Abstract
Hemoglobin (Hb) uniquely associates with proinflammatory HDL in atherogenic mice and coronary heart disease (CHD) patients. In this paper, we report that Hb and its scavenger proteins, haptoglobin (Hp) and hemopexin (Hx) are significantly increased in apoA-1-containing particles of HDL both in mouse models of hyperlipidemia and in CHD patients, when compared with wild type mice and healthy donors, respectively. We further demonstrate that the association of Hb, Hp, and Hx proteins with HDL positively correlates with inflammatory properties of HDL and systemic inflammation in CHD patients. Interestingly, HDL from Hp−/− mice under atherogenic conditions does not accumulate Hb and is anti-inflammatory, suggesting that (i) Hp is required for the association of Hb with HDL and (ii) Hb·Hp complexes regulate the inflammatory properties of HDL. Moreover, treatment of apoE−/− mice with an apoA-1 mimetic peptide resulted in significant dissociation of Hb·Hp complexes from HDL and improvement of HDL inflammatory properties. Our data strongly suggest that HDL can become proinflammatory via the Hb·Hp pathway in mice and humans, and dissociation of Hb·Hp·Hx complexes from apoA-1-containing particles of HDL may be a novel target for the treatment of CHD.
Atherosclerosis is the leading cause of morbidity and mortality in Western society. The inverse relationship between HDL2 cholesterol and the risk of atherosclerosis is well established. Although HDL cholesterol is an epidemiological predictor of risk for coronary heart disease (CHD) (1), a significant number of CHD events occur in patients with normal LDL and HDL cholesterol levels (1, 2). Based on a number of recent studies in both animal models and human samples, it appears that the anti- or proinflammatory nature of HDL may be a more sensitive indicator of the presence or absence of atherosclerosis than HDL cholesterol levels. HDL exerts anti-inflammatory functions by promoting reverse cholesterol transport and preventing the oxidation of LDL (3, 4). We have previously shown that the anti-inflammatory functions of HDL can be impaired in humans (5) rabbits (6), and mice (7) during inflammatory processes. This impaired HDL is proinflammatory in nature, as characterized by (i) decreased levels and activity of anti-inflammatory, antioxidant factors, including apolipoprotein A1 (apoA-1) and PON1 (paraoxonase 1) (8); (ii) gain of proinflammatory proteins, such as serum amyloid A and ceruloplasmin (6); (iii) increased lipid hydroperoxide (LOOH) content (9); (iv) reduced potential to efflux cholesterol (10); and (v) diminished ability to prevent LDL oxidation (11). The molecular changes and mechanisms that promote anti-inflammatory HDL conversion to proinflammatory HDL are currently not well understood.
We recently reported the identification and characterization of Hb associated with proinflammatory HDL in atherogenic/hyperlipidemic mice and in human CHD patients (12). We demonstrated that under normal circumstances, a small amount of Hb is always found outside of red blood cells (RBC) in the non-lipoprotein fractions of serum (on the order of 10 μm compared with the >1 m concentration of Hb in RBC). We further demonstrated that under conditions of hyperlipidemia in mice and in CHD patients, the non-RBC Hb moves out of the non-lipoprotein fractions and associates with HDL. This HDL-associated Hb was shown to play an important role in the modulation of HDL function, suggesting that Hb is not only a novel biomarker but may also serve as a therapeutic target for atherosclerosis (12). We therefore sought to determine the molecular mechanisms that play a role in the association of Hb with HDL.
Hp and Hx are plasma proteins with the highest binding affinity for Hb (Kd ≈ 1 pm) and heme (Kd < 1 pm), respectively. They are expressed mainly in the liver and belong to the family of acute phase proteins, whose synthesis is induced during inflammatory processes (13, 14). Under conditions of increased hemolysis, Hb becomes highly toxic because of the oxidative properties of heme, which participates in the Fenton reaction to produce reactive oxygen species causing cell injury (15). Under these conditions, Hb is known to be scavenged by Hp·Hx complexes that utilize specific receptor pathways, thus protecting the body against the harmful effects of excess free Hb. We set out to determine whether the Hb·Hp·Hx system (i) also participates in the association of Hb with proinflammatory HDL and (ii) plays a role in the inflammatory properties of HDL.
In this paper, we demonstrate that (i) Hb·Hp·Hx complexes associate with HDL in CHD patients and mouse models of hyperlipidemia but not in healthy human donors and wild type mice, and (ii) Hb·Hp·Hx association with HDL positively correlates with proinflammatory properties of HDL. We further show that HDL from Hp−/− mice on an atherogenic diet is anti-inflammatory and did not contain any Hb, suggesting that (i) Hp is required for the association of Hb with HDL, and (ii) Hp regulates the inflammatory properties of HDL. In contrast to HDL from Hp−/− mice, HDL from Hx−/− mice on normal chow was proinflammatory and associated with Hb and Hp, suggesting a novel protective role for Hx in HDL function. When apoE−/− mice were treated in vivo with an apoA-1 mimetic peptide, 4F, Hb·Hp·Hx dissociated from HDL. Our data strongly suggest that the association of Hb·Hp·Hx with HDL plays an important role in the functional status and inflammatory properties of HDL.
EXPERIMENTAL PROCEDURES
Animal Experiments
All animal experiments presented here were approved by the UCLA animal research committee. C57BL/6J, apoE−/−, Hp−/−, and Hx−/− female mice between the ages of 8 and 12 weeks were used in all experiments. Mice were fed either a chow diet (Ralston Purina Mouse Chow) or an atherogenic diet containing 15.8% fat, 1.25% cholesterol, and 0.5% cholate (Harlan Teklad, Madison, WI). For short term studies, mice were fed the diet for 7 days, and for long term studies, mice were fed the diet for 15 weeks. Serum samples were isolated from mice fasted overnight, cryopreserved in 10% sucrose, and freshly frozen at −80 °C until use.
Human Samples
After informed consent, human plasma samples were obtained from healthy donors and donors with stable CHD or CHD equivalents, as defined by the National Cholesterol Education Program Adult Treatment Panel III criteria.
Total and Lipoprotein Cholesterol Levels
Human plasma or mouse serum cholesterol levels were determined by commercially available kits (Thermo, Louisville, CO). HDL from each individual serum sample was freshly isolated with LipiDirect HDL reagent (Polymedco, Cortland Manor, NY) according to the manufacturer's protocol. The supernatant containing HDL was assayed for cholesterol and protein (Promega, Madison, WI) within 48 h after isolation. very low density lipoprotein/LDL cholesterol was determined by subtracting HDL cholesterol from total cholesterol.
Detection of Reactive Intermediate Species in Plasma
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) contents in individual plasma or serum samples were determined with 2,7,7′-dichlorofluorescein diacetate (H2DCFDA; Invitrogen) and 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (Invitrogen), respectively. Briefly, individual plasma or serum samples (10 μl) were diluted at 1:5 with 1× phosphate-buffered saline and incubated with H2DCFDA or 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (10 μg/ml) in methanol for 30 min at 37 °C. The products of ROS and RNS were determined by measuring fluorescence intensity at 485 nm/525 nm.
Lipoprotein Isolation
Pooled human plasma or mouse serum samples were fractionated by a gel permeation fast protein liquid chromatography (FPLC) system consisting of dual Superose 6 columns in series (Amersham Biosciences), as described previously (12). Briefly, the column was eluted with 1× phosphate-buffered saline at a flow rate of 0.5 ml/min and fractionated every 1 ml. The first 10 fractions of the FPLC runs were discarded prior to analysis in all experiments. Individual fractions were assayed for cholesterol (Thermo, Louisville, CO), protein (Promega, Madison, WI), and heme content by measuring A410, and ROS content was determined with H2DCFDA (16).
Detection of Non-RBC Hb Associated with HDL by SELDI ProteinChip Analysis
Non-RBC Hb associated with HDL in individual HDL samples was determined as described previously (12). Briefly, individual HDL samples diluted with binding buffer (phosphate-buffered saline with 0.1% Triton X-100, pH 7.0) were subjected to surface-enhanced laser desorption/ionization (SELDI) analysis with strong anion exchange (Q10) ProteinChip arrays. Q10 arrays were equilibrated with binding buffer prior to use to select proteins with a pI value of <7. The data were analyzed with ProteinChip data analysis software version 3.2 (Ciphergen Biosystems), as described previously (17). Sample statistics were performed on groups of profiles (apoE−/− versus apoE−/− with d-4F treatment). Protein differences (-fold changes of relative intensity) were calculated among the various groups. A protein was considered differentially associated between two groups if, when compared with the other group, statistically significant differences in its intensity were observed (p < 0.05).
