Abstract
Amyloid-β (Aβ) peptides, generated by the proteolysis of β-amyloid precursor protein by β- and γ-secretases, play an important role in the pathogenesis of Alzheimer disease. Inflammation is also important. We recently reported that prostaglandin E2 (PGE2), a strong inducer of inflammation, stimulates the production of Aβ through EP2 and EP4 receptors, and here we have examined the molecular mechanism. Activation of EP2 and EP4 receptors is coupled to an increase in cellular cAMP levels and activation of protein kinase A (PKA). We found that inhibitors of adenylate cyclase and PKA suppress EP2, but not EP4, receptor-mediated stimulation of the Aβ production. In contrast, inhibitors of endocytosis suppressed EP4, but not EP2, receptor-mediated stimulation. Activation of γ-secretase was observed with the activation of EP4 receptors but not EP2 receptors. PGE2-dependent internalization of the EP4 receptor was observed, and cells expressing a mutant EP4 receptor lacking the internalization activity did not exhibit PGE2-stimulated production of Aβ. A physical interaction between the EP4 receptor and PS-1, a catalytic subunit of γ-secretases, was revealed by immunoprecipitation assays. PGE2-induced internalization of PS-1 and co-localization of EP4, PS-1, and Rab7 (a marker of late endosomes and lysosomes) was observed. Co-localization of PS-1 and Rab7 was also observed in the brain of wild-type mice but not of EP4 receptor null mice. These results suggest that PGE2-stimulated production of Aβ involves EP4 receptor-mediated endocytosis of PS-1 followed by activation of the γ-secretase, as well as EP2 receptor-dependent activation of adenylate cyclase and PKA, both of which are important in the inflammation-mediated progression of Alzheimer disease.
Alzheimer disease (AD)2 is the most common neurodegenerative disorder of the central nervous system and the leading cause of adult onset dementia. AD is characterized pathologically by the accumulation of tangles and senile plaques. Senile plaques are composed of the amyloid-β (Aβ) peptides Aβ40 and Aβ42 (1, 2). To generate Aβ, β-amyloid precursor protein (APP) is first cleaved by β-secretase and then by γ-secretase. Cleavage of APP by α-secretase produces non-amyloidogenic peptides (3, 4). The γ-secretase is an aspartyl protease complex composed of four core components, including presenilin (PS) 1 and PS2 (5). Early onset familial AD is linked to three genes, APP, PS1, and PS2 (5, 6), strongly suggesting that γ-secretase-dependent production of Aβ is a key factor in the pathogenesis of AD. Therefore, cellular factors that affect the γ-secretase-dependent production of Aβ may be good targets for the development of drugs to prevent and treat AD.
Both APP and PS-1 are transmembrane proteins, and their intracellular localization is controlled by secretory and endocytic pathways. These proteins are modified in the endoplasmic reticulum and trafficked to the cell surface through the trans-Golgi network (TGN). Then, they are internalized again and trafficked to early endosomes. Next, they are trafficked to late endosomes and lysosomes (LEL), which are recycling endosomes that are targeted to the cell surface or the TGN (7–11). The production of Aβ seems to occur in a broad range of cellular compartments including the cell surface, TGN, and endosomes (12). Abnormalities of secretory and endocytic pathways have been observed in the brains of AD patients (9, 13). Importantly, factors that control these vesicle transport systems affect the production of Aβ. For example, overproduction of Rab5, a factor essential for traffic of vesicles to early endosomes, has been shown to stimulate the production of Aβ (14), and SorL1 has been shown to reduce the production of Aβ by stimulating the traffic of APP in early endosomes to the TGN (15, 16).
It has been suggested that inflammation is important in the pathogenesis of AD; chronic inflammation has been observed in the brains of AD patients, and trauma to the brain and ischemia, both of which can activate inflammation, are major risk factors for AD (17–19). Cyclooxygenase (COX) is essential for the synthesis of prostaglandin E2 (PGE2), a potent inducer of inflammation and has two subtypes, COX-1 and COX-2. COX-1 is expressed constitutively, whereas expression of COX-2 is induced under inflammatory conditions and is responsible for the progression of inflammation (20–22). The following evidences of the involvement of PGE2 (and COX-2) in the progression of AD suggest that they are good targets for the development of AD drugs: (i) Elevated levels of PGE2 and overexpression of COX-2 have been observed in the brains of AD patients (23–25); (ii) the extent of COX-2 expression correlates with the amount of Aβ and the degree of progression of AD pathogenesis (26); (iii) transgenic mice constitutively overexpressing COX-2 show aging-dependent neural apoptosis and memory dysfunction (27); (iv) prolonged use of nonsteroidal anti-inflammatory drugs, inhibitors of COX, delays the onset and reduces the risk of AD (28); (v) PGE2 stimulates the production of reactive oxygen species in microglia cells, resulting in activation of β-secretase (29).
We recently reported that PGE2 stimulates the production of Aβ in human embryonic kidney (HEK) 293 and human neuroblastoma (SH-SY5Y) cells that stably express a form of APP with two mutations (K651N/M652L) (APPsw) that elevate cellular and secreted levels of Aβ (30). Similar results were reported by another group (31). Using agonists and antagonists specific for each of the four PGE2 receptors (EP1, EP2, EP3, and EP4), we found that EP4 receptors alone and also both EP2 and EP4 receptors are involved in PGE2-stimulated production of Aβ in HEK293 or SH-SY5Y cells, respectively (30). Furthermore, experiments with transgenic mice suggest that EP2 and EP4 receptors are involved in the production of Aβ in vivo (30). Based on these results, we propose that antagonists of the EP2 and/or EP4 receptors may be therapeutically beneficial for the treatment of AD. Understanding the mechanism governing EP2 and EP4 receptor-mediated stimulation of production of Aβ by PGE2 will be important for such drug development.
Activation of EP2 and EP4 receptors causes activation of adenylate cyclase and an increase in the cellular level of cAMP (32). We have shown that an EP4 receptor agonist or both EP2 and EP4 receptor agonists increase the cellular level of cAMP in HEK293 or SH-SY5Y cells, respectively, and that a cAMP analogue, 8-(4-chlorophenylthio)-cAMP (pCPT-cAMP), increases the level of Aβ in HEK293 cells (30). These findings suggest that the cellular level of cAMP is important for PGE2-stimulated production of Aβ. An increase in the cellular level of cAMP is known to activate protein kinase A (PKA), which is important for cAMP-regulated intracellular signal transduction (33). However, an inhibitor of PKA, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinoline-sulfonamide (H-89), does not block PGE2-stimulated production of Aβ in HEK293 cells (30). Other cAMP-regulated signal transduction factors, such as phosphatidylinositol 3-kinase and Epac (exchange protein directly activated by cAMP), were also shown not to be involved in PGE2-stimulated production of Aβ in HEK293 cells (30). Thus, the mechanism whereby the activation of EP2 and EP4 receptors stimulates the production of Aβ has remained unknown. In this study, by using inhibitors of adenylate cyclase and PKA, we found that activation of the EP2 receptor stimulates production of Aβ through activation of adenylate cyclase and PKA. We also propose that activation of the EP4 receptor causes its co-internalization with PS-1 (γ-secretase) into endosomes and that this co-internalization is important for EP4 receptor-mediated stimulation of Aβ production by PGE2 through the activation of γ-secretase.
