Abstract
Zinc ions play indispensable roles in biological chemistry. However, bacteria have an impressive ability to acquire Zn2+ from the environment, making it exceptionally difficult to achieve Zn2+ deficiency, and so a comprehensive understanding of the importance of Zn2+ has not been attained. Reduction of the Zn2+ content of Escherichia coli growth medium to 60 nm or less is reported here for the first time, without recourse to chelators of poor specificity. Cells grown in Zn2+-deficient medium had a reduced growth rate and contained up to five times less cellular Zn2+. To understand global responses to Zn2+ deficiency, microarray analysis was conducted of cells grown under Zn2+-replete and Zn2+-depleted conditions in chemostat cultures. Nine genes were up-regulated more than 2-fold (p < 0.05) in cells from Zn2+-deficient chemostats, including zinT (yodA). zinT is shown to be regulated by Zur (zinc uptake regulator). A mutant lacking zinT displayed a growth defect and a 3-fold lowered cellular Zn2+ level under Zn2+ limitation. The purified ZinT protein possessed a single, high affinity metal-binding site that can accommodate Zn2+ or Cd2+. A further up-regulated gene, ykgM, is believed to encode a non-Zn2+ finger-containing paralogue of the Zn2+ finger ribosomal protein L31. The gene encoding the periplasmic Zn2+-binding protein znuA showed increased expression. During both batch and chemostat growth, cells “found” more Zn2+ than was originally added to the culture, presumably because of leaching from the culture vessel. Zn2+ elimination is shown to be a more precise method of depleting Zn2+ than by using the chelator N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine.
Almost all biological interactions depend upon contacts between precisely structured protein domains, and Zn2+ may be used to facilitate correct folding and stabilize the domain (1, 2). Zn2+ also plays an indispensable catalytic role in many proteins (1). Although normally classed as a trace element, Zn2+ accumulates to the same levels as calcium and iron in the Escherichia coli cell (3); predicted Zn2+-binding proteins account for 5–6% of the total proteome (4).
However, despite its indispensable role in biology, as with all metals, Zn2+ can become toxic if accumulated to excess. With no subcellular compartments to deposit excess metal, Zn2+ homeostasis in bacteria relies primarily on tightly regulated import and export mechanisms (5). The major inducible high affinity Zn2+ uptake system is the ABC transporter ZnuABC. ZnuA is important for growth (6) and Zn2+ uptake (7) and is thought to pass Zn2+ to ZnuB for transport through the membrane. Zn2+-bound Zur represses transcription of znuABC, whereas the addition of the metal chelator TPEN3 de-represses expression from a promoterless lacZ gene inserted into znuA, znuB, and znuC (8). Zur can sense subfemtomolar concentrations of cytosolic Zn2+, implying that cellular Zn2+ starvation commences at exceptionally low Zn2+ concentrations (3). Outten and O'Halloran (3) found that the minimal Zn2+ content required for growth in E. coli is 2 × 105 atoms/cell, which corresponds to a total cellular Zn2+ concentration of 0.2 mm, ∼2000 times the Zn2+ concentration found in the medium. A similar cellular concentration of Zn2+ was found in cells grown in LB medium.
Thus, E. coli has an impressive ability to acquire and concentrate Zn2+ (3), making the task of depleting this organism of Zn2+ very difficult. Nevertheless, during the course of this work, a paper was published (9) in which the authors conclude that ZinT (formerly YodA) “is involved in periplasmic zinc binding and either the subsequent import or shuttling of zinc to periplasmic zinc-containing proteins under zinc-limiting conditions.” Surprisingly, this conclusion was drawn from experiments in which Zn2+ levels in the medium were lowered only by reducing the amount of Zn2+ added, without metal extraction or chelation.
Only a few attempts have been made to study the global consequences of metal deficiency using “omic” technologies. A study using TPEN (10) found 101 genes to be differentially regulated in E. coli. However, the authors note that TPEN has been reported to bind Cd2+, Co2+, Ni2+, and Cu2+ more tightly than it binds Zn2+, and indeed, 34 of the 101 differentially regulated genes are transcriptionally regulated by Fur (the iron (Fe) uptake regulator) or involved in iron or copper metabolism. Thus, the transcriptome of E. coli associated with Zn2+ deficiency alone has not been elucidated. Most genome-wide microarray studies of the effects of metal stresses to date have been carried out in batch culture, but continuous culture offers major benefits for such studies. The greater biological homogeneity of continuous cultures and the ability to control all of the relevant growth conditions, such as pH and especially growth rate, eliminate the masking effects of secondary stresses and growth rate changes, allowing more precise delineation of the response to an individual stress (11, 12). In the case of transcriptomics, it has been demonstrated that the reproducibility of analyses between different laboratories is greater when chemostat cultures are used than when identical analyses are performed with batch cultures (13). Some studies have exploited continuous culture to examine the effects of metal stresses, such as that of Lee et al. (14) in which E. coli cultures grown in continuous culture at a fixed specific growth rate, temperature, and pH were used to assay the transcriptional response to Zn2+ excess. In the present study, E. coli was grown in continuous culture in which severe depletion was achieved without recourse to chelating agents in the medium by thorough extraction and scrupulous attention to metal contamination. Microarray analysis identifies only nine genes that respond significantly to Zn2+ starvation. We demonstrate here for the first time that one such gene, zinT, is up-regulated in response to extreme Zn2+ deprivation by Zur and that ZinT has a high affinity for Zn2+. We also reveal roles for Zn2+ redistribution in surviving Zn2+ deficiency.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Growth Conditions
Bacterial strains used in this study are listed in Table 1. The cells were grown in glycerol-glycerophosphate medium (GGM), slightly modified from Beard et al. (15). GGM is buffered with MES, which has minimal metal chelating properties, and uses organic phosphate as the phosphate source to minimize formation of insoluble metal phosphates (16). The final concentrations are: MES (40.0 mm), NH4Cl (18.7 mm), KCl (13.4 mm), β-glycerophosphate (7.64 mm), glycerol (5.00 mm), K2SO4 (4.99 mm), MgCl2 (1.00 mm), EDTA (134 μm), CaCl2·2H2O (68.0 μm), FeCl3·6H2O (18.5 μm), ZnO (6.14 μm), H3BO3 (1.62 μm), CuCl2·2H2O (587 nm), Co(NO3)2·6H2O (344 nm), and (NH4)6Mo7O24·4H2O (80.9 nm) in MilliQ water (Millipore). Bulk elements (MES, NH4Cl, KCl, K2SO4, and glycerol in MilliQ water at pH 7.4 (batch growth) or 7.6 (continuous culture)) were passed through a column containing Chelex-100 ion exchange resin (Bio-Rad) to remove contaminating cations. Trace elements (with or without Zn2+ as necessary) and a CaCl2 solution were then added to give the final concentrations shown above prior to autoclaving. After autoclaving, MgCl2 and β-glycerophosphate were added at the final concentrations shown. All of the chemicals were of AnalaR grade purity or higher. Chelex-100 was packed into a Bio-Rad Glass Econo-column (∼120 × 25 mm) that had previously been soaked in 3.5% nitric acid for 5 days.
TABLE 1.
List of strains used
| Strain | Genotype | Source |
|---|---|---|
| AL6 | MC4100 λΦ(PzinT-lacZ) | Ref. 25 |
| FB20133 | MG1655 ykgM ::kan | University of Wisconsin Genome Project |
| FB23354 | MG1655 znuA ::kan | University of Wisconsin Genome Project |
| MC4100 | F−araD139 Δ(argF-lac)U169 rpsL150 relA1 flbB5301 deoC1 ptsF25 rbsR | Ref. 25 |
| MG1655 | F− λ−ilvG rfb-50 rph-1 | Laboratory stock |
| SIP812 | MC4100 zur ::Spcr | Ref. 8 |
| RKP5082 | MG1655/pKD46 (Ampr) | This work |
| RKP5456 | MG1655 zinT ::cam | This work |
| RKP5466 | BL21(DE3) pLysS pET28a-zinT | This work |
| RKP5475 | AL6 with zur ::Spcr | This work |
Creating Zn2+-deficient Conditions and Establishing Zn2+-limited Cultures
Culture vessels and medium were depleted of Zn2+ by extensive acid washing of glassware, the use of a chemically defined minimal growth medium, chelation of contaminating cations from this medium using Chelex-100, and the use of newly purchased high purity chemicals and metal-free pipette tips. Plastics that came into contact with the medium (e.g. bottles, tubes, and tubing) were selected on the basis of their composition and propensity for metal leaching, and included polypropylene, polyethylene, polytetrafluoroethylene (PTFE), or polyvinyl chloride. Dedicated weigh boats, spatulas, measuring cylinders, PTFE-coated stir bars, and a pH electrode were used. PTFE face masks, polyethylene gloves, and a PTFE-coated thermometer were also used. The solutions were filter-sterilized using polypropylene syringes with no rubber seal, in conjunction with syringe filters with a PTFE membrane and polypropylene housing. Vent filters contained a PTFE membrane in polypropylene housing. The cells were grown in continuous culture in a chemostat that was constructed entirely of nonmetal parts as detailed below.
Continuous Culture of E. coli Strain MG1655
E. coli strain MG1655 was grown in custom-built chemostats made entirely of nonmetal parts essentially as described by Lee et al. (14) with some modifications. Glass growth vessels and flow-back traps were soaked extensively (approximately two months) in 10% nitric acid before rinsing thoroughly in MilliQ water. Vent filters (Vent Acro 50 from VWR) were connected to the vessel using PTFE tubing. Metal-free pipette tips were used (MAXYMum Recovery Filter Tips from Axygen). Culture volume was maintained at 120 ml using an overflow weir in the chemostat vessel (14). The vessel was inoculated using one of the side arms. Flasks were stirred on KMO 2 Basic IKA-Werke stirrers at 437 rpm determined using a handheld laser tachometer (Compact Instruments Ltd). The use of a vortex impeller suspended from above the culture avoided grinding of the glass vessel that would occur if a stir bar were used. The samples were taken from the culture vessel as in Lee et al. (14). The dilution rate (and hence the specific growth rate) was 0.1 h−1 (which is below the maximal specific growth rate μmax for this strain (17)). No washout was observed in long term chemostat cultures in Zn2+-depleted medium. One chemostat was fed medium that contained “adequate” Zn2+ (i.e. normal GGM concentration), whereas the other contained no added Zn2+ and had been depleted of Zn2+ as above. Chemostats were grown for 50 h to allow five culture volumes to pass through the vessel and allow an apparent (pseudo-)steady state to be reached. More prolonged growth was avoided to minimize the formation of mutations in the rpoS gene (18). Samples were taken throughout to check pH, A600, glycerol content and for contaminants. Steady state values for pH and A600 were 6.9 and 0.6, respectively. Glycerol assays (19) showed cultures to be glycerol-limited.