Assays to Determine the Inflammatory Properties of HDL
HDL properties were determined as described previously by measuring ROS content with H2DCFDA (12), paraoxonase activity (6), LOOH content by Auerbach assay (18), and monocyte chemotactic activity (4, 5).
Electrophoresis and Immunoblots
Isoelectric focusing (IEF) Tris/HCl gels and all other reagents for electrophoresis were purchased from Bio-Rad. Mouse serum (2 μl) was analyzed by IEF-Tris/HCl native two-dimensional gels, as described previously (12). Each lane from the IEF (pH 3–10) gels was cut and inserted into 4–20% Tris/HCl native gels. Samples were loaded on gels and transferred to nitrocellulose membrane (Amersham Biosciences). The membrane was immunoblotted against mouse Hb at 1:1000 (MP Biomedicals, Irvine, CA), mouse apoA-1 at 1:10,000 (Biodesign, Saco, ME), and mouse Hp and Hx at 1:5000 (ICL, Newberg, OR). HRP-conjugated anti-rabbit antibody (Amersham Biosciences) or anti-chicken antibody (ICL) was used at 1:10,000, and the bands were visualized with ECL detection reagent (Amersham Biosciences).
Association of Hb with HDL
Individual plasma or serum samples from mice and humans were assayed for apoA-1, Hb, Hp, Hx, IL-6, and IL-10 in plasma/serum and HDL by direct and sandwich ELISA, respectively, as described previously (12). The following antibodies were used in the ELISA experiments reported in this paper: mouse apoA-1 (Biodesign); mouse Hb (MB Biomedicals); mouse Hp and Hx (ICL); human HDL, apoA-1, Hb, Hp, and Hx (Abcam); human IL-6 and IL-10 (eBioscience, San Diego, CA); and HRP-conjugated detection secondary antibodies for anti-rabbit and mouse antibodies (Amersham Biosciences) and anti-chicken antibody (ICL).
Proteins in Serum and HDL
ApoA-1, Hb, Hp, Hx, IL-6, and IL-10 levels in individual plasma, serum, or HDL were quantified by direct ELISA, as described previously (12). Briefly, plasma or serum at 1:20 or HDL at 1:2 was coated on 96-well PVC microfilter plates (BD Biosciences) at 4 °C overnight. Primary antibodies used were as follows: mouse apoA-1 at 1:5000, mouse Hb at 1:2000, and mouse Hp and Hx at 1:5000 and human apoA-1, Hb, Hp, and Hx at 1:5000 and human IL-6 and IL-10 at 1:1000. The primary antibodies were detected by HRP-conjugated secondary anti-rabbit, chicken, or mouse antibodies (used at a 1:5000 dilution). Following incubation with TMB solution (KPL, Gaithersburg, MD), HRP activity was measured at A450. HRP-conjugated detection antibody was used as an internal standard to convert A450 of each sample to the concentration of detection antibody. The value for each protein from a given sample was calculated relative to the average of that protein concentration in mice on a chow diet or healthy donors, which were set to 1. In some experiments, commercial ELISA kits from ICL were used to measure Hp and Hx in mouse serum.
Proteins Associated with HDL
The association of Hb, Hp, and Hx with HDL containing apoA-1 was determined by sandwich ELISA, as described previously (12). Briefly, 96-well PVC microfilter plates (BD Biosciences) were precoated with 1–5 μg/ml of goat anti-mouse or human apoA-1 or chicken anti-human HDL (Abcam) at 4 °C, overnight. Following incubation of the precoated plates with individual plasma, serum at 1:20 dilution, or HDL at 1:2 dilution, the plates were washed thoroughly and further incubated with corresponding primary antibodies to mouse Hb at 1:2000; mouse apoA-1, Hp, and Hx at 1:2500; and human apoA-1, Hb, Hp, and Hx at 1:2500. The primary antibodies were detected by HRP-conjugated secondary antibodies (used at a 1:2500 dilution). Following incubation with TMB solution (KPL, Gaithersburg, MD), HRP activity was measured at A450. HRP-conjugated detection antibody was used as an internal standard to convert A450 of each sample to the concentration of detection antibody. The value for each protein from a given sample was calculated relative to the average of that protein concentration in mice on a chow diet or healthy donors, which was set to 1.
Statistical Analysis
All data were statistically analyzed by t test or linear regression. Significance was determined as p < 0.05.
RESULTS
Lipid Profiles in Plasma from CHD Patients
Patient plasma samples were obtained from 42 men and postmenopausal women between the ages of 21 and 75 with stable CHD or CHD equivalents, as defined by the National Cholesterol Education Program Adult Treatment Panel III criteria. All of the CHD patients were taking statins, as required by current guidelines. Plasma samples were also obtained from 29 healthy blood donors. Cholesterol levels and paraoxonase activity in the healthy donors and CHD patients were not significantly different (Table 1).
TABLE 1.
Lipid profiles and inflammatory properties of plasma samples from healthy donors and CHD patients
Healthy donors (n = 29) and CHD patients (n = 42) were compared. Each number represents the average ± S.D. A t test was performed for statistical analysis. RFU, relative fluorescence units. NS, not significant.
| Healthy | CHD | p | |
|---|---|---|---|
| Total cholesterol (mg/dl) | 136.4 ± 31.0 | 134.4 ± 43.7 | NS |
| HDL cholesterol (mg/dl) | 55.0 ± 10.1 | 51.4 ± 11.4 | NS |
| LDL/VLDL cholesterol (mg/dl) | 81.4 ± 28.7 | 83.0 ± 38.6 | NS |
| Paraoxonase (units/ml) | 224.6 ± 124.7 | 178.4 ± 134.1 | NS |
| Heme (A410) | 0.26 ± 0.07 | 0.66 ± 0.78 | 0.002 |
| ROS (RFU) | 172.5 ± 77.1 | 236.7 ± 73.3 | 0.001 |
| RNS (RFU) | 63.1 ± 5.2 | 66.4 ± 6.1 | 0.02 |
| Detection | Direct ELISA (relative concentration) |
||
|---|---|---|---|
| Healthy | CHD | p | |
| IL-6 | 1.00 ± 0.31 | 1.69 ± 0.81 | <0.00001 |
| IL-10 | 1.00 ± 0.39 | 1.84 ± 0.63 | <0.00001 |
Markers of Inflammatory Profiles in Plasma from CHD Patients
Individual plasma samples were tested for heme content, oxidative stress, and systemic inflammation. The levels of heme (measured at 410 nm and including heme content from all heme-containing proteins, including Hb), ROS, RNS IL-6, and IL-10 contents were significantly higher in plasma from the CHD patients than in plasma from healthy donors (Table 1).
Hb, Hp, and Hx Protein Levels Are Significantly Increased in HDL from CHD Patients Compared with Healthy Donors
Total Hb content was constant in normal and atherogenic serum from mouse models of atherosclerosis (12). To determine Hb content as well as its scavenger proteins (Hp and Hx) in plasma from healthy donors and CHD patients, direct ELISA was performed on individual human plasma (Table 2). Direct ELISA analysis showed significant increases in Hb, Hp, and Hx levels in plasma from CHD patients compared with the healthy donors, whereas there was no difference in plasma apoA-1 levels (Table 2). To determine whether Hb·Hp·Hx complexes are preferentially associated with apoA-1-containing particles or HDL in plasma samples from CHD patients, the association of Hb, Hp, and Hx with apoA-1-containing particles or HDL was determined by sandwich ELISA with plates precoated with antibody against either apoA-1 or HDL (Table 2). Hb, Hp, and Hx levels in apoA-1-containing particles (i.e. particles captured with antibody to apoA-1) and HDL (i.e. particles captured with antibody against whole human HDL) in the plasma of CHD patients were significantly higher than those in healthy donors, as measured by all three assays.
TABLE 2.