EXPERIMENTAL PROCEDURES
Materials
Dulbecco's modified Eagle's medium (DMEM) and Ham-F12 medium were obtained from Nissui Pharmaceutical Co. The first-strand cDNA synthesis kit was from GE Healthcare. SQ22536 and fluorescent β-secretase substrate (H2N-Arg-Glu-(EDANS)-Glu-Val-Asn-Leu-Asp-Ala-Glu- Phe-Lys-(DABCYL)-Arg-OH) were from Calbiochem. Lipofectamine (TM2000), Alexa Fluor 488 goat anti-rat immunoglobulin G, Alexa Fluor 594 goat anti-rabbit immunoglobulin G, and Alexa Fluor 488 goat anti-rabbit immunoglobulin G were purchased from Invitrogen. The plasmid pEGFP-N1 was obtained from Clontech. An antibody against actin was obtained from Santa Cruz Biotechnology. Fetal bovine serum, PGE2, pCPT-cAMP, G418, H-89, concanavalin A, and antibodies against the C-terminal fragment (CTF) of APP, hemagglutinin (HA), Rab5, and Rab7 were from Sigma. An antibody against the N-terminal fragment (NTF) of PS-1 was from Chemicon or Immuno-Biological Laboratories, Inc., and the antibody against clathrin was from BD Biosciences. An antibody against EP4 was from Cayman Chemical. The RNeasy kit and HiPerFect transfection reagent were from Qiagen. The APP-derived fluorescent substrate of γ-secretase (Nma-Gly- Gly-Val-Val-Ile-Ala-Thr-Val-Lys(Dnp)-d-Arg-d-Arg-d-Arg-NH2) was from Peptide Institute Inc. Sulfo-NHS-S-S-biotin and UltraLink immobilized Neutravidin beads were from Pierce.
Animals
APP23 transgenic mice, a gift from Dr. M. Staufenbiel, were generated as described previously (34). APPsw/EP4−/− and APPsw/EP4+/+ mice were generated as described previously (30). The experiments and procedures described here were carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institute of Health and were approved by the Animal Care Committee of Kumamoto University.
Cell Culture
HEK293, SH-SY5Y, and Chinese hamster ovary (CHO)-K1 cells were cultured in DMEM, DMEM/Ham-F12, and Ham-F12 medium, respectively, supplemented with 10% fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin in a humidified atmosphere of 95% air with 5% CO2 at 37 °C. HEK293 and SH-SY5Y cells expressing APPsw were from our laboratory stocks (35). CHO-K1 cells were from the RIKEN BioResource Center.
For transient expression, cells were seeded 24 h before transfection in 24-well plates at a density of 1.5 × 105 cells/well. Transfections were carried out using Lipofectamine (TM2000) according to the manufacturer's instructions. Cells were used for experiments after a 24-h recovery period. Transfection efficiency was determined in parallel plates by transfection of cells with pEGFP-N1 control vector. Transfection efficiencies were greater than 90% in all experiments. The stable transfectants expressing each gene were selected by immunoblotting or real-time reverse transcription-PCR analyses. Positive clones were maintained in the presence of 200 μg/ml G418.
Immunoblotting Analysis
Whole cell extracts were prepared as described previously (36). For detection of CTFα and CTFβ, membrane fractions were prepared as described previously (37). The protein concentration of each sample was determined by the Bradford method (38). Samples were applied to polyacrylamide-SDS gels (Tris-Tricine gel for the detection of CTFα and CTFβ or Tris-glycine gel for other proteins) and subjected to electrophoresis, after which proteins were immunoblotted with each antibody.
Sandwich Enzyme-linked Immunosorbent Assay for Aβ and γ-Secretase- or β-Secretase-mediated Peptide Cleavage Assay
Cells were cultured for 24 h, and the conditioned medium was subjected to a sandwich enzyme-linked immunosorbent assay using three types of specific monoclonal antibodies as described previously (35, 39).
We monitored the activity of γ- and β-secretase as reported previously (40, 41). Solubilized membranes were incubated overnight at 37 °C in 200 μl of 50 mm Tris-HCl (pH 6.8) buffer containing 2 mm EDTA, 0.25% CHAPSO (w/v), and 10 μm fluorescent substrate of γ-secretase or were incubated for 1 h at 37 °C in 200 μl of 50 mm acetate buffer (pH 4.1) containing 100 mm sodium chloride, 0.025% bovine serum albumin, and 10 μm fluorescent β-secretase substrate. We measured fluorescence using a plate reader (Fluostar Galaxy) with an excitation wavelength of 355 nm and an emission wavelength of 440 nm (for the γ-secretase) or 510 nm (for the β-secretase).
Small Interfering RNA (siRNA) Targeting of Genes
We used siRNA with the sequence 5′-gcugggaaaacucuucagadTdT-3′ and 5′- ucugaagaguuuucccagcdTdT-3′, 5′- gcaagcaaguccuaacauudTdT-3′ and 5′-aauguuaggacuugcuugcdTdT-3′, or 5′-cgguuccagucucucggugdAdG-3′and 5′-caccgagagacuggaaccgdAdT-3′ as annealed oligonucleotides for repressing clathrin, Rab5, or Rab7 expression, respectively. Cells were transfected with siRNA using HiPerFect transfection reagent according to the manufacturer's instructions. Non-silencing siRNA (5′-uucuccgaacgugucacgudTdT-3′and 5′-acgugacacguucggagaadTdT-3′) was used as a negative control.
Immunostaining Microscopy
Cells or mouse brain sections were incubated with antibody against each protein for 30 min before (for HA or EP4) or after (for PS-1 and Rab7) treatment with PGE2. Samples were fixed and incubated with the respective secondary antibody. We acquired images with a confocal fluorescence microscope (Olympus FV500).
Co-immunoprecipitation Assay
Immunoprecipitation was carried out as described previously (35), with some modifications. Cells were harvested, lysed with buffer containing 1% CHAPSO, and centrifuged. The antibody against HA or EP4 was added to the supernatant, and the samples were incubated for 12 h at 4 °C with rotation. Dynabeads-Protein G was added and incubated for 2 h at 4 °C with rotation. Beads were washed four times, and the proteins were eluted by boiling in SDS sample buffer.
