Abstract
Objective
High-density lipoproteins (HDL) have antiinflammatory effects on the vascular endothelium. Because bone morphogenetic proteins (BMP) are known to be inflammatory mediators, we examined the effect of HDL on BMP signaling.
Methods and Results
Increasing concentrations of HDL progressively enhanced expression of the activin-like kinase receptor (ALK)1 and ALK2 in human aortic endothelial cells as determined by real-time PCR and immunoblotting. Induction of ALK1 was a result of enhanced ALK2 expression as determined by siRNA interference, and was associated with increased levels of vascular endothelial growth factor (VEGF) and matrix Gla protein (MGP). The HDL-induction of ALK2 was dependent on BMP-signaling, and affected co-regulation of the ALK2 gene by the homeodomain proteins MSX2, DLX3 and DLX5, as determined by reporter gene assays, siRNA interference and chromatin immunoprecipitation. Apolipoprotein A-I transgenic mice, known to have high HDL and inhibition of atherogenesis, exhibited similar changes in aortic gene expression as seen in endothelial cells treated with HDL in vitro.
Conclusions
We conclude that HDL benefits the arterial wall by allowing for enhanced ALK1 and ALK2 signaling, resulting in an increase of VEGF and MGP, essential for endothelial cell survival and prevention of vascular calcification, respectively.
Keywords: High-density lipoproteins (HDL), bone morphogenetic proteins (BMP), BMP-receptors, endothelial cells, homeodomain proteins
INTRODUCTION
High-density lipoproteins (HDL) are well recognized to be antiatherogenic and to play a role in mediating cholesterol efflux from cells1, 2. In addition, HDL has multiple endothelial and antithrombotic actions that may add to its protective and anti-inflammatory effects, such as stimulation of endothelial NO synthase, maintenance of the lipid environment in caveolae, activation of prostacyclin synthesis and modulation of inflammatory cytokines1, 2.
Bone morphogenetic proteins (BMP)-2, -4 and -6 have been detected in atherosclerotic plaques3-5, and are believed to contribute to their formation. In endothelial cells (EC), BMP-2 and -4 have been identified as genes mediating the response to inflammation and oscillatory shear stress6, 7. Noggin and matrix Gla protein (MGP), both able to inhibit BMP, are also induced by oscillatory shear stress8 suggesting that the balance of BMP signaling is essential in the vascular endothelium. BMP-2 and -4 may contribute to the progression of vascular calcification due to their osteoinductive properties9. Indeed, BMP-2 has been shown to augment signaling through MSH homeodomain 2 (MSX2)-Wnt signaling in aortic myofibroblasts, thereby stimulating osteogenic differentiation and calcification10. In osteoblastic cells, MSX2 and distal-less homeodomains 3 and 5 (DLX3 and DLX5) have been shown to co-regulate genes that are essential in bone development, including the RUNX2 and the osteocalcin genes11.
The BMPs belong to the transforming growth factor (TGF)-ß superfamily of growth factors12. These growth factors elicit their responses via two types of serine/threonine receptors, termed type I and type II receptors. BMP binds to the type I receptor, and this complex then binds the type II receptor with increased affinity12. The receptor complex phosphorylates specific receptor-regulated (R)-SMAD proteins, which associate with a common partner (Co)-SMAD4 and translocate into the nucleus, where they regulate target gene expression12.
Activin-like kinase receptor (ALK)2, ALK3 and ALK6 are categorized as type I BMP receptors, which interact with the BMP type II receptor (BMPRII) to phosphorylate SMAD1/5/812. ALK1 is also a type I receptor, which resembles ALK2, but was initially reported to be a receptor for TGF-ß1 and -3. However, BMP-9 and -10 were recently identified as ligands, and ALK1 was shown to interact with BMPRII similar to other BMP receptors13, 14. We previously showed that ALK1 expression is dose-dependently regulated by BMP-2 and -4 in vascular cells15, 16, suggesting regulatory links between the BMP receptors. ALK1 also mediates stimulatory effects of BMP-4 on expression of the vascular endothelial growth factor (VEGF), essential for EC survival17, and MGP, an inhibitor of vascular calcification18. However, the relationship between the respective BMP receptors in atherogenesis is incompletely understood.
Although HDL has multiple endothelial effects, its effect on BMP signaling is not known. In this study, we demonstrate that HDL promotes expression of ALK2 in EC, allowing for induction of ALK1, VEGF and MGP. We show that HDL-induction of ALK2 is dependent on BMP-signaling, and affects co-regulation of the ALK2 gene by MSX2, a repressive homeodomain protein, and DLX3 and DLX5, activating homeodomain proteins11, 19. The importance of our in vitro findings was confirmed in apolipoprotein (apo) A-I transgenic mice with high HDL levels and resistance to atherogenesis20. These mice exhibited similar changes in aortic gene expression as seen in EC in vitro. Thus, HDL benefits the arterial wall by enhancing a response system for BMP that allows for increased VEGF, which is essential for EC survival17, and MGP, which prevents excess BMP-activity and vascular calcification18.
METHODS
Cell Culture and Transfection Assays
Human aortic EC (HAEC) and bovine aortic EC (BAEC) were obtained and cultured as previously described15. Recombinant human BMP-4 and Noggin (both from R&D Systems) were added as indicated in the results section. HDL was prepared as previously described21, and treatment with HDL was performed in M199 medium supplemented with 2% fetal bovine serum as previously described21. Transient transfections of HAEC with siRNA were performed as previously described15. Gene-specific SiRNAs (Silencer® Validated siRNA, Ambion) and scrambled siRNAs with the same nucleotide contents were used. Transient transfections of BAEC and luciferase assays were performed as previously described15.
Transgenic mice
Apolipoprotein (apo)-AI transgenic mice on C57BL/6 background20 (derived from founder line A2) were obtained from Jackson Labs.
Vector constructions
For construction and mutagenesis of the pGL2ALK2pro reporter gene, see supplemental data. The MSX2 expression construct was kindly provided by Dr. Dwight A. Towler, Washington University, St. Louis.
RNA analysis
Real-time PCR assays were performed as previously described15. The following primers and probes were used: human ALK1 (hALK1) forward (F) (5′-AGGGCAAACCAGCCATTG-3′), hALK1 reverse (R) (5′-GGTTGCTCTTGACCAGCACAT-3′), hALK1 Taqman probe (FAM-CACCGCGACTTCAAGAGCCGC-TAMRA. Primers and probes for other human and mice genes were obtained from Applied Biosystems.
