Summary
To facilitate the discovery of new therapeutics for Burkholderia pseudomallei infections, we have developed cellular reporter screens for inhibitors of B. pseudomallei targets in the surrogate host Pseudomonas aeruginosa. P. aeruginosa strains carrying deletions of essential genes were engineered to be dependent on the IPTG-regulated expression of their B. pseudomallei orthologs on a broad-host-range plasmid. P. aeruginosa genes which are upregulated in response to depletion of each target gene product were fused to the Photorhabdus luminescens luxCDABE operon via pGSV3-lux-SpR to generate reporter strains with increased bioluminescence upon target inhibition. A total of 11 of 19 B. pseudomallei genes complemented deletions of their orthologs in P. aeruginosa. The dependence of growth on IPTG levels varied from complete dependence (ftsQ, gyrA, glmU, secA), to slower growth in the absence of IPTG (coaD, efp, mesJ), to apparently normal growth in the absence of IPTG (ligA, lpxA, folA, ipk). Reporter screening strains have been constructed for three gene targets (gyrA, glmU, secA), and one (gyrA) has been applied to 68,000 compounds resulting in a primary hit rate of 0.5% and a confirmed hit rate of 0.06% including several fluoroquinolones. These results provide proof of principle for surrogate cellular reporter screens as a useful approach to identify inhibitors of essential gene products.
Keywords: bioluminescence, reporter, screen
Introduction
Burkholderia pseudomallei is a Gram-negative bacterium that causes melioidosis in humans and animals. Transmission to humans occurs through skin abrasion, ingestion, and inhalation. The disease often presents as a serious pneumonia or septicemic infection, and lethality is typically as high as 40%, even with the use of third-generation cephalosporins (Currie et al., 2000; White et al., 1989).
Current therapies are based on existing drugs which happen to have a broad enough spectrum to show some efficacy against B. pseudomallei. Carbapenems, third generation cephalosporins, chloramphenicol, and tetracyclines are usually effective, but the best current therapies utilize combinations of antibiotics such as ceftazidime-cotrimoxazole or amoxicillin-clavulanate (Brett and Woods, 2000). Clinical isolates of B. pseudomallei are consistently resistant to a wide variety of antibiotics (Gilligan and Whittier, 1999; Jenney et al., 2001; Piliouras et al., 2002; Vorachit et al., 2000; Wiedemann and Grimm, 1991). The bacterium is well-armed with drug-resistance mechanisms, including a nearly impermeable outer membrane (Burtnick and Woods, 1999) and the action of several broad-acting efflux pumps (Chan et al., 2004; Moore et al., 1999). The most effective antibiotics in use today for B. pseudomallei target only cell wall biosynthesis, protein synthesis, and co-factor biosynthesis.
In order to facilitate the discovery of new classes of anti-B. pseudomallei agents, we have developed a platform approach for screening B. pseudomallei targets directly for inhibitors by using whole cell reporter screens in the surrogate host P. aeruginosa (Moir et al., 2007). These screens depend upon transcription and translation of a reporter as an indirect measure of target inhibition based on the response of the cellular regulatory network to depletion of the target. Suitable reporter promoters were discovered by expression profiling of engineered P. aeruginosa strains grown under conditions which are limiting for the B. pseudomallei target gene expression. Appropriate promoters were fused to the P. luminescens luxCDABE operon to provide a luminescence report without the need to permeabilize cells and add a substrate. In vivo reporter screens of this type offer substantial benefits including, for example (a) preselection for permeable compounds, (b) ability to monitor multiple metabolic steps simultaneously (e.g., pathway screens), (c) sensitivity (e.g., often superior to assays that simply detect growth inhibition), (d) applicability to biochemically intractable targets (e.g., those with no known function or functions that are difficult to assay), as well as (e) a safer approach to high throughput, cell-based screening of targets from BSL-3 organisms.
As a proof of principle, we describe here the development of P. aeruginosa bioluminescent reporter screening strains for inhibitors of B. pseudomallei gyrA, glmU, and secA gene products. In addition, we provide details on the implementation of a high throughput screen for B. pseudomallei gyrase inhibitors.
Experimental/Materials and methods
Strains, plasmids, and growth media
P. aeruginosa strains and plasmids are described in Table 1. E. coli TOP10 (Invitrogen®), E. coli DB3.1 (Gateway® host, Invitrogen®), E. coli SM10 (de Lorenzo and Timmis, 1994), and E. coli S17-1 (ATCC 47055) were used as hosts for molecular cloning. VBMMG is VBMM medium (Vogel and Bonner, 1956) containing 0.3% trisodium citrate and 30 μg/ml gentamicin. Luria-Bertani (LB) medium (liquid and agar) was purchased from Difco. LB was supplemented with 10 μg/ml gentamicin (LBG) or both 10 μg/ml gentamicin and 200 μg/ml spectinomycin (LBGS) and various concentrations of isopropyl-β-D-thiogalactopyranoside (IPTG) as indicated. Opaque, white, flat-bottom, 96-well microplates (Nunc Cat. No. 236108; VWR International) were covered with gas permeable adhesive seals (Abgene, Inc., Cat. No. AB-0718) for reporter screens. RNA Protect Bacteria Reagent was purchased from Qiagen, Inc.
TABLE 1.
