Abstract
Objectives
This study sought to evaluate the natural history of occult cardiac dysfunction in Duchenne Muscular Dystrophy (DMD).
Background
DMD is characterized by progressive cardiac dysfunction and myocardial fibrosis late in the disease process. We hypothesized that left ventricular myocardial peak circumferential strain (εcc) would decrease in DMD prior to global systolic functional abnormalities regardless of age or ventricular ejection fraction (EF).
Methods
We evaluated cardiac magnetic resonance image (CMR) data from 70 DMD patients and 16 aged-matched control subjects. Standard imaging data included steady-state free precession (SSFP) short-axis cine stack images, cine myocardial tagged images and myocardial delayed enhancement (MDE, an indicator of myocardial fibrosis) sequences. Analysis was performed using QMASS® and HARP® softwares. DMD patient data was subdivided by age (< 10 years or > 10 years), EF (> 55% or <55%) and the presence or absence of MDE.
Results
DMD patients with normal EF had reduced εcc at an early age (<10 years) compared to control subjects (p< 0.01). DMD patients >10 years with normal EF had further decline in εcc compared to younger DMD patients (p<0.01). There was further decline in εcc with age in patients with reduced EF (p<0.01) without MDE. The oldest patients, with both reduced EF and positive MDE, exhibited the lowest εcc. None of the patients had ventricular hypertrophy.
Conclusions
Myocardial strain abnormalities are prevalent in young DMD patients despite normal EF, and these strain values continue to decline with advancing age. Strain analysis in combination with standard CMR and MDE imaging provides a means to stratify DMD cardiomyopathy.
Keywords: Duchenne Muscular Dystrophy, Cardiac Magnetic Resonances Imaging, Circumferential Strain
INTRODUCTION
Duchenne muscular dystrophy (DMD), an X-linked recessive disorder affecting approximately 1 in 3,500 males (1), results from a mutation in the gene which encodes dystrophin, a sarcolemmal protein abundant in skeletal and cardiac muscle cells (1). DMD is characterized by progressive skeletal muscle weakness, with loss of ambulation between the ages of 7 and 13 years. Death secondary to cardiac or respiratory failure typically occurs in the second or third decade. Cardiac disease manifests as a dilated cardiomyopathy (2,3). End stage cardiac pathology consists of alternating areas of myocyte hypertrophy, atrophy and fibrosis (3,4).
Use of corticosteroids and supportive respiratory care (5–7) have improved outcomes in DMD patients such that cardiomyopathy is now the leading cause of death (8). The progression of cardiomyopathy does not correlate to the severity of skeletal muscle weakness, and early manifestations of heart failure in DMD patients often go unrecognized due to lack of classic signs and symptoms (9). Previous investigators have demonstrated that cardiac disease is underway long before symptoms appear (10–12).
Traditionally, assessment of global cardiac function has been evaluated via transthoracic echocardiography (TTE) (2,13,14). However, this modality has proved challenging in the DMD population. TTE rarely detects functional abnormalities during the first decade (15), and acoustic windows in DMD patients tend to be limited due to altered body habitus, including scoliosis and significant chest wall adiposity. To overcome these limitations, our center and others have turned to cardiac magnetic resonance imaging (CMR) for primary screening of global cardiac function in DMD patients (16–18). Recent reports have shown that occult cardiac dysfunction (19) and myocardial fibrosis (16) can be diagnosed by CMR in DMD patients. However, the natural history of the cardiac dysfunction, manifest as reduction in peak left ventricular myocardial circumferential strain (εcc), has not been reported. We hypothesized that abnormalities of εcc would exist early in the course of DMD cardiomyopathy despite normal ejection fraction (EF) and would be progressive during the course of the disease, as cardiac dysfunction becomes more generalized.
METHODS
Study Population
Data was analyzed from records of DMD patients followed at Cincinnati Children’s Hospital Medical Center. The diagnosis of DMD was confirmed by a skeletal muscle biopsy showing absent dystrophin and/or DNA analysis demonstrating a characteristic dystrophin mutation in all patients.
CMR Inclusion and Exclusion Criteria
DMD patients who underwent clinical CMR studies between September 2005 and September 2007 were included in this analysis. Only good quality (confirmed by three independent expert readers, RJF, WMG, KNH) tagged cine MR images were included for analysis. An age-matched control group underwent an identical protocol. All subjects (controls and DMD patients) were > 5 years of age, thereby eliminating the need for sedation. CMR studies were performed on 97 DMD patients between September 2005 to September 2007. Data from 27/97 patients was excluded due to absence of tagged images (n = 18) or poor tag quality secondary to breathing artifact or patient movement (n = 9). The Institutional Review Board at the Cincinnati Children’s Hospital Medical Center approved the study.