Surface Biotinylation Assay
This assay was carried out as described previously (42) with some modifications. Proteins on the cell surface were biotinylated with a reversible membrane-impermeable derivative of biotin (sulfo-NHS-S-S-biotin). Internalization of proteins was allowed to occur by incubation at 37 °C for 1 h. The remaining cell surface biotin was cleaved by glutathione, and the cells were lysed. Biotinylated proteins were precipitated using UltraLink immobilized Neutravidin beads and eluted by boiling in SDS sample buffer.
Statistical Analysis
All values are expressed as the mean ± S.D. Two-way analysis of variance followed by the Tukey test or the Student's t test for unpaired results was used to evaluate differences between more than three groups or between two groups, respectively. Differences were considered to be significant for values of p < 0.05.
RESULTS
Mechanism for EP2 Receptor-mediated Stimulation of Aβ Production by PGE2
Although primary neurons should be used for this type of experiments, we used an immortalized cell line in this study, because we used stable and transient transfection for the experiments in this study (see below). We confirmed our previous results that H-89 did not block PGE2-stimulated production of Aβ in HEK293 cells and found that an inhibitor of adenylate cyclase, SQ22536, also did not block this stimulation (supplemental Fig. S1, A and B), suggesting that an increase in the cellular level of cAMP is not involved in PGE2-stimulated production of Aβ in HEK293 cells. In contrast, in SH-SY5Y cells, both H-89 and SQ22536 decreased the level of Aβ in the presence, but not in the absence, of PGE2 (supplemental Fig. S1, C and D). These inhibitors at the concentrations used did not affect cell viability (data not shown). Therefore, the results shown in Fig. 1, C and D, suggest that an increase in the cellular level of cAMP and the resulting activation of PKA are involved in PGE2-stimulated production of Aβ in SH-SY5Y cells. In a previous report (30), we used HEK293 cells that express only APPsw stably. In the current study, we used CHO and HEK293 cells that express each EP receptor transiently in addition to the stable expression of APPsw (see below). For these types of cells, 10 nm PGE2, which had been used for experiments in the previous study (30), was not enough to stimulate the production of Aβ clearly (supplemental Figs. S7–S10), and thus we used 1 μm PGE2 in all experiments described in this article.
FIGURE 1.
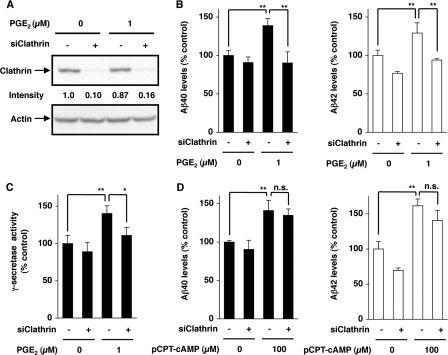
Effect of inhibitors of endocytosis on PGE2-stimulated production of Aβ in HEK293 cells. HEK293 cells expressing APPsw were preincubated for 1 h with 0.5 m sucrose (A and B) or 0.25 mg/ml concanavalin A (C and D) and further incubated for 24 h with or without 1 μm PGE2 in the presence of the same concentration of each inhibitor as in the preincubation step. The amounts of Aβ40 and Aβ42 in the conditioned medium were determined by sandwich enzyme-linked immunosorbent assay and expressed relative to the control (without PGE2) (A and C). After incubation with or without PGE2 for 1 h, membrane fractions were prepared and subjected to γ-secretase-mediated peptide cleavage assay as described under “Experimental Procedures” (B and D). Values are given as means ± S.D. (n = 3). **, p < 0.01; *, p < 0.05.
As the EP2 receptor is involved in PGE2-stimulated production of Aβ in SH-SY5Y but not in HEK293 cells (30), the results shown in supplemental Fig. S1 suggest that the increase in the cellular level of cAMP and resulting activation of PKA contributes to EP2 receptor-dependent (but not EP4 receptor-dependent) stimulation of Aβ production by PGE2. To confirm this proposition, we examined the effects of these inhibitors on the production of Aβ in CHO cells artificially expressing APPsw and EP2 or EP4 receptor tagged with HA. Lack of functional endogenous EP2 and EP4 receptors in cells has been reported previously (43). Expression of these receptors was confirmed by immunoblotting (data not shown) and immunostaining (Fig. 3A). Both H-89 and SQ22536 suppressed PGE2-stimulated production of Aβ in cells expressing the EP2 receptor but not in cells expressing the EP4 receptor (supplemental Fig. S2, A–D), supporting our theory described above. We reported previously that treatment of HEK293 cells with PGE2 increases γ-secretase activity in extracts of these cells (30). Here we found that PGE2 treatment increased γ-secretase activity in extracts prepared from CHO cells expressing the EP4 receptor but not in cells expressing the EP2 receptor and that it did not affect β-secretase activity in either of these cell types (supplemental Fig. S2, E and F). Furthermore, pCPT-cAMP did not affect γ-secretase activity in HEK293 cells (data not shown). On the other hand, treatment of cells with PGE2 did not affect the expression of γ-secretase (PS-1-NTF) (supplemental Fig. S3). These results suggest that the EP2 receptor mediates PGE2-stimulated production of Aβ through activation of cAMP and PKA without an increase in β- and γ-secretase activity and that the EP4 receptor mediates PGE2-stimulated Aβ production through different mechanisms, which involve an activation rather than induction of expression of γ-secretase.
FIGURE 3.
PGE2-dependent internalization of the EP4 receptor and its contribution to PGE2-stimulated production of Aβ. CHO-K1 cells expressing APPsw were transiently transfected with expression plasmid encoding EP2 (A), EP4 (A–C), or EP4-t369 (A–C) or with control vector (B and C). Cells were preincubated for 1 h with antibody against HA and further cultured for 1 h (A and C) or 24 h (B) with or without 1 μm PGE2. After incubation with secondary antibody, the cells were inspected using fluorescence microscopy. Pictures of both high (right) and low (left) magnification are shown (scale bar, 20 μm) in each of the three panels. (A) The amounts of Aβ (B) or γ-secretase activity (C) were determined and expressed as described in the legend for Fig. 1. Values are given as means ± S.D. (n = 3). **, p < 0.01; n.s., not significant.
Mechanism for EP4 Receptor-mediated Stimulation of Aβ Production by PGE2
Agonist-dependent internalization (endocytosis) of the EP4 receptor (but not the EP2 receptor) has been described: The binding of PGE2 to the EP4 receptor induces formation of vesicles that contain the receptor and the vesicles are trafficked to endosomes (44, 45). Thus, we used inhibitors of endocytosis (sucrose and concanavalin A) to test whether agonist-dependent internalization of the EP4 receptor is involved in PGE2-stimulated production of Aβ. As shown in Fig. 1, both sucrose and concanavalin A suppressed PGE2 (1 μm)-stimulated production of Aβ and decreased γ-secretase activity in HEK293 cells. Similar results were obtained with 10 nm PGE2 in HEK293 cells (supplemental Fig. S7) and in CHO cells expressing the EP4 receptor but not in those expressing the EP2 receptor (data not shown). These inhibitors, at the concentrations specified in Fig. 1, did not affect cell viability (data not shown). These results suggest that agonist-dependent internalization of the EP4 receptor is involved in PGE2-stimulated Aβ production.