Strains and Plasmids
| Straina or Plasmid | Relevant Genotypeb | Source |
|---|---|---|
| P. aeruginosa strains: | ||
| PAO-LAC | Wild type strain PAO1 with lacIQ lacZ′ inserted at attB in chromosome via mini-CTX-lacM15 | (Hoang et al., 2000) |
| Complemented P. aeruginosa deletions: | ||
| MDM271 | PAO-LAC Δ gyrA::TcR / pUCP24GW-lacPO-Pae-gyrA | This study |
| MDM275 | PAO-LAC Δ coaD::TcR / pUCP24GW-lacPO-Bpseu-coaD | This study |
| MDM339 | PAO-LAC Δ ftsQ::TcR / pUCP24GW-lacPOBpseu-ftsQ | This study |
| MDM349 | PAO-LAC Δ gyrA::TcR / pUCP24GW-lacPO-Bpseu-gyrA | This study |
| MDM359 | PAO-LAC Δ lpxA::TcR / pUCP24GW-lacPO-Bpseu-lpxA | This study |
| MDM363 | PAO-LAC Δ mesJ::TcR / pUCP24GW-lacIQ-lacPO-Bpseu-mesJ | This study |
| MDM498 | PAO-LAC Δ efp::TcR / pUCP24GW-lacIQ-lacPO-Bpseu-efp | This study |
| MDM502 | PAO-LAC Δ glmU::TcR / pUCP24GW-lacIQ-lacPO-Bpseu-glmU | This study |
| MDM504 | PAO-LAC Δ ligA::TcR / pUCP24GW-lacIQ-lacPO-Bpseu-ligA | This study |
| MDM510 | PAO-LAC Δ secA::TcR / pUCP24GW-lacIQ-lacPO-Bpseu-secA | This study |
| MDM882 | PAO-LAC Δ folA::TcR / pUCP24GW-lacPO-Bpseu-folA | This study |
| MDM949 | PAO-LAC Δ ipk::TcR / pUCP24GW-lacIQ-lacPO-Bpseu-ipk | This study |
| MDM366 | PAO-LAC Δ dnaA::TcR / pUCP24GW-lacPO-Bpseu-dnaA | This study |
| MDM924 | PAO-LAC Δ ftsA::TcR / pUCP24GW-lacIQ-lacPO-Bpseu-ftsA | This study |
| Reporter strains and complemented P. aeruginosa deletion reporter strains: | ||
| MDM977 | PAO-LAC Δ gyrA::TcR::pGSV3-‘PA0614’-luxCDABE / pUCP24GW-lacPO-Pae-gyrA | This study |
| MDM981 | PAO-LAC Δ gyrA::TcR::pGSV3-‘PA0614’-luxCDABE / pUCP24GW-lacPO-Bpseu-gyrA | This study |
| MDM1141 | PAO-LAC Δ coaD::TcR::pGSV3-‘PA4607’-luxCDABE / pUCP24GW-lacPO-Bpseu-coaD | This study |
| MDM1142 | PAO-LAC Δ glmU::TcR::pGSV3-‘PA1494’-luxCDABE / pUCP24GW-lacIQ-lacPO-Bpseu-glmU | This study |
| MDM1143 | PAO-LAC Δ secA::TcR::pGSV3-‘PA2403’-luxCDABE / pUCP24GW-lacIQ-lacPO-Bpseu-secA | This study |
| MDM1144 | PAO-LAC Δ secA::TcR::pGSV3-‘PA2408’-luxCDABE / pUCP24GW-lacIQ-lacPO-Bpseu-secA | This study |
| MDM1145 | PAO-LAC Δ secA::TcR::pGSV3-‘PA1365’-luxCDABE / pUCP24GW-lacIQ-lacPO-Bpseu-secA | This study |
| MDM1164 | PAO-LAC Δ glmU::TcR::pGSV3-‘PA1471’-luxCDABE / pUCP24GW-lacIQ-lacPO-Bpseu-glmU | This study |
| MDM1166 | PAO-LAC Δ glmU::TcR::pGSV3-‘PA2358’-luxCDABE / pUCP24GW-lacIQ-lacPO-Bpseu-glmU | This study |
| MDM1167 | PAO-LAC Δ secA::TcR::pGSV3-‘PA2404’-luxCDABE / pUCP24GW-lacIQ-lacPO-Bpseu-secA | This study |
| MDM1168 | PAO-LAC Δ coaD::TcR::pGSV3-‘PA2030’-luxCDABE / pUCP24GW-lacIQ-lacPO-Bpseu-coaD | This study |
| MDM1169 | PAO-LAC Δ secA::TcR::pGSV3-‘PA1494’-luxCDABE / pUCP24GW-lacIQ-lacPO-Bpseu-secA | This study |
| MDM1170 | PAO-LAC Δ glmU::TcR::pGSV3-‘PA1365’-luxCDABE / pUCP24GW-lacIQ-lacPO-Bpseu-glmU | This study |
| MDM1184 | PAO-LAC Δ secA::TcR::pGSV3-‘PA1471’-luxCDABE / pUCP24GW-lacIQ-lacPO-Bpseu-secA | This study |
| Plasmids: | ||
| pEX18ApGW | pEX18Ap modified with Invitrogen Gateway Vector Conversion kit | (Choi and Schweizer, 2005) |
| pUCP24GW | pUCP24 modified with Invitrogen Gateway Vector Conversion Kit | (Moir et al., 2007) |
| pUCP24GW-lacIQ | pUCP24GW modified by addition of lacIQ at BspHI site | (Moir et al., 2007) |
| pGSV3-lux-SpR | pGSV3-lux modified by replacement of GmR with SpR element at SacI sites | This study |
Commonly used E. coli hosts and cloning vectors are described and referenced in Materials and Methods
ApR, SpR, GmR, and TcR: ampicillin, spectinomycin, gentamicin, and tetracycline resistance, respectively.
PCR and Primers
Synthetic oligonucleotide primers (from Operon, Inc.) were designed using the published genome sequences for P. aeruginosa (Stover et al., 2000) and B. pseudomallei (Holden et al., 2004) and web-based PRIMER3 (Whitehead Institute)(see electronic Supplementary Information for a list of primer sequences). Primers were used at 10 μM in PCR amplifications with Failsafe polymerase (Epicentre®), Buffer G (Epicentre®), and 4% DMSO for P. aeruginosa and B. pseudomallei chromosomal DNA templates.
Generation of complemented deletions of essential genes
Knock-out pEX18ApGW vectors were built for 19 P. aeruginosa loci by means of splicing by overlap extension (SOE) PCR and used successfully to create merodiploids which were resolved in the presence of a complementing gene copy on the extrachromosomal plasmid pUCP24GW (with or without the lacIQ gene) as described previously (Moir et al., 2007). The pEX18ApGW vectors carried a TcR element flanked by about 1 kb of homology on both sides of the locus to be deleted. Presumed deletions were confirmed by PCR with flanking primers outside the regions cloned into pEX18ApGW.
Expression profiling with microarrays
Complemented deletion strains were grown overnight in 5 ml of LBG with 1 mM IPTG and diluted 100-fold into 7 ml VBMMG with (≥0.1 mM) or without (≤0.025 mM) IPTG. Cells were harvested by centrifugation for 5 min at 5,000 x g about 120 minutes after detectable effects on growth were observed in the limiting IPTG cultures. Cells were resuspended in a 2:1 mixture of RNA Protect Bacteria Reagent:VBMM, incubated at room temperature for 5 min, re-centrifuged in a microfuge, decanted and stored at −70°C until used for RNA isolation. RNA isolation, cDNA production, labeling, fragmentation, hybridzation to GeneChip® P. aeruginosa Genome Arrays (Cat. No. 900340, Affymetrix, Inc.), washing and reading of signals were done as described by Wolfgang et al (Wolfgang et al., 2003). About 80% of the genes were called “present” in each hybridization, consistent with adequate elimination of DNA in the RNA preparations. Each deleted target gene was called “absent” by the Affymetrix analysis software as expected due to negligible cross-hybridization of the complementing B. pseudomallei ortholog gene with the array probes. Each complemented deletion strain was analyzed in duplicate. To enable comparisons between arrays, we normalized the signal intensity data for each gene in the array to the total signal from that array and then calculated the ratio of intensity at limiting and excess IPTG levels for each locus in each complemented deletion strain.
Generation of transcriptional fusions to P. luminescens luxCDABE for reporter evaluation
Luciferase reporter fusions were added to the complemented deletions in an improved version of the method described previously (Moir et al., 2007). To permit the addition of reporters directly to the complemented deletion strains which carry TcR and GmR markers, we modified pGSV3-lux (Moore et al., 2004) to carry SpR instead of GmR. This was accomplished by restriction digestion of pGSV3-lux with SacI, gel purification of the larger fragment followed by ligation to a SpR cassette which was PCR amplified from pVLT35 (de Lorenzo et al., 1993) with primers carrying SacI-tails (Spec+SacI-F/Spec+SacI-R), and cut with SacI. Internal fragments from genes whose expression level was up-regulated in response to target depletion were amplified with primers carrying EcoRI tails, cut with EcoRI and ligated to EcoRI-cut and alkaline phosphatase-treated pGSV3-lux-SpR. Resulting gene fragment-luciferase operon transcriptional fusions on pGSV3-lux-SpR were introduced into E. coli SM10 by electroporation and conjugated with the complemented deletions to generate single cross-over insertions placing the P. luminescens luxCDABE operon under regulation of the target-depletion-responsive gene and its promoter region. Insertion at the correct genomic locus was confirmed by PCR with a primer outside of the cloned gene fragment (see “out-F” primers for each locus in the primer list) and a primer within the luxC gene (LuxC-R).