Subject Stratification
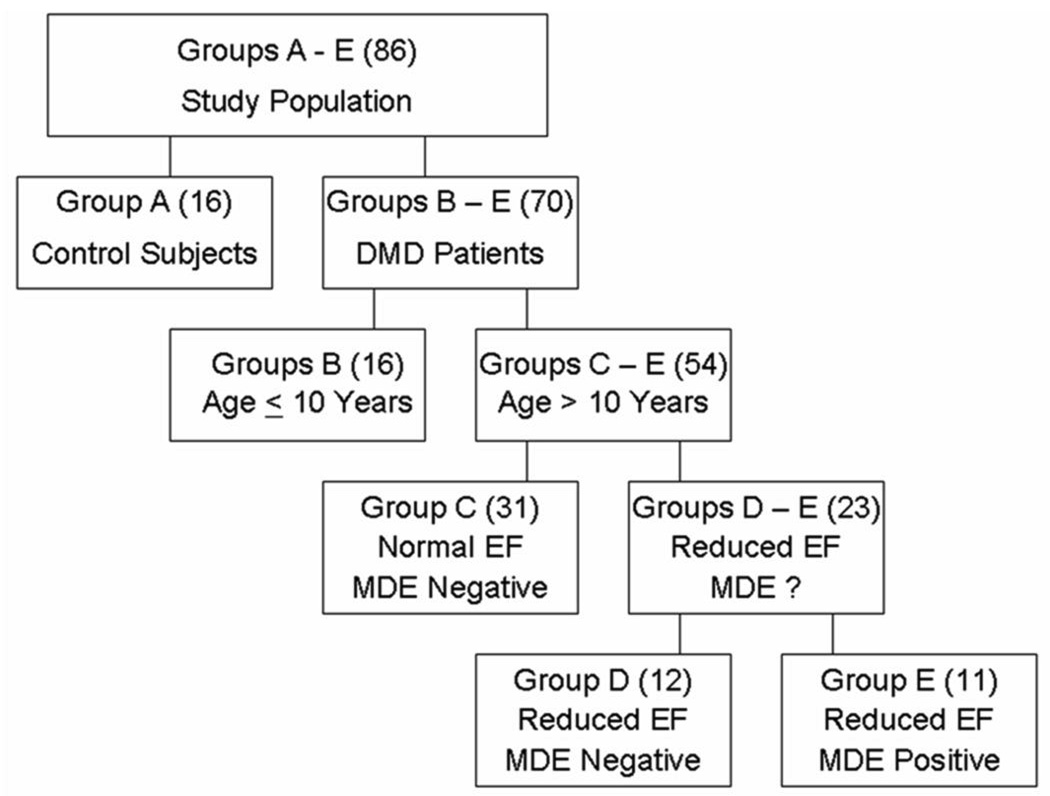
The subject data was stratified into 5 groups: Group A (controls); Groups B-E (DMD patients) grouped according to age, EF and presence of myocardial fibrosis based on positive myocardial delayed enhancement (MDE) imaging (Figure 1). Because prior studies have rarely identified cardiac dysfunction before age10 years (15), we stratified DMD patients ≤ 10 years or > 10 years. As such, Group B were DMD patients age < 10 years with normal EF and negative MDE. Since MDE has usually been associated with advanced cardiac disease, patients > 10 years were further stratified by MDE status, i.e. with or without MDE. Lastly, we stratified the patients > 10 years without MDE into those with normal EF and those with reduced EF. Thus Group C were DMD patients > 10 years with normal EF and negative MDE. Group D were DMD patients > 10 years with reduced EF (<55%) but negative MDE. Finally Group E patients were DMD patients > 10 years with reduced EF and positive MDE.
Figure 1. Group stratification.
Stratification of DMD patients is based on age, ejection fraction (EF) and presence or absence of delayed enhancement (MDE).
CMR and HARP® Analyses
Scanner Specifics
CMR studies were conducted either on a Siemens 3 Tesla Trio (Siemens Medical Solutions, Malvern, PA/Erlangen, Germany) or on a 1.5 Tesla GE Signa Excite (General Electric Healthcare; Milwaukee, WI). The type of machine used on the DMD patients and controlled subjects was based solely on clinical availability, independent of the patient’s clinical status.
Imaging Protocols
Ventricular Volumetry and Global Functional Imaging
Cardiac functional imaging was performed using retrospective ECG-gating, segmented Steady State Free Precession (SSFP) technique after localized shimming and/or frequency adjusting. Subjects were breath-held as tolerated; for those subjects who could not adequately breath-hold, a free breathing technique with multiple signal averaging was used. Standard imaging included a short axis stack of cine SSFP images from cardiac base to apex; the short axis was prescribed as the perpendicular plane to the left ventricular long axis in 2 and 4 chamber views as previously described (20,21). Typical scan parameters included FOV= 32–38 cm, slice thickness= 5–6 mm, gap = 1–2 mm, NEX=2 (breath hold; 4–5 for free breathing), TE/TR=1.4/2.8 (Siemens), TE/TR=2.0/4.0 (GE), in-plane resolution =1.2– 2.2 mm. A minimum of 12 slices were performed, with 20 phases/slice. The typical temporal resolution of the cine SSFP images was 30–40 ms and were adjusted according to the patient’s heart rate and ability to breath-hold. The RF flip angles were set between 50°–70° dependent on the patient weight, height and the SAR level.
