Abstract
Tumor-derived growth factors and cytokines stimulate neoangiogenesis from surrounding capillaries in order to support tumor growth. Recent studies reveal that macrophage migration inhibitory factor (MIF) expression is increased in lung cancer, particularly non-small cell lung carcinomas (NSCLC). Because MIF has important autocrine effects on normal and transformed cells, we investigated whether autocrine MIF and its only known family member, D-dopachrome tautomerase (D-DT), promote the expression of pro-angiogenic factors CXCL8 and VEGF in NSCLC cells. Our results demonstrate that the expression of CXCL8 and VEGF are strongly reliant upon both the individual and cooperative activities of the two family members. CXCL8 transcriptional regulation by MIF and D-DT appears to involve a signaling pathway that includes the activation of c-Jun-N-terminal Kinase (JNK), c-jun phosphorylation and subsequent AP-1 transcription factor activity. Importantly, human umbilical vein endothelial cell (HUVEC) migration and tube formation induced by supernatants from lung adenocarcinoma cells lacking either or both MIF and D-DT are substantially reduced when compared to normal supernatants. Finally, we demonstrate that the cognate MIF receptor, CD74, is necessary for both MIF and D-DT-induced JNK activation and CXCL8 expression, suggesting its potential involvement in angiogenic growth factor expression. This is the first demonstration of a biological role for D-DT and its synergism with MIF suggests that the combined therapeutic targeting of both family members may enhance current anti-MIF based therapies.
Keywords: Angiogenesis, HUVEC, CXCL8, Migration, NSCLC, VEGF
INTRODUCTION
Lung cancer is one of the leading causes of cancer-related deaths in the US. Overall, survival is poor and has not improved substantially over the last half century1,2 Of the three types of lung cancer, the most common are non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) which together account for over 90% of all pulmonary malignant diseases. Tumor growth, invasion, and metastasis are dependent on a pro-angiogenic environment. In normal tissues, angiogenesis is carefully regulated by maintaining an even balance between pro and anti-angiogenic factors. However, within a tumor microenvironment, the balance is tipped in favor of pro-angiogenic growth factors that serve to promote a constant, disorganized, state of blood vessel formation3.
Vascular endothelial growth factor (VEGF) is a key mediator of tumor-associated angiogenesis and is thought to support neovascularization by inducing endothelial cell migration, and proliferation leading to vascular permeability4. In addition to VEGF, CXCL8 (formerly referred to as interleukin-8 or IL-8) supports angiogenesis directly through the induction of endothelial cell chemotaxis and proliferation and indirectly by recruiting inflammatory effector cells to tumor tissue where they secrete additional pro-angiogenic factors5. A member of the CXC chemokine family, CXCL8 binds to and signals from CXCR2 receptors which are reportedly the primary functional chemokine receptor in endothelial cell chemotaxis6. Like VEGF, inflammatory cell-derived CXCL8 has been shown to be associated with promoting angiogenesis in NSCLC7–9.
One of the first cytokine activities ever described, macrophage migration inhibitory factor (MIF) is a pro-inflammatory cytokine that is released from activated macrophages and other immune effector cells10,11. Several recent studies have shown that MIF is over-expressed in a variety of human tumors, most notably non-small cell lung cancers7,12–15.
From crystallographic studies, MIF exists as a homotrimer and shares structural homology with two bacterial enzymes16. Extracellular MIF signals through a CD74/CD44 cell surface receptor complex that serves to initiate MIF-dependent activation of several signaling pathways17,18. Interestingly, a recent study revealed that MIF is also a non-cognate ligand for both CXCR2 and CXCR4 chemokine receptors19.
Unlike other cytokines, MIF has the unique ability to catalyze a non-physiologic enzymatic reaction. MIF converts D-Dopachrome into 5, 6-dihydroxyindole-2-carboxylic acid. The only known MIF homolog, D-dopachrome tautomerase (D-DT), retains this tautomerase activity but also de-carboxylates the D-dopachrome substrate to give a 5, 6-dihydroxyindole product20. While D-DT retains only 38% identity and 49% homology to MIF, the tertiary structure of D-DT is remarkably similar21. Despite these intriguing similarities to the very well studied MIF, there have been no reports to date on the biologic function(s) of D-DT.
Several studies have shown that MIF promotes both the expression and secretion of CXCL8 and VEGF from an array of different cell types22–25. Interestingly, one such study reported that lung adenocarcinoma-derived MIF induces the expression of stromal macrophage CXCL826. A recent investigation from our laboratory reveals that in human lung adenocarcinoma cells, autocrine-acting MIF is necessary for Rho GTPase Rac1 effector binding, c-Jun-N-terminal Kinase (JNK) activation and subsequent cell migratory and invasive properties12. Not coincidentally, the JNK activation pathway has been demonstrated to be responsible for the transcription of several gene products induced by MIF27–29,30.
Because the importance of MIFs contributions to many tumor-associated processes is generally accepted, we sought to determine whether the only known MIF homolog functionally regulates, or similarly contributes to, MIF-dependent tumor angiogenesis. We now present evidence that MIF and D-DT, individually and additively, promote VEGF and CXCL8 expression in human lung adenocarcinoma cell lines. Moreover, both MIF family members are required for maximal NSCLC-induced endothelial cell migration and vascular tube formation. Finally, our data indicates that MIF and D-DT signaling to angiogenic growth factor production is initiated by the MIF receptor CD74 and converges upon JNK activation and AP-1-dependent transcription.
MATERIALS AND METHODS
Cell Culture
Murine embryonic fibroblast and A549 lung adenocarcinoma (ATTC, Manassas, VA) cell lines were maintained in Dulbecco's Modified Eagle Medium (DMEM) and H23 was cultured in RPMI media (Invitrogen, Carlsbad, CA). All media was supplemented with 10% FBS, L-Glutamate, and Gentamycin. Human umbilical vein endothelial cells (HUVECs), (Cambrex, Walkersville, MD) were maintained in EGM media (Cambrex) supplemented with growth factors and Gentamicin Amphotericin-B and passaged on gelatin coated plates.
siRNA
A549 and H23 lung cancer cells were plated at ~20% confluency and incubated overnight at 37°C in a 5% CO2 incubator. Cells were transfected with 50 nM annealed siRNA oligos using Oligofectamine reagent (Invitrogen) following the manufacturer’s protocol. The targeted base sequence for human MIF was: 5’-CCTTCTGGTGGGGAGAAAT-3’ (Dharmacon, Lafayette, CO). The targeted base sequence for human D-DT was: 5’-CTGGCAGATTGGCAAGATA-3’ (Dharmacon, Lafayette, CO). Scrambled oligos for MIF and D-DT were 5 ’- ACGATCCGGATGTGAGTGT-3’ and 5’-TGACGCAGTATCGATGCCA-3’, respectively. The targeted base sequence for human CD74 was: 5’-AAACTGACAGTCACCTCCCAG-3’. A commercially available siRNA referred to as non-specific (NS) oligo (Dharmacon) was used as an internal siRNA control for CD74 experiments.
