Abstract
In a world without numbers, we would be unable to build a skyscraper, hold a national election, plan a wedding, or pay for a chicken at the market. The numerical symbols used in all these behaviors build on the approximate number system (ANS) which represents the number of discrete objects or events as a continuous mental magnitude. In this review, we first discuss evidence that the ANS bears a set of behavioral and brain signatures that are universally displayed across animal species, human cultures, and development. We then turn to the question of whether the ANS constitutes a specialized cognitive and neural domain--a question central to understanding how this system works, the nature of its evolutionary and developmental trajectory, and its physical instantiation in the brain.
Universality and domain-specificity
The case that the approximate number system (ANS) is cognitively universal is based on four sources of evidence: cognitive development [1, 2], comparative cognition [3–5], cross-cultural cognition [6], and neurobiology [7–9]. Collectively, these four types of evidence have established a case for a culturally, developmentally, and evolutionarily universal system for representing ‘numbers’ as mental magnitudes. Less clear, however, is whether the neural system supporting the ANS is exclusive for numerical representation.
Numerical cognition is often considered to be a quintessential cognitive domain [5, 7–11]. What constitutes a cognitive “domain”, however, remains controversial. Most would agree that a domain defines a set of specialized (though not necessarily modular) processing mechanisms that become engaged only when presented with particular types of information [12]. In addition, domain-specific cognitive faculties are typically hypothesized to require specialized neural architecture [13]. The degree to which numerical cognition satisfies either of these criteria of domain-specificity is currently unresolved [14–16].
In what follows, we discuss evidence supporting the developmental and evolutionary primacy of the ANS. We then weigh evidence for and against the idea that there are unique behavioral and neural signatures for approximate numerical processing. In particular, we consider whether non-numerical magnitudes such as time, size, ordinal position, and brightness recruit the same cognitive and neural machinery as numerical magnitudes. Endorsing and extending prior reviews [17, 18], we conclude that the cognitive and neural systems mediating the ANS are largely co-extensive with those mediating other types of quantitative judgments, thereby calling into question the notion of a domain-specific system devoted to numerical processing.
A primitive system for representing ‘number’
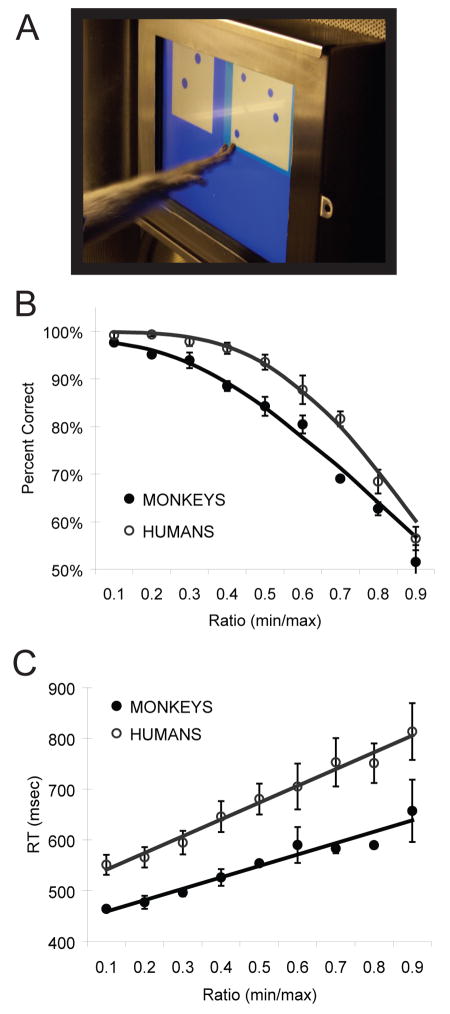
The primary behavioral signature of the ANS is Weber’s law (Box 1), which holds universally across species, human development, and human cultures [1, 2, 8, 9]. For example, when monkeys and college students are tested in the same numerosity comparison and addition tasks, Weber’s law similarly predicts their performance (Figure 1) [4, 19]. Moreover, numerical discrimination in infancy is reliably predicted by numerical ratio [20, 21], indicating that the behavioral signatures of the ANS emerge within the first year of human life. Finally, Amazonian peoples without verbal counting systems show ratio dependence when comparing relative numerosity despite the lack of an exact appreciation for large numerical quantities [6]. Ratio-dependent nonverbal number discrimination, evident across species, cultures, and development, suggests a universal analog mental code for representing number approximately.
Box 1: Analog representation of approximate number
Weber’s law states that the difference in intensity needed to discriminate two stimuli is proportional to their objective intensities or, ΔI/I = k, where I is stimulus intensity and k is a constant that signifies the resulting sensitivity to changes (or differences) in stimulus intensity. Under Weber’s law, discrimination performance is modulated by the ratio of the intensities rather than their absolute difference.
Weber’s law adequately predicts performance on numerical tasks but, it does not address the initial process that translates a set of objects into a numerical representation. Currently, two competing models attempt to explain the process of forming an approximate numerical representation. In the mode-control model [40], an accumulator is serially incremented by a constant amount for each object or event, and the accumulated signal indexes the total number of objects or events in the set. A second model [100] invokes a parallel summation mechanism in which the objects in a set are detected in parallel and passed to a summation stage that accumulates signals across the object detection stage, ultimately indexing the total number of objects in the set. In their accumulation stages, both models postulate that objective, discrete numerical value is analogically translated into a continuous subjective representation of numerical value or, an analog magnitude representation.
Figure 1.
Numerical judgments in monkeys and humans follow Weber’s Law. (A) In a recent study from our lab, monkeys and adults were trained to discriminate stimuli based on their best estimate of numerical value. Adults were specifically instructed to avoid verbally counting and to respond as rapidly as possible. For both groups, accuracy (B) and response times (C) were modulated by the numerical ratio between the stimuli. In addition, accuracy performance is well-fit by a mathematical model of performance that adheres to Weber’s law. Reprinted with permission from [19].