Myocardial Strain Imaging
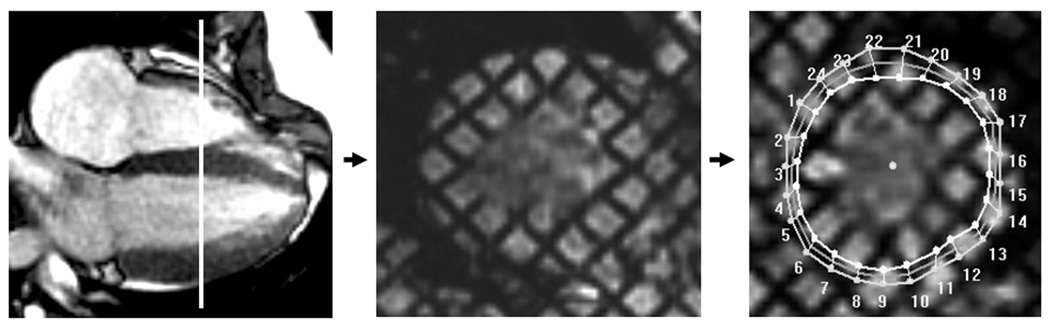
Tagged cine MR images were acquired in the short axis of the midventricle at the level of the papillary muscles (Figure 1) using an ECG-triggered segmented k-space fast gradient echo sequence with spatial modulation of magnetization in orthogonal planes. Tag imaging was performed prior to administration of Gadolinium. Grid tag spacing was 7–8 mm. The scan parameters used were: field of view = (30–32)×(25–26) cm2, slice thickness = 6 mm, flip = 20°, TE/TR = 3 ms/6.6 ms (GE) , =3 ms/4.2 ms (Siemens), views per segment = 8 (GE), =7–9 (Siemens).
Myocardial Delayed Enhancement
MDE imaging was performed on DMD patients when intravenous access was obtained (n = 54); no MDE imaging was performed in the control group. MDE imaging was performed via a FLASH inversion sequence recovery protocol 10 minutes after 0.2 mmol/kg Gadolinium (Gd-DTPA) injection (16,22,23). MDE imaging was considered positive if any area of the mid-myocardium showed hyperenhancement (Figure 2) as assessed by three independent expert observers (RJF, WMG, KNH) (24).
Figure 2. CMR cardiac image.
The short axis of the mid-ventricle is obtained from the four-chamber view at the level of the papillary muscles with a tag sequence. Mesh overlaying of the tag image using a harmonic phase (HARP) software (Diagnosoft Inc.). Both the four-chamber and the tag images are shown during early systole.
Data Analysis
Ventricular Volumetry, Global Functional Data, and MDE Status
Ventricular volumes, mass and global function were assessed via standard planimetry techniques using semi-automated computer software (QMASS v.6.1.5, Medis Medical Imaging Systems, Netherlands) by expert readers (RJF, WMG, KNH) (25,26). This assessment was performed on DICOM images from either scanner, independent of vendor or field strength (27,28). MDE status, ventricular volumes, mass, and EF were tabulated for each subject, and then exported to a spreadsheet file.
Myocardial Strain Analysis
Tagged images were analyzed using the HARmonic Phase (HARP, Diagnosoft, CA, USA) technique (19,29–33). Only the mid-ventricular slice was analyzed, based on our experience and others’ (19) of limited reproducibility of the basal and apical slices. Details of εcc analysis are described in Supplemental Methods. The εcc data was exported to a spreadsheet file for analysis. An average of all the regional values per subject was calculated as a composite regional strain value, to allow comparison of single index of regional strain value (19,30–32,34). The average εcc of all subjects were than tabulated and grouped according to the Group stratification criteria (METHODS, Figure 1). All HARP strain analyses were performed by an expert reader (KNH). To assess interobserver variability of HARP strain analyses, a second expert reader (WMG) performed the same analysis on subsets of patient (n=10) and control (n=5) data.
Statistical Analyses
All statistical analyses were performed using SPSS software v16 (SPSS Inc, Chicago, IL). Differences in the means between the groups for all paremetric data were assessed by ANOVA. Due to unequal variance, post hoc analysis was performed using the Games-Howell procedure to determine significance. For non-parametric data, Mann Whitney-U was performed. Probability values < 0.05 were considered statistically significant.
RESULTS
Study Population
Data from 70 DMD patients (ages 7 to 26 years) and 16 controls (age 6 to 34 years) were included in the study. MDE sequence imaging was performed in 54/70 DMD patients; MDE imaging was not performed in 16/70 patients (9 from Group B, 5 from Group C and 2 from Group D) lacking intravenous access. Patient stratification (Figure 1) revealed the following: Group A (controls, n = 16); Group B (DMD patients ≤10 years, n = 16), Group C (DMD patients > 10 years with normal EF, n = 31), Group D (DMD patients > 10 years with low EF but no MDE, n = 12), and Group E (DMD patients >10 years with low EF and positive MDE, n = 11). Demographic data of the DMD and control groups were not significantly different (Table 1). ECG findings of relative tachycardia were found in DMD patients, consistent with prior publications (35). None of the patients had ventricular hypertrophy as evidenced by a normal mass-volume ratio and normal wall thicknesses (Supplemental Table 1).
Table 1.