ELISAs
Cytokines were measured by ELISA from supernatants of lung adenocarcinoma cells that were subjected to siRNA and allowed to incubate 3–6 days depending on the experiment. ELISA kits used were human CXCL8 ELISA DuoSet system Elisa Development kit and human VEGF Elisa kit (R&D Systems, Minneapolis, MN). Assays and measurements were performed according to the manufacturer’s protocols.
Human Umbilical Vein Endothelial Cell Migration Assay
Modified Boyden chambers (Millicell-PCF, 8 µm pore size; Millipore, Bedford MA) were placed in a 24 well plate and coated with 10 µg/ml rat tail collagen (Roche Diagnostics, Mannheim, Germany) for 16 h at 37° C. After removal of collagen and washing with PBS, 0.4 ml of conditioned media was placed in the bottom chamber. In some cases, neutralizing mAbs against human CXCL8 and VEGF (R & D Systems) were added to conditioned supernatants prior to co-culture with HUVECs. 2×105 HUVECs were re-suspended in 0.3 mls serum-free media, plated in the top of the transwell chambers and incubated for 24 hours at 37°C with 5% CO2. Cells were removed from the upper membrane surface with a cotton tip applicator, washed with PBS and cells on the lower membrane surface were fixed with 4% formaldehyde. HUVEC migration was quantified by manually counting the number of cells on the inserts under low power at (40x) magnification.
Human Umbilical Vein Endothelial Tube Formation
Plates (48-well) were coated with 200 µl of an equal mixture of Growth Factor-Depleted Matrigel + serum-free DMEM and incubated overnight at 37°C. The following day, 7 × 105 HUVECs were resuspended in 0.3 mls of the appropriate conditioned media, dispensed onto GF-depleted Matrigel coated wells and incubated for 24 hrs. Tubes were quantified by counting the number of connecting branches between discrete endothelial cells. Individual wells were photographed with a Nikon Act 1 phase-contrast microscope at (10x) magnification.
Luciferase Assay
CXCL8 wt and mutant promoter luciferase reporter plasmids were kindly provided by Dr. Allan Brasier (University of Texas Medical Branch). 0.4 × 105 cells/ml were plated in a 24 well plate and transfected with siRNA the following day for 48 hours. Cells were then transiently co-transfected with 0.125 µg/well of CXCL8 WT or CXCL8 mutant luciferase promoter plasmid together with 0.0125 µg/well Renilla pRL-null plasmid (Promega) using Lipofectamine (Invitrogen) transfection reagent. After 24 hrs, Firefly and Renilla luciferase activities were measured by the Dual Luciferase Reporter Assay System (Promega, Madison, WI) on a TD-20/20 luminometer (Turner Designs).
Adenovirus
Adenoviruses for MIF and D-DT were generated using the Gateway cloning system (Invitrogen). Briefly, human MIF and D-DT were PCR amplified and TOPO cloned into the pENTR D-TOPO plasmid. LR Recombinase (Invitrogen) was used to shuttle inserts into the pAd/CMV/V5-DEST destination vector and subclones were confirmed by sequencing. Adenoviral vectors were digested with PacI, ethanol precipitated and transfected into 293A adenoviral packaging cells using Lipofectamine (Invitrogen). After amplifying viral supernatants, virus was purified using Virabind purification columns (Cell Biolabs, San Diego, CA) and tested for expression efficiency vs. toxicity.
Quantitative PCR Analysis
Total RNA was extracted from the cells using RNeasy Mini Kit (Qiagen, Valencia, CA). The RNA was transcribed into the first strand cDNA with the Omniscript RT kit (Qiagen) according to the manufacturer’s description. Reactions were performed using a DNA Engine Opticon (Biorad) and amplifications were carried out at a final volume of 25 µl containing 1.5 µl of cDNA, 5 µl of Takara PCR mix 5X (Takara Bio Inc), forward and reverse primers at a 0.3 µM final concentration, and SYBr Green (Molecular Probes) at a final dilution ratio of 25,000. The specific primers were forward 5’-AGAACCGCTCCTACAGCAAG-3 ’ and reverse 5 ’- TAGGCGAAGGTGGAGTTGTT 3’, for MIF; forward 5’-TCTCCTCCATCGGCGTAGTG-3’ and reverse 5’-AATCTGCCAGGACTCCAAG-3’ for D-DT; 5'-ATGACTTCCAAGCTGGCC GTGGCT and reverse 5'-TCTCAGCCCTCTTCAAAAACTTCTC for CXCL8; forward 5’ CAAGGCCAACCGCGAGAAGA 3’ and reverse 5’ GGATAGCACAGCCTGGATAG 3’ for β- actin. Relative expression was determined using the delta Ct method.
Western blotting
A 1x RIPA buffer containing Na3VO4, NaF and a protease inhibitor cocktail was used to lyse cells. Adherent NSCLC cells were scraped and homogenized with a 23 gauge needle. Protein concentration was measured using the DC Protein Assay (Biorad, Hercules, CA). Equal amounts of Laemmli sample buffer and protein lysate were added together, boiled for 10 minutes, and loaded on 4–20 % SDS-polyacrylamide gel and blotted to PVDF. Blots were probed with antibodies that recognize: MIF (pAb, Santa Cruz), D-DT (rabbit polyclonal – see below), total JNK (pAb, Cell Signaling), phospho-JNK (pAb, Cell Signaling) and phospho-c-jun (pAb, Cell Signaling) followed by horseradish peroxidase-conjugated anti-rabbit antibody and detected using enhanced chemiluminescence. β-actin immunoblotting (mAb, Sigma) was used to assure equal protein loading.
Recombinant D-DT and D-DT antibody production
Recombinant His-tagged D-DT fusion protein was prepared by PCR amplifying the ORF of human D-DT using BamH1 and Xho1 linked primers respectively: 5’-CACCATGCCGTTCCTGGAGCTGGAC-3’ and 5’-TAAAAAAGTCATGACCGTC-3’. PCR amplicon and pET 21b vector (Novagen, Madison, WI) were digested with BamH1 and Xho1, purified by GeneClean II (QBiogene, Carlsbad, CA) and ligated. pET21b/D-DT subclones were confirmed by sequencing and transformed into BL21(DE3) competent bacteria (Novagen) and rD-DT was induced by 0.4 mM IPTG for 4 hours followed by bacterial disruption and purification using Ni-NTA sepharose (Novagen). Rabbit polyclonal anti-human D-DT was prepared by immunizing an NZW rabbit with 200 µg recombinant D-DT in suspension with Complete Freund’s Adjuvant (Sigma, St. Louis, MO) followed by boosts at weeks 2 and 4 with 200 µg recombinant D-DT suspended in Incomplete Freund’s Adjuvant (Sigma). Blood was collected by ear bleeds at week 6 and tested for reactivity of human rD-DT by western blotting.
Statistical Analyses
Results were expressed as mean ± S.D. Multiple data comparisons were derived by one way ANOVA followed by Tukey’s post hoc test using GraphPad Prism 4.1 statistical program. P values < 0.05 were considered significant.