The Zn2+-free chemostat was inoculated with cells that had been subcultured in Zn2+-free medium. A 0.25-ml aliquot of a saturated culture of strain MG1655 grown in LB was centrifuged, and the pellet was used to inoculate 5 ml of GGM that was incubated overnight at 37 °C with shaking. A 2.4-ml (i.e. 2% of chemostat volume) aliquot of this was then used to inoculate the chemostat. The adequate Zn2+ chemostat was inoculated with cells treated in essentially the same way but grown in GGM containing adequate Zn2+. The two cultures (±Zn2+) used to inoculate the chemostats had A600 readings within 2.5% of each other. Aliquots from the chemostat were used to harvest RNA and for metal analysis by inductively coupled plasma-atomic emission spectroscopy (ICP-AES; see below).
Batch Growth of E. coli Strains in GGM ± Zn2+
A saturated culture was grown in LB (with antibiotics as appropriate). To minimize carry-over of broth, the cells were collected from ∼0.25 ml of culture by centrifugation, and the pellet was resuspended in a 5-ml GGM starter culture (with Zn2+ and antibiotics as appropriate) for 24 h. Side arm flasks containing 25 ml GGM with Zn2+ were then inoculated with the equivalent of 1 ml of a culture with A600 of 0.6. For these experiments, cultures with zinc were grown in medium containing adequate Zn2+ where no special precautions were taken in preparing the medium. Zinc-depleted cultures were grown in side arm flasks that had been soaked extensively in 10% nitric acid before being rinsed thoroughly in MilliQ water. Growth was measured over several hours using a Klett colorimeter and a red filter (number 66; Manostat Corporation). The colorimeter was blanked using GGM. No antibiotics were present in the growth medium used for batch growth curves because they can act as chelators (20–23), but cultures were spotted onto solid LB plates with and without antibiotics at the end of the growth curve to verify that antibiotic resistance was retained. At the end of the growth curve, aliquots of the culture were combined and pelleted for ICP-AES analysis (see below).
RNA Isolation and Microarray Procedures
These were conducted as described by Lee et al. (14). RNA was quantified using a BioPhotometer (Eppendorf). E. coli K-12 V2 OciChip microarray slides were purchased from Ocimum Biosolutions Ltd. (previously MWG Biotech). Biological experiments (i.e. comparison of low Zn2+ versus adequate Zn2+ in chemostat culture) were carried out three times, and a dye swap was performed for each experiment, providing two technical repeats for each of the three biological repeats. The data were analyzed as before (14). Spots automatically flagged as bad, negative, or poor in the Imagene software were removed before the statistical analysis was carried out in GeneSight.
zinT Gene Inactivation
The zinT gene was functionally inactivated by the insertion of a chloramphenicol resistance cassette using the method of Datsenko and Wanner (24). The pACYC184 chloramphenicol resistance cassette was amplified by PCR using primers that have 40 bases of identity at their 5′ ends to regions within the zinT gene. The forward primer was 5′-GCATGGTCATCACTCACACGGCAAACCCTTAACAGAGGTCAAGCCACTGGAGCACCTCAA-3′ and the reverse was 5′-CAATGCCGTCCTCAATGCCAATCATCTCGATATCTGTTGCACGGGGAGAGCCTGAGCAAA-3′ (the regions homologous to zinT are underlined). The linear DNA was used to transform strain RKP5082 by electroporation. This strain contains pKD46, which overexpresses the phage λ recombination enzymes when arabinose is present. Bacteria were grown to an A600 of 0.6 in 500 ml of LB containing ampicillin (final concentration, 150 μg/ml) and arabinose (final concentration, 1 mm) at 30 °C. The cells were then pelleted and made electrocompetent by washing the pellet three times in ice-cold 10% glycerol. The last pellet was not resuspended but vortexed into a slurry. Aliquots of cells (50–100 μl) were electroporated with 1–10% linear DNA (v/v) at 1800 V. The cells were recovered by the addition of 1 ml of LB and incubation at 37 °C for 90 min. The cells were then pelleted and plated onto LB containing chloramphenicol at 34 μg/ml (final concentration). Loss of pKD46 plasmid was checked by streaking transformants on LB agar plates containing ampicillin (final concentration, 150 μg/ml). Insertion of the chloramphenicol cassette was checked by DNA sequencing. The zinT::cam (chloramphenicol resistance cassette) mutant strain was named RKP5456.
Construction of a λΦ(PzinT-lacZ) zur::Spcr Strain
The zur::Spcr (spectinomycin resistance cassette) mutation in strain SIP812 (8) was moved into strain AL6, which harbors the λΦ(PzinT-lacZ) fusion (25), by P1 transduction (26). The strain was named RKP5475.
Quantitative Real Time (qRT)-PCR
This was carried out on RNA samples harvested from the chemostats exactly as described in Lee et al. (14). The mRNA levels of holB were unchanged as determined by array analysis and were thus used as an internal control.
ICP-AES
Cells (from 25 ml (batch) or ∼85 ml of culture (chemostat)) were harvested by centrifugation at 5000 × g for 5 min (Sigma 4K15) in polypropylene tubes from Sarstedt (catalogue numbers 62.547.004 (50 ml) or 62.554.001 (15 ml)). Culture supernatants were retained for analysis. The pellets were washed three times in 0.5 ml of 0.5% HNO3 (Aristar nitric acid, 69% v/v) to remove loosely bound elements. Supernatants collected from the washes were also retained for analysis.
The pellets were resuspended in 0.5 ml of HNO3 (69%) before transfer to nitric acid-washed test tubes (previously dried). The samples were placed in an ultrasonic bath for ∼30 min to break the cells. The resultant digest was then quantitatively transferred to a calibrated 15-ml tube and made up to 5 ml with 1% HNO3. The samples were analyzed using a SpectrocirosCCD (Spectroanalytical) inductively coupled plasma-atomic emission spectrometer using background correction. Analyte curves were created for each element to be tested using multi-element standard solutions containing 0.1, 0.2, 1, 5, and 10 mg liter−1. The wavelengths (nm) for each element were as follows: calcium, 183.801; cobalt, 228.616; copper, 324.754 and 327.396; iron, 259.941; magnesium, 279.079; molybdenum, 202.030; sodium, 589.592; and zinc, 213.856. A 1% nitric acid solution in MilliQ water was used as a blank and to dilute cell digests before ICP-AES analysis. Concentrations of each element in each sample (pellets, culture supernatants, and wash supernatants) were calculated using the standard curves. The measurements obtained were the means of five replicate integrations. The limit of Zn2+ detection was 0.001 mg liter−1 (i.e. 1 ppb). In the “simple” low matrix solutions analyzed here, the wavelength used for Zn2+ detection is interference-free and specific for Zn2+.
Elemental recoveries were calculated from these samples. Two different recovery calculations were performed: 1) the percentage of an element in the culture that was subsequently recovered in the washed cell pellet, wash supernatants, and culture supernatant, and 2) the percentage of an element recovered in the unwashed pellet and culture supernatant. The former was used for batch and chemostat samples, and the latter was used for chemostat only. In some samples, element concentrations were below the calculated limit of detection (LOD) for the method. LOD is calculated from the calibration curve based on three σ of a blank signal. Where the signal is at or below the LOD, the instrument reports a <LOD value. In these cases, the LOD is used in subsequent calculations, so it will be an overestimation. Detection of Zn2+ was further complicated because, in many cases, Zn2+ concentrations were close to unavoidable background levels.
Calculation of Dry Cell Weight
Cellular metal contents were expressed on a dry cell mass basis. This was determined by filtering known volumes of culture (10, 20, and 30 ml) through preweighed cellulose nitrate filters (47-mm diameter and pore size of 0.2 μm; Millipore). The filters had previously been dried at 105 °C for 18–24 h to constant weight. The filters were again dried at 105 °C until a constant weight was attained, which was recorded.
β-Galactosidase Activity Assay
For β-galactosidase assays with strains AL6 (λΦ(PzinT-lacZ)) and RKP5475, a saturated culture was grown in LB with or without spectinomycin (final concentration, 50 μg/ml) as appropriate, and cells from ∼0.25 ml of culture were collected and resuspended in 5 ml GGM with or without Zn2+ and spectinomycin as appropriate. This was incubated overnight at 37 °C with shaking. A 1-ml aliquot of this was then used to inoculate several cultures (15 ml) as described in the text. The cultures were harvested when an A600 of 0.2–0.4 was reached. Immediately prior to harvesting, 5 μl was spotted onto solid LB plates with and without antibiotics to check that resistance was retained. Separate flasks were set up and used to grow the strains under each of the conditions mentioned above for ICP-AES analysis.
β-Galactosidase activity was measured in CHCl3- and SDS-permeabilized cells by monitoring the hydrolysis of o-nitrophenyl-β-d-galactopyranoside. Cell pellets were resuspended in ∼15 ml of Z buffer (26). Each culture was assayed in triplicate. Absorbance (A) at 420, 550, and 600 nm was measured to allow β-galactosidase activity (Miller units) to be calculated as described in Ref. 26.