Comparison of CMR Findings Between Control and DMD Groups
| Patient Groups Parameters | A ( n = 16) | B (n = 16) | C (n = 31) | D (n =12) | E (n = 11) |
|---|---|---|---|---|---|
| Age (years) | 14.5±8.4* | 8.4±0.84* | 13.0±2.9* | 15.8±4.5 | 17.3±5.3 |
| Heart Rate (bpm) | 81±15* | 106±15 | 101±16 | 103±6 | 112±19 |
| LVM/BSAZ | −2.0±1.2 | −2.0±0.76* | −1.5±0.60 | −0.88±1.7* | 0.46±1.1 |
| LVEDV/BSAZ | −2.0±0.96 | −1.6±0.70 | −2.1±1.4 | −0.20±3.9 | 2.6±3.8 |
| EDMass/LHT2.7 | 18.5±6.3* | 25.8±5.0 | 26.7±4.2 | 28.8±9.2* | 37±10 |
| EF (%) | 65.1±3.7 | 65.5±3.7 | 64.1±5.5* | 47.4±7.4* | 32.7±14.9 |
| εcc (%) | −18.6±2.0* | −14.4±1.1* | −12.4±2.1* | −10±1.9* | −6.5±1.2 |
| MDE Performed | 0/16 | 7/16 | 26/31 | 10/12 | 11/11 |
| MDE Result | --------------- | Negative | Negative | Negative | Negative |
Abbreviations: BPM = beat per minute, CMR = Cardiac Magnetic Resonance Imaging, DMD = Duchenne Muscular Dystrophy, ECG = Electrocardiogram, εcc = Circumferential Strain, EDMass/HT2.7 = Left Ventricular Endiastolic Mass ÷ height2.7, EF = Ejection Fraction, Group A = Control, Group B = DMD age ≤10, Group C = DMD age >10 Normal EF and MDE negative, Group D = DMD age >10 Reduced EF and MDE negative, Group E = DMD age >10 Reduced EF and MDE positive, LVM/BSAZ = Left Ventricular Mass Z-score adjusted to Body Surface Area, LVEDV/BSAZ = Left Ventricular Endiastolic Volume Z-score adjusted to Body Surface Area, MDE = Myocardial Delayed Enhancement, n = Number of patient in each group
P value (<0.05) is significant compared to the preceding group.
Circumferential Strain Values
Control Subject Strain Data
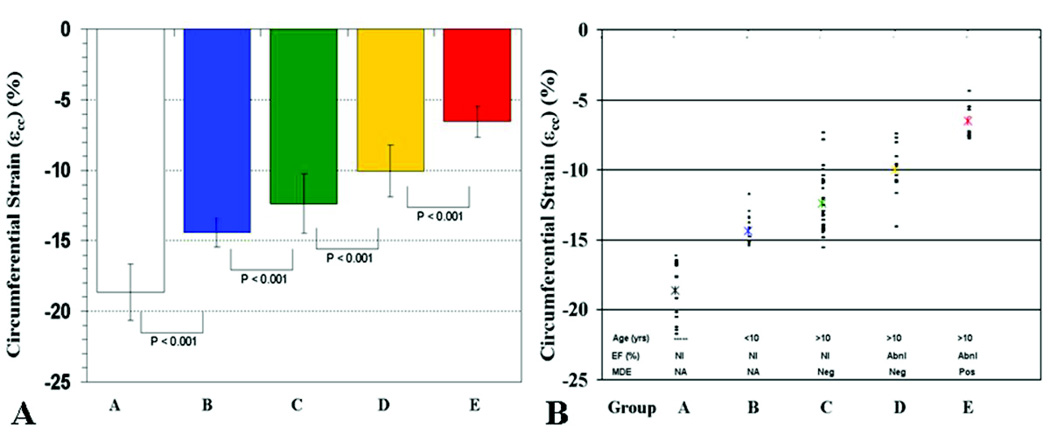
All Group A subjects had εcc < −16% (Figure 5B). Given the wide range of age and mean heart rate of control subjects, we performed an analysis of the effect of heart rate on εcc. We divided control subjects into age ≤10 years (n = 7, mean = 8±1.3) years or age > 10 years (n = 9, mean = 19.6±8). There was no significant difference in εcc (−19.3% vs. −18.2%, p = NS) or EF (66.3% vs. 64.2%, p=NS) between the subgroups, despite significant differences in heart rate between the groups (90 bpm vs. 75 bpm, p = 0.03).
Figure 5. Graphs of εcc values per strata.
(A). Bar graph shows statistically significant (p< 0.05) progressive reduction in εcc for each strata (Groups B–E) compared to controls (Group A). In addition, each strata is statistically different from other strata (B vs C, C vs D, D vs E). (B). Scatter graph of εcc of control (A) and DMD patients (B–E). No control subjects have εcc < −16% and no DMD subjects have εcc > − 16%. The asterisk (*) indicates the mean εcc of each group.