RESULTS
MIF and D-DT expression in NSCLC cells is attenuated by siRNA
MIF is over-expressed in non-small cell lung cancers and modulates tumor progression by stimulating both autocrine and paracrine-associated processes7,12,30. However, very little is known about D-DT, particularly its biological functions. Similar to MIF, D-DT is expressed at relatively high levels in both human lung adenocarcinoma cell lines we examined (Figs. 1A and 1B). Because of the structural and enzymatic similarities between MIF and D-DT, we hypothesized that D-DT also participates in tumorigenic processes and may act additively with MIF. Therefore, we silenced both MIF and D-DT, individually and in combination, using small-interfering RNA (siRNA) transfection. siRNA effectively abrogated the protein expression of both MIF and D-DT, in A549 (Fig. 1A) and H23 (Fig. 1B) lung adenocarcinoma cells.
FIGURE 1.
Additive contributions by MIF family members to lung adenocarcinoma cell angiogenic growth factor expression. A, A549 lung adenocarcinoma cells were oligo-transfected with 50 nM scrambled MIF + 50 nM scrambled D-DT siRNA (Scr), 50 nM MIF + 50 nM Scr MIF siRNA (MIF), 50 nM D-DT + 50 nM Scr D-DT siRNA (D-DT) and 50 nM MIF + 50 nM D-DT siRNA (MIF + D-DT) or 50 nM scrambled MIF + 50 nM scrambled D-DT siRNA (Scr) and 50 nM MIF + 50 nM D-DT siRNA (MIF + D-DT) in duplicate for H23 cells (B). Cells were harvested, lysed and immunoblotted for MIF, D-DT and β-actin as indicated. A549 cells C, E and H23 cells D, were oligofected as in A. 48 hours later, media was changed and supernatants were collected the next day and analyzed by ELISA for CXCL-8 (C, D), or VEGF (E). ELISA results are expressed ± S.D. of the average of data from duplicate siRNA transfected samples and are representative of at least 2 independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 by one way ANOVA analysis is shown for control and individual group comparisons while statistical significance between relevant groups was found by Tukey’s post hoc test.
Loss of MIF and D-DT reduces basal CXCL8 and VEGF levels in NSCLC cells
We next sought to determine whether there was a functional correlation between MIF, D-DT and CXCL8 levels in NSCLC cells. We consistently observed a 40–50% reduction of basal CXCL8 levels in both A549 (Fig. 1C) and H23 (Fig. 1D) cells subjected to either MIF or D-DT siRNA alone. Intriguingly, combined MIF and D-DT deficiency resulted in ~ 70–80% reductions in basal CXCL8 levels as compared to the control (Figs. 1C, 1D). These results suggest that MIF and D-DT play an important and additive functional role in modulating steady state CXCL8 expression in human non-small cell lung cancer cell lines.
Because VEGF is an important regulator of both normal and dysregulated angiogenesis and MIF has been reported to modulate its expression31–33, we sought to determine whether MIF and D-DT functionally regulate VEGF in lung adenocarcinoma cells. Similar to CXCL8, basal VEGF levels were reduced when MIF and D-DT levels were attenuated individually and, in an additive fashion, when depleted together (Fig. 1E). Combined with results demonstrating both individual and additive requirements for MIF and D-DT in CXCL8 production, (Figs. 1C, 1D) our results support the conclusion that both of these family members are important autocrine contributors to angiogenic growth factor expression in lung adenocarcinoma cells.
MIF and D-DT depletion results in the loss of CXCL8 transcription corresponding to decreased JNK activation, c-jun phosphorylation and AP-1 activity
We next sought to determine whether the loss of CXCL8 expression/secretion was due to defective CXCL8 transcription in cells lacking MIF and D-DT. From Fig. 2A, qPCR analysis of MIF and D-DT-deficient cells resulted in a nearly complete loss of both MIF and D-DT message consistent with the previously observed decreases in protein expression in A549 cells (Fig. 1A). Accompanying decreased MIF and D-DT mRNA was a nearly uniform loss of CXCL8 mRNA, consistent with the reduction of CXCL8 protein secretion observed in MIF and D-DT-deficient cells (Figs. 1C, 1D).
FIGURE 2.
MIF and D-DT additively regulate CXCL-8 transcription and JNK activation. A, MIF, D-DT, IL-8 and β-actin mRNA from A549 cells transfected for 48 hours with either scrambled RNA oligos or MIF + D-DT siRNA oligos was analyzed by quantitative PCR (qPCR). Data shown represents the delta Ct of the average of duplicate reactions for each condition between target mRNA (MIF, D-DT or CXCL-8) and β-actin and is representative of 2 independent experiments. B, A549 lung adenocarcinoma cells were oligo transfected as indicated and 48 hours later cells were harvested, lysed and immunoblotted for phospho-JNK, phosphor-c-jun and total JNK. C, A549 cells were oligofected for 48 hours with either scrambled RNA oligos or MIF + D-DT siRNA oligos and then co-transfected with null Renilla luciferase plasmid (pRL-null Renilla) and either wildtype (IL-8-WT) or mutant CXCL-8 firefly luciferase promoter constructs. 20 hours post transfection, Firefly and Renilla luciferase activities were measured by the Dual Luciferase Reporter Assay System. Results are expressed as fold increase/decrease over control after normalizing ratios of luciferase/Renilla luciferase from triplicate samples.
Several studies have demonstrated an important role for c-Jun-N-Terminal kinase (JNK)-dependent AP-1 activation in MIFs contribution to inflammatory and mesenchymal cell matrix metalloprotease production27–29. Because AP-1 is also found to be an important contributor to both basal and induced CXCL8 in normal34,35 and transformed cells36,37, we investigated whether JNK and/or AP-1 was involved in the regulation of CXCL8 by MIF and D-DT. As shown in Fig. 2B, analysis of phosphorylated JNK in lysates from cells deficient in either MIF or D-DT alone revealed significant reductions in each. More importantly, MIF + D-DT depletion in A549 cells resulted in a synergistic and nearly complete loss of JNK phosphorylation. Consistent with a loss of JNK phosphorylation/activation we observed a very similar pattern of defective c-jun phosphorylation with individual MIF or D-DT knockdown and a nearly complete loss of steady state c-jun phosphorylation with the combined loss of MIF/D-DT (Fig. 2B, bottom panel).
We next used CXCL8 promoter-driven luciferase constructs to further analyze the transcriptional contribution of MIF family members to CXCL8 expression. Using these CXCL8 reporter plasmids we tested MIF/D-DT-deficient cells for their relative CXCL8 driven transcription from wildtype promoters compared to promoters harboring mutated binding sites for NF-IL-6, NF-kB and AP-1. As shown in Fig. 2C, luciferase activity from wildtype CXCL8 promoter was ~ 50% reduced in cells lacking both MIF and D-DT when compared to cells transfected with scrambled oligos only. Interestingly, relative differences in CXCL8 promoter-driven luciferase between MIF/D-DT containing and MIF/D-DT-deficient cells were relatively unchanged by mutation of either NF-IL6 or NF-kB binding sites when compared to wildtype promoters suggesting that these transcription factors are unaffected by loss of MIF and D-DT (Fig. 2C). In contrast, when the consensus sequence for AP-1 was mutated in the CXCL8 promoter, reduction of wildtype luciferase was decreased to the levels of MIF/D-DT knockdown cells indicating that AP-1 activity is necessary for maximal CXCL8 transcription in these cells and that AP-1 activity is defective in MIF/D-DT-deficient cells. Combined with the requirements for both MIF family members in steady state JNK and c-jun phosphorylation, these results suggest that MIF and D-DT may functionally regulate CXCL8 transcription, at least in part, through modulation of the JNK pathway.