Immunoblotting and Immunocytochemistry
Immunoblotting was performed as previously described15. Blots were incubated with antibodies to ALK1, ALK2, ALK3 or ALK6 (all 0.4 μg/ml; Santa Cruz Biotechnology), BMPRII (2 μg/ml; R&D Systems), SMAD1 or SMAD2 (both 1 μg/ml; Upstate Biotechnology), MSX2 (0.2 μg/ml; Abcam), DLX3 or DLX5 (0.2 μg/ml; Santa Cruz Biotechnology). ß-Actin (1:5000 dilution; Sigma) was used as loading control. Immunocytochemistry was performed as previously described15, with goat polyclonal antibodies to ALK1 and ALK2 from Santa Cruz Biotechnology.
Chromatin Immunoprecipitation (ChIP) Assays
A commercially available kit (USB Corporation) was used to perform ChIP assays in HAEC. The assays were performed as per the manufacturer’s protocol. Immunoprecipitation was performed using the same antibodies used for immunoblotting. PCR was performed using the following primers from the ALK2 promoter (surrounding MSX1.2 site at -2206): (F) 5′-CATGAAACAATCTCCCCAAA-3′, and (R) 5′-TTTCTGATGCACCTCAATG-3′ (94°C denaturation, 55°C annealing, 72°C extension, 35 cycles, 253 bp product).
Statistics
Data was analyzed for statistical significance by ANOVA with post-hoc Scheffe’s analysis, using StatView, version 4.51 (Abacus Concepts). Experiments were repeated a minimum of three times.
RESULTS
HDL induces expression of ALK1, ALK2, VEGF and MGP in EC
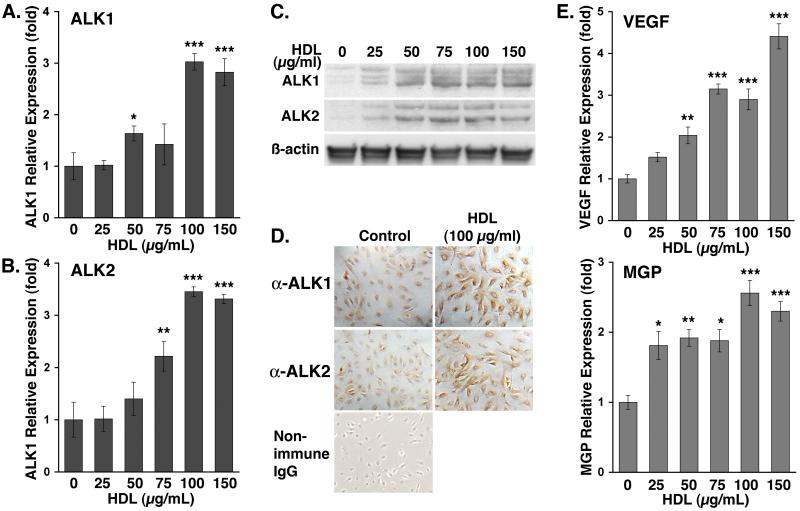
To determine the effect of HDL on the expression of components of BMP signaling pathways, we treated HAEC with HDL (0-150 μg/ml) for 24 hours. We analyzed changes in expression of BMP ligands and receptors. Our results showed that expression of ALK1 and ALK2 was significantly enhanced as determined by real-time PCR, immunoblotting and immunocytochemistry (Fig. 1A-D). Treatment with bovine serum albumin or LDL had no effect on expression of ALK1 and ALK2 (supplemental Fig. I), suggesting that the effect was specific for HDL. However, HDL had no significant effect on the expression of ALK3, ALK6, BMPRII, or BMP-2, -4, -6 and -7 (data not shown). Since our previous studies showed that activated ALK1 induced expression of VEGF and MGP15, we also determined the effect of HDL on VEGF and MGP. As expected, the results showed that HDL significantly increased both VEGF and MGP as determined by real-time PCR (Fig. 1E), suggesting a beneficial effect on the vascular wall.
Figure 1. HDL induces expression of ALK1 and ALK2 in HAEC.
HAEC were treated for 24 hours with increasing concentrations of HDL (0-150 μg/ml).
Expression of ALK1 and ALK2 was determined by real-time PCR (A, B), immunoblotting (C), and immunohistochemistry (D). Expression of VEGF and MGP was determined by real-time PCR (E).
Asterisks indicate statistically significant differences compared to control (no HDL). *, p<0.05; **, p<0.01; ***, p < 0.001; Scheffe’s test.
HDL-induction of ALK1 expression requires the ALK2 receptor
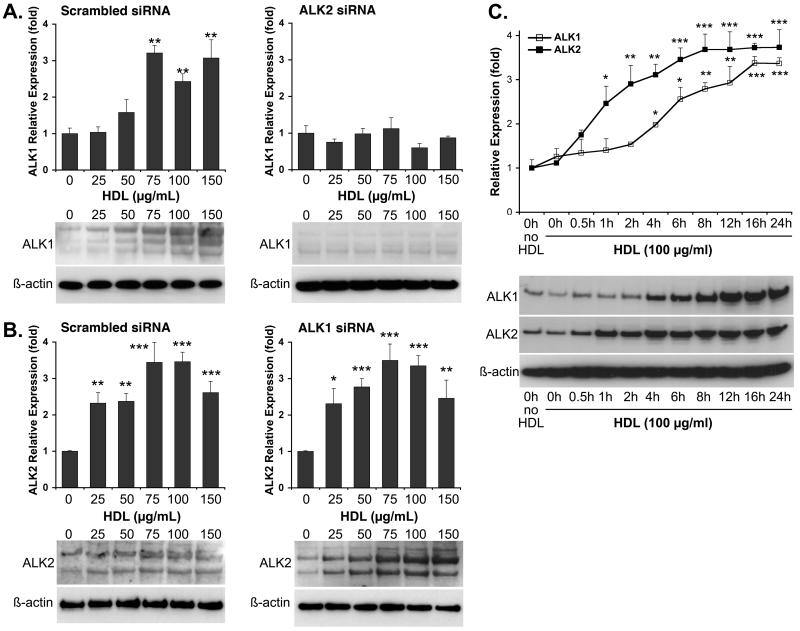
To determine whether the HDL-induction of ALK1 was dependent on ALK2 or vice versa, we transfected HAEC with either ALK1 or ALK2 siRNA, which reduced RNA and protein levels up to 95%15 (supplemental Fig. II). The cells were treated with HDL (0-150 μg/ml) for 24 hours, and expression of ALK1 and ALK2 was determined. The results showed that depletion of ALK2 blocked HDL-induction of ALK1 (Fig. 2A), whereas depletion of ALK1 did not affect HDL-induction of ALK2 (Fig. 2B), suggesting that HDL-induction of ALK1 depends on ALK2. We also performed a time course where HAEC were treated with HDL (100 μg/ml) for up to 24 hours. The results showed that ALK2 expression increased after one hour, whereas ALK1 expression increased after 4 hours as determined by real-time PCR and immunoblotting (Fig. 2C) supporting that ALK1 expression depends on ALK2.
