Abstract
In spite of significant advances in treatment of patients with schizophrenia and continued efforts towards their deinstitutionalization, a considerable group of patients remain chronically hospitalized or otherwise dependent on others for basic necessities of life. It has been proposed that these patients belong to a distinct etiopathological subgroup, termed Kraepelinian, whose course of illness may be progressive and resistant to treatment. Indeed, longitudinal studies appear to show that elderly Kraepelinian patients follow a course of rapid cognitive and functional deterioration, commensurate with a dementing process, and that their poor functional status is closely correlated with the cognitive deterioration. Recent neuroimaging studies described a pattern of posteriorization of grey and white matter deficits with poor outcome in schizophrenia, and produced a constellation of findings implicating primary processing of visual and auditory information as central to the impaired functional status in this patient group. These studies are summarized in detail in this review and future directions for neuroimaging assessment of very poor outcome patients with schizophrenia are suggested.
Introduction
In spite of considerable and increasingly concerted scientific effort, the nature of genetic susceptibility to schizophrenia remains elusive. One of the main strategies to overcome this quandary may be identification of endophenotypes aimed at reducing the likely heterogeneity of the illness thus rendering it more amenable to genetic analysis (Jablensky, 2006). This may include a clinical and neuromorphological isolation of more homogeneous schizophrenia subtypes (Buchsbaum & Haier, 1978), based on the predominance of certain clinical descriptors, severity or functional outcome of its course (Roy, Merette, & Maziade, 2001). This latter strategy of identifying a very poor outcome subgroup on the basis of the longitudinal course of the illness was employed by Richard Keefe and colleagues (originally at Bronx Veterans’ Affairs Medical Center) and may hold a special promise (Keefe et al., 1987, 1988, 1990, 1991).
It has long been suggested that there may be a subgroup of patients with schizophrenia, who follow a progressively deteriorating course conforming to the original views of Emil Kraepelin on the natural course of dementia praecox. Beginning from the 1950s, these patients were colloquially referred to as ‘Kraepelinian’. The longitudinal criteria, proposed by Keefe and colleagues in 1987 were aimed at a valid isolation of this very poor outcome subgroup of patients with schizophrenia, which have been incapable of independent living and followed an unremitting clinical course for at least a five-year period. In order to fulfil the inclusion criteria for the poor-outcome, or Kraepelinian, schizophrenia subtype, patients must display for preceding 5 years: (1) continuous hospitalization or complete dependence on others for obtaining basic necessities of life, including food, clothing, and shelter; (2) no evidence of useful employment; and (3) no evidence for symptomatic remission. Societal and scientific significance of this classification lies in the fact that these criteria by definition incorporate into the Kraepelinian schizophrenia subtype the vast majority of chronically institutionalized patients that linger in state psychiatric facilities despite a continuous three-decade effort at their deinstitutionalization.
From the first clinical description of the Kraepelinian schizophrenia subtype (Keefe et al., 1987), several groups in the United States, Canada, France, and Japan have published clinical, neuro-psychological and neuroimaging assessments of such patients. These assessments include clinical validations of the classificatory criteria on independent samples by the original group of Keefe et al. (1996) and by Roy et al. (2001), and on a cohort of French patients with Kraepelinian schizophrenia by Bralet, Loas, Yon and Marechal (2002). More recently, a clinical study in Japan (Nakaya & Ohmori, 2006) found that although there may be a significant overlap between the Kraepelinian/non-Kraepelinian classification, based on the longitudinal course, and deficit/nondeficit schizophrenia classification, based on the prominence of the negative symptoms of the illness (Carpenter, Heinrichs, & Wagman, 1988), the two classificatory schemes select distinctly differing subgroups of patients: despite the overlap, there are Kraepelinian patients with and without deficit syndrome and the same holds true for the non-Kraepelinian group.
Clinical and neuropsychological characteristics
The clinical picture and course of the poor-outcome (Kraepelinian) schizophrenia is characterized by severe dysfunctions in self-care (Keefe et al., 1987, 1996), disturbance in premorbid sociosexual functioning (Keefe et al., 1989, 1990), more severe negative symptoms and formal thought disorder (Bralet et al., 2002; Brickman et al., 2004; Keefe et al., 1989; Stephens, 1978), presence of delusional passivity phenomena (Keks et al., 1992), lower association with affective symptomatology (Kilzieh, Wood, Erdmann, Raskind, & Tapp, 2003; Rieckmann et al., 2005), excessive summertime (July) clustering of birthdates and association with morbid polydypsia (Bralet et al., 2002, Bralet, Ton, & Falissard, 2007), and more extensive family history of schizophrenia spectrum disorders (Keefe et al., 1996). Some of these poor-outcome characteristics are known to be interrelated, e.g. nonresponsiveness to antipsychotic treatment and increased familiality of the illness (Joober et al., 2005) or poor premorbid sociosexual functioning and severity of the negative symptoms (Salokangas, 2003). Some (but not all) authors report more severe positive symptoms in the Kraepelinian subgroup as well (Brickman et al., 2004; Roy et al., 2001). Finally, patients with Kraepelinian schizophrenia may also exhibit resistance to antipsychotic (haloperidol) treatment (Keefe et al., 1987) with no changes in symptomatology upon discontinuation of treatment (Harvey et al., 1991) and blunted prolactin response to haloperidol challenge (Keks et al., 1992). Neuropsychological studies suggest rather significant and apparently multilobar impairments in this group of patients, including impaired performance on visual-motor processing, abstraction/flexibility (Albus et al., 1996), fine motor dexterity, and executive functioning tests (Roy et al., 2003).
Some of these poor-outcome characteristics are shared by patients with deficit schizophrenia subtype, e.g. higher severity of negative and disorganization symptoms, poorer premorbid adjustment and social functioning, high degree of temporal stability of subgrouping diagnosis, lower prevalence of depressive symptomatology, and excess of summertime births (see Roy et al., 2001 for review). Other characteristics, like poor response to antipsychotic treatment (Harvey et al., 1991; Keefe et al., 1987), possibly more severe positive symptoms (Brickman et al., 2004, but not in somewhat smaller samples of Keefe et al., 1987, 1996 and Bralet et al., 2002) and earlier age of onset (Brickman et al., 2004; Roy et al., 2001, 2003), may only be peculiar to the Kraepelinian subtype.
Longitudinal course of the illness
The course of the illness in poor-outcome, chronically institutionalized patients with schizophrenia has over the last decade been evaluated in both cross-sectional and longitudinal studies. These studies indicate that unlike schizophrenia with favourable outcome, there may be a progressive process and continued clinical deterioration in the poor-outcome institutionalized subgroup of patients (Arnold et al., 1995a; Davidson et al., 1995; Harvey et al., 1999a, 1999b). Elderly institutionalized patients with poor outcomes, regardless of gender (Moriarty et al., 2001) or treatment location (Harvey, Leff, Trieman, Anderson, & Davidson, 1997a; Harvey et al., 1998), display an array of severe adaptive and cognitive deficits (e.g. in Mini-Mental State examination, clock-drawing test as a nonspecific measure of global cognitive and visual-analytic functioning, as well as on the phonemic tests of verbal fluency) that are not grossly dissimilar to those seen in Alzheimer’s disease (Arnold et al., 1995a; Bozikas et al., 2002; Gabrovska-Johnson et al., 2003; Heinik, Lahav, Drummer, Vainer-Benaiah, & Lin, 2000; Kosmidis et al., 2005; Lowery et al., 2003; McBride et al., 2002; Owens & Johnstone, 1980). Recognition memory has been reported a single most impaired domain of cognitive functioning in this patient group (Harvey et al., 2000a). Some authors do in fact talk of a true dementia complicating the course of chronic schizophrenia in a considerable subgroup of institutionalized patients (de Vries, Honer, Kemp, & McKenna, 2001; Goldberg, Weinberger, Berman, Pliskin, & Podd, 1987; Johnstone et al., 1978; Marsden, 1976). Indeed, age disorientation, generally associated with (and predictive of) global intellectual impairment (Harvey et al., 1995; Liddle & Crow, 1984), may be seen in approximately a quarter of chronically hospitalized patients (Goldberg et al., 1988; Manschreck et al., 2000; Stevens, Crow, Bowman, & Coles, 1978; Tapp, Tandon, Scholten, & Dudley, 1993) and may be not related to premorbid intellectual functioning or prior treatment (Buhrich, Crow, Johnstone, & Owens, 1988). Yet, unlike patients with Alzheimer’s disease and most other known dementias, who show consistent and linear longitudinal decline in cognitive functions, patients with poor-outcome schizophrenia show a pattern whereby the rate of their cognitive deficits accelerates with age (Friedman et al., 2001; Waddington & Youssef, 1996). Thus, their cognitive functioning appears to be fairly stable until late life (about 65 years of age), followed by rapid deterioration at a rate of approximately 15% a year (Friedman et al., 2001; Harvey et al., 1999a). The risk for this cognitive and functional decline does not appear to be associated with gender, severity of the negative symptoms, or even vicissitudes of contemporaneous neuroleptic treatment (Harvey et al., 1999a).
This pattern of differential age-related decline in cognitive functioning suggests that chronically hospitalized patients with poor-outcome schizophrenia may represent a diagnostic group, distinct from both the better-outcome schizophrenia and known types of dementing disorders. In the longitudinal studies, the cognitive declines in these patients do not appear to be strongly related to the baseline cognitive and functional status, or to the baseline clinical, medical and demographic variables (Friedman et al., 2002; Harvey et al., 2003), but may be associated with orofacial tardive dyskinesia (Byne et al., 1998; Waddington & Youssef, 1996). Declines in the adaptive functioning, on the other hand, may be predicted by the course of the cognitive decline and, independently, by the baseline severity of the negative symptoms (Harvey, Sukhodolsky, Parrella, White, & Davidson, 1997c, Harvey et al., 1999b, 2003). Overall, functional (social and adaptive life skills) outcome in elderly poor-outcome patients is strongly correlated with cognitive status and the negative syndrome, regardless of its severity (McGurk et al., 2000). Still, even within this severely impaired group of patients, affective symptomatology (depressed mood) may be associated with somewhat better outcome (Rieckmann et al., 2005).