Cloning of zinT for Protein Purification
Primers 5′-CTCCTGCCTTTCATATGGGTCATCAC-3′ (forward) and 5′-CATAGTGATGAGCTCGTCTGTAGC-3′ (reverse) were used to amplify the zinT coding region minus the sequence that encodes the 24-amino acid periplasmic signaling sequence (27) from MG1655 genomic DNA. An NdeI site was engineered into the forward primer and a SacI site into the reverse primer (underlined above), which, following enzymic digestion, allowed the 684-bp product to be ligated into pET28a (Novagen). The translated protein is produced with an N-terminal His tag and thrombin cleavage site. This allowed the protein to be purified using TALON metal affinity resin (Clontech), which uses immobilized Co2+ ions to trap polyhistidine tags with high affinity, followed by cleavage with thrombin to release the pure protein. Insertion of the correct fragment was verified by digestion with restriction endonucleases. pET28a containing the zinT gene fragment (pET28a-zinT) was used to transform E. coli overexpression strain BL21(DE3) pLysS and named strain RKP5466.
Overexpression and Purification of Recombinant ZinT
Strain RKP5466 was grown in LB containing kanamycin (50 μg/ml, to maintain pET28a-zinT) and chloramphenicol (34 μg/ml, to maintain pLysS) at 37 °C with shaking to an A600 of 0.6, at which point isopropyl β-d-thiogalactopyranoside was added to a final concentration of 1 mm. The cells were harvested after a further 4 h of incubation. The pellets were stored at −80 °C for later use; a cell pellet derived from 1 liter of culture was resuspended in ∼15 ml of buffer P (50 mm Tris/MOPS, 100 mm KCl, pH 8) and sonicated on ice to break the cells. Cell debris was pelleted by centrifugation for 30 min at 12 000 × g at 4 °C, whereupon the supernatant was removed and further centrifuged for 15 min at 27 000 × g. The cleared lysate was then loaded into a 5-ml TALON resin column, washed with 50 ml of buffer P, followed by 50 ml of buffer P containing 20 mm imidazole. Thrombin (60–80 units in 3–4 ml of buffer P) was pipetted onto the column, allowed to soak into the resin, and incubated overnight at room temperature. Ten 1-ml fractions were eluted using buffer P. Recombinant ZinT was determined to be >95% pure by SDS-PAGE. Protein was quantified using its absorbance at 280 nm and the theoretical extinction coefficient of 35995 m−1 cm−1 (estimated using the web-based program ProtParam at ExPASy), which assumes that all cysteines in the protein appear as half-cysteines using information based on (28). The theoretical extinction coefficient is based on the protein sequence minus the periplasmic targeting sequence.
N-terminal Protein Sequencing
Following SDS-PAGE, purified YodA was blotted onto a polyvinylidene fluoride membrane. The fragment of interest was excised from the membrane, and the sequence was determined using an Applied Biosystems Procise 392 protein sequencer.
Assays of Metal Binding to Purified ZinT
Purified recombinant ZinT was exchanged into buffer D (20 mm MOPS, pH 7) using a PD-10 desalting column (GE Healthcare). ZinT (1 ml) was incubated with various concentrations of ZnSO4·7H2O (ACS grade reagent) and/or CdCl2·2½H2O (AnalaR grade) for 1 h at room temperature. The protein/metal mixture was then loaded onto a PD-10 column and eluted in 7 × 0.5-ml fractions using buffer D. The fractions were assayed for A280 and for metal content using ICP-AES. Quantification of some elements was below the LOD in a limited number of samples that do not affect the overall interpretation of the experiment. In these cases the value for the LOD was used for subsequent calculations and thus will be an overestimation.
Mag-fura-2 Binding Experiments
Purified recombinant ZinT was exchanged into buffer M (140 mm NaCl, 20 mm Hepes, pH 7.4) using a PD-10 desalting column. Absorption spectra were collected using a Varian Cary 50 Bio UV-visible spectrophotometer at 37 °C. Buffer composition and experimental conditions were taken from Simons (47). ZinT (500 μl; ∼15 μm) was placed in a quartz cuvette, and a spectrum was taken from which the concentration of ZinT was determined. Difference spectra were recorded in which the reference sample was buffer M. Equimolar mag-fura-2 (MF; Molecular Probes, catalogue number M-1290) was then added. Aliquots of ZnSO4·7H2O (ACS grade reagent) and/or CdCl2·2½H2O (AnalaR grade) in buffer M were added, mixed, and incubated for 1 min before the spectra were collected. The equilibrium was established within 1 min of Zn2+ being added.
RESULTS
Creating Zn2+-deficient Conditions
Several precautions, based on normal analytical practice and the findings of Kay (29) regarding Zn2+ contamination, were taken to ensure that culture vessels and medium were depleted of Zn2+ where necessary. Table 2 shows typical values for the amounts of various metals in GGM as analyzed by ICP-AES. Both Zn2+-depleted and -replete media show good correlation with the expected values. In various batches of media analyzed, Zn2+ concentrations in Zn2+-depleted medium ranged from <0.001 to 0.004 mg liter−1 (<15–60 nm Zn2+). The variation in Zn2+ depletion achieved is a result of the difficulty in excluding Zn2+ from all sources that come into contact with the medium and culture. Sodium was used as the exchanging ion on Chelex-100, but excess sodium was not detected in the medium following chelation (data not shown).
TABLE 2.
Expected and representative measured amounts of elements in Zn2+-sufficient and -depleted GGM
| Element | Predicted from medium composition | Measured by ICP-AES |
|
|---|---|---|---|
| Zn2+-sufficient | Zn2+-depleted | ||
| mg liter−1 | mg liter−1 | ||
| Zinc | 0.401/0 (+zinc/−zinc) | 0.340 | 0.004 |
| Iron | 1.045 | 0.886 | 0.878 |
| Copper | 0.037 | 0.033 | 0.034 |
| Cobalt | 0.0257 | 0.018 | 0.019 |
| Molybdenum | 0.054 | 0.068 | 0.059 |
| Calcium | 2.24 | 2.83 | 2.85 |
| Magnesium | 24.0 | 24.2 | 25.0 |
Growth in Zn2+-depleted Batch Cultures
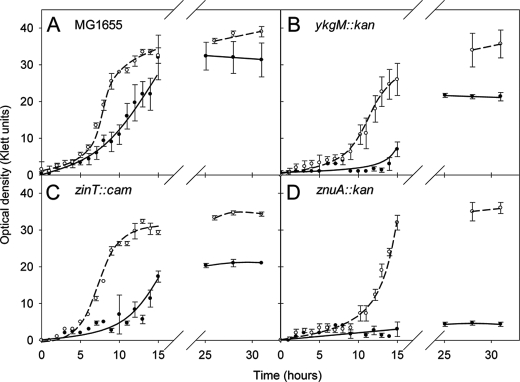
E. coli strain MG1655 was grown in GGM with or without Zn2+ (Fig. 1A). The Zn2+-limited culture showed a lag in entering the exponential phase, and a semi-logarithmic analysis of growth (not shown) revealed that the Zn2+-limited culture had an increased doubling time (159.0 min) compared with the Zn2+-replete culture (125.4 min) and reached a lower final A value. Because A measurements may reflect cell size changes (30), the samples were taken at the end of growth for electron microscopy, but no discernible size difference was seen between E. coli cells grown with or without Zn2+ in GGM (not shown). Cells grown in GGM (±Zn2+) were, however, smaller (length, width, and volume) than cells grown in rich medium (LB), presumably because of a slower growth rate (31).
FIGURE 1.
Growth of wild-type and isogenic mutant E. coli strains in Zn2+-depleted (filled circles, solid line) and Zn2+-replete (open circles, dashed line) GGM in batch culture. In each case, the means and standard deviations of three flasks are plotted. The doubling times of the strains during exponential growth, calculated from semi-logarithmic plots, were as follows: MG1655 replete, 125 min; MG1655 deplete, 159 min; ykgM::kan replete, 211 min; ykgM::kan deplete, 885 min; zinT::cam replete, 124 min; zinT::cam replete, 193 min; znuA::kan replete 134 min; znuA::kan deplete, 492 min. A, MG1655 wild type; B, ykgM::kan (FB20133); C, zinT::cam (RKP5456); D, znuA::kan (FB23354).
GGM contains EDTA, which prevents precipitation of the trace elements present. This is a well established and common practice (17). However, to investigate whether this EDTA was itself creating Zn2+ depletion, we cultured MG1655 in GGM with and without EDTA (supplemental Fig. S1). When grown in GGM without EDTA, MG1655 displayed a longer lag phase and reduced growth yield. The growth rate was also affected; the doubling time during exponential growth increased from 125.5 (with EDTA) to 131.5 min (without EDTA). Thus, EDTA is not creating a state of Zn2+ depletion but rather is a beneficial component of the medium.
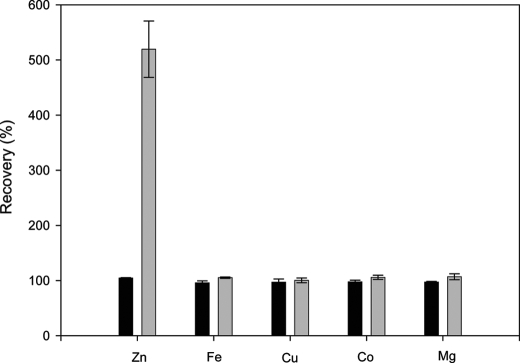
As well as growing at a reduced rate, cells grown in Zn2+-depleted medium had ∼1.8–5.0-fold less cellular Zn2+ than those grown in Zn2+-replete medium (based on three separate experiments). For example, at the end of the growth curve shown in Fig. 1A, the cells cultured in Zn2+-replete medium contained 1.12 × 10−5 mg of Zn2+/mg of dry weight cells and the cells grown in Zn2+-depleted medium contained 3.40 × 10−6 mg of Zn2+/mg of dry weight cells (a 3.3-fold difference). Here, “cellular Zn” is defined as that which cannot be removed by three successive washes with 0.5% nitric acid. To verify the reliability of the metal analyses, elemental recoveries were calculated from these samples. Fig. 2 shows that, for cells grown in Zn2+-replete medium, Zn2+ recovery was between 90 and 110%, and for cells grown in Zn2+-replete and Zn2+-deplete medium, the recovery of iron, copper, cobalt, and magnesium was also between 90 and 110%. For these elements, therefore, the metal content in the washed pellet and the culture supernatant and the wash supernatants fully accounts for the metal initially added to the culture in the medium. However, this was not true for Zn2+ recovery in cells grown in Zn2+-deficient medium. Zn2+ in these cells, together with that in the culture supernatant and wash supernatants, was 5-fold higher than the amount originally added to the culture in the medium. This suggests an avid Zn2+ sequestering ability of cells cultured under limiting Zn2+ conditions. Details of the analyses of individual pellets, wash solutions, supernatants, and media for Zn2+ are found in supplemental Table S1. We conclude that Zn2+ limitation can be achieved in batch culture without resorting to chelators despite effective bacterial Zn2+ scavenging mechanisms.
