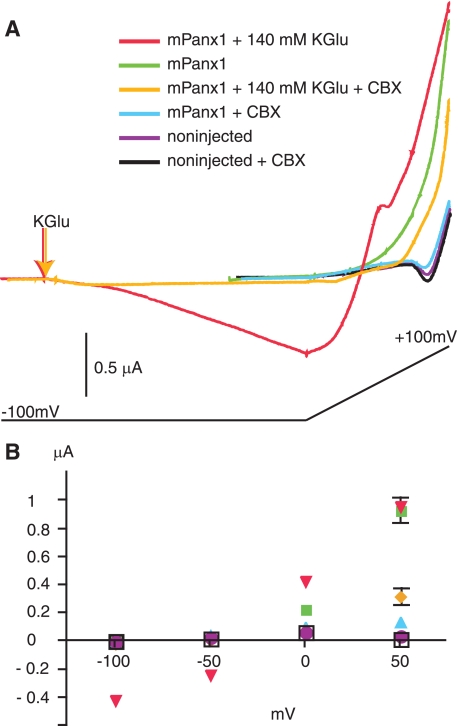
Voltage dependence of activation of pannexin 1 channels by high extracellular K+. A, currents from oocytes held in OR2 or perfused with 140 mm potassium gluconate (KGlu). Cells were held at −100 mV and subjected to a voltage ramp lasting 1 min from −100 mV to + 100 mV as indicated below the current traces. In OR2, cells expressing mPanx1 showed much larger current than noninjected cells, and addition of carbenoxolone (CBX) (100 μm) reduced mPanx1 currents to noninjected levels. Carbenoxolone had no effect on noninjected cells. Addition of potassium gluconate to mPanx1-expressing cells resulted in inward currents at −100 mV and increased outward currents at positive potentials. Carbenoxolone dramatically reduced both the inward and outward currents. B, quantification of currents recorded at −100, −50, 0, and +50 mV. Data are mean ± S.E.; n = 4–5. Where not visible, error bars are smaller than symbols.
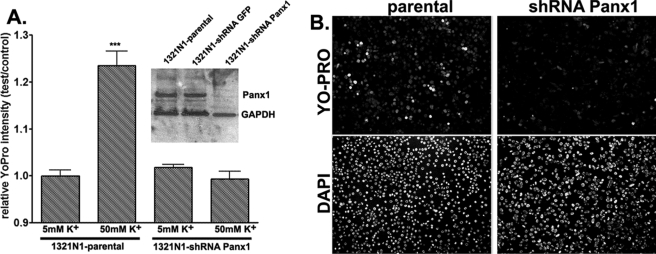
The activation of pannexin 1 currents by high extracellular K+ was observed over a wide range of voltages (Fig. 2) with depolarization facilitating the process. Only at high positive potentials was the effect of high K+ minimal, probably because of exhaustion of recruitable channels. Taken together, these data support the hypothesis that high extracellular K+ opens pannexin 1 channels at resting potentials at which the channel is normally closed. To test whether K+ also stimulates pannexin 1-mediated dye uptake, we used the astrocytoma cell line 1321N1. Thirty-minute exposure of 1321N1 cells to a depolarizing solution (50 mm K+) caused a significant influx of YoPro1 into cells compared with those bathed in normal (5 mm K+) solution (control, 0.99 ± 0.012-fold; high K+, 1.24 ± 0.031-fold; n = 6 fields from three experiments) (Fig. 3). High K+-induced dye uptake was significantly attenuated (0.99 ± 0.017-fold, n = 6 fields from three experiments) when Panx1 was knocked down with Panx1 shRNA (Fig. 3).
FIGURE 3.