DMD Patient Strain Data
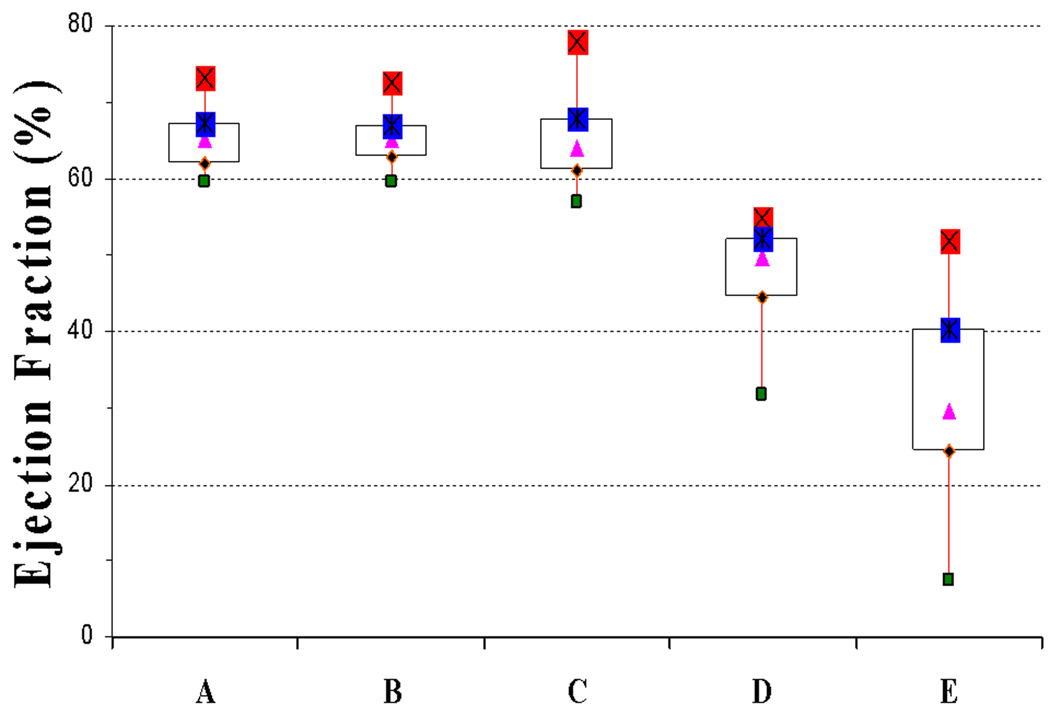
No DMD patient had εcc < − 16%, even in the youngest DMD patients (Group B) with normal EF (Figure 5B). Compared to the control subjects, two DMD groups (Group B and C) had similar EF (65.5% vs. 64.1% v, p = NS, Table 1). However, despite similarity of EF, εcc was significantly decreased in both Group B (−14.4%) vs. −18.6%, p<0.001) and Group C (−12.4% vs. −14.4%, p<0.001) compared to the control group. Furthermore, the presence of reduced EF (47.4%, Group D) was associated with a further reduction in εcc (−10%) vs. −12.4%), p< 0.001) compared to age-matched DMD cohort with normal EF (64.1%o, Group C). The presence of overt ventricular dysfunction (EF = 32.7%) and MDE (Group E) was associated with significantly decreased εcc compared to Group D (−6.5% vs. −10%, p<0.0001) (Figure 4 and Figure 5A).
Figure 4. Box plot of ejection fraction (EF) per strata.
Normal EF is seen in controls (Group A) and also in DMD patients (Groups B and C). Progressive decline in EF is seen in older patients (Group D), with further decline once MDE is present (Group E).
Variability of HARP Strain Measurements
Two independent observers (WMG, KNH) blindly performed separate quantitative strain analyses of myocardial cine MR-tagging images in 10 DMD patients and 5 control subjects. As previously shown (36), interobserver variability was low with a mean difference of 0.009%±0.05%.
DISCUSSION
A major finding of this study is the detection of abnormal εcc in young (< 10 years, Group B) DMD patients despite normal EF. There was further reduction in εcc in older DMD patients with normal EF (Group C), a finding similar to that previously reported by Ashford et al in a cohort of DMD patients similar in mean age to Group C (19). It is important to note that the mean age of Groups B and C differs by almost 5 years, again placing emphasis on the young age at which reduced strain can be observed. In addition, with advancing age and reduced EF, there was further reduction in εcc. A relationship between strain reduction and disease severity was further exemplified when MDE, the CMR marker of myocardial fibrosis, was considered. Taken together, we concluded that εcc may be of value in defining the natural history of cardiac dysfunction in DMD and be a useful marker to assess therapeutic efficacy in young patients with normal global cardiac function.
It is not surprising to find abnormal εcc in relatively young DMD boys. DMD results from mutation in dystrophin, a large cytoskeletal protein localized to the inner surface of the sarcolemma membrane (1). Dystrophin mutation results in greatly reduced or absent dystrophin leading to a weakened sarcolemma that is more easily damaged during muscle contraction. A longstanding hypothesis regarding DMD disease pathogenesis is that loss of membrane integrity is a primary event leading to degeneration of myocytes. Intermittent tears in the cell membrane permit influx of calcium that then functions as a primary inducer of a destructive cascade culminating in myocyte necrosis and replacement fibrosis (37–39). Recent observations that the Angiotensin II receptor blocker (ARB), losartan, reduces fibrotic disease in the mdx mouse implicates involvement of the TGFβ1 and angiotensin II effector pathways in DMD pathogenesis (40). Collectively, these processes lead to necrosis, inflammation and fibrosis manifested clinically by a progressive cardiac dysfunction (37,38,40,41). As these processes are ongoing even in early stages of disease, abnormal εcc should be expected.
There has long been interest in identification of early indicators of abnormal cardiac function in the DMD population. However, TTE evidence of cardiac dysfunction is not evident until late in the disease course (2,3,13,14,41,42). Studies utilizing tissue Doppler imaging and strain rate imaging may detect early alteration in systolic and/or diastolic function compared to conventional imaging indexes such as EF and ventricular dimensions in DMD boys. In addition, TTE-based ultrasonic tissue characterization has been advocated as a means to characterize preclinical myocardial changes in DMD patients via integrated backscatter indices (10,43). Although ultrasonic tissue characterization may prove to be useful for myocardial assessment, it too is limited by acoustic windows and angle dependence (10,43–45).
CONCLUSION
CMR strain analysis in DMD patients demonstrates occult cardiovascular dysfunction in the presence of normal global function. The occult dysfunction progresses to global dysfunction with advancing age. Detection of such strain abnormalities may allow a better definition of the natural history of DMD cardiac dysfunction, and also may provide a useful surrogate index to assess therapeutic efficacy.