Detection of bioluminescence of reporter strains
Complemented deletion strains carrying luxCDABE transcriptional fusions were grown overnight at 37°C on LBGS plates containing various concentrations of IPTG and then subcultured at 30°C into LBGS without IPTG (except the control which was maintained in ≥0.05 mM IPTG) at an initial OD600 ~0.03). Relative light units (RLU) were measured in white opaque microplates in a Perkin Elmer Envision Multilabel Reader periodically throughout a 7 hour period, and one final measurement was made the next day. In some cases, relative luminescence units (RLU) were normalized to cell number by using OD600 measured in a Perkin Elmer Victor3V 1420 Multilabel HTS counter.
High-throughput screening with reporter strains
Screening was carried out essentially as previously described (Moir et al., 2007) but with the following modifications. The screening strain, MDM981, consisted of a gyrA deletion in P. aeruginosa complemented by a lac-regulated copy of B. pseudomallei gyrA on pUCP24GW and carrying an insertion of pGSV3-lux-SpR at the PA0614 locus. It was grown overnight from a frozen stock at 37°C on LB agar containing 1 mM IPTG, 10 μg/ml gentamicin and 200 μg/ml spectinomycin. Cells from the agar plate were used to inoculate liquid LB medium containing the same concentrations of IPTG, gentamicin and spectinomycin at an OD600 ~0.05. Cultures were grown for about two hours to an OD600 = 0.4, and then 200 μl of culture was added to each well of a 96-well microtiter dish containing compound to initiate the screen. Compounds for screening were purchased from the following vendors – Chembridge (San Diego, CA), Timtec (Newark, DE), and ChemDiv (San Diego, CA). Compounds were diluted in 96-well master plates at 2.5 mM in DMSO at −20°C. Master plates were thawed at room temperature on the day of the screen, and 2 μl of compound was added to the screening plates by means of a Sciclone ALH 3000 liquid handling robot (Caliper, Inc.) and a Twister II Microplate Handler (Caliper, Inc.). The first column of wells contained only culture (negative control), and the last column contained culture plus 0.5-fold MIC of ciprofloxacin (0.03 μg/ml) (positive control). Plates were sealed with a gas permeable sealant (see Materials). Luminescence was measured after 16h incubation at 30°C by using a Perkin Elmer Envision Multilabel Reader. Z′ values were calculated as previously described (Zhang et al., 1999) based on the positive and negative controls in order to monitor the reproducibility of the screen; plate Z′ values were typically >0.5. A z-score for each sample was derived by subtracting the sample RLU from the mean negative control RLU and dividing the difference by the negative control standard deviation. Screening hits generated luminescence with a z-score >3. Confirmed hits exhibited luminescence with z-score >3 in at least three of four re-tests.
Results
Evaluation of B. pseudomallei orthologs in P. aeruginosa
Heterospecific complementation
Predicted orthologs for 19 P. aeruginosa genes (TABLE 2) which had been demonstrated previously to be essential in P. aeruginosa or in other Gram-negative species (Gerdes et al., 2003; Gerdes et al., 2002; Jacobs et al., 2003) were identified in the B. pseudomallei strain K96243 genomic sequence (Holden et al., 2004) by using standard BLAST tools (Wheeler et al., 2002). Knock-out pEX18ApGW vectors were constructed for all 19 P. aeruginosa loci and used successfully to create merodiploids in the P. aeruginosa strain PAO-LAC which carries a copy of the lac repressor lacIQ at the φCTX chromosomal locus (Hoang et al., 2000). All 19 P. aeruginosa merodiploids were electroporated with the corresponding B. pseudomallei orthologs on pUCP24GW and tested for the formation of deletions by selecting for sucrose resistance and screening for carbenicillin-sensitivity and tetracycline-resistance. Presumed deletions were confirmed by PCR with outer flanking primers as described previously (Moir et al., 2007). In 11 of the 19 cases, the B. pseudomallei ortholog complemented sufficiently to permit deletion of the P. aeruginosa gene (TABLE 2).
Table 2.
Complementation of P. aeruginosa Genes with B. pseudomallei Orthologs
| Gene Name | Paer PAO1 ID#a | Bpseu K96243 ID#b | Functional Annotation (Pathway) | Similarityc | Complementation | Strain | Growth Dependence on Inducer |
|---|---|---|---|---|---|---|---|
| gyrA | PA3168 | BPSL2521 | DNA gyrase subunit A (DNA replication) | 0 | yes | MDM271 | complete |
| secA | PA4403 | BPSL3016 | Secretion protein SecA (Protein secretion) | 0 | yes | MDM410 | complete |
| ligA | PA1529 | BPSL2164 | DNA ligase (DNA replication) | −213 | yes | MDM504 | no detectable dependence |
| glmU | PA5552 | BPSL0313 | Glucosamine-1-phosphate acetyltransferase/N-acetylglucosamine-1-phosphate uridyltransferasen (Cell wall biosynthesis) | −169 | yes | MDM502 | complete |
| ftsA | PA4408 | BPSL3021 | Cell division protein FtsA (Cell division) | −163 | unknown | MDM924 | over-expression sensitive |
| dnaA | PA0001 | BPSL0075 | Chromosomal replication initiator protein DnaA (DNA replication) | −150 | unknown | MDM366 | over-expression sensitive |
| accA | PA3639 | BPSL2241 | Acetyl-coenzyme A carboxylase carboxyl transferase (alpha subunit) (Fatty acid synthesis) | −130 | no | n/a | |
| accD | PA3112 | BPSS1696 | Acetyl-CoA carboxylase beta subunit (Fatty acid synthesis) | −123 | no | n/a | |
| lpxA | PA3644 | BPSL2147 | UDP-N-acetylglucosamine acyltransferase (Fatty acid synthesis) | −83 | yes | MDM359 | no detectable dependence |
| ipk | PA4669 | BPSL0523 | Isopentenyl monophosphate kinase (ipk, ispE) (Cofactor biosynthesis) | −76 | yes | MDM949 | no detectable dependence |
| efp | PA2851 | BPSL2422 | Translation elongation factor P (Translation) | −73 | yes | MDM498 | partial |
| coaD | PA0363 | BPSL0516 | Phosphopantetheine adenylyltransferase (Cofactor biosynthesis) | −62 | yes | MDM275 | partial |
| ftsW | PA4413 | BPSL3026 | Cell division protein FtsW (Cell division0 | −56 | no | n/a | |
| holA | PA3989 | BPSL2936 | DNA polymerase III, delta subunit (DNA replication) | −49 | no | n/a | |
| fabZ | PA3645 | BPSL2148 | (3R)-Hydroxymyristoyl-[acyl carrier protein] dehydratase (Fatty acid synthesis) | −44 | no | n/a | |
| folA | PA0350 | BPSL2476 | Dihydrofolate reductase (Cofactor biosynthesis) | −33 | yes | MDM882 | no detectable dependence |
| ftsQ | PA4409 | BPSL3022 | Cell division protein FtsQ (Cell division) | −27 | yes | MDM339 | complete |
| lolA | PA2614 | BPSL2603 | OM lipoproteins carrier protein (Chaperones) | −22 | no | n/a | |
| mesJ | PA3638 | BPSL2240 | Conserved hypothetical protein (Hypothetical, unknown) | −17 | yes | MDM363 | partial |
Paer, Pseudomonas aeruginosa; genes numbered according to (Stover et al., 2000)
Bpseu, Burkholderia pseudomallei; genes numbered according to (Holden et al., 2004)
The value of the exponent of the P-value from the BLAST analysis is shown
We were not able to generate complemented deletions for 8 of the 19 genes (TABLE 2) despite the fact that the complementing B. pseudomallei orthologs on pUCP24GW were verified to be of accurate nucleotide sequence. Two of the genes that did not support deletions (ftsA, dnaA) inhibited the growth of their respective merodiploids when IPTG levels were increased above 0.025 mM. While the merodiploids containing the B. pseudomallei ftsA and dnaA genes on pUCP24GW could be grown in low IPTG levels, we were not able to generate complemented deletions for either of them.