Pannexin 1-mediated membrane permeabilization. A, relative YoPro fluorescence intensity (test/control, mean ± S.E., n = 6, two fields from three independent experiments) measured from parental and shRNA-Panx1 1321N1 cells treated for 30 min at 37 °C with normal (5 mm) and high (50 mm) K+ solutions (osmolalities adjusted by reducing equimolar amounts of NaCl). After treatments, cells were fixed and counterstained with 4′,6-diamidino-2-phenylindole. Fluorescence intensity was measured from the whole field of view (10× objective) and normalized to that obtained under control (5 mm K+) conditions. Inset, Western blot showing expression of Panx1 in parental 1321N1 cells and in two stable clones, one expressing an irrelevant shRNA (shRNA-green fluorescent protein (GFP)) and another shRNA-Panx1. B, representative images of YoPro- and 4′,6-diamidino-2-phenylindole (DAPI)-stained parental and shRNA-Panx1 1321N1 cells exposed to 50 mm K+ solution. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Probenecid Is a “Specific” Inhibitor of Pannexin
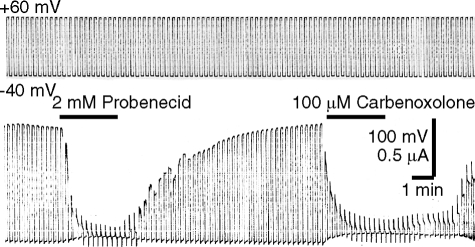
Distinguishing the functions of pannexin 1 channels from connexin channels has been hampered by the lack of specific inhibitors able to discriminate between them. Despite the lack of any sequence homology between pannexins and connexins, known inhibitors of connexins also inhibit pannexin channels. Carbenoxolone demonstrates some specificity as its dose dependence for inhibition of pannexin and connexins differs by a factor of ∼3 (30). We have reported that a more specific inhibitor of pannexin 1 is probenecid, a common drug used to treat gout and gouty arthritis (29). Application of probenecid rapidly inhibited voltage-induced pannexin 1 currents in oocytes as did carbenoxolone (Fig. 4). We also found that probenecid significantly reduced the currents in response to application of 130 mm extracellular K+ (supplemental Fig. 2), providing additional evidence that pannexin 1 is responsible for the currents in response to the extracellular K+ application.
FIGURE 4.
Probenecid inhibits pannexin 1 currents. Oocytes expressing pannexin 1 were held at −40 mV, and pulses to +60 mV were applied to open pannexin 1 channels. Channel activity was inhibited by probenecid and by carbenoxolone.
K+ Activation of Inflammasomes Is Blocked by the Pannexin 1 Inhibitor Probenecid
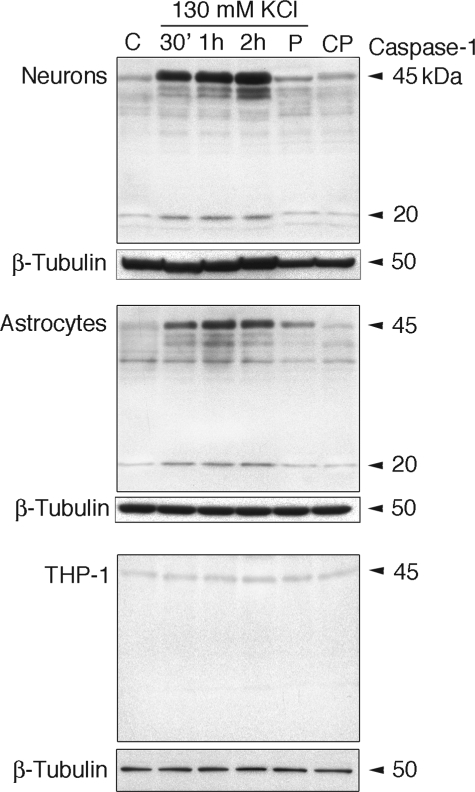
Neurons in culture express the NLRP1 inflammasome (21). To test whether high extracellular K+ activates inflammasomes and caspase-1, we treated rat cortical neurons and astrocytes and human THP-1 cells grown in culture with 130 mm KCl for 30 min, 1 h, and 2 h and assayed protein lysates for caspase-1 activation. As shown in Fig. 5, neurons and astrocytes exposed to high extracellular K+ showed an increase in caspase-1 activation when compared with untreated controls.
FIGURE 5.