STUDY LIMITATIONS
Although significant differences in εcc were demonstrated between young DMD patients with normal EF and older patients with reduced EF, this is a cross-sectional and not a serial study. Accordingly, repeat serial examinations would provide a more robust analysis of longitudinal εcc in this patient population. In the current study, only the mid-ventricular slice was analyzed secondary to our experience of limited reproducibility of the basal and apical slices. Studies were performed with two different vendors and magnetic field strengths, but we do not believe this confounds our data (27). Further MDE imaging was not performed in some younger patients with normal EF, nor in any of the control subjects. In our experience, MDE occurs late in the course of the disease, so it can reasonably be expected to have been absent in those individuals. Lastly, due to the size of our study population we were not able to stratify εcc based on dystrophin genotype; such stratification may require multicenter studies. Despite these limitations, we proposed that using, εcc, determined by HARP® analysis, appears to be a sensitive indicator of cardiac dysfunction in DMD patients.
Supplementary Material
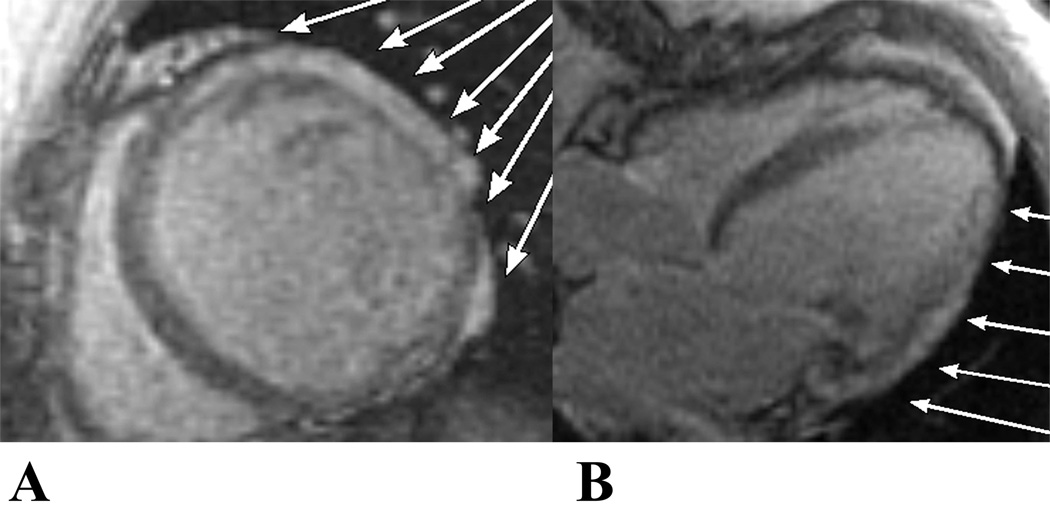
Figure 3. Delayed enhancement (MDE), a CMR marker of myocardial fibrosis.
MDE in the short axis (A) and long axis (B) planes indicates myocardial fibrosis in a 20 year old DMD patient as shown by the white arrows.
ACKNOWLEDGEMENTS
We wish to recognize additional members of the CCHMC CMR Team (Eric Crotty MD, Kathy Helton MD, Amy Tipton, BFA. at some point you will need their written permission to acknowledge them) for clinical data acquisition and analysis
Supported in part by the Children’s Heart Association of Cincinnati (WMG) and the National Institutes of Health HL069712 (DWB) Bethesda, MD.
ABBREVIATION LIST
- BPM
beat per minute
- CMR
cardiac magnetic resonance imaging
- DMD
Duchenne Muscular Dystrophy
- ECG
electrocardiogram
- εcc
circumferential strain
- EDMass/HT2.7
left ventricular endiastolic mass ÷ height2.7
- EF
ejection fraction
- LVM/BSAZ
left ventricular mass z-score adjusted to body surface area
- LVEDV/BSAZ
left ventricular end diastolic volume z-score adjusted to body surface area
- MDE
myocardial delayed enhancement
- TTE
transthoracic echocardiogram
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest and Financial Disclosure: None
References
- 1.McKusick V. Online Mendelian Inheritance in Man, OMIM (TM) Johns Hopkins University; 2005. [Google Scholar]
- 2.de Kermadec JM, Becane HM, Chenard A, Tertrain F, Weiss Y. Prevalence of left ventricular systolic dysfunction in Duchenne muscular dystrophy: an echocardiographic study. Am Heart J. 1994;127:618–623. doi: 10.1016/0002-8703(94)90672-6. [DOI] [PubMed] [Google Scholar]
- 3.Angermann C, Spes C, Pongratz D. Cardiac manifestation of progressive muscular dystrophy of the Duchenne type. Z Kardiol. 1986;75:542–551. [PubMed] [Google Scholar]
- 4.Moriuchi T, Kagawa N, Mukoyama M, Hizawa K. Autopsy analyses of the muscular dystrophies. Tokushima J Exp Med. 1993;40:83–93. [PubMed] [Google Scholar]
- 5.Bushby K, Muntoni F, Urtizberea A, Hughes R, Griggs R. Report on the 124th ENMC International Workshop. Treatment of Duchenne muscular dystrophy; defining the gold standards of management in the use of corticosteroids. 2–4 April 2004, Naarden, The Netherlands. Neuromuscul Disord. 2004;14:526–534. doi: 10.1016/j.nmd.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Markham LW, Spicer RL, Cripe LH. The heart in muscular dystrophy. Pediatr Ann. 2005;34:531–535. doi: 10.3928/0090-4481-20050701-10. [DOI] [PubMed] [Google Scholar]
- 7.Finder JD, Birnkrant D, Carl J, et al. Respiratory care of the patient with Duchenne muscular dystrophy: ATS consensus statement. Am J Respir Crit Care Med. 2004;170:456–465. doi: 10.1164/rccm.200307-885ST. [DOI] [PubMed] [Google Scholar]
- 8.Eagle M, Baudouin S, Chandler C, Giddings D, Bullock R, Bushby K. Survival in Duchenne muscular dystrophy:Improvements in life expectancy since 1967 and the impact of home nocturnal ventilaton. Neuromuscul Disord. 2002;12:926–929. doi: 10.1016/s0960-8966(02)00140-2. [DOI] [PubMed] [Google Scholar]