Hb, Hp, and Hx complexes in individual plasma or HDL from healthy donors and CHD patients
Healthy donors (n = 29) and CHD patients (n = 42) were compared. Each number represents the average ± S.D. A t test was performed for statistical analysis.
| Detection | Healthy | CHD | p |
|---|---|---|---|
| Direct ELISA (relative concentration) | |||
| ApoA-1 | 1.00 ± 0.49 | 1.09 ± 0.63 | NS |
| Hemoglobin | 1.00 ± 0.41 | 4.33 ± 3.75 | <0.00001 |
| Haptoglobin | 1.00 ± 0.48 | 2.15 ± 0.88 | <0.00001 |
| Hemopexin | 1.00 ± 0.25 | 1.59 ± 0.66 | <0.00001 |
| ApoA-1-captured ELISA (relative concentration) | |||
| ApoA-1 | 1.00 ± 0.81 | 1.10 ± 0.81 | NS |
| Hemoglobin | 1.00 ± 0.38 | 3.79 ± 3.51 | <0.00001 |
| Haptoglobin | 1.00 ± 0.33 | 1.50 ± 0.82 | <0.001 |
| Hemopexin | 1.00 ± 0.41 | 1.58 ± 1.18 | 0.005 |
| HDL-captured ELISA (relative concentration) | |||
| ApoA-1 | 1.00 ± 0.24 | 1.22 ± 0.42 | <0.01 |
| Hemoglobin | 1.00 ± 0.37 | 1.60 ± 1.19 | <0.005 |
| Haptoglobin | 1.00 ± 0.35 | 1.40 ± 0.55 | 0.0004 |
| Hemopexin | 1.00 ± 0.74 | 1.16 ± 0.76 | NS |
Lipid Hydroperoxide Content in HDL and HDL Inflammatory Index Are Positively Correlated with ApoA-1-associated Hb, Hp, and Hx Proteins in CHD Patients
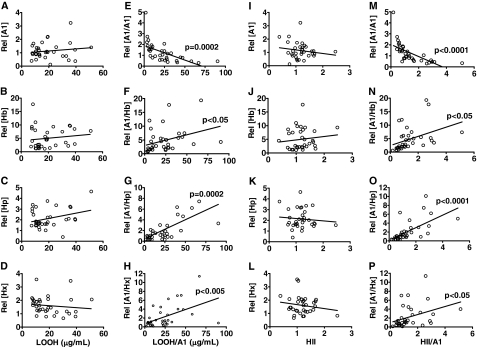
Previous studies (5) showed that proinflammatory HDL is characterized by both accumulation of LOOHs and an HDL inflammatory index (HII) of ≥1.0. To determine whether Hb, Hp, and Hx complexes are indices of proinflammatory HDL, (i) total Hb, Hp, and Hx levels and (ii) apoA-1-associated (i.e. particles captured with antibody to apoA-1) Hb, Hp, and Hx levels were correlated with LOOH contents in HDL (Fig. 1, A–H) and HII (Fig. 1, I–P) in patient samples. ApoA-1-associated Hb correlated positively with LOOH levels and HII (Fig. 1, F and N, respectively), whereas total plasma non-RBC Hb was not (Fig. 1, B and J, respectively), suggesting a potential link between Hb accumulation on HDL, generation of LOOH, and HDL inflammatory properties. Furthermore, apoA-1-associated Hp and Hx also showed positive correlation with LOOH (Fig. 1, G and H) and HII (Fig. 1, O and P), suggesting an important role for apoA-1-associated Hp and Hx complexes in HDL inflammatory properties. There was no correlation for any of these measurements in samples from healthy donors (data not shown).
FIGURE 1.
Correlation of LOOH content in HDL and HII with Hb, Hp, and Hx proteins in HDL from CHD patients. ApoA-1 (A1), Hb, Hp, and Hx contents in individual plasma (A–D) or in apoA-1-containing particles (E–H) were correlated with LOOH content in individual HDL. Hb, Hp, and Hx contents in individual plasma (I–L) or in apoA-1-containing particles (M–P) were correlated with individual HII determined by monocyte chemotactic assay, as described under “Experimental Procedures.” Individual HDL from CHD patients (n = 42) were tested for each assay. Circles, individual data; linear regression was performed for the statistical analysis on each group. p < 0.05 shows the significance of slope to the background. Rel followed by brackets, relative concentration of target protein. LOOH/A1 (E–H) and HII/A1 (M–P), each data set normalized to the apoA-1 content in each sample.
Heme Content, ROS Accumulation, and IL-6/IL-10 Ratio Are Positively Correlated with ApoA-1-associated Hb but Not RNS
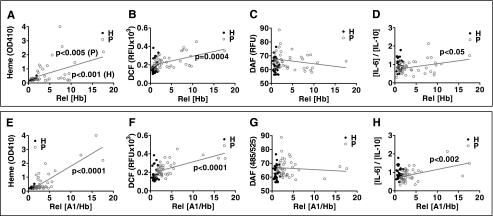
Hb preferentially associates with proinflammatory HDL from CHD patients (Fig. 1). We next examined whether HDL-associated Hb influences heme content, ROS, RNS, and the IL-6/IL-10 ratio, which is a measure of systemic inflammation. Total plasma Hb and apoA-1-associated Hb (i.e. Hb in particles captured with an antibody to apoA-1) were positively correlated with heme (Fig. 2, A and E), ROS (Fig. 2, B and F), and the ratio of IL-6 to IL-10 (as a marker of systemic inflammation) (Fig. 2, D and H) but not RNS (Fig. 2, C and G) in CHD patients. These correlations were not observed in plasma from healthy donors.
FIGURE 2.
Hb-associated HDL positively correlates with heme, ROS contents, and systemic inflammation in plasma from CHD patients. Hb, Hp, and Hx contents in individual plasma (A–D) or in apoA-1-containing particles (E–H) were correlated with heme (A and E), ROS (B and F), or RNS contents (C and G) and the ratio of IL-6 to IL-10 (D and H) in plasma. Individual plasma from healthy donors (dark circles, n = 29) and CHD patients (gray circles, n = 42) were tested. Circles, individual data; linear regression was performed for the statistical analysis on each group. Rel followed by brackets, relative concentration of target protein. H, healthy donors; P, patients.
Hb, Hp, and Hx Complexes Are Associated with HDL from Hyperlipidemic/Atherogenic Mice
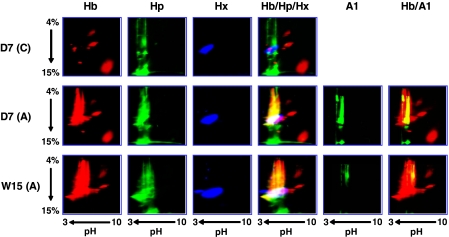
We previously reported that Hb complexes associate with HDL in C57BL/6J mice on an atherogenic diet but not on a normal chow diet (12). HDL-associated Hb has distinct properties; particularly, its pI is around 4, whereas the pI of non-HDL Hb is above 7. The pI values of Hp, Hx, and apoA-1 are around 5 (Swiss-Prot data base; available on the World Wide Web). Therefore, we sought to determine whether the low pI of Hb complexes in HDL is due to the association with Hp, Hx, or apoA-1. Serum and HDL samples from C57BL/6J mice on either a normal chow or an atherogenic diet were analyzed by IEF/native two-dimensional gels and ELISA for Hb, Hp, Hx, and apoA-1 contents, and their association with apoA-1 was determined (Figs. 3 and 4). On an atherogenic diet (both short and long term feeding regimens), complexes immunoreactive to Hb, Hp, Hx, and apoA-1 were observed to coincide on the two-dimensional gels (Fig. 3) but not in serum from mice on a normal chow. Direct ELISA showed that the Hb content in HDL (Fig. 4D) was significantly induced by an atherogenic diet, whereas there was no difference in total Hb content in serum (Fig. 4B). Sandwich ELISA with apoA-1-capturing antibody showed that Hb, Hp, and Hx complexes were exclusively associated with apoA-1 in HDL from mice on the atherogenic diet but not in mice on a normal chow diet (Fig. 3), suggesting that Hb·Hp·Hx complexes associate with HDL through apoA-1.
FIGURE 3.
Association of Hb, Hp, and Hx complexes with HDL in mice fed an atherogenic diet. Pooled serum samples from C57BL/6J mice (n = 8/group) on either a normal chow (C) or atherogenic diet (A) for 7 days (D7) or 15 weeks (W15) were loaded on IEF/native two-dimensional gels. Gels were immunoblotted for Hb, Hp, Hx, and apoA-1 (A1). The images of Hb, Hp, Hx, and apoA-1 bands in atherogenic serum were reversed and superimposed, as shown in red, green, blue, and green, respectively. The Hb panel on the left is similar to the published results from Watanabe et al. (12). The merged images of the first three columns of the Hb, Hp, and Hx panels are shown in the fourth column (Hb/Hp/Hx). The merged images of the first (Hb) and fifth column of panels (A1) are shown in the sixth column (Hb/A1).
FIGURE 4.
Hb, Hp, and Hx proteins are significantly elevated in HDL from atherogenic mice. Total apoA-1 (A1) and Hb in individual serum (A and B) (n = 16/group) or in HDL (C and D) (n = 4/group) or apoA-1-associated Hb (E), Hp (F), and Hx (G) contents in HDL (n = 4/group) from C57BL/6J mice on chow (C) or atherogenic diet (A) for 7 days were determined by ELISA, as described under “Experimental Procedures.” Each bar represents the average concentration relative to WT (C) ± one S.D. value. A t test was performed for a statistical analysis. Relative followed by brackets, relative concentration of the targeted protein.
A Novel Role for Hp in the Formation of Hb-associated HDL
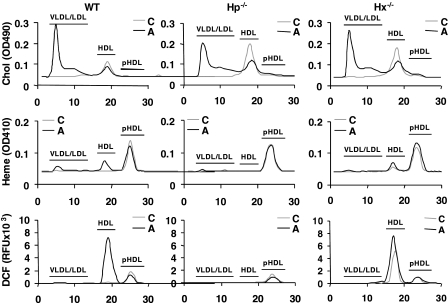
We previously reported that HDL from C57BL/6J mice on an atherogenic diet was proinflammatory, whereas HDL from mice on a normal chow diet was normal/anti-inflammatory (12). To determine if the association of Hb with HDL is mediated by Hp and Hx, we tested whether Hp and Hx are necessary for the association of Hb with HDL. Serum and HDL samples from wild-type, Hp−/−, and Hx−/− mice on a normal chow or an atherogenic diet were assayed for (i) cholesterol content (Table 3 and Fig. 5, top), (ii) the accumulation of heme (Fig. 5, middle), ROS (Fig. 5, bottom), and Hb·Hp·Hx complexes in HDL (Fig. 6). It should be noted that the heme content in the FPLC fractions measured in Fig. 5 reflects the sum of all heme-containing proteins and not just Hb.
TABLE 3.
Serum and lipoprotein cholesterol levels (mg/dl) in wild type, Hp−/−, and Hx−/− mice on an atherogenic diet for 7 days
Individual serum samples from wild-type (WT), Hp−/−, and Hx−/− mice (n = 10–12/group) were compared. Each number represents the average ± S.D. A t test was performed for statistical analysis. WT and C, p < 0.05 compared with wild-type or chow-fed mice of each group, respectively.
| Normal chow |
Atherogenic diet |
|||||
|---|---|---|---|---|---|---|
| Total | HDL | VLDL/LDL | Total | HDL | VLDL/LDL | |
| WT | 63.2 ± 6.4 | 55.3 ± 7.3 | 7.9 ± 4.5 | 176.9 ± 22.8 (C) | 47.9 ± 3.4 (C) | 129.0 ± 23.5 (C) |
| Hp−/− | 61.0 ± 6.7 | 48.0 ± 4.5 (WT) | 13.0 ± 6.6 (WT) | 154.7 ± 43.9 (C) | 46.6 ± 13.4 | 108.1 ± 35.1 (C) |
| Hx−/− | 71.9 ± 16.6 | 53.5 ± 6.1 | 18.4 ± 12.6 (WT) | 142.1 ± 59.4 (C) | 50.2 ± 12.8 | 91.9 ± 61.2 (C) |
FIGURE 5.
Hp, but not Hx, is required for the accumulation of heme and ROS in HDL. Individual FPLC fractions from pooled serum samples from C57BL/6J wild-type (WT), Hp−/−, or Hx−/− mice (n = 10–12/group) on a normal chow (C) or atherogenic diet (A) for 7 days were assayed for cholesterol, heme content, or ROS content by dichlorofluorescein, as described under “Experimental Procedures.” pHDL, post-HDL.
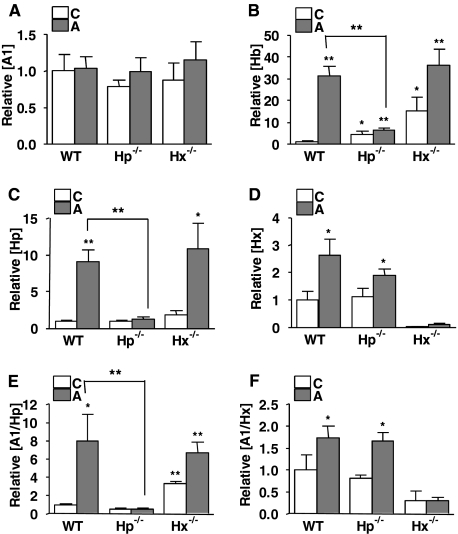
FIGURE 6.
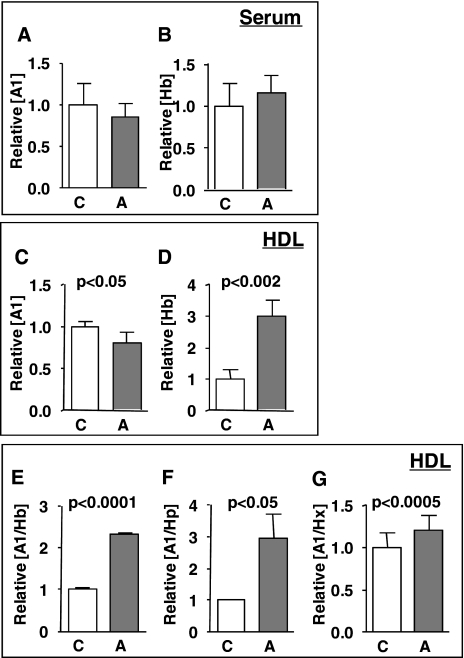
Hp, but not Hx, is required for the accumulation of Hb in HDL. HDL (n = 4/group) was fractionated by FPLC from pooled serum from C57BL/6J wild-type (WT), Hp−/−, or Hx−/− mice (n = 10–12/group) on a normal chow (C) or atherogenic diet (A) for 7 days. ApoA-1 (A), Hb (B), Hp (C), and Hx (D) contents in HDL from C57BL/6J wild-type, Hp−/−, or Hx−/− mice on a normal chow (C) or atherogenic diet (A) for 7 days were assayed by direct ELISA. ApoA-1-associated Hp and Hx proteins were determined by apoA-1 capture sandwich ELISA with detection antibodies for Hp (A1/Hp) (E) and Hx (A1/Hx) (F). Each bar represents the average concentration relative to WT (C) ± one S.D. value. A t test was performed for the statistical analysis. *, p < 0.05; **, p < 0.005.
The cholesterol levels in very low density lipoprotein/LDL were significantly higher in Hp−/− and Hx−/− mice on a normal chow than those of wild type, whereas no difference in very low density lipoprotein/LDL cholesterol was observed among groups on an atherogenic diet (Table 3). Although both Hp and Hx were associated with HDL from wild-type mice on the atherogenic diet, elevated levels of Hb associated with HDL were only seen when Hp was present in HDL (Figs. 5 and 6B), suggesting that Hp is necessary for the association of Hb with HDL. Furthermore, sandwich ELISA of HDL captured by its apoA-1 content showed that Hp only associated with HDL containing apoA-1 in wild-type mice on the atherogenic diet (Fig. 6E). Interestingly, apoA-1-associated Hp content in HDL from Hx−/− mice on a normal chow diet was significantly higher than the wild-type mice, suggesting that Hx may inhibit the formation of Hb·Hp complexes in HDL (Fig. 6E), or it is possible that higher Hp levels in the Hx−/− mice on chow are simply due to more base-line inflammation. There were no significant differences in the apoA-1 content between wild type, Hp−/−, and Hx−/− mice (Fig. 6A).
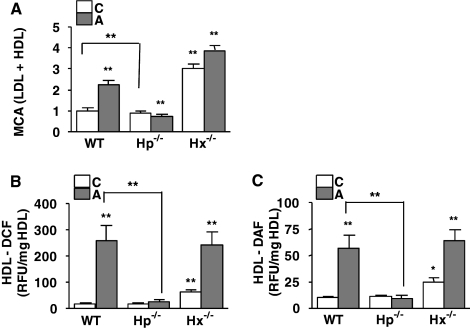
Hp, but Not Hx, Is Required for the Accumulation of ROS and RNS in Proinflammatory HDL
To determine the role of Hp and Hx in the association of Hb with HDL and HDL inflammatory properties, HDL from wild type, Hp−/−, and Hx−/− mice on a normal chow or atherogenic diet were tested in a monocyte chemotactic assay. HDL from Hp−/− on an atherogenic diet was anti-inflammatory (Fig. 7A) and contained very low levels of Hb and low levels of Hx on the atherogenic diet (Fig. 6, B and D, respectively). Interestingly, in contrast, HDL from Hx−/− mice on both the chow and atherogenic diets was proinflammatory (Fig. 7A), consistent with the fact that it contained significantly more Hb and Hp (Fig. 6, B and C).
FIGURE 7.
Role of Hb·Hp·Hx on the inflammatory properties of HDL. Pooled HDL (A) from C57BL/6J wild-type (WT), Hp−/−, or Hx−/− mice (n = 10–12/group) on a normal chow (C; white bars) or atherogenic diet (A; gray bars) for 7 days were assayed by monocyte chemotactic assay, as described previously (4, 5), using autologous LDL. The contents of ROS (B) and RNS (C) in HDL were measured by dichlorofluorescein or difluorofluorescein fluorescence dyes, respectively. Each bar represents the average concentration relative to wild type (C) ± one S.D. value. A t test was performed for the statistical analysis. *, p < 0.05; **, p < 0.005; NS, not significant.
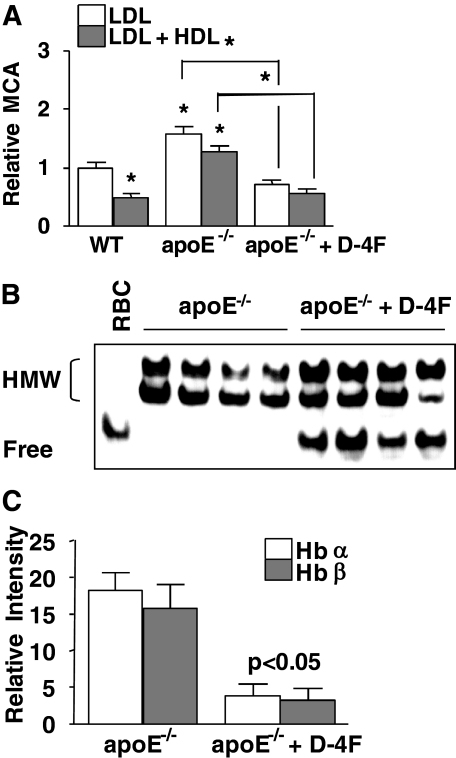
ApoA-1 Mimetic Peptide Converts Proinflammatory HDL to Anti-inflammatory HDL by Lowering Hb-associated HDL
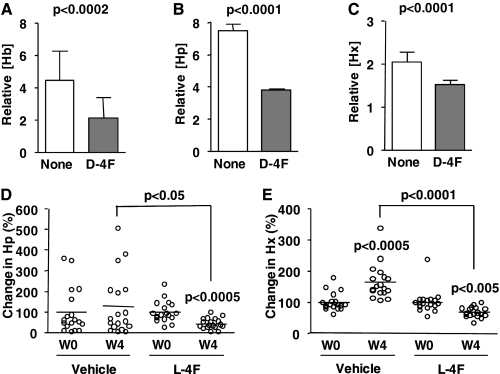
Hb·Hp·Hx complexes associate with proinflammatory HDL but not anti-inflammatory HDL. To further validate that HDL-inflammatory properties are dependent on HDL-associated Hb·Hp·Hx, we examined the effect of the apoA-1 mimic peptide, 4F, on Hb·Hp·Hx complexes in HDL. Our previous studies demonstrated that d-4F and l-4F (the d- and l-amino acid form, respectively) convert proinflammatory HDL to anti-inflammatory HDL in animals and humans (18–21). HDL from apoE−/− mice on a normal chow diet was proinflammatory and converted to anti-inflammatory by d-4F treatment (Fig. 8A). d-4F treatment resulted in partial dissociation of Hb from HDL (i.e. after d-4F treatment, some Hb was seen to migrate on the gel similarly to Hb from RBC lysates; Fig. 8B). After d-4F treatment, there was also a significant decrease in Hb polypeptides with abnormal pI (Fig. 8C). HDL samples from apoE−/− mice treated with vehicle only (drinking water) or d-4F (provided in drinking water) were assayed for Hb, Hp, and Hx contents (Fig. 9, A–C). HDL from apoE−/− mice contained significantly higher Hb, Hp, and Hx (Fig. 9, A–C) compared with d-4F-treated mice. In addition, l-4F (l-amino acid form) also caused a significant reduction in the contents of Hp and Hx in HDL (Fig. 9, D and E) in apoE null mice. Our results suggest that the conversion of proinflammatory HDL to anti-inflammatory HDL by 4F, in part, involves a reduction in the content of Hb, Hp, and Hx proteins in HDL.
FIGURE 8.
ApoA-1 mimetic peptide, d-4F, rescues HDL anti-inflammatory functions and promotes the dissociation of Hb from HDL. A, the inflammatory properties of HDL and LDL from C57BL/6J wild-type (WT) and apoE−/− mice treated with vehicle (drinking water) or d-4F (10 μg/mouse/day provided in drinking water) for 4 weeks (n = 12/group) were determined by a monocyte chemotactic assay as described previously (4, 5). B, individual serum samples were loaded on native-PAGE (4–15%) and immunoblotted for Hb. RBC lysates were used as a standard for free Hb that was not associated with HDL. HMW, Hb complexes at high molecular weight. C, HDL from each of the four individual apoE−/− mice without or with d-4F treatment were isolated by LipiDirect HDL reagent and subjected to SELDI analysis with Q10 (pI < 7) ProteinChip arrays, as described previously (12). The two SELDI peaks of interest, Hb α chain (m/z 14,900) and β chain (m/z 15,600), in HDL samples from apoE−/− mice were compared with those of HDL from apoE−/− mice treated with d-4F. For C, each bar represents the average ± one S.D. value in each peak of Hb α chain (white) and β chain (gray). The resulting intensities were statistically analyzed. For A, each bar represents the average of number of migrated monocytes relative to wild type ± one S.D. A t test was performed for the statistical analysis on each group. *, p < 0.001.
FIGURE 9.
Effect of apoA1 mimetic peptide, 4F, administration on the association of Hb, Hp, and Hx with proinflammatory HDL in mice. ApoE−/− mice (n = 4/group) on a normal chow diet were treated with vehicle (drinking water) or d-4F (10 μg/mouse/day provided in drinking water) for 4 weeks. Changes in Hb (A), Hp (B), and Hx (C) contents in HDL from apoE−/− mice treated with vehicle (None) or d-4F were determined by ELISA. Each bar represents the average concentration relative to WT (C) ± one S.D. value. Individual serum samples (n = 17/group) from apoE−/− before (W0) or after 4-week treatment (W4) with vehicle (administered subcutaneously daily) or l-4F (1 mg/kg/day administered subcutaneously in vehicle) were tested for Hp (D) and Hx (E) contents. Circles and bars represent individual data and averages of changes in Hp and Hx in percentages compared with those of week 0, respectively. A t test was performed for a statistical analysis, and p values are shown.
DISCUSSION
Oxidation of LDL is a major factor in human atherosclerosis (22, 23). Entrapment and oxidation of LDL in the subendothelial space and the subsequent interactions between endothelial cells and monocytes is a key process in the initiation of atherosclerotic lesion development (24, 25). The inverse relationship between HDL and the risk of atherosclerosis is well established. Although there does not appear to be a single explanation for the antiatherogenic role of HDL, it has become clear that the functional status of HDL, which is largely dependent on its protein components, is probably an important determinant of CHD (9). PON1, lecithin-cholesterol acyltransferase, platelet-activating factor acetyl hydrolase, proteinase (elastase-like), phospholipase D, albumin, apoJ, and apoA-1 are proteins in HDL with antiatherogenic properties capable of preventing LDL oxidation. HDL was shown to inhibit the mild oxidation of LDL and consequently inhibit the production of the potent monocyte chemoattractant MCP-1 by human artery wall cells (26).
Van Lenten et al. (6) were the first to demonstrate that HDL could exist as an anti-inflammatory molecule or a proinflammatory molecule, depending on the context and environment. Van Lenten et al. (6) showed that the acute phase reaction in rabbits and humans converts HDL from anti-inflammatory to proinflammatory (i.e. HDL loses its ability to inhibit LDL-induced MCP-1 in cultures of human artery wall cells). Indeed, in its proinflammatory state, HDL actually promotes this LDL-induced inflammatory response. The authors concluded that under basal conditions, HDL serves an anti-inflammatory role, but during the acute phase reaction there is loss of anti-oxidant enzyme activities and displacement and/or exchange of proteins associated with HDL resulting in a proinflammatory HDL. Subsequently, it was shown that an atherogenic diet in mice that are genetically susceptible to atherosclerosis (but not in mice genetically resistant to atherosclerosis) converts HDL from anti-inflammatory to proinflammatory (27). Several studies have shown the presence and nature of proinflammatory HDL in animal models (11, 28) of atherosclerosis, suggesting that the anti- or proinflammatory nature of HDL function is a more sensitive indicator of the presence or absence of atherosclerosis than HDL cholesterol levels. Knowledge of protein profiles that distinguish proinflammatory HDL from normal/anti-inflammatory HDL will probably allow the development of novel biomarkers for the early detection of atherosclerosis and provide new strategies for therapeutic interventions (5, 29). We have recently utilized protein-profiling methods and identified Hb as a major protein associated with proinflammatory HDL (12). We have demonstrated that accumulation of Hb in HDL plays a role in the proinflammatory properties of HDL in atherogenic mice and in CHD patients. In this paper, we set out to determine the mechanisms behind the association of Hb with proinflammatory HDL.
Humans metabolize more than 6 g of Hb everyday (30). Although under conditions of hemolysis, more than one-tenth of Hb can be released into the plasma compartment (30), the presence of scavenger proteins (Hp and Hx) helps to protect humans from the highly toxic effects of free Hb (31, 32). In humans and in mice, there is always a small amount of Hb outside of RBC. The non-RBC Hb was found associated with HDL in mouse models of hyperlipidemia and atherosclerosis but not in normal mice, where it was almost exclusively found in the non-lipoprotein fractions (12). In the current studies, we found that the total Hb content of serum was significantly higher in CHD patients compared with healthy subjects (Table 2). The samples were devoid of any noticeable hemolysis that could potentially account for higher Hb levels. Moreover, we also found that total Hp and Hx contents were also significantly elevated in these CHD patients compared with healthy donors (Table 2). In both humans with CHD and mice on an atherogenic diet, Hb·Hp·Hx complexes were significantly elevated and associated with HDL (12) (Table 2 and Figs. 3 and 4).
Metabolism of plasma Hb is considered a major function of tissue macrophages, which can take up free Hb or Hb·Hp complexes produced in vitro through the macrophage scavenger receptor CD163 (33, 34) and internalize them for degradation (35). The low density lipoprotein receptor-related protein/CD91 has been identified (36) as the receptor responsible for scavenging Hx·heme complexes through receptor-mediated endocytosis (37). In this paper, we have shown that Hb, Hp, and Hx are associated with HDL in both CHD patients and mouse models of hyperlipidemia/atherosclerosis. Hp has been reported to associate with apoA-1, the major protein component of HDL (37–40), and alter HDL function by impairing lecithin-cholesterol acyltransferase activation (41).
To our knowledge, this is the first report on the association of Hb·Hp·Hx complexes with HDL. Recent studies demonstrated the non-inflammatory clearance of Hb·Hp complexes formed in vitro (35, 42–45). Schaer et al. (42) examined by gene array analysis the transcriptional response of human monocyte-macrophages to Hb·Hp complexes formed in vitro. The macrophage response was non-inflammatory and was characterized by antioxidative and anti-inflammatory gene expression with prominent induction of HO-1. We show that Hb·Hp·Hx complexes associate with HDL in CHD patients and in mouse models of hyperlipidemia/atherosclerosis but not in healthy blood donors or in wild type mice on a chow diet (Table 2 and Figs. 3 and 4). HDL from the patients and the mice on the atherogenic diet has been shown to be proinflammatory (Figs. 1 and 7), and it remains to be determined whether Hb·Hp·Hx complexes in such proinflammatory HDL are cleared via pathways involving CD163 (Hp), CD91 (Hx), or classic scavenger receptors SR-B1 and CD36. Moreover, it will be interesting to determine the type of inflammatory responses mediated by macrophages or hepatocytes that are exposed to proinflammatory HDL.
Our results show that Hp is required for the association of Hb with proinflammatory HDL (Figs. 6 and 7). Interestingly, neither Hp knock-out mice (46) nor humans with anhaptoglobinemia (47) display compromised Hb clearance either in the absence or presence of severe hemolysis. Schaer et al. (34) demonstrated that Hb without Hp readily interacts with CD163 on human monocyte-derived macrophages, resulting in the non-inflammatory removal of Hb. Moreover, free Hb is actually taken up by hepatic cells faster than Hb·Hp complexes (48, 49). However, in the presence of severe hemolysis, the absence of Hp was associated with greater oxidative tissue damage, and Lim et al. (46) concluded that Hp evolved to protect against hemolytically induced tissue damage. Thus, it is possible that the small amounts of non-RBC Hb found in the non-lipoprotein fractions of serum in the absence of increased hemolysis or hyperlipidemia/atherosclerosis are cleared by the anti-inflammatory CD163 pathway. HDL-mediated clearance of Hb may be via another pathway and therefore may not be anti-inflammatory. The induction of an acute phase response in hyperlipidemia/atherosclerosis may lead to elevated levels of Hp that associate with HDL and result in the binding of Hb to HDL. If the resulting HDL-associated complexes do not interact with macrophages via the anti-oxidant CD163 pathway, the clearance of the HDL-associated Hb might result in further oxidative stress and cytokine production. Thus, one of the mechanisms by which HDL may become proinflammatory may well be related to its Hb content, as suggested by the data presented here.
Our results are also consistent with the work of Levy et al. (50). Humans have two classes of alleles for Hp, designated 1 and 2. The Hp 2 allele is found only in humans. All other mammals, including mice, have only the Hp 1 allele and therefore the Hp 1-1 genotype. The Hp 2-2 genotype has been associated with increased risk of CHD. The Hp 1-1 genotype produces a monomer that is monovalent and thus can only associate with one other haptoglobin molecule to create dimers. In contrast, the Hp 2-2 genotype is bivalent and can associate with two different haptoglobin monomers and thus can form cyclic polymers. The Hb/Hp 1-1 complex was found to stimulate macrophages to secrete anti-inflammatory cytokines much more than the Hp 2-2 complex (44, 51). Levy et al. (50) generated mice that express the Hp 2 allele by homologous recombination. Lesions from Hp 2-2 mice on a C57BL/6 apoE−/− background had increased iron, LOOH, and macrophage accumulation, as compared with plaques from C57BL/6 apoE−/− Hp 1-1 mice (50). The work of Levy and colleagues taken in the context of our report suggests that individuals with the Hp 2-2 genotype may have proinflammatory HDL with increased Hb content compared with individuals with the Hp 1-1 genotype. More recently, Levy and co-workers (52) found that in Hp 2-2 diabetic humans and Hp 2-2 diabetic mice, Hb and lipid peroxides associated with HDL were increased, and the HDL was dysfunctional in its ability to promote cholesterol efflux from macrophages compared with Hp 1-1 subjects. Our results are in agreement with the findings of Levy and co-workers (52). Unfortunately, in the present study design, we did not have access to the genotypes of the human samples. We plan to pursue the correlation between Hp genetic predisposition and HDL inflammatory properties in a separate study.
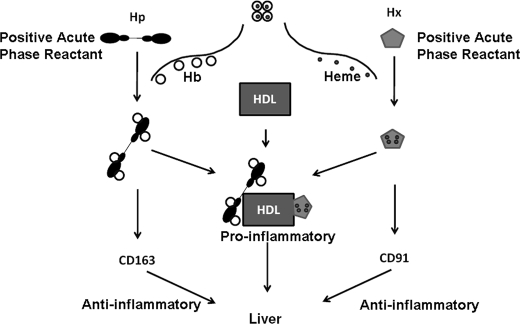
The data presented here suggest that the ability of apoA-1 mimetic peptides to render proinflammatory HDL anti-inflammatory is probably related in part to their ability to decrease the content of Hb in HDL (Fig. 8). The decrease in Hp and Hx levels induced by treatment with the apoA-1 mimetic peptide (Fig. 9) may have been due to a peptide-mediated reduction of tissue inflammation, which in turn reduced Hp and Hx levels, thus preventing Hb from associating with HDL. After treatment of apoE−/− mice with the apoA-1 mimetic peptide, there appeared to be a return of some of the non-RBC Hb to the non-lipoprotein fractions of serum (Fig. 8B). The clearance of this non-lipoprotein-bound Hb could occur via the CD163 pathway and thus be anti-inflammatory. The anti-inflammatory properties of apoA-1 mimetic peptides could in part be due to a favorable action on macrophage iron metabolism. Fig. 10 shows a proposed model for the clearance of Hb·Hp·Hx complexes based on whether or not the complexes associate with HDL. These interesting possibilities are amenable to experimental testing in future studies.
FIGURE 10.
Proposed model for the clearance of Hb·Hp·Hx complexes. Hp and Hx are positive acute phase reactants that are induced during the course of an acute phase response. CD163 and CD91 are receptors on macrophage for Hp and Hx proteins, respectively, and aid in the removal of Hb (large open circles) and heme (small closed circles), respectively, via macrophages in the liver. As Hp and Hx concentrations increase in the plasma during an acute phase reaction, there is increased association of these proteins with HDL. The result is that HDL-associated Hp and Hx compete with non-HDL-associated Hp and Hx for the binding of Hb and heme. Hb and heme that bind to non-HDL-associated Hp and Hx, respectively, are cleared by anti-inflammatory macrophage receptor pathways (CD163 and CD91), mainly in the liver. In this proposed model, Hb and heme that bind to HDL-associated Hp and Hx, respectively, as a result of their binding to HDL are hypothesized to be unable to bind to the anti-inflammatory CD163 and CD91 receptors on macrophages. Consequently, Hb and heme associated with HDL enter macrophage by pathways that are yet to be determined but which are presumed to be proinflammatory.
In conclusion, we have shown that Hb·Hp·Hx complexes associate with HDL in CHD patients and mouse models of atherosclerosis. The association of Hb·Hp·Hx complexes correlates positively with the proinflammatory properties of HDL in mice and humans. We have further shown that, under hyperlipidemia/atherogenic conditions in mice, Hp is required for both the association of Hb with HDL and the accumulation of ROS in HDL. The studies reported here strongly suggest that the proinflammatory nature of HDL is at least in part due to its Hb content and that apoA-1 mimetic peptides may render HDL anti-inflammatory by reducing HDL-associated Hb levels.
This work was supported, in whole or in part, by National Institutes of Health, NHLBI, Grants HL-30568 (to A. M. F., M. N., and S. T. R.) and 1R01HL082823 (to S. T. R.). This work was also supported by the Laubisch, Castera, and M.K. Grey Funds at UCLA. M. N., S. T. R., and A. M. F. are principals in Bruin Pharma, and A. M. F. is an officer in Bruin Pharma.
- HDL
- high density lipoprotein(s)
- apo
- apolipoprotein
- CHD
- coronary heart diseases
- ELISA
- enzyme-linked immunosorbent assay
- Hb
- hemoglobin
- Hp
- haptoglobin
- Hx
- hemopexin
- LDL
- low density lipoprotein
- LOOH
- lipid hydroperoxide
- RBC
- red blood cell(s)
- RNS
- reactive nitrogen species
- ROS
- reactive oxygen species
- H2DCFDA
- 2,7,7′-dichlorofluorescein diacetate
- FPLC
- fast protein liquid chromatography
- IEF
- isoelectric focusing
- HRP
- horseradish peroxidase
- IL
- interleukin
- HII
- HDL inflammatory index
- SELDI
- surface-enhanced laser desorption/ionization.
REFERENCES
- 1.Gordon T., Castelli W. P., Hjortland M. C., Kannel W. B., Dawber T. R. ( 1977) Am. J. Med. 62, 707– 714 [DOI] [PubMed] [Google Scholar]
- 2.Castelli W. P., Garrison R. J., Wilson P. W., Abbott R. D., Kalousdian S., Kannel W. B. ( 1986) J. Am. Med. Assoc. 256, 2835– 2838 [PubMed] [Google Scholar]
- 3.Navab M., Hama S. Y., Anantharamaiah G. M., Hassan K., Hough G. P., Watson A. D., Reddy S. T., Sevanian A., Fonarow G. C., Fogelman A. M. ( 2000) J. Lipid Res. 41, 1495– 1508 [PubMed] [Google Scholar]
- 4.Navab M., Hama S. Y., Cooke C. J., Anantharamaiah G. M., Chaddha M., Jin L., Subbanagounder G., Faull K. F., Reddy S. T., Miller N. E., Fogelman A. M. ( 2000) J. Lipid Res. 41, 1481– 1494 [PubMed] [Google Scholar]
- 5.Ansell B. J., Navab M., Hama S., Kamranpour N., Fonarow G., Hough G., Rahmani S., Mottahedeh R., Dave R., Reddy S. T., Fogelman A. M. ( 2003) Circulation 108, 2751– 2756 [DOI] [PubMed] [Google Scholar]
- 6.Van Lenten B. J., Hama S. Y., de Beer F. C., Stafforini D. M., McIntyre T. M., Prescott S. M., La Du B. N., Fogelman A. M., Navab M. ( 1995) J. Clin. Invest. 96, 2758– 2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Lenten B. J., Wagner A. C., Nayak D. P., Hama S., Navab M., Fogelman A. M. ( 2001) Circulation 103, 2283– 2288 [DOI] [PubMed] [Google Scholar]
- 8.Mackness M. I., Durrington P. N., Mackness B. ( 2004) Am. J. Cardiovasc. Drugs 4, 211– 217 [DOI] [PubMed] [Google Scholar]
- 9.Navab M., Berliner J. A., Subbanagounder G., Hama S., Lusis A. J., Castellani L. W., Reddy S., Shih D., Shi W., Watson A. D., Van Lenten B. J., Vora D., Fogelman A. M. ( 2001) Arterioscler. Thromb. Vasc. Biol. 21, 481– 488 [DOI] [PubMed] [Google Scholar]
- 10.Hayek T., Oiknine J., Brook J. G., Aviram M. ( 1994) Biochem. Biophys. Res. Commun. 205, 1072– 1078 [DOI] [PubMed] [Google Scholar]
- 11.Navab M., Ananthramaiah G. M., Reddy S. T., Van Lenten B. J., Ansell B. J., Hama S., Hough G., Bachini E., Grijalva V. R., Wagner A. C., Shaposhnik Z., Fogelman A. M. ( 2005) Ann. Med. 37, 173– 178 [DOI] [PubMed] [Google Scholar]
- 12.Watanabe J., Chou K. J., Liao J. C., Miao Y., Meng H. H., Ge H., Grijalva V., Hama S., Kozak K., Buga G., Whitelegge J. P., Lee T. D., Farias-Eisner R., Navab M., Fogelman A. M., Reddy S. T. ( 2007) J. Biol. Chem. 282, 23698– 23707 [DOI] [PubMed] [Google Scholar]
- 13.Bowman B. H., Kurosky A. ( 1982) Adv. Hum. Genet. 12, 189– 261, 453– 4 [DOI] [PubMed] [Google Scholar]
- 14.Altruda F., Poli V., Restagno G., Argos P., Cortese R., Silengo L. ( 1985) Nucleic Acids Res. 13, 3841– 3859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puppo A., Halliwell B. ( 1988) Biochem. J. 249, 185– 190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navab M., Hama S. Y., Hough G. P., Subbanagounder G., Reddy S. T., Fogelman A. M. ( 2001) J. Lipid Res. 42, 1308– 1317 [PubMed] [Google Scholar]
- 17.Kozak K. R., Amneus M. W., Pusey S. M., Su F., Luong M. N., Luong S. A., Reddy S. T., Farias-Eisner R. ( 2003) Proc. Natl. Acad. Sci. U.S.A. 100, 12343– 12348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navab M., Anantharamaiah G. M., Reddy S. T., Hama S., Hough G., Grijalva V. R., Wagner A. C., Frank J. S., Datta G., Garber D., Fogelman A. M. ( 2004) Circulation 109, 3215– 3220 [DOI] [PubMed] [Google Scholar]
- 19.Navab M., Anantharamaiah G. M., Reddy S. T., Van Lenten B. J., Datta G., Garber D., Fogelman A. M. ( 2004) Curr. Opin. Lipidol. 15, 645– 649 [DOI] [PubMed] [Google Scholar]
- 20.Navab M., Anantharamaiah G. M., Reddy S. T., Hama S., Hough G., Grijalva V. R., Yu N., Ansell B. J., Datta G., Garber D. W., Fogelman A. M. ( 2005) Arterioscler. Thromb. Vasc. Biol. 25, 1325– 1331 [DOI] [PubMed] [Google Scholar]
- 21.Navab M., Anantharamaiah G. M., Reddy S. T., Fogelman A. M. ( 2006) Nat. Clin. Pract. Cardiovasc. Med. 3, 540– 547 [DOI] [PubMed] [Google Scholar]
- 22.Witztum J. L., Steinberg D. ( 2001) Trends Cardiovasc. Med. 11, 93– 102 [DOI] [PubMed] [Google Scholar]
- 23.Witztum J. L., Steinberg D. ( 1991) J. Clin. Investig. 88, 1785– 1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Navab M., Berliner J. A., Watson A. D., Hama S. Y., Territo M. C., Lusis A. J., Shih D. M., Van Lenten B. J., Frank J. S., Demer L. L., Edwards P. A., Fogelman A. M. ( 1996) Arterioscler. Thromb. Vasc. Biol. 16, 831– 842 [DOI] [PubMed] [Google Scholar]
- 25.Berliner J. A., Navab M., Fogelman A. M., Frank J. S., Demer L. L., Edwards P. A., Watson A. D., Lusis A. J. ( 1995) Circulation 91, 2488– 2496 [DOI] [PubMed] [Google Scholar]
- 26.Navab M., Imes S. S., Hama S. Y., Hough G. P., Ross L. A., Bork R. W., Valente A. J., Berliner J. A., Drinkwater D. C., Laks H. ( 1991) J. Clin. Investig. 88, 2039– 2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Navab M., Hama-Levy S., Van Lenten B. J., Fonarow G. C., Cardinez C. J., Castellani L. W., Brennan M. L., Lusis A. J., Fogelman A. M., La Du B. N. ( 1997) J. Clin. Investig. 99, 2005– 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Lenten B. J., Wagner A. C., Navab M., Anantharamaiah G. M., Hama S., Reddy S. T., Fogelman A. M. ( 2007) J. Lipid Res. 48, 2344– 2353 [DOI] [PubMed] [Google Scholar]
- 29.Ridker P. M. ( 2002) Circulation 105, 2– 4 [PubMed] [Google Scholar]
- 30.Garby L., Noyes W. D. ( 1959) J. Clin. Investig. 38, 1479– 1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sadrzadeh S. M., Graf E., Panter S. S., Hallaway P. E., Eaton J. W. ( 1984) J. Biol. Chem. 259, 14354– 14356 [PubMed] [Google Scholar]
- 32.Rother R. P., Bell L., Hillmen P., Gladwin M. T. ( 2005) JAMA 293, 1653– 1662 [DOI] [PubMed] [Google Scholar]
- 33.Fabriek B. O., Dijkstra C. D., van den Berg T. K. ( 2005) Immunobiology 210, 153– 160 [DOI] [PubMed] [Google Scholar]
- 34.Schaer D. J., Schaer C. A., Buehler P. W., Boykins R. A., Schoedon G., Alayash A. I., Schaffner A. ( 2006) Blood 107, 373– 380 [DOI] [PubMed] [Google Scholar]
- 35.Kristiansen M., Graversen J. H., Jacobsen C., Sonne O., Hoffman H. J., Law S. K., Moestrup S. K. ( 2001) Nature 409, 198– 201 [DOI] [PubMed] [Google Scholar]
- 36.Hvidberg V., Maniecki M. B., Jacobsen C., Højrup P., Møller H. J., Moestrup S. K. ( 2005) Blood 106, 2572– 2579 [DOI] [PubMed] [Google Scholar]
- 37.Hunt R. C., Hunt D. M., Gaur N., Smith A. ( 1996) J. Cell. Physiol. 168, 71– 80 [DOI] [PubMed] [Google Scholar]
- 38.Kunitake S. T., Carilli C. T., Lau K., Protter A. A., Naya-Vigne J., Kane J. P. ( 1994) Biochemistry 33, 1988– 1993 [DOI] [PubMed] [Google Scholar]
- 39.Porta A., Cassano E., Balestrieri M., Bianco M., Picone R., De Stefano C., Abrescia P. ( 1999) Zygote 7, 67– 77 [DOI] [PubMed] [Google Scholar]
- 40.Rademacher B. E., Steele W. J. ( 1987) Anal. Biochem. 160, 119– 126 [DOI] [PubMed] [Google Scholar]
- 41.Spagnuolo M. S., Cigliano L., D'Andrea L. D., Pedone C., Abrescia P. ( 2005) J. Biol. Chem. 280, 1193– 1198 [DOI] [PubMed] [Google Scholar]
- 42.Schaer C. A., Schoedon G., Imhof A., Kurrer M. O., Schaer D. J. ( 2006) Circ. Res. 99, 943– 950 [DOI] [PubMed] [Google Scholar]
- 43.Cigliano L., Spagnuolo M. S., Dale B., Balestrieri M., Abrescia P. ( 2001) Steroids 66, 889– 896 [DOI] [PubMed] [Google Scholar]
- 44.Philippidis P., Mason J. C., Evans B. J., Nadra I., Taylor K. M., Haskard D. O., Landis R. C. ( 2004) Circ. Res. 94, 119– 126 [DOI] [PubMed] [Google Scholar]
- 45.Abraham N. G., Drummond G. ( 2006) Circ. Res. 99, 911– 914 [DOI] [PubMed] [Google Scholar]
- 46.Lim S. K., Kim H., Lim S. K., bin Ali A., Lim Y. K., Wang Y., Chong S. M., Costantini F., Baumman H. ( 1998) Blood 92, 1870– 1877 [PubMed] [Google Scholar]
- 47.Delanghe J., Langlois M., De Buyzere M. ( 1998) Blood 91, 3524. [PubMed] [Google Scholar]
- 48.Weinstein M. B., Segal H. L. ( 1984) Biochem. Biophys. Res. Commun. 123, 489– 496 [DOI] [PubMed] [Google Scholar]
- 49.Keene W. R., Jandl J. H. ( 1965) Blood 26, 705– 719 [PubMed] [Google Scholar]
- 50.Levy A. P., Levy J. E., Kalet-Litman S., Miller-Lotan R., Levy N. S., Asaf R., Guetta J., Yang C., Purushothaman K. R., Fuster V., Moreno P. R. ( 2007) Arterioscler. Thromb. Vasc. Biol. 27, 134– 140 [DOI] [PubMed] [Google Scholar]
- 51.Guetta J., Strauss M., Levy N. S., Fahoum L., Levy A. P. ( 2007) Atherosclerosis 191, 48– 53 [DOI] [PubMed] [Google Scholar]
- 52.Asleh R., Blum S., Kalet-Litman S., Alshiek J., Miller-Lotan R., Asaf R., Rock W., Aviram M., Milman U., Shapira C., Abassi Z., Levy A. P. ( 2008) Diabetes 57, 2794– 2800 [DOI] [PMC free article] [PubMed] [Google Scholar]