Figure 2. HDL-induction of ALK1 is dependent on ALK2, but ALK2 is not dependent on ALK1.
HAEC were transfected with scrambled siRNA or siRNA to ALK2 (A) or ALK1 (B), and treated with HDL (0-150 μg/ml) for 24 hours starting the day after transfection. Expression of ALK1 and ALK2 was determined by real-time PCR and immunoblotting. (C) To determine the time course for expression, HAEC were treated for up to 24 hours with HDL (100 μg/ml). Expression of ALK1 and ALk2 was determined by real-time PCR and immunoblotting.
Asterisks indicate statistically significant differences compared to control (no HDL). *, p<0.05; **, p<0.01; ***, p < 0.001; Scheffe’s test.
We also determined if BMP receptors other than ALK2 and SMAD signaling were involved in HDL-induction of ALK1 expression by using siRNA to ALK3, ALK6, BMPRII, and SMAD1 or SMAD2, which mediate BMP and TGF-ß signaling, respectively12. The results showed that BMPRII and SMAD1, but not ALK3, ALK6 or SMAD2, were necessary for HDL-induction of ALK1 (supplemental Fig. III). We have previously shown that ALK1 is dose-dependently induced by BMP-2/4 in EC 15. Experiments using the same siRNA showed that BMP-4-induction of ALK1 also requires ALK2, BMPRII and SMAD1 (supplemental Fig. IV), which suggests that HDL and BMP-4 may use the same pathway to induce ALK2.
HDL and BMP-4 activate the ALK2 promoter
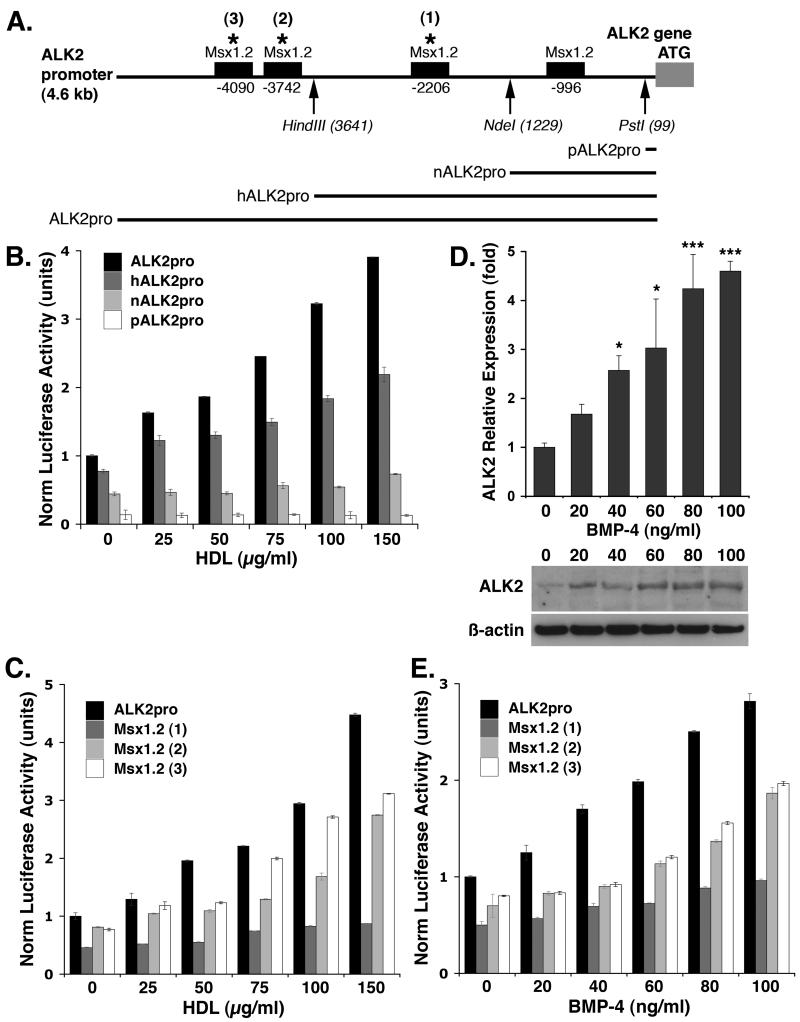
To determine if HDL affected the activity of the promoter of ALK2, a 4.6kb genomic DNA fragment from the promoter region of the human ALK2 gene (nucleotides -1 to -4625) was subcloned into a luciferase reporter gene. Three deletions were made in the ALK2 promoter, generating reporter genes with promoter fragments of 3.6, 1.2 and 0.1 kb (Fig. 3A). The resulting vectors were transfected into BAEC since HAEC transfected poorly with expression vectors, and the cells were treated with HDL (0-150 μg/ml). After 24 hours, the luciferase activity was determined. The results showed that the activity of the ALK2 promoter decreased progressively as the promoter was shortened (Fig. 3B).
Figure 3. HDL- and BMP-4-induction of ALK2 is dependent on the presence of MSX1.2 sites in the ALK2 promoter.
(A) Schematic representation of MSX1.2 sites in the ALK2 promoter. Sites of trunctions (arrows) and mutagenesis (stars 1, 2 and 3) of the ALK2 promoter are indicated.
(B) ALK2 promoter activity in response to HDL (0-150 μg/ml) using 4.6 kb (ALK2pro), 3.6 kb (hALK2pro), 1.2 kb (nALK2pro) and 0.1 kb (pALK2pro) promoter lengths, as determined by luciferase activity.