In the clinical domain, these patients display longitudinal deterioration in the negative syndrome, especially in the deficit symptomatology (alogia, poverty of speech); positive symptoms and disturbances in the train of thought, on the other hand, appear to display a modest improvement with age (Bowie et al., 2005; Davidson et al., 1995; Harvey et al., 1997b; Mancevski et al., 2007a; Putnam et al., 1996). At the same time, the structure and inter-relatedness of the psychopathology, if not the relative severity of the individual symptoms, remain longitudinally stable (Reichenberg, Rieckmann, & Harvey, 2005). Similarly, neurological soft signs in these patients appear to be stable over time (Smith, Hussain, Chowdhury, & Stearns, 1999), but their relationship to poor outcome remains inconclusive (Kolakowska, Williams, Jambor, & Ardern, 1985b)
It has been suggested that the very chronic hospitalization and the resistance of these patients to repeated drives at de-institutionalization may be related to the cognitive and functional deficits, unremitting and severe illness, volitional and especially activating symptomatology, which includes agitation and aggressive behaviours (Arnold et al., 1995a; Bowie et al., 2001; Johnstone, Owens, Gold, Crow, & MacMillan, 1981; Krasik & Logvinovich, 1977; Perlick, Mattis, Stastny, & Teresi, 1992; White et al., 1997, White, Opler, Harvey, Parrella, & Friedman, 2004). Accordingly, it had been shown that progression of the patients’ self-care abilities and cognitive deficits was not related to the severity of positive symptoms, but that the course of cognitive decline and to a lesser degree the severity and course of the negative syndrome were predictive of the decline in the patients’ abilities for self-care. Similarly, in a less impaired, outpatient group of elderly schizophrenia patients severity of the negative symptoms and cognitive functioning were the main determinants of the likelihood of independent living and gainful employment (Hofer et al., 2005a, 2005b). Visual vigilance (ability to sustain selective attention) was associated with independent living whereas visual and working memory was associated with employment status. This is in line with several prior studies placing more emphasis on cognitive deficits, rather than clinical semiotics of the illness, in its social and functional outcome (reviewed in Green, 1996, Green, Kern, Braff, & Mintz, 2000, and Stip, 2006; see also Velligan et al., 1997, 2000), as well as reports of improvements in the clinical symptomatology but not in adaptive functioning in the aftermath of typical antipsychotic treatment (Velligan, Mahurin, True, Lefton, & Flores, 1996; Velligan & Miller, 1999). Negative symptoms have also been shown to be associated with adaptive functioning, both concurrent and longitudinal, but to a much lesser degree than with the cognitive functioning (Ho, Nopoulos, Flaum, Arndt, & Andreasen, 1998; Herbener & Harrow, 2004). Although there have been reports that atypical antipsychotic agents may have a more favourable effect on certain domains of cognitive performance of outpatients with schizophrenia (Harvey & Keefe, 2001; Velligan et al., 2002, 2003), these agents do not appear to have a differential impact on their concurrent adaptive functioning (Velligan et al., 2003) or eventual symptomatic, cognitive and functional outcome of the chronically hospitalized schizophrenia patients (White et al., 2006).
Neuroimaging findings in poor-outcome schizophrenia
Ventricular system
Several lines of earlier neuroimaging evidence alluded to a relationship between posterior cortical regions (in particular temporal and parietal cortices) and clinical outcome in schizophrenia, starting with the pneumoencephalographic study by Matthew T. Moore and colleagues (1933) of the Temple University School of Medicine in Philadelphia, which noted an association between cognitive deterioration and degree of parietal lobe and insular atrophy in a sample of 60 schizophrenia patients. Further pneumoencephalographic studies similarly reported strong correlations among cognitive/clinical deterioration in schizophrenia patients and degree (Asano, 1967; Nagy, 1963) or frequency (Haug, 1982; Huber, 1957) of ventricular enlargement, especially in the third ventricles in both hemispheres, thus indirectly implicating temporal lobes in the cognitive and clinical deterioration seen in a subgroup of patients with schizophrenia.
Tomographic imaging techniques introduced a quantitative and three-dimensional morphometric approach which strengthened these findings. Over the last three decades, earlier computerized tomography and then MRI studies found that in comparison to patients with good outcomes, poor outcome was associated with relatively larger ventricular asymmetry (Losonczy et al., 1986), as well as size (Galderisi et al., 2000; Johnstone, Crow, Frith, Husband, & Kreel, 1976; Johnstone et al., 1989; Katsanis, Iacono, & Beiser, 1991; Kolakowska et al., 1985a; Owens et al., 1985; Pearlson, Garbacz, Breakey, Ahn, & DePaulo, 1984; Rossi et al., 2000; Staal et al., 2001). Ventricular size was predictive of poor outcome (DeLisi et al., 1992) and continued to show progressive enlargement in these patients over an up to five-year follow-up period (Davis et al., 1998; Ho et al., 2003; Lieberman et al., 1996, 2001). DeLisi, Sakuma, Maurizio, Relja and Hoff (2004) found that the rate of change in ventricular volume over time was significantly correlated with total time spent in hospital. Temporal lobe volume at onset was predictive of unremitting positive symptomatology as an outcome measure at five-year follow-up (Milev et al., 2003) while cerebellar, but not temporal or ventricular, volumes were predictive of psychosocial and clinical outcome at seven-year follow-up (Wassink, Andreasen, Nopoulos, & Flaum, 1999). Some of the findings were suggestive of a relationship between the larger lateral ventricular size and cognitive impairment in institutionalized (Gabrovska-Johnson et al., 2003; Johnstone et al., 1976; Owens et al., 1985) and noninstitutionalized chronic patients (Goldberg et al., 1988; Keilp et al., 1988; Lawson, Waldman, & Weinberger, 1988; Roccatagliatta, Gandolfo, Ruffinengo, Scotto, & Bacigalupo, 1986). Longitudinal outcome measures were employed only in some of these studies that assessed the overtly Kraepelinian schizophrenia subtype (Davis et al., 1998; Johnstone et al., 1976, 1989; Losonczy et al., 1986; Owens et al., 1985; Staal et al., 2001). While not all shared identical clinical assessments, the thread of ventricular abnormalities and associated poorer outcome measures is clearly discernable. Search for morphometric predictors of outcome in two other, prospective MRI studies, however, proved more inconclusive, with one report finding no such predictors on two-year follow-up (van Haren et al., 2003) and another reporting no relationship between clinical characteristics and rate of longitudinal progression of ventricular size or grey matter volume on 2-to-4-year follow-up (Whitworth et al., 2005). This may be explained by the relatively short periods to follow-up in the samples consisting of patients close to their first psychotic outbreak, whereas cognitive and functional deterioration tend to occur much later into the course of the illness (Friedman et al., 2001).
Cerebral grey matter
The first MRI study to specifically evaluate poor-outcome, institutionalized patients with schizophrenia (Marsh et al., 1997) found grey matter reductions in the temporal lobe, superior temporal gyrus, and frontoparietal regions in comparison to healthy comparison subjects; good-outcome comparison group was not included in the analyses. Analogously, Staal et al. (2001), who did include a better-outcome comparison group, found smaller total grey matter volumes, especially in the frontal lobe, in chronically hospitalized and not in good-outcome schizophrenia patients in comparison to healthy subjects, but differences between the two schizophrenia groups did not reach statistical significance. Our group has over the past several years completed several neuroimaging assessments on a large cohort of middle-aged poor-outcome (Kraepelinian, N=54), good-outcome (non-Kraepelinian, N=52), and healthy comparison (N=42) subjects, the former two patient groups recruited primarily from the Pilgrim State Psychiatric Center and Bronx Veterans’ Affairs Hospital of the Mount Sinai School of Medicine in New York. A study, comparing clinical, cognitive, and functional characteristics of long-stay schizophrenia patients between these two facilities (Harvey et al., 2000b) found that Pilgrim State Medical Center patients had more severe functional deficits, negative and cognitive symptoms than similarly institutionalized patients at the Bronx VA Hospital, but that relationship between the different variables of the illness was similar amongst the two groups. The primary discriminator between these patient groups was deficits in self-care, significantly more severe in the state hospital patients, and this is precisely the criterion that we used in selecting our poor-outcome (Kraepelinian) patient group, therefore ensuring its homogeneity. These two long-term care facilities were also the primary patient recruitment centers for the original researches by Richard Keefe and colleagues (1987, 1996), wherein the concept of Kraepelinian schizophrenia was operationalized and validated.
The main finding from a subsample of 37 patients with schizophrenia and 37 healthy comparison subjects (Mitelman, Shihabuddin, Brickman, Hazlett, & Buchsbaum, 2003), more recently confirmed in the whole cohort (Mitelman et al., 2007b), was the posteriorization, or more posterior distribution, of cortical grey matter deficits in patients with poor outcomes. That is, while all patients with schizophrenia regardless of outcome had dorsolateral prefrontal and temporal grey matter deficits, it was more posterior – parietal, but particularly temporal and occipital – changes in grey matter volumes that differentiated between patients with varying outcomes (Figure 1). A similar pattern of posteriorization was observed in the anteroposterior arch of the cingulate gyrus, where too the main intergroup differences were registered in the posterior cingulate (including retrosplenial) regions (Mitelman, Shihabuddin, Brickman, Hazlett, & Buchsbaum, 2005a; Mitelman et al., 2007b).
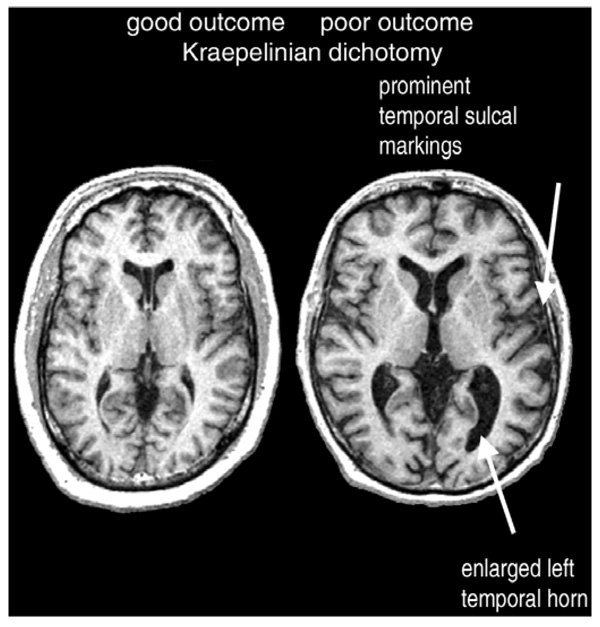
Figure 1.