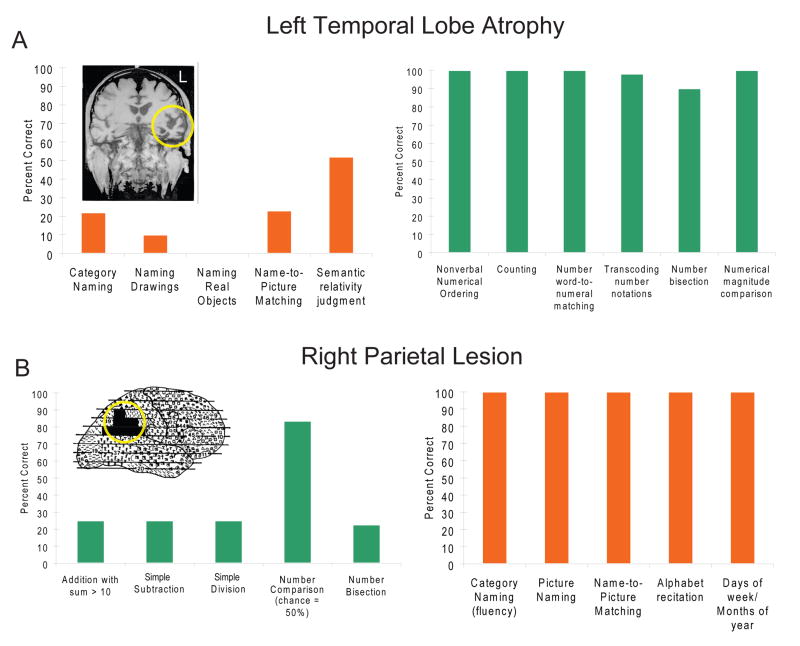
Neurobiological studies of the ANS in adult humans have yielded a highly reproducible set of brain areas that contribute to numerical cognition. Specifically, regions of the parietal cortex along the intraparietal sulcus (IPS) appear critical for approximate numerical cognition. Patients with parietal lesions can be impaired in making numerical judgments (Figure 2) while other cognitive abilities remain normal [8, 9, 22, 23]. Furthermore, studies of patients with semantic dementia (and anterior temporal lobe atrophy) have shown that numerical skills can be spared in cases where other semantically demanding tasks such as picture categorization or picture naming are impaired [24]. Approximate numerical judgments thus require an intact parietal lobe, and doubly dissociate from other types of semantic judgments, which require temporal lobe processing. Human neuroimaging studies converge with the neuropsychological evidence and implicate the IPS specifically, and parietal cortex more generally, in numerical processing [8, 9]. In adulthood, the IPS shows a ratio-dependent BOLD response to numerical stimuli [25, 26] and is more engaged during numerical processing than shape and color judgments [27].
Figure 2.
Brain damage reveals distinct functional localization of numerical and nonnumerical semantic judgments. (A) Patients with temporal lobe atrophy can show impairments in categorization and recognition tasks involving animals, objects, or letters but be unimpaired on tasks requiring numerical computation, recognition, and counting. (B) By contrast, patients with right parietal lesions can be impaired at tasks involving mathematical operations such as addition and subtraction as well as number comparison and bisection but be unimpaired in categorization and recognition of non-numerical stimuli. Drawn with permission from [23, 24].
Adult-like neural signatures of approximate numerical processing emerge as early as four years of age, in children who are not yet proficient verbal counters [14, 28]. Moreover, measurements of stimulus-evoked electrical activity on the scalp (event-related potentials, or ERPs) suggest that these signatures may develop even earlier, within the first months of life [29, 30]. In short, while there are likely developmental changes in the neural processes underlying the ANS, the IPS appears to be involved throughout development.
In parallel, neurophysiological studies in monkeys have identified populations of neurons in parietal cortex that are sensitive to the cardinal value of elements in a visual array or sequence of elements [31–34] or the number of times an action is performed [35]. This line of research suggests that ventral intraparietal (VIP) neurons may provide a numerical “readout” of accumulated sets of objects, events, or actions. A recent neurophysiological study has demonstrated that, in contrast to the cardinal value response of VIP neurons, number-sensitive neurons in the lateral intraparietal area (LIP) respond to the number of elements in their receptive fields in a monotonic fashion, with firing rate increasing or decreasing with numerical magnitude[34]. These observations suggest the hypothesis that the representation of cumulative numerical magnitude is maintained by the monotonic LIP population and translated into cardinal representations of total numerosity in VIP. Alternatively, differences in the neural responses of these two intraparietal regions may reflect differences in the behavioral or neural protocols employed in the experiments. Yet, in either case, the numerical tuning functions of neurons in VIP and LIP provide evidence of a neural foundation for the behavioral distance and magnitude effects characteristic of the ANS.
The evidence for similar numerical processes, from a diverse set of methods, populations, and species, makes a strong case that the basis of numerical representation is a primitive cognitive and neural system. But, is there neural machinery dedicated to number representation that is not used for representing size, length, time or other continuous variables? For that matter, are the behavioral signatures of numerical judgments common to judgments of other magnitudes such as size, length, or time? In what follows we explore the possibility that the cognitive and neural processes involved in representing and manipulating numerical information reflect operations that are broadly available for processing other types of quantitative information.
Cognitive similarities between number and other magnitudes
Magnitude judgments are those that invoke questions such as ‘Which is more?’ or ‘Which is bigger?’ Judgments of this nature can be applied to size, length, time, loudness, number, or any uni-dimensional property of an object or set. In fact, several cognitive signatures of magnitude processing, are common to numbers and many other quantitative dimensions. The shared cognitive signatures of quantitative judgments implicate both a common mental code for quantitative representation and a common mental comparison process for judging their magnitude.
Despite the prevalent view that numerical cognition operates within its own domain [5, 7–11], the notion of a generalized magnitude system for representing number and non-numerical quantities is not new [1, 17, 18, 36–43]. For example, Walsh [17] synthesized the behavioral and neurobiological evidence that time, space, and number share common processing mechanisms and proposed that they are linked to guide action because the computations necessary to determine the spatial location of an object depend critically on quantitative computations such as ‘how far’, ‘how many’, and ‘how long.’ According to Walsh’s [17] account, mental magnitudes are bound to one another through the computational demands of the motor control system.
One possible point of convergence in the cognitive processing of mental magnitudes is in the format of their underlying mental code. Psychophysical data collected over the past two centuries demonstrate that Weber’s law characterizes a wide variety of magnitude judgments [36, 37, 40]. Beyond numerosity, time, and spatial extent, Weber’s law characterizes judgments of loudness, pitch, warmth, weight, brightness, perceived difficulty, and many other continua. We can infer from Weber’s law that the psychological format of these quantities takes the form of an analog magnitude (Box 1). And, if these quantities share a common representational format, we might expect that mental transformations across quantitative dimensions are feasible. Indeed, humans are facile at measuring one magnitude as a function of another magnitude; adults can measure brightness in terms of handgrip pressure, line length, number, or loudness [44], providing evidence of a translational mechanism between quantitative representations.
Representations of time and number in particular have long been hypothesized to originate from a common representational system. A seminal study by Meck and Church [40] showed that rats can simultaneously estimate time and number and their estimates of these quantities shift to the same degree when they are administered methamphetamine. Since the Meck and Church study, a great deal of work has revealed parallel sensitivity for time and number discrimination in non-human animals and adult humans [40, 45, 46]
Beyond time and number, dual representation studies testing the simultaneous quantitative representation of dimensions such as size, brightness, and angle also have reported parallel cognitive effects (e.g., interference) among distinct quantities [47–50]. The overall implication is that a common analog magnitude code underlies a wide variety of quantitative representations, thereby causing these representations to interact during simultaneous judgments.