Caspase-1 maturation induced by high extracellular K+ in neurons and astrocytes, but not in THP-1 cells, is blocked by probenecid. Primary cortical neurons, astrocytes, and THP-1 cells were maintained in culture and treated for 30 min (30′), 1 h, and 2 h with 130 mm KCl. Other cultures (P) were pretreated with 1 mm probenecid for 10 min, and the medium was removed and replaced with medium containing probenecid and 130 mm KCl for 30 min, whereas controls (CP) received probenecid alone. β-Tubulin was used as an internal standard and control for protein loading. C, media change only.
As shown by others (20), human THP-1 cells were refractory to high extracellular K+ exposure and therefore served as control. Pretreatment with the pannexin channel blocker probenecid (1 mm) prior to high K+ treatment suppressed caspase-1 induction in neurons and astrocytes (Fig. 5). Thus, inflammasome activation and caspase-1 processing induced by high extracellular K+ are inhibited by blocking the pannexin 1 channel, suggesting a role for pannexin 1 in the activation of the inflammasome in neurons and astrocytes but not THP-1 cells.
Stimulation of the inflammasome in neurons and astrocytes also led to the release of IL-1β (supplemental Fig. 3). Curiously the supernatant contained more of the unprocessed than the mature form of IL-1β. The rapid increase in caspase-1 expression after stimulation is in agreement with observations made in CNS injury models (21, 31, 32). A similar rapid increase of caspase-1 is also observed in macrophages exposed to Gram-negative bacteria or other agents like pathogen-associated molecular patterns (9, 33).
For an alternate test for a role of pannexin 1 in the activation of the inflammasome by K+ we used the 1321N1 cell line. The parental cell line exhibited an activation pattern of caspase-1 similar to that of primary astrocytes and neurons in response to stimulation with high K+. In contrast, caspase-1 in the pannexin 1 knockdown line was barely detectable and did not show increased activation (Fig. 6). The reduced expression of caspase-1 could be due to either transcriptional/translational effects or stability of the various components of the inflammasome multiprotein complex. It is conceivable that unassembled caspase-1 is degraded faster than the complexed protein.
FIGURE 6.
Caspase-1 activation is attenuated in pannexin 1 knockdown cells. A, representative immunoblot of 1321N1 parental cells and knockdown cells probed for pannexin 1. B, stimulation of both cell types with 130 mm KCl for the indicated times and immunoblot for caspase-1. 30′, 30 min; C, media change only.
The NLRP1 Inflammasome Complex in Neurons Interacts with Pannexin 1 and the P2X7 Receptor
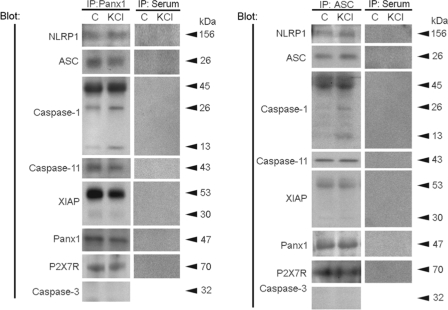
To characterize associations of inflammasome proteins in cortical neurons in culture, co-immunoprecipitations of neuronal lysates exposed to 130 mm KCl for 1 h were performed using anti-pannexin 1 antibody (Fig. 7, left). Anti-pannexin 1 immunoprecipitated NLRP1, ASC, caspase-1 and -11, XIAP, pannexin 1, and the P2X7 receptor. Caspase-1 was processed into its fragments (p26 and p13), and full-length XIAP was cleaved into a 30-kDa fragment. Thus, exposure to high extracellular K+ activates inflammasomes. Anti-pannexin 1 did not immunoprecipitate caspase-3, serving as a control. Caspase-3, however, was expressed in neurons as indicated by its presence in the lysate (supplemental Fig. 4). Like caspase-1, caspase-3 levels increased with exposure to extracellular K+. In reciprocal co-immunoprecipitation experiments, anti-ASC immunoprecipitated NLRP1, ASC and caspase-1 and -11 as well as XIAP, pannexin 1, and the purinergic receptor P2X7 (Fig. 7, right). These findings demonstrate that the NLRP1 inflammasome in neurons associates with pannexin 1 and the P2X7 receptor.