Temporal lobe volume loss and lateral ventricular enlargement in a schizophrenia patient with poor outcome (right) in comparison to a patient with good outcome (left).
In the temporal lobes, differential grey matter reductions in association with poor outcome were noted in the lateral (Brodmann’s areas 21 and 22, i.e. superior and middle temporal gyri), medial (Brodmann’s areas 27, 28, and 36), and polar (Brodmann’s area 38) cortices, and at the temporal-occipital junction (Brodmann’s area 37). Unlike the temporal deficits, which were seen in all schizophrenia patients and were only more pronounced in those with poor outcomes, grey matter reductions in the occipital (Brodmann’s areas 17, 18, and 18) and – to a lesser extent – parietal (areas 7b, 31, 39, 40, 43) and posterior frontal (motor-premotor) cortical regions were unique to the poor-outcome, Kraepelinian schizophrenia subtype. In concert with this posteriorized pattern of structural deficits, a PET study in a subsample of these same participants found that poor-outcome patients have lower frontal/occipital ratios and metabolic rates in the temporal lobe and cingulate gyrus, as well as striatum, than patients with non-Kraepelinian schizophrenia (Buchsbaum et al., 2002). Overall, the areas with preferential grey matter reductions in those with poor outcomes tended to involve predominantly visual unimodal association and paralimbic cortices, in the left hemisphere showing predilection for the agranular cortex. Finally, similar to the findings of Staal and coworkers (2001), whole-brain volume in patients with poor outcomes was found to be significantly lower than in normal comparison subjects, but did not differ significantly from that in the good-outcome schizophrenia group (Mitelman et al., 2007b).
Selective assessment of several subcortical structures revealed that regional volumes in the ventral thalamus (occupied primarily by the pulvinar complex) were decreased more significantly in the poor-outcome rather than good-outcome patient group (Brickman et al., 2004). Interestingly, volumes of the putamen, especially dorsal and in the right hemisphere, showed increases in non-Kraepelinian patients with better outcomes, whereas putamen volumes in the Kraepelinian patients did not differ from those in healthy comparison subjects (Buchsbaum et al., 2003). Expansion of the putamen is known to occur as a result of antipsychotic treatment, so that failure to expand in patients with poor outcome may be related to their well-documented resistance to treatment.
Correlational analyses for regional grey matter volumes uncovered a pattern of abnormal thalamocortical intercorrelations in patients with poor outcome, which was not seen not only in the good-outcome group, but also in healthy comparison subjects (Mitelman et al., 2005b). This abnormal correlational network comprised dorsal thalamus (occupied predominantly by the dorsomedial nucleus) and the orbital cortex in the right hemisphere and somewhat more dorsal prefrontal areas 8, 9, 10, 45 and 47 in the left hemisphere. Given that dorsal thalamic and prefrontal volumes were not differentially decreased in the poor-outcome group (thus excluding a possibility of a correlated volume loss in these regions), this finding of abnormal regional intercorrelatedness was interpreted as reflecting a pathological thalamocortical network, whose sustained and consistent recruitment is somehow associated with poor functional outcome to the illness. The network itself may not be pathological, yet pathologically mobilized in, say, a compensatory effort. Considering the role of the dorsomedial nucleus and ventrolateral prefrontal cortex in the processes of selective attention and visual information encoding, the pathologically excessive reliance on this prefrontothalamic network may represent an alternative strategy for visual information-processing.
Analogous correlational analyses for the cortical surface parcellated into the individual Brodmann’s areas (see Mitelman, Buchsbaum, Brickman, & Shihabuddin, 2005c and Mitelman, Shihabuddin, Brickman, & Buchsbaum, 2005d for detailed review) revealed specific frontotemporal dissociations (lack of normal interregional correlatedness) between grey matter volumes in Brodmann’s area 9 and both medial and lateral temporal cortex in patients with poor outcome. Negative regional intercorrelations, interpreted as excessive reliance on one region (increased volume) to compensate for malfunction in the other (decreased volume), were seen in the good-outcome, but not poor-outcome group between the primary auditory (temporal areas 41 and 42) and prefrontal (areas 10 and 46) cortices in the left hemisphere, thus potentially indicating an alternative strategy to compensate for impaired auditory information processing in those with good outcomes but not in those with poor outcomes. Abnormally tight intercorrelations were noted in the poor-outcome group between the primary visual (occipital) versus prefrontal and medial temporal cortical regions, conceivably pertinent to primary visual information processing. Therefore, a common thread to these correlational findings is a possible disturbance in networks subserving various aspects of visual and auditory information processing in patients with poor functional outcomes.
Cerebral white matter
Volumetric analysis of white matter volumes, partitioned by their association with overlying cortical Brodmann’s areas (Mitelman et al., 2007b), revealed that whereas patients with good outcomes exhibited frontoparietal white matter expansion, Kraepelinian, poor-outcome subjects failed to evidence such expansion, at least in the right hemisphere, and may even display white matter shrinkage. White matter volumes in the latter group were smaller than in patients with good outcomes in the right temporal, parietal, and occipital lobes, as well as – more localized – in the node underlying lateral prefrontal areas 45, 46, and 47. That is, similar to the pattern of posteriorized grey matter deficits in the poor-outcome group, white matter deficits likewise follow a posteriorized distribution, largely sparing the frontal lobe.
Analyses of overall differences in patterns of interareal correlations within a lobe, utilizing the Kullback test for correlation matrices (Mitelman et al., 2005c, 2005d), also provide support for right hemispheric abnormalities in the poor-outcome group, with significant intergroup differences between patients with different outcomes found for both grey and white matter intercorrelations within the right temporal lobe, and only for white matter intercorrelations within the right frontal lobe. Follow-up correlational analyses for individual Brodmann’s areas provided more details for these differences: intratemporal positive correlations of white matter volumes were significantly weaker in patients with poor outcomes, implying disordered volumetric interrelationships within the temporal lobe in this patient group. Frontotemporal white matter intercorrelations in Kraepelinian patients were stronger in the right hemisphere and weaker in the left hemisphere, than in the good-outcome group, perhaps reflecting a more severe frontotem-poral dissociation.
Diffusion tensor imaging in the same cohort of patients and healthy subjects (Mitelman et al., 2006) found that like white matter volumes, fractional anisotropy values averaged across identically parcel-lated overlying Brodmann’s areas were lower in patients with poor outcome than in the non-Kraepelinian group. In the right hemisphere, these reductions in fractional anisotropy followed the topography of co-occurring differences in white matter volumes, i.e. were seen in the temporal, parietal, and occipital lobes, and in the node underlying lateral prefrontal cortex. Interestingly, both patient groups had lower anisotropy in these regions than healthy comparison subjects. In contrast, in the left hemisphere decreases in anisotropy values were more extensive in patients with poor outcomes than in the good-outcome group, involving almost symmetrical temporal, parietal, occipital and prefrontal regions as seen in the right hemisphere. The conclusion that can be drawn from these two (volumetric and diffusion tensor imaging) studies is that patients with poor outcome display more severe and more extensive reductions in anisotropy (i.e. structural white matter disturbance) than patients with good outcomes, and that they fail to invoke compensatory expansion in co-territorial white matter volumes which in the right hemisphere is seen in the good-outcome group and may thus be instrumental in better outcome. The latter claim may also be supported by the inverse relationship between white matter volumes and anisotropy values (lower anisotropy correlated with larger volumes), seen in the good-outcome but not in the poor-outcome patient group (Mitelman et al., 2006). Further separation between the good and poor-outcome patient groups in this study was evident from the significantly different patterns of intercorrelations amongst regional fractional anisotropy values in each lobe, as analyzed with the Kullback test for correlation matrices.
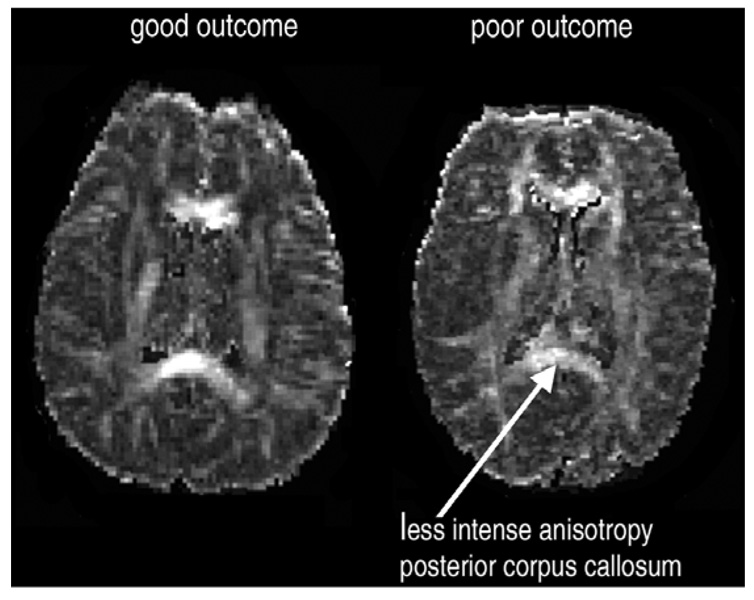
A survey of anisotropy values in an array of white matter fibre tracts (Mitelman et al., 2007a) showed that poor outcome in patients with schizophrenia was primarily associated with reduced anisotropy in the whole extent of the corpus callosum (unlike only genual decreases seen in patients with good outcome), as well as in the bilateral occipitofrontal fasciculus, left internal capsule and left optic radiation (Figure 2). Corpus callosum has previously been associated with poor outcome as reflected by its lower density in those with longer cumulative periods of hospitalization by anamnesis (Hulshoff Pol et al., 2004) and by association of its length with global prognosis (Uematsu & Kaiya, 1988) and GAF score severity (Colombo, Bonfanti, & Scarone, 1994). Anterior limbs of the internal capsules in this very cohort were also evaluated in a morphometric study (Brickman et al., 2006) and found smaller in absolute volume in poor-outcome patients in comparison to healthy subjects and the good-outcome group (the latter two groups showing no differences in volumes). Analysis of relative (to total brain) volumes recorded intergroup differences in shape, whereby anterior limbs in poor-outcome patients had smaller volumes dorsally and larger volumes ventrally, again these differences not seen between non-Kraepelinian patients and healthy subjects.
Figure 2.
Lower fractional anisotropy in the posterior callosal regions in a patient with poor outcome (right) in comparison to a patient with good outcome (left).