Commonalities in an underlying mental code may also underlie the well-established link between quantitative and one-dimensional spatial representations [51]. Adult humans appear to intrinsically map numerical representations onto a unidimensional spatial mental number line [18]. This spatial mapping of number has been termed the Spatial-Numerical Association in Response Code (SNARC). However, beyond number-space relations, other research has demonstrated spatial mappings of non-numerical quantitative information such as pitch and ordinal position [43, 52, 53]. Further, some evidence suggests that ordinal positions in arbitrary lists may be encoded as analog magnitudes [42, 54]. One possibility, then, is that one-dimensional spatial representations and magnitude representations interact because they are encoded in a common analog mental currency.
Neuropsychological studies provide further evidence that multiple quantities share cognitive representations. [55]. Patients who neglect the left side of space similarly neglect the left side of the mental number line. When the same patients are asked to indicate the spatial or numerical mid-point of two anchor values, their answers systematically shift in the same direction. A similar finding has been reported for left neglect patients on an interval timing bisection task [17, 56]. Moreover, a recent study of color-number synaesthetes showed that the irrepressible perceptual bond that synaesthetes experience between specific colors and numbers can be explained by a systematic association between numerical magnitude and luminance intensity [57]. Thus, non-idiosyncratic intrinsic associations between number and other continuous dimensions may also occur, perhaps as a result of their common underlying code.
A second possible point of convergence among quantitative judgments is the mental comparison process. Dual-task studies have revealed significant overlap in the cognitive resources utilized in quantitative judgments of time, size, and number but considerably less overlap between quantitative and non-quantitative tasks such as color or shape judgments, rotary tracking, phonological memory, and visuospatial search [17, 47, 58, 59]. Studies of this nature support the idea that global cognitive resources are shared to a greater degree among quantitative judgments than between quantitative and non-quantitative judgments. A common mental comparison process may be a cognitive source of these dual-task interference effects.
Several studies have examined the algorithm underlying magnitude comparison [38, 39, 60–62]. Rather than focusing on the mental currency or global resources of quantitative representations, these studies have examined the computations necessary to relate mental magnitudes to one another along a continuum. The mechanisms that mediate magnitude comparisons are hypothesized to operate on analog magnitude representations like those described for numerical values. The dominant theory of the cognitive processes underlying magnitude comparisons asserts that reference points of extreme values (i.e., the smallest and largest values) are used as anchors for comparisons along a given continuum. Each pair of stimuli encountered is compared to the reference points in order to determine which of the pair is closer to the smaller end, in the case of ‘Which is smaller?’, or the larger end, for ‘Which is larger?’. Response time in the decision process is determined by evidence accrual toward the target end of the continuum.
The primary evidence for the reference-point model of magnitude comparison is the semantic congruity effect (Box 2), which is observed when adult humans compare numerical values [60]. Semantic congruity effects, however, are not unique to the numerical domain and are instead found when a wide variety of stimuli are compared along a single dimension [38, 39, 60–62]. Additionally, the semantic congruity effect is not a uniquely human phenomenon since monkeys show semantic congruity effects for magnitude judgments [3]. The fact that a semantic congruity effect emerges in non-humans and holds for comparisons of many different uni-dimensional properties suggests that mental comparisons of numerical values recruit an evolutionarily primitive cognitive algorithm that applies to a wide variety of quantities. Together with the ubiquitous analog format of mental magnitude representations, this generalized comparison process may contribute to the common cognitive signatures of quantitative processing.
Box 2: The semantic congruity effect
It seems intuitive that judging whether a mouse is larger than a squirrel should be psychologically equivalent to judging which is smaller. But, surprisingly, people are actually faster to report that a mouse is smaller than a squirrel than to answer that a squirrel is larger than a mouse! Conversely, people are faster to report the larger of two large animals such as an elephant and a horse. Thus, for identical stimuli, the speed of comparison depends on both the direction of the comparison and the size of the stimuli. This effect has been termed the semantic congruity effect.
Semantic congruity effects emerge in performance whenever people compare two things along a single dimension and have been reported for judging the distance between two cities, line length, weight, brightness, animal size, the intelligence of animals, size, number, and many other dimensions [38, 39, 60–62]. These findings implicate a generalized comparison process for relative judgments of quantities.
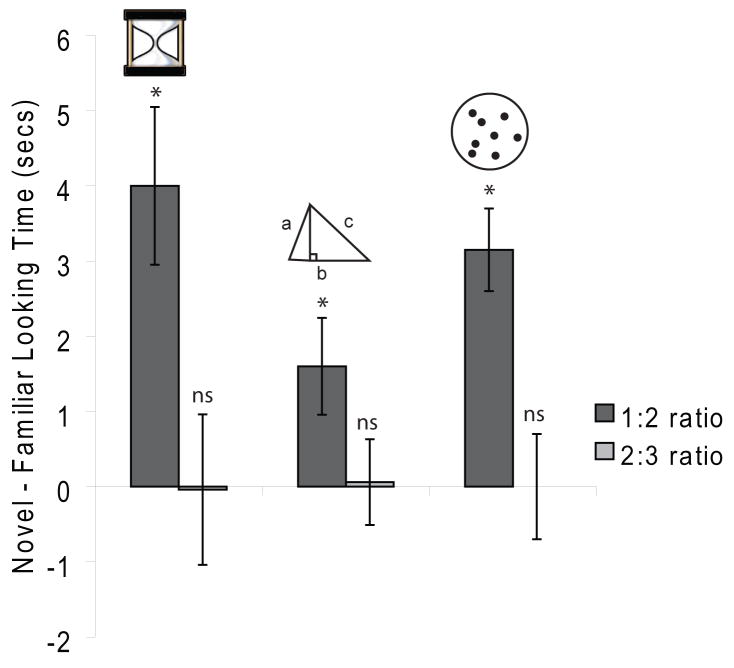
Current evidence from human developmental studies implicates similar developmental trajectories for discriminating quantities [63, 64]. Infants of a given age show similar precision at representing magnitudes such as surface area, time, and number [20, 21, 65–67] (Figure 3). Furthermore, the ratio that infants require to successfully discriminate a change in time and number systematically declines over the first year at the same rate. The degree to which judgments of different quantities overlap in infancy will have important implications for the origins of the common cognitive signatures of quantitative judgments observed in adulthood.
Figure 3.
Infants can discriminate multiple quantities. Six-month-old infants show similar sensitivity to discriminations to time, surface area, and number. During looking-time studies testing discrimination of each of these dimensions, infants look longer at novel values when they are in a 1:2 ratio to the familiar stimuli but they do not look longer to novel stimuli presented in a 2:3 ratio to the familiar stimuli. These findings suggest that infants can discriminate time, surface area, and number at a 1:2 ratio but not at a 2:3 ratio. But, while at 6 months of age babies require a 2-fold change in number, time, or surface area to detect a change, by 9 or 10 months of age a 2:3 ratio is sufficient for discrimination. Drawn with permission from [20, 65, 66].