FIGURE 7.
The NLRP1 inflammasome in neurons interacts with pannexin 1 and the P2X7 receptor. Left, co-immunoprecipitation (IP) with pannexin 1 of lysates of primary neurons (left lane; C) and neurons treated with 130 mm KCl for 30 min (right lane; KCl). Pannexin 1 immunoprecipitates were blotted for NLRP1, ASC, caspase-1, caspase-11, XIAP, pannexin 1 (Panx1), P2X7 receptor (P2X7R), and caspase-3 (control). Pannexin 1 immunoprecipitated NLRP1, ASC, caspase-1, XIAP, and caspase-11, thus indicating association of these proteins in a multiprotein complex. In reciprocal co-immunoprecipitations (right), anti-ASC immunoprecipitated NLRP1, ASC, caspase-1, XIAP, caspase-11, Panx1, and P2X7R, indicating protein interactions. Preimmune serum did not immunoprecipitate inflammasome proteins, Panx1, or P2X7R and was used as control.
The co-immunoprecipitation pattern was similar in unstimulated and stimulated neurons, suggesting that the inflammasome complex is preassembled. This observation is consistent with findings that the NLRP1 inflammasome is preassembled in motor neurons of the spinal cord (21). Thus, signaling within the chain of inflammasome components in neurons probably results from allosteric events rather than assembly of the NLRP1 complex.
Inhibition of the Inflammasome Prevents Cell Death in Pannexin 1 + P2X7-co-expressing Cells
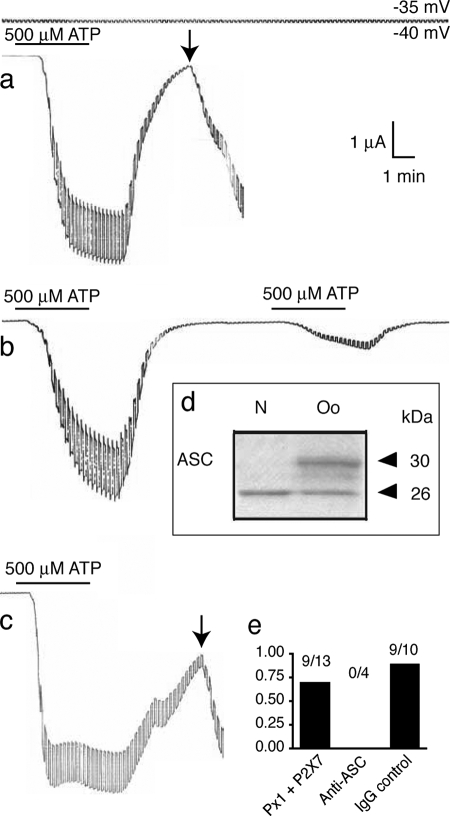
Repetitive or sustained application of ATP to cells co-expressing the P2X7 purinergic receptor and pannexin 1 causes cell death (supplemental Fig. 5) (8). Oocytes expressing either pannexin 1 or the P2X7 receptor alone did not lose membrane integrity when subjected to the same stimulus protocol (4, 8). ATP-induced oocyte death is characterized by an irreversible electrical breakdown of the cell membrane even after ATP has been removed (Fig. 8). To determine whether activation of the inflammasome is necessary for this ATP-induced cell death, we first tested oocytes for the presence of the inflammasome protein ASC. Fig. 8d shows that a protein of appropriate size (26 kDa) is recognized by an anti-ASC antibody in an oocyte lysate. For functional testing, we injected 60 nl of anti-ASC antibody (diluted 1:10 in PBS) into oocytes 1 h before electrophysiological analysis. As shown previously (8), cells expressing human pannexin 1 and P2X7 exposed to 500 μm ATP for 4 min subsequently underwent cell death (Fig. 8a). In contrast, all cells injected with anti-ASC fully recovered from prolonged ATP exposure and also recovered from a second 4-min ATP exposure (Fig. 8b). Injection of a nonspecific immunoglobulin (IgG) did not protect cells (Fig. 8c), demonstrating that anti-ASC was responsible for cell survival and suggesting that ASC is necessary for ATP-induced cell death.
sFIGURE 8.