(C) ALK2 promoter activity in response to HDL (0-150 μg/ml) using ALK2 promoter constructs with mutated MSX1.2, site 1, 2 and 3, as determined by luciferase activity.
(D) ALK2 expression in HAEC in response to treatement with BMP-4 (0-100 ng/ml) for 24 hours as determined by real-time PCR and immunoblotting.
(E) ALK2 promoter activity in response to BMP-4 (0-100 ng/ml) using ALK2 promoter constructs with mutated MSX1.2, site 1, 2 and 3, as determined by luciferase activity.
Asterisks indicate statistically significant differences compared to control (no HDL). *, p<0.05; ***, p < 0.001; Scheffe’s test.
When analyzing the sequence of the ALK2 promoter, four MSX1.2 sites were detected within the 4.6 kb promoter fragment (Fig. 3A). MSX1 and MSX2 are well-known transcription factors targeted by BMP/SMAD signaling11, 19. The 3.6 and the 1.2 kb promoter fragments contained two and one MSX1.2 sites, respectively (Fig. 3A). The 0.1 kb fragment contained no MSX1.2 sites. We hypothesized that the MSX1.2 sites were essential for HDL-induction of ALK2, and mutated the sites marked 1-3 in figure 4A. We transfected BAEC with the resultant three reporter genes, treated with HDL (0-150 μg/ml), and determined the luciferase activity 24 hours later. The results showed that mutagenesis of any of the three MSX1.2 sites, in particular the site at -2206, reduced luciferase activity (Fig. 3C). For unclear reasons, we were unable to mutate and test the MSX1.2 site at -996. Mutation of a DLX5 site at -1099 had no effect on luciferase activity after HDL treatment (supplemental Fig. VA). Together, the results suggested that the MSX1.2 sites are essential for HDL-induction of ALK2.
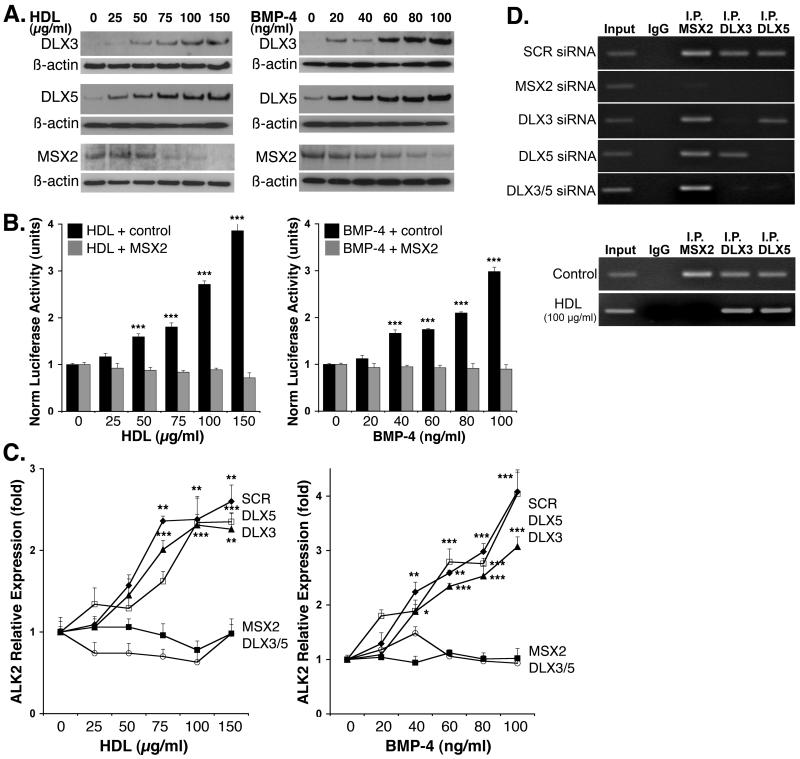
Figure 4. HDL and BMP-4 modulate expression of MSX2, DLX3 and DLX5.
(A) HAEC were treated with HDL (0-150 μg/ml) or BMP-4 (0-100 ng/ml) for 24 hours. Expression of MSX2, DLX3 and DLX5 was determined by immunoblotting of cell lysates.
(B) BAEC were co-transfected with the 4.6 kb ALK2 promoter reporter gene and increasing amounts of an MSX2-expression construct or empty vector. The cells were treated with HDL (0-150 μg/ml) or BMP-4 (0-100 ng/ml) and luciferase activity was determined after 24 hours.
(C) HAEC were transfected with scrambled siRNA or siRNA to MSX2, DLX3, DLX5, or DLX3 and DLX5, and treated with HDL (0-150 μg/ml) or BMP-4 (0-100 ng/ml) for 24 hours starting the day after transfection. Expression of ALK2 was determined by real-time PCR (and immunoblotting, see supplemental Fig. VI).
(D) ChIP assays at MSX1.2. site at -2206 were performed using untreated HAEC transfected with scrambled siRNA or siRNA to MSX2, DLX3, DLX5, or DLX3 and DLX5 (top) or HAEC that were either control-treated or treated with HDL (100 μg/ml) for 24 hours (bottom).
Asterisks indicate statistically significant differences compared to control (no HDL or BMP-4). *, p<0.05; ***, p < 0.001; Scheffe’s test.
Since BMP targets MSX1.2 sites, we tested if BMP-4 also induced ALK2 expression. HAEC were treated with BMP-4 (0-100 ng/ml) for 24 hours, and ALK2 expression was determined by real time PCR and immunoblotting. The results showed that BMP-4 increased ALK2 expression up to 4-5-fold (Fig. 3D). We also transfected BAEC with the mutated promoter constructs and treated the cells for 24 hours with BMP-4 (0-100 ng/ml), before determining the luciferase activity. As expected, the activity was diminished after mutation of MSX1.2 sites (Fig. 3E). BMP-4 concentrations above 100 ng/ml had no further stimulatory effect (supplemental Fig. VB). Thus, both HDL and BMP-4 are dependent on MSX1.2 sites for efficient induction of ALK2 expression.