Neural overlap between number and other magnitudes
Multiple sources of evidence including neuropsychological and neuroimaging studies of humans as well as neurophysiological studies of non-human primates have converged on parietal cortex, particularly the IPS, as a substrate for numerical cognition. Classically, however, parietal cortex is associated with attention, visuo-spatial reasoning, and the visual guidance of motor behavior [68, 69] [70]. Damage to parietal cortex can lead to hemi-spatial neglect and/or extinction as well as an inability to use visual information to guide reaching, grasping, or orienting.
Numerical cognition appears to depend on many of these same structures within the parietal lobe. In fact, patients with the neurological disorder Gerstmann’s Syndrome present with an array of co-morbid cognitive impairments including numerical, visuospatial, and motor deficits [71]. However, as indicated above, neuropsychological and neuroimaging studies have demonstrated that the neural processes related to numerical processing can be dissociated from other forms of semantic processing [22–24, 25, 27, 28] as well as general cognitive operations such as finger and eye movements, working memory, and attention (but see [72]) [25, 26, 28, 73–76].
Although neuropsychological and neuroimaging studies have reported dissociations between numerical performance and performance during other semantic and cognitive operations, the critical question is whether similar dissociations can be found between numerical processing and processing of other magnitudes such as brightness or size. To our knowledge, such data are not yet available from neuropsychological studies. In fact, association rather than dissociation among quantitative deficits seems more likely: the extant neuropsychological literature suggests that some neurological deficits in quantitative representation (particularly time, space, and number), resulting from parietal lesions, may co-occur [17, 55, 56].
A few neuroimaging studies have directly compared brain responses to different types of quantitative information [15]. Collectively, these studies do not provide a compelling case for the specificity of numerical processing in the brain. For instance, Pinel and colleagues [77] compared brain responses during numerical processing with other types of quantitative processing. Subjects were given a task in which they had to judge the approximate brightness, size, or numerical value of two Arabic numerals that were presented simultaneously on a computer screen. The values for each dimension were varied to equate difficulty. Under these conditions, all three tasks (brightness, size, & number) activated a broad swath of the cortex along the IPS relative to baseline and each of the three tasks evoked distance-related activations in this area. More importantly, activation associated with each task varied along adjacent segments of the IPS and only partially overlapped. Anterior portions of the horizontal segment of the IPS responded more strongly during numerical comparisons than during either of the other two types of comparisons. The authors argued that this pattern of activity reflected “distributed but overlapping” organization of quantitative processing in the IPS.
Several other fMRI studies using a similar design have reported somewhat conflicting results [75, 78–80]. None of these studies reported the same anterior-posterior organization of quantitative selectivity observed by Pinel and colleagues. Taken together, observations using the same basic technique suggest significant overlap among brightness, size, and numerical representations in parietal cortex, thus raising the question of whether representation of these dimensions is truly independent at the neural level.
Additional studies have identified regions of parietal cortex that respond equally to judgments of line length, size, and number [81, 82]. For example, Fias and colleagues [81] demonstrated a convergence of activity in the IPS for judgments of line length, angle aperture, and number. Similar findings have been reported for ordinal judgments of the positions of the letters in the alphabet [82] and the positions of arbitrarily ordered items from a memorized list [42, 83]. Judgments of such ordinal, but discrete, information can also be interpreted as a magnitude judgment [54] and may be accomplished in much the same way as comparisons of numerical values.
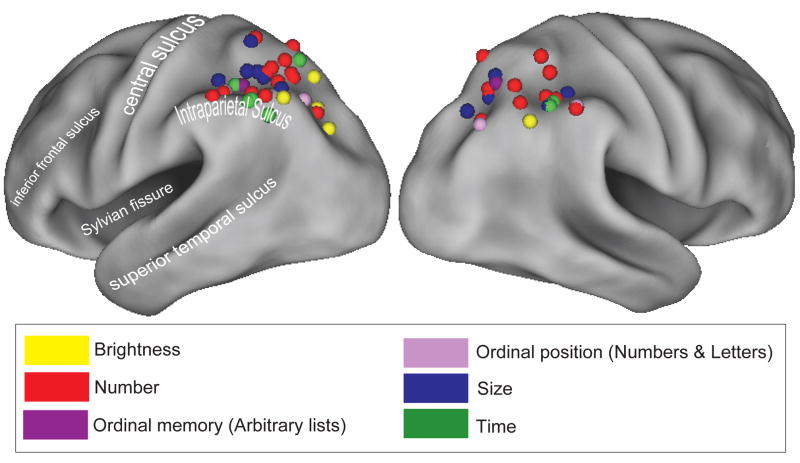
A similar case can be made for timing judgments. Interval timing is hypothesized to recruit a widely distributed cortico-striatal circuit including the thalamus, striatum, and frontal and parietal cortices [84]. Although within-subject data are necessary to determine whether neural loci are identical across tasks, similar posterior parietal regions to those reported for numerical judgments have also been implicated in judgments of time [84–90]. For example, in an fMRI study by Rao and colleagues [88], posterior parietal activity during the comparison of the duration of two tones was greater than during the comparison of their pitch. Consistent with this claim, patients with damage to right posterior parietal cortex can exhibit impairments in judging the relative duration of two tones but remain unimpaired in judging tone pitch [91]. Although the specific role of parietal cortex in the cortico-striatal circuit underlying interval timing is unknown, it is possible that it is important for comparisons of temporal intervals. Notably, a common feature of interval timing tasks and numerical, size, ordinal position, and brightness judgment tasks described above is that they all require comparisons of mental magnitudes (Figure 4).
Figure 4.
Activation of parietal cortex by magnitude processing. Neuroimaging studies have reported activity in parietal cortex, including regions along the IPS, in response to stimuli from a variety of magnitudes including number, size, time, brightness, and ordinal position judgments. Each point on the cortical template represents peak parietal coordinates from studies of: Number: [26–28, 73–78, 80, 82, 97, 98]; Time: [85, 87, 88, 90]; Brightness: [77, 78, 90]; Size: [75, 78, 80, 81]; Ordinal memory: [83]; Ordinal position: [82]. Maps generated using Caret software (http://brainmap.wustl.edu/caret; [99]).