FIGURE 2.
Recovery of elements following growth of strain MG1655 in batch culture. The means and standard deviations of three flasks are plotted. The black and gray bars represent the percentage of added elements recovered from cells grown in Zn2+-replete and -deplete conditions, respectively. See text for details of calculation.
Cells Grown in Continuous Culture “Find” Extra Zn2+
To explore Zn2+ acquisition and localization at constant growth rates and defined conditions for a detailed transcriptomic study, E. coli strain MG1655 was grown in parallel glycerol-limited chemostats, one fed with medium that contained “adequate” Zn2+ and one that had been rigorously depleted of Zn2+. For the majority of elements assayed (iron, copper, cobalt, magnesium, molybdenum, potassium, sodium, phosphorus, and sulfur), the percentage recoveries were 90–110% (data not shown). However, more Zn2+ was recovered from the cells grown in the Zn2+-deficient chemostat than was originally added to the culture (Table 3), as in batch culture (Fig. 2). This is presumed to be due to active leaching from glassware or carry-over from the inoculum. Interestingly, this percentage markedly decreased with successive experiments in the same chemostat apparatus, suggesting that there is less Zn2+ able to be leached after repeated runs of culture in the same chemostat vessel (Table 3). Details of the analyses of individual pellets, wash solutions, supernatants, and media are found in supplemental Table S2.
TABLE 3.
Recovery of Zn2+ from E. coli strain MG1655 growing in a Zn2+-limited chemostat (Run 1) followed by successive cultures in the same chemostat under the same conditions (Runs 2–5)
A run is an experiment conducted after terminating a chemostat experiment and re-establishing a new culture in the same apparatus. ND, not determined. See text for details of calculation.
| Run | Recovery of Zn2+ in medium |
|||
|---|---|---|---|---|
| Washed cell pellet + wash solutions + supernatant |
Unwashed cell pellet + supernatant |
|||
| +Zinc | −Zinc | +Zinc | −Zinc | |
| % | % | % | % | |
| 1 | 104 | 1858 | ND | ND |
| 2 | 110 | 1676 | ND | ND |
| 3 | 105 | 559 | ND | ND |
| 4 | 104 | 493 | 102 | 454 |
| 5 | 103 | 248 | 103 | 254 |
Cells grown in the Zn2+-deficient chemostat consistently contained less cellular Zn2+ than those grown in Zn2+-replete medium (e.g. 2.94 × 10−5 mg of Zn2+/mg of cells for cells grown in adequate Zn2+ and 0.536 × 10−5 mg of Zn2+/mg of cells for cells harvested from run 5 of the Zn2+-limited chemostat (a 5.5-fold decrease)).
Transcriptome Changes Induced by Zn2+ Deficiency
The genome-wide mRNA changes of strain MG1655 grown in continuous culture with adequate or limiting Zn2+ were probed using microarray technology. Commonly applied criteria to determine the significance in transcriptomic studies are a fold change of more than 2 and a p value of less than 0.05. Using these criteria, of the 4288 genes arrayed, only nine showed significant changes (an increase in all cases) in mRNA levels and are listed in Table 4. Genes not meeting these criteria may be biologically significant but are not studied further here. It should be noted that microarrays measure the relative abundance of mRNA but cannot inform as to whether changes occur because of changes in the rate of transcription or because of changes in the stability of the transcript. Zn2+ has been reported to affect the stability of the mRNA of a human Zn2+ transporter (32). The full data set has been deposited in GEO (accession number GSE11894) (33). Three genes were chosen for further study based on known links to Zn2+ homeostasis. The remaining six genes were not studied further. In total, 21 genes displayed a greater than 2-fold increase in mRNA levels, 13 displayed a decrease, and the mRNA changes from 140 genes had a p value of <0.05. No genes exhibited a 2-fold or greater decrease in mRNA levels with a p value of less than 0.05.
TABLE 4.
Genes with a significant change in mRNA level in response to Zn2+ deficiency
Only genes with a fold increase of more than 2 and a p value of less than 0.05 are included. The gene names are the primary names on Ecogene. The gene descriptions are from Ecogene.
| Gene | b number | Gene product | Fold increase | p value (<0.05) |
|---|---|---|---|---|
| zinT | b1973 | Periplasmic cadmium-binding protein; induced by cadmium and peroxide; binds zinc, nickel, and cadmium; SoxS- and Fur-regulated | 8.07 | 0.0001 |
| znuA | b1857 | High affinity ABC transport system for zinc, periplasmic | 2.88 | 0.00117 |
| fdnG | b1474 | Formate dehydrogenase-N, selenopeptide, anaerobic; periplasmic | 2.86 | 0.00386 |
| emtA | b1193 | Membrane-bound transglycosylase E, lipoprotein; involved in limited murein hydrolysis | 2.86 | 0.00998 |
| ykgM | b0296 | RpmE paralogue, function unknown | 2.64 | 0.03647 |
| mdtD | b2077 | Putative transporter, function unknown; no MDR phenotype when mutated or cloned; fourth gene in mdtABCDbaeRS operon | 2.46 | 0.01614 |
| ribA | b1277 | GTP cyclohydrolase II, riboflavin biosynthesis | 2.36 | 0.02506 |
| ydfE | b1577 | Pseudogene, N-terminal fragment, Qin prophage | 2.17 | 0.00452 |
| aslA | b3801 | Suppresses gpp mutants; putative arylsulfatase | 2.15 | 0.02660 |
The gene exhibiting the greatest change in transcription (and lowest p value) was zinT (up-regulated 8.07-fold), previously known as yodA. ZinT was initially identified in a global study of E. coli defective in the histone-like nucleoid-structuring protein H-NS (34). Levels of ZinT increase when cells are grown in the presence of Cd2+ (27) and at pH 5.8 (35). More recently, it has been suggested that the abundance of yodA mRNA changes in response to cytoplasmic pH stress (36). Transcription of zinT is increased by the addition of Cd2+, but not Zn2+, Cu2+, Co2+, and Ni2+, to growing cells (25), even though Cd2+, Zn2+, and Ni2+ were found in crystals of ZinT (37, 38) (see “Discussion”). Further evidence for the binding of Cd2+ to ZinT was presented by Stojnev et al. (39), who found that γ-labeled 109Cd2+-bound proteins could be detected in wild-type E. coli but not a mutant lacking zinT (39), suggesting a specific role for ZinT in Cd2+ accumulation. ZinT is found primarily in the cytoplasm in unstressed cells but is exported to the periplasm upon Cd2+ stress (25). The mature, periplasmic form of ZinT is thought to form a disulfide bond, because it is a substrate of DsbA (40). A recent paper (9) suggests a role for ZinT in periplasmic zinc binding under zinc-limiting conditions, but no direct evidence for zinT up-regulation in response to rigorous exclusion of zinc has been previously reported.
The znuA gene was also up-regulated in response to Zn2+ depletion (Table 4). ZnuA is the soluble periplasmic metallochaperone component of the ZnuABC Zn2+ importer and was up-regulated 2.88-fold. In this complex, ZnuB is the integral membrane protein, and ZnuC is the ATPase component. The znuB and znuC genes were up-regulated by 1.34- and 1.36-fold, respectively (with p values of >0.05 and thus are not shown in Table 4). No other genes that encode proteins involved in Zn2+ transport (specifically zupT, zur, zitB, zntA, zntR, zraS, zraR, and zraP) were more than 1.4-fold up-regulated or 1.2-fold down-regulated, and all had p values of >0.05. The changes in the mRNA levels of a number of genes involved in Zn2+ metabolism are shown in Table 5.
TABLE 5.
Changes in the mRNA levels from a number of genes in response to Zn2+ deficiency
The gene names are the primary names on Ecogene. The gene descriptions are from Ecogene.
| Gene | b number | Gene product | Fold change | p value |
|---|---|---|---|---|
| yodB | b1974 | Function unknown | 2.38 | 0.0725 |
| zur | b4046 | Repressor for znuABC, the zinc high affinity transport genes; dimer; binds two Zn(II) ions per monomer | 1.37 | 0.9578 |
| znuC | b1858 | High affinity ABC transport system for zinc | 1.36 | 0.2294 |
| znuB | b1859 | High affinity ABC transport system for zinc | 1.34 | a |
| zntR | b3292 | Zinc-responsive activator of zntA transcription | 1.34 | 0.4857 |
| zraS | b4003 | Two component sensor kinase for ZraP; responsive to Zn2+ and Pb2+; autoregulated; regulation of Hyd-3 activity is probably due to cross-talk of overexpressed protein | 1.32 | 0.1109 |
| zraP | b4002 | Zinc-binding periplasmic protein; responsive to Zn2+ and Pb2+; regulated by zraSR two-component system; rpoN-dependent | 1.25 | 0.9322 |
| yiiP | b3915 | Iron and zinc efflux membrane transporter; cation diffusion facilitator family; dimeric | 1.17 | 0.2742 |
| zitB | b0752 | Zn(II) efflux transporter; zinc-inducible | 1.09 | 0.9571 |
| zntA | b3469 | Zn(II), Cd(II), and Pb(II) translocating P-type ATPase; mutant is hypersensitive to Zn2+ and Cd2+ salts | 1.07 | 0.9285 |
| spy | b1743 | Periplasmic protein induced by zinc and envelope stress, part of cpxR and baeSR regulons | 1.03 | 0.8314 |
| zraR | b4004 | Two component response regulator for zraP; responsive to Zn2+ and Pb2+; autoregulated; regulation of Hyd-3 activity is probably due to cross-talk of overexpressed protein | 0.95 | 0.9315 |
| zupT | b3040 | Zinc and other divalent cation uptake transporter | 0.88 | 0.3258 |
a Insufficient data available to obtain a p value.