Growth dependence on ortholog expression
In order to identify suitable promoters to express the luciferase operon in reporter screens, we used expression profiling by microarrays to survey the expression response of all genes to depletion of each target. Complemented deletions were first tested for growth impairment in low IPTG concentrations as a means to assess their suitability for expression profiling. We used detectable effects on growth due to repression of the lac promoter driving the complementing target copy as an indication that cells were responding to target depletion in a manner that would be sensed by the cellular regulatory circuitry and revealed in expression profiling. The response to limiting IPTG concentrations varied widely, from complete dependence on IPTG for growth (glmU, secA, gyrA, ftsQ), to significant impairment of growth in the absence of IPTG (coaD, efp, mesJ), to apparently normal growth in the absence of IPTG (ligA, lpxA, folA, ipk) (TABLE 2). These results appear to reflect a varying requirement by the cell for these specific gene products.
Expression profiling of complemented deletions under target-depletion conditions
In order to identify target-depletion responsive genes and promoters, expression profiles were generated for four complemented deletions (glmU, secA, coaD and gyrA ) which demonstrated adequate growth dependence on IPTG levels. Due to poor growth of the B. pseudomallei gyrA complemented strain in excess IPTG, we complemented the gyrA deletion with a copy of the P. aeruginosa gyrA gene for the purposes of expression profiling. We then used the B. pseudomallei gyrA gene to complement the deletion in the reporter strain used for screening (see below). Expression profiles were examined from cells grown in excess IPTG, providing nearly wild-type growth, and for cells grown in limiting IPTG, causing impaired growth and early saturation. Signals from the corresponding microarrays were processed (see Methods) and analyzed as the ratio for each gene of its signal under inducing conditions to its signal under repressing conditions.
As a control, gene expression in the wild-type parental PAO-LAC strain was profiled from cells grown in the absence of IPTG and in 0.1 mM IPTG. The signal ratios for all genes called “present” averaged 1.0 (S.D. ± 0.27) reflecting the absence of any significant effect of IPTG on gene expression in PAO-LAC. In order to prioritize genes which could represent suitable sources of reporter promoters, we required that the normalized signal intensity ratio of limiting to excess IPTG levels be >5, that the intensity in excess IPTG be >25, and that the intensity in limiting IPTG be >100. These criteria were designed to ensure that up-regulated genes were statistically significant and that signals generated by reporter fusions would be easily detectable. Next, we prioritized the genes that met these criteria for each depleted target to determine which up-regulated genes responded specifically to reduction in the amount of that target. In addition, to further ensure specificity, we compared the target depletion gene expression responses to published expression profiling results from cells grown under a variety of conditions – hydrogen peroxide stress (Palma et al., 2004); nitrosative stress (Firoved et al., 2004); imipenem (Bagge et al., 2004); Ca++/EGTA (Wolfgang et al., 2003); stationary phase (S. Lory, personal communication); iron deprivation (Ochsner et al., 2002; Palma et al., 2003); and biofilm/quorum sensing (Wagner et al., 2004; Whiteley et al., 2001).
Comparison of the gene expression profiles of coaD, glmU, gyrA, and secA complemented deletions growing at limiting versus excess IPTG concentrations revealed several P. aeruginosa genes whose expression levels increased significantly and specifically in response to depletion of the essential gene product (TABLE 3). Some of the responding genes appear to be in operons, for example, PA2030 and PA2031 for coaD, PA4181 and PA4182 for glmU, PA2403 through PA2410 for secA, and PA0612 through PA0648 for gyrA. Of particular note, the gyrA-depletion-responsive operon has been shown previously to respond to ciprofloxacin treatment (Brazas and Hancock, 2005; Cirz et al., 2006). The present discovery that it also responds to GyrA depletion supports the hypothesis that cellular regulatory changes in response to target depletion mimic the regulatory changes expected for antibiotic inhibition of the same target. Identification of multiple genes in an apparent operon as up-regulated in response to target depletion provides assurance of the reproducibility of detection by microarray-based expression profiling. Note that none of the up-regulated genes has any obvious mechanistic involvement in the depletion of the target. In most cases, they are hypothetical or conserved hypothetical genes. However, it is not necessary that there be a mechanistic relationship between the target and responsive gene product as long as the promoter responds specifically and potently to depletion of the target.
TABLE 3.