Adenoviral delivery of MIF family members rescues JNK activation and CXCL8 expression in MIF/D-DT-deficient cells
In order to test for specificity of MIF and/or D-DT in defective CXCL8 expression, we asked whether forced overexpression of MIF family members can rescue CXCL8 levels in their respective siRNA-knocked down cells. To achieve this, we made adenoviral constructs of both MIF and D-DT and generated high titer adenoviral supernatants of both. To accomplish efficient rescue in cells transfected with MIF and D-DT siRNA, we utilized siRNA that targets the 3’ UTR of MIF mRNA allowing for efficient expression of the MIF open-reading frame. Because D-DT siRNA targets sequences within the open-reading frame of human D-DT (Materials and Methods) we used the murine homolog of D-DT for adenoviral expression because the sequence is not conserved within this siRNA targeting domain.
As shown in Fig. 3B, combined adenoviral transduction of MIF and D-DT efficiently rescues defective JNK phosphorylation concomitant with the restoration of MIF and D-DT expression. To determine if adenoviral MIF family members rescue CXCL8 expression in a manner consistent with their rescue of JNK activation, scrambled and MIF siRNA transfected cells were infected with MIF adenovirus, D-DT siRNA transfected cells were infected with D-DT adenovirus and MIF + D-DT siRNA transfected cells received both adenoviruses. As shown in Fig. 3B, defective CXCL8 expression associated with either loss of MIF or D-DT, was fully rescued by MIF and D-DT adenovirus respectively, while combined MIF + D-DT adenoviruses fully rescued CXCL8 in cells lacking both MIF family members.
FIGURE 3.
Rescue of defective JNK activation and CXCL-8 expression in MIF/D-DT-deficient cells by adenoviral reconstitution of MIF and D-DT. A, A549 lung adenocarcinoma cells were oligo transfected and 48 hours later GFP adenovirus or MIF + D-DT adenovirus was added as indicated. 24 hours after adenovirus addition, cells were lysed and immunoblotted for MIF, D-DT, p-JNK, total JNK and β-actin. B, A549 cells were transfected with scrambled, MIF, D-DT or MIF + D-DT siRNA oligos for 48 hours and adenoviral MIF and/or D-DT was added to cells. 24 hours later, media was replaced and collected the following day for CXCL-8 ELISA measurements. ELISA results are expressed ± S.D. of the average of data from two samples and are representative of 2 independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 by one way ANOVA analysis is shown for control and individual group comparisons while statistical significance between relevant groups was found by Tukey’s post hoc test.
Maximal angiogenic potential of lung adenocarcinoma cells requires both MIF and D-DT
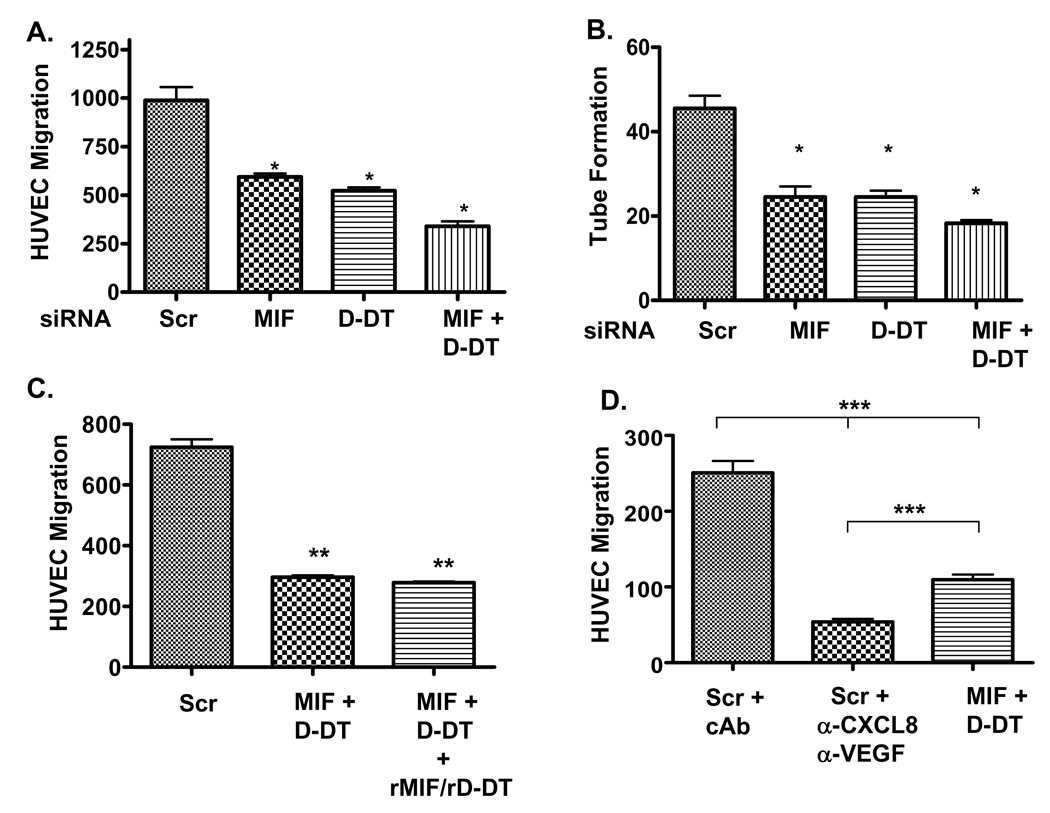
Tumor-initiated angiogenesis arises as a result of secreted, paracrine-acting angiogenic growth factors eliciting responses from stromal endothelial cells and surrounding vasculature. To further study the impact of MIF and D-DT on lung adenocarcinoma-initiated angiogenesis we tested the effects of MIF and/or D-DT depletion on the potential of NSCLC-conditioned supernatants to induce human umbilical endothelial cell (HUVEC) migration and vascular tube formation. Endothelial cells co-cultured with supernatants from control siRNA transfected NSCLC cells were 40–50% more efficient at migrating than those cells co-cultured with supernatants from MIF or D-DT siRNA transfected cells. Additionally, consistent with an additive function for these family members, supernatants from cells deficient in both MIF and D-DT were more than 70% defective in inducing HUVEC migration compared to control cells (Fig. 4A). Mirroring almost exactly the results from endothelial cell migration, loss of MIF and/or D-DT individually and additively resulted in significant reductions in NSCLC supernatant-induced HUVEC tube formation (Fig. 4B). Note also that defects observed in MIF and/or D-DT-deficient cell supernatants correlate closely with defective CXCL8 and VEGF levels in the same supernatants (Fig. 1).
FIGURE 4.