Prevention of cell death by anti-ASC antibody. a, stimulation of oocytes co-expressing pannexin 1 and P2X7 receptor with 500 μm ATP for 4 min resulted in membrane breakdown (arrow) at a time when pannexin 1 and P2X7 currents had dissipated. b, preinjection (1 h) of 6 ng of anti-ASC prevented membrane breakdown, and oocytes survived repeated applications of ATP. c, preinjection of an unrelated rabbit IgG (Cx43; 1.5 ng) did not prevent membrane breakdown following ATP application. d, immunoblotting of oocyte lysates (Oo) with ASC antibody reveals the presence of ASC. The identity of the second band reacting with the anti-ASC antibody is not known. Neuronal lysates (N) served as control. e, quantitative analysis of cell death. The fraction of oocytes with membrane breakdown is plotted.
DISCUSSION
The results of the present study show that pannexin 1 channels as well as the inflammasome can be activated by high extracellular K+. Activation of pannexin 1 channels by extracellular K+ is not due to the depolarization resulting from the elimination of the transmembrane K+ gradient because it occurred in cells voltage-clamped to the resting membrane potential. Consequently the ions must bind to an extracellular moiety that is either part of the pannexin 1 sequence or of an auxiliary molecule. This activation mechanism by an extracellular ion as a ligand is unusual as channel activation by ions is typically observed intracellularly. Only protons are also known to induce activity of the sodium channel ENaC from the extracellular side (34) apparently by relieving self-inhibition of the channel by Na+ (35). Protons also activate acid-sensing ion channels (36).
Whether K+ activation of pannexin 1 channels occurs in vivo is unclear. The high concentrations of K+ ions required for channel activation (>20 mm) might only come about in the vicinity of dying cells releasing their cytoplasmic contents into a confined space. Alternatively efflux of K+ through pannexin 1 channels could create a locally high K+ concentration sufficient to activate the channel. This mechanism would represent an additional positive feedback loop of pannexin 1 activation. Pannexin 1 channels are prime candidates to serve as ATP release channels. When co-expressed with P2Y or with P2X7 receptors, extracellular application of ATP results in pannexin 1-mediated membrane currents (8, 37). This type of positive feedback is consistent with the observation of ATP-induced ATP release (38). Repetitive or sustained application of ATP to cells co-expressing P2X7 receptor with pannexin 1 results in cell death (8). Thus, there must be regulatory mechanisms that keep this potentially deadly channel in check. Indeed ATP has recently been identified as a pannexin 1 channel inhibitor and thus acts as a permeant regulating its own permeation pore (39).
A role of K+ ions in the activation of the inflammasome is well documented (20, 40). The mechanism of action, however, is unclear. Based on experiments with potassium ionophores it has been proposed that K+ efflux with commensurate reduction in cytoplasmic K+ concentration is the trigger for the various components of the inflammasome to assemble into the active macromolecular complex. However, it is debatable whether this mechanism is operative in vivo. In particular, in tissues where the K+ efflux is into confined spaces then such a mechanism seems unlikely. As is well known from excitable cells, thousands of action potentials can be fired without significant changes in ion concentrations even when the transporters responsible for the ion gradients are blocked. In the case of K+ flux into confined spaces, the driving force of the flux would soon decline. Furthermore the target of the inflammasome, caspase-1-mediated conversion of pro-IL-1β, can be blocked without changes of K+ fluxes (41, 42). Thus, there should be another pathway for activation of the inflammasome.