HDL and BMP-4 affect expression of MSX2, DLX3 and DLX5, which co-regulate ALK2 expression
MSX1 and MSX2 are classical repressor proteins11, 19 that are expressed in endothelial cells22. A regulatory mechanism involving MSX2, DLX3 and DLX5 has been reported in the bone-related Runx2 and osteocalcin promoters, where MSX2 has a repressive function and DLX3 and DLX5 have an activating function11. To determine if HDL and BMP-4 affected the levels of MSX2, DLX3 and DLX5 in HAEC, we treated cells with HDL (0-150 μg/ml) or BMP-4 (0-100 ng/ml) for 24 hours and determined protein levels with immunoblotting. The results showed that both HDL and BMP-4 reduced MSX2 and increased DLX3 and DLX5 (Fig. 4A). To confirm that MSX2 was a repressor of ALK2 promoter activity, we co-transfected BAEC with the 4.6 kb ALK2 promoter reporter gene and an expression construct for MSX2 or control vector. The cells were treated with HDL (0-150 μg/ml) or BMP-4 (0-100 ng/ml) for 24 hours before luciferase activity was determined. The results showed that overexpression of MSX2 abolished the effect of HDL and BMP-4 on the ALK2 promoter (Fig. 4B).
To determine the importance of the homeodomain proteins for ALK2 expression, we transfected HAEC with siRNA to MSX2, DLX3, DLX5, or DLX3 and DLX5, which reduced RNA and protein levels up to 95% (supplemental Fig. II). The cells were treated with HDL (0-150 μg/ml) or BMP-4 (0-100 ng/ml) for 24 hours and expression of ALK2 was determined. The results showed that depletion of either MSX2 or DLX3 and DLX5 blocked HDL and BMP-4 induction of ALK2 as determined by real-time PCR (Fig. 4C) and immunoblotting (supplemental Fig. VI). Depeletion of just one DLX did not inhibit ALK2 expression. We then performed ChIP assays using untreated HAEC transfected with the respective siRNA and primers surrounding the MSX1.2 site at -2206. Loss of this site significantly reduced stimulatory effects of HDL (Fig. 3C,E). The results showed that MSX2, DLX3 and DLX5 all bound to this site, but that the binding of DLX3 and DLX5 was dependent on MSX2 (Fig. 4D, top). We then compared control- and HDL-treated HAEC. The results showed that HDL caused release of MSX2, allowing DLX3 and DLX5 to bind without MSX2 (Fig. 4D, bottom). This suggests that the MSX1.2 site represent a DLX-binding cognate that is activated by DLX and suppressed by MSX2 in a similar fashion to what has been described in great detail for the Runx2 promoter when responding to BMP-211.
The HDL effect on ALK2 expression is BMP-dependent and is affected by changes in cellular cholesterol
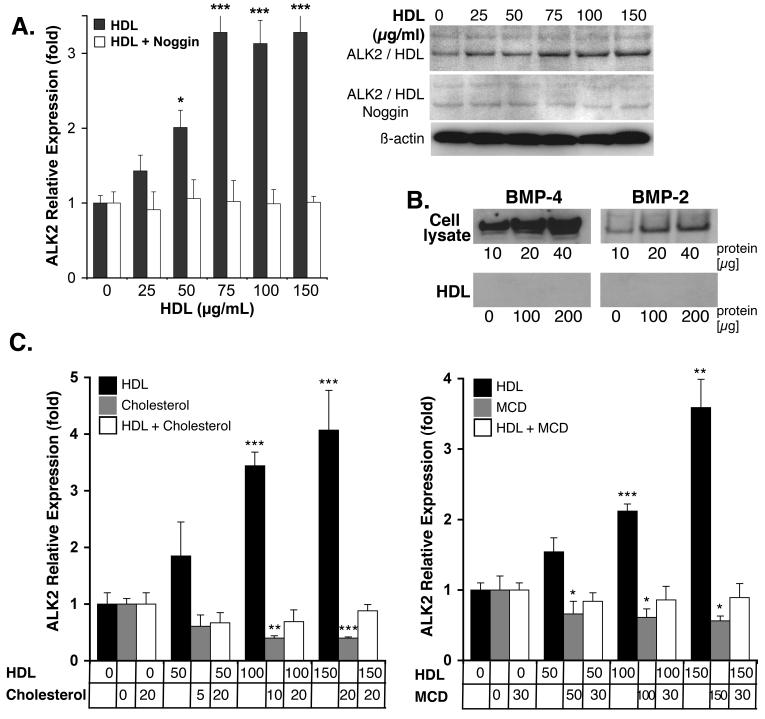
Since the HDL effect on ALK2 is similar to that of BMP-4, we hypothesized that HDL may act through BMP to induce ALK2. To test this, we treated HAEC with HDL (0-150 μg/ml) in presence of absence of Noggin (300 ng/ml), a BMP-inhibitor. After 24 hours of treatment, expression of ALK2 was determined by real-time PCR and immunoblotting. The results showed that HDL-induced ALK2 expression was inhibited by Noggin (Fig 5A), suggesting that the HDL enhanced BMP-signaling. Since no exogenous BMP was added, the change in BMP signaling was presumably due to a change in the response to endogenously expressed BMP-2 or -4 (Fig. 5B), no BMP was detected in HDL (Fig. 5B).
Figure 5. Noggin and changes in cellular cholesterol neutralize the effect of HDL on ALK2 expression.
(A) HAEC were treated for 24 hours with increasing concentrations of HDL (0-150 μg/ml) in absence or presence of Noggin (300 ng/ml), and ALK2 expression was determined by real-time PCR and immunoblotting.
(B) BMP-2 and -4 in EC lysates and HDL as determined by immunoblotting.
(C) HAEC were treated with HDL (0-150 μg/ml), cholesterol (0-20 μg/ml), or HDL (0-150 μg/ml) with cholesterol (20 μg/ml). ALK2 expression was determined by real-time PCR (and immunoblotting, see supplemental Fig. VII).
(D) HAEC were treated with HDL (0-150 μg/ml), methyl-ß-cyclodextrin (MCD) (0-30 mM), or HDL (0-150 μg/ml) with MCD (0-30 mM). ALK2 expression was determined by real-time PCR (and immunoblotting, see supplemental Fig. VII).
Asterisks indicate statistically significant differences compared to control (no HDL). *, p<0.05; ***, p < 0.001; Scheffe’s test.