Agonist-dependent internalization is initiated by the formation of clathrin-coated vesicles, and thus clathrin is essential for this internalization (46). We examined the effect of siRNA targeting the clathrin heavy chain on PGE2-stimulated production of Aβ in HEK293 cells. Transfection with siRNA inhibited clathrin expression in the presence and absence of PGE2 (Fig. 2A). This siRNA suppressed PGE2-stimulated production of Aβ and γ-secretase activity (Fig. 2, B and C), suggesting that clathrin-dependent vesicle formation at the cell surface and their subsequent internalization are involved in EP4 receptor-mediated stimulation of Aβ production by PGE2. siRNA did not affect Aβ production in the absence of PGE2 (Fig. 2B) and its pCPT-cAMP-dependent stimulation (Fig. 2D), suggesting that siRNA specifically affects EP4 receptor-mediated stimulation of Aβ production by PGE2.
FIGURE 2.
Effect of siRNA for clathrin on PGE2-stimulated production of Aβ. HEK293 cells expressing APPsw were transiently transfected with siRNA for clathrin (siClathrin) (+) or non-silencing siRNA (−) (A–D). Cells were incubated with 1 μm PGE2 (A–C) or 100 μm pCPT-cAMP (D) for 24 h (A, B, and D) or 1 h (C). Whole cell extracts were analyzed by immunoblotting with an antibody against clathrin or actin. The band intensity was determined and expressed relative to the control (A). The amounts of Aβ (B and D) and the γ-secretase activity (C) were determined and expressed as described in the legend for Fig. 1. Values are given as means ± S.D. (n = 3). **, p < 0.01; *, p < 0.05; n.s., not significant.
By immunostaining we observed PGE2 (1 μm)-dependent internalization of EP4 receptors (Fig. 3A). In contrast, EP2 receptors remained localized to the cell surface even after PGE2 treatment (Fig. 3A). It has been reported that the C-terminal region of the EP4 receptor is required for its agonist-dependent internalization (45). We confirmed that a mutant form of the EP4 receptor, which is truncated after Thr-369 (EP4-t369) (47), does not exhibit agonist-dependent internalization (Fig. 3A). As shown in Fig. 3, B and C, in contrast to CHO cells expressing the wild-type EP4 receptor, in CHO cells expressing EP4-t369, PGE2 did not stimulate the production of Aβ and γ-secretase activity. Similar results were obtained with 10 nm PGE2; however, the effects were not so apparent, and some of them were not statistically significant (supplemental Fig. S8). This suggests that agonist-dependent internalization of the EP4 receptor is essential for EP4 receptor-mediated and PGE2-dependent stimulation of Aβ production and γ-secretase activation.
In clathrin-dependent endocytosis, vesicles formed at the cell surface are trafficked first to early endosomes and then to LEL. Rab5 and Rab7 are essential for the traffic to early endosomes and LEL, respectively (48). To examine the role of the traffic in PGE2-stimulated Aβ production, the effects of siRNA for Rab5 and Rab7 on the production of Aβ were examined. Each siRNA clearly inhibited the expression of their target protein (Fig. 4, A and D). Furthermore, siRNA for Rab5 or Rab7 suppressed the PGE2-stimulated production of Aβ and γ-secretase activity in HEK293 cells (Fig. 4, B, C, E, and F), suggesting that traffic of vesicles containing the EP4 receptor to LEL is important for PGE2-stimulated Aβ production and γ-secretase activity.
FIGURE 4.
Effect of siRNA for Rab5 and Rab7 on PGE2-stimulated production of Aβ. HEK293 cells expressing APPsw were transiently transfected with siRNA for Rab5 (siRab5) (A–C) or Rab7 (siRab7) (D–F), or with non-silencing siRNA (−) (A–F). Cells were incubated with 1 μm PGE2 for 24 h (A, B, D, and E) or 1 h (C and F). Whole cell extracts were analyzed by immunoblotting as described in the legend for Fig. 2 (A and D). The amounts of Aβ (B and E) and γ-secretase activity (C and F) were determined and expressed as described in the legend for Fig. 1. Values are given as means ± S.D. (n = 3). **, p < 0.01; *, p < 0.05.
Contribution of Co-internalization of PS-1 with the EP4 Receptor to PGE2-stimulated Production of Aβ
It was reported recently that γ-secretase is activated when it is trafficked into endosomes via agonist-dependent internalization of the β-adrenergic receptor, which interacts with γ-secretase (49). Thus, we hypothesized that the EP4 receptor also interacts with γ-secretase and that γ-secretase is trafficked to LEL in a PGE2-dependent manner, resulting in activation of γ-secretase and stimulation of Aβ production. To test this theory, we first examined the interaction between the EP4 receptor and PS-1 by a co-immunoprecipitation assay. As shown in Fig. 5A, efficient immunoprecipitation of PS-1-NTF with antibody against was dependent on the expression of the HA-tagged EP4 receptor. On the other hand, PS-1-NTF was not immunoprecipitated with antibody against HA in cells expressing HA-tagged EP2 receptor (supplemental Fig. S4). These results suggest that the EP4 receptor can physically and specifically interact with PS-1-NTF (γ-secretase). The physical interaction between EP4 receptor and PS-1-NTF was also observed in SH-SY5Y cells without artificial overexpression of EP4 receptor (supplemental Fig. S11A). PS-1 is cleaved to produce PS-1-NTF and PS-1-CTF in cells, and both of PS-1-NTF and PS-1-CTF are included in γ-secretase complex. The results in Fig. 5 also show that EP4-t369 can interact with PS-1-NTF, suggesting that the interaction of PS-1 (γ-secretase) with the EP4 receptor alone is not sufficient for PGE2-stimulated production of Aβ and γ-secretase activity (see Fig. 3, B and C). It has been reported that a general acceleration of cellular endocytic pathways and agonist-induced endocytosis of some receptors (such as the angiotensin II receptor) does not affect the production of Aβ and γ-secretase activity (49, 50). Thus, internalization of PS-1 (γ-secretase) with the EP4 receptor seems to enhance production of Aβ and γ-secretase activity specifically.
FIGURE 5.