MIF and/or D-DT deficiency renders lung adenocarcinoma supernatants defective in induced endothelial cell migration and tube formation. A, A549 cells were transfected with scrambled, MIF, D-DT or MIF + D-DT siRNA for 48 hours, replaced with fresh media and supernatants were collected 48 hours later. 2 × 105 HUVECs were plated in serum free media in the top chamber and cell free supernatants were added to the bottom chamber of collagen-coated transwell chambers. 24 hours later cells were fixed, stained and enumerated. Results represent the ± S.D. of the average of parallel co-culture assays and are representative of 3 independent experiments. B, Cell free supernatants from (A) were used to re-suspend 7 × 105 HUVECs which were plated onto Growth Factor-Depleted Matrigel coated 48 well plates. 24 hours later, tubes were quantified by counting the number of connecting branches between discrete endothelial cells. Results represent the ± S.D. of the average of parallel co-culture assays and are representative of 4 independent experiments. C, HUVEC migration was determined as in (A) using cell free supernatants from cells transfected with scrambled and MIF + D-DT siRNAs. In one set of supernatants from MIF + D-DT siRNA transfected lung adenocarcinoma cells, rMIF and rD-DT were added at a final concentration of 50 ng/ml of each. Results represent the ± S.D. of the average of parallel co-culture assays and are representative of 3 independent experiments. D, HUVEC migration was determined as in (A) using cell free supernatants from cells transfected with scrambled and MIF + D-DT siRNAs. Neutralizing monoclonal antibodies against CXCL8 and VEGF were added at a final concentration of 10 µg/ml each to supernatants from scrambled siRNA transfected A549 cells. For control, 20 µg/ml isotype control antibody was added (cAb) to supernatants from scrambled siRNA transfected A549 cells. Results represent the ± S.D. of the average of parallel co-culture assays and are representative of 2 independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 by one way ANOVA analysis is shown for control and individual group comparisons while statistical significance between relevant groups was found by Tukey’s post hoc test.
In order to determine whether decreased MIF and/or D-DT levels in the NSCLC supernatants were partially or wholly responsible for the defective phenotypes observed in endothelial cell migration, we added back recombinant MIF and D-DT to the endothelial cell/MIF + D-DT-depleted supernatant co-culture experiment. As shown in Fig. 4C, HUVEC migration was unaffected by the reconstitution of MIF and D-DT in lung adenocarcinoma supernatants suggesting that the defective HUVEC migration observed in these co-culture experiments was likely due to loss of angiogenic growth factors. Finally, because we hypothesized that loss of CXCL8 and VEGF in MIF/D-DT-deficient NSCLC supernatants is responsible for the defects observed in migration and tube formation, we assessed the relative contribution of CXCL8 and VEGF to NSCLC-supernatant-induced endothelial cell migration. As shown in Fig. 4C, supernatants from control siRNA-transfected A549 cells co-incubated with neutralizing antibodies to CXCL8 and VEGF resulted in the reduction of NSCLC supernatant-induced EC migration by nearly 80% while supernatants from MIF/D-DT-deficient cells were ~ 60% less efficient than controls. These findings are consistent with our findings that CXCL8 and VEGF levels are only ~ 60% reduced in MIF/D-DT-deficient cells (Fig. 1) while neutralizing antibodies should effectively render all CXCL8 and VEGF inactive. Combined, these results imply that CXCL8 and VEGF secretion from NSCLC cells account for the vast majority of the soluble angiogenic inducing capacity of these cells and suggest that MIF and D-DT-dependent regulation of CXCL8 and VEGF expression is of central importance in NSCLC-associated angiogenesis.
Crossover rescue by MIF family members of HUVEC tube formation
Data from Fig. 4 suggested to us that the requirement for MIF family members in NSCLC supernatant-induced HUVEC migration and tube formation was at the level of CXCL8 and VEGF expression. If this were the case, then adenoviral rescue of MIF family members resulting in rescue of CXCL8 (from Fig. 3B) should result in the rescue of HUVEC angiogenic phenotypes. To investigate this possibility, we tested supernatants from combined MIF/D-DT siRNA transfected cells that had been adenovirally infected with either, MIF, D-DT or both. Interestingly, both MIF and D-DT were fully capable of rescuing defective tube formation from supernatants lacking both MIF family members (Figs. 5A and 5B). Although co-cultures with supernatants from cells with add-back of both family members resulted in higher levels of tube formation than those from control cells (Figs. 5A and 5B), our findings that one family member can fully compensate for the loss of both indicate that there is a common mechanism of action shared by these two proteins.
FIGURE 5.
Adenoviral reconstitution of MIF and/or D-DT rescues defective tube formation from supernatants derived from MIF + D-DT-deficient cells. A549 lung adenocarcinoma cells were oligo transfected and 48 hours later GFP, MIF, D-DT or MIF + D-DT adenovirus was added as indicated. After overnight incubation, media was replaced and 48 hours later conditioned media was collected for HUVEC tube formation. Representative GF-depleted Matrigel coated wells were photographed (A) and enumerated, (B). Results from panel B are tubes/transwell and represent the ± S.D. of the average of parallel co-culture assays and are representative of 3 independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 by one way ANOVA analysis is shown for control and individual group comparisons while statistical significance between relevant groups was found by Tukey’s post hoc test.
A role for the MIF receptor, CD74, in MIF and D-DT-induced JNK activation
Thus far our findings had suggested that MIF and D-DT are necessary for maximal CXCL8 expression and likely activate signaling to CXCL8 expression in a mechanistically similar fashion. To investigate whether the MIF cell surface receptor CD74 is involved in this shared signaling pathway, A549 cells were transfected with CD74 siRNA and evaluated for CXCL8 induction by MIF and D-DT over-expression. As shown in Fig. 6A, significant increases in CXCL8 secretion were observed in both MIF and D-DT infected cells and a corresponding additive induction was found with MIF and D-DT combined expression in control siRNA transfected cells (NS). In contrast, CD74 siRNA transfected cells produced ~ 50% less steady state CXCL8 and were completely resistant to MIF, D-DT and MIF/D-DT-induced CXCL8 expression. To confirm and extend the observed requirements for CD74 in MIF and D-DT induced CXCL8 expression, we next tested whether CD74 was necessary for MIF family member-induced JNK pathway of signal transduction using CD74+/+ and CD74−/− murine embryonic fibroblasts. As shown in Fig. 6B, c-Jun-N-terminal kinase (JNK) activation in GFP, MIF, D-DT or MIF/D-DT infected lysates revealed that MIF family members alone or combined, strongly induced JNK and c-jun phosphorylation in CD74+/+, but not CD74−/−, cells. Combined, these studies suggest that MIF and D-DT are both sufficient and necessary for maximal CXCL8 expression in NSCLC cell lines and CD74 is likely responsible for initiating signaling by both MIF family members resulting in JNK activation and subsequent CXCL8 expression.
FIGURE 6.
MIF and D-DT are individually and additively sufficient to induce CXCL-8 from lung adenocarcinoma cells and CD74 is necessary for maximal JNK activation in mouse fibroblasts. A, A549 lung adenocarcinoma cells were oligo transfected with either Control siRNA (NS) or CD74 siRNA and 48 hours later GFP, MIF, D-DT or MIF + D-DT adenovirus was added for 16 hours. After media was replaced, cells were incubated for an additional 24 hours and supernatants were collected and analyzed by CXCL-8 ELISA. Results are the ± S.D. of the average of CXCL-8 ELISA readings from supernatants from two parallel infections and are representative of 2 independent experiments. B, CD74+/+ and CD74−/− murine embryonic fibroblasts were infected with GFP, MIF, D-DT or MIF + D-DT for 48 hours. Cell lysates were examined for pJNK, p-c-jun and total JNK. Results are representative of 2 independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 by one way ANOVA analysis is shown for control and individual group comparisons while statistical significance between relevant groups was found by Tukey’s post hoc test.