Recent neurophysiological data from non-human primates further implicate a common, evolutionarily primitive neural substrate in the representation of multiple distinct magnitudes [92]. In one study, monkeys were trained to perform a line length matching task and a numerical matching task. During stimulus presentation or a subsequent delay, single neurons in area VIP responded selectively to visual stimuli based on their numerosity or length. Although some neurons responded only to numerosity and others only to line length, a subset of cells (~20%) responded to the magnitudes of both the line lengths and the numerical values. These data support a case, originally proposed by Pinel and colleagues [77], for ‘distributed but overlapping’ neural coding of quantitative dimensions in the IPS.
One implication of these results is that different quantitative dimensions can be represented by generic magnitude-coding neurons. A second implication is that, at a more global level, the IPS is recruited during judgments of quantitative dimensions beyond number alone. Finally, continuity across species in the overlapping neural representation of different quantities in parietal cortex indicates that primitive magnitude processing algorithms originate from an evolutionarily primitive neural source that is common to humans and other primates.
Conclusions
A wealth of empirical data demonstrates that numerical cognition is a primitive system that is shared with other animal species and emerges early in development. These data support the hypothesis that ‘number’ is a fundamental and universal cognitive capacity. Yet, several other cognitive functions for discriminating magnitudes also appear to be widely shared with other species and develop early in ontogeny. These capacities include judgments of size, time, brightness, and other continuous quantities.
Our review highlights the commonalities in the cognitive and neural signatures of number and continuous magnitudes. However, one point of hesitation in ruling out the cognitive specialization of numerical processes is that the computational problem an organism faces in representing number intuitively differs from the problem of representing brightness or total surface area, for example, at least in terms of the input variables that are drawn upon. The problem of representing number requires individuating objects or events and normalizing over their non-numerical features. It thus seems likely that a unique set of neural processes provides the basis for the formation of numerical representations even if the hardware underlying the remainder of the quantitative judgment process is generalized.
A second issue in characterizing the specificity of numerical cognition is that no region of the IPS has been consistently identified as exclusively involved in numerical processing. But, while our review indicates little evidence for neural processes specific to numerical representation or comparison, there are at least four possibilities that would not preclude such neural specificity. Namely, the lack of evidence for distinct numerical processes may result from 1) complete intermingling of neural populations that discretely encode numerosity and other magnitudes [77], 2) inter-individual variability in the regions used to represent different magnitudes that hinders identification of number-specific processes at the group level, 3) a lack of spatial resolution necessary to identify small but spatially dissociable patches of cortex used for processing different magnitudes, and 4) convergence of neural mechanisms of quantitative processes at a late stage in the processing stream, such as response selection. This lattermost possibility has received some support in recent fMRI and computational modeling studies [15, 79, 93].
Finally, much of the literature on the neural basis of numerical cognition has focused on parietal cortex [9, 17]. The functional roles of other brain regions engaged by numerical tasks, such as frontal cortex, remain to be determined [14, 16, 26]. A closer investigation of activity in regions outside parietal cortex may reveal different processes for different quantities. Additionally, differences among quantitative processes may also be evident in the lateralization of activity across brain regions [17]. However, across studies, a clearly defined pattern of lateralization remains elusive in quantity-related brain activity, particularly in ‘number’ studies.
Why might time, number, space, and other continuous quantities be linked in cortex? Walsh [17] has raised the possibility that time, space, and number might share resources because these dimensions each contain information that can be used to direct action. Our review, however, illustrates that continua beyond number, space, and time share the same psychophysical signatures, comparison processes, and neural substrate. It is unclear how the computational demands of the action system unify all of these ordinal continua.
Rather than reflecting the computational demands of the action control system, the common algorithms and neural processes underlying magnitude judgments may instead derive from a shared evolutionary heritage. For example, the processes underlying different quantitative judgments may have evolved from a single magnitude system. Under this scenario, a system that once computed one magnitude (e.g., size) may have been hi-jacked to perform judgments along a new dimension (e.g., number) [94]. Alternatively, different magnitude judgments may have evolved simultaneously and independently, but obey common principles because they are simply the most efficient [95] or the most probable [96] under the pre-existing constraints of the nervous system. Computational modeling studies can play a useful role in defining the potentially adaptive features of a common magnitude code and comparison algorithm for number and other quantities. Additionally, studies of the structure and function of quantitative abilities in developing humans and non-human animals will further illuminate convergence and divergence in the origins of different quantitative abilities as well as the contribution of experience toward relating or delineating quantities in the mind.
In conclusion, the ANS might be best characterized as part of a broad, evolutionarily primitive domain in which all quantitative dimensions share computational mechanisms, despite likely differences in their initial encoding processes. In this view, the characterization of the nature of numerical cognition may be best approached independently of issues of domain-specificity. New cognitive and neurobiological data are needed to better characterize the sources of the common signatures observed among distinct magnitudes such as numerosity, brightness, size, and time. Currently, neuroimaging and neurophysiological data implicate convergence of magnitude processing in parietal cortex. The challenge, then, is to discover the cognitive and neural processes underlying quantitative judgments that lead to the indisputable experience of different quantities as distinct properties.
Box 3: Questions for future research
What cognitive and neural processes distinguish numerical processing from processing related to size, brightness, and other quantities at the level of the initial encoding of the representation?
Can the magnitude representation process be psychologically and neurally distinguished from the magnitude comparison process?
Can analyses of individual subject data in fMRI studies reveal individual differences in associations or dissociations among quantitative processes?
What is the contribution of brain regions other than parietal cortex to approximate numerical processing?
Do neuropsychological impairments in a variety of ordinal quantitative judgments co-occur?
What clues can the origins of numerical processing as revealed by human infant and non-human animal studies provide about the cognitive and neural relationships among quantities?
Acknowledgments
We thank Sara Cordes, Emily Hopkins, Melissa Libertus, Brad Mahon, Dustin Merritt, David Paulsen, and the members of the Brannon lab for comments on this manuscript. This work was supported by Grants an NSF CAREER award and a McDonnell Scholar Award to EMB in addition to an NRSA postdoctoral fellowship to JFC. We also wish to thank David Van Essen for advice in using Caret (http://brainmap.wustl.edu/caret) to generate Figure 4.