Finally, several groups reported abnormalities in distribution of interstitial white matter neurons, suggestive of disturbance in the second trimester neuronal migration, hence inferring neuro-developmental basis of hypothesized dysconnectivity in patients with schizophrenia. For methodological reasons, many, if not most, of these studies used post-mortem tissue collected from aged, institutionalized patients (as acknowledged in Arnold, Lee, Gur, & Trojanowski, 1991 and Rioux, Nissanov, Lauber, Bilker, & Arnold, 2003), still others found the abnormalities only in those with prominent negative symptoms (Eastwood & Harrison, 2003; Kirkpatrick, Conley, Kakoyannis, Reep, & Roberts, 1999; Kirkpatrick, Messias, Conley, & Roberts, 2003). These abnormalities may in fact be characteristic only of those with poor outcomes and at present it is not clear whether they are generalizable to schizophrenia in toto; there have indeed been negative studies as well (Beasley, Cotter, & Everall, 2002).
Summary
Kraepelinian patients represent a very poor outcome, treatment resistant subgroup of patients with schizophrenia, which follows a rapid, progressively deteriorating clinical, cognitive, and functional course late in life, resembling that of a dementing illness.
Adaptive self-care deficits (i.e. the very Kraepelinian status) in these patients are strongly associated with cognitive deficits and to a much lesser degree with the severity of negative symptoms.
Expansion of the ventricular system (lateral and third ventricles) is either more pronounced in patients with poor outcome or is specific to this patient group, may be associated with cognitive deficits and may be progressive.
Poor outcome is associated with posteriorization of grey matter deficits, uniquely involving occipital cortex.
Expansion of right hemispheric white matter volumes may be associated with better outcome, may be important in successful compensation for diffusely reduced anisotropy, and fails to occur in the Kraepelinian patient group; moreover, these patients appear to display reductions in white matter volume, as well as more widespread and more severe decreases in white matter anisotropy in both hemispheres.
Interhemispheric disconnectivity in posterior brain regions, as inferred from the observed reductions in the callosal body and splenium, may play a role in poor outcome in these patients.
Several observations appear to point to disordered processing of primary sensory information as central to poor functional outcome in this group of patients; these include grey matter volume loss in the pulvinar complex, unimodal auditory and particularly visual cortices, dyscorrelations involving thalamocortical and corticocortical networks that may be subservient to audiovisual information processing, and uniquely reduced anisotropy in the optic radiation.
Conclusions and future directions
Two fundamental, if related, questions on nature of the very poor outcome schizophrenia remain to be answered:
Is it continuous with schizophrenic illness of better outcome, i.e. occupies an extreme position along a certain outcome or some other functional dimension? If indeed so and Kraepelinian is simply an extreme course variant of the illness, what factors then mediate or incur such a dire outcome? The alternative would be a dichotomous view, whereby Kraepelinian schizophrenia would represent a distinct pathogenetic (or even etiological) patient group with distinctive longitudinal trajectory. At present, there appears to have been accumulating enough evidence to buttress the latter view: complete inability to support one’s own independent existence, peculiar clinical course leading to rapid cognitive deterioration in old age, and distinct pattern of posteriorized cerebral deficits way in advance of the cognitive decline, – all seem to sway toward a dichotomous view on the Kraepelinian/ non-Kraepelinian division.
What is the nature of late-life dementia in institutionalized Kraepelinian patients? This question is all the more baffling given that post-mortem investigations of clearly demented and prospectively identified schizophrenic patients have failed to yield any specific results (Arnold et al., 1995a; Golier et al., 1995; Martin-Ruiz et al., 2003), with a possible exception of reactive astrocytosis (Arnold et al., 1996b) and abnormal myelination (Mancevski et al., 2007b), both as yet unreplicated. Nonetheless, there are four conceivable views on this issue.
First, dementia in these patients may be epiphenomenal (superimposed and coincidental) and pathogenetically unrelated to schizophrenia proper, as concluded by Harrison (1995) in his decade-old review of a century of neuropathological studies of the illness.
More plausible (and having won more subscribers – cf., inter alia, de Vries et al., 2001; Goldberg et al., 1987; Johnstone et al., 1978; Marsden, 1976) is the view that dementia in these patients is a complication of the very illness in question, that occurs in a vulnerable subgroup of patients. This view is in fact indistinguishable from the classical concept of vesanic dementia (Gombault, 1900) as an end-product of pernicious chronic psychosis.
Some authors (Harvey et al., 2003; Mitelman et al., 2005a) with equal plausibility appear to envisage dementia in these patients as inherent in the very Kraepelinian subtype of the illness, an invariable part of its natural clinical course.
As a more radical form of the previous view, we would like to propose here a fourth possibility that may be deserving of closer scrutiny, namely that the very Kraepelinian subtype may in fact be a variant of a progressive dementing illness. Thus, the clinicopathological process in these patients may be a type of biphasically progressive dementia that begins early in life, manifests itself first with frontotemporal schizophrenia-like clinical and morphological features, which are then superseded by insidious posteriorization of cortical grey matter deficits, concurrent failure to evoke compensatory white matter changes, functional decline, and eventual rapid cognitive deterioration late in life. That this may be an as yet uncharacterized form of dementia and inaccuracies in early-life diagnosis may be in part to blame for the atypical (for schizophrenia) course of the illness in these patients has been previously suggested (Arnold & Trojanowski, 1996). Psychotic symptomatology, resembling that of schizophrenia, has indeed been described in both Pick’s and non-Pick’s variants of frontotemporal dementia with early onset (Lamote, Tan, & Verhoeven, 1998; Stone, Griffiths, Rastogi, Perry, & Cleland, 2003; Vanderveyzen et al., 2003; Waddington, Youssef, Farrell, & Toland, 1995) and with late onset (Kerssens, Pijnenburg, Schouws, Eikelenboom, & van Tilburg, 2006; Reischle, Sturm, Schuierer, & Ibach, 2003). In all of these case reports, retrospective diagnosis was made possible only because of the progression of frontotemporal atrophy and associated identifiable cognitive impairments. It could be speculated that if the frontal impairments remained static, with pathological progression in the more posterior regions of the brain, the longitudinal clinical presentation would not be dissimilar to the one described in the chronically institutionalized Kraepelinian schizophrenics.
At any measure, the latter two views (3 and 4) may be best supported by available evidence, since the late-life dementing process clearly seems to occur in an early identifiable group of patients with severe deficits in self-care, semiotics marked by prominent deficit syndrome and progressive cognitive abnormalities, rather distinct neuroimaging presentation, reactive astrocytosis, and very similar natural course. To differentiate among these plausible scenarios, future neuroimaging studies should concentrate on longitudinal course of the illness, include elderly institutionalized patients, good-outcome controls, prospective follow-up of first-outbreak cohorts, and correlate neuroimaging findings with specific neuropsychological impairments in this very poor outcome group of patients with schizophrenia.
Acknowledgements
his work was supported by NIMH grants P50 MH 66392-01, MH 60023, and MH 56489 to Dr Buchsbaum and by NARSAD Young Investigator and NIMH MH 077146 awards to Dr Mitelman.
Footnotes
Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
References
- Albus M, Hubmann W, Ehrenberg C, Forcht U, Mohr F, Sobizack N, et al. Neuropsychological impairment in first-episode and chronic schizophrenic patients. European Archives of Psychiatry Clinical Neurosciences. 1996;246:249–255. doi: 10.1007/BF02190276. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Lee VM, Gur RE, Trojanowski JQ. Abnormal expression of two microtubule-associated proteins (MAP2 and MAP5) in specific subfields of the hippocampal formation in schizophrenia. Proceedings of the National Academy of Sciences USA. 1991;88:10850–10854. doi: 10.1073/pnas.88.23.10850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SE, Gur RE, Shapiro RM, Fisher KR, Moberg PJ, Gibney MR, et al. Prospective clinicopathologic studies of schizophrenia: Accrual and assessment of patients. American Journal of Psychiatry. 1995a;152:731–737. doi: 10.1176/ajp.152.5.731. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Franz BR, Trojanowski JQ, Moberg PJ, Gur RE. Glial fibrillary protein-immunoreactive astrocytosis in elderly patients with schizophrenia and dementia. Acta Neuropathologica. 1995b;91:269–277. doi: 10.1007/s004010050425. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Trojanowski JQ. Cognitive impairment in elderly schizophrenia: A dementia (still) lacking distinctive histopathology. Schizophrenia Bulletin. 1996;22:5–9. doi: 10.1093/schbul/22.1.5. [DOI] [PubMed] [Google Scholar]