DISCUSSION
We report for the first time that the MIF homolog, D-dopachrome tautomerase, supports CXCL8 expression and secretion from lung adenocarcinoma cells. Moreover, D-DT functionally cooperates with MIF in promoting angiogenic growth factor expression and subsequent paracrine-induced endothelial cell angiogenic phenotypes. Several studies have demonstrated an important contribution by MIF to CXCL8 and VEGF expression and maintenance of angiogenic phenotypes in malignant cells and tissue7,22,24,38,39,40. However, this is the first demonstration of a functional overlap between MIF and its only known homolog, D-DT.
Our results suggest that MIF and D-DT modulate JNK-dependent AP-1 transactivation and subsequent CXCL8 transcription in lung adenocarcinoma cells. Perhaps more importantly, the cognate MIF receptor, CD74, is necessary for both CXCL8 expression and maximal JNK and c-jun phosphorylation induced by MIF and D-DT alone and in combination. These findings are consistent with a recent study demonstrating that CD74 is necessary for MIF-dependent contributions to prostatic adenocarcinoma cell invasion, anchorage-independence and tumor-associated neo-vascularization40. What is less clear is how JNK is activated by CD74. An earlier study from our laboratory revealed that MIF functionally regulates Rac1 effector binding by stabilizing cholesterol-enriched membrane microdomains12. Although JNK is a well known effector of Rac1, we have no evidence as yet that the defective JNK observed with loss of MIF is linked to Rac1. Studies are currently underway to determine whether Rac1 is necessary for MIF and D-DT-dependent, CD74-mediated JNK activation. It is also interesting to note that JNK activation has been linked to MIF activity by virtue of MIF binding to and regulating the activity of Jab1/CSN541. Currently, it is unknown whether D-DT functionally interacts with Jab1/CSN5 but this pathway may represent an alternative pathway for MIF family member-dependent regulation of JNK activity.
Although our results indicate that AP-1 activity is important for MIF and D-DT contributions to CXCL8 expression, it is possible that other signaling pathways may be involved. Of note, MIF:CD74 signaling has recently been suggested to modulate CXCL8 expression in an NF-κB-dependent manner38. While we have not observed any correlation between MIF or D-DT and NF-κB activation (Fig. 2B and unpublished observations), we cannot rule out the possible contribution of these family members to other pathways reported to be important for CXCL8 and VEGF expression25,35. Nonetheless, our studies do reveal an important and previously undescribed requirement by CD74 for both MIF and D-DT-induced JNK activation and c-jun phosphorylation.
Our studies with HUVECs reveal that supernatants from lung adenocarcinoma cells deficient in MIF and/or D-DT results in significant reductions in both endothelial cell migration and tube formation. While our findings indicate that the depleted CXCL8 and VEGF levels present in these supernatants is responsible for this defective angiogenic phenotype we can’t rule out the possibility that endothelial cell-derived MIF and/or D-DT may influence endothelial cell activation in an autocrine manner as has been reported42. This possibility, coupled with the fact that tumor-derived MIF stimulates CXCL8 and VEGF from tumor stromal macrophages26, suggest that MIF family members modulate intratumoral neoangiogenesis on several different levels.
Our laboratory recently discovered a novel role for MIF in pancreatic ductal adenocarcinoma (PDAC) cell hypoxic adaptation43,44. Specifically, endogenous PDAC MIF was found to be necessary for maximal HIF-1α stabilization and subsequent transcription of VEGF. Intriguingly, CXCL8 is also a transcriptional target of hypoxia and HIF-1α45–47 and as such, it is likely that MIF is similarly necessary for HIF-dependent CXCL8 expression in hypoxic regions of tumors. Because our current studies indicate that both MIF and D-DT functionally regulate both CXCL8 and VEGF in normoxic conditions, it is not unreasonable to conclude that MIF family members are necessary for both HIF-dependent and HIF-independent maximal expression of these angiogenic growth factors. This is an important distinction as HIF-independent CXCL8 was recently shown to compensate for loss of HIF-1α in tumor-associated angiogenesis and co-neutralization of HIF-dependent and HIF-independent CXCL8 and VEGF markedly reduces tumor angiogenesis and corresponding tumor burdens48. Although studies are ongoing to determine whether D-DT is similarly important for HIF-dependent regulation of angiogenic growth factor expression, our current findings support a strategy of dual targeting of MIF and D-DT in patients with established tumors. Further in vivo studies to evaluate the individual versus combined influence of MIF and D-DT in intratumoral angiogenesis and malignant disease progression may well lead to a reassessment of current MIF therapeutic targeting strategies for lung cancer.
ACKNOWLEDGMENTS
The authors wish to acknowledge Dr. Lin Leng (Yale University, New Haven, CT) for his contribution of CD74+/+ and CD74−/− murine fibroblasts and Dr. Allan Brasier (UTMB, Galveston, TX) for providing CXCL-8 luciferase constructs.
Footnotes
This work was supported in part by NIH CA102285-S (A.M.C), NIH CA102285 (R.A.M.), NIH AI042310 (R.B) and a grant from Philip Morris USA Inc. and Philip Morris International (R.A.M.).
DISCLOSURES
R.A.M. and R.B. are co-inventors on patents and patent applications describing the therapeutic value of MIF antagonists.