Works Cited
- 1.Gallistel CR, Gelman R. Non-verbal numerical cognition: From reals to integers. Trends in Cognitive Sciences. 2000;4(2):59–65. doi: 10.1016/s1364-6613(99)01424-2. [DOI] [PubMed] [Google Scholar]
- 2.Feigenson L, Dehaene S, Spelke ES. Core systems of number. Trends in Cognitive Sciences. 2004;8(7):307–314. doi: 10.1016/j.tics.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Cantlon JF, Brannon EM. Semantic congruity affects numerical judgments similarly in monkeys and humans. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(45):16507–16511. doi: 10.1073/pnas.0506463102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cantlon JF, Brannon EM. Basic math in monkeys and college students. PLoS Biology. 2007;5(12):e328. doi: 10.1371/journal.pbio.0050328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hauser MD, Spelke ES. Evolutionary and developmental foundations of human knowledge: A case study of mathematics. In: Gazzaniga MS, editor. The cognitive neurosciences. The MIT Press; Cambridge, MA: 2004. pp. 853–864. [Google Scholar]
- 6.Pica P, et al. Exact and approximate arithmetic in an Amazonian indigene group. Science. 2004;306(5695):499–503. doi: 10.1126/science.1102085. [DOI] [PubMed] [Google Scholar]
- 7.Butterworth B. The mathematical brain. London: Macmillan; 1999. [Google Scholar]
- 8.Dehaene S, et al. Arithmetic and the brain. Current Opinion in Neurobiology. 2004;14(2):218–224. doi: 10.1016/j.conb.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Dehaene S, et al. Three parietal circuits for number processing. Cognitive Neuropsychology. 2003;20(3–6):487–506. doi: 10.1080/02643290244000239. [DOI] [PubMed] [Google Scholar]
- 10.Carey S, Spelke ES. Domain-specific knowledge and conceptual change. In: Hirschfeld LA, Gelman SA, editors. Mapping the mind: Domain specificity in cognition and culture. Cambridge University Press; New York, NY: 1994. pp. 169–200. [Google Scholar]
- 11.Kinzler KD, Spelke ES. Core systems in human cognition. Progress in Brain Research. 2007;164:257–264. doi: 10.1016/S0079-6123(07)64014-X. [DOI] [PubMed] [Google Scholar]
- 12.Fodor JA. The modularity of mind:An essay on faculty psychology. Cambridge, MA: The MIT Press; 1983. [Google Scholar]
- 13.Kanwisher N. Domain specificity in face perception. Nature Neuroscience. 2000;3(8):759–763. doi: 10.1038/77664. [DOI] [PubMed] [Google Scholar]
- 14.Ansari D. Effect of development and enculturation on number representation in the brain. Nature Reviews Neuroscience. 2008;9(4):278–291. doi: 10.1038/nrn2334. [DOI] [PubMed] [Google Scholar]
- 15.Cohen Kadosh R, Lammertyn J, Izard V. Are numbers special? An overview of chronometric, neuroimaging, developmental and comparative studies of magnitude representation. Progress in Neurobiology. 2008;84(2):132–147. doi: 10.1016/j.pneurobio.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Nieder A. The number domain: Can we count on parietal cortex? Neuron. 2004;44(3):407–409. doi: 10.1016/j.neuron.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 17.Walsh V. A theory of magnitude: Common cortical metrics of time, space, and quantity. Trends in Cognitive Sciences. 2003;7(11):483–488. doi: 10.1016/j.tics.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Hubbard EM, et al. Interactions between number and space in parietal cortex. Nature Reviews Neuroscience. 2005;6(6):435–448. doi: 10.1038/nrn1684. [DOI] [PubMed] [Google Scholar]
- 19.Cantlon JF, Brannon EM. Shared system for ordering small and large numbers in monkeys and humans. Psychological Science. 2006;17(5):401–406. doi: 10.1111/j.1467-9280.2006.01719.x. [DOI] [PubMed] [Google Scholar]
- 20.Lipton JS, Spelke ES. Origins of number sense: Large-number discrimination in human infants. Psychological Science. 2003;14(5):396–401. doi: 10.1111/1467-9280.01453. [DOI] [PubMed] [Google Scholar]
- 21.Xu F, Spelke ES. Large number discrimination in 6-month-old infants. Cognition. 2000;74(1):B1–B11. doi: 10.1016/s0010-0277(99)00066-9. [DOI] [PubMed] [Google Scholar]
- 22.Cipolotti L, Butterworth B, Denes G. A specific deficit for numbers in a case of dense acalculia. Brain. 1991;114(6):2619–2637. doi: 10.1093/brain/114.6.2619. [DOI] [PubMed] [Google Scholar]
- 23.Dehaene S, Cohen L. Cerebral pathways for calculation: Double dissociation between rote verbal and quantitative knowledge of arithmetic. Cortex. 1997;33(2):219–250. doi: 10.1016/s0010-9452(08)70002-9. [DOI] [PubMed] [Google Scholar]
- 24.Cappelletti M, et al. Dissociations in numerical abilities revealed by progressive cognitive decline in a patient with semantic dementia. Cognitive Neuropsychology. 2005;22(7):771–793. doi: 10.1080/02643290442000293. [DOI] [PubMed] [Google Scholar]
- 25.Piazza M, et al. Tuning curves for approximate numerosity in the human intraparietal cortex. Neuron. 2004;44(3):547–555. doi: 10.1016/j.neuron.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Piazza M, et al. A magnitude code common to numerosities and number symbols in human intraparietal cortex. Neuron. 2007;53(2):293–305. doi: 10.1016/j.neuron.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 27.Eger E, et al. A supramodal number representation in human intraparietal cortex. Neuron. 2003;37(4):719–725. doi: 10.1016/s0896-6273(03)00036-9. [DOI] [PubMed] [Google Scholar]
- 28.Cantlon JF, et al. Functional imaging of numerical processing in adults and 4-year-old children. PLoS Biology. 2006;4(5):e125. doi: 10.1371/journal.pbio.0040125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Libertus ME, et al. Electrophysiological markers of number processing in 7-month-old infants. submitted. [Google Scholar]
- 30.Izard V, Dehaene-Lambertz G, Dehaene S. Distinct cerebral pathways for object identity and number in human infants. PLoS Biology. 2008;6(2):e11. doi: 10.1371/journal.pbio.0060011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diester I, Nieder A. Semantic associations between signs and numerical categories in the prefrontal cortex. PLoS Biology. 2007;5(11):e294. doi: 10.1371/journal.pbio.0050294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nieder A, Miller EK. A parieto-frontal network for visual numerical information in the monkey. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(19):7457–7462. doi: 10.1073/pnas.0402239101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nieder A, Diester I, Tudusciuc O. Temporal and spatial enumeration processes in the primate parietal cortex. Science. 2006;313(5792):1431–1435. doi: 10.1126/science.1130308. [DOI] [PubMed] [Google Scholar]
- 34.Roitman J, Brannon EM, Platt ML. Monotonic coding of numerosity in macaque lateral intraparietal area. PLoS Biology. 2007;5(8):e208. doi: 10.1371/journal.pbio.0050208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sawamura H, Shima K, Tanji J. Numerical representation for action in the parietal cortex of the monkey. Nature. 2002;415(6874):918–922. doi: 10.1038/415918a. [DOI] [PubMed] [Google Scholar]
