Abstract
Natural product extracts have proven to be a rich source of small molecules that potently inhibit the catalytic activity of certain PPP-family ser/thr protein phosphatases. To date, the list of inhibitors includes, okadaic acid (produced by marine dionoflagelates, Prorocentrum sp. and Dinophysis sp.), calyculin A, dragmacidins (isolated from marine sponges), microcystins, nodularins (cyanobacteria, Microcystis sp. and Nodularia sp.), tautomycin, tautomycetin, cytostatins, phospholine, leustroducsins, phoslactomycins, fostriecin (soil bacteria, Streptomyces sp.) and cantharidin (blister beetles, ~1500 species). Many of these compounds share structural similarities, and several have become readily available for research purposes. Here we will review the specificity of available inhibitors, and present methods for their use in studying sensitive phosphatases. Common mistakes in the employment of these compounds will also be addressed briefly, notably the widespread misconception that they only inhibit the activity of PP1 and PP2A. Inhibitors of PP2B (calcineurin) will only be mentioned in passing, except to state that in our hands cypermethrin, deltamethrin, and fenvalerate, which are sold as potent inhibitors of PP2B, do not inhibit the catalytic activity of PP2B.
Keywords: okadaic acid, fostriecin, calyculin A, microcystin, cantharidin, protein phosphatase inhibitors, natural products
1. Introduction
In a landmark study Bialojan and Takai revealed that okadaic acid, a complex polyether identified as the causative agent of diarrhetic shellfish poisoning, acts as a potent inhibitor of PP1 and PP2A (1). This opened the door for the use of small molecule inhibitors to study the roles of sensitive protein phosphatases. Indeed to date, although a more selective inhibitor has been identified (fostriecin) okadaic acid is still the most widely used inhibitor in studies designed to provide insight into the biological actions of PP1 and PP2A. However, okadaic acid also potently inhibits the activity of PP4, PP5 and likely PP6 (See Note 1), a fact that is often missed or ignored by investigators using this compound. In actuality, it appears that most, if not all, of the above-mentioned inhibitors act against PP1, PP2A, PP4, PP5 and PP6 (2). It should also be mentioned that in humans there are four isoforms of the catalytic subunit of PP1 (α, β/δ, γ1, and γ2) and two isoforms of PP2Ac (α and β). Therefore, to construct valid arguments for a role of a particular phosphatase based on studies using these inhibitors, it is important to understand their specificity and the limitations of their use.
For the sake of brevity the focus of this chapter will be on inhibitors that are commercially available (i.e. okadaic acid, calyculin A, microcystin-LR, tautomycin, fostriecin and cantharidin). Based on published studies conducted using purified catalytic subunits of the indicated phosphatases and phosphoproteins as substrates, the relative sensitivity of PP1-PP7 to commonly used inhibitors is summarized in Table 1.
Table 1*.
Natural compounds that inhibit PPP-family ser/thr protein phosphatases
| Inhibition of Ser/thr Protein Phosphatase activity (IC50**) | ||||||
|---|---|---|---|---|---|---|
| Compound | PP1 | PP2A | PP2B (calcineurin) |
PP4 | PP5 | PP7 |
| Okadaic acid | 15–50 | 0.1–0.3 | ~4,000 | 0.1 | 3.5 | >1,000 |
| Microcystin-LR | 0.3–1 | <0.1–1 | ~1,000 | 0.15 | 1.0 | >1,000 |
| Nodularin | 2.4 | 0.3 | >1,000 | ND | ~4 | >1,000 |
| Calyculin A | 0.4 | 0.25 | >1,000 | 0.4 | 3 | >1,000 |
| Tautomycin | 0.23–22 | 0.94–32 | >1,000 | 0.2 | 10 | ND |
| Cantharidin | 1,100 | 194 | >10,000 | 50 | 600 | ND |
| Fostriecin | 45,000–58,000 | 1.5–5.5 | >100,000 | 3.0 | 50,000–70,000 | ND |
ND, not determined
This table is adapted from Honkanen and Golden (2).