- Asano N. Pneumoencephalographic study of schizophrenia. In: Mitsuda H, editor. Clinical genetics in psychiatry: Problems in neurological classification. Tokyo: IgakuShoin; 1967. [Google Scholar]
- Beasley CL, Cotter DR, Everall IP. Density and distribution of white matter neurons in schizophrenia, bipolar disorder and major depressive disorder: No evidence for abnormalities of neuronal migration. Molecular Psychiatry. 2002;7:564–570. doi: 10.1038/sj.mp.4001038. [DOI] [PubMed] [Google Scholar]
- Bowie CR, Moriarty PJ, Harvey PD, Parrella M, White L, Davis KL. Aggression in elderly schizophrenia patients: A comparison of nursing home and state hospital residents. Journal of Neuropsychiatry and Clinical Neurosciences. 2001;13:357–366. doi: 10.1176/jnp.13.3.357. [DOI] [PubMed] [Google Scholar]
- Bowie CR, Tsapelas I, Friedman JI, Parrella M, White L, Harvey PD. The longitudinal course of thought disorder in geriatric patients with chronic schizophrenia. American Journal of Psychiatry. 2005;162:793–795. doi: 10.1176/appi.ajp.162.4.793. [DOI] [PubMed] [Google Scholar]
- Bozikas VP, Kosmidis MH, Kourtis A, Gamvrula K, Melissidis P, Tsolaki M, et al. Clock drawing test in institutionalized patients with schizophrenia compared with Alzheimer’s disease patients. Schizophrenia Research. 2002;59:173–179. doi: 10.1016/s0920-9964(01)00335-8. [DOI] [PubMed] [Google Scholar]
- Bralet MC, Loas G, Yon V, Marechal V. Clinical characteristics and risk factors for Kraepelinian subtype of schizophrenia: Replication of previous findings and relation to summer birth. Psychiatry Research. 2002;111:147–154. doi: 10.1016/s0165-1781(02)00148-8. [DOI] [PubMed] [Google Scholar]
- Bralet MC, Ton T, Falissard B. Schizophrenic patients with polydypsia and water intoxication more often have a form of schizophrenia first described by Kraepelin. Psychiatry Research. 2007 doi: 10.1016/j.psychres.2006.11.009. in press. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Buchsbaum MS, Shihabuddin L, Byne W, Newmark RE, Brand J, et al. Thalamus size and outcome in schizophrenia. Schizophrenia Research. 2004;71:473–484. doi: 10.1016/j.schres.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Buchsbaum MS, Ivanov Z, Borod JC, Foldi NS, Hahn E, et al. Internal capsule size in good and poor outcome schizophrenia. Journal of Neuropsychiatry and Clinical Neurosciences. 2006;18:364–376. doi: 10.1176/jnp.2006.18.3.364. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Haier RJ. Biological homogeneity, symptom heterogeneity and the diagnosis of schizophrenia. Schizophrenia Bulletin. 1978;4:473–475. doi: 10.1093/schbul/4.4.473. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Shihabuddin L, Hazlett EA, Schroeder J, Haznedar MM, Powchik P, et al. Kraepelinian and non-Kraepelinian schizophrenia subgroup differences in cerebral metabolic rate. Schizophrenia Research. 2002;55:25–40. doi: 10.1016/s0920-9964(01)00206-7. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Shihabuddin L, Brickman AM, Miozzo R, Prikryl R, Shaw R, et al. Caudate and putamen volumes in good and poor outcome patients with schizophrenia. Schizophrenia Research. 2003;64:53–62. doi: 10.1016/s0920-9964(02)00526-1. [DOI] [PubMed] [Google Scholar]
- Buhrich N, Crow TJ, Johnstone EC, Owens DGC. Age disorientation in chronic schizophrenia is not associated with pre-morbid intellectual impairment or past physical treatment. British Journal of Psychiatry. 1988;152:466–469. doi: 10.1192/bjp.152.4.466. [DOI] [PubMed] [Google Scholar]
- Byne W, White L, Parrella M, Adams R, Harvey PD, Davis KL. Tardive dyskinesia in a chronically institutionalized population of elderly schizophrenic patients: Prevalence and association with cognitive impairment. International Journal of Geriatric Psychiatry. 1998;13:473–479. doi: 10.1002/(sici)1099-1166(199807)13:7<473::aid-gps800>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Carpenter WT, Jr, Heinrichs DW, Wagman AMI. Deficit and nondeficit forms of schizophrenia: The concept. American Journal of Psychiatry. 1988;145:578–583. doi: 10.1176/ajp.145.5.578. [DOI] [PubMed] [Google Scholar]
- Colombo C, Bonfanti A, Scarone S. Anatomical characteristics of the corpus callosum and clinical correlates in schizophrenia. European Archives of Psychiatry and Clinical Neurosciences. 1994;243:244–248. doi: 10.1007/BF02191582. [DOI] [PubMed] [Google Scholar]
- Davidson M, Harvey PD, Powchik P, Parrella M, White L, Knobler HY, et al. Severity of symptoms in chronically institutionalized geriatric schizophrenic patients. American Journal of Psychiatry. 1995;152:197–207. doi: 10.1176/ajp.152.2.197. [DOI] [PubMed] [Google Scholar]
- Davis KL, Buchsbaum MS, Shihabuddin L, Spiegel-Cohen J, Metzger M, Frecska E, et al. Ventricular enlargement in poor-outcome schizophrenia. Biological Psychiatry. 1998;43:783–793. doi: 10.1016/s0006-3223(97)00553-2. [DOI] [PubMed] [Google Scholar]
- De Vries PJ, Honer WG, Kemp PM, McKenna PJ. Dementia as a complication of schizophrenia. Journal of Neurology Neurosurgery and Psychiatry. 2001;70:588–596. doi: 10.1136/jnnp.70.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisi LE, Stritzke P, Riordan H, Holan V, Boccio A, Kushner M, et al. The timing of brain morphological changes in schizophrenia and their relationship to clinical outcome. Biological Psychiatry. 1992;31:241–254. doi: 10.1016/0006-3223(92)90047-4. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Sakuma M, Maurizio AM, Relja M, Hoff AL. Cerebral ventricular change over the first 10 years after the onset of schizophrenia. Psychiatry Research: Neuroimaging. 2004;130:57–70. doi: 10.1016/j.pscychresns.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ. Interstitial white matter neurons express less reelin and are abnormally distributed in schizophrenia: Towards an integration of molecular and morphologic aspects of the neurodevelopmental hypothesis. Molecular Psychiatry. 2003;769:821–831. doi: 10.1038/sj.mp.4001399. [DOI] [PubMed] [Google Scholar]
- Friedman JI, Harvey PD, Coleman T, Moriarty PJ, Bowie C, Parrella M, et al. Six-year follow-up study of cognitive and functional status across the lifespan in schizophrenia: A comparison with Alzheimer’s disease and normal aging. American Journal of Psychiatry. 2001;158:1441–1448. doi: 10.1176/appi.ajp.158.9.1441. [DOI] [PubMed] [Google Scholar]
- Friedman JI, Harvey PD, McGurk SR, White L, Parrella M, Raykov T, et al. Correlates of change in functional status of institutionalized geriatric schizophrenic patients: Focus on medical comorbidity. American Journal of Psychiatry. 2002;159:1388–1394. doi: 10.1176/appi.ajp.159.8.1388. [DOI] [PubMed] [Google Scholar]
- Gabrovska-Johnson VS, Scott M, Jeffries S, Thacker N, Baldwin RC, Burns A, et al. Right-hemisphere encephalopathy in elderly subjects with schizophrenia: Evidence from neuropsychological and brain imaging studies. Psychopharmacology. 2003;169:367–375. doi: 10.1007/s00213-003-1524-9. (Berlin). [DOI] [PubMed] [Google Scholar]
- Galderisi S, Vita A, Rossi A, Stratta P, Leonardi M, Maj M, et al. Qualitative MRI findings in patients with schizophrenia: A controlled study. Psychiatry Research. 2000;98:117–126. doi: 10.1016/s0925-4927(00)00047-0. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Weinberger DR, Berman KF, Pliskin NH, Podd MH. Further evidence for dementia of the prefrontal type in schizophrenia? A controlled study of teaching the Wisconsin Card Sorting Test. Archives of General Psychiatry. 1987;44:1008–1014. doi: 10.1001/archpsyc.1987.01800230088014. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Kleinman JE, Daniel DG, Myslobodsky MS, Ragland JD, Weinberger DR. Dementia Praecox revisited. Age disorientation, mental status, and ventricular enlargement. British Journal of Psychiatry. 1988;153:187–190. doi: 10.1192/bjp.153.2.187. [DOI] [PubMed] [Google Scholar]
- Golier JA, Davidson M, Haroutunian VH, Powchik P, Purohit D, Perl D, et al. Neuropathological study of 101 elderly schizophrenics: Preliminary findings. Schizophrenia Research. 1995;15:120. [Google Scholar]
- Gombault N. La démence terminale dans les psychoses. Annales Médico-Psychologiques. 1900;58:213–249. [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? American Journal of Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: Are we measuring the ‘right stuff’? Schizophrenia Bulletin. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Harrison P. On the neuropathology of schizophrenia and its dementia: Neurodevelopmental, neurodegenerative or both? Neurodegeneration. 1995;4:1–12. doi: 10.1006/neur.1995.0001. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Putnam KM, Davidson M, Kahn RS, Powchik P, McQueeney R, et al. Brief neuroleptic discontinuation and clinical symptoms in Kraepelinian and non-Kraepelinian chronic schizophrenic patients. Psychiatry Research. 1991;38:285–292. doi: 10.1016/0165-1781(91)90018-k. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Lombardi J, Kincaid MM, Parrella M, White L, Powchik P, et al. Cognitive functioning in chronically hospitalized schizophrenic patients: Age-related changes and age disorientation as a predictor of impairment. Schizophrenia Research. 1995;17:15–24. doi: 10.1016/0920-9964(95)00026-i. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Leff J, Trieman N, Anderson J, Davidson M. Cognitive impairment and adaptive deficit in geriatric chronic schizophrenic patients: A cross-national study in New York and London. International Journal of Geriatric Psychiatry. 1997a;12:1001–1007. doi: 10.1002/(sici)1099-1166(199710)12:10<1001::aid-gps674>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Lombardi J, Leibman M, Parrella M, White L, Powchik P, et al. Age-related differences in formal thought disorder in chronically hospitalized schizophrenic patients: A cross-sectional study across nine decades. American Journal of Psychiatry. 