Interaction between the EP4 receptor and PS-1 and their PGE2-dependent co-internalization. HEK293 cells expressing APPsw were transiently transfected with expression plasmid encoding the EP4 receptor or EP4-t369 or with control vector. Whole cell extracts were immunoprecipitated with antibody against HA. A, whole cell extracts (WCE) and the immunoprecipitates (IP) were analyzed by immunoblotting with antibody against HA or PS-1-NTF as described in the legend for Fig. 2 (n.d., not detectable). B, cells were surface-biotinylated and incubated with or without 1 μm PGE2. Then, cells were treated with glutathione to cleave biotin from the surface proteins. Biotinylated proteins present in the cell lysates were precipitated with Neutravidin, and the precipitates were analyzed by immunoblotting with HA or PS-1-NTF as described in the legend for Fig. 2.
Next, we tested co-internalization of PS-1-NTF with the EP4 receptor using a surface biotinylation assay. Cells were surface-biotinylated, and after induction of internalization, biotinylated proteins remaining on the cell surface were cleaved by glutathione. The biotinylated proteins (internalized proteins) were precipitated, and the presence of each protein in the precipitates was monitored by immunoblotting. As shown in Fig. 5B, the wild-type EP4 receptor and EP4-t369 bands were not apparent after glutathione cleavage, whereas preincubation of cells with PGE2 (1 μm) prior to cleavage gave rise to the wild-type EP4 receptor band but not the EP4-t369 band. This shows that the wild-type EP4 receptor, but not EP4-t369, internalizes in a PGE2-dependent manner. The PS-1-NTF band was also not apparent following cleavage; preincubation with PGE2 recovered the band in cells expressing wild-type EP4 receptor but not in cells expressing EP4-t369 (Fig. 5B). Similar results were obtained with 10 nm PGE2 in HEK293 cells (supplemental Fig. S9) and in SH-SY5Y cells without artificial overexpression of EP4 receptor (supplemental Fig. S11B). We also examined the effect of sucrose and concanavalin A on a surface biotinylation assay for EP4 receptor and PS-1-NTF. As shown in supplemental Fig. S5, preincubation of cells with PGE2 prior to cleavage did not restore the band of either the EP4 receptor or PS-1-NTF in the presence of sucrose or concanavalin A, confirming that these inhibitors suppressed the endocytosis. Similar results were observed for transfection of siRNA for clathrin or Rab5 but not for Rab7 (supplemental Fig. S6). These results suggest that internalization of PS-1 as a result of PGE2 treatment is dependent on internalization of the EP4 receptor.
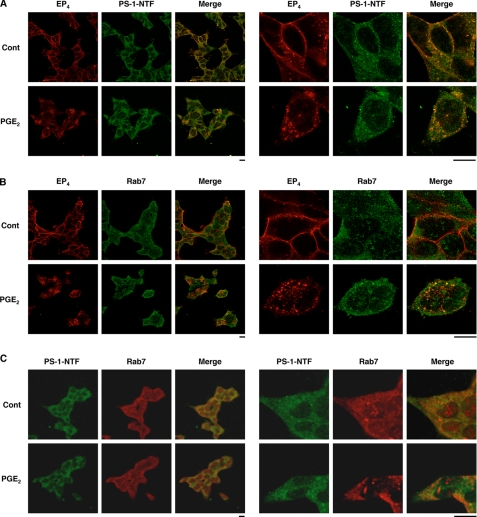
For further confirmation of PGE2-dependent co-internalization of the EP4 receptor and PS-1, we performed a co-immunostaining assay. Similar to what was observed for the EP4 receptor, PS-1-NTF was localized to the cell surface in the absence of PGE2; however, strong staining of PS-1-NTF in intracellular components was observed after PGE2 treatment (Fig. 6A). As shown in Fig. 6A (see merged panel), localizations of the EP4 receptor and PS-1-NTF were well matched, suggesting that PS-1-NTF co-internalizes with the EP4 receptor.
FIGURE 6.
Co-localization of EP4 receptors, PS-1, and Rab7 in cells. HEK293 cells expressing APPsw were transiently transfected with an expression plasmid encoding the EP4 receptor. Cells were preincubated with antibody against HA (A, B) for 1 h and further incubated for 1 h with or without (Cont, control) 1 μm PGE2. After fixation, samples were incubated with antibody against PS-1-NTF (A, C) or Rab7 (B, C). After incubation with the respective secondary antibody, cells were inspected using fluorescence microscopy as described in the legend for Fig. 3.
To identify the intracellular components where both PS-1-NTF and the EP4 receptor localize after PGE2 treatment, we performed a co-immunostaining assay for these factors with Rab7, an LEL marker. As shown in Fig. 6, B and C, intracellular localizations of the EP4 receptor (and PS-1-NTF) and Rab7 were well matched in PGE2-treated cells but not in control cells, suggesting that PS-1 is trafficked into LEL with the EP4 receptor in a PGE2 treatment-dependent manner. Results similar to those in Fig. 6 (in HEK293 cells with 1 μm PGE2) were obtained with 10 nm PGE2 in HEK293 cells (supplemental Fig. S10) and in SH-SY5Y cells without artificial overexpression of EP4 receptor (supplemental Fig. S12).
Finally, we tested the in vivo relevance of our in vitro results using transgenic mice expressing APPsw (APP23) that were crossed to EP4−/− mice (APPsw/EP4−/− mice). We had previously reported that the amount of Aβ in the brains of APPsw/EP4−/− mice was lower than in APPsw/EP4+/+ mice (30). As shown in Fig. 7A, the γ-secretase activity in extracts prepared from the brains of APPsw/EP4−/− mice was very slightly, but significantly, lower than in those from APPsw/EP4+/+ mice. This is consistent with the in vitro results, which showed that PGE2 increases γ-secretase activity in extracts in an EP4 receptor-dependent manner (supplemental Fig. 2F). There was no significant difference of the expression of PS-1-NTF between APPsw/EP4−/− and APPsw/EP4+/+ mice (Fig. 7B), suggesting that activation rather than expression of γ-secretase is important for the decrease in the amount of Aβ in the brains of APPsw/EP4−/−. We previously reported that in HEK293 cells where the EP4 but not the EP2 receptor is functional, PGE2 does not affect the expression and maturation of APP or α- and β-secretase activities (30). In this study, we showed that the expression and maturation of APP (the ratio of the mature form of APP (mAPP) to the immature form of APP (imAPP)) and the α- and β-secretase activities in extracts prepared from the brains of APPsw/EP4−/− mice were similar to those from APPsw/EP4+/+ mice (Fig. 7B and C). (CTFs of APP that are generated by α- or β-secretase (CTFα or CTFβ, respectively) are used as an indirect index of secretase activity.) Furthermore, we compared the co-localization of PS-1-NTF with Rab7 in brain sections prepared from these mice. As shown in Fig. 7, D and E, yellows spots (index of the intracellular co-localization of PS-1-NTF with Rab7) were more apparent in brain sections prepared from APPsw/EP4+/+ mice than in sections from APPsw/EP4−/− mice, suggesting that even in vivo PS-1 is trafficked to LEL in an EP4 receptor-dependent manner. The results shown in Fig. 7 suggest that our in vitro results are relevant in vivo and that PGE2-dependent traffic of PS-1 into LEL contributes to the observed increase in γ-secretase activity and resulting stimulation of Aβ production by expression of the EP4 receptor in vivo.