The ykgM gene was up-regulated 2.64-fold in this study (Table 4) and has been identified previously by bioinformatics as the non-Zn2+ ribbon-containing paralogue of the ribosomal protein L31 that normally contains a Zn2+ ribbon motif and is thus predicted to bind Zn2+ (41). Panina et al. (41) predicted (but did not show) that ykgM would be up-regulated upon Zn2+ starvation and then displace the Zn2+-containing version of L31 in the ribosome, thus liberating Zn2+ for use by Zn2+-containing enzymes. However, no previous study has attained the degree of Zn2+ limitation reported here, and the role of ykgM has not been further explored.
To verify the results obtained by microarray experiments, several genes that were induced by Zn2+ depletion were examined by qRT-PCR to determine independently relative mRNA levels. The levels of up-regulation determined by qRT-PCR (mean ± normalized standard deviation) were as follows: yodA, 7.77 ± 0.63; ykgM, 2.83 ± 0.61; and znuA, 2.34 ± 0.58. These values correspond closely to increases in the microarray analysis of 8.07-, 2.64-, and 2.88-fold, respectively. Similar qRT-PCR values were obtained on one (ykgM and znuA) or two (yodA) other occasions. The mRNA levels of holB (internal control) were unchanged as determined by qRT-PCR and array analysis.
Hypersensitivity of Selected Strains to Zn2+ Deficiency
To assess the importance of the ykgM, zinT, and znuA genes in surviving Zn2+ deficiency, mutants were used in which each gene are inactivated by insertion of an antibiotic resistance cassette; the growth of these isogenic strains was compared in Zn2+-depleted and Zn2+-replete liquid cultures (Fig. 1). Each strain (wild type and mutants) grew more poorly in the absence of Zn2+ than in its presence. Also, in Zn2+-depleted medium, the ykgM::kan (kanamycin resistance cassette), zinT::cam, and znuA::kan mutants consistently grew more poorly than MG1655 in the same medium. We were unable to culture the znuA::kan mutant to >5 Klett units in the severely Zn2+-depleted conditions achieved here (Fig. 1D). All of the experiments were carried out in triplicate, and similar results were seen on at least two separate occasions. We confirmed by qRT-PCR that the genes downstream of ykgM, zinT, and znuA (i.e. ykgO, yodB and yebA, respectively) were in all cases transcribed in the mutant strains.
We measured cellular Zn2+ levels in bacteria grown in conditions of severe Zn2+ limitation in batch culture. The levels of Zn2+ detected in cell digests on analysis by ICP-AES were exceedingly low. Nevertheless, the zinT::cam strain contained ∼9-fold less cellular Zn2+ when cultured under Zn2+ limitation (1.28 × 10−6 mg of Zn2+/mg of cells) than when grown in Zn2+-replete (1.16 × 10−5 mg of Zn2+/mg of cells) conditions. Also, under Zn2+-deficient conditions, the zinT::cam strain contained nearly 3-fold less cellular Zn2+ than MG1655 wild-type cells grown under similar conditions (1.28 × 10−6 mg of Zn2+/mg of cells and 3.40 × 10−6 mg of Zn2+/mg of cells, respectively). These data are the first to demonstrate a role for ZinT in Zn2+ acquisition under strictly Zn2+-limited conditions. When the znuA::kan mutant was assayed after growth in Zn2+ depleted conditions, the measurement of cellular Zn2+ was below the LOD. Similar results were seen on at least one other occasion.
Transcriptional Regulation of zinT under Various Zn2+ Concentrations
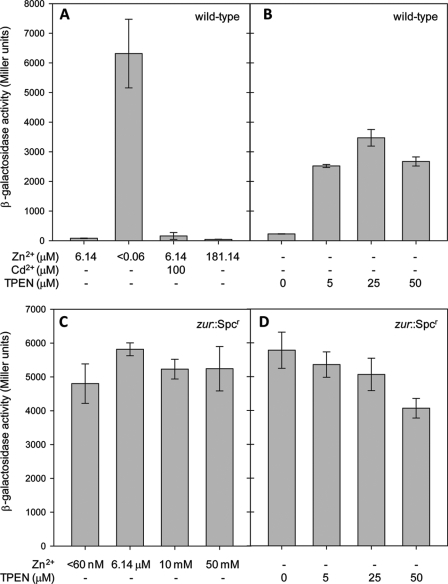
Having established that zinT transcription was elevated on Zn2+ depletion, a PzinT-lacZ transcriptional fusion (25), in which lacZ is transcribed from the zinT promoter, was used to investigate an alternative Zn2+ removal method and the effects of added Cd2+ and Zn2+. Fig. 3A shows that λΦ(PzinT-lacZ) activity was highly up-regulated under the Zn2+-deficient conditions created here (in which Zn2+ is excluded from the medium). These data were compared with cultures treated with TPEN (Fig. 3B), which is widely used as a Zn2+ chelator (3, 7, 32, 42, 43, 45). Fig. 3B shows that expression from λΦ (PzinT-lacZ) increases with increasing TPEN concentrations in the growth medium. Although expression from λΦ (PzinT-lacZ) was higher in cells grown in medium containing TPEN than in cells grown in adequate Zn2+, it was lower than that of cells grown in medium from which Zn2+ has been rigorously eliminated (Fig. 3A). In LB medium, the PzinT-lacZ fusion strain has previously been shown to respond to elevated levels of Cd2+ but not of Zn2+ (25). In GGM, the construct was again unresponsive to elevated Zn2+, but no response was seen to elevated Cd2+ (Fig. 3A), although this may be due to difficulties in growing cells at high levels of Cd2+, which were near its maximum permissive concentration.
FIGURE 3.
β-Galactosidase activity of λΦ(PzinT-lacZ) under various conditions. A and B, β-galactosidase activity of λΦ(PzinT-lacZ) (strain AL6) grown in GGM containing the concentrations of Zn2+, Cd2+, and TPEN shown. The Zn2+ concentrations can be interpreted as follows: 6.14 μm is GGM in which the bulk elements were Chelex-100-treated and then trace elements containing Zn2+ were added back; <0.06 μm is GGM in which extreme precautions were taken to exclude Zn2+ (see text). The cultures were harvested when the A600 reached 0.2–0.4. The means ± standard deviation for three technical replicates are shown. The same results were seen on at least one other occasion. C and D, β-galactosidase activity of λΦ(PzinT-lacZ) in a zur::Spcr background (strain RKP5475) grown in GGM containing the Zn2+ and TPEN concentrations shown. The cultures were harvested when the A600 reached 0.2–0.4. The means and standard deviations of three technical replicates are shown. The same results were seen on at least one other occasion.
A Zur-binding site has been reported in the zinT promoter (41), and Zn2+-bound Zur represses the transcription of znuABC (8). Therefore, to test the hypothesis that Zur also negatively regulates zinT, λΦ(PzinT-lacZ) activity was monitored in a strain lacking zur. Fig. 3 (C and D) shows that, in a zur mutant, λΦ(PzinT-lacZ) activity was not dependent on the extracellular Zn2+ concentration under any condition tested. Thus, Zur is a negative regulator of zinT transcription.
Stoichiometric Binding of Zn2+ and Cd2+ by ZinT
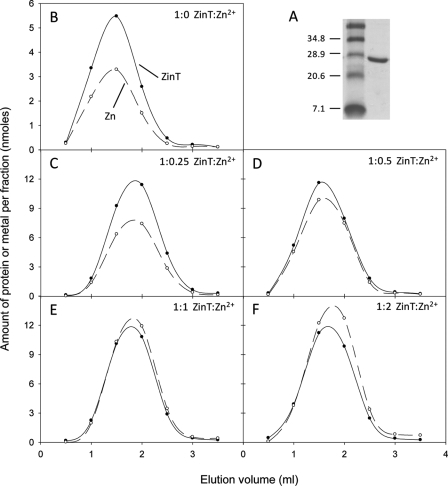
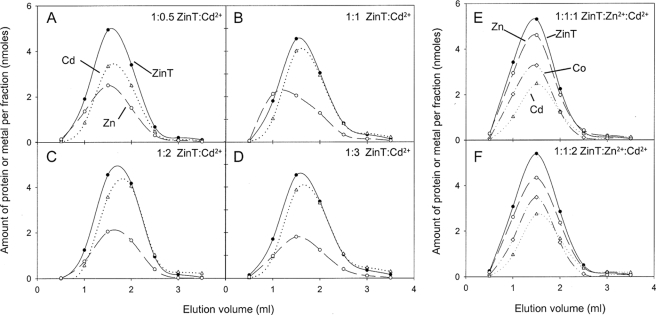
To investigate the possible role of ZinT in metal binding as suggested by the transcription and growth studies reported here, the zinT gene was cloned into pET28a such that the translated protein lacked the periplasmic signal sequence but was fused to a polyhistidine tag and thrombin cleavage site to aid purification. The polyhistidine tag was removed by cleavage with thrombin to minimize the danger of the protein adopting aberrant conformations. The sequence of the resultant protein, which was used to calculate the extinction coefficient, mimics the form of the protein found in the periplasm. Residual imidazole in the final ZinT preparation was avoided by using only a single wash step containing imidazole (20 mm) during purification and exchange into a buffer lacking imidazole before final use. The effective removal of the polyhistidine tag was confirmed by N-terminal sequencing. The pure recombinant protein (Fig. 4A) was incubated with different molar ratios of Zn2+ and then subjected to size exclusion chromatography to assess co-elution of Zn2+ with ZinT. Fig. 4 shows the elution profiles of ZinT and Zn2+ following incubation of ZinT with 0, 0.25, 0.5, 1, and 2 molar equivalents of Zn2+. Fig. 4B (and Fig. 5, A–D) shows that, even when no Zn2+ is added, ZinT co-eluted from the size exclusion column with Zn2+. The occupancy of Zn2+ observed under these conditions (0.6 mol of Zn2+/mol of ZinT) was approximately half that observed at superstoichiometric Zn2+/ZinT ratios (Fig. 4F), and so we conclude that the Zn2+ content shown in Fig. 4B represents ∼0.5 Zn2+/ZinT. This suggests a high affinity of ZinT for Zn2+ and is reminiscent of the crystallization of ZinT (38); crystals formed in the absence of added metals contained Zn2+ or Ni2+, indicative of high metal affinity (see “Discussion”). When ZinT was incubated with 0.25 or 0.5 molar equivalents of Zn2+ (Fig. 4, C and D) more Zn2+ co-eluted with ZinT than was originally added. However, when 1 (Fig. 4E), 2 (Fig. 4F), or 3 (data not shown) molar equivalents Zn2+ were incubated with ZinT, approximately one equivalent eluted from the column with the protein. These data provide evidence that ZinT binds one Zn2+ ion with high affinity.