1997b;154:205–210. doi: 10.1176/ajp.154.2.205. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Sukhodolsky D, Parrella M, White L, Davidson M. The association between adaptive and cognitive deficits in geriatric chronic schizophrenic patients. Schizophrenia Research. 1997c;27:211–218. doi: 10.1016/S0920-9964(97)00068-6. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Howanitz E, Parrella M, White L, Davidson M, Mohs RC, et al. Symptoms, cognitive functioning, and adaptive skills in geriatric patients with lifelong schizophrenia: A comparison across treatment sites. American Journal of Psychiatry. 1998;155:1080–1086. doi: 10.1176/ajp.155.8.1080. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Silverman JM, Mohs RC, Parrella M, White L, Powchik P, et al. Cognitive decline in late-life schizophrenia: A longitudinal study of geriatric chronically hospitalized patients. Biological Psychiatry. 1999a;45:32–40. doi: 10.1016/s0006-3223(98)00273-x. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Parrella M, White L, Mohs RC, Davidson M, Davis KL. Convergence of cognitive and adaptive decline in late-life schizophrenia. Schizophrenia Research. 1999b;35:77–84. doi: 10.1016/s0920-9964(98)00109-1. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Moriarty PJ, Friedman JI, White L, Parrella M, Mohs RC, et al. Differential preservation of cognitive functions in geriatric patients with lifelong chronic schizophrenia: Less impairment in reading compared with other skill areas. Biological Psychiatry. 2000a;47:962–968. doi: 10.1016/s0006-3223(00)00245-6. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Jacobsen H, Mancini D, Parrella M, White L, Haroutunian V, et al. Clinical, cognitive and functional characteristics of long-stay patients with schizophrenia: A comparison of VA and state hospital patients. Schizophrenia Research. 2000b;43:3–9. doi: 10.1016/s0920-9964(99)00182-6. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Keefe RSE. Studies of cognitive change in patients with schizophrenia following novel anti-psychotic treatment. American Journal of Psychiatry. 2001;158:176–184. doi: 10.1176/appi.ajp.158.2.176. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Bertisch H, Friedman JI, Marcus S, Parrella M, White L, et al. The course of functional decline in geriatric patients with schizophrenia: Cognitive-functional and clinical symptoms as determinants of change. American Journal of Geriatric Psychiatry. 2003;11:610–619. doi: 10.1176/appi.ajgp.11.6.610. [DOI] [PubMed] [Google Scholar]
- Haug JO. Pneumoencephalographic evidence of brain atrophy in acute and chronic schizophrenic patients. Acta Psychiatrica Scandinavica. 1982;66:374–383. doi: 10.1111/j.1600-0447.1982.tb06719.x. [DOI] [PubMed] [Google Scholar]
- Heinik J, Lahav D, Drummer D, Vainer-Benaiah Z, Lin R. Comparison of a clock drawing test in elderly schizophrenia and Alzheimer’s disease patients: A preliminary study. International Journal of Geriatric Psychiatry. 2000;15:638–643. doi: 10.1002/1099-1166(200007)15:7<638::aid-gps166>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Herbener ES, Harrow M. Are negative symptoms associated with functioning deficits in both schizophrenia and nonschizophrenia patients? A 10-year longitudinal analysis. Schizophrenia Bulletin. 2004;30:813–825. doi: 10.1093/oxfordjournals.schbul.a007134. [DOI] [PubMed] [Google Scholar]
- Ho BC, Nopoulos P, Flaum M, Arndt S, Andreasen NC. Two-year outcome in first-episode schizophrenia: Predictive value of symptoms for quality of life. American Journal of Psychiatry. 1998;155:1196–1201. doi: 10.1176/ajp.155.9.1196. [DOI] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Nopoulos P, Magnotta V, Arndt S, Flaum M. Progressive structural brain abnormalities and their significance on outcome: A longitudinal MRI study early in the course of schizophrenia. Archives of General Psychiatry. 2003;60:585–594. doi: 10.1001/archpsyc.60.6.585. [DOI] [PubMed] [Google Scholar]
- Hofer A, Baumgartner S, Edlinger M, Hummer M, Kemmler G, Rettenbacher MA, et al. Patient outcomes in schizophrenia I: Correlates with sociodemographic variables, psychopathology, and side effects. European Psychiatry. 2005a;20:386–394. doi: 10.1016/j.eurpsy.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Hofer A, Baumgartner S, Bodner T, Edlinger M, Kemmler G, Rettenbacher MA, et al. Patient outcomes in schizophrenia II: The impact of cognition. European Psychiatry. 2005b;20:395–402. doi: 10.1016/j.eurpsy.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Huber G. Pneumencephalographische und psychopathologische Bilder bei endogenen Psychosen. Berlin: Springer; 1957. [Google Scholar]
- Hulshoff Pol HE, Schnack HG, Mandl RCW, Cahn W, Collins DL, Evans AC, et al. Focal white matter density changes in schizophrenia: Reduced inter-hemispheric connectivity. NeuroImage. 2004;21:27–35. doi: 10.1016/j.neuroimage.2003.09.026. [DOI] [PubMed] [Google Scholar]
- Jablensky A. Subtyping schizophrenia: Implications for genetic research. Molecular Psychiatry. 2006;11:815–836. doi: 10.1038/sj.mp.4001857. [DOI] [PubMed] [Google Scholar]
- Johnstone EC, Crow TJ, Frith CD, Husband J, Kreel L. Cerebral ventricular size and cognitive impairment in chronic schizophrenia. Lancet. 1976;2:924–926. doi: 10.1016/s0140-6736(76)90890-4. [DOI] [PubMed] [Google Scholar]
- Johnstone EC, Crow TJ, Frith CD, Stevens M, Kreel L, Husband J. The dementia of dementia praecox. Acta Psychiatrica Scandinavica. 1978;57:305–327. doi: 10.1111/j.1600-0447.1978.tb06899.x. [DOI] [PubMed] [Google Scholar]
- Johnstone EC, Owens DGC, Gold A, Crow TJ, MacMillan JF. Institutionalization and the defects of schizophrenia. British Journal of Psychiatry. 1981;139:195–203. doi: 10.1192/bjp.139.3.195. [DOI] [PubMed] [Google Scholar]
- Johnstone EC, Owens DG, Bydder GM, Colter N, Crow TJ, Frith CD. The spectrum of structural brain changes in schizophrenia: Age of onset as a predictor of cognitive and clinical impairments and their cerebral correlates. Psychological Medicine. 1989;19:91–103. doi: 10.1017/s0033291700011053. [DOI] [PubMed] [Google Scholar]
- Joober R, Rouleau GA, Lal S, Bloom D, Lalonde P, Labelle A, et al. Increased prevalence of schizophrenia spectrum disorders in relatives of neuroleptic-nonresponsive schizophrenic patients. Schizophrenia Research. 2005;77:35–41. doi: 10.1016/j.schres.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Katsanis J, Iacono WG, Beiser M. Relationship of lateral ventricular size to psychophysiological measures and short-term outcome. Psychiatry Research. 1991;37:115–129. doi: 10.1016/0165-1781(91)90069-2. [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Mohs RC, Losonczy MF, Davidson M, Silverman JM, Kendler KS, et al. Characteristics of very poor outcome schizophrenia. American Journal of Psychiatry. 1987;144:889–895. doi: 10.1176/ajp.144.7.889. [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Mohs RC, Davidson M, Losonczy MF, Silverman JM, Lesser JC, et al. Kraepelinian schizophrenia: A subgroup of schizophrenia? Psychopharmacological Bulletin. 1988;24:56–61. [PubMed] [Google Scholar]
- Keefe RSE, Mohs RC, Losonczy MF, Davidson M, Silverman JM, Horvath TB, et al. Premorbid sociosexual functioning and long-term outcome in schizophrenia. American Journal of Psychiatry. 1989;146:206–211. doi: 10.1176/ajp.146.2.206. [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Mohs RC, Silverman JM, Losonczy MF, Davidson M, Horvath TB, et al. Characteristics of Kraepelinian schizophrenia and their relation to premorbid sociosexual functioning. In: Angrist B, Schulz SC, editors. The neuroleptic nonresponsive patients: Characterization and treatment. Wahington DC: American Psychiatric Press; 1990. [Google Scholar]
- Keefe RSE, Lobel DS, Mohs RC, Silverman JM, Harvey PD, Davidson M, et al. Diagnostic issues in chronic schizophrenia: Kraepelinian schizophrenia, undifferentiated schizophrenia, and state-independent negative symptoms. Schizophrenia Research. 1991;4:71–79. doi: 10.1016/0920-9964(91)90026-n. [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Frescka E, Apter SH, Davidson M, Macaluso JM, Hirschowitz J, et al. Clinical characteristics of Kraepelinian schizophrenia: Replication and extension of previous findings. American Journal of Psychiatry. 1996;153:806–811. doi: 10.1176/ajp.153.6.806. [DOI] [PubMed] [Google Scholar]
- Keilp JG, Sweeney JA, Jacobsen P, Solomon C, St Louis L, Deck M, et al. Cognitive impairment in schizophrenia: Specific relations to ventricular size and negative symptomatology. Biological Psychiatry. 1988;24:47–55. doi: 10.1016/0006-3223(88)90120-5. [DOI] [PubMed] [Google Scholar]
- Keks NA, McKenzie DP, Low LH, McGorry PD, Hill C, Kulkarni J, Singh BS, Copolov DL. Multidiagnostic Biological evaluation of prolactin response to haloperidol challenge in schizophrenia: Maximal blunting in Kraepelinian patients. Bio Psychiatry. 1992;32:426–437. doi: 10.1016/0006-3223(92)90130-r. [DOI] [PubMed] [Google Scholar]
- Kerssens CJ, Pijnenburg YAL, Schouws S, Eikelenboom P, van Tilburg W. Het ontstaan psychotische verschijnselen op latere leeftijd. Laat-ontstane schizofrenie of frontotemporale dementie? [The development of psychotic symptoms in later life: Late-onset schizophrenia or frontotemporal dementia? A case study.] Tijdschrift Voor Psychiatrie. 2006;48:739–744. [PubMed] [Google Scholar]