Since HDL has been shown to maintain the lipid environment in caveolae1, 2, we hypothesized the cholesterol balance might affect BMP signaling. To determine if cellular cholesterol affected HDL-induction of ALK2, HAEC were loaded with cholesterol (0-20 μg/ml) or treated with methyl-ß-cyclodextrin (MCD, 0-30 mM), a cholesterol binder, prior to HDL treatment. The cells were subsequently treated with HDL (0-150 μg/ml) for 24 hours prior to determination of ALK2 expression with real time PCR and immunoblotting. The results showed that both cholesterol loading and MCD significantly decreased ALK2 expression, and abolished induction of ALK2 when combined with HDL treatment (Fig.5C, real-time PCR, supplemental Fig. VII, immunoblotting). However, MCD had the same effect (Fig. 5C, supplemental Fig. VII). It is still possible that the effect of HDL is related to the removal of cellular cholesterol, if the manner in which HDL removes cholesterol is of importance. Alternatively, HDL affects ALK2 through a mechanism unrelated to cholesterol.
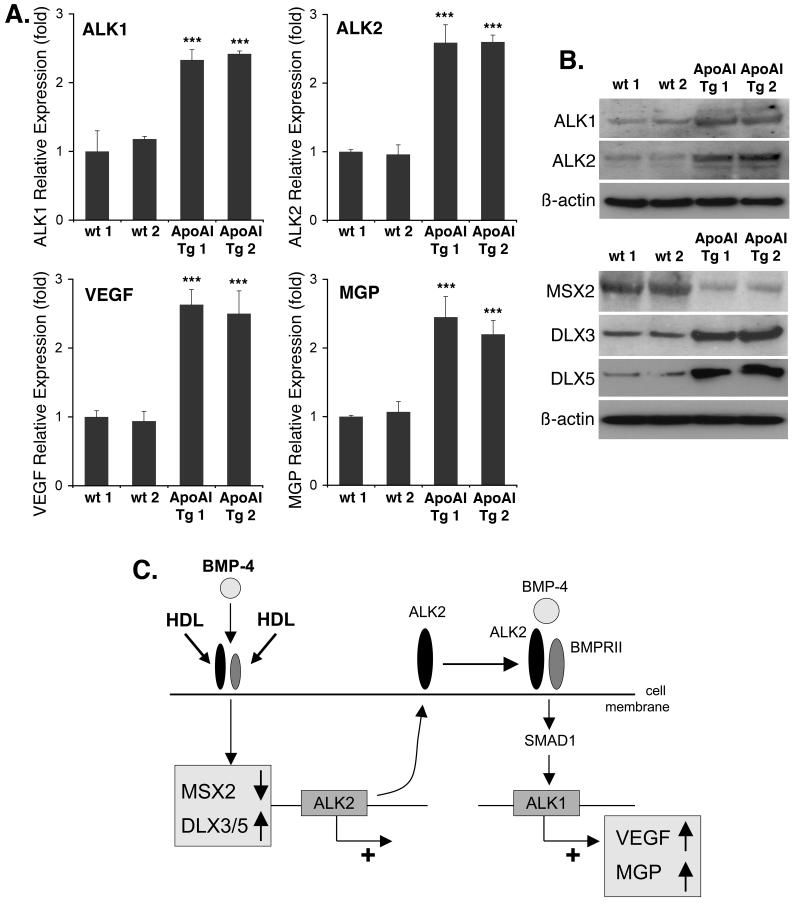
High HDL in vivo enhances aortic expression of ALK1, ALK2, VEGF and MGP
To confirm the effect of HDL on gene expression in vivo, we prepared RNA and protein from aortas of apo A-I transgenic mice, known to have a 2-fold increase in HDL compared to control mice20. The results showed a 2-3-fold increase in expression of ALK1, ALK2, VEGF and MGP as determined by real-time PCR (Fig. 6A). Immunoblotting confirmed the increased in ALK1 and ALK2, and also showed a decrease in MSX2 and an increase in DLX3 and DLX5 (Fig. 6B). Together the results support that high HDL affects BMP-signaling in vivo and benefits the vascular wall.
Figure 6. Aortic gene expression in apo A-I transgenic mice with high HDL is similar in to that in HAEC treated with HDL.
(A) Aortic expression in wild type (wt) and apo A-I transgenic (tg) mice of ALK1, ALk2, VEGF and MGP as determined by real-time PCR.
(B) Aortic expression in wt and apo A-I tg mice of ALK1, ALk2, MSX2, DLX3 and DLX5 as determined by immunoblotting.
(C) Schematic working model of induction of ALK1 and ALK2 by HDL and BMP-4.
Asterisks indicate statistically significant differences compared to control (no HDL). ***, p < 0.001; Scheffe’s test.
DISCUSSION
In this study, we demonstrate that HDL enhances the BMP-signaling system in EC by promoting the expression of ALK2, which together with BMPRII, SMAD1 and endogenous BMP allows for induction of ALK1. The HDL-induction of ALK2 expression is dependent on BMP-signaling and intact MSX1.2 sites in the ALK2 promoter. We show that HDL and BMP-4 modulate the levels of MSX2, DLX3 and DLX5 in HAEC, and promote DLX-activation of the ALK2 gene similarly to the Runx2 gene responding to BMP-211. The increase in ALK1 is associated with enhanced expression of VEGF, essential for EC survival 17, and MGP, which effectively prevents excess BMP-activity and vascular calcification18. The results were confirmed in vivo, and provide evidence for a novel role for HDL in benefiting the vascular wall (see Fig. 6C for schematic working model).
Cellular cholesterol or lipid content has previously been implicated in BMP receptor function. For instance, Hartung et al. 23 showed that localization of BMP receptors in distinct membrane domains was a prerequisite for taking different endocytosis routes with specific signaling cascades. Although HDL did not affect TGF-ß signaling through ALK5 in our experiments (data not shown), HDL and hypercholesterolemia have previously been shown to affect TGF-ß and TGF-ß signaling. HDL and hypercholesterolemia, respectively, induced endothelial expression of TGF-ß2 and endoglin, a so-called TGF-ß type III receptor 24, 25, and cholesterol suppressed cellular TGF-ß responsiveness by altering TGF-ß binding to TGF-ß receptors 26. Since our studies showed that both cholesterol loading and cholesterol depletion by MCD abolished the HDL effect, it is possible that the effect of HDL is specific for the manner in which cholesterol depletion occurs or is due to factors unrelated to cholesterol.