FIGURE 4.
Metal binding to purified ZinT. A, purified recombinant ZinT (right lane) on an SDS-PAGE gel. Size markers (left lane) are shown in kDa. The elution profiles of ZinT and Zn2+ are from a PD-10 column following incubation of protein and metal ions. B, elution following incubation of 13.3 nmol of ZinT with no added metal. C–F, elution following incubation of 28.6 nmol of ZinT with 0.25, 0.5, 1, or 2 molar equivalents of Zn2+. Filled circles with solid line, ZinT; open circles with dashed line, Zn2+.
FIGURE 5.
Elution profiles of ZinT, Zn2+, and Cd2+ from a PD-10 column following incubation of protein and metal ions. A–D, elution following incubation of 17.8 nmol of ZinT with 0.5, 1, 2, or 3 molar equivalents of Cd2+. Filled circles with solid line, ZinT; open circles with dashed line, Zn2+; open triangles with dotted line, Cd2+. E and F, elution following incubation of 13.3 nmol of ZinT with 1 molar equivalent of Zn2+ and 1 molar equivalent of Cd2+ or with 1 molar equivalent of Zn2+ and two molar equivalents of Cd2+. Filled circles with solid line, ZinT; open circles with dashed line, Zn2+; open diamonds with dotted and dashed line, Co2+; open triangles with dotted line, Cd2+.
Previous work (38) has suggested that ZinT is able to bind Cd2+, and so the experiment was also carried out using Cd2+. ZinT co-elutes from a size exclusion column with up to 1 molar equivalent of Cd2+, even when initially incubated with more (Fig. 5, A–D). When 13.3 nmol of ZinT was incubated without Cd2+ prior to size exclusion chromatography, the eluate contained less than 18 pmol of Cd2+/fraction (not shown). It should be noted that, in the case of Cd2+, the Cd2+/ZinT ratio was ∼0.9 but never exceeded 1 (Fig. 5D) unlike the case with Zn2+ (Fig. 4F). This is attributable to the inevitable contamination of reagents and materials with Zn2+ but not Cd2+.
To investigate competition of Zn2+ and Cd2+ for site(s) in ZinT, the protein was incubated with both metals, and co-elution of metals and protein was assayed. ZinT co-eluted with almost 1 molar equivalent of Zn2+ and ∼0.5 molar equivalents of Cd2+ (Fig. 5E). These ratios were similar when the Cd2+:Zn2+ ratio was increased to 2:1 (Fig. 5F), indicating that ZinT preferentially binds Zn2+ over Cd2+. Multi-element analysis of the eluate also revealed ∼0.5 molar equivalents of Co2+ with ZinT. This was seen in all experiments, and the reasons for this are discussed below. Two metal ions/ZinT protein would match previous structural data (38).
Mag-fura-2 (MF) and ZinT Competitive Metal Binding
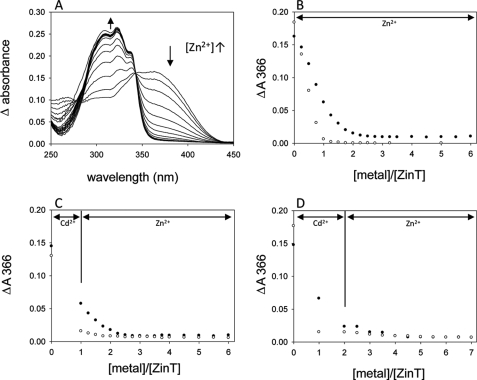
To estimate the affinity of ZinT for Zn2+, Mag-fura-2, a chromophore that binds Zn2+ in a 1:1 ratio (46) and with a Kd of 20 nm (47), was used. Its absorption maximum shifts from 366 to 325 nm on Zn2+ binding, which is accompanied by a decrease in its extinction coefficient from 29,900 m−1 cm−1 (MF) to 1880 m−1 cm−1 (Zn2+-MF) (46). Therefore Zn2+ binding to MF can be tracked by examining the absorbance at 366 nm (Fig. 6A). Fig. 6B shows a titration of a 1:1 ZinT:MF mixture (filled circles) and MF alone (open circles) with Zn2+. When ZinT was not present, the ΔA366 decreased to zero when 1 molar equivalent of Zn2+ had been added. When ZinT was present, however, incremental additions of Zn2+ gave smaller decreases in MF absorbance reaching a plateau at 2 molar equivalents of Zn2+. This provides good evidence that, although the affinity of ZinT for Zn2+ is not high enough to completely outstrip MF of Zn2+, ZinT competes with MF for binding of Zn2+. The Kd for Zn2+ binding by ZinT is therefore not less than 20 nm, but of an order that is able to compete with MF for Zn2+.
FIGURE 6.
Titration of ZinT and/or MF with Zn2+ and/or Cd2+. A, representative difference spectra (i.e. minus the protein-only spectrum) of a titration of 14.5 μm ZinT and 14.5 μm MF with Zn2+ (0.25–3.5 molar equivalents Zn2+ in 0.25 steps and then 4–6 molar equivalents in 0.5 steps). The arrows indicate the direction of absorbance changes as Zn2+ is added. B, titration of 14.5 μm ZinT and 14.5 μm MF with Zn2+. C, titration of 14.3 μm ZinT and 14.3 μm MF with 1 molar equivalent of Cd2+, then Zn2+ in 0.5 molar equivalent steps to 4 molar equivalents and then Zn2+ in 0.5 molar equivalent steps to 6 molar equivalents. D, titration of 14.1 μm ZinT and 14.1 μm MF with 2 molar equivalents of Cd2+ and then Zn2+ in 0.5 molar equivalent steps. In B–D, absorbance change at 366 nm is plotted against molar equivalents of metal added. The filled circles are in the presence of ZinT; open circles are in the absence of ZinT (MF and buffer only). The lines indicate whether the added metal was Zn2+ or Cd2+.
MF also binds Cd2+ in a 1:1 ratio and has a Kd for Cd2+ of 126 nm (48). The addition of Cd2+ to MF and ZinT (Fig. 6, C and D) elicited a smaller decrease in absorbance than with MF alone, again indicating the ability of ZinT to compete with MF for Cd2+. Without protein, the decrease in absorbance at 366 nm plateaued at 1 molar equivalent of metal added, whereas when ZinT was present, this shifted to 2. These data together suggest that ZinT has one binding site for metal that can be occupied by Cd2+ or Zn2+ and that the site has a sufficiently low Kd to be able to compete with MF for these metals.
DISCUSSION
The manipulation of metal ion concentrations in biological systems, so that the consequences of metal excess and limitation may be studied, is a major challenge. Global responses to elevated levels of Ag2+, Cd2+, Cu2+, Ni2+, Zn2+, and arsenic (14, 49–54) have been reported. However, constituents of complex growth medium can bind to metal ions and result in the metal ion concentration available to the cells being orders of magnitude lower than that added (16). For the first time, we have grown Zn2+-depleted E. coli in batch and chemostat culture in defined medium, without recourse to chelating agents, and defined the transcriptome associated with severe Zn2+ limitation. In batch culture, wild-type E. coli MG1655 cells grown in Zn2+-depleted cultures showed an increased doubling time (Fig. 1A) and a reduction in Zn2+ content compared with Zn2+-replete cultures. Thus, in the face of extreme Zn2+ depletion in the extracellular medium, homeostatic mechanisms ensure adequate cellular zinc contents.
Zn2+-depleted medium was successfully prepared by eliminating Zn2+ during medium preparation and culture. In contrast, chelators can be unspecific, strip metals from exposed sites and increase the availability of certain metals (16). The major disadvantage of using chelators is that the metal is still present in the medium to be picked up by proteins with a higher affinity for the metal than that exhibited by the chelator. For example, ZnuA is able to compete with EDTA for Zn2+ (6). Fig. 3A highlights the disadvantage of using chelators to study Zn2+ deficiency; the widely used chelator TPEN was less effective than Zn2+ elimination, as judged by λΦ(PzinT-lacZ) activity. Although neither are specific to Zn2+, both TPEN and EDTA have been used in studies focusing on Zn2+ depletion (see earlier references and Ref. 55).
Fig. 2 and Table 3 show that cells grown in Zn2+-depleted medium accumulate Zn2+ that cannot be accounted for by the medium constituents. Table 3 shows that the extent of leaching decreased with successive experiments in the same chemostat apparatus. The most likely explanation is that metal is actively leached from the glassware (flasks or chemostat vessel). Kay (29) notes that acid washing removes only surface Zn2+, which can be replaced from deeper within the glass. Previous studies have shown that growing cells in medium deficient in one nutrient can lead to cells evolving mechanisms to increase the uptake of that nutrient (56).
In contrast to Ref. 10, this study found only nine genes to be differentially regulated in response to Zn2+ starvation after careful metal avoidance and extraction. The small number of differentially regulated genes suggests that, because of the ubiquity of Zn2+ in the environment, the cells have not evolved elaborate mechanisms to cope with extreme Zn2+ deficiency. Interestingly, computational analysis found only three candidate Zur sites in the E. coli genome, and these sites were immediately upstream of three genes identified here: zinT, ykgM, and znuA (41).