FIGURE 7.
Mechanism for EP4 receptor-mediated stimulation of production of Aβ in vivo. Membrane fractions (A and C) or whole cell extracts (B) were prepared from the brains of 3-month-old APPsw/EP4+/+ and APPsw/EP4−/− mice and subjected to a γ-secretase-mediated peptide cleavage assay (A) or immunoblotting with antibody against APP, PS-1-NTF or actin (B and C). Values are given as means ± S.D. (n = 6). **, p < 0.01 (A). The brain sections were prepared from the same mice and subjected to immunostaining as described in the legend for Fig. 6 (D). The ratio of cells with yellow spots (positive cells) to total cells in the brain sections (three sections/brain) was determined. Values are given as means ± S.D. (n = 8). **, p < 0.01 (E).
DISCUSSION
We recently reported the importance of PGE2 as a factor that connects inflammation and AD; PGE2 stimulates production of Aβ and this stimulation is mediated by EP2 and EP4 receptors. We also suggested the importance of EP2 and EP4 receptors in production of Aβ in vivo by showing that the amount of Aβ in the brains of APPsw/EP2−/− and APPsw/EP4−/− mice was lower than in the respective control mice. Based on these results, we proposed that antagonists for EP2 and/or EP4 receptor would be therapeutically beneficial for AD (30). To determine the potential drug target (whether it is the EP2 receptor, the EP4 receptor, or both), it is important to understand the molecular mechanism for intracellular signal transduction governing EP2 (or EP4) receptor-mediated stimulation of Aβ production by PGE2. Because activation of both EP2 and EP4 receptors is coupled to activation of the cAMP-PKA pathway, we speculated that this pathway might be involved in the signal transduction. However, our attempts to prove this conjecture failed in our previous study (30). Furthermore, our finding that both EP2 knock-out mice and EP4 receptor knock-out mice showed decreased levels of Aβ in the brain could not be explained by the hypothesis that the cAMP-PKA pathway is responsible for both EP2 receptor- and EP4 receptor-mediated signal transduction pathways for PGE2 stimulated Aβ production. In the current study, using inhibitors for adenylate cyclase and PKA, we showed that the cAMP-PKA pathway is involved in EP2-mediated but not EP4-mediated stimulation of Aβ production by PGE2. As the EP4 receptor is not linked to the cAMP-PKA pathway for PGE2-stimulated Aβ production, EP4 receptor antagonists may be therapeutically beneficial for the treatment of AD. It has been reported that the cAMP-PKA pathway is important for long-term potentiation (51, 52) so EP2 receptor, but not EP4 receptor, antagonists may have side effects on the memory system by inhibiting long-term potentiation. On the other hand, deletion of EP2 receptor in mice was shown to decrease oxidative damage and inhibit β-secretase, resulting in a decrease in the level of Aβ in brain (29), or to enhance Aβ phagocytosis in the brain (53). It has also been suggested that PGE2-dependent activation of cAMP-PKA pathway causes induction of expression of APP (54, 55), indicating that the EP2 receptor antagonists may be therapeutically beneficial for the treatment of AD. The fact that EP2 and EP4 receptors stimulate the production of Aβ through different mechanisms could explain why single knock-outs of either the EP2 or EP4 receptor reduce Aβ production in vivo.
Much attention has been paid to agonist-dependent internalization (endocytosis) of receptors, including the EP4 receptor, because this causes receptor desensitization. Furthermore, this internalization was also recently reported to be important for signal transduction (44). From the current study, we propose that the EP4 receptor mediates the PGE2 signal through its co-internalization with PS-1 (γ-secretase). This suggestion is based on the following results: The immunoprecipitation assay revealed a physical interaction between the EP4 receptor and PS-1-NTF; PGE2-stimulated production of Aβ was not observed under conditions in which co-internalization of the EP4 receptor and PS-1 is inhibited (such as in the presence of endocytosis inhibitors, in cells transfected with siRNA for clathrin, and in cells expressing EP4-t369); internalization of PS-1-NTF was observed to be dependent on both PGE2 and expression of the wild-type EP4 receptor in the surface biotinylation assay. This is the first demonstration of the EP4 receptor mediating signal transduction through co-internalization with other molecules. Similar mechanisms may be involved in EP4 receptor-mediated signal transduction for other responses.
We have also concluded that PS-1 (γ-secretase) is trafficked to LEL in a PGE2- and EP4 receptor-dependent manner and that this traffic is important for PGE2-stimulated production of Aβ and γ-secretase activity. This conclusion is based on the following results: PGE2-dependent co-localization of PS-1-NTF, EP4, and Rab7 was observed in vitro, and co-localization of PS-1-NTFand Rab7 was observed in APPsw/EP4+/+ mice but not as distinctly in APPsw/EP4−/− mice; and transfection of siRNA for Rab5 or Rab7 inhibited PGE2-stimulated production of Aβ and γ-secretase activity. A similar mechanism was proposed for β-adrenergic receptor-mediated stimulation of production of Aβ and γ-secretase activity (49). They also showed the enhancement of γ-secretase activity and elevation of Aβ production in endosomes. It is well known that the pH in endosomes is relatively low and that as a result γ-secretase is more active in endosomes (56–58). Thus, the relatively low pH value in endosomes may contribute to EP4 receptor-mediated stimulation of Aβ production. However, we found that the γ-secretase activity in extracts decreased when the extracts were prepared from cells cultured under conditions in which the traffic of PS-1 (γ-secretase) into endosomes is inhibited, even though the γ-secretase assay was carried out at the same pH. Thus, something other than the lower pH of endosomes (such as induction of expression and post-translational modification of γ-secretase) may also contribute to the stimulation of Aβ production by internalization of PS-1 (γ-secretase) into endosomes. It is also possible that internalization of the EP4 receptor stimulates the traffic of β-secretase to endosomes, resulting in its activation, as is the case for apolipoprotein receptor-2 (59), because β-secretase is also more active at lower pH (60). However, we reported previously that the activity of β-secretase (estimated from the amounts of CTFβ) is not enhanced by PGE2 (30). Thus, the EP4 receptor may not interact with β-secretase.
In summary, we have shown that EP2 and EP4 receptors mediate stimulation of production of Aβ by PGE2 through distinct mechanisms. This finding is important for understanding the mechanisms underlying the inflammation-mediated progression of AD and the regulation of vesicle transport of PS-1 (γ-secretase) and for identifying potential targets for the development of drugs to treat AD.
Supplementary Material
Acknowledgments
We thank Dr. M. Staufenbiel (Novartis Pharma Ltd.) and Dr. B. Ashby (Temple University) for providing the APP23 mice and plasmids expressing EP2, EP4, and EP4-t369, respectively.