Promoter Response to Target Depletion
| Gene Source of luxCDABE Promoter |
Promoter Gene Annotation |
coaD Signal Ratioa |
glmU Signal Ratioa |
secA Signal Ratioa |
gyrA Signal Ratioa |
Ciprofloxacin Signal Ratiob |
Reporter Strain RLU Signal Ratio |
Reporter Strain |
Control RLU Ratio |
Control Strain & Condition |
|---|---|---|---|---|---|---|---|---|---|---|
| PA2030 | hypothetical protein | 10.59 | 1.23 | 1.33 | 0.45 | 0.73 | 7.4 | MDM1168 | ||
| PA2031 | hypothetical protein | 12.53 | 1.82 | 1.48 | 0.60 | 0.72 | n.d. | |||
| PA3361 | hypothetical protein | 15.68 | 0.20 | 0.44 | 0.14 | 0.64 | n.d. | |||
| PA4607 | hypothetical protein | 8.46 | 0.71 | 1.14 | 0.21 | 1.12 | 2.5 | MDM1141 | ||
| PA1471 | hypothetical protein | 3.10 | 12.35 | 1.16 | 0.93 | 0.78 | 12.7 | MDM1164 | 0.7 | MDM1184; limiting [IPTG] |
| PA1494 | conserved hypothetical protein | 1.64 | 18.55 | 0.51 | 0.94 | 1.10 | 8.0 | MDM1142 | 0.5 | MDM1169; limiting [IPTG] |
| PA2358 | hypothetical protein | 0.40 | 6.17 | 1.50 | 0.77 | 1.99 | 16.0 | MDM1166 | ||
| PA4181 | hypothetical protein | 1.40 | 15.28 | 3.03 | 1.03 | 1.07 | n.d. | |||
| PA4182 | hypothetical protein | 1.92 | 10.64 | 3.93 | 0.82 | 0.91 | n.d. | |||
| PA1365 | probable siderophore receptor | 0.94 | 0.66 | 16.69 | 0.74 | 1.44 | 7.9 | MDM1145 | 0.3 | MDM1170; limiting [IPTG] |
| PA2403 | hypothetical protein | 0.36 | 2.52 | 6.87 | 0.07 | 1.07 | 1.9 | MDM1143 | ||
| PA2404 | hypothetical protein | 0.53 | 1.61 | 7.93 | 0.06 | 0.71 | 3.3 | MDM1167 | ||
| PA2405 | hypothetical protein | 0.45 | 1.69 | 8.22 | 0.03 | 1.03 | n.d. | |||
| PA2406 | hypothetical protein | 0.28 | 1.40 | 4.88 | 0.07 | 1.52 | n.d. | |||
| PA2407 | probable adhesion protein | 0.35 | 1.30 | 5.92 | 0.07 | 0.92 | n.d. | |||
| PA2408 | probable component of ABC transporter | 0.56 | 1.31 | 9.85 | 0.07 | 1.17 | 5.6 | MDM1144 | ||
| PA2409 | probable permease of ABC transporter | 0.44 | 1.05 | 6.16 | 0.07 | 0.66 | n.d. | |||
| PA2410 | hypothetical protein | 0.62 | 1.15 | 5.09 | 0.11 | 0.99 | n.d. | |||
| PA0612 | hypothetical protein | 0.78 | 1.54 | 1.37 | 6.00 | 4.43 | n.d. | |||
| PA0613 | hypothetical protein | 1.84 | 1.74 | 1.17 | 6.94 | 3.79 | n.d. | |||
| PA0614 | hypothetical protein | 1.64 | 0.45 | 0.72 | 7.39 | 5.44 | 6.0/7.5 | MDM981/MDM977 | 25/43 | MDM981/MDM977 +/− 0.03 µg/ml FQc |
| PA0615 | hypothetical protein | 3.32 | 0.33 | 0.91 | 6.09 | 3.11 | n.d. | |||
| PA0616 | hypothetical protein | 1.69 | 0.39 | 0.93 | 7.18 | 6.51 | n.d. | |||
| PA0617 | probable bacteriophage protein | 1.20 | 0.39 | 0.58 | 8.09 | 7.49 | n.d. |
Ratio of normalized signal intensities in limiting IPTG/excess IPTG
Ratio of normalized signal intensities from growth in 0.006 µg/ml ciprofloxacin (~0.1x MIC) vs. growth in the absence of ciprofloxacin
FQ, fluoroquinolone (ciprofloxacin was used in these experiments at ~0.5x MIC)
Response of luxCDABE fusions to target depletion
In order to convert the gene expression response to target depletion into a report for target inhibition, we created specific transcriptional fusions to the P. luminescens luxCDABE operon. This was accomplished by cloning internal fragments of several target-depletion responsive genes into the EcoRI site of pGSV3-lux-SpR and integrating them into the chromosome of each corresponding complemented deletion strain (Moore et al., 2004). Resulting strains were grown under limiting IPTG conditions, transferred to medium lacking IPTG, and bioluminescence was measured over several hours to assess the response of the reporter construct to loss of the target gene product by dilution and by repression of further expression (see Methods). In general, bioluminescence intensity was indirectly dependent on the initial IPTG level, directly dependent on the time of growth in the absence of IPTG up to a limit of 5–7 h and specific for depletion of the target. Detailed results are shown in Fig. 1 for the GlmU-depletion-sensitive reporter fusion PA1494-luxCDABE in both glmU and secA complemented deletion strains MDM1142 and MDM1169, respectively, grown in limiting IPTG concentrations. Cell growth is reduced markedly by either GlmU or SecA depletion (Fig. 1A and B), but bioluminescence increases significantly only under GlmU depletion conditions (Fig. 1C). The maximum ratio of bioluminescence intensity in limiting versus excess IPTG for several reporter strains for the targets coaD, glmU, secA, and gyrA are shown in Table 3. In general, the mRNA ratios measured in the expression profiling experiments are good predictors of the bioluminescence response, but there are several exceptions presumably due to the additional steps of translation, protein stability and light production not accounted for in expression profiling. It is also interesting to note that use of an inhibitor may be more effective than limiting IPTG to deplete essential gene products from these complemented deletions. For example, the bioluminescence ratios for both the P. aeruginosa gyrA and the B. pseudomallei gyrA complemented deletions (MDM977 and MDM981, respectively) +/− limiting IPTG concentrations and +/− 0.5x MIC of ciprofloxacin are shown in Table 3. The source of the complementing gene has little effect on the RLU ratios, but addition of the known gyrase inhibitor ciprofloxacin appears to be much more effective than the use of limiting IPTG to deplete functional gyrase in both strains, producing RLU ratios over 4-fold greater than those observed +/− IPTG.
Figure 1.
Comparison of growth and bioluminescence of ΔglmU and ΔsecA strains carrying the GlmU-depletion responsive promoter from PA1494 fused to P. luminescens luxCDABE. Bioluminescent reporter strains MDM1142 (ΔglmU) and MDM1169 (ΔsecA), both containing the PA1494-luxCDABE element, were grown overnight in LBGS containing 0.05 mM IPTG (■)(control culture), 0.025 mM ITPG (△), or 0.0125 mM IPTG (◇), then subcultured into LBGS without IPTG, except for the control, which was maintained at 0.05 mM IPTG. Growth (OD600) was followed for 7.5 h for (A) MDM1142 (solid lines) and (B) MDM1169 (dashed lines). (C) Cells (200 μl) from each culture were added to 96-well opaque white microplates after 3 h, and relative light units (RLU) were measured for an additional 4.5 h.
High throughput screening – gyrA proof of principle
For proof of principle for these reporter screens, we chose to use strain MDM981, a P. aeruginosa gyrA deletion strain complemented by B. pseudomallei gyrA on pUCP24GW and carrying a pGSV3-lux-SpR insertion at PA0614. This strain was chosen for two reasons – (a) a successful pilot screen was done previously on 2,000 compounds using a similar strain except that it was complemented by P. aeruginosa gyrA (Moir et al., 2007), and (b) the existence of known inhibitors of GyrA, the fluoroquinolones, provided accessible positive controls. We screened 68,000 compounds using reporter strain MDM981 (see Methods) and identified 328 primary hits which exhibited z-scores >3 (compared to the negative control) for a primary hit rate of 0.48%. About 13% of the primary hits, or 43 compounds, were confirmed in quadruplicate assays with the same reporter strain. The best 34 of those, as judged by having drug-like scaffolds, were re-ordered from vendors, re-examined, and 16 exhibited z-scores >3 when re-assayed.