- Kilzieh N, Wood AE, Erdmann J, Raskind M, Tapp A. Depression in Kraepelinian schizophrenia. Comprehensive Psychiatry. 2003;44:1–6. doi: 10.1053/comp.2003.50002. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Conley RC, Kakoyannis A, Reep RL, Roberts RC. Interstitial cells of the white matter in the inferior parietal cortex in schizophrenia: An unbiased cell-counting study. Synapse. 1999;34:95–102. doi: 10.1002/(SICI)1098-2396(199911)34:2<95::AID-SYN2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Messias NC, Conley RR, Roberts RC. Interstitial cells of the white matter in the dorsolateral prefrontal cortex in deficit and nondeficit schizophrenia. The Journal of Nervous and Mental Disease. 2003;191:563–567. doi: 10.1097/01.nmd.0000087181.61164.e1. [DOI] [PubMed] [Google Scholar]
- Kitabayashi Y, Otakara C, Hirosawa R, Narumoto J, Fukui K. Frontotemporal dementia complicated with schizophrenia. Psychiatry and Clinical Neurosciences. 2005;59:749–750. doi: 10.1111/j.1440-1819.2005.01449.x. [DOI] [PubMed] [Google Scholar]
- Kolakowska T, Williams AO, Ardern M, Revely MA, Jambor K, Gelder MG, et al. Schizophrenia with good and poor outcome. I: Early clinical features, response to neuroleptics and signs of organic dysfunction. British Journal of Psychiatry. 1985a;146:229–239. doi: 10.1192/bjp.146.3.229. [DOI] [PubMed] [Google Scholar]
- Kolakowska T, Williams AO, Jambor K, Ardern M. Schizophrenia with good and poor outcome. III: Neurological ‘soft’ signs, cognitive impairment and their clinical significance. British Journal of Psychiatry. 1985b;146:348–357. doi: 10.1192/bjp.146.4.348. [DOI] [PubMed] [Google Scholar]
- Kosmidis MH, Bozikas VP, Vlahou CH, Kiosseoglou G, Giaglis G, Karavatos A. Verbal fluency in institutionalized patients with schizophrenia: Age-related performance decline. Psychiatry Research. 2005;134:233–240. doi: 10.1016/j.psychres.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Krasik ED, Logvinovich GV. Клиническаяструктура госпитализма у больньӀх шизофрениенией (реабилитационньӀе аспектьӀ) [Clinical structure of institutionalism in schizophrenic patients (rehabilitation aspect)] Zhurnal Nevropatologii i Psikhiatrii Imeni S. S. Korsakova. 1977;77:1711–1715. [PubMed] [Google Scholar]
- Lamote H, Tan KL, Verhoeven WMA. Frontotemporale dementie bij een jonge vrouw met ogenschijnlijk schizofrenie. [Frontotemporal dementia in a young woman with apparent schizophrenia] Nederlands Tijdschrift Voor Geneeskunde. 1998;142:1962–1965. [PubMed] [Google Scholar]
- Lawson WB, Waldman IN, Weinberger DR. Schizophrenic dementia. Clinical and computed axial tomography correlates. The Journal of Nervous and Mental Disease. 1988;176:207–212. [PubMed] [Google Scholar]
- Liddle PF, Crow TJ. Age disorientation in chronic schizophrenia is associated with global intellectual impairment. British Journal of Psychiatry. 1984;144:193–199. doi: 10.1192/bjp.144.2.193. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Alvir JM, Koreen A, Geisler S, Chakos MH, Sheitman B, et al. Psychobiologic correlates of treatment response in schizophrenia. Neuropsychopharmacology. 1996;14 Suppl:13S–21S. doi: 10.1016/0893-133X(95)00200-W. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Chakos MH, Wu H, Alvir JM, Hoffman E, Robinson D, et al. Longitudinal study of brain morphology in first episode schizophrenia. Biological Psychiatry. 2001;49:487–499. doi: 10.1016/s0006-3223(01)01067-8. [DOI] [PubMed] [Google Scholar]
- Losonczy MF, Song IS, Mohs RC, Small NA, Davidson M, Johns CA, et al. Correlates of lateral ventricular size in chronic schizophrenia, I: Behavioral and treatment response measures. American Journal of Psychiatry. 1986;143:976–981. doi: 10.1176/ajp.143.8.976. [DOI] [PubMed] [Google Scholar]
- Lowery N, Giovanni L, Mozley LH, Arnold SE, Bilker WB, Gur RE, et al. Relationship between clock-drawing and neuropsychological and functional status in elderly institutionalized patients with schizophrenia. American Journal of Geriatric Psychiatry. 2003;11:621–628. doi: 10.1176/appi.ajgp.11.6.621. [DOI] [PubMed] [Google Scholar]
- Mancevski B, Keilp J, Kurzon M, Berman RM, Ortakov V, Harkavy-Friedman J, et al. Lifelong course of positive and negative symptoms in chronically institutionalized patients with schizophrenia. Psychopathology. 2007a;40:83–92. doi: 10.1159/000098488. [DOI] [PubMed] [Google Scholar]
- Mancevski B, Ilievski B, Trencevska I, Ortakov V, Serafimova T, Rosoklija G, et al. Frontal myelin histology and cognitive function in schizophrenia. Schizophrenia Bulletin. 2007b;33:270–271. [Google Scholar]
- Manschreck TC, Maher BA, Winzig L, Candela SF, Beaudette S, Boshes R. Age disorientation in schizophrenia. Journal of Neuropsychiatry and Clinical Neurosciences. 2000;12:350–358. doi: 10.1176/jnp.12.3.350. [DOI] [PubMed] [Google Scholar]
- Marsden CD. Cerebral atrophy and cognitive impairment in chronic schizophrenia. Lancet. 1976;2:1079. doi: 10.1016/s0140-6736(76)90984-3. [DOI] [PubMed] [Google Scholar]
- Marsh L, Harris D, Lim KO, Beal M, Hoff AL, Minn K, et al. Structural magnetic resonance imaging abnormalities in men with severe chronic schizophrenia and an early age at clinical onset. Archives of General Psychiatry. 1997;54:1104–1112. doi: 10.1001/archpsyc.1997.01830240060009. [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz CM, Haroutunian VH, Long P, Young AH, Davis KL, Perry EK, et al. Dementia rating and nicotinic receptor expression in the prefrontal cortex in schizophrenia. Biological Psychiatry. 2003;54:1222–1233. doi: 10.1016/s0006-3223(03)00348-2. [DOI] [PubMed] [Google Scholar]
- McBride T, Moberg PJ, Arnold SE, Mozley LH, Mahr RN, Gibney M, et al. Neuropsychological functioning in elderly patients with schizophrenia and Alzheimer’s disease. Schizophrenia Research. 2002;55:217–227. doi: 10.1016/s0920-9964(01)00232-8. [DOI] [PubMed] [Google Scholar]
- McGurk SR, Moriarty PJ, Harvey PD, Parrella M, White L, Friedman JI, et al. Relationship of cognitive functioning, adaptive life skills, and negative symptom severity in poor-outcome geriatric schizophrenia patients. Journal of Neuropsychiatry and Clinical Neurosciences. 2000;12:257–264. doi: 10.1176/jnp.12.2.257. [DOI] [PubMed] [Google Scholar]
- Milev P, Ho BC, Arndt S, Nopoulos P, Andreasen NC. Initial magnetic resonance imaging volumetric brain measurements and outcome in schizophrenia: A prospective longitudinal study with 5-year follow-up. Biol Psychiatry. 2003;54:608–615. doi: 10.1016/s0006-3223(03)00293-2. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Shihabuddin L, Brickman AM, Hazlett EA, Buchsbaum MS. MRI assessment of gray and white matter distribution in Brodmann’s areas of the cortex in patients with schizophrenia with good and poor outcomes. American Journal of Psychiatry. 2003;160:2154–2168. doi: 10.1176/appi.ajp.160.12.2154. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Shihabuddin L, Brickman AM, Hazlett EA, Buchsbaum MS. Volume of the cingulate and outcome in schizophrenia. Schizophrenia Research. 2005a;72:91–108. doi: 10.1016/j.schres.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Brickman AM, Shihabuddin L, Newmark RE, Chu KW, Buchsbaum MS. Correlations between MRI-assessed volumes of the thalamus and cortical Brodmann’s areas in schizophrenia. Schizophrenia Research. 2005b;75:265–281. doi: 10.1016/j.schres.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Buchsbaum MS, Brickman AM, Shihabuddin L. Cortical intercorrelations of frontal area volumes in schizophrenia. NeuroImage. 2005c;27:753–770. doi: 10.1016/j.neuroimage.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Shihabuddin L, Brickman AM, Buchsbaum MS. Cortical intercorrelations of temporal area volumes in schizophrenia. Schizophrenia Research. 2005d;76:207–229. doi: 10.1016/j.schres.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Newmark RE, Torosjan Y, Chu KW, Brickman AM, Haznedar MM, et al. White matter fractional anisotropy and outcome in schizophrenia. Schizophrenia Research. 2006;87:138–159. doi: 10.1016/j.schres.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Torosjan Y, Newmark RE, Schneiderman JS, Chu KW, Brickman AM, et al. Internal capsule, corpus callosum and long associative fibers in good and poor outcome schizophrenia: A diffusion tensor imaging survey. Schizophrenia Research. 2007a;92:211–224. doi: 10.1016/j.schres.2006.12.029. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Brickman AM, Shihabuddin L, Newmark RE, Hazlett EA, Haznedar MM, et al. A comprehensive assessment of gray and white matter volumes and their relationship to outcome and severity in schizophrenia. NeuroImage. 2007b doi: 10.1016/j.neuroimage.2007.04.070. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty PJ, Lieber D, Bennett A, White L, Parrella M, Harvey PD, et al. Gender differences in poor outcome patients with lifelong schizophrenia. Schizophrenia Bulletin. 2001;27:103–113. doi: 10.1093/oxfordjournals.schbul.a006850. [DOI] [PubMed] [Google Scholar]
- Moore MT, Nathan D, Elliott AR, Laubach C. Encephalographic studies in schizophrenia (Dementia Praecox): Report of sixty cases. American Journal of Psychiatry. 1933;89:801–810. [Google Scholar]
- Nagy K. Pneumoencephalographische Befunde bei Endogenen Psychosen. Nervenarzt. 1963;34:543–548. [PubMed] [Google Scholar]
- Nakaya M, Ohmori K. Kraepelinian subtype and deficit syndrome in chronic schizophrenia. Psychiatry Research. 2006;144:221–225. doi: 10.1016/j.psychres.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Owens DGC, Johnstone EC. The disabilities of chronic schizophrenia–their nature and the factors contributing to their development. British Journal of Psychiatry. 1980;136:384–395. doi: 10.1192/bjp.136.4.384. [DOI] [PubMed] [Google Scholar]
- Owens DGC, Johnstone EC, Crow TJ, Frith CD, Jagoe JR, Kreel L. Lateral ventricular size in schizophrenia: Relationship to the disease process and its clinical manifestations. Psychological Medicine. 1985;15:27–41. doi: 10.1017/s0033291700020900. [DOI] [PubMed] [Google Scholar]