The IC50 values provided are nM and represent the amount of inhibitor needed to inhibit 50% of the activity in an assay. Because IC50 values are influenced by the concentration of inhibitor, the mode of inhibition, and the amount of both enzyme and substrate employed in an assay, the different values shown likely reflect variations in the assay conditions used by the labs reporting these values. Details of the structure activity relationship for okadaic acid, calyculin A, microcystin-LR, cantharidin and tautomycin can be found in a recent review by Sakoff and McClusky (3). Details of the structure activity relationship for fostriecin has been published recently (4), and more information about the “fostriecin like” compounds produced by Streptomyces can be found in a review by Lewy et al. (5)
Comparison of the relative IC50 values reveals that the most toxic inhibitors (microcystin-LR, calyculin A and nodularin) are all very potent inhibitors of PP1, PP2A, PP4 and PP5, markedly inhibiting the activity of each enzyme at nanomolar concentrations. They have very limited activity against PP2B or PP7 and virtually no effect on PPM-family ser/thr phosphatases or phosphotyrosine phosphatases. To date >50 variants of microcystin and ~10 variants of nodularin have been identified. The microcystins/nodularins are cyclic-peptides, and many (i.e. microcystin-LR) demonstrate substantial solubility in aqueous solutions. Still, they are not readily taken up by most cell types, with the notable exception of hepatocytes and intestinal epithelial cells from the distal ileum that are capable of actively transporting these molecules across their plasma membranes (likely via the bile acid transporter). Accordingly, microcystins/nodularins are most useful as inhibitors when added to cell homogenates or extracts, where they rapidly diffuse in an aqueous environment and potently inhibit the activity of PP1, −2A, −4, −5 and likely −6. Due to its very limited membrane permeability, microcystin-LR is also useful for patch-clamp studies when it is desirable to restrict an inhibitor to a particular side of a lipid membrane (6).
In contrast to the microcysitins, calyculin A readily partitions into cell membranes. However, calyculin A is essentially insoluble in aqueous solutions. Therefore, when added to a living cell culture most of the calyculin A ends up in an “oil-slick” on the surface of the culture media, separated from the cells by an “ocean” of media in which it has very limited solubility. The same thing, to a slightly lesser extent, occurs with the other hydrophobic inhibitors (i.e. okadaic acid, tautomycin and to a much lesser extent cantharidin). As a result the uptake of a hydrophobic inhibitor by cells is influenced by, 1) the partitioning of the inhibitor from the “oil slick” into the aqueous media (i.e. water solubility), 2) the passive diffusion through the aqueous media, 3) the partitioning into the cell membrane, and 4) the partitioning from the membrane into the cytoplasm of the cells where it binds with high affinity to sensitive phosphatases. This makes it very difficult to determine how much inhibitor actually enters a cell. Nonetheless, although limited by it very low water solubility, due to its high affinity for PPases and ability to cross cell membranes, calyculin A will enter living cells and can be used in a limited fashion as an inhibitor of PP1, −2A, −4 and −5. For such studies calyculin A is most useful for distinguishing the actions of calyculin A-sensitive PPases from the actions of PP2B/calcineurin, PP2C and PTPases. When employed alone calyculin A cannot be used to distinguish the actions of the sensitive PPase from each other. It should also be noted that the concentration commonly employed (50–100 nM) will kill most, if not all, human cells when free inhibitor concentrations inside a cell approach 10 nM (See Note 2).
The most selective inhibitors disclosed to date are fostriecin, okadaic acid, and tautomycetin, with fostriecin by far demonstrating the most selectivity (PP2A/PP4 vs PP1/PP5 selectivity ≥ 104). In comparison the PP2A/PP4 vs PP1/PP5 selectivity of okadaic acid is <102, and the PP1 vs PP2A/PP4 selectivity of tautomycin is ~5 (tautomycetin ~40). All three compounds readily enter living cells. Both okadaic acid and tautomycin/tautomycetin are fairly hydrophobic compounds that readily partition into cell membranes but have low (pM to low nM) water solubility. Fostriecin, which demonstrates substantial water solubility (µM to low mM), appears to be capable of entering cells via a carrier mediated transporter, likely the reduced folate carrier system (7). This makes fostriecin expecially attractive for studies conducted with living cells. Like microcystin and calyculin A, fostriecin, okadaic acid, and tautomycin demonstrate little inhibitory activity against PP2B or PP7 and have virtually no effect on PP2C or PTPases. Cantharidin also demonstrates some selectivity (PP2A/PP4> PP5>PP1). However the selectivity of cantharidin is less than that of okadaic acid or fostriecin. It should also be noted that okadaic acid is a fairly stable compound. In contrast the lactone ring of fostriecin, which is necessary for selectivity (4) is sensitive to oxidation, light and changes in pH. Fostriecin is not stable above pH 8.0 and very labile in dilute acid. Therefore, fostriecin should be kept in solutions buffered with ascorbate to protect from oxidation and with buffers adjusted to maintain a pH range of 5.5–7.5.