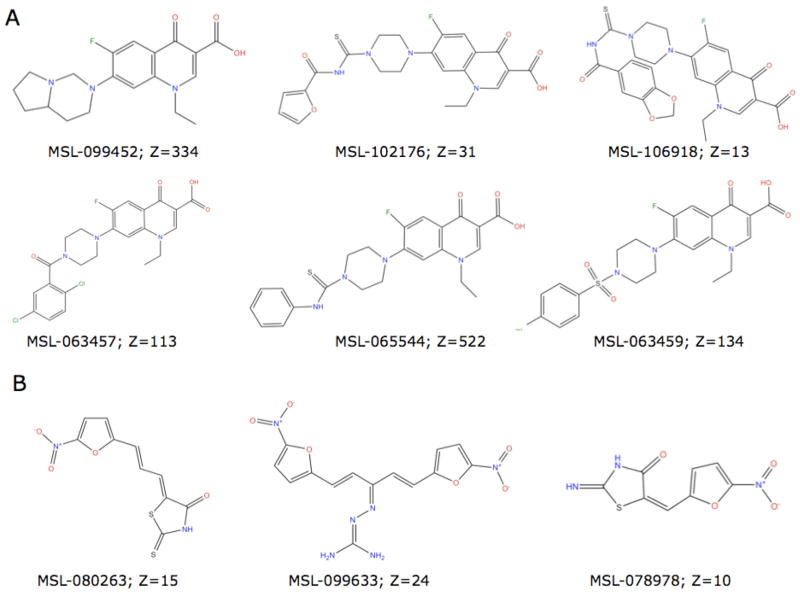
Examples of two chemotypes of confirmed hits are shown in Fig. 2. The screen identified 51% (20 of 39) of the fluoroquinolones in the screening library as hits. All 20 contain the piperazine ring recognized as important for anti-pseudomonas activity (Neu, 1989), while only 5 of the 19 fluoroquinolones, which were not identified as hits, contain the piperazine ring. Several nitrofurans were also identified as hits. These may be activated by a nitro-reductase in the bacterial cell to generate DNA alkylating agents as has been described previously for metronidazole (Goodwin et al., 1998). Both chemotypes were expected as hits based on the known response of the reporter promoter from gene PA0614 (Matsui et al., 1993; Moir et al., 2007) to DNA damage.
Figure 2.
Chemical structures of representative (A) fluoroquinolones and (B) nitrofurans identified in a high throughput screen with reporter strain MDM981. The corporate compound identifier (MSL#) and the z-score are shown for each confirmed hit.
Discussion
In this study, heterospecific complementation of essential P. aeruginosa genes by B. pseudomallei orthologs was feasible for 11 of 19 of genes attempted, and down-regulation of the complementing ortholog affected cell growth significantly for 7 of 11 genes tested. Apparently specific effects on the regulatory circuitry of the cell were observed in expression profiles of 4 complemented deletions examined in excess and limiting inducer concentrations. These properties have permitted the development of homogeneous, whole-cell bioluminescent screening assays for inhibitors of B. pseudomallei targets in P. aeruginosa. Similar bioluminescent reporter assays have been described in B. subtilis based on the cellular regulatory response to antibiotic treatments and conditional mutants (Freiberg et al., 2005; Hutter et al., 2004). However, those assays utilized firefly luciferase as a reporter and required the addition of exogenous substrate. The assays described here are simpler and homogeneous, requiring no substrate addition, operate in a less-permeable Gram-negative species, P. aeruginosa and are designed to identify inhibitors of heterologous targets in a surrogate species. We discuss a few aspects of the approach below.
Complementation efficiency of orthologs
Slightly over half (11/19) of the B. pseudomallei genes tested were capable of complementing deletions of their P. aeruginosa orthologs. It is interesting to note that the success in complementation did not correlate with the overall sequence similarity (see P-value exponents in TABLE 2). However, all of these proteins are highly homologous, and sequence similarity within functional motifs might be a better predictor of cross-species complementation. There are several possible reasons for the eight complementation failures. In most cases, failure is probably because the B. pseudomallei gene product is not capable of performing all of the functions of the P. aeruginosa ortholog. We verified the accuracy of the sequences of the B. pseudomallei genes cloned into pUCP24GW; thus, sequence errors introduced by PCR cannot explain the complementation failures. Another possibility is that the lac regulatory control may be an inadequate substitute for native regulation of these genes, and/or the fusion of the lac promoter to the gene fragment may have been suboptimal for expression. Other promoters could be tested, and we are interested in experimenting with other promoters for a second reason as well. We would like to reduce transcription of complementing B. pseudomallei genes such as ligA, lpxA, folA and ipk more completely in order to detect target-depletion-responsive genes in transcription profiling experiments with those complemented deletions.
Over-expression lethals
Over-expression of the B. pseudomallei dnaA and ftsA genes in P. aeruginosa inhibited the growth of the merodiploids carrying them, judging from the fact that P. aeruginosa cells carrying these genes on pUCP24GW grew normally in LB medium containing ≤0.025 mM IPTG but failed to grow in LB with more IPTG. Over-expression of the E. coli dnaA gene in E. coli has been reported as lethal (Weigel et al., 1999). In contrast, the P. aeruginosa ftsA could be over-expressed in E. coli, but its accumulation as inclusion bodies does not indicate whether functional FtsA can be over-produced in E. coli (Paradis-Bleau et al., 2005). We note that over-expression growth inhibition might be used to select for compounds which inhibit the offending gene product, and this is an area of current research.
Dependence of growth on IPTG as a validation of drug targets
The sensitivity of cell growth to reduction of lac-regulated B. pseudomallei gyrA, secA, glmU, efp, coaD, ftsQ and mesJ gene expression when IPTG levels are reduced suggests that cells require a relatively high minimal level of expression of these genes for normal growth. The most dramatic cases are the glmU and secA complemented deletion strains which fail to grow at all in the absence of IPTG. Apparently, substantial quantities of these gene products are required for growth, certainly more than the basal level produced from the uninduced lac promoter. Thus, even inefficient inhibition of these gene products should be adequate to arrest cell growth, making these attractive drug discovery targets. By contrast, Korycka-Machala et al. (Korycka-Machala et al., 2007) demonstrated that the minimum amount of DNA ligase required for growth of Mycobacterium smegmatis is quite low, suggesting that this gene product is not an ideal target for drug discovery.
Specificity of reporter strains
Our confidence in the specificity of the up-regulation is limited to the range of conditions included in our analysis, but the inclusion of a variety of stresses from published sources insures that we will avoid selecting reporter fusions responding to these common perturbations. Clearly, secondary assays will be required to further qualify the hits from the reporter screens. Nevertheless, the reporter screens provide a sensitive means to detect inhibitors of targets inside intact cells, which is quite useful since many biochemical screening hits fail at the level of inhibiting whole cells (Payne et al., 2007). We observed some lack of specificity for GyrA depletion -- a partial overlap in response from depletion of GyrA and treatment with ciprofloxacin (DTM and MD, unpublished observations, and TABLE 3). The overlap includes the PA0614 gene, which is the source of the promoter used for the reporter strain, and this supports the hypothesis that depletion of an antibiotic target will cause a gene expression response similar to that of inhibition of the target with a drug. The lack of a complete overlap in the responses probably reflects the fact that ciprofloxacin acts on an additional target in P. aeruginosa – topoisomerase IV (parE + parC gene product)(Lee et al., 2005; Oh et al., 2003) as well as gyrase, and the lac-regulated control of gyrA may not reduce GyrA activity as substantially as does treatment with ciprofloxacin.