This work was supported by grants-in-aid for scientific research from the Ministry of Health, Labor, and Welfare, Japan and the Ministry of Education, Culture, Sports, Science, and Technology, Japan and by the Japan Science and Technology Agency.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S12.
- AD
- Alzheimer disease
- Aβ
- amyloid-β
- APP
- β-amyloid precursor protein
- CHO
- Chinese hamster ovary
- COX
- cyclooxygenase
- CTF
- C-terminal fragment
- DMEM
- Dulbecco's modified Eagle's medium
- H-89
- N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinoline-sulfonamide
- HA
- hemagglutinin
- HEK
- human embryonic kidney
- LEL
- late endosomes and lysosomes
- NTF
- N-terminal fragment
- pCPT-cAMP
- 8-(4-chlorophenylthio)-cAMP
- PKA
- protein kinase A
- PGE2
- prostaglandin E2
- PS
- presenilin
- siRNA
- small interfering RNA
- TGN
- trans-Golgi network
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine
- CHAPSO
- 3-[(3-cholamidopropyl)dimethylammonio]-2-hydroxy-1-propanesulfonic acid.
REFERENCES
- 1.Hardy J., Selkoe D. J. ( 2002) Science 297, 353– 356 [DOI] [PubMed] [Google Scholar]
- 2.Mattson M. P. ( 2004) Nature 430, 631– 639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sisodia S. S., St George-Hyslop P. H. ( 2002) Nat. Rev. Neurosci. 3, 281– 290 [DOI] [PubMed] [Google Scholar]
- 4.Selkoe D. J. ( 1999) Nature 399, A23– 31 [DOI] [PubMed] [Google Scholar]
- 5.Haass C. ( 2004) EMBO J. 23, 483– 488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Price D. L., Sisodia S. S., Borchelt D. R. ( 1998) Science 282, 1079– 1083 [DOI] [PubMed] [Google Scholar]
- 7.Pasternak S. H., Bagshaw R. D., Guiral M., Zhang S., Ackerley C. A., Pak B. J., Callahan J. W., Mahuran D. J. ( 2003) J. Biol. Chem. 278, 26687– 26694 [DOI] [PubMed] [Google Scholar]
- 8.Small S. A., Gandy S. ( 2006) Neuron 52, 15– 31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cataldo A. M., Peterhoff C. M., Troncoso J. C., Gomez-Isla T., Hyman B. T., Nixon R. A. ( 2000) Am. J. Pathol. 157, 277– 286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haass C., Selkoe D. J. ( 1993) Cell 75, 1039– 1042 [DOI] [PubMed] [Google Scholar]
- 11.Koo E. H., Squazzo S. L. ( 1994) J. Biol. Chem. 269, 17386– 17389 [PubMed] [Google Scholar]
- 12.De Strooper B., Annaert W. ( 2000) J. Cell Sci. 113, 1857– 1870 [DOI] [PubMed] [Google Scholar]
- 13.Nixon R. A. ( 2005) Neurobiol. Aging 26, 373– 382 [DOI] [PubMed] [Google Scholar]
- 14.Grbovic O. M., Mathews P. M., Jiang Y., Schmidt S. D., Dinakar R., Summers-Terio N. B., Ceresa B. P., Nixon R. A., Cataldo A. M. ( 2003) J. Biol. Chem. 278, 31261– 31268 [DOI] [PubMed] [Google Scholar]
- 15.Rogaeva E., Meng Y., Lee J. H., Gu Y., Kawarai T., Zou F., Katayama T., Baldwin C. T., Cheng R., Hasegawa H., Chen F., Shibata N., Lunetta K. L., Pardossi-Piquard R., Bohm C., Wakutani Y., Cupples L. A., Cuenco K. T., Green R. C., Pinessi L., Rainero I., Sorbi S., Bruni A., Duara R., Friedland R. P., Inzelberg R., Hampe W., Bujo H., Song Y. Q., Andersen O. M., Willnow T. E., Graff-Radford N., Petersen R. C., Dickson D., Der S. D., Fraser P. E., Schmitt-Ulms G., Younkin S., Mayeux R., Farrer L. A., St George-Hyslop P. ( 2007) Nat. Genet. 39, 168– 177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersen O. M., Reiche J., Schmidt V., Gotthardt M., Spoelgen R., Behlke J., von Arnim C. A., Breiderhoff T., Jansen P., Wu X., Bales K. R., Cappai R., Masters C. L., Gliemann J., Mufson E. J., Hyman B. T., Paul S. M., Nykjaer A., Willnow T. E. ( 2005) Proc. Natl. Acad. Sci. U. S. A. 102, 13461– 13466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Townsend K. P., Praticò D. ( 2005) FASEB J. 19, 1592– 1601 [DOI] [PubMed] [Google Scholar]
- 18.Ikonomovic M. D., Uryu K., Abrahamson E. E., Ciallella J. R., Trojanowski J. Q., Lee V. M., Clark R. S., Marion D. W., Wisniewski S. R., DeKosky S. T. ( 2004) Exp. Neurol. 190, 192– 203 [DOI] [PubMed] [Google Scholar]
- 19.Wyss-Coray T. ( 2006) Nat. Med. 12, 1005– 1015 [DOI] [PubMed] [Google Scholar]
- 20.Vane J. ( 1994) Nature 367, 215– 216 [DOI] [PubMed] [Google Scholar]
- 21.Smith C. J., Zhang Y., Koboldt C. M., Muhammad J., Zweifel B. S., Shaffer A., Talley J. J., Masferrer J. L., Seibert K., Isakson P. C. ( 1998) Proc. Natl. Acad. Sci. U. S. A. 95, 13313– 13318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srinivasan B. D., Kulkarni P. S. ( 1989) Prog. Clin. Biol. Res. 312, 229– 249 [PubMed] [Google Scholar]
- 23.Kitamura Y., Shimohama S., Koike H., Kakimura J., Matsuoka Y., Nomura Y., Gebicke-Haerter P. J., Taniguchi T. ( 1999) Biochem. Biophys. Res. Commun. 254, 582– 586 [DOI] [PubMed] [Google Scholar]
- 24.Yasojima K., Schwab C., McGeer E. G., McGeer P. L. ( 1999) Brain Res. 830, 226– 236 [DOI] [PubMed] [Google Scholar]
- 25.Montine T. J., Sidell K. R., Crews B. C., Markesbery W. R., Marnett L. J., Roberts L. J., 2nd, Morrow J. D. ( 1999) Neurology 53, 1495– 1498 [DOI] [PubMed] [Google Scholar]
- 26.Ho L., Purohit D., Haroutunian V., Luterman J. D., Willis F., Naslund J., Buxbaum J. D., Mohs R. C., Aisen P. S., Pasinetti G. M. ( 2001) Arch. Neurol. 58, 487– 492 [DOI] [PubMed] [Google Scholar]