Given the above, the known inhibitors are very powerful tools for studying the actions of PP1, PP2A, PP4, PP5 and PP6. They can be most effectively employed in studies conducted using appropriately diluted cell extracts, (including immunoprecipitations) to gain insight into the involvement of a sensitive PPase and in vivo studies to distinguish the actions of broad classes of phosphatases (PP1/2A/4/5/6 vs PP2B/7/PP2Cs/PTPases). With care fostriecin can be employed in vivo to distinguish between PP2A/4/6 and PP1/PP5. However, for proper interpretation of the data it is necessary to first determine the total amount of sensitive PPase(s) contained in a give preparation. This can be accomplished using a microcystin-LR titration assay, described below. Methods to produce phosphoprotein substrates with very high specific activity, and a very sensitive phosphatase assay are also provided, which when used in combination with fostriecin and okadaic acid can be employed to obtain insight into the involvement of PP2A/PP4 in a give event.
2. Materials
2.1 Inhibitors: source, solubility, storage and stability
1. Okadaic acid (C44 H68 O13; M.W. 804.5)
Principal source: marine dinoflagelates; Prorocentrum lima, Prorocentrum elegans, and Prorocentrum concavum. Also produced by several species of Dinophysis.
Okadaic acid is complex hydrophobic polyether that comes as a white crystalline solid. It is readily soluble in many organic solvents and degrades in acid or base. Although soluble in DMSO and a number of organic solvents, the recommended solvent is N,N-dimethylformamide (DMF). When stored in DMF at −20°C okadaic acid is stable for years (>5).
2. Microcystin-LR (C49 H74 N10 O12; M.W. 995)
Principal source: cyanobacteria (blue-green algae) Microcystis aeruginosa
Microcystins are cyclic heptapeptides that come as a dry white powder. Microcystin-LR is soluble in most aqueous solutions, DMSO and DMF (recommended). It is unstable at pH >7.7 and can be stored at −20°C in DMF for ~1 year.
3. Nodularin (C41 H60 N8 O10; M.W. 824)
Principal source: cyanobacteria; Nodularia spumigena.
Nodularin is a cyclic pentapeptide with similar properties to microcystin-LR. It is readily soluble in most aqueous solutions, DMSO and DMF. Nodularin is unstable at pH >7.7 and can be stored at −20°C in DMF for ~1 year.
4. Calyculin A (C50 H81 N4 O15 P; M.W. 1009)
Principle source: unknown; purified from a marine sponge, Discodermia calyx
Calyculin A is a novel spiro ketal bearing phosphate, oxazole, nitrile and amide functionalities. It comes as a white solid and is soluble in DMSO, ethanol and DMF. Calyculin A is virtually insoluble in water. During storage it should be protected from light and moisture. As a concentrated solution in DMF calyculin A is fairly stable and can be stored for >6 months at −20°C.
5. Fostriecin (C19H26O9PNa; M.W. 452.4)
Principal source: Streptomyces pulveraceus ( subspecies fostreus).
Fostriecin is a structurally novel phosphate ester that comes as a colorless or yellow solid. It is soluble in water, methanol, ethanol and DMF and should be protected from light both as a powder and in solution. It is recommended to store the dry powder at −80°C. Concentrated stock solutions can be made by dissolving fostriecin in DMF. In solution fostriecin should be stored in a tightly sealed vial under argon. DMF stocks can be stored under argon for ~2 months at −20°C with minimal loss of potency. When diluted in aqueous solution fostriecin should be used fresh. It is sensitive to oxidation and MUST be maintain at a pH range between 5.5–7.5. Even brief exposure to weak acids or bases will result in the loss of inhibitory activity. Buffered or pH adjusted freshly prepared ascorbic acid (1 mM) can be added to aqueous solutions to help protect against oxidation (See Note 3). Although concentrated stocks solutions in DMF can be stored at −20°C for two to three months, even highly concentrated aqueous stocks cannot be stored at +4°C for more then a few days.
6. Tautomycin (C41H66O13; M.W. 767.0)
Principal source: Streptomyces sp. (i.e. S. spiroverticillatus).