Essentiality of target genes in B. pseudomallei
Several of these gene targets including gyrA, glmU, secA and coaD, have been shown to be essential in B. pseudomallei by the failure to resolve pKAS46-generated merodiploids into deletions (RAM and DEW, unpublished observations). Thus, the reporter screens described here offer an opportunity to identify novel anti-B. pseudomallei compounds for further development as antibacterials.
Conclusions
This study demonstrates the feasibility of constructing and using whole cell reporter screens to identify inhibitors of targets from a BSL-3 species, B. pseudomallei, in a surrogate BSL-2 species, P. aeruginosa. The principles are generally transferable to other related sets of species, and the resulting reporter strains provide a relatively safe and inexpensive means for high throughput screening for inhibitors of biothreat species.
Supplementary Material
Acknowledgments
We gratefully acknowledge helpful discussions with Dr. Stephen Lory (Harvard Medical School) on the development of these reporter assays. We thank Dr. Jonathan Segal for help with BLAST analysis of orthologs, Dr. Shauna Reckseidler-Zenteno and Ms. Patricia Baker for assistance with cloning B. pseudomallei genes, Dr. Timothy Opperman for advice and Gateway modification of the pUCP24 plasmid, and Terry Bowlin for helpful scientific suggestions.
Funding. This research was funded by SBIR grant AI056644 awarded by NIAID of the US NIH. DEW holds a Canada Research Chair in Microbiology
Abbreviations
- Bpseu
B. pseudomallei
- Paer
P. aeruginosa
Footnotes
A manuscript submitted to “Transactions of the Royal Society of Tropical Medicine and Hygiene” and presented at the “World Melioidosis Congress 2007” in Khon Kaen, Thailand
Declarations:
Conflicts of interest: DTM and MD are employed by Microbiotix, Inc. HPS and DEW are paid consultants to Microbiotix, Inc. RAM received funding for this research from Microbiotix, Inc. under a subcontract.
Ethical approval: Not required.
Authors’ contributions. DTM, HPS, and DEW designed the experimental strategy. RAM and MD carried out the experimental approach. DTM drafted the manuscript. All authors read and approved the final manuscript.
References
- Bagge N, Schuster M, Hentzer M, Ciofu O, Givskov M, Greenberg EP, et al. Pseudomonas aeruginosa biofilms exposed to imipenem exhibit changes in global gene expression and beta-lactamase and alginate production. Antimicrob Agents Chemother. 2004;48:1175–87. doi: 10.1128/AAC.48.4.1175-1187.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazas MD, Hancock RE. Ciprofloxacin induction of a susceptibility determinant in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2005;49:3222–7. doi: 10.1128/AAC.49.8.3222-3227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett PJ, Woods DE. Pathogenesis of and immunity to melioidosis. Acta Trop. 2000;74:201–10. doi: 10.1016/s0001-706x(99)00071-6. [DOI] [PubMed] [Google Scholar]
- Burtnick MN, Woods DE. Isolation of polymyxin B-susceptible mutants of Burkholderia pseudomallei and molecular characterization of genetic loci involved in polymyxin B resistance. Antimicrob Agents Chemother. 1999;43:2648–56. doi: 10.1128/aac.43.11.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YY, Tan TM, Ong YM, Chua KL. BpeAB-OprB, a multidrug efflux pump in Burkholderia pseudomallei. Antimicrob Agents Chemother. 2004;48:1128–35. doi: 10.1128/AAC.48.4.1128-1135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KH, Schweizer HP. An improved method for rapid generation of unmarked Pseudomonas aeruginosa deletion mutants. BMC Microbiol. 2005;5:30. doi: 10.1186/1471-2180-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirz RT, O’Neill BM, Hammond JA, Head SR, Romesberg FE. Defining the Pseudomonas aeruginosa SOS response and its role in the global response to the antibiotic ciprofloxacin. J Bacteriol. 2006;188:7101–10. doi: 10.1128/JB.00807-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie BJ, Fisher DA, Howard DM, Burrow JN, Selvanayagam S, Snelling PL, et al. The epidemiology of melioidosis in Australia and Papua New Guinea. Acta Trop. 2000;74:121–7. doi: 10.1016/s0001-706x(99)00060-1. [DOI] [PubMed] [Google Scholar]
- de Lorenzo V, Eltis L, Kessler B, Timmis KN. Analysis of Pseudomonas gene products using lacIq/Ptrp-lac plasmids and transposons that confer conditional phenotypes. Gene. 1993;123:17–24. doi: 10.1016/0378-1119(93)90533-9. [DOI] [PubMed] [Google Scholar]
- de Lorenzo V, Timmis KN. Analysis and construction of stable phenotypes in gram-negative bacteria with Tn5- and Tn10-derived minitransposons. Methods Enzymol. 1994;235:386–405. doi: 10.1016/0076-6879(94)35157-0. [DOI] [PubMed] [Google Scholar]
- Firoved AM, Wood SR, Ornatowski W, Deretic V, Timmins GS. Microarray analysis and functional characterization of the nitrosative stress response in nonmucoid and mucoid Pseudomonas aeruginosa. J Bacteriol. 2004;186:4046–50. doi: 10.1128/JB.186.12.4046-4050.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiberg C, Fischer HP, Brunner NA. Discovering the mechanism of action of novel antibacterial agents through transcriptional profiling of conditional mutants. Antimicrob Agents Chemother. 2005;49:749–59. doi: 10.1128/AAC.49.2.749-759.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes SY, Scholle MD, Campbell JW, Balazsi G, Ravasz E, Daugherty MD, et al. Experimental determination and system level analysis of essential genes in Escherichia coli MG1655. J Bacteriol. 2003;185:5673–84. doi: 10.1128/JB.185.19.5673-5684.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes SY, Scholle MD, D’Souza M, Bernal A, Baev MV, Farrell M, et al. From genetic footprinting to antimicrobial drug targets: examples in cofactor biosynthetic pathways. J Bacteriol. 2002;184:4555–72. doi: 10.1128/JB.184.16.4555-4572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilligan PH, Whittier S. Burkholderia, Stenotrophomonas, Ralstonia, Brevundimonas, Comamonas, and Acidovorax. In: Murray PR, et al., editors. Manual of Clinical Microbiology. ASM Press; Washington, DC: 1999. [Google Scholar]
- Goodwin A, Kersulyte D, Sisson G, Veldhuyzen van Zanten SJ, Berg DE, Hoffman PS. Metronidazole resistance in Helicobacter pylori is due to null mutations in a gene (rdxA) that encodes an oxygen-insensitive NADPH nitroreductase. Mol Microbiol. 1998;28:383–93. doi: 10.1046/j.1365-2958.1998.00806.x. [DOI] [PubMed] [Google Scholar]
- Hoang TT, Kutchma AJ, Becher A, Schweizer HP. Integration-proficient plasmids for Pseudomonas aeruginosa: site-specific integration and use for engineering of reporter and expression strains. Plasmid. 2000;43:59–72. doi: 10.1006/plas.1999.1441. [DOI] [PubMed] [Google Scholar]