Al-Aly et al.10 showed in their recent studies that BMP-2 and MSX2 were induced by TNF-alpha, an inflammatory cytokine that is upregulated by high fat diet in LDL null mice, and that triggering of the BMP-2-MSX2 program led to increased vascular calcification. In osteoblastic cells, MSX2 is known to be part of a BMP-dependent molecular switch that regulates the osteocalcin and Runx2 genes, and also involves DLX3 and DLX511. MSX2 acts as a suppressor of gene expression, whereas DLX3 and DLX5 act as activating factors. Our results from ChIP assays support this model, in that HDL-activation of ALK2 expression released MSX2 from the promoter allowing DLX3 and DLX5 to bind and drive transcription suggesting that the MSX1.2 sites are DLX-binding cognates. The results prompt the question whether HDL and cholesterol levels have an effect on bone formation and osteoporosis. Although early studies suggested that so-called statins may exert an anabolic effect on bone, and conversely, that hypercholesterolemia may have an adverse effect on osteoporotic bone loss 27, recent clinical studies have not shown significant effects of statins on bone mineral density 28-30. Thus, the connections between lipid status and bone health remain unclear.
Although ALK2 upregulates ALK1, gene deletion produces different phenotypes and their respective target genes are presumably different. ALK1 deficiency is associated with hereditary hemorrhagic telangiectasia (HHT) and formation of arterio-venous malformations13, 14, whereas ALK2 affects the development of the cardiac outflow tract and aortic arch derivatives31. Overall, the roles of the BMP-receptors are unclear in vascular inflammation and atherogenesis. ALK1 expression is induced in atherosclerotic lesions and may affect proliferation and aggregation of vascular cells16. Based on our data, a high level of ALK2 relative to ALK3 and ALK6 may be beneficial and alter inflammatory effects of BMP-2/4 in the endothelium6, 7. However, further studies are needed to identify an optimal ratio between various BMP receptors in the endothelium.
Although HDL and BMP-4 are dependent on the same signaling pathway to induce expression of ALK1, the patterns of induction appear to be different. BMP-4-induction of ALK1 is dose-dependent with a narrow range of optimal concentrations15 (supplemental Fig. IV), whereas HDL-induction increases with dose up to a plateau level (supplemental Fig. III). Since BMP-4 and HDL affects the levels of MSX2, DLX3 and DlX5 similarly, we presume that other mechanisms or feedback loops are involved in determining the exact pattern of ALK1 expression.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING
This work was funded in part by the International HDL Awards Program (Pfizer), NIH grants HL30568 and HL81397, and the American Heart Association (Western Affiliate).
Footnotes
DISCLOSURES
None.
Publisher's Disclaimer: This is an un-copyedited author manuscript that was accepted for publication in Arteriosclerosis, Thrombosis, and Vascular Biology, copyright The American Heart Association. This may not be duplicated or reproduced, other than for personal use or within the “Fair Use of Copyrighted Materials” (section 107, title 17, U.S. Code) without prior permission of the copyright owner, The American Heart Association. The final copyedited article, which is the version of record, can be found at Arteriosclerosis, Thrombosis, and Vascular Biology. The American Heart Association disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties.
REFERENCES
- 1.Mineo C, Deguchi H, Griffin JH, Shaul PW. Endothelial and antithrombotic actions of HDL. Circ Res. 2006;98:1352–1364. doi: 10.1161/01.RES.0000225982.01988.93. [DOI] [PubMed] [Google Scholar]
- 2.Norata GD, Catapano AL. Molecular mechanisms responsible for the antiinflammatory and protective effect of HDL on the endothelium. Vasc Health Risk Manag. 2005;1:119–129. doi: 10.2147/vhrm.1.2.119.64083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bostrom K, Watson KE, Horn S, Wortham C, Herman IM, Demer LL. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest. 1993;91:1800–1809. doi: 10.1172/JCI116391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhore CR, Cleutjens JP, Lutgens E, Cleutjens KB, Geusens PP, Kitslaar PJ, Tordoir JH, Spronk HM, Vermeer C, Daemen MJ. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001;21:1998–2003. doi: 10.1161/hq1201.100229. [DOI] [PubMed] [Google Scholar]
- 5.Schluesener HJ, Meyermann R. Immunolocalization of BMP-6, a novel TGF-beta-related cytokine, in normal and atherosclerotic smooth muscle cells. Atherosclerosis. 1995;113:153–156. doi: 10.1016/0021-9150(94)05438-o. [DOI] [PubMed] [Google Scholar]
- 6.Sorescu GP, Sykes M, Weiss D, Platt MO, Saha A, Hwang J, Boyd N, Boo YC, Vega JD, Taylor WR, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress stimulates an inflammatory response. J Biol Chem. 2003;278:31128–31135. doi: 10.1074/jbc.M300703200. [DOI] [PubMed] [Google Scholar]
- 7.Csiszar A, Ahmad M, Smith KE, Labinskyy N, Gao Q, Kaley G, Edwards JG, Wolin MS, Ungvari Z. Bone morphogenetic protein-2 induces proinflammatory endothelial phenotype. Am J Pathol. 2006;168:629–638. doi: 10.2353/ajpath.2006.050284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang K, Weiss D, Suo J, Vega JD, Giddens D, Taylor WR, Jo H. Bone morphogenic protein antagonists are coexpressed with bone morphogenic protein 4 in endothelial cells exposed to unstable flow in vitro in mouse aortas and in human coronary arteries: role of bone morphogenic protein antagonists in inflammation and atherosclerosis. Circulation. 2007;116:1258–1266. doi: 10.1161/CIRCULATIONAHA.106.683227. [DOI] [PubMed] [Google Scholar]