Tautomycin exists as a tautomeric mixture in solution (hence the name) and comes as a lyophilized solid. It is soluble in ethanol, ethyl acetate, DMSO and DMF. Tautomycin is fairly stable and can be stored for ~1 year at −20°C as a lyophilized solid or in a concentrated DMF solution. Dilute solutions in DMF can be stored at −20°C for ~3–6 months.
7. Cantharidin (C10H12O4; M. W. 196.2)
Principal source; blister beetles (i.e. Cantharis vesicatoria.)
Cantharidin (left) comes a solid white powder, that can be stored at room temperature (+20°C). It should be protected from light and moisture. Cantharidin is soluble in DMSO and DMF (>25 mg/ml) and in ethanol (1.0 mg/mL). As a dry powder it is quite stable and can be stored for up to 5 years. Concentrated stock solutions should be stored at −20°C and are stable for ~3–6 months). Cantharidic acid (right) may be the active form of the inhibitor in a cell, for SAR studies indicate that derivatives that prevent ring opening have markedly lower inhibitory activity (3). It should also be noted that Mn2+, which is commonly employed in assays using recombinant PPases expressed in bacteria, blocks the inhibitory activity of cantharidin, likely by inhibiting is binding to the catalytic site (Swingle, Li and Honkanen, unpublished observation).
3. Methods
Of the above mentioned inhibitors fostriecin, okadaic acid, tautomycin and calyculin A are most useful for distinguishing the activity of the “toxin-sensitive PPP-family phosphatases (PP1, PP2A, PP4, PP5 and likely PP6) from the toxin-insensitive PPP-phosphatases (PP2B/calcineurin, PP7), PPM-family phosphatases, phosphotyrosine phosphatases (PTPases) and “dual-specific” phosphatases. For this purpose, any of these compounds can be added to an assay (or to cells in culture) at an appropriate concentration to test the hypothesis that a “toxin-sensitive” phosphatase participates in a given event. The identification of a toxin-sensitive PPase in a particular event can most easily be accomplished by conducting dose-response studies using assays conducted with appropriately diluted (see below) cell extracts, immunoprepipitates or reconstituted systems, and the ability of any of these inhibitors to affect a given response in a crude cell homogenate at a concentration close to the IC50 values listed in Table 1 is a reasonable indication that PP1, PP2A, PP4, PP5 or PP6 is involved. Similarly, when applied at a sub-lethal concentration to cells, the ability of any of these inhibitors to affect a given response in living cells is also a good indication that at least one of the toxin-sensitive PPases is involved. However, demonstrating the involvement of a particular “toxin-sensitive” PPase is more difficult, and in living cells may not be possible with a high degree of certainty.
Both calyculin A and okadaic acid are commonly used both in vitro and in vivo to study the actions of PP1 and PP2A, and the conclusions drawn from such studies are often inaccurate. Most notably, in the literature it is often incorrectly reported that the ability of calyculin A to affect a given response that is not influenced by 5–10 nM okadaic acid indicates the involvement of PP1. This argument is flawed in several ways. First and foremost, the argument ignores the possible involvement of PP4, PP5 or PP6. Second, such studies usually assume “instant equilibrium” of the inhibitors. The problem is that different cell-permiant compounds do not necessarily diffuse into cells at the same rate (See Note 2), especially if the compounds are added at different concentrations (8). Third, organic anion efflux pumps (i.e. the multidrug resistance pump) may use these compounds as substrates. Finally, okadaic acid, like all of the above mentioned inhibitors, binds to PP2A, PP4, PP5 and PP1 with high affinity at a molar ratio of 1:1, and most of the okadaic acid-sensitive PPase are expressed at fairly high levels in eukaryotic cells. Therefore, unless the assays using okadaic acid are conducted with dilute samples, 5–10 nM okadaic acid may not be sufficient to fully inhibit the most OA-sensitive PPases (PP2Aalpha/beta, PP4, PP5 and probally PP6) present in the assays, because undiluted samples may contain concentrations of these PPases near to, or in excess of 10 nM (≥ their respective inhibitor dissociation constants). In such a regime, IC50 values deviate substantially from the Ki values because the free inhibitor concentrations is reduced significantly through binding and sequestration of inhibitors by the PPases (i.e. “titration”). Indeed, when [E]≫ki, essentially all inhibitor is bound and the IC50 is simply [E]/2. This phenomenom forms the basis for the microcystin-LR titration assay descrived below. Accordingly, to appropriately interpret data obtained with okadaic acid, calyculin A or tautomycin, it is necessary to first determine the amount of toxin-sensitive PPase contained in a given preparation and then dilute the extracts so that such titration effects are minimized.