There is a precedent in Bacillus for redistribution of Zn2+ under conditions of Zn2+ starvation, involving the synthesis of non-Zn2+ finger homologues of Zn2+-binding ribosomal proteins. Makarova et al. (57) searched sequenced genomes and found that genes encoding some ribosomal proteins were present as two copies: one, designated C+, containing a Zn2+-binding motif and a second, designated C−, in which this motif is missing. In the case of the E. coli ribosomal protein L31, the C+ form is encoded by rpmE and the C− form by ykgM (41) identified in the present study. Based on the present results, we hypothesize that non-Zn2+-containing L31 proteins displace the Zn2+-containing form in ribosomes, and subsequent degradation of the latter form would release Zn2+ for use by other proteins. The number of ribosomes in the cell would make this a significant Zn2+ reserve. Such a model has been experimentally proven for L31 proteins in Bacillus subtilis (58, 59) and Streptomyces coelicolor (60, 61).
The present study shows that zinT expression is increased most dramatically, not by Cd2+ addition as reported previously (27), but by Zn2+ removal. However, the present and past findings are reconciled by the fact that Cd2+ may displace other metals from enzymes, such as Zn2+ from alkaline phosphatase in E. coli (2, 25), so that Cd2+ exposure mimics Zn2+ depletion. Panina et al. (41) reported a Zur-binding site in the zinT promoter. Monitoring expression from λΦ(PzinT-lacZ) in a strain lacking zur showed constitutive de-repression, regardless of extracellular Zn2+ concentration, confirming that Zur is involved in the regulation of zinT (Fig. 3). This was also reported in an unpublished thesis cited in a review (62).
Based on the established link between ZinT and Cd2+, David et al. (38) included the metal (20 mm) in crystallization trials and obtained a crystal form distinct from that obtained under crystallization conditions that included 200 mm Zn2+ or no added metal. The crystal structure reveals a principal metal-binding site (common to all crystallized forms) that binds one Cd2+ or two Zn2+ ions. Further metal ions are found at the protein surface at intermolecular, negatively charged sites formed by residues from neighboring ZinT molecules. The crystal form prepared in the absence of exogenous metal also revealed one metal ion bound in the central, common, metal-binding site; this metal was positioned similarly to Cd2+ and coordinated by the three same His residues. The buried metal-binding site must be of high affinity, because no divalent cations were included in crystallization of the native form. The binding geometry suggests that the metal in the native form is Zn2+, although contamination by Ni2+ from the affinity chromatography or other metal ions could not be excluded, and x-ray fluorescence suggested the presence of Ni2+, albeit in an unusual distorted tetrahedral geometry. Fig. 5 (E and F) shows that, in our hands, ∼0.5 molar equivalents Co2+ co-elute with the ZinT protein. It is likely that this Co2+ has been picked up from the TALON column used during purification, again providing evidence for a high affinity metal-binding site within ZinT. No Ni2+ was found in eluting samples (data not shown).
On the basis of the crystallography, David et al. (38) could not conclude which metal would bind to ZinT under physiological conditions. The present study shows clearly that ZinT binds both Zn2+ and Cd2+ with high affinity. The direct binding experiments (Fig. 5, E and F) show that more Zn2+ remains bound to ZinT after size exclusion chromatography than Cd2+, providing evidence that Zn2+ binds to ZinT more tightly than Cd2+. Also, the Kd of MF for Cd2+ is greater than for Zn2+, so somewhat weaker binding by Cd2+ would not be detected in the Mag-Fura-2 competition experiments. Fig. 5 (E and F) shows that more than 1 molar equivalent of metal can bind to the protein. This is consistent with the crystal structure proposed by David et al. (38), which suggests that at least two Zn2+ ions can bind in the vicinity of the high affinity site, and that there is additional capacity for further Zn2+, up to 4, although this may be due to intermolecular contacts formed during crystallization. The finding that one Zn2+ ion is needed to saturate the protein, as assessed by competition with Mag-Fura-2, is entirely consistent with the crystallographic data because this experiment can only report on metal binding to ZinT that is tighter than 20 nm. Although this site in ZinT accommodates different metal ions, the marked accumulation of zinT mRNA by extreme Zn2+ limitation strongly suggests that the physiological role of ZinT is ferrying Zn2+ ions in the periplasm. Indeed, David et al. (38) suggested that the binding of a second metal, possibly at a lower affinity site, could trigger a conformational change that promotes transport across the membrane or interaction with an unidentified ABC-type transporter. In support of this is the fact that ZinT shows sequence similarity to a number of periplasmic metal-binding receptors of ABC metal transport systems that have been shown to bind Zn2+.
In a recent paper (9), growth in media with various Zn2+ supplements, or none, was purported to show “dependence of the ΔzinT mutant strain on zinc for growth.” Zn2+-limited conditions were those in which reduced growth yields (A595) were observed relative to growth at 0.6–1 mm added Zn2+. In defined medium containing less than 0.4 mm Zn2+, the mutant grew to lower A levels after 10 h than the wild type, but at high Zn2+ (0.6–1 mm), the zinT mutant grew to higher A values than the wild-type strain. This is in conflict with the present work (Fig. 1, A and C), which shows that the zinT mutant and wild-type strains grew similarly, even at only 60 nm Zn2+. Surprisingly, Kershaw et al. (9) also found that even growth of the wild-type strain was impaired at low Zn2+ concentrations (0.4, 0.05 mm added Zn2+); with no added Zn2+, growth was barely detectable. The claim that E. coli shows a strict dependence on added Zn2+ is, to our knowledge, unprecedented in the literature. Considerations of biomass composition suggest that the Zn2+ concentration in the medium used by Kershaw et al. (9) (0.5 mg liter−1) should support growth to a yield of 2.5 g of dry weight liter−1 (17), well in excess of the A595 of ∼0.5 or lower reported (9). Furthermore, inspection of the responses of both wild-type and zinT mutant strains to metals reveals that the experiments (9) to define the Zn2+ response were conducted at limiting copper concentrations; the basic defined medium contained 0.62 μm copper (0.1 mg CuSO4 liter−1), ∼1000-fold lower than the required copper concentration for optimal growth of both strains. Similarly, experiments to define the copper response were conducted at limiting Zn2+ concentrations; the basic defined medium contained 3.1 μm Zn2+ (0.5 mg ZnSO4 liter−1), i.e. much lower than the concentration at which both strains showed reduced cell yield. These calculations may explain why the cell yields at saturating copper concentrations (0.6–1.0 mm) were significantly lower than those at saturating Zn2+ concentrations (0.6–1.0 mm). Thus, the data of Kershaw et al. (9) do not provide robust evidence that the zinT mutant shows a growth disadvantage at low Zn2+ ion concentrations and conflict with previous work demonstrating the exceedingly low copper concentrations required for Cu-limited growth (3, 63).
Kershaw et al. (9) reported that ZinT binds metal ions. Cd2+ binding was observed when Cd2+ was incubated with the protein in a 1:1 ratio (0.1 mm ZinT:0.1 mm Cd2+), although the resolution of a peak corresponding to mass 22,450 (ZinT plus 1 Cd2+) is poor. The mass of the ZinT-Cd peak varied by 2 Da (as did the mass of apo-ZinT). The authors were only able to detect binding of Zn2+ to ZinT when 5 or more molar equivalents were added, although their other experiments detected binding when ZinT was incubated with less than 0.1 molar equivalent of Zn2+. In Figs. 4 and 5 of the present study, we show binding of Zn2+ to ZinT when no metal is added because of the high affinity of ZinT for contaminating Zn2+ in the buffers.
In addition to the need to sense Zn2+ levels to maintain homeostasis for all cellular systems, the lack of Zn2+ may be sensed by pathogens as indicative of entry into the host and thus trigger expression of virulence factors. Indeed, several studies in different bacteria have established that ZnuA or ZnuABC (or homologues) are required for bacterial replication in the infected host (see Refs. 44 and 55, among others).
In conclusion, we propose that, when cells are severely starved of Zn2+, the response is to increase Zn2+ uptake into the cell and redistribute nonessential Zn2+. The rpmE gene expresses the Zn2+ finger L31 protein that is incorporated into the ribosome. Upon Zn2+ depletion, the ykgM-encoded L31 protein is expressed (probably de-repressed by Zur) and becomes preferentially bound to the ribosome (the exact mechanism is unclear), allowing Zn2+ within the rpmE-encoded L31 to be recycled. The physiological role of ZinT remains to be fully established, but it may function as a Zn2+ chaperone to the membrane-bound Zn2+ importer ZnuBC (or a different importer) or mediate direct transport from the periplasm to the cytoplasm. Zn2+ is the metal that binds most tightly. This study provides a new appreciation of the regulation of zinT and the role of ZinT in protecting cells from Zn2+ depletion.
Supplementary Material
Acknowledgment
We thank Dr. A. J. G. Moir (Krebs Institute Sequencing and Synthesis Facility, University of Sheffield, Sheffield, United Kingdom) for carrying out the N-terminal protein sequencing.
This work was supported by the Biotechnology and Biological Sciences Research Council, UK.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1 and S2 and Fig. S1.
- TPEN
- N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine
- ICP-AES
- inductively coupled plasma-atomic emission spectroscopy
- LOD
- limit of detection
- MES
- 2-(N-morpholino)ethanesulfonic acid
- MF
- mag-fura-2
- PTFE
- polytetrafluoroethylene (Teflon®)
- qRT
- quantitative real time
- GGM
- glycerol-glycerophosphate medium
- MOPS
- 4-morpholinepropanesulfonic acid.