Recent evidence indicates that pannexin 1 plays a crucial role in inflammation explicitly through its association with the P2X7 receptor (7, 43). Knockdown of pannexin 1 by short interfering RNA attenuates the release of IL-1β from stimulated macrophages, and pannexin 1 currents can be induced by ATP in oocytes co-expressing pannexin 1 with the P2X7 receptor (7, 8). As in macrophages, repetitive or sustained stimulation of the oocytes with ATP or its analogues causes cell death. This appears to become detached from pannexin 1 channel activity because membrane breakdown was observed after ATP was removed and pannexin 1 channels were closing (Fig. 8; also see Fig. 4 in Ref. 8). The present results suggest that protein-protein interactions could be responsible for this phenomenon. Pannexin 1 appears to be part of a large protein-signaling cascade that links the P2X7 receptor to the components of the inflammasome resulting in caspase activation and eventual release of cytokines. Thus, not only did a neutralizing antibody to ASC inhibit ATP-induced cell death in oocytes co-expressing pannexin 1 and P2X7 receptors, but probenecid attenuated caspase-1 activation. Probenecid is known as an inhibitor of organic anion transporters (44), but it inhibits pannexin 1 channels with the same potency. Probenecid is specific among “gap junction protein” inhibitors as channels composed of pannexins are affected, whereas connexins are insensitive to the drug (29).
Probenecid has been used for decades in the treatment of gout, a condition characterized by inflammatory responses to the formation of urate crystals that activate the NLRP3 inflammasome (45). The therapeutic action of probenecid in treatment of gout was thought to be exclusively by inhibition of an organic anion transporter, thereby affecting urate excretion in the kidney by blocking urate reuptake. The present findings suggest an additional site of action for probenecid by attenuating signals in the inflammatory response, possibly inhibiting activation of the inflammasome.
Disturbed ionic and neurotransmitter homeostasis contributes to secondary damage induced by traumatic brain injury, spinal cord injury, and stroke (46). ATP and K+ released from injured cells mediate a variety of toxic metabolic disturbances often leading to cell death. ATP acting on P2X7 receptors is a potent stimulus for caspase-1 activation within the NLRP3 inflammasome (47). Apparently the ATP-bound receptor unmasks an ATPase activity of NLRP3 with specificity for ATP or dATP. NLRP3-catalyzed nucleotide hydrolysis is vital for protein function and is required for NLRP3 self-association, interaction with ASC and caspase-1, and IL-1 cytokine release (47). In contrast, the NLRP1 inflammasome exhibits little nucleotide specificity. Because neurons contain the NLRP1 inflammasome (21) and not the NLRP3 inflammasome, it does not appear that ATP released after CNS injury activates the NLRP1 inflammasome in neurons contributing to increased IL-1β. However, we cannot rule out the possibility that inflammasomes present in other CNS cell types are activated by ATP, thus contributing to increased IL-1β production following injury. We present data that support an alternative model for caspase-1 activation where high extracellular K+ is a trigger for a pathway leading to the processing of IL-1β in both neurons and astrocytes. Thus, it will be of interest to determine whether blocking the opening of pannexin 1 channels in response to high extracellular K+ caused by traumatic injury inhibits inflammasome activation and release of IL-1β.
Strategies now aimed at treating CNS trauma focus on neuroprotection, enhanced regeneration, or treatment of demyelination. Our study shows that neutralization of ASC significantly blocks ATP-induced cell death. Moreover we have recently shown that neutralization of ASC significantly reduces caspase-1 activation and processing of IL-1β and IL-18, resulting in significant improvement in tissue sparing and functional recovery after spinal cord injury (21). Thus, pannexin 1 and inflammasome inhibition might offer a new therapy for treatment of ATP-induced cell death observed in a variety of CNS insults and diseases.
Supplementary Material
Acknowledgments
We thank Dr. K. Muller for critically reading the manuscript and Dr. Minh Tran for astrocyte cultures.
This work was supported, in whole or in part, by National Institutes of Health Grants GM48610 (to G. D.) and NS052245 (to E. S.) and a training grant (to W. S.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–5.
- IL
- interleukin
- NLR
- NOD (nucleotide-binding oligomerization domain)-like receptor
- ASC
- apoptosis-associated specklike protein containing a CARD (caspase recruitment domain)
- CNS
- central nervous system
- XIAP
- X-linked inhibitor of apoptosis protein
- PBS
- phosphate-buffered saline
- sh
- short hairpin.