REFERENCES
- 1.Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 2.Carney DN. Lung cancer--time to move on from chemotherapy. N.Engl.J Med. 2002;346:126–128. doi: 10.1056/NEJM200201103460211. [DOI] [PubMed] [Google Scholar]
- 3.Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, Dzuiba J, Van Damme J, Walz A, Marriott D. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995;270:27348–27357. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- 4.Nor JE, Christensen J, Mooney DJ, Polverini PJ. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am.J Pathol. 1999;154:375–384. doi: 10.1016/S0002-9440(10)65284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rot A. Binding of neutrophil attractant/activation protein-1 (interleukin 8) to resident dermal cells. Cytokine. 1992;4:347–352. doi: 10.1016/1043-4666(92)90077-5. [DOI] [PubMed] [Google Scholar]
- 6.Addison CL, Daniel TO, Burdick MD, Liu H, Ehlert JE, Xue YY, Buechi L, Walz A, Richmond A, Strieter RM. The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. J Immunol. 2000;165:5269–5277. doi: 10.4049/jimmunol.165.9.5269. [DOI] [PubMed] [Google Scholar]
- 7.White ES, Flaherty KR, Carskadon S, Brant A, Iannettoni MD, Yee J, Orringer MB, Arenberg DA. Macrophage migration inhibitory factor and CXC chemokine expression in non-small cell lung cancer: role in angiogenesis and prognosis. Clin.Cancer Res. 2003;9:853–860. [PubMed] [Google Scholar]
- 8.Chen JJ, Yao PL, Yuan A, Hong TM, Shun CT, Kuo ML, Lee YC, Yang PC. Up-regulation of tumor interleukin-8 expression by infiltrating macrophages: its correlation with tumor angiogenesis and patient survival in non-small cell lung cancer. Clin.Cancer Res. 2003;9:729–737. [PubMed] [Google Scholar]
- 9.Arenberg DA, Keane MP, DiGiovine B, Kunkel SL, Morris SB, Xue YY, Burdick MD, Glass MC, Iannettoni MD, Strieter RM. Epithelial-neutrophil activating peptide (ENA-78) is an important angiogenic factor in non-small cell lung cancer. J Clin.Invest. 1998;102:465–472. doi: 10.1172/JCI3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bloom BR, Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966;153:80–82. doi: 10.1126/science.153.3731.80. [DOI] [PubMed] [Google Scholar]
- 11.Calandra T, Bernhagen J, Mitchell RA, Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J.Exp.Med. 1994;179:1895–1902. doi: 10.1084/jem.179.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rendon BE, Roger T, Teneng I, Zhao M, Al Abed Y, Calandra T, Mitchell RA. Regulation of human lung adenocarcinoma cell migration and invasion by macrophage migration inhibitory factor. J Biol Chem. 2007;282:29910–29918. doi: 10.1074/jbc.M704898200. [DOI] [PubMed] [Google Scholar]
- 13.Howard BA, Zheng Z, Campa MJ, Wang MZ, Sharma A, Haura E, Herndon JE, Fitzgerald MC, Bepler G, Patz EF., Jr Translating biomarkers into clinical practice: prognostic implications of cyclophilin A and macrophage migratory inhibitory factor identified from protein expression profiles in non-small cell lung cancer. Lung Cancer. 2004;46:313–323. doi: 10.1016/j.lungcan.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 14.Kettunen E, Anttila S, Seppanen JK, Karjalainen A, Edgren H, Lindstrom I, Salovaara R, Nissen AM, Salo J, Mattson K, Hollmen J, Knuutila S, Wikman H. Differentially expressed genes in nonsmall cell lung cancer: expression profiling of cancer-related genes in squamous cell lung cancer. Cancer Genet.Cytogenet. 2004;149:98–106. doi: 10.1016/S0165-4608(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 15.Tomiyasu M, Yoshino I, Suemitsu R, Okamoto T, Sugimachi K. Quantification of macrophage migration inhibitory factor mRNA expression in non-small cell lung cancer tissues and its clinical significance. Clin.Cancer Res. 2002;8:3755–3760. [PubMed] [Google Scholar]
- 16.Sun HW, Bernhagen J, Bucala R, Lolis E. Crystal structure at 2.6-A resolution of human macrophage migration inhibitory factor. Proc.Natl.Acad.Sci.U.S.A. 1996;93:5191–5196. doi: 10.1073/pnas.93.11.5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leng L, Metz CN, Fang Y, Xu J, Donnelly S, Baugh J, Delohery T, Chen Y, Mitchell RA, Bucala R. MIF Signal Transduction Initiated by Binding to CD74. J Exp.Med. 2003;197:1467–1476. doi: 10.1084/jem.20030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi X, Leng L, Wang T, Wang W, Du X, Li J, McDonald C, Chen Z, Murphy JW, Lolis E, Noble P, Knudson W, Bucala R. CD44 is the signaling component of the macrophage migration inhibitory factor-CD74 receptor complex. Immunity. 2006;25:595–606. doi: 10.1016/j.immuni.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, Dewor M, Georgiev I, Schober A, Leng L, Kooistra T, Fingerle-Rowson G, Ghezzi P, Kleemann R, McColl SR, Bucala R, Hickey MJ, Weber C. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat.Med. 2007;13:587–596. doi: 10.1038/nm1567. [DOI] [PubMed] [Google Scholar]
- 20.Rosengren E, Bucala R, Aman P, Jacobsson L, Odh G, Metz CN, Rorsman H. The immunoregulatory mediator macrophage migration inhibitory factor (MIF) catalyzes a tautomerization reaction. Mol.Med. 1996;2:143–149. [PMC free article] [PubMed] [Google Scholar]
- 21.Sugimoto H, Taniguchi M, Nakagawa A, Tanaka I, Suzuki M, Nishihira J. Crystal structure of human D-dopachrome tautomerase, a homologue of macrophage migration inhibitory factor, at 1.54 A resolution. Biochemistry. 1999;38:3268–3279. doi: 10.1021/bi982184o. [DOI] [PubMed] [Google Scholar]
- 22.Xu X, Wang B, Ye C, Yao C, Lin Y, Huang X, Zhang Y, Wang S. Overexpression of macrophage migration inhibitory factor induces angiogenesis in human breast cancer. Cancer Lett. 2007 doi: 10.1016/j.canlet.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 23.Fan J, Chen Y, Chan HM, Tam PK, Ren Y. Removing intensity effects and identifying significant genes for Affymetrix arrays in macrophage migration inhibitory factor-suppressed neuroblastoma cells. Proc.Natl.Acad.Sci.U.S.A. 2005;102:17751–17756. doi: 10.1073/pnas.0509175102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren Y, Chan HM, Li Z, Lin C, Nicholls J, Chen CF, Lee PY, Lui V, Bacher M, Tam PK. Upregulation of macrophage migration inhibitory factor contributes to induced N-Myc expression by the activation of ERK signaling pathway and increased expression of interleukin-8 and VEGF in neuroblastoma. Oncogene. 2004;23:4146–-4154. doi: 10.1038/sj.onc.1207490. [DOI] [PubMed] [Google Scholar]
- 25.Ren Y, Chan HM, Fan J, Xie Y, Chen YX, Li W, Jiang GP, Liu Q, Meinhardt A, Tam PK. Inhibition of tumor growth and metastasis in vitro and in vivo by targeting macrophage migration inhibitory factor in human neuroblastoma. Oncogene. 2006 doi: 10.1038/sj.onc.1209395. [DOI] [PubMed] [Google Scholar]