- 9.Nigro G, Comi LI, Politano L, Bain RJ. The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy. Int J Cardiol. 1990;26:271–277. doi: 10.1016/0167-5273(90)90082-g. [DOI] [PubMed] [Google Scholar]
- 10.Giglio V, Pasceri V, Messano L, et al. Ultrasound tissue characterization detects preclinical myocardial structural changes in children affected by Duchenne muscular dystrophy. J Am Coll Cardiol. 2003;42:309–316. doi: 10.1016/s0735-1097(03)00581-3. [DOI] [PubMed] [Google Scholar]
- 11.Sasaki K, Sakata K, Kachi E, Hirata S, Ishihara T, Ishikawa K. Sequential changes in cardiac structure and function in patients with Duchenne type muscular dystrophy: a two-dimensional echocardiographic study. Am Heart J. 1998;135:937–944. doi: 10.1016/s0002-8703(98)70057-2. [DOI] [PubMed] [Google Scholar]
- 12.Takenaka A, Yokota M, Iwase M, Miyaguchi K, Hayashi H, Saito H. Discrepancy between systolic and diastolic dysfunction of the left ventricle in patients with Duchenne muscular dystrophy. Eur Heart J. 1993;14:669–676. doi: 10.1093/eurheartj/14.5.669. [DOI] [PubMed] [Google Scholar]
- 13.Danilowicz D, Rutkowski M, Myung D, Schively D. Echocardiography in duchenne muscular dystrophy. Muscle Nerve. 1980;3:298–303. doi: 10.1002/mus.880030405. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg SJ, Stern LZ, Feldman L, Allen HD, Sahn DJ, Valdes-Cruz LM. Serial two-dimensional echocardiography in Duchenne muscular dystrophy. Neurology. 1982;32:1101–1105. doi: 10.1212/wnl.32.10.1101. [DOI] [PubMed] [Google Scholar]
- 15.Jefferies JL, Eidem BW, Belmont JW, et al. Genetic predictors and remodeling of dilated cardiomyopathy in muscular dystrophy. Circulation. 2005;112:2799–2804. doi: 10.1161/CIRCULATIONAHA.104.528281. [DOI] [PubMed] [Google Scholar]
- 16.Silva MC, Meira ZM, Gurgel Giannetti J, et al. Myocardial delayed enhancement by magnetic resonance imaging in patients with muscular dystrophy. J Am Coll Cardiol. 2007;49:1874–1879. doi: 10.1016/j.jacc.2006.10.078. [DOI] [PubMed] [Google Scholar]
- 17.White JA, Patel MR. The role of cardiovascular MRI in heart failure and the cardiomyopathies. Cardiol Clin. 2007;25:71–95. doi: 10.1016/j.ccl.2007.02.003. vi. [DOI] [PubMed] [Google Scholar]
- 18.Macedo R, Schmidt A, Rochitte CE, Lima JA, Bluemke DA. MRI to assess arrhythmia and cardiomyopathies: relationship to echocardiography. Echocardiography. 2007;24:194–206. doi: 10.1111/j.1540-8175.2007.00376.x. [DOI] [PubMed] [Google Scholar]
- 19.Ashford MW, Jr, Liu W, Lin SJ, et al. Occult cardiac contractile dysfunction in dystrophin-deficient children revealed by cardiac magnetic resonance strain imaging. Circulation. 2005;112:2462–2467. doi: 10.1161/CIRCULATIONAHA.104.516716. [DOI] [PubMed] [Google Scholar]
- 20.Pennell DJ, Sechtem UP, Higgins CB, et al. Clinical indications for cardiovascular magnetic resonance (CMR): Consensus Panel report. J Cardiovasc Magn Reson. 2004;6:727–765. doi: 10.1081/jcmr-200038581. [DOI] [PubMed] [Google Scholar]
- 21.Pohost GM, Hung L, Doyle M. Clinical use of cardiovascular magnetic resonance. Circulation. 2003;108:647–653. doi: 10.1161/01.CIR.0000083233.86078.3E. [DOI] [PubMed] [Google Scholar]
- 22.Flacke SJ, Fischer SE, Lorenz CH. Measurement of the gadopentetate dimeglumine partition coefficient in human myocardium in vivo: normal distribution and elevation in acute and chronic infarction. Radiology. 2001;218:703–710. doi: 10.1148/radiology.218.3.r01fe18703. [DOI] [PubMed] [Google Scholar]
- 23.Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 24.McCrohon JA, Moon JC, Prasad SK, et al. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54–59. doi: 10.1161/01.CIR.0000078641.19365.4C. [DOI] [PubMed] [Google Scholar]
- 25.van der Geest RJ, Buller VG, Jansen E, et al. Comparison between manual and semiautomated analysis of left ventricular volume parameters from short-axis MR images. J Comput Assist Tomogr. 1997;21:756–765. doi: 10.1097/00004728-199709000-00019. [DOI] [PubMed] [Google Scholar]
- 26.van der Geest RJ, Reiber JH. Quantification in cardiac MRI. J Magn Reson Imaging. 1999;10:602–608. doi: 10.1002/(sici)1522-2586(199911)10:5<602::aid-jmri3>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 27.Valeti VU, Chun W, Potter DD, et al. Myocardial tagging and strain analysis at 3 Tesla: comparison with 1.5 Tesla imaging. J Magn Reson Imaging. 2006;23:477–480. doi: 10.1002/jmri.20527. [DOI] [PubMed] [Google Scholar]