- 9.Hruska KA, Mathew S, Saab G. Bone morphogenetic proteins in vascular calcification. Circ Res. 2005;97:105–114. doi: 10.1161/01.RES.00000175571.53833.6c. [DOI] [PubMed] [Google Scholar]
- 10.Al-Aly Z, Shao JS, Lai CF, Huang E, Cai J, Behrmann A, Cheng SL, Towler DA. Aortic Msx2-Wnt calcification cascade is regulated by TNF-alpha-dependent signals in diabetic Ldlr-/- mice. Arterioscler Thromb Vasc Biol. 2007;27:2589–2596. doi: 10.1161/ATVBAHA.107.153668. [DOI] [PubMed] [Google Scholar]
- 11.Hassan MQ, Tare RS, Lee SH, Mandeville M, Morasso MI, Javed A, van Wijnen AJ, Stein JL, Stein GS, Lian JB. BMP2 commitment to the osteogenic lineage involves activation of Runx2 by DLX3 and a homeodomain transcriptional network. J Biol Chem. 2006;281:40515–40526. doi: 10.1074/jbc.M604508200. [DOI] [PubMed] [Google Scholar]
- 12.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 13.David L, Mallet C, Mazerbourg S, Feige JJ, Bailly S. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood. 2007;109:1953–1961. doi: 10.1182/blood-2006-07-034124. [DOI] [PubMed] [Google Scholar]
- 14.Scharpfenecker M, van Dinther M, Liu Z, van Bezooijen RL, Zhao Q, Pukac L, Lowik CW, ten Dijke P. BMP-9 signals via ALK1 and inhibits bFGF-induced endothelial cell proliferation and VEGF-stimulated angiogenesis. J Cell Sci. 2007;120:964–972. doi: 10.1242/jcs.002949. [DOI] [PubMed] [Google Scholar]
- 15.Yao Y, Zebboudj AF, Shao E, Perez M, Bostrom K. Regulation of bone morphogenetic protein-4 by matrix GLA protein in vascular endothelial cells involves activin-like kinase receptor 1. J Biol Chem. 2006;281:33921–33930. doi: 10.1074/jbc.M604239200. [DOI] [PubMed] [Google Scholar]
- 16.Yao Y, Zebboudj AF, Torres A, Shao E, Bostrom K. Activin-like kinase receptor 1 (ALK1) in atherosclerotic lesions and vascular mesenchymal cells. Cardiovasc Res. 2007;74:279–289. doi: 10.1016/j.cardiores.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 17.Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao Y, Shahbazian A, Bostrom KI. Proline and gamma-carboxylated glutamate residues in matrix Gla protein are critical for binding of bone morphogenetic protein-4. Circ Res. 2008;102:1065–1074. doi: 10.1161/CIRCRESAHA.107.166124. [DOI] [PubMed] [Google Scholar]
- 19.Cohen MM., Jr. The new bone biology: pathologic, molecular, and clinical correlates. Am J Med Genet A. 2006;140:2646–2706. doi: 10.1002/ajmg.a.31368. [DOI] [PubMed] [Google Scholar]
- 20.Rubin EM, Krauss RM, Spangler EA, Verstuyft JG, Clift SM. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature. 1991;353:265–267. doi: 10.1038/353265a0. [DOI] [PubMed] [Google Scholar]
- 21.Gharavi NM, Gargalovic PS, Chang I, Araujo JA, Clark MJ, Szeto WL, Watson AD, Lusis AJ, Berliner JA. High-density lipoprotein modulates oxidized phospholipid signaling in human endothelial cells from proinflammatory to anti-inflammatory. Arterioscler Thromb Vasc Biol. 2007;27:1346–1353. doi: 10.1161/ATVBAHA.107.141283. [DOI] [PubMed] [Google Scholar]
- 22.Murthi P, Hiden U, Rajaraman G, Liu H, Borg AJ, Coombes F, Desoye G, Brennecke SP, Kalionis B. Novel Homeobox Genes are Differentially Expressed in Placental Microvascular Endothelial Cells Compared with Macrovascular Cells. Placenta. 2008 doi: 10.1016/j.placenta.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Hartung A, Bitton-Worms K, Rechtman MM, Wenzel V, Boergermann JH, Hassel S, Henis YI, Knaus P. Different routes of bone morphogenic protein (BMP) receptor endocytosis influence BMP signaling. Mol Cell Biol. 2006;26:7791–7805. doi: 10.1128/MCB.00022-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norata GD, Callegari E, Marchesi M, Chiesa G, Eriksson P, Catapano AL. High-density lipoproteins induce transforming growth factor-beta2 expression in endothelial cells. Circulation. 2005;111:2805–2811. doi: 10.1161/CIRCULATIONAHA.104.472886. [DOI] [PubMed] [Google Scholar]
- 25.Nachtigal P, Pospisilova N, Jamborova G, Pospechova K, Solichova D, Andrys C, Zdansky P, Semecky V. Endothelial expression of endoglin in normocholesterolemic and hypercholesterolemic C57BL/6J mice before and after atorvastatin treatment. Can J Physiol Pharmacol. 2007;85:767–773. doi: 10.1139/y07-068. [DOI] [PubMed] [Google Scholar]
- 26.Chen CL, Huang SS, Huang JS. Cholesterol modulates cellular TGF-beta responsiveness by altering TGF-beta binding to TGF-beta receptors. J Cell Physiol. 2008;215:223–233. doi: 10.1002/jcp.21303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McFarlane SI, Muniyappa R, Shin JJ, Bahtiyar G, Sowers JR. Osteoporosis and cardiovascular disease: brittle bones and boned arteries, is there a link? Endocrine. 2004;23:1–10. doi: 10.1385/ENDO:23:1:01. [DOI] [PubMed] [Google Scholar]
- 28.Solomon DH, Avorn J, Canning CF, Wang PS. Lipid levels and bone mineral density. Am J Med. 2005;118:1414. doi: 10.1016/j.amjmed.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 29.Bone HG, Kiel DP, Lindsay RS, Lewiecki EM, Bolognese MA, Leary ET, Lowe W, McClung MR. Effects of atorvastatin on bone in postmenopausal women with dyslipidemia: a double-blind, placebo-controlled, dose-ranging trial. J Clin Endocrinol Metab. 2007;92:4671–4677. doi: 10.1210/jc.2006-1909. [DOI] [PubMed] [Google Scholar]
- 30.Tekin GO, Kekilli E, Yagmur J, Uckan A, Yagmur C, Aksoy Y, Turhan H, Yetkin E. Evaluation of cardiovascular risk factors and bone mineral density in post menopausal women undergoing coronary angiography. Int J Cardiol. 2008 doi: 10.1016/j.ijcard.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Kaartinen V, Dudas M, Nagy A, Sridurongrit S, Lu MM, Epstein JA. Cardiac outflow tract defects in mice lacking ALK2 in neural crest cells. Development. 2004;131:3481–3490. doi: 10.1242/dev.01214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.