REFERENCES
- 1.Berg J. M., Shi Y. ( 1996) Science 271, 1081– 1085 [DOI] [PubMed] [Google Scholar]
- 2.Fraústo da Silva J. J. R., Williams R. J. P. ( 2001) The Biological Chemistry of the Elements: The Inorganic Chemistry of Life, Oxford University Press, Oxford [Google Scholar]
- 3.Outten C. E., O'Halloran T. V. ( 2001) Science 292, 2488– 2492 [DOI] [PubMed] [Google Scholar]
- 4.Andreini C., Banci L., Bertini I., Rosato A. ( 2006) J Proteome Res 5, 3173– 3178 [DOI] [PubMed] [Google Scholar]
- 5.Blencowe D. K., Morby A. P. ( 2003) FEMS Microbiol. Rev. 27, 291– 311 [DOI] [PubMed] [Google Scholar]
- 6.Berducci G., Mazzetti A. P., Rotilio G., Battistoni A. ( 2004) FEBS Lett. 569, 289– 292 [DOI] [PubMed] [Google Scholar]
- 7.Patzer S. I., Hantke K. ( 1998) Mol. Microbiol. 28, 1199– 1210 [DOI] [PubMed] [Google Scholar]
- 8.Patzer S. I., Hantke K. ( 2000) J. Biol. Chem. 275, 24321– 24332 [DOI] [PubMed] [Google Scholar]
- 9.Kershaw C. J., Brown N. L., Hobman J. L. ( 2007) Biochem. Biophys. Res. Commun. 364, 66– 71 [DOI] [PubMed] [Google Scholar]
- 10.Sigdel T. K., Easton J. A., Crowder M. W. ( 2006) J. Bacteriol. 188, 6709– 6713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayes A., Zhang N., Wu J., Butler P. R., Hauser N. C., Hoheisel J. D., Lim F. L., Sharrocks A. D., Oliver S. G. ( 2002) Methods 26, 281– 290 [DOI] [PubMed] [Google Scholar]
- 12.Hoskisson P. A., Hobbs G. ( 2005) Microbiology 151, 3153– 3159 [DOI] [PubMed] [Google Scholar]
- 13.Piper M. D., Daran-Lapujade P., Bro C., Regenberg B., Knudsen S., Nielsen J., Pronk J. T. ( 2002) J. Biol. Chem. 277, 37001– 37008 [DOI] [PubMed] [Google Scholar]
- 14.Lee L. J., Barrett J. A., Poole R. K. ( 2005) J. Bacteriol. 187, 1124– 1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beard S. J., Hashim R., Membrillo-Hernández J., Hughes M. N., Poole R. K. ( 1997) Mol. Microbiol. 25, 883– 891 [DOI] [PubMed] [Google Scholar]
- 16.Hughes M. N., Poole R. K. ( 1991) J. Gen. Microbiol. 137, 725– 734 [Google Scholar]
- 17.Pirt S. J. ( 1975) Principles of Microbe and Cell Cultivation, Blackwell Scientific Publications, Oxford [Google Scholar]
- 18.Ferenci T. ( 2008) Adv. Microb. Physiol. 53, 169– 229 [DOI] [PubMed] [Google Scholar]
- 19.Garland P. B., Randle P. J. ( 1962) Nature 196, 987– 988 [DOI] [PubMed] [Google Scholar]
- 20.Cherny R. A., Atwood C. S., Xilinas M. E., Gray D. N., Jones W. D., McLean C. A., Barnham K. J., Volitakis I., Fraser F. W., Kim Y., Huang X., Goldstein L. E., Moir R. D., Lim J. T., Beyreuther K., Zheng H., Tanzi R. E., Masters C. L., Bush A. I. ( 2001) Neuron 30, 665– 676 [DOI] [PubMed] [Google Scholar]
- 21.Lin P. S., Kwock L., Hefter K., Misslbeck G. ( 1983) Cancer Res. 43, 1049– 1053 [PubMed] [Google Scholar]
- 22.Mukherjee G., Ghosh T. ( 1995) J. Inorg. Biochem. 59, 827– 833 [DOI] [PubMed] [Google Scholar]
- 23.Sebat J. L., Paszczynski A. J., Cortese M. S., Crawford R. L. ( 2001) Appl. Environ. Microbiol. 67, 3934– 3942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Datsenko K. A., Wanner B. L. ( 2000) Proc. Natl. Acad. Sci. U. S. A. 97, 6640– 6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puskárová A., Ferianc P., Kormanec J., Homerová D., Farewell A., Nyström T. ( 2002) Microbiology 148, 3801– 3811 [DOI] [PubMed] [Google Scholar]
- 26.Miller J. H. ( 1972) Experiments in Molecular Genetics, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 27.Ferianc P., Farewell A., Nyström T. ( 1998) Microbiology 144, 1045– 1050 [DOI] [PubMed] [Google Scholar]
- 28.Gill S. C., von Hippel P. H. ( 1989) Anal. Biochem. 182, 319– 326 [DOI] [PubMed] [Google Scholar]
- 29.Kay A. R. ( 2004) BMC Physiol. 4, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koch A. L. ( 1961) Biochim. Biophys. Acta 51, 429– 441 [DOI] [PubMed] [Google Scholar]
- 31.Neidhardt F. C., Ingraham J. L., Schaechter M. ( 1990) Physiology of the Bacterial Cell: A Molecular Approach, pp. 418– 441, Sinauer Associates, Inc., Sunderland, MA [Google Scholar]
- 32.Jackson K. A., Helston R. M., McKay J. A., O'Neill E. D., Mathers J. C., Ford D. ( 2007) J. Biol. Chem. 282, 10423– 10431 [DOI] [PubMed] [Google Scholar]
- 33.Barrett T., Troup D. B., Wilhite S. E., Ledoux P., Rudnev D., Evangelista C., Kim I. F., Soboleva A., Tomashevsky M., Edgar R. ( 2007) Nucleic Acids Res. 35, D760– D765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laurent-Winter C., Ngo S., Danchin A., Bertin P. ( 1997) Eur. J. Biochem. 244, 767– 773 [DOI] [PubMed] [Google Scholar]
- 35.Birch R. M., O'Byrne C., Booth I. R., Cash P. ( 2003) Proteomics 3, 764– 776 [DOI] [PubMed] [Google Scholar]
- 36.Kannan G., Wilks J. C., Fitzgerald D. M., Jones B. D., Bondurant S. S., Slonczewski J. L. ( 2008) BMC Microbiol. 8, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.David G., Blondeau K., Renouard M., Lewit-Bentley A. ( 2002) Acta Crystallogr. D Biol. Crystallogr. 58, 1243– 1245 [DOI] [PubMed] [Google Scholar]
- 38.David G., Blondeau K., Schiltz M., Penel S., Lewit-Bentley A. ( 2003) J. Biol. Chem. 278, 43728– 43735 [DOI] [PubMed] [Google Scholar]
- 39.Stojnev T., Harichová J., Ferianc P., Nyström T. ( 2007) Curr. Microbiol. 55, 99– 104 [DOI] [PubMed] [Google Scholar]
- 40.Kadokura H., Tian H., Zander T., Bardwell J. C., Beckwith J. ( 2004) Science 303, 534– 537 [DOI] [PubMed] [Google Scholar]
- 41.Panina E. M., Mironov A. A., Gelfand M. S. ( 2003) Proc. Natl. Acad. Sci. U. S. A. 100, 9912– 9917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai F., Adrion C. B., Keller J. E. ( 2006) Infect. Immun. 74, 5617– 5624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fekkes P., de Wit J. G., Boorsma A., Friesen R. H., Driessen A. J. ( 1999) Biochemistry 38, 5111– 5116 [DOI] [PubMed] [Google Scholar]
- 44.Ammendola S., Pasquali P., Pistoia C., Petrucci P., Petrarca P., Rotilio G., Battistoni A. ( 2007) Infect. Immun. 75, 5867– 5876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scott C., Rawsthorne H., Upadhyay M., Shearman C. A., Gasson M. J., Guest J. R., Green J. ( 2000) FEMS Microbiol. Lett. 192, 85– 89 [DOI] [PubMed] [Google Scholar]
- 46.Yatsunyk L. A., Easton J. A., Kim L. R., Sugarbaker S. A., Bennett B., Breece R. M., Vorontsov, Tierney D. L., Crowder M. W., Rosenzweig A. C. ( 2008) J. Biol. Inorg. Chem. 13, 271– 288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simons T. J. ( 1993) J. Biochem. Biophys. Methods 27, 25– 37 [DOI] [PubMed] [Google Scholar]
- 48.de Seny D., Heinz U., Wommer S., Kiefer M., Meyer-Klaucke W., Galleni M., Frere J. M., Bauer R., Adolph H. W. ( 2001) J. Biol. Chem. 276, 45065– 45078 [DOI] [PubMed] [Google Scholar]
- 49.Brocklehurst K. R., Morby A. P. ( 2000) Microbiology 146, 2277– 2282 [DOI] [PubMed] [Google Scholar]
- 50.Kershaw C. J., Brown N. L., Constantinidou C., Patel M. D., Hobman J. L. ( 2005) Microbiology 151, 1187– 1198 [DOI] [PubMed] [Google Scholar]
- 51.Moore C. M., Gaballa A., Hui M., Ye R. W., Helmann J. D. ( 2005) Mol. Microbiol. 57, 27– 40 [DOI] [PubMed] [Google Scholar]
- 52.Wang A., Crowley D. E. ( 2005) J. Bacteriol. 187, 3259– 3266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamamoto K., Ishihama A. ( 2005) Mol. Microbiol. 56, 215– 227 [DOI] [PubMed] [Google Scholar]
- 54.Yamamoto K., Ishihama A. ( 2005) J. Bacteriol. 187, 6333– 6340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davis L. M., Kakuda T., DiRita V. J. ( 2009) J. Bacteriol. 191, 1631– 1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Notley-McRobb L., Ferenci T. ( 1999) Environ. Microbiol. 1, 45– 52 [DOI] [PubMed] [Google Scholar]
- 57.Makarova K. S., Ponomarev V. A., Koonin E. V. ( 2001) Genome Biol. 2, research 0033.1–0033.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Akanuma G., Nanamiya H., Natori Y., Nomura N., Kawamura F. ( 2006) J. Bacteriol. 188, 2715– 2720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nanamiya H., Akanuma G., Natori Y., Murayama R., Kosono S., Kudo T., Kobayashi K., Ogasawara N., Park S. M., Ochi K., Kawamura F. ( 2004) Mol. Microbiol. 52, 273– 283 [DOI] [PubMed] [Google Scholar]
- 60.Owen G. A., Pascoe B., Kallifidas D., Paget M. S. ( 2007) J. Bacteriol. 189, 4078– 4086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shin J. H., Oh S. Y., Kim S. J., Roe J. H. ( 2007) J. Bacteriol. 189, 4070– 4077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hantke K. ( 2005) Curr. Opin. Microbiol. 8, 196– 202 [DOI] [PubMed] [Google Scholar]
- 63.Ciccognani D. T., Hughes M. N., Poole R. K. ( 1992) FEMS Microbiol. Lett. 73, 1– 6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.