REFERENCES
- 1.Panchin Y., Kelmanson I., Matz M., Lukyanov K., Usman N., Lukyanov S. ( 2000) Curr. Biol. 10, R473– 474 [DOI] [PubMed] [Google Scholar]
- 2.Dahl G., Locovei S. ( 2006) IUBMB Life 58, 409– 419 [DOI] [PubMed] [Google Scholar]
- 3.Huang Y., Grinspan J. B., Abrams C. K., Scherer S. S. ( 2007) Glia 55, 46– 56 [DOI] [PubMed] [Google Scholar]
- 4.Locovei S., Bao L., Dahl G. ( 2006) Proc. Natl. Acad. Sci. U.S.A. 103, 7655– 7659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iglesias R., Locovei S., Roque A., Alberto A. P., Dahl G., Spray D. C., Scemes E. ( 2008) Am. J. Physiol. Cell Physiol. 295, C752– 760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scemes E., Suadicani S. O., Dahl G., Spray D. C. ( 2007) Neuron Glia Biol. 3, 199– 208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pelegrin P., Surprenant A. ( 2006) EMBO J. 25, 5071– 5082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Locovei S., Scemes E., Qiu F., Spray D. C., Dahl G. ( 2007) FEBS Lett. 581, 483– 488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinon F., Burns K., Tschopp J. ( 2002) Mol. Cell 10, 417– 426 [DOI] [PubMed] [Google Scholar]
- 10.Martinon F., Tschopp J. ( 2007) Cell Death Differ. 14, 10– 22 [DOI] [PubMed] [Google Scholar]
- 11.Ogura Y., Sutterwala F. S., Flavell R. A. ( 2006) Cell 126, 659– 662 [DOI] [PubMed] [Google Scholar]
- 12.Ting J. P., Willingham S. B., Bergstralh D. T. ( 2008) Nat. Rev. Immunol. 8, 372– 379 [DOI] [PubMed] [Google Scholar]
- 13.Carneiro L. A., Magalhaes J. G., Tattoli I., Philpott D. J., Travassos L. H. ( 2008) J. Pathol. 214, 136– 148 [DOI] [PubMed] [Google Scholar]
- 14.Taniguchi S., Sagara J. ( 2007) Semin. Immunopathol. 29, 231– 238 [DOI] [PubMed] [Google Scholar]
- 15.Matzinger P. ( 2002) Ann. N.Y. Acad. Sci. 961, 341– 342 [DOI] [PubMed] [Google Scholar]
- 16.Matzinger P. ( 2007) Nat. Immunol. 8, 11– 13 [DOI] [PubMed] [Google Scholar]
- 17.Perregaux D., Gabel C. A. ( 1994) J. Biol. Chem. 269, 15195– 15203 [PubMed] [Google Scholar]
- 18.Walev I., Reske K., Palmer M., Valeva A., Bhakdi S. ( 1995) EMBO J. 14, 1607– 1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colomar A., Marty V., Médina C., Combe C., Parnet P., Amédée T. ( 2003) J. Biol. Chem. 278, 30732– 30740 [DOI] [PubMed] [Google Scholar]
- 20.Pétrilli V., Papin S., Dostert C., Mayor A., Martinon F., Tschopp J. ( 2007) Cell Death Differ. 14, 1583– 1589 [DOI] [PubMed] [Google Scholar]
- 21.de Rivero Vaccari J. P., Lotocki G., Marcillo A. E., Dietrich W. D., Keane R. W. ( 2008) J. Neurosci. 28, 3404– 3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tedeschi B., Barrett J. N., Keane R. W. ( 1986) J. Cell Biol. 102, 2244– 2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keane R. W., Tallent M. W., Podack E. R. ( 1992) Transplantation 54, 520– 526 [DOI] [PubMed] [Google Scholar]
- 24.Kawamoto J. C., Barrett J. N. ( 1986) Brain Res. 384, 84– 93 [DOI] [PubMed] [Google Scholar]