- 36.Stevens SS. Mathematics, measurement, and psychophysics. In: Stevens SS, editor. Handbook of experimental psychology. Wiley; Oxford, England: 1951. pp. 1–49. [Google Scholar]
- 37.Moyer RS, Landauer TK. Time required for judgments of numerical inequality. Nature. 1967;215(5109):1519–1520. doi: 10.1038/2151519a0. [DOI] [PubMed] [Google Scholar]
- 38.Holyoak KJ, Walker JH. Subjective magnitude information in semantic orderings. Journal of Memory and Language. 1976;15(3):287–299. [Google Scholar]
- 39.Petrusic WM. Semantic congruity effects and theories of the comparison process. Journal of Experimental Psychology: Human Perception and Performance. 1992;18(4):962–986. doi: 10.1037//0096-1523.18.4.962. [DOI] [PubMed] [Google Scholar]
- 40.Meck WH, Church RM. A mode control model of counting and timing processes. Journal of Experimental Psychology: Animal Behavior Processes. 1983;9(3):320–334. [PubMed] [Google Scholar]
- 41.Dehaene S, Bossini S, Giraux P. The mental representation of parity and number magnitude. Journal of Experimental Psychology: General. 1993;122(3):371–396. [Google Scholar]
- 42.Marshuetz C, et al. Order information in working memory: fMRI evidence for parietal and prefrontal mechanisms. Journal of Cognitive Neuroscience. 2000;12(2):130–144. doi: 10.1162/08989290051137459. [DOI] [PubMed] [Google Scholar]
- 43.Gevers W, Reynvoet B, Fias W. The mental representation of ordinal sequences is spatially organized. Cognition. 2003;87(3):B87–B95. doi: 10.1016/s0010-0277(02)00234-2. [DOI] [PubMed] [Google Scholar]
- 44.Stevens JC, Marks LE. Cross-modality matching of brightness and loudness. Proceedings of the National Academy of Sciences of the United States of America. 1965;54(2):407–411. doi: 10.1073/pnas.54.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dormal V, Seron X, Pesenti M. Numerosity-duration interference: A Stroop experiment. Acta Psychologica. 2006;121(2):109–124. doi: 10.1016/j.actpsy.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 46.Roitman J, et al. Nonverbal representation of time and number in adults. Acta Psychologica. 2007;124:296–318. doi: 10.1016/j.actpsy.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 47.Fias W, Lauwereyns J, Lammertyn J. Irrelevant digits affect feature-based attention depending on the overlap of neural circuits. Cognitive Brain Research. 2001;12(3):415–423. doi: 10.1016/s0926-6410(01)00078-7. [DOI] [PubMed] [Google Scholar]
- 48.Hurewitz F, Gelman R, Schnitzer B. Sometimes area counts more than number. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(51):19599–19604. doi: 10.1073/pnas.0609485103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oliveri M, et al. Perceiving numbers alters time perception. Neuroscience Letters. 2008;438(3):308–311. doi: 10.1016/j.neulet.2008.04.051. [DOI] [PubMed] [Google Scholar]
- 50.Xuan B, et al. Larger stimuli are judged to last longer. Journal of Vision. 2007;7(10):1–5. doi: 10.1167/7.10.2. [DOI] [PubMed] [Google Scholar]
- 51.Cantlon JF, et al. Comment on ‘Log or linear? Distinct intuitions of the number scale in Western and Amazonian indigene cultures’. Science. doi: 10.1126/science.1164878. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Repp BH, Knoblich G. Action can affect auditory perception. Psychological Science. 2007;18(1):6–7. doi: 10.1111/j.1467-9280.2007.01839.x. [DOI] [PubMed] [Google Scholar]
- 53.Rusconi E, et al. Spatial representation of pitch height: The SMARC effect. Cognition. 2006;99(2):113–129. doi: 10.1016/j.cognition.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 54.Terrace HS. The simultaneous chain: A new approach to serial learning. Trends in Cognitive Sciences. 2005;9(4):202–210. doi: 10.1016/j.tics.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 55.Zorzi M, Priftis K, Umiltà C. Neglect disrupts the mental number line. Nature. 2002;417(6885):138–139. doi: 10.1038/417138a. [DOI] [PubMed] [Google Scholar]
- 56.Basso G, et al. Time perception in a neglected space. Neuroreport. 1996;7(13):2111–2114. doi: 10.1097/00001756-199609020-00009. [DOI] [PubMed] [Google Scholar]
- 57.Cohen Kadosh R, Henik A, Walsh V. Small is bright and big is dark in synaesthesia. Current Biology. 2007;17(19):R834–R835. doi: 10.1016/j.cub.2007.07.048. [DOI] [PubMed] [Google Scholar]
- 58.Brown SW. Attentional resources in timing: Interference effects in concurrent temporal and nontemporal working memory tasks. Perception & Psychophysics. 1997;59(7):1118–1140. doi: 10.3758/bf03205526. [DOI] [PubMed] [Google Scholar]
- 59.Herrera A, Macizo P, Semenza C. The role of working memory in the association betwee number magnitude and space. Acta Psychologica. 2008;128(2):225–237. doi: 10.1016/j.actpsy.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 60.Holyoak KJ. Comparative judgments with numerical reference points. Cognitive Psychology. 1978;10(2):203–243. [Google Scholar]
- 61.Holyoak KJ, Mah WA. Cognitive reference points in judgments of symbolic magnitude. Cognitive Psychology. 1982;14(3):328–352. [Google Scholar]
- 62.Shaki S, Algom D. The locus and nature of semantic congruity in symbolic comparison: Evidence from the Stroop effect. Memory & Cognition. 2002;20(1):3–17. doi: 10.3758/bf03195260. [DOI] [PubMed] [Google Scholar]
- 63.Bryant PE, Squire S. Children’s mathematics: Lost and found in space. In: Gattis M, editor. Spatial schemas and abstract thought. The MIT Press; Cambridge, MA: 2001. pp. 175–200. [Google Scholar]
- 64.Feigenson L. The equality of quantity. Trends in Cognitive Sciences. 2007;11(5):185–187. doi: 10.1016/j.tics.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 65.Brannon EM, Lutz D, Cordes S. The development of area discrimination and its implications for number representation in infancy. Developmental Science. 2006;9:F59–F64. doi: 10.1111/j.1467-7687.2006.00530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brannon EM, Suanda S, Libertus K. Temporal discrimination increases in precision over development and parallels the development of numerosity discrimination. Developmental Science. 2007;10(6):770–777. doi: 10.1111/j.1467-7687.2007.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.vanMarle K, Wynn K. Six-month-old infants use analog magnitudes to represent duration. Developmental Science. 2006;9(5):F41–F49. doi: 10.1111/j.1467-7687.2006.00508.x. [DOI] [PubMed] [Google Scholar]
- 68.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 69.Milner AD, Goodale MA. Oxford Psychology Series. New York: Oxford University Press; 1995. The visual brain in action; p. 248. [Google Scholar]