To date the most selective inhibitor that has been disclosed is fostriecin, and although its use is somewhat hampered by the above mentioned storage stability issues, when handled properly fostriecin can be employed in combination with parallel studies using calyculin A, cantharidin or okadaic acid to distinguishing the actions of PP2A/PP4/PP6 from those of PP1/PP5 in assays that are conducted in vitro and possibly in vivo as well. The major advantages of fostriecin are it high selectivity for PP2A/PP4, its water solubility, and its ability to enter cells via the reduced folate transporter.
At present inhibitors capable of distinguishing the actions of PP2A from PP4/PP6 have not been reported. Therefore, currently it is not possible to use small molecule inhibitors alone to distinguish the actions of PP2A from that of PP4/PP6 or the actions of PP1 from the actions of PP5. Nonetheless, fostriecin can be used for in vitro characterization of the toxin sensitive PPases that contribute to a given response and to indicate the involvement of PP2A/PP4/PP6 in vivo. However, since the inhibition of PP1/PP2A/PP4 and PP5 is lethal to most, if not all, human cells, despite numerous claims in the literature it is actually very difficult to use either okadaic acid or fostriecin to attribute any given observation to the suppression of PP1 or PP5. To do so, numerous additional experiments are needed using a combination of small molecule inhibitors and protein inhibitors (i.e. inhibitors 1), time-course studies, and phosphatase assays using different substrates. An excellent example of such studies using MCF-7 cells can be found in a paper by Favre et al. (8). Fortunately, recent advances using antisense oligonucleotides and siRNA indicate that virtually any protein that is expressed in a cell can be suppressed. Thus the development of siRNA for each PPase should greatly aid in the assignment of roles to a given PPase. Indeed methods for suppressing the expression of human PP5 have already been reported (9). Still small molecule inhibitors are very useful in “culling experiments”, which are designed to eliminate PPases that are not involved in a given response. In addition, the actions of the small molecules are more rapid and reversible.
3.1 Microcystin-LR Titration assay
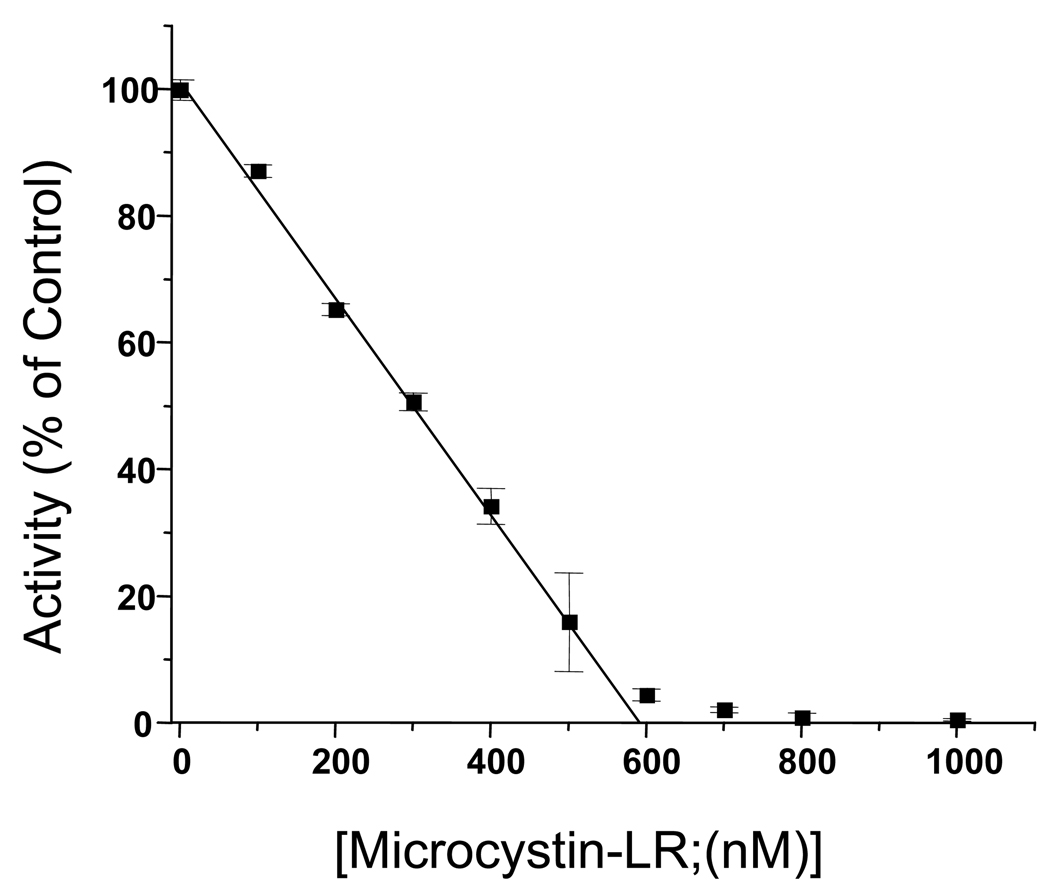
Since microcystin-LR binds sensitive phosphatase with high affinity and with a molar ratio of 1:1, it can be employed to determine the concentrations of sensitive PPases in partially purified protein preparations and crude cell extracts by conducting detailed dose-response inhibition studies (Figure 1). Since enzyme concentrations are often high in these assays, it is important to ensure that substrate depletion is negligible when compared to total substrate concentration. For the example given below the assay is conducted using partially purified PP5 and p-Nitrophenyl Phosphate (pNPP; 50 mM) as the substrate. For use in crude cell homogenates, phosphoproteins substrates, such as phosphohistone (methods for production given below) can be employed. For all substrates, with the microcystin-LR titration assay lower temperature and shorter reaction times help reduce substrate depletion and product inhibition.
Aliquots of extracts (~20 µl) are diluted to 27.5 µl with water whereupon 2.5 µl of microcystin-LR or vehicle (DMF) is added and mixed by pipetting. For such studies concentrated microcystin-LR stocks are diluted in DMF using serial dilutions to produce stocks solutions that will produce the concentrations indicated when 2.5 µl of the stocks added to the assays (See Figure 1).
The enzyme/extract is pre-incubated with microcystin-LR for 10 minutes at room temperature and reactions are started by the addition of 10 µl of 4× assay buffer (200 mM tris pH 8.3 at 37° C, 200 mM MgCl2, 200 mM pNPP, and 0.4% 2-mercaptoethanol) followed by incubation at 37° C for 10 minutes.
Reactions are stopped by the addition of 100 µl of 1M EDTA pH 10 and absorbance is measured at 400 nm using a spectrophotometer. The data is plotted as shown and the intercept of the linear portion of the curve with the x-axis provides a fairly accurate indication of the amount of toxin-sensitive PPase present. In the example provided, there is nearly 600 nM microcystin-LR sensitive PPase in the assay.
Fig. 1.
Inhibition of PP5 activity by microcystin-LR. For this reaction it is necessary that the concentration of enzymes [E] is greater then the Ki, [E]≫Ki. Thus, for a mixture of PPases the Kis and differences in Kis become irrelevant.
3.2 Preparation of Phosphohistone Substrate
For microcystin-titration assays conducted in crude cell homogenates, pNPP is not the substrate of choice because microcystin-LR insensitive phosphatases that can use pNPP as a substrate are often present (i.e. PP2B, PP2C and PTPase) which will result in a high background. Therefore, a phosphoproteins substrate may be more useful. In addition, substrates with very high specificity, when combined with a sensitive phosphatase assay, can be employed along with fostriecin and okadaic acid to detect changes in the activity of PP2A/PP4 in dilute crude cell homogenates and immunoprecipitations. Histone phosphorylated with cAMP-dependent protein kinase (PKA) is a good substrate for PP1, PP2A, PP4 and PP5 (See Note 4) and phosphohistone with specific activity > 5 × 106 dpm/nmole incorporated phosphate can be produced as follows:
Histone (type-2AS) is phosphorylated with cAMP-dependent protein kinase (PKA) in a reaction containing 10 mg/ml histone, 2 mg/ml PKA, 6 mCi/ml [γ-32P]ATP (200 mM ATP), 10 mM cAMP, 40 mM PIPES pH 6.8 (at 37° C), 7 mM MgCl2, 0.1 mM EDTA, and 5 mM DTT. The reaction volume is 1.5 ml, and the reaction is conducted in a 15 ml conical polypropylene test tube (See Note 5). Aliquots (useally 2 × 5 µl) are removed for use in the calculation of specific activity, and the reaction is carried out overnight at 37° C.
The reaction is terminated by the addition of ice cold TCA to 25%. The tube is placed on ice for at least 10 minutes, and the precipitated phosphohistone is collected by centrifugation (~3,000 × G for 7 minutes). The supernatant is discarded, the pellet resuspended in 1M tris pH 8.5, and the phosphohistone is precipitated again by the addition of ice cold TCA to 25%.
The precipitation-resuspension cycle is repeated 5 times, which is the minimum number of washes that are required to wash free [32P], ATP and free phosphate from the preparation (See Note 6).
To remove the TCA the pellet produced upon the sixth TCA precipitation is washed 4 times with ethanol/ethyl ether (1:4, v:v) then twice with acidified ethanol/ethyl ether (1:4, v:v ; 0.1 N HCl). Between each wash, the pellet is mixed using a small glass rod to break up any clumps that are observed. Prior to removal of the wash, each time the pellet is stabilized by centrifugation as above.
The washed histone pellet is then air-dried and dissolved in 5 mM tris pH 7.4 (See Note 7). After the histone is dissolved, aliquots can be stored at −20 °C. If performed correctly, the above procedure generates sufficient substrate for ~3,000 assasy with ~500,000 CPM of phosphohistone and a background (free 32P) of ~300.
-
Calculations:
- ATP incorporation ratio; R
- Concentration of incorporated phosphate: Cp
3.3 Measurement of phosphatase activity
To take advantage of substrates with very high specific activity, a very sensitive assay is often needed. The following phosphatase assay can be employed.
With this method phosphatase activity against phosphoproteins is determined by the quantification of [32P] liberated from phosphohistone, prepared as described above. The assay (80 µl final volume) is conducted in 50 mM Tris-HCL (pH 7.4), 0.5 mM DTT, 0.1 mM EDTA, phosphoprotein (2 µM incorporated PO4) (assay buffer; final concentration). The assay is conducted in 1.5 ml microcentrifuge tube at 30 °C for 10 minutes, and is initiated by adding 30 µl of phosphohistone substrate (dissolved in 5 mM Tris- HCL, pH 7.4) to diluted crude cell extracts containing 4 X assay buffer and water. Typically a reaction contains 20 µl 4 X assay buffer, 5–10 µl dilute cell extract, water to bring the volume to 50 µl, and 30 µl substrate).
The dephosphorylation reaction is stopped by the addition of 100 µl of acid (1N H2SO4) containing 1 mM K2H PO4 (H3PO4 at pH 1)
[32P]liberated by the enzymes is then extracted as a phosphomolybdate complex and measured based on the methods of Killilea et al. (10). Free phosphate is extracted by adding 20 µl of ammonium molybdate (7.5% w/v in 1.4 N H2SO4) and 250 µl of isobutanol:benzine (1:1, v/v) to each tube. The tubes are mixed vigorously for ~10 seconds (vortex set at maximum speed), followed by centrifugation at 14,000 × G for 2 minutes. This results in a bi-phasic solution. The radioactivity (phosphate liberated from the phosphohistone) is contained in the upper phase, is quantified by counting 100 µl aliquots in a scintillation counter (See Note 8).
For inhibition studies, inhibitors are generally dissolved and diluted in DMF (See Note 9) and added to the enzyme mixture 10 minutes before the initiation of the reaction with the addition of substrate. Controls receive solvent alone, and, in all experiments the amount of enzyme is diluted to ensure that the samples are below the titration endpoint. The titration endpoint is defined as the concentration of enzyme after which further dilution no longer affects the IC50 of the inhibitor and can be determined using the microcystin-titration assay described above. It represents a point where the concentration of enzyme used in the assay no longer large in comparison to its inhibitor dissociation constant. This ensures that the IC50 represents the potency of the inhibitor alone and is not representative of a combination of potency of the inhibitor and titration artifacts of the assay system. Preliminary assays must also be performed to ensure the dephosphorylation reaction is linear with respect to enzyme concentration and time.
3.4 Identification of the activity of PP2A/PP4 using fostriecin and okadaic acid