- 25.Keane R. W., Srinivasan A., Foster L. M., Testa M. P., Ord T., Nonner D., Wang H. G., Reed J. C., Bredesen D. E., Kayalar C. ( 1997) J. Neurosci. Res. 48, 168– 180 [DOI] [PubMed] [Google Scholar]
- 26.Doucet J. P., Pierce G. N., Hertzberg E. L., Tuana B. S. ( 1992) J. Biol. Chem. 267, 16503– 16508 [PubMed] [Google Scholar]
- 27.Dahl G. ( 1992) in Cell-cell Interactions. A Practical Approach ( Stevenson B., Gallin W., Paul D. eds) pp. 143– 165, IRL Press, Oxford [Google Scholar]
- 28.Bao L., Locovei S., Dahl G. ( 2004) FEBS Lett. 572, 65– 68 [DOI] [PubMed] [Google Scholar]
- 29.Silverman W., Locovei S., Dahl G. ( 2008) Am. J. Physiol. Cell Physiol. 295, C761– 767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruzzone R., Barbe M. T., Jakob N. J., Monyer H. ( 2005) J. Neurochem. 92, 1033– 1043 [DOI] [PubMed] [Google Scholar]
- 31.Pineau I., Lacroix S. ( 2007) J. Comp. Neurol. 500, 267– 285 [DOI] [PubMed] [Google Scholar]
- 32.Abulafia D. P., de Rivero Vaccari J. P., Lozano J. D., Lotocki G., Keane R. W., Dietrich W. D. ( 2009) J. Cereb. Blood Flow Metab. 29, 534– 544 [DOI] [PubMed] [Google Scholar]
- 33.Mariathasan S., Newton K., Monack D. M., Vucic D., French D. M., Lee W. P., Roose-Girma M., Erickson S., Dixit V. M. ( 2004) Nature 430, 213– 218 [DOI] [PubMed] [Google Scholar]
- 34.Ji H. L., Benos D. J. ( 2004) J. Biol. Chem. 279, 26939– 26947 [DOI] [PubMed] [Google Scholar]
- 35.Collier D. M., Snyder P. M. ( 2009) J. Biol. Chem. 284, 792– 798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waldmann R., Champigny G., Bassilana F., Heurteaux C., Lazdunski M. ( 1997) Nature 386, 173– 177 [DOI] [PubMed] [Google Scholar]
- 37.Locovei S., Wang J., Dahl G. ( 2006) FEBS Lett. 580, 239– 244 [DOI] [PubMed] [Google Scholar]
- 38.Bodin P., Burnstock G. ( 1996) J. Cardiovasc. Pharmacol. 27, 872– 875 [DOI] [PubMed] [Google Scholar]
- 39.Qiu F., Dahl G. ( 2009) Am. J. Physiol. Cell Physiol. 296, C250– 255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franchi L., Kanneganti T. D., Dubyak G. R., Núñez G. ( 2007) J. Biol. Chem. 282, 18810– 18818 [DOI] [PubMed] [Google Scholar]
- 41.Kahlenberg J. M., Dubyak G. R. ( 2004) Am. J. Physiol. Cell Physiol. 286, C1100– 1108 [DOI] [PubMed] [Google Scholar]
- 42.Carta S., Tassi S., Semino C., Fossati G., Mascagni P., Dinarello C. A., Rubartelli A. ( 2006) Blood 108, 1618– 1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanneganti T. D., Lamkanfi M., Kim Y. G., Chen G., Park J. H., Franchi L., Vandenabeele P., Núñez G. ( 2007) Immunity 26, 433– 443 [DOI] [PubMed] [Google Scholar]
- 44.Deuticke B. ( 1970) Naturwissenschaften 57, 172– 179 [DOI] [PubMed] [Google Scholar]
- 45.Gutman A. B. ( 1951) Bull. N.Y. Acad. Med. 27, 144– 164 [PMC free article] [PubMed] [Google Scholar]
- 46.Mautes A. E., Weinzierl M. R., Donovan F., Noble L. J. ( 2000) Phys. Ther. 80, 673– 687 [PubMed] [Google Scholar]
- 47.Duncan J. A., Bergstralh D. T., Wang Y., Willingham S. B., Ye Z., Zimmermann A. G., Ting J. P. ( 2007) Proc. Natl. Acad. Sci. U.S.A. 104, 8041– 8046 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.