- 28.Hinton DP, Wald LL, Pitts J, Schmitt F. Comparison of cardiac MRI on 1.5 and 3.0 Tesla clinical whole body systems. Invest Radiol. 2003;38:436–442. doi: 10.1097/01.RLI.0000067489.31556.70. [DOI] [PubMed] [Google Scholar]
- 29.Gotte MJ, Germans T, Russel IK, et al. Myocardial strain and torsion quantified by cardiovascular magnetic resonance tissue tagging: studies in normal and impaired left ventricular function. J Am Coll Cardiol. 2006;48:2002–2011. doi: 10.1016/j.jacc.2006.07.048. [DOI] [PubMed] [Google Scholar]
- 30.Osman NF, Kerwin WS, McVeigh ER, Prince JL. Cardiac motion tracking using CINE harmonic phase (HARP) magnetic resonance imaging. Magn Reson Med. 1999;42:1048–1060. doi: 10.1002/(sici)1522-2594(199912)42:6<1048::aid-mrm9>3.0.co;2-m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osman NF, McVeigh ER, Prince JL. Imaging heart motion using harmonic phase MRI. IEEE Trans Med Imaging. 2000;19:186–202. doi: 10.1109/42.845177. [DOI] [PubMed] [Google Scholar]
- 32.Osman NF, Prince JL. Regenerating MR tagged images using harmonic phase (HARP) methods. IEEE Trans Biomed Eng. 2004;51:1428–1433. doi: 10.1109/TBME.2004.827932. [DOI] [PubMed] [Google Scholar]
- 33.Garot J, Bluemke DA, Osman NF, et al. Fast determination of regional myocardial strain fields from tagged cardiac images using harmonic phase MRI. Circulation. 2000;101:981–988. doi: 10.1161/01.cir.101.9.981. [DOI] [PubMed] [Google Scholar]
- 34.Osman NF, Prince JL. Visualizing myocardial function using HARP MRI. Phys Med Biol. 2000;45:1665–1682. doi: 10.1088/0031-9155/45/6/318. [DOI] [PubMed] [Google Scholar]
- 35.Bhattacharyya KB, Basu N, Ray TN, Maity B. Profile of electrocardiographic changes in Duchenne muscular dystrophy. J Indian Med Assoc. 1997;95:40–42. 47. [PubMed] [Google Scholar]
- 36.Castillo E, Osman NF, Rosen BD, et al. Quantitative assessment of regional myocardial function with MR-tagging in a multi-center study: interobserver and intraobserver agreement of fast strain analysis with Harmonic Phase (HARP) MRI. J Cardiovasc Magn Reson. 2005;7:783–791. doi: 10.1080/10976640500295417. [DOI] [PubMed] [Google Scholar]
- 37.Fong PY, Turner PR, Denetclaw WF, Steinhardt RA. Increased activity of calcium leak channels in myotubes of Duchenne human and mdx mouse origin. Science. 1990;250:673–676. doi: 10.1126/science.2173137. [DOI] [PubMed] [Google Scholar]
- 38.Shigihara-Yasuda K, Tonoki H, Goto Y, et al. A symptomatic female patient with Duchenne muscular dystrophy diagnosed by dystrophin-staining: a case report. Eur J Pediatr. 1992;151:66–68. doi: 10.1007/BF02073897. [DOI] [PubMed] [Google Scholar]
- 39.Williams IA, Allen DG. The role of reactive oxygen species in the hearts of dystrophin-deficient mdx mice. Am J Physiol Heart Circ Physiol. 2007;293:H1969–H1977. doi: 10.1152/ajpheart.00489.2007. [DOI] [PubMed] [Google Scholar]
- 40.Habashi JP, Judge DP, Holm TM, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Markham LW, Michelfelder EC, Border WL, et al. Abnormalities of diastolic function precede dilated cardiomyopathy associated with Duchenne muscular dystrophy. J Am Soc Echocardiogr. 2006;19:865–871. doi: 10.1016/j.echo.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Backman E, Nylander E. The heart in Duchenne muscular dystrophy: a noninvasive longitudinal study. Eur Heart J. 1992;13:1239–1244. doi: 10.1093/oxfordjournals.eurheartj.a060343. [DOI] [PubMed] [Google Scholar]
- 43.Mori K, Manabe T, Nii M, Hayabuchi Y, Kuroda Y, Tatara K. Myocardial integrated ultrasound backscatter in patients with Duchenne's progressive muscular dystrophy. Heart. 2001;86:341–342. doi: 10.1136/heart.86.3.341a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chetboul V, Escriou C, Tessier D, et al. Tissue Doppler imaging detects early asymptomatic myocardial abnormalities in a dog model of Duchenne's cardiomyopathy. Eur Heart J. 2004;25:1934–1939. doi: 10.1016/j.ehj.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 45.Towbin JA. A noninvasive means of detecting preclinical cardiomyopathy in Duchenne muscular dystrophy? J Am Coll Cardiol. 2003;42:317–318. doi: 10.1016/s0735-1097(03)00580-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.