- 26.White ES, Strom SR, Wys NL, Arenberg DA. Non-small cell lung cancer cells induce monocytes to increase expression of angiogenic activity. J.Immunol. 2001;66:7549–7555. doi: 10.4049/jimmunol.166.12.7549. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe H, Shimizu T, Nishihira J, Abe R, Nakayama T, Taniguchi M, Sabe H, Ishibashi T, Shimizu H. Ultraviolet A-induced production of matrix metalloproteinase-1 is mediated by macrophage migration inhibitory factor (MIF) in human dermal fibroblasts. J Biol Chem. 2004;279:1676–1683. doi: 10.1074/jbc.M303650200. [DOI] [PubMed] [Google Scholar]
- 28.Hagemann T, Wilson J, Kulbe H, Li NF, Leinster DA, Charles K, Klemm F, Pukrop T, Binder C, Balkwill FR. Macrophages induce invasiveness of epithelial cancer cells via NF-kappa B and JNK. J Immunol. 2005;175:1197–1205. doi: 10.4049/jimmunol.175.2.1197. [DOI] [PubMed] [Google Scholar]
- 29.Pakozdi A, Amin MA, Haas CS, Martinez RJ, Haines GK, III, Santos LL, Morand EF, David JR, Koch AE. Macrophage migration inhibitory factor: a mediator of matrix metalloproteinase-2 production in rheumatoid arthritis. Arthritis Res.Ther. 2006;8:R132. doi: 10.1186/ar2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell RA, Liao H, Chesney J, Fingerle-Rowson G, Baugh J, David J, Bucala R. Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response. Proc.Natl.Acad.Sci.U.S.A. 2002;99:345–350. doi: 10.1073/pnas.012511599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim HR, Park MK, Cho ML, Yoon CH, Lee SH, Park SH, Leng L, Bucala R, Kang I, Choe J, Kim HY. Macrophage migration inhibitory factor upregulates angiogenic factors and correlates with clinical measures in rheumatoid arthritis. J Rheumatol. 2007;34:927–936. [PubMed] [Google Scholar]
- 32.Ren Y, Law S, Huang X, Lee PY, Bacher M, Srivastava G, Wong J. Macrophage migration inhibitory factor stimulates angiogenic factor expression and correlates with differentiation and lymph node status in patients with esophageal squamous cell carcinoma. Ann.Surg. 2005;242:55–63. doi: 10.1097/01.sla.0000168555.97710.bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ren Y, Tsui HT, Poon RT, Ng IO, Li Z, Chen Y, Jiang G, Lau C, Yu WC, Bacher M, Fan ST. Macrophage migration inhibitory factor: roles in regulating tumor cell migration and expression of angiogenic factors in hepatocellular carcinoma. Int.J Cancer. 2003;107:22–29. doi: 10.1002/ijc.11287. [DOI] [PubMed] [Google Scholar]
- 34.Natarajan R, Gupta S, Fisher BJ, Ghosh S, Fowler AA., III Nitric oxide suppresses IL-8 transcription by inhibiting c-Jun N-terminal kinase-induced AP-1 activation. Exp.Cell Res. 2001;266:203–212. doi: 10.1006/excr.2001.5218. [DOI] [PubMed] [Google Scholar]
- 35.Li J, Kartha S, Iasvovskaia S, Tan A, Bhat RK, Manaligod JM, Page K, Brasier AR, Hershenson MB. Regulation of human airway epithelial cell IL-8 expression by MAP kinases. Am.J Physiol Lung Cell Mol.Physiol. 2002;283:L690–L699. doi: 10.1152/ajplung.00060.2002. [DOI] [PubMed] [Google Scholar]
- 36.Henriquet C, Gougat C, Combes A, Lazennec G, Mathieu M. Differential regulation of RANTES and IL-8 expression in lung adenocarcinoma cells. Lung Cancer. 2007;56:167–174. doi: 10.1016/j.lungcan.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Wang Q, Ives KL, Evers BM. Curcumin inhibits neurotensin-mediated interleukin-8 production and migration of HCT116 human colon cancer cells. Clin.Cancer Res. 2006;12:5346–5355. doi: 10.1158/1078-0432.CCR-06-0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Binsky I, Haran M, Starlets D, Gore Y, Lantner F, Harpaz N, Leng L, Goldenberg DM, Shvidel L, Berrebi A, Bucala R, Shachar I. IL-8 secreted in a macrophage migration-inhibitory factor- and CD74-dependent manner regulates B cell chronic lymphocytic leukemia survival. Proc.Natl.Acad.Sci.U.S.A. 2007;104:13408–13413. doi: 10.1073/pnas.0701553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ren Y, Law S, Huang X, Lee PY, Bacher M, Srivastava G, Wong J. Macrophage migration inhibitory factor stimulates angiogenic factor expression and correlates with differentiation and lymph node status in patients with esophageal squamous cell carcinoma. Ann.Surg. 2005;242:55–63. doi: 10.1097/01.sla.0000168555.97710.bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer-Siegler KL, Iczkowski KA, Leng L, Bucala R, Vera PL. Inhibition of macrophage migration inhibitory factor or its receptor (CD74) attenuates growth and invasion of DU-145 prostate cancer cells. J Immunol. 2006;177:8730–8739. doi: 10.4049/jimmunol.177.12.8730. [DOI] [PubMed] [Google Scholar]
- 41.Kleemann R, Hausser A, Geiger G, Mischke R, Burger-Kentischer A, Flieger O, Johannes FJ, Roger T, Calandra T, Kapurniotu A, Grell M, Finkelmeier D, Brunner H, Bernhagen J. Intracellular action of the cytokine MIF to modulate AP-1 activity and the cell cycle through Jab1. Nature. 2000;408:211–216. doi: 10.1038/35041591. [DOI] [PubMed] [Google Scholar]
- 42.Chesney J, Metz C, Bacher M, Peng T, Meinhardt A, Bucala R. An essential role for macrophage migration inhibitory factor (MIF) in angiogenesis and the growth of a murine lymphoma. Mol Med. 1999;5:181–191. [PMC free article] [PubMed] [Google Scholar]
- 43.Winner M, Koong AC, Rendon BE, Zundel W, Mitchell RA. Amplification of tumor hypoxic responses by macrophage migration inhibitory factor-dependent hypoxia-inducible factor stabilization. Cancer Res. 2007;67:186–193. doi: 10.1158/0008-5472.CAN-06-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Winner M, Leng L, Zundel W, Mitchell RA. Macrophage migration inhibitory factor manipulation and evaluation in tumoral hypoxic adaptation. Methods Enzymol. 2007;435:355–369. doi: 10.1016/S0076-6879(07)35018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim KS, Rajagopal V, Gonsalves C, Johnson C, Kalra VK. A novel role of hypoxia-inducible factor in cobalt chloride- and hypoxia-mediated expression of IL-8 chemokine in human endothelial cells. J Immunol. 2006;177:7211–7224. doi: 10.4049/jimmunol.177.10.7211. [DOI] [PubMed] [Google Scholar]
- 46.Desbaillets I, Diserens AC, de Tribolet N, Hamou MF, Van Meir EG. Regulation of interleukin-8 expression by reduced oxygen pressure in human glioblastoma. Oncogene. 1999;18:1447–1456. doi: 10.1038/sj.onc.1202424. [DOI] [PubMed] [Google Scholar]
- 47.Maxwell PJ, Gallagher R, Seaton A, Wilson C, Scullin P, Pettigrew J, Stratford IJ, Williams KJ, Johnston PG, Waugh DJ. HIF-1 and NF-kappaB-mediated upregulation of CXCR1 and CXCR2 expression promotes cell survival in hypoxic prostate cancer cells. Oncogene. 2007;26:7333–7345. doi: 10.1038/sj.onc.1210536. [DOI] [PubMed] [Google Scholar]
- 48.Mizukami Y, Jo WS, Duerr EM, Gala M, Li J, Zhang X, Zimmer MA, Iliopoulos O, Zukerberg LR, Kohgo Y, Lynch MP, Rueda BR, Chung DC. Induction of interleukin-8 preserves the angiogenic response in HIF-1alpha-deficient colon cancer cells. Nat.Med. 2005;11:992–997. doi: 10.1038/nm1294. [DOI] [PubMed] [Google Scholar]