- Holden MT, Titball RW, Peacock SJ, Cerdeno-Tarraga AM, Atkins T, Crossman LC, et al. Genomic plasticity of the causative agent of melioidosis, Burkholderia pseudomallei. Proc Natl Acad Sci U S A. 2004;101:14240–5. doi: 10.1073/pnas.0403302101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter B, Fischer C, Jacobi A, Schaab C, Loferer H. Panel of Bacillus subtilis reporter strains indicative of various modes of action. Antimicrob Agents Chemother. 2004;48:2588–94. doi: 10.1128/AAC.48.7.2588-2594.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs MA, Alwood A, Thaipisuttikul I, Spencer D, Haugen E, Ernst S, et al. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 2003;100:14339–44. doi: 10.1073/pnas.2036282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenney AW, Lum G, Fisher DA, Currie BJ. Antibiotic susceptibility of Burkholderia pseudomallei from tropical northern Australia and implications for therapy of melioidosis. Int J Antimicrob Agents. 2001;17:109–13. doi: 10.1016/s0924-8579(00)00334-4. [DOI] [PubMed] [Google Scholar]
- Korycka-Machala M, Rychta E, Brzostek A, Sayer HR, Rumijowska-Galewicz A, Bowater RP, et al. Evaluation of NAD(+) -dependent DNA ligase of mycobacteria as a potential target for antibiotics. Antimicrob Agents Chemother. 2007;51:2888–97. doi: 10.1128/AAC.00254-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Lee YS, Park YK, Kim BS. Alterations in the GyrA and GyrB subunits of topoisomerase II and the ParC and ParE subunits of topoisomerase IV in ciprofloxacin-resistant clinical isolates of Pseudomonas aeruginosa. Int J Antimicrob Agents. 2005;25:290–5. doi: 10.1016/j.ijantimicag.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Matsui H, Sano Y, Ishihara H, Shinomiya T. Regulation of pyocin genes in Pseudomonas aeruginosa by positive (prtN) and negative (prtR) regulatory genes. J Bacteriol. 1993;175:1257–63. doi: 10.1128/jb.175.5.1257-1263.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir DT, Di M, Opperman T, Schweizer HP, Bowlin TL. A high-throughput, homogeneous, bioluminescent assay for Pseudomonas aeruginosa gyrase inhibitors and other DNA-damaging agents. J Biomol Screen. 2007;12:855–64. doi: 10.1177/1087057107304729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RA, DeShazer D, Reckseidler S, Weissman A, Woods DE. Efflux-mediated aminoglycoside and macrolide resistance in Burkholderia pseudomallei. Antimicrob Agents Chemother. 1999;43:465–70. doi: 10.1128/aac.43.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RA, Reckseidler-Zenteno S, Kim H, Nierman W, Yu Y, Tuanyok A, et al. Contribution of gene loss to the pathogenic evolution of Burkholderia pseudomallei and Burkholderia mallei. Infect Immun. 2004;72:4172–87. doi: 10.1128/IAI.72.7.4172-4187.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu HC. Chemical evolution of the fluoroquinolone antimicrobial agents. Am J Med. 1989;87:2S–9S. [PubMed] [Google Scholar]
- Ochsner UA, Wilderman PJ, Vasil AI, Vasil ML. GeneChip expression analysis of the iron starvation response in Pseudomonas aeruginosa: identification of novel pyoverdine biosynthesis genes. Mol Microbiol. 2002;45:1277–87. doi: 10.1046/j.1365-2958.2002.03084.x. [DOI] [PubMed] [Google Scholar]
- Oh H, Stenhoff J, Jalal S, Wretlind B. Role of efflux pumps and mutations in genes for topoisomerases II and IV in fluoroquinolone-resistant Pseudomonas aeruginosa strains. Microb Drug Resist. 2003;9:323–8. doi: 10.1089/107662903322762743. [DOI] [PubMed] [Google Scholar]
- Palma M, DeLuca D, Worgall S, Quadri LE. Transcriptome analysis of the response of Pseudomonas aeruginosa to hydrogen peroxide. J Bacteriol. 2004;186:248–52. doi: 10.1128/JB.186.1.248-252.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma M, Worgall S, Quadri LE. Transcriptome analysis of the Pseudomonas aeruginosa response to iron. Arch Microbiol. 2003;180:374–9. doi: 10.1007/s00203-003-0602-z. [DOI] [PubMed] [Google Scholar]
- Paradis-Bleau C, Sanschagrin F, Levesque RC. Peptide inhibitors of the essential cell division protein FtsA. Protein Eng Des Sel. 2005;18:85–91. doi: 10.1093/protein/gzi008. [DOI] [PubMed] [Google Scholar]
- Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat Rev Drug Discov. 2007;6:29–40. doi: 10.1038/nrd2201. [DOI] [PubMed] [Google Scholar]
- Piliouras P, Ulett GC, Ashhurst-Smith C, Hirst RG, Norton RE. A comparison of antibiotic susceptibility testing methods for cotrimoxazole with Burkholderia pseudomallei. Int J Antimicrob Agents. 2002;19:427–9. doi: 10.1016/s0924-8579(02)00016-x. [DOI] [PubMed] [Google Scholar]
- Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, et al. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature. 2000;406:959–64. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- Vogel HJ, Bonner DM. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956;218:97–106. [PubMed] [Google Scholar]
- Vorachit M, Chongtrakool P, Arkomsean S, Boonsong S. Antimicrobial resistance in Burkholderia pseudomallei. Acta Trop. 2000;74:139–44. doi: 10.1016/s0001-706x(99)00063-7. [DOI] [PubMed] [Google Scholar]
- Wagner VE, Gillis RJ, Iglewski BH. Transcriptome analysis of quorum-sensing regulation and virulence factor expression in Pseudomonas aeruginosa. Vaccine. 2004;22 Suppl 1:S15–20. doi: 10.1016/j.vaccine.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Weigel C, Schmidt A, Seitz H, Tungler D, Welzeck M, Messer W. The N-terminus promotes oligomerization of the Escherichia coli initiator protein DnaA. Mol Microbiol. 1999;34:53–66. doi: 10.1046/j.1365-2958.1999.01568.x. [DOI] [PubMed] [Google Scholar]
- Wheeler DL, Church DM, Lash AE, Leipe DD, Madden TL, Pontius JU, et al. Database resources of the National Center for Biotechnology Information: 2002 update. Nucleic Acids Res. 2002;30:13–6. doi: 10.1093/nar/30.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NJ, Dance DA, Chaowagul W, Wattanagoon Y, Wuthiekanun V, Pitakwatchara N. Halving of mortality of severe melioidosis by ceftazidime. Lancet. 1989;2:697–701. doi: 10.1016/s0140-6736(89)90768-x. [DOI] [PubMed] [Google Scholar]
- Whiteley M, Bangera MG, Bumgarner RE, Parsek MR, Teitzel GM, Lory S, et al. Gene expression in Pseudomonas aeruginosa biofilms. Nature. 2001;413:860–4. doi: 10.1038/35101627. [DOI] [PubMed] [Google Scholar]
- Wiedemann B, Grimm H. Susceptibility to Antibiotics: Species Incidence and Trends. In: Lorian V, editor. Antibiotics in Laboratory Medicine. Williams & Wilkins; Baltimore: 1991. [Google Scholar]
- Wolfgang MC, Lee VT, Gilmore ME, Lory S. Coordinate regulation of bacterial virulence genes by a novel adenylate cyclase-dependent signaling pathway. Dev Cell. 2003;4:253–63. doi: 10.1016/s1534-5807(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Chung TD, Oldenburg KR. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.