- 27.Andreasson K. I., Savonenko A., Vidensky S., Goellner J. J., Zhang Y., Shaffer A., Kaufmann W. E., Worley P. F., Isakson P., Markowska A. L. ( 2001) J. Neurosci. 21, 8198– 8209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.in t' Veld B. A., Ruitenberg A., Hofman A., Launer L. J., van Duijn C. M., Stijnen T., Breteler M. M., Stricker B. H. ( 2001) N. Engl. J. Med. 345, 1515– 1521 [DOI] [PubMed] [Google Scholar]
- 29.Liang X., Wang Q., Hand T., Wu L., Breyer R. M., Montine T. J., Andreasson K. ( 2005) J. Neurosci. 25, 10180– 10187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoshino T., Nakaya T., Homan T., Tanaka K., Sugimoto Y., Araki W., Narita M., Narumiya S., Suzuki T., Mizushima T. ( 2007) J. Biol. Chem. 282, 32676– 32688 [DOI] [PubMed] [Google Scholar]
- 31.Qin W., Ho L., Pompl P. N., Peng Y., Zhao Z., Xiang Z., Robakis N. K., Shioi J., Suh J., Pasinetti G. M. ( 2003) J. Biol. Chem. 278, 50970– 50977 [DOI] [PubMed] [Google Scholar]
- 32.Coleman R. A., Smith W. L., Narumiya S. ( 1994) Pharmacol. Rev. 46, 205– 229 [PubMed] [Google Scholar]
- 33.Regan J. W. ( 2003) Life Sci. 74, 143– 153 [DOI] [PubMed] [Google Scholar]
- 34.Sturchler-Pierrat C., Abramowski D., Duke M., Wiederhold K. H., Mistl C., Rothacher S., Ledermann B., Bürki K., Frey P., Paganetti P. A., Waridel C., Calhoun M. E., Jucker M., Probst A., Staufenbiel M., Sommer B. ( 1997) Proc. Natl. Acad. Sci. U. S. A. 94, 13287– 13292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoshino T., Nakaya T., Araki W., Suzuki K., Suzuki T., Mizushima T. ( 2007) Biochem. J. 402, 581– 589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoshino T., Tsutsumi S., Tomisato W., Hwang H. J., Tsuchiya T., Mizushima T. ( 2003) J. Biol. Chem. 278, 12752– 12758 [DOI] [PubMed] [Google Scholar]
- 37.Gu Y., Misonou H., Sato T., Dohmae N., Takio K., Ihara Y. ( 2001) J. Biol. Chem. 276, 35235– 35238 [DOI] [PubMed] [Google Scholar]
- 38.Bradford M. M. ( 1976) Anal. Biochem. 72, 248– 254 [DOI] [PubMed] [Google Scholar]
- 39.Tomita S., Kirino Y., Suzuki T. ( 1998) J. Biol. Chem. 273, 19304– 19310 [DOI] [PubMed] [Google Scholar]
- 40.Farmery M. R., Tjernberg L. O., Pursglove S. E., Bergman A., Winblad B., Näslund J. ( 2003) J. Biol. Chem. 278, 24277– 24284 [DOI] [PubMed] [Google Scholar]
- 41.Fukumoto H., Cheung B. S., Hyman B. T., Irizarry M. C. ( 2002) Arch. Neurol. 59, 1381– 1389 [DOI] [PubMed] [Google Scholar]
- 42.Gardner L. A., Delos Santos N. M., Matta S. G., Whitt M. A., Bahouth S. W. ( 2004) J. Biol. Chem. 279, 21135– 21143 [DOI] [PubMed] [Google Scholar]
- 43.Bastepe M., Ashby B. ( 1997) Mol. Pharmacol. 51, 343– 349 [DOI] [PubMed] [Google Scholar]
- 44.González-Gaitán M., Stenmark H. ( 2003) Cell 115, 513– 521 [DOI] [PubMed] [Google Scholar]
- 45.Desai S., Ashby B. ( 2001) FEBS Lett. 501, 156– 160 [DOI] [PubMed] [Google Scholar]
- 46.Ferguson S. S., Downey W. E., 3rd, Colapietro A. M., Barak L. S., Ménard L., Caron M. G. ( 1996) Science 271, 363– 366 [DOI] [PubMed] [Google Scholar]
- 47.Desai S., April H., Nwaneshiudu C., Ashby B. ( 2000) Mol. Pharmacol. 58, 1279– 1286 [DOI] [PubMed] [Google Scholar]
- 48.Seachrist J. L., Ferguson S. S. ( 2003) Life Sci. 74, 225– 235 [DOI] [PubMed] [Google Scholar]
- 49.Ni Y., Zhao X., Bao G., Zou L., Teng L., Wang Z., Song M., Xiong J., Bai Y., Pei G. ( 2006) Nat. Med. 12, 1390– 1396 [DOI] [PubMed] [Google Scholar]
- 50.Guo D. F., Sun Y. L., Hamet P., Inagami T. ( 2001) Cell Res. 11, 165– 180 [DOI] [PubMed] [Google Scholar]
- 51.Vitolo O. V., Sant'Angelo A., Costanzo V., Battaglia F., Arancio O., Shelanski M. ( 2002) Proc. Natl. Acad. Sci. U. S. A. 99, 13217– 13221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sang N., Zhang J., Marcheselli V., Bazan N. G., Chen C. ( 2005) J. Neurosci. 25, 9858– 9870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shie F. S., Breyer R. M., Montine T. J. ( 2005) Am. J. Pathol. 166, 1163– 1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee R. K., Knapp S., Wurtman R. J. ( 1999) J. Neurosci. 19, 940– 947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pooler A. M., Arjona A. A., Lee R. K., Wurtman R. J. ( 2004) Neurosci. Lett. 362, 127– 130 [DOI] [PubMed] [Google Scholar]
- 56.Vetrivel K. S., Cheng H., Lin W., Sakurai T., Li T., Nukina N., Wong P. C., Xu H., Thinakaran G. ( 2004) J. Biol. Chem. 279, 44945– 44954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takahashi R. H., Almeida C. G., Kearney P. F., Yu F., Lin M. T., Milner T. A., Gouras G. K. ( 2004) J. Neurosci. 24, 3592– 3599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Langui D., Girardot N., El Hachimi K. H., Allinquant B., Blanchard V., Pradier L., Duyckaerts C. ( 2004) Am. J. Pathol. 165, 1465– 1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.He X., Cooley K., Chung C. H., Dashti N., Tang J. ( 2007) J. Neurosci. 27, 4052– 4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin X., Koelsch G., Wu S., Downs D., Dashti A., Tang J. ( 2000) Proc. Natl. Acad. Sci. U. S. A. 97, 1456– 1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.