- 70.Grefkes C, Fink GR. The functional organization of the intraparietal sulcus in humans and monkeys. Journal of Anatomy. 2005;207(1):3–17. doi: 10.1111/j.1469-7580.2005.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gerstmann J. Syndrome of finger agnosia, disorientation for right and left, agraphia and acalculia. Archives of Neurology and Psychiatry. 1940;44:398–408. [Google Scholar]
- 72.Shuman M, Kanwisher N. Numerical magnitude in the human parietal lobe: Tests of representational generality and domain specificity. Neuron. 2004;44(3):557–569. doi: 10.1016/j.neuron.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 73.Simon O, et al. Topographical layout of hand, eye, calculation, and language-related areas in the human parietal lobe. Neuron. 2002;33(3):475–487. doi: 10.1016/s0896-6273(02)00575-5. [DOI] [PubMed] [Google Scholar]
- 74.Naccache L, Dehaene S. The priming method: Imaging unconscious repetition priming reveals an abstract representation of number in the parietal lobes. Cerebral Cortex. 2001;11(10):966–974. doi: 10.1093/cercor/11.10.966. [DOI] [PubMed] [Google Scholar]
- 75.Cohen Kadosh R, et al. Notation-dependent and -independent representations of number in the parietal lobes. Neuron. 2007;53(2):307–314. doi: 10.1016/j.neuron.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 76.Ansari D, Dhital B, Siong SC. Parametric effects of numerical distance on the intraparietal sulcus during passive viewing of rapid numerosity changes. Brain Research. 2006;1067(1):181–188. doi: 10.1016/j.brainres.2005.10.083. [DOI] [PubMed] [Google Scholar]
- 77.Pinel P, et al. Distributed and overlapping cerebral representations of number, size, and luminance during comparative judgments. Neuron. 2004;41(6):983–993. doi: 10.1016/s0896-6273(04)00107-2. [DOI] [PubMed] [Google Scholar]
- 78.Cohen Kadosh R, et al. Are numbers special? The comparison systems of the human brain investigated by fMRI. Neuropsychologia. 2005;43(9):1238–1248. doi: 10.1016/j.neuropsychologia.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 79.Gobel SM, et al. Response-selection-related parietal activation during number comparison. Journal of Cognitive Neuroscience. 2004;16(9):1536–1551. doi: 10.1162/0898929042568442. [DOI] [PubMed] [Google Scholar]
- 80.Kaufmann L, et al. Neural correlates of distance and congruity effects in a numerical Stroop task: An event-related fMRI study. Neuroimage. 2005;25(3):888–898. doi: 10.1016/j.neuroimage.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 81.Fias W, et al. Parietal representation of symbolic and nonsymbolic magnitude. Journal of Cognitive Neuroscience. 2003;15(1):47–56. doi: 10.1162/089892903321107819. [DOI] [PubMed] [Google Scholar]
- 82.Fias W, et al. Processing of abstract ordinal knowledge in the horizontal segment of the intraparietal sulcus. Journal of Neuroscience. 2007;27(33):8952–8956. doi: 10.1523/JNEUROSCI.2076-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marshuetz C, et al. Working memory for order and the parietal cortex: An event-related functional magnetic resonance imaging study. Neuroscience. 2006;139(1):311–316. doi: 10.1016/j.neuroscience.2005.04.071. [DOI] [PubMed] [Google Scholar]
- 84.Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nature Reviews Neuroscience. 2005;6(10):755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- 85.Harrington DL, et al. Neural representation of interval encoding and decision making. Cognitive Brain Research. 2004;21(2):193–205. doi: 10.1016/j.cogbrainres.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 86.Hinton SC, et al. Neural systems supporting timing and chronometric counting: An fMRI study. Cognitive Brain Research. 2004;21(2):183–192. doi: 10.1016/j.cogbrainres.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 87.Pouthas V, et al. Neural network involved in time perception: An fMRI study comparing long and short interval estimation. Human Brain Mapping. 2005;25(4):433–441. doi: 10.1002/hbm.20126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rao SM, Mayer AR, Harrington DL. The evolution of brain activation during temporal processing. Nature Neuroscience. 2001;4(3):317–323. doi: 10.1038/85191. [DOI] [PubMed] [Google Scholar]
- 89.Alexander I, Cowey A, Walsh V. The right parietal cortex and time perception: Back to Critchley and the Zeitraffer Phenomenon. Cognitive Neuropsychology. 2005;22(34):306–315. doi: 10.1080/02643290442000356. [DOI] [PubMed] [Google Scholar]
- 90.Maquet P, et al. Brain activation induced by estimation of duration: a PET study. Neuroimage. 1996;3(2):119–126. doi: 10.1006/nimg.1996.0014. [DOI] [PubMed] [Google Scholar]
- 91.Harrington DL, Haaland KY, Hermanowicz N. Temporal processing in the basal ganglia. Neuropsychology. 1998;12(1):3–12. doi: 10.1037//0894-4105.12.1.3. [DOI] [PubMed] [Google Scholar]
- 92.Tudusciuc O, Nieder A. Neuronal population coding of continuous and discrete quantity in the primate posterior parietal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(36):14513–14518. doi: 10.1073/pnas.0705495104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zorzi M, Butterworth B. A computational model of number comparison. In: Hahn, Stoness, editors. Proceedings of the 21st Annual Meeting of the Cognitive Science Society; 1999. pp. 778–783. [Google Scholar]
- 94.Gould SJ, Vrba ES. Exaptation-A missing term in the science of form. Paleobiology. 1982;8(1):4–15. [Google Scholar]
- 95.Gallistel CR. Learning, development, and conceptual change. Cambridge, MA: The MIT Press; 1990. The organization of learning; p. 648. [Google Scholar]
- 96.Gould SJ, Lewontin RC. The spandrels of San Marco and the Panglossian paradigm: A critique of the adaptationist programme. Proceedings of the Royal Society of London, Series B. 1979;205(1161):581–598. doi: 10.1098/rspb.1979.0086. [DOI] [PubMed] [Google Scholar]
- 97.Castelli F, Glaser DE, Butterworth B. Discrete and analogue quantity processing in the parietal lobe: A functional MRI study. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(12):4693–4698. doi: 10.1073/pnas.0600444103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chochon F, et al. Differential contributions of the left and right inferior parietal lobules to number processing. Journal of Cognitive Neuroscience. 1999;44(6):617–630. doi: 10.1162/089892999563689. [DOI] [PubMed] [Google Scholar]
- 99.Van Essen DC, et al. An integrated software suite for surface-based analyses of cerebral cortex. Journal of the American Medical Informatics Association. 2001;8(5):443–459. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dehaene S. Symbols and quantities in parietal cortex: Elements of a mathematical theory of number representation and manipulation. In: Haggard P, Rossetti Y, Kawato M, editors. Attention and Performance XXII. Oxford University Press; London: 2008. [Google Scholar]