- Pearlson GD, Garbacz DJ, Breakey WR, Ahn HS, DePaulo JR. Lateral ventricular enlargement associated with persistent unemployment and negative symptoms in both schizophrenia and bipolar disorder. Psychiatry Research. 1984;12:1–9. doi: 10.1016/0165-1781(84)90133-1. [DOI] [PubMed] [Google Scholar]
- Perlick D, Mattis S, Stastny P, Teresi J. Neuropsychological discriminators of long-term inpatient or outpatient status in chronic schizophrenia. Journal of Neuropsychiatry and Clinical Neurosciences. 1992;4:428–434. doi: 10.1176/jnp.4.4.428. [DOI] [PubMed] [Google Scholar]
- Putnam KM, Harvey PD, Parrella M, White L, Kincaid M, Powchik P, et al. Symptom stability in geriatric chronic schizophrenic inpatients: A one-year follow-up study. Biological Psychiatry. 1996;39:92–99. doi: 10.1016/0006-3223(95)00105-0. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Rieckmann N, Harvey PD. Stability in schizophrenia symptoms over time: Findings from the Mount Sinai Pilgrim Psychiatric Center longitudinal study. Journal of Abnormal Psychology. 2005;114:363–372. doi: 10.1037/0021-843X.114.3.363. [DOI] [PubMed] [Google Scholar]
- Reischle E, Sturm K, Schuierer G, Ibach B. Ein Fall von schizophrenieformer Störung bei frontotemporaler Demenz (FTD). [A case of schizophreniform disorder in frontotemporal dementia (FTD).] Psychiatrische Praxis. 2003;30:S78–S82. [PubMed] [Google Scholar]
- Rieckmann N, Reichenberg A, Bowie CR, Parrella M, White L, Friedman JI, et al. Depressed mood and its functional correlates in institutionalized schizophrenia patients. Schizophrenia Research. 2005;77:179–187. doi: 10.1016/j.schres.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Rioux L, Nissanov J, Lauber K, Bilker WB, Arnold SE. Distribution of microtubule-associated protein MAP2-immunoreactive interstitial neurons in the parahippo-campal white matter in subjects with schizophrenia. American Journal of Psychiatry. 2003;160:149–155. doi: 10.1176/appi.ajp.160.1.149. [DOI] [PubMed] [Google Scholar]
- Roccatagliatta G, Gandolfo C, Ruffinengo U, Scotto P, Bacigalupo F. [Enlargement of the cerebral ventricles and cognitive efficiency in chronic schizophrenia.] Rivista di Neurologia. 1986;56:158–167. [PubMed] [Google Scholar]
- Rossi A, Bustini M, Prosperini P, Marinangeli MG, Splendiani A, Daneluzzo E, et al. Neuromorphological abnormalities in schizophrenic patients with good and poor outcome. Acta Psychiatrica Scandinavica. 2000;101:161–166. doi: 10.1034/j.1600-0447.2000.900666.x. [DOI] [PubMed] [Google Scholar]
- Roy M, Lehoux C, Brassard A, Rene L, Trepanier J, Merette C, et al. Kraepelninian and non-Kraepelinian schizophrenia: Replication and extension of previous findings [abstract] Schizophrenia Research. 2001;49 Suppl:21. [Google Scholar]
- Roy MA, Merette C, Maziade M. Subtyping schizophrenia according to outcome or severity: A search for homogeneous subgroups. Schizophrenia Bulletin. 2001;27:115–138. doi: 10.1093/oxfordjournals.schbul.a006851. [DOI] [PubMed] [Google Scholar]
- Roy MA, Lehoux C, Emond C, Laplante L, Bouchard RH, Everett J, et al. A pilot neuropsychological study of Kraepelinian and non-Kraepelinian schizophrenia. Schizophrenia Research. 2003;62:155–163. doi: 10.1016/s0920-9964(02)00481-4. [DOI] [PubMed] [Google Scholar]
- Salokangas RKR. Symptom dimensions and outcome in schizophrenia. World Psychiatry. 2003;2:172–178. [PMC free article] [PubMed] [Google Scholar]
- Smith RC, Hussain MI, Chowdhury SA, Stearns A. Stability of neurological soft signs in chronically hospitalized schizophrenic patients. Journal of Neuropsychiatry and Clinical Neurosciences. 1999;11:91–96. doi: 10.1176/jnp.11.1.91. [DOI] [PubMed] [Google Scholar]
- Staal WG, Hulshoff Pol HE, Schnack HG, van Haren NEM, Seifert N, Kahn RS. Structural brain abnormalities in chronic schizophrenia at the extremes of the outcome spectrum. American Journal of Psychiatry. 2001;158:1140–1142. doi: 10.1176/appi.ajp.158.7.1140. [DOI] [PubMed] [Google Scholar]
- Stephens JH. Long-term prognosis and followup in schizophrenia. Schizophrenia Bulletin. 1978;4:25–47. doi: 10.1093/schbul/4.1.25. [DOI] [PubMed] [Google Scholar]
- Stevens M, Crow TJ, Bowman MJ, Coles EC. Age disorientation in schizophrenia: A constant prevalence of 25 per cent in a chronic mental hospital population? British Journal of Psychiatry. 1978;133:130–136. doi: 10.1192/bjp.133.2.130. [DOI] [PubMed] [Google Scholar]
- Stip E. Cognition, schizophrénie et effet des antipsychotiques [Cognition, schizophrenia and the effect of antipsychotics.] Encephale. 2006;32:341–350. doi: 10.1016/s0013-7006(06)76162-0. [DOI] [PubMed] [Google Scholar]
- Stone J, Griffiths TD, Rastogi S, Perry RH, Cleland PG. Non-Pick’s frontotemporal dementia imitating schizophrenia in a 22-year-old man. Journal of Neurology. 2003;250:360–370. doi: 10.1007/s00415-003-0989-0. [DOI] [PubMed] [Google Scholar]
- Tapp A, Tandon R, Scholten R, Dudley E. Age disorientation in Kraepelinian schizophrenia: Frequency and clinical correlates. Psychopathology. 1993;26:225–228. doi: 10.1159/000284826. [DOI] [PubMed] [Google Scholar]
- Uematsu M, Kaiya H. The morphology of the corpus callosum in schizophrenia. An MRI study. Schizophrenia Research. 1988;1:391–398. doi: 10.1016/0920-9964(88)90020-5. [DOI] [PubMed] [Google Scholar]
- Vanderzeypen F, Bier JC, Genevrois C, Mendlewicz J, Lotstra F. Démence frontale ou ‘démence précoce’? A propos de l’observation d’un trouble psychotique associé à une détérioration severe. [Frontal dementia or dementia praecox? A case report of a psychotic disorder with a severe decline.] Encephale. 2003;29 272–180. [PubMed] [Google Scholar]
- Van Haren NEM, Cahn W, Hulshoff Pol HE, Schnack HG, Caspers E, Lemstra A, et al. Brain volumes as predictor of outcome in recent-onset schizophrenia: A multi-center MRI study. Schizophrenia Research. 2003;64:41–52. doi: 10.1016/s0920-9964(03)00018-5. [DOI] [PubMed] [Google Scholar]
- Velligan DI, Mahurin RK, True JE, Lefton RS, Flores CV. Preliminary evaluation of cognitive adaptation training to compensate for cognitive deficits in schizophrenia. Psychiatric Services. 1996;47:415–417. doi: 10.1176/ps.47.4.415. [DOI] [PubMed] [Google Scholar]
- Velligan DI, Mahurin RK, Diamond PL, Hazleton BC, Eckert SL, Miller AL. The functional significance of symptomatology and cognitive function in schizophrenia. Schizophrenia Research. 1997;25:21–31. doi: 10.1016/S0920-9964(97)00010-8. [DOI] [PubMed] [Google Scholar]
- Velligan DI, Miller AL. Cognitive dysfunction in schizophrenia and its importance to outcome: The place of atypical antipsychotics in treatment. Journal of Clinical Psychiatry. 1999;60(S23):25–28. [PubMed] [Google Scholar]
- Velligan DI, Bow-Thomas CC, Mahurin RK, Miller AL, Halgunseth LC. Do specific neurocognitive deficits predict specific domains of community function in schizophrenia? The Journal of Nervous and Mental Disease. 2000;1888:518–524. doi: 10.1097/00005053-200008000-00007. [DOI] [PubMed] [Google Scholar]
- Velligan DI, Newcomer J, Pultz J, Csernansky J, Hoff AL, Mahurin RK, et al. Does cognitive function improve with quetiapine in comparison to haloperidol? Schizophrenia Research. 2002;53:239–248. doi: 10.1016/s0920-9964(01)00268-7. [DOI] [PubMed] [Google Scholar]
- Velligan DI, Prihoda TJ, Sui D, Ritch JL, Maples N, Miller AL. The effectiveness of quetiapine versus conventional antipsychotics in improving cognitive and functional outcomes in standard treatment settings. Journal of Clinical Psychiatry. 2003;64:524–531. doi: 10.4088/jcp.v64n0505. [DOI] [PubMed] [Google Scholar]
- Waddington JL, Youssef HA, Farrell MA, Toland J. Initial ‘schizophrenia-like’ psychosis in Pick’s disease: Case study with neuroimaging and neuropathology, and implications for frontotemporal dysfunction in schizophrenia. Schizophrenia Research. 1995;18:79–82. doi: 10.1016/0920-9964(95)00064-x. [DOI] [PubMed] [Google Scholar]
- Waddington JL, Youssef HA. Cognitive dysfunction in chronic schizophrenia followed prospectively over 10 years and its longitudinal relationship to the emergence of tardive dyskinesia. Psychological Medicine. 1996;26:681–688. doi: 10.1017/s0033291700037697. [DOI] [PubMed] [Google Scholar]
- Wassink TH, Andreasen NC, Nopoulos P, Flaum M. Cerebellar morphology as predictor of symptom and psychosocial outcome in schizophrenia. Biological Psychiatry. 1999;45:41–48. doi: 10.1016/s0006-3223(98)00175-9. [DOI] [PubMed] [Google Scholar]
- White L, Parrella M, McCrystal-Simon J, Harvey PD, Masiar SJ, Davidson M. Characteristics of elderly psychiatric patients retained in a state hospital during downsizing: A prospective study with replication. International Journal of Geriatric Psychiatry. 1997;12:474–480. doi: 10.1002/(sici)1099-1166(199704)12:4<474::aid-gps530>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- White L, Opler LA, Harvey PD, Parrella M, Friedman JI. Activation symptoms and discharge in elderly chronic schizophrenic inpatients. The Journal of Nervous and Mental Disease. 2004;192:880–883. doi: 10.1097/01.nmd.0000146883.74872.07. [DOI] [PubMed] [Google Scholar]
- White L, Friedman JI, Bowie CR, Evers M, Harvey PD, Parrella M, et al. Long-term outcomes in chronically hospitalized geriatric patients with schizophrenia: Retrospective comparison of first generation and second generation anti-psychotics. Schizophrenia Research. 2006;88:127–134. doi: 10.1016/j.schres.2006.06.038. [DOI] [PubMed] [Google Scholar]
- Whitworth AB, Kemmler G, Honeder M, Kremser C, Felber S, Hausmann A, et al. Longitudinal volumetric MRI study in first-and multiple-episode male schizophrenia patients. Psychiatry Research. 2005;140:225–237. doi: 10.1016/j.pscychresns.2005.07.006. [DOI] [PubMed] [Google Scholar]