Abstract
The endoplasmic reticulum (ER) is a key organelle regulating intracellular Ca2+ homeostasis. Oxidants and mitochondria-derived free radicals can target ER-based Ca2+ regulatory proteins and cause uncontrolled Ca2+ release that may contribute to protracted ER stress and apoptosis. Several ER stress proteins have been suggested to counteract the deregulation of ER Ca2+ homeostasis and ER stress. Here we showed that knockdown of Herp, an ubiquitin-like domain containing ER stress protein, renders PC12 and MN9D cells vulnerable to 1-methyl-4-phenylpyridinium-induced cytotoxic cell death by a mechanism involving up-regulation of CHOP expression and ER Ca2+ depletion. Conversely, Herp overexpression confers protection by blocking 1-methyl-4-phenylpyridinium-induced CHOP up-regulation, ER Ca2+ store depletion, and mitochondrial Ca2+ accumulation in a manner dependent on a functional ubiquitin-proteasomal protein degradation pathway. Deletion of the ubiquitin-like domain of Herp or treatment with a proteasomal inhibitor abolished the central function of Herp in ER Ca2+ homeostasis. Thus, elucidating the underlying molecular mechanism(s) whereby Herp counteracts Ca2+ disturbances will provide insights into the molecular cascade of cell death in dopaminergic neurons and may uncover novel therapeutic strategies to prevent and ameliorate Parkinson disease progression.
Parkinson disease (PD)2 is the second most common age-related neurodegenerative disorder that results in the selective degeneration of dopaminergic neurons of the substantia nigra pars compacta (1, 2). The proximate cause of selective degeneration of dopaminergic neurons in PD has not been clearly elucidated. Several mechanisms are inferred to play a role in the pathogenesis of PD based on studies from animals or in vitro studies using dopaminergic neurotoxins. These include mitochondrial dysfunction, oxidative stress, and impairment of the ubiquitin-proteasomal pathway (UPP) (1–3). It has been shown that several genes that are mutated in familial PD encode for proteins that have functions linked to UPP and mitochondria (1–3). The UPP plays a critical role in ER-associated protein degradation (ERAD), a protein quality control system of the ER that eliminates misfolded proteins in the ER lumen (4). UPP dysfunction results in the accumulation of misfolded or unfolded proteins within the ER, which induces ER stress (5).
Important roles for ER stress and ER stress-induced cell death have been reported in a broad spectrum of pathological conditions (6). To alleviate ER stress and enhances cell survival, cells launch the unfolded protein response (UPR), an adaptive response to minimize accumulation of misfolded proteins that would otherwise be toxic to the cell (7). The biological objectives of the UPR are to reduce the overall protein translation, increase the production of ER localized chaperones, and increase the clearance of unfolded proteins by UPP (7). Although short time UPR activation serves to reduce the unfolded protein load, a protracted activation of UPR, as the result of either severe or prolonged ER dysfunction, activates the cell death program (7). Important mediators of ER stress-associated death include the activation of the ER-associated procaspase-12 (in mouse) or procaspase-4 (in human) and increased expression of the pro-apoptotic transcription factor CCAAT enhancer-binding protein homologous protein (CHOP, also termed as growth arrest-DNA damage response protein or Gadd153) (8).
Recent studies have demonstrated hallmarks of ER stress in several experimental models of PD (9–12) and in dopaminergic neurons in the substantia nigra of PD subjects (13). Although these studies indicate that ER stress is closely associated with PD, it is yet not clear whether and how ER stress contributes to the degenerative cascades in PD. Cells that fail to respond to ER stress are more sensitive to neurotoxin-induced death (9), suggesting that up-regulation of ER stress proteins, at least during the early phase of the ER stress response, is important to restore ER homeostasis and to prevent activation of the ER stress-induced apoptotic program. Consistent with this notion, preconditioning with a sublethal level of ER stress has been shown to protect cells, in part through up-regulation of ER stress proteins. Hence, understanding the molecular mechanisms by which ER stress proteins overcome ER stress may help to uncover novel approaches to block the ER stress-associated pathological processes in cell culture and animal models of PD (9–12).
Herp (homocysteine-inducible ER stress protein) is a membrane-bound, ubiquitin-like protein that is located in the ER (14). Herp expression is strongly up-regulated in cultured primary neurons exposed to proteasomal inhibitors or pharmacological agents that selectively induce ER dysfunction (14–16). We previously reported that overexpression of Herp promotes neuronal survival, whereas knockdown of Herp protein by small interference RNA enhances vulnerability to ER stress- and amyloid β-peptide-induced apoptosis (16). The ability of Herp to prevent ER stress-induced death was correlated with its ability to stabilize cellular Ca2+ homeostasis (16, 17). Here, we investigated the role of Herp in the cellular response to 1-methyl-4-phenylpyridinium (MPP+), a neuro-toxicant commonly used to elicit experimental models of PD (18). Because disturbances in intracellular Ca2+ homeostasis have been implicated in oxidative cell injury (19), we test the hypothesis of whether Herp may play a role in counteracting MPP+-induced disturbances in intracellular Ca2+ homeostasis. Our results indicate that knockdown of Herp increases MPP+-induced CHOP expression, ER Ca2+ leakage, and vulnerability to MPP+-induced cytotoxic cell death, suggesting that Herp is critical for survival adaptation to this PD neurotoxin.
EXPERIMENTAL PROCEDURES
Materials
1-Methyl-4-phenylpyridinium (MPP+) and tunicamycin were purchased from Sigma. Lactacystin and LLVY-amino-4-methylcoumarin were obtained from BioMol. The antibodies for Herp and CHOP were obtained from BioMol and Santa Cruz Biotechnology. The antibody for ERK1 was obtained from Cell Signaling. The antibodies to Grp78 and Bcl-2 were purchased from Stressgen and Millipore, respectively. Secondary antibodies conjugated to horseradish peroxidase were from Jackson Immunoresearch, respectively. 7-Dichloro-dihydrofluorescein diacetate was obtained from Molecular Probes.
Generation of DNA Constructs
Plasmids containing the full-length or mutant deletion human Herp cDNA were constructed as described previously (16). Site-directed mutagenesis was performed to generate by a PCR-based primer overlap extension method. In brief, the same pair of flanking primers and two different mutant overlapping primers were synthesized as described (16). The PCR products that contained the mutant sequence were subcloned into the PCR4 TOPO TA cloning vector (Stratagene), which was then amplified and digested with BamHI and EcoRII and subcloned into the pcDNA3.1 vector. The mutation was confirmed by automated DNA sequencing.
Generation of Stably Transfected Cell Lines
Transfection of PC12 cells was carried out using the Lipofectamine reagent (Invitrogen) as previously described (16). Stably expressing clones were obtained after selection for growth in the presence of geneticin (500 mg/liter) and characterized for Herp expression by immunoblot analysis. For experiments, PC12 cells were plated onto glass coverslips and used between 18 and 48 h after plating. Cells stably transfected with the empty vector were used as controls.
Experimental Treatments
PC12 and MN9D cells were treated with MPP+ (1 mm), tunicamycin (5 μg/ml), and lactacystin (5 μm) in OPTI-MEM (Invitrogen). Each of these compounds was prepared in Me2SO immediately before applying them to the cultures. When Me2SO was used as the solvent, their final concentration did not exceed 0.1%. At the end of each treatment, the cultures were processed for immunoblotting and for evaluating the extent of cell death.
RNA Interference
Herp and CHOP siRNA duplexes are designed to specifically target the 21-nucleotide region 5′-CGCAACAAATAGTCGGAACATC-3′ of the Herp gene (NM_004562.1) and 5′-CTCTTGACCCTGCATCCCTA-3′ of the CHOP gene (nucleotides 270–291; NM_024134). These target sequences were chosen based on previous experiments testing the gene-silencing effectiveness of three to four siRNA duplexes (16, 20). Blast searches confirmed that these sequences were not homologous to any genes. A previously described scrambled sequence (20) is used as siRNA-Control. The cells were transfected with the siRNA duplexes using Lipofectamine 2000 (Invitrogen) in Opti-MEM according to manufacturer's protocol. After overnight incubation, the cultures were washed and replaced with 2 ml of fresh serum containing Dulbecco's modified Eagle's medium to allow recovery for 24 h. To monitor knockdown, the cells were harvested and processed for RT-PCR and/or Western blot analyses.
Quantification of Cell Survival
Cell viability was assessed by the trypan blue exclusion method and the lactate dehydrogenase release assay as described previously (16, 20). Cell viability was evaluated in triplicates for each treatment. All of the experiments were repeated at least three times.
RNA Isolation and RT-PCR
Total RNA from cells grown on 100-mm dishes was isolated with TRIzol (Invitrogen), and 2 μg of RNA was reverse transcribed with Superscript II reverse transcriptase and an oligo(dT) primer (Invitrogen). Semi-quantitative RT-PCR analyses of Herp, CHOP, and glyceraldehyde-3-phosphate dehydrogenase were performed using the following pairs of primers: rat Herp, 5′-CCACTACCACAACTACCACTG-3′ (forward) and 5′-CCTCTCTTTGGCTTTCTGGAA-3′ (reverse); rat glyceraldehyde-3-phosphate dehydrogenase, 5′-TGTGATGGACTCCGGTGACGG-3′ (forward) and 5′-ACAGCTTCTCTTTGATGTCACGC-3′ (reverse); rat CHOP, 5′-AAGGTCTACGAAGGTGAACGACCCC-3′ (forward) and 5′-GACCCCAAGACACGTGAGCAACTGC-3′ (reverse); rat Grp78/Bip, 5′-CCACAAGGATGCAGACATTG-3′ (forward) and 5′-AGGGCCTCCACTTCCATAGA-3′ (reverse); and rat glyceraldehyde-3-phosphate dehydrogenase (which served as an internal control), 5′-CCACAAGGATGCAGACATTG-3′ (forward) and 5′-AGGGCCTCCACTTCCATAGA-3′ (reverse).
Measurement of ER and Mitochondrial Ca2+ Concentrations
Free Ca2+ levels in the ER ([Ca2+]ER) will be evaluated using the ER-targeted YC4 (YC4-ER; gift of Dr. W. F. Graier, University of Graz), a low affinity ratiometric “cameleon” indicator with a KDEL sequence and a calreticulin signal peptide, as previously described (21). For measurement of mitochondria Ca2+ level ([Ca2+]M), the mitochondria-targeted ratiometric-pericam (RP-mt; gift of Dr. A. Miyawaki, RIKEN Brain Science Institute) was used as described previously (22). Briefly, cells plated at 60% confluency on glass coverslips were transiently transfected with 2 μg of p-BudCR4.1-YC4-ER or pcDNA3-Rp-mt using Lipofectamine. Twenty-four hours after transfection, the cells were incubated with MPP+, and changes in [Ca2+]ER and [Ca2+]M were monitored by confocal laser scanning imaging system with excitation set at 440 nm (for YC4-ER) or at 433 and 485 nm (for Rp-mt). Emission was monitored at 485 and 535 nm (for YC4-ER) or at 539 nm (for Rp-mt). Measurements were performed in Locke's buffer containing: 154 mm NaCl, 5.6 mm KCl, 2.3 mm CaCl2, 1.0 mm MgCl2, 3.6 mm NaHCO3, 5 mm HEPES, and 10 mm d-glucose, pH 7.2 (16, 20). The data were expressed as the ratios of the fluorescence in treated relative to untreated cultures.
Protein Extraction, Immunoprecipitation, and Western Blotting
Cell lysates for Western blotting were prepared in T-PER lysis buffer (Pierce). In all of the experiments, the same amount of total protein was loaded for each sample. The membranes were probed with the primary antibodies: Herp, Grp78/Bip, CHOP, and ERK1, followed by peroxidase-conjugated secondary antibody and developed with the Super Signal West Pico Chemiluminescent substrate (Pierce). For immunoprecipitation, aliquots of cell lysates containing 300 μg of protein were incubated with rabbit polyclonal Herp antibody in immunoprecipitation buffer (150 mm NaCl, 2 mm EDTA, 1% Nonidet P-40, 5 μg/ml leupeptin, 5 μg/ml aprotinin, 2 μg/ml pepstatin A, 0.25 mm phenylmethylsulfonyl fluoride, 50 mm Tris, pH 7.6). Antigen-antibody complexes were precipitated with immobilized protein A, washed three times in immunoprecipitation buffer, and solubilized by heating in Laemmli buffer containing 2-mercaptoethanol at 100 °C for 4 min. The solubilized proteins were separated by SDS-polyacrylamide gel and then immunoblotted with a polyclonal antibody to Bcl-2.
Proteasomal Activity
Chymotrypsin-like activity of proteasome was assayed using the fluorogenic peptide Suc-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin according to the method reported previously (15). Briefly, after the treatment with MG132 or lactacystin for 30 min, cultures were harvested, lysed in proteasome buffer (10 mmol/liter Tris-HCl, pH 7.5, 1 mmol/liter EDTA, 2 mmol/liter adenosine-5′-triphosphate, 20% glycerol, and 4 mmol/liter dithiothreitol), and centrifuged at 13,000 × g at 4 °C for 10 min. The supernatant (20 μg of protein) was then incubated with proteasome activity assay buffer (0.05 mol/liter Tris-HCl, pH 8.0, 0.5 mmol/liter EDTA, 40 μmol/liter LLVY-amino-4-methylcoumarin) for 1 h at 37 °C. The reaction was stopped by adding 0.9 ml of cold water and placing the reaction mixture on ice for at least 10 min. Subsequently, the fluorescence of the solution was measured with a fluorescence microplate reader with excitation at 380 nm and emission at 440 nm. All of the readings were standardized relative to the fluorescence intensity of an equal volume of free 7-amino-4-methylcoumarin solution.
Statistical Analysis
Comparison between two groups was performed using Student's t test, whereas multiple comparisons between more than two groups was analyzed by one-way ANOVA and post hoc tests. The data evaluated for the effects of two variables were analyzed using two-way ANOVA. The results are presented as the means ± standard deviation. For all analyses, statistical significance is defined as p value of ≤0.05.
RESULTS
Herp Is Required for Survival Adaptation to MPP+-induced ER Stress
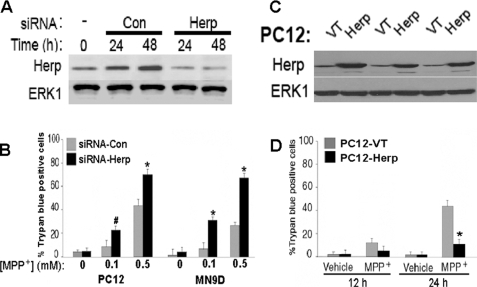
Several ER stress inducible proteins such as Grp78 and Herp are constitutively expressed. To address the role of Herp in the MPP+-induced cell death model, we used RNA interference to knockdown endogenous Herp expression. Transfection of PC12 cells with a siRNA that targets Herp (siRNA-Herp) resulted in a substantial reduction in the level Herp protein (Fig. 1A). To evaluate the effect of Herp knockdown on neuronal vulnerability to MPP+ toxicity, we assessed cell viability by using the trypan blue exclusion (Fig. 1B) and lactate dehydrogenase release (not shown). Exposure of cultures to 0.5 mm MPP+ induced ∼45–50% cell death within 24 h. Depletion of Herp protein markedly enhanced the vulnerability of PC12 cells to MPP+ toxicity. Compared with cultures transfected with a scramble control siRNA (siRNA-Con), there were significantly more dead cells in cultures treated with siRNA-Herp, indicating that down-regulation of Herp sensitizes PC12 cells to MPP+-induced death (Fig. 1B). Similar results were obtained in MN9D, a midbrain-derived dopaminergic neuronal cell line (Fig. 1B). Next, we determined whether PC12 cells stably overexpressing Herp (PC12-Herp) are resistant to MPP+-induced death. Herp protein overexpression was confirmed in three independent clones by immunoblotting (Fig. 1C). Compared with PC12-VT, PC12-Herp cells were significantly more resistant to 0.5 mm MPP+ (Fig. 1D), a dose that yielded ∼50% cell death 24 h post-treatment (Fig. 1B). Collectively, the results indicate that survival adaptation to MPP+ is dependent on Herp function.
FIGURE 1.
Herp protects from MPP+ toxicity. A, PC12 cells were transfected with siRNA targeting Herp (Herp; 100 nm) or nonsilencing control siRNA (Con; 100 nm) for 24 and 48 h. For sequences of each siRNA-Herp or siRNA-Con, see “Experimental Procedures.” Total protein lysates were prepared and analyzed by immunoblotting using an anti-Herp antibody. Equal protein loading was confirmed by reprobing immunoblots with an anti-ERK1 antibody. B, PC12 cells and MN9D were transfected with siRNA-Herp or siRNA-Con (100 nm) for 24 h prior to exposure to 0.5 mm MPP+ or vehicle. At the indicated time points, the percentage of trypan blue-positive cells in each culture was quantified. The values are the means and S.D. of three independent experiments. *, p < 0.01; #, p < 0.05 (ANOVA with Scheffe post-hoc tests) compared with vehicle-treated cultures. C, PC12 clones were stably transfected with plasmid containing Herp (Herp) or the empty plasmid (VT). Total protein lysates were analyzed in three independent clones by Western blotting using an anti-Herp antibody. Equal protein loading was confirmed by reprobing the immunoblots with an anti-ERK1 antibody. D, cultures of PC12-VT and PC12-Herp were left untreated or treated for the indicated time points with 0.5 mm MPP+ or vehicle, and cell viability was assessed. The results were expressed as percentages of trypan blue-positive cells in each culture, normalized to untreated cultures. The values represent the means ± S.D. of three independent experiments. *, p < 0.01 (ANOVA with Scheffe post-hoc tests).
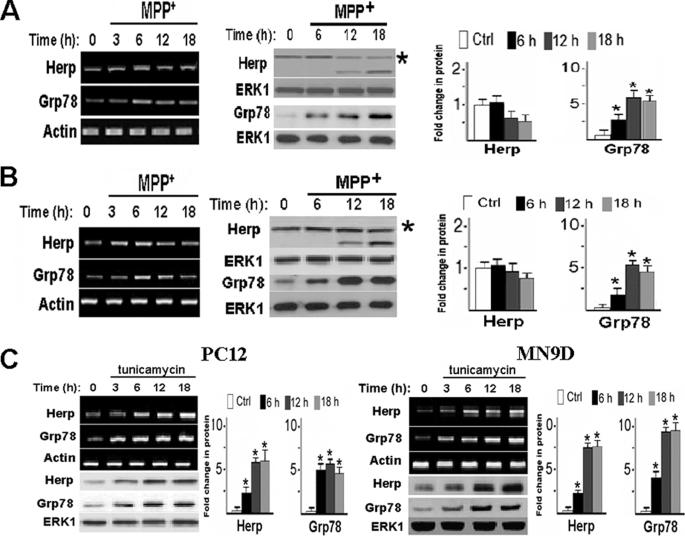
Given that Herp expression is responsive to ER stress (14–16) and that ER stress has been shown to accompany neurotoxin-induced death (9–11), we next evaluated whether MPP+ may induce the expression of Herp. Levels of Herp mRNA and protein in PC12 cells were not markedly increased after exposure to MPP+ (Fig. 2A). The same dose of MPP+ also failed to robustly increase Herp protein expression in MN9D cells (Fig. 2B), thus excluding the possibility that the observed anomaly in Herp induction was cell type-specific. By contrast, the protein level of Grp78, a marker of ER stress, was transiently up-regulated in PC12 and MN9D cells by MPP+ (Fig. 2, A and B), indicating activation of the ER stress response by MPP+. For comparison, we treated sister cultures with tunicamycin, a known pharmacological ER stressor that causes protein accumulation in the ER by inhibiting protein glycosylation (16). Levels of both Herp and Grp78 protein were robustly up-regulated in both PC12 and MN9D cells after treatment with tunicamycin (Fig. 2C). Taken together, the above results suggest that cells are unable to induce Herp protein expression in response to MPP+.
FIGURE 2.
MPP+ and tunicamycin induce Herp and CHOP expression with different kinetics. PC12 (A) and MN9D (B) cells were treated with 0.5 mm MPP+ for the indicated time points. Total RNA and cell lysates were prepared and analyzed by semi-quantitative RT-PCR and immunoblotting for Herp and Grp78, respectively. As control (Ctrl) for equal loading, actin mRNA and ERK1 protein were determined. Densitometric analyses of protein bands are shown next to each panel. *, p < 0.01 (ANOVA with Scheffe post-hoc tests) compared with untreated cultures. Asterisks indicated the full-length Herp protein. C, PC12 and MN9D cells were treated with 5 μg/ml tunicamycin for the indicated time points. Total lysates were prepared and analyzed by semi-quantitative RT-PCR and immunoblotting for Herp and Grp78, respectively. Quantitation of the density of the protein bands is shown next to each panel. *, p < 0.01 (ANOVA with Scheffe post-hoc tests) compared with untreated cells.
Herp Counteracts MPP+-induced Perturbation of ER Ca2+ Homeostasis
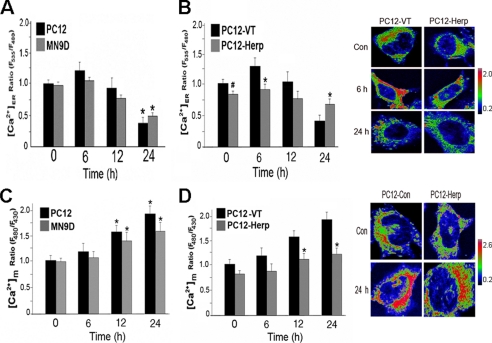
Oxidative stress is an important factor implicated in the disruption of neuronal Ca2+ homeostasis (23). Consistent with previous reports (24, 25), MPP+ increased the intracellular accumulation of hydroxyl and peroxynitrite in PC12 and MN9D cells (supplemental Fig. S1). Given that perturbations of intracellular Ca2+ homeostasis have been implicated in oxidative stress-induced cell death (19), we next examined the effects of MPP+ on ER Ca2+ handling. To this end, we monitor changes in the Ca2+ concentration in the ER lumen ([Ca2+]ER) of PC12 and MND9D cells at various time points after treatment with MPP+. The early rise in [Ca2+]ER was quickly followed by a gradual and progressive decline in MPP+-treated cells (Fig. 3A), suggesting that MPP+ increased Ca2+ leakage from the ER.
FIGURE 3.
Herp counteracts MPP+-induced depletion of ER Ca2+ store. Statistical evaluations of the changes in the Ca2+ concentration in the ER ([Ca2+]ER) (A and B) and mitochondria ([Ca2+]M) (C and D) in PC12 and MN9D cells (A and C) and in the indicated stably transfected PC12 clones (B and D) after treatment with MPP+. The indicated cultures were transiently transfected with 2 μg of YC4-ER or RP-mt for 24 h prior to incubation with 0.5 mm MPP+. At the indicated time points, [Ca2+]ER and [Ca2+]M were measured as described under “Experimental Procedures.” The results were expressed as the ratios of the YC4-ER or RP-mt fluorescence signals in MPP+-treated relative to untreated cultures. The values are the means and S.D. of measurements made in three or four cultures (n = 4 dishes, 4–6 microscopic fields/dish, 25–30 cells/field). *, p < 0.01; #, p < 0.05 (ANOVA with Scheffe post-hoc tests) compared with either untreated cultures or PC12-VT cultures. Representative pseudocolored images of the indicated PC12 clones at base line and after exposure to MPP+ are shown in B and D. The pseudocolor bar shows the ratio range. Con, control.
Because Herp functions to stabilize ER Ca2+ homeostasis during ER stress (16, 17), we next evaluated whether overexpression of Herp prevents MPP+-induced perturbations of [Ca2+]ER. Compared with PC12-VT cells, PC12-Herp cells exhibited reduced ER Ca2+ leakage and were able to maintain [Ca2+]ER (Fig. 3B). Because uncontrolled ER Ca2+ release can lead to protracted rise in the Ca2+ concentration of the mitochondria ([Ca2+]M) (8, 29) and because aberrant mitochondrial Ca2+ handling has been shown to be involved in MPP+-induced toxicity (30, 31), we next assessed changes in [Ca2+]M in PC12 and MN9D cells at various time points after treatment with MPP+. The decrease of [Ca2+]ER was accompanied by an increase in [Ca2+]M in MPP+-treated cells (Fig. 3C), indicating that MPP+ induces Ca2+ mobilization from the ER to mitochondria. As expected, the magnitude of the increase in [Ca2+]M was significantly attenuated in PC12-Herp (Fig. 3D), suggesting that Herp likely inhibits the toxic Ca2+ transfer from the ER to mitochondria under oxidant-induced ER stress. Representative pseudocolored images of the indicated PC12 clones expressing the fluorescent Ca2+ indicators, YC4-ER and pericam-mt, are shown in Fig. 3 (B and D), respectively. Co-localization studies confirmed that the indicated fluorescent indicators are properly targeted and expressed either in the ER (YC4-ER) or in the mitochondria (pericam-mt) (supplemental Fig. S3).
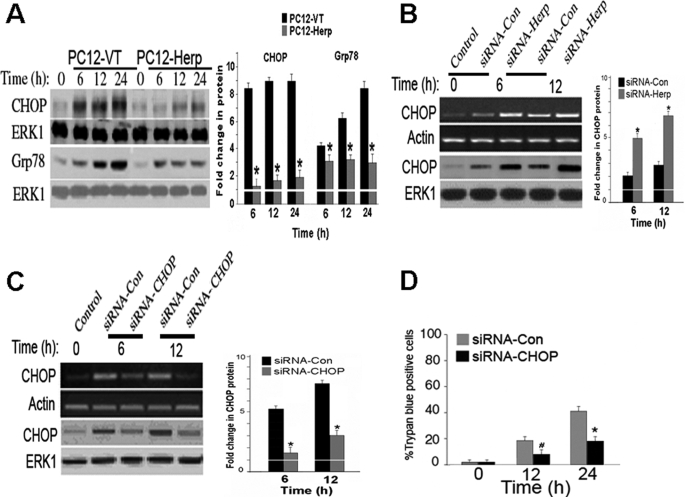
Herp Blocks MPP+-induced Activation of CHOP
CHOP has been implicated as a mediator of apoptosis in the context of ER and oxidative stress (32, 33). ER stress-induced cell death in cultures occurs only when CHOP is permanently up-regulated but not when the increase in CHOP is transient, suggesting that CHOP contributes to the activation of ER-initiated apoptosis signaling (31–34). Given that store depletion has been associated with CHOP up-regulation (20, 35) and that overexpression of Herp counteracts MPP+-induced ER Ca2+ store depletion (Fig. 3B), we next evaluate whether CHOP is differentially induced by MPP+ in PC12-VT and PC12-Herp clones. The magnitude of the MPP+ induced increase in CHOP protein level was significantly lower in PC12-Herp when compared with PC12-VT (Fig. 4A). The Herp-mediated suppression of CHOP was also associated with reduced Grp78 induction (Fig. 4A), suggesting that Herp restores ER homeostasis in MPP+-treated cells by inhibiting downstream events caused by CHOP.
FIGURE 4.
Herp counteracts MPP+-induced up-regulation of CHOP. A, time course of CHOP and Grp78 protein levels in cultures of PC12-VT and PC12-Herp before and after incubation with 0.5 mm MPP+. As control for equal loading, immunoblots were reprobed with an antibody to ERK1. Densitometric analysis of protein bands normalized to untreated control cultures is shown in the right panel. *, p < 0.01 (ANOVA with Scheffe post-hoc tests) compared with PC12-VT. B, time course of MPP+-induced CHOP mRNA and protein levels in cultures of PC12 cells transfected with siRNA-Herp or siRNA-Con (100 nm). The cultures were incubated with 0.5 mm MPP+ 24 h after transfection. Actin and ERK1 were used as loading controls. Densitometric analysis of protein bands normalized to untreated control cultures is shown in the right panel. *, p < 0.01 (ANOVA with Scheffe post-hoc tests) compared with cultures transfected with siRNA-Con. C and D, PC12 cells were transfected with siRNA-CHOP or siRNA-Con (100 nm). One day after transfection, PC12 cells were exposed to 0.5 mm MPP+ or vehicle for the indicated time points and subsequently harvested for detection of Herp mRNA and protein (C) or fixed for quantitation of cell death (D). Actin and ERK1 were used as loading controls. Densitometric analysis of protein bands normalized to untreated control cultures is shown in the right panel. *, p < 0.01 (ANOVA with Scheffe post-hoc tests) compared with cultures transfected with siRNA-Con. For quantitation of cell death, the percentages of trypan blue-positive cells in each treated culture, normalized to vehicle-treated cultures, were shown. The values are the means and S.D. of three dishes/group for each time point. *, p < 0.01; #, p < 0.05 (ANOVA with Scheffe post-hoc tests) compared with cultures transfected with siRNA-Con.
By contrast, suppression of endogenous Herp by RNA interference potentiates CHOP induction in MPP+-treated PC12 cells (Fig. 4B). Treatment with siRNA-Con, which had no effect on Herp expression (Fig. 1A), did not enhance MPP+-induced CHOP up-regulation (Fig. 4B). These data are consistent with a previous study showing that Herp null cells displayed aberrant ER stress signaling with increased level of CHOP transcript when compared with wild-type control cells (15).
Next, we determined whether suppression of CHOP induction by RNA interference is sufficient to inhibit MPP+ toxicity. siRNA targeting CHOP (siRNA-CHOP) (20) was used to inhibit the MPP+-induced CHOP expression in PC12 cells (Fig. 4C). Cell viability assessed by trypan blue exclusion assay showed that siRNA-induced silencing of CHOP provided significant protection against MPP+ toxicity (Fig. 4D). By contrast, the siRNA-Con did not rescue PC12 cells from MPP+ toxicity (Fig. 4D). Interestingly, knockdown of CHOP did not further rescue PC12-Herp cells from MPP+-induced toxicity (supplemental Fig. S2), suggesting that Herp promotes cell survival in large by suppressing CHOP-dependent pro-apoptotic signaling. Collectively, the above results indicate that Herp attenuates MPP+-induced cell death by inhibiting CHOP up-regulation.
CHOP Contributes to MPP+-induced Perturbation of ER Ca2+ Homeostasis
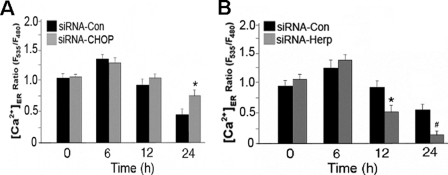
To determine whether CHOP up-regulation in MPP+-treated cells is causally linked to ER Ca2+ store depletion, we measured the MPP+-induced perturbation in ER Ca2+ homeostasis in PC12 cells transfected with siRNA-CHOP. Compared with siRNA-Control, siRNA-CHOP substantially reduced ER Ca2+ leakage in MPP+-treated PC12 cells. CHOP knockdown also attenuates MPP+-induced ER Ca2+ store depletion (Fig. 5A). Because Herp suppressed CHOP induction in MPP+-treated PC12 cells, we examined whether knockdown of Herp expression exacerbated MPP+-induced ER Ca2+ store depletion. As expected, knockdown of Herp exacerbated ER Ca2+ leakage (Fig. 5B) in MPP+-treated PC12 cells (Fig. 5C). These data indicate that Herp prevents CHOP-mediated ER Ca2+ store depletion in MPP+-treated PC12 cells.
FIGURE 5.
CHOP and Herp modulates ER Ca2+ homeostasis in MPP+-treated cells. Knockdown of CHOP (A) and Herp (B) alters ER Ca2+ store contents in MPP+-treated PC12 cells. Twenty-four hours after co-transfection with 2 μg of pBudCE4.1-YC4-ER with the indicated siRNA duplexes (100 nm), cultures were incubated with 0.5 mm MPP+ or vehicle control (Con). Changes in ER Ca2+ concentration ([Ca2+]ER) were recorded at the indicated time points as described under “Experimental Procedures” and presented as the ratios of the YC4-ER fluorescence signal in MPP+-treated relative to untreated cultures. The values are the means and S.D. of measurements made in three separate cultures (n = 4 dishes, 4–6 microscopic fields/dish, 25–30 cells/field). *, p < 0.01; #, p < 0.05 (ANOVA with Scheffe post-hoc tests) compared with cultures treated with siRNA-Con.
The Herp-dependent Protective Mechanism Is Not Mediated by the Anti-apoptotic Bcl-2 Protein
Because CHOP has been shown to down-regulate the expression of Bcl-2 protein (36) and because overexpression of Bcl-2 affects Ca2+ handling by the ER (8, 37), we next determined whether Bcl-2 protein may be acting downstream of Herp to maintain ER Ca2+ homeostasis in MPP+-treated PC12 cells. Levels of Bcl-2 protein in PC12-VT and PC12-Herp were not significantly different before and after exposure to MPP+ (supplemental Fig. S4). To rule out the possibility that Herp might interact with Bcl-2 and therefore facilitated Bcl-2 association with the ER membrane, we measured the amounts of Bcl-2 protein in isolated microsomes. Levels of Bcl-2 protein in the microsomes prepared from PC12-Herp were comparable with those from PC12-VT and were nearly unchanged following exposure to MPP+ (supplemental Fig. S4). No interaction between Bcl-2 and Herp was detected by co-immunoprecipitation analysis (supplemental Fig. S4). All in all, although Herp affects Ca2+ handling by the ER in a manner resembling the effects of Bcl-2 overexpression, data from these experiments excluded a role for Bcl-2 in the Herp-dependent stabilization of ER Ca2+ homeostasis in MPP+-treated cells.
The Ubiquitin-like (UBL) Domain Is Essential for Herp-mediated ER Ca2+ Stabilization and Protection from MPP+-induced Toxicity
Previous studies established a critical role of the N-terminal UBL domain in the cytoprotective action of Herp (15–17). Overexpression of a mutant Herp deletion construct lacking the UBL domain (ΔUBL-Herp; Fig. 6A) failed to protect PC12 cells from ER stress-induced cell death (15–17). To determine whether the Herp-dependent protection against MPP+ toxicity may also require the UBL domain, we generated PC12 clones stably overexpressing ΔUBL-Herp. Stable transfection inducing overexpression of ΔUBL-Herp was verified by immunoblotting (Fig. 6A). Compared with PC12-VT, PC12-ΔUBL-Herp clones were not more resistant to MPP+-induced cell death (Fig. 6B), suggesting that the UBL domain is required for Herp-dependent cytoprotective action.
FIGURE 6.
The ubiquitin-like (UBL) domain is essential for Herp-mediated stabilization of ER Ca2+ homeostasis and rescue from MPP+ toxicity. A, schematic diagram of full-length Herp protein and a deletion mutant lacking the N-terminal UBL domain (ΔUBL-Herp). Expression of Herp and ΔUBL-Herp protein in stably transfected PC12 clones is shown in the inset. ERK1 was used as the loading control. B, ΔUBL-Herp fails to rescue PC12 cells from MPP+ toxicity. The indicated PC12 clones were exposed to 0.5 mm MPP+ for the indicated time points. The results were expressed as percentage of trypan blue-positive cells in each culture, normalized to vehicle-treated cultures, from three independent experiments. *, p < 0.01; #, p < 0.05 (ANOVA with Scheffe post-hoc tests) compared with PC12-VT. C, ΔUBL-Herp fails to stabilize ER Ca2+ homeostasis. Twenty-four hours after transfection of pBudCE4.1-YC4-ER, the indicated PC12 clones were incubated with 0.5 mm MPP+. Changes in ER Ca2+ concentration ([Ca2+]ER) were recorded at the indicated time points as described under “Experimental Procedures” and presented as the ratio of the fluorescence signals in MPP+-treated relative to untreated cultures. The values are the means and S.D. of measurements made in three or four cultures (n = 4 dishes, 4–6 microscopic fields/dish, 25–30 cells/field). *, p < 0.01 (ANOVA with Scheffe post-hoc tests) compared with PC12-VT and PC12-ΔUBL-Herp; #, p < 0.05 (ANOVA with Scheffe post-hoc tests) compared with PC12-VT. D, ΔUBL-Herp fails to suppress MPP+ induced up-regulation of CHOP. Time course of MPP+-induced increase in CHOP protein level in the indicated stably transfected PC12 clones. As control for equal loading, immunoblots were reprobed with an antibody to ERK1. Densitometric analysis of protein bands normalized to untreated control cultures is shown in the right panel. *, p < 0.01 (ANOVA with Scheffe post-hoc tests) compared with PC12-VT and PC12-ΔUBL-Herp.
To determine whether the UBL domain is essential for the stabilization of ER Ca2+ homeostasis, we measured [Ca2+]ER in PC12-ΔUBL-Herp. The magnitude of the MPP+-induced ER Ca2+ leakage was indistinguishable in PC12-VT and PC12-ΔUBL-Herp, suggesting that the UBL domain is required for the ability of Herp to maintain ER Ca2+ homeostasis (Fig. 6C). We also found that CHOP mRNA and protein levels in PC12-VT clones are not significantly different in PC12-ΔUBL-Herp clones (Fig. 6D), which further supports the notion that a functional Herp protein is required to suppress CHOP induction and to stabilize ER Ca2+ homeostasis.
Herp-dependent Stabilization of ER Ca2+ Homeostasis Requires a Functional UPP
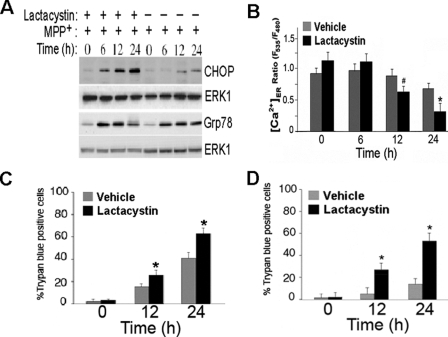
Herp has recently been implicated in the regulation of ERAD (38, 39), a protein quality control system of the ER, which eliminates misfolded proteins by UPP-dependent degradation (4). To determine whether ERAD is involved in the Herp-dependent stabilization of ER Ca2+ homeostasis, we blocked ERAD with the proteasomal inhibitor lactacystin. Treatment of PC12 cells with lactacystin for 12 and 24 h significantly reduced proteasomal activity (supplemental Fig. S5). Inhibition of proteasome function reversed the Herp-dependent suppression of CHOP induction (Fig. 7A) and accelerates ER Ca2+ depletion (Fig. 7B), suggesting that proteosomal activity is required for Herp-dependent stabilization of ER Ca2+ homeostasis. Consistent with the notion that the proteasomal function is required for the neuroprotective action of Herp, we found that lactacystin not only increased the vulnerability of PC12 cells (Fig. 7C) but also restores the sensitivity of PC12-Herp clones to MPP+ toxicity (Fig. 7D).
FIGURE 7.
Proteasomal-mediated degradation is essential for Herp-dependent stabilization of ER Ca2+ homeostasis and rescue from MPP+ toxicity. A, the proteasomal inhibitor lactacystin abolishes Herp-mediated suppression of CHOP in MPP+-treated PC12 cells. Time course of CHOP and Grp78 protein levels in PC12-Herp cultures incubated with 0.5 mm MPP+ in the presence or absence of lactacystin (5 μm). As control for equal loading, immunoblots were reprobed with an antibody to ERK1. B, lactacystin abolishes the Herp-dependent suppression of ER Ca2+ store depletion in MPP+-treated PC12 cells. Twenty-four hours after transfection with 2 μg of pBudCE4.1-YC4-ER, the cultures of PC12-Herp were incubated with 0.5 mm MPP+ in the presence of lactacystin (5 μm) or its vehicle control. Changes in ER Ca2+ concentration ([Ca2+]ER) were recorded at the indicated time points as described under “Experimental Procedures” and presented as the ratio of the YC4-ER fluorescence signal in MPP+-treated relative to untreated cultures. The values are the means and S.D. of measurements made in three or four cultures (n = 4 dishes, 4–6 microscopic fields/dish, 25–30 cells/field). *, p < 0.01 (ANOVA with Scheffe post-hoc tests). C and D, lactacystin enhances MPP+ toxicity (C) and reverses the Herp-dependent cell death rescue from MPP+ toxicity (D). Cultures of PC12 (C) and PC12-Herp clones (D) were exposed to 0.5 mm MPP+ in the presence of lactacystin (5 μm) or vehicle control for the indicated time points. Shown is the percentage of trypan blue-positive cells in each culture, normalized to untreated cultures, from three independent experiments. *, p < 0.01 (ANOVA with Scheffe post-hoc tests) compared with vehicle-treated cultures.
DISCUSSION
Elucidating the specific and sequential molecular events induced by MPP+ will provide a better understanding of the molecular basis of dopaminergic cell death. MPP+ is selectively toxic to dopaminergic neurons and has been studied extensively as an etiologic model of PD because mitochondrial dysfunction is implicated in both MPP+ toxicity and the pathogenesis of PD. MPP+ toxicity has been attributed to the generation of reactive oxygen species (ROS) (24, 25). ROS generated from mitochondrial appear to be a main contributor of oxidative stress-mediated neurodegeneration in PD models (40).
Oxidative stress is an important factor implicated in the disruption of neuronal Ca2+ homeostasis (19). In this study we showed that MPP+ induces the deregulation of ER Ca2+ homeostasis. There is a growing body of evidence that the ER can play pivotal roles in regulating cell survival and apoptosis in a variety of cell types including neurons. The ER serves many specialized functions in the cells including the biosynthesis of membrane and secretory proteins (7) and maintenance of neuronal Ca2+ homeostasis (41). Dysregulation of ER Ca2+ homeostasis occurs as an early event during many forms of apoptosis and has been implicated in the pathophysiology of several acute and chronic neurodegenerative diseases, including ischemic injury, trauma, and Alzheimer, Huntington, and prion diseases (41–43). Uncontrolled Ca2+ release from the ER is a key proapoptotic event, as indicated by the ability of blockers of ER Ca2+ release to reduce the extent of ischemic injury (44) and to protect cultured neurons against cell death induced by glutamate, mutant huntingtin, Aβ, and prion peptides (21, 45). Whether dysregulation of ER Ca2+ homeostasis contributes to PD initiation and progression has not yet been established.
Disturbances in intracellular Ca2+ homeostasis could play a role in dopaminergic degeneration because treatment with various PD neurotoxins has been shown to perturb intracellular Ca2+ homeostasis (26–28). The MPP+-induced cell death was inhibited by co-expression of calbindin-D28K or co-treatment with BAPTA (1,2-bis-(o-aminophenoxy)-ethane-N,N,N′,N′-tetracidic acid), suggesting a critical role for intracellular Ca2+ loads in MPP+-induced toxicity (46). The pertinent mechanism whereby neurotoxins disrupt intracellular Ca2+ homeostasis remains poorly understood. Antagonists of glutamate and Ca2+ channels (47) have been reported ineffective in preventing MPP+ toxicity, suggesting that the Ca2+ perturbations induced by MPP+ are likely attributed to deregulated ER Ca2+ release. Consistent with the latter notion, inhibition of ER Ca2+ release prevents the MPP+-induced perturbations of intracellular Ca2+ (47).
Various pathological conditions that induce ER stress have been shown to perturb ER Ca2+ homeostasis (8, 16, 29), but the underlying mechanisms remain poorly characterized. Oxidative damage to the ER can lead to perturbations in ER Ca2+ homeostasis. Protein folding in the ER can generate ROS, which in turn may exacerbate ER stress by perturbing the function of ER foldases and/or chaperones (48). ROS could also sensitize ryanodine receptor- and inositol triphosphate receptor-mediated ER Ca2+ release (49, 50) or block sarcoplasmic/endoplasmic reticulum Ca2+-ATPase-mediated Ca2+ sequestration by the ER (51). During ER stress, increased Ca2+ transfer from the ER to mitochondria leads to mitochondria Ca2+ overload and generation of mitochondrial-derived ROS, which could further disrupt protein folding in the ER and potentiate ER Ca2+ release through a positive feed-forward mechanism (52, 53).
Recent studies show that neurotoxins induce ER stress via the generation of ROS (54, 55), suggesting that ER stress may be involved downstream of ROS. The biological relevance of the neurotoxin induced-ER stress is still unknown. Given that ER is an important regulator of intracellular Ca2+ homeostasis, oxidant-induced deregulation of ER Ca2+ homeostasis could contribute to dopaminergic degeneration.
Induction of ER stress proteins during oxidative and ER stress seems to be important to remedy the perturbations of ER Ca2+ homeostasis. We previously reported that Herp is essential for cell survival in response to ER stress (16). Here, we found that Herp is critical for cellular stress adaptation in response to MPP+. Consistent with this notion, we found that overexpression of Herp attenuated MPP+-induced toxicity, whereas knockdown of Herp increased not only CHOP expression but also ER Ca2+ leakage and mitochondrial Ca2+ accumulation, resulting in cell death (Fig. 1). Notably, we found that MPP+ failed to up-regulate Herp expression in dopaminergic cells in vitro (Fig. 2, A and B). Failure to induce a compensatory increase in Herp expression may deteriorate ER function in MPP+-treated cells. Hence, exploring ways to increase Herp expression can increase the ability of dopaminergic cells to cope with ER stress and to protect from MPP+ toxicity.
Given that Herp plays a crucial role in stabilizing ER Ca2+ homeostasis (16, 17), we determined whether stable expression of Herp counteracts MPP+-induced toxicity by inhibiting Ca2+ transfer from ER to mitochondria. Time course analysis of the MPP+-induced alterations in [Ca2+]ER and [Ca2+]M revealed that knockdown of Herp accelerates ER Ca2+ store depletion with a time course that parallels [Ca2+]M accumulation (Fig. 3B) and that overexpression of Herp reversed the toxic Ca2+ transfer between the ER and mitochondria (Fig. 3, A and B).
The mitochondrial apoptotic pathway is an integral part of MPP+-induced apoptosis (24, 25). Excessive accumulation of [Ca2+]M causes collapse of the mitochondria membrane potential, which results in mitochondrial transition pore opening and release of pro-apoptogenic factors including cytochrome c that promotes downstream caspase activation (53). Because mitochondria are linked to the ER both by proximity and through Ca2+ signaling (52, 56), various pathological conditions that perturb ER Ca2+ homeostasis could adversely impact the function of the mitochondria. Hence, oxidant-induced damage to the ER could cause a protracted elevation in [Ca2+]M that could enhance generation of mitochondrial-derived ROS that through a feed forward mechanism exacerbates the loss of Ca2+ from the ER (53).
The underlying molecular and cellular mechanisms whereby Herp stabilizes ER Ca2+ homeostasis and preserves mitochondrial function in MPP+-treated cells remain to be established. It is unlikely that the ER membrane-associated Herp functions as a calcium-binding chaperone analogous to Grp78 and calreticulin (14). We excluded a role for Bcl-2 in the Herp-mediated stabilization of ER Ca2+ homeostasis based on the findings that Herp fails to bind to Bcl-2 and that total levels of Bcl-2 in the microsome fractions were not significantly different in PC12-VT and PC12-Herp cells (supplemental Fig. S4). Hence, the mechanism by which Herp maintains ER Ca2+ homeostasis appears to be different from the proposed anti-apoptotic action of Bcl-2 (37). Because the UBL domain is essential for Herp-mediated protection against neurotoxins (Fig. 7B), we determined that the UBL domain is essential for the ability of Herp to maintain ER Ca2+ homeostasis. Expression of Herp lacking the UBL domain fails to stabilize intracellular Ca2+ and to suppress the induction of CHOP in MPP+-treated cells (Fig. 7, C and D), indicating that UBL is critical for the cytoprotective function of Herp.
How the UBL domain is involved in the protective function of Herp is not clear. The presence of the UBL domain, which faces into the cytosol (14), suggests that Herp may function as a proteasome-interacting domain, as has recently been demonstrated for Parkin (57). Several recent studies support a role of Herp in ERAD (38, 39). Herp interacts with Hrd1p, a membrane-anchored E3 ligase (38), and with ubiquilin, a shuttle protein that delivers ubiquitinated substrates to the proteasome for degradation (58). Overexpression of Herp enhances the degradation of the ERAD substrate CD3δ, whereas siRNA-mediated reduction of Herp expression stabilized the ERAD substrate CD3δ but did not alter or increased degradation of non-ERAD substrates tested (38). It is possible that Herp may target yet to be identified CHOP-regulated ERAD substrates whose accumulation results in perturbations in ER Ca2+ homeostasis. Hence, elucidating the precise role of Herp in ERAD and the identity of the ERAD substrates that accumulate in Herp knockdown cells will likely provide clues to the mechanisms of Herp-mediated ER Ca2+ stabilization and protection from MPP+-induced toxicity.
Supplementary Material
This work was supported by funds from the American Federation on Aging Research (to S. L. C.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S5.
- PD
- Parkinson disease
- ER
- endoplasmic reticulum
- MPP+
- 1-methyl-4-phenylpyridinium
- UPP
- ubiquitin-proteasomal pathway
- ERAD
- ER-associated protein degradation
- UPR
- unfolded protein response
- CHOP
- CCAAT enhancer-binding protein homologous protein
- siRNA
- small interfering RNA
- RT
- reverse transcription
- ERK
- extracellular signal-regulated kinase
- ANOVA
- analysis of variance
- UBL
- ubiquitin-like
- ROS
- reactive oxygen species.
REFERENCES
- 1.Dawson T. M., Dawson V. L. ( 2003) Science 302, 819– 822 [DOI] [PubMed] [Google Scholar]
- 2.Cookson M. R. ( 2005) Annu. Rev. Biochem. 74, 29– 52 [DOI] [PubMed] [Google Scholar]
- 3.McNaught K. S., Olanow C. W., Halliwell B., Isacson O., Jenner P. ( 2001) Nat. Rev. Neurosci. 2, 589– 594 [DOI] [PubMed] [Google Scholar]
- 4.Hiller M. M., Finger A., Schweiger M., Wolf D. H. ( 1996) Science 273, 1725– 1728 [DOI] [PubMed] [Google Scholar]
- 5.Boyce M., Yuan J. ( 2006) Cell Death Differ. 13, 363– 373 [DOI] [PubMed] [Google Scholar]
- 6.Lindholm D., Wootz H., Korhonen L. ( 2006) Cell Death Differ. 13, 385– 392 [DOI] [PubMed] [Google Scholar]
- 7.Harding H. P., Calfon M., Urano F., Novoa I., Ron D. ( 2002) Annu. Rev. Cell Dev. Biol. 18, 575– 599 [DOI] [PubMed] [Google Scholar]
- 8.Breckenridge D. G., Germain M., Mathai J. P., Nguyen M., Shore G. C. ( 2003) Oncogene. 22, 8608– 8618 [DOI] [PubMed] [Google Scholar]
- 9.Ryu E. J., Harding H. P., Angelastro J. M., Vitolo O. V., Ron D., Greene L. A. ( 2002) J. Neurosci. 22, 10690– 10698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holtz W. A., O'Malley K. L. ( 2003) J. Biol. Chem. 278, 19367– 19377 [DOI] [PubMed] [Google Scholar]
- 11.Yamamuro A., Yoshioka Y., Ogita K., Maeda S. ( 2006) Neurochem. Res. 31, 657– 664 [DOI] [PubMed] [Google Scholar]
- 12.Smith W. W., Jiang H., Pei Z., Tanaka Y., Morita H., Sawa A., Dawson V. L., Dawson T. M., Ross C. A. ( 2005) Hum. Mol. Genet. 14, 3801– 3811 [DOI] [PubMed] [Google Scholar]
- 13.Hoozemans J. J., van Haastert E. S., Eikelenboom P., de Vos R. A., Rozemuller J. M., Scheper W. ( 2007) Biochem. Biophys. Res. Commun. 354, 707– 711 [DOI] [PubMed] [Google Scholar]
- 14.Kokame K., Agarwala K. L., Kato H., Miyata T. ( 2000) J. Biol. Chem. 275, 32846– 32853 [DOI] [PubMed] [Google Scholar]
- 15.Hori O., Ichinoda F., Yamaguchi A., Tamatani T., Taniguchi M., Koyama Y., Katayama T., Tohyama M., Stern D. M., Ozawa K., Kitao Y., Ogawa S. ( 2004) Genes Cells 9, 457– 469 [DOI] [PubMed] [Google Scholar]
- 16.Chan S. L., Fu W., Zhang P., Cheng A., Lee J., Kokame K., Mattson M. P. ( 2004) J. Biol. Chem. 279, 28733– 28743 [DOI] [PubMed] [Google Scholar]
- 17.Tuvia S., Taglicht D., Erez O., Alroy I., Alchanati I., Bicoviski V., Dori-Bachash M., Ben-Avraham D., Reiss Y. ( 2007) J. Cell Biol. 177, 51– 61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dauer W., Przedborski S. ( 2003) Neuron 39, 889– 909 [DOI] [PubMed] [Google Scholar]
- 19.Kim J., Choi T. G., Ding Y., Kim Y., Ha K. S., Lee K. H., Kang I., Ha J., Kaufman R. J., Lee J., Choe W., Kim S. S. ( 2008) J. Cell Sci. 121, 3636– 3648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan S. L., Liu D., Kyriazis G. A., Ouyang X., Mattson M. P. ( 2006) J. Biol. Chem. 281, 37391– 37403 [DOI] [PubMed] [Google Scholar]
- 21.Malli R., Frieden M., Osibow K., Zoratti C., Mayer M., Demaurex N., Graier W. F. ( 2003) J. Biol. Chem. 278, 44769– 44779 [DOI] [PubMed] [Google Scholar]
- 22.Nagai T., Sawano A., Park E. S., Miyawaki A. ( 2001) Proc. Natl. Acad. Sci. U.S.A. 98, 3197– 3202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattson M. P., Chan S. L. ( 2001) J. Mol. Neurosci. 17, 205– 224 [DOI] [PubMed] [Google Scholar]
- 24.Cassarino D. S., Fall C. P., Swerdlow R. H., Smith T. S., Halvorsen E. M., Miller S. W., Parks J. P., Parker W. D., Jr., Bennett J. P., Jr. ( 1997) Biochim. Biophys. Acta 1362, 77– 86 [DOI] [PubMed] [Google Scholar]
- 25.Kalivendi S. V., Kotamraju S., Cunningham S., Shang T., Hillard C. J., Kalyanaraman B. ( 2003) Biochem. J. 371, 151– 164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee D. H., Han Y. S., Han E. S., Bang H., Lee C. S. ( 2006) Neurochem. Res. 31, 851– 860 [DOI] [PubMed] [Google Scholar]
- 27.Lee C. S., Park S. Y., Ko H. H., Song J. H., Shin Y. K., Han E. S. ( 2005) Neurochem. Int. 46, 169– 178 [DOI] [PubMed] [Google Scholar]
- 28.Kass G. E., Wright J. M., Nicotera P., Orrenius S. ( 1988) Arch. Biochem. Biophys. 260, 789– 797 [DOI] [PubMed] [Google Scholar]
- 29.Deniaud A., Sharaf el dein O., Maillier E., Poncet D., Kroemer G., Lemaire C., Brenner C. ( 2008) Oncogene 27, 285– 299 [DOI] [PubMed] [Google Scholar]
- 30.Lee C. S., Song E. H., Park S. Y., Han E. S. ( 2003) Neurochem. Int. 43, 147– 154 [DOI] [PubMed] [Google Scholar]
- 31.Packer M. A., Miesel R., Murphy M. P. ( 1996) Biochem. Pharmacol. 51, 267– 273 [DOI] [PubMed] [Google Scholar]
- 32.Oyadomari S., Mori M. ( 2004) Cell Death Differ. 11, 381– 389 [DOI] [PubMed] [Google Scholar]
- 33.Wang X. Z., Lawson B., Brewer J. W., Zinszner H., Sanjay A., Mi L. J., Boorstein R., Kreibich G., Hendershot L. M., Ron D. ( 1996) Mol. Cell Biol. 16, 4273– 4280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benavides A., Pastor D., Santos P., Tranque P., Calvo S. ( 2005) Glia 52, 261– 275 [DOI] [PubMed] [Google Scholar]
- 35.Copanaki E., Schürmann T., Eckert A., Leuner K., Müller W. E., Prehn J. H., Kögel D. ( 2007) Biochim. Biophys. Acta 1773, 157– 165 [DOI] [PubMed] [Google Scholar]
- 36.McCullough K. D., Martindale J. L., Klotz L. O., Aw T. Y., Holbrook N. J. ( 2001) Mol. Cell Biol. 21, 1249– 1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinton P., Ferrari D., Rapizzi E., Di Virgilio F., Pozzan T., Rizzuto R. ( 2001) EMBO J. 20, 2690– 2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schulze A., Standera S., Buerger E., Kikkert M., van Voorden S., Wiertz E., Koning F., Kloetzel P. M., Seeger M. ( 2005) J. Mol. Biol. 354, 1021– 1027 [DOI] [PubMed] [Google Scholar]
- 39.Okuda-Shimizu Y., Hendershot L. M. ( 2007) Mol. Cell 28, 544– 554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jenner P. ( 2003) Ann. Neurol. 53, S26– 38 [DOI] [PubMed] [Google Scholar]
- 41.Mattson M. P., LaFerla F. M., Chan S. L., Leissring M. A., Shepel P. N., Geiger J. D. ( 2000) Trends Neurosci. 23, 222– 229 [DOI] [PubMed] [Google Scholar]
- 42.Tang T. S., Tu H., Chan E. Y., Maximov A., Wang Z., Wellington C. L., Hayden M. R., Bezprozvanny I. ( 2003) Neuron. 39, 227– 239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hetz C., Russelakis-Carneiro M., Maundrell K., Castilla J., Soto C. ( 2003) EMBO J. 22, 5435– 5445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei H., Perry D. C. ( 1996) J. Neurochem. 67, 2390– 2398 [DOI] [PubMed] [Google Scholar]
- 45.Ferreiro E., Resende R., Costa R., Oliveira C. R., Pereira C. M. ( 2006) Neurobiol. Dis. 23, 669– 678 [DOI] [PubMed] [Google Scholar]
- 46.Choi W. S., Lee E., Lim J., Oh Y. J. ( 2008) Biochem. Biophys. Res. Commun. 371, 127– 131 [DOI] [PubMed] [Google Scholar]
- 47.Lotharius J., Dugan L. L., O'Malley K. L. ( 1999) J. Neurosci. 19, 1284– 1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haynes C. M., Titus E. A., Cooper A. A. ( 2004) Mol. Cell 15, 767– 776 [DOI] [PubMed] [Google Scholar]
- 49.Suzuki Y. J., Ford G. D. ( 1992) Am. J. Physiol. 262, H114– 116 [DOI] [PubMed] [Google Scholar]
- 50.Madesh M., Hawkins B. J., Milovanova T., Bhanumathy C. D., Joseph S. K., Ramachandrarao S. P., Sharma K., Kurosaki T., Fisher A. B. ( 2005) J. Cell Biol. 170, 1079– 1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boraso A., Williams A. J. ( 1994) Am. J. Physiol. 267, H1010– 1016 [DOI] [PubMed] [Google Scholar]
- 52.Hajnóczky G., Csordás G., Madesh M., Pacher P. ( 2000) Cell Calcium. 28, 349– 363 [DOI] [PubMed] [Google Scholar]
- 53.Jacobson J., Duchen M. R. ( 2002) J. Cell Sci. 115, 1175– 1188 [DOI] [PubMed] [Google Scholar]
- 54.Holtz W. A., Turetzky J. M., Jong Y. J., O'Malley K. L. ( 2006) J. Neurochem. 99, 54– 69 [DOI] [PubMed] [Google Scholar]
- 55.Yokouchi M., Hiramatsu N., Hayakawa K., Okamura M., Du S., Kasai A., Takano Y., Shitamura A., Shimada T., Yao J., Kitamura M. ( 2008) J. Biol. Chem. 283, 4252– 4260 [DOI] [PubMed] [Google Scholar]
- 56.Pinton P., Giorgi C., Siviero R., Zecchini E., Rizzuto R. ( 2008) Oncogene 27, 6407– 6418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kahns S., Kalai M., Jakobsen L. D., Clark B. F., Vandenabeele P., Jensen P. H. ( 2003) J. Biol. Chem. 278, 23376– 23380 [DOI] [PubMed] [Google Scholar]
- 58.Kim T. Y., Kim E., Yoon S. K., Yoon J. B. ( 2008) Biochem. Biophys. Res. Commun. 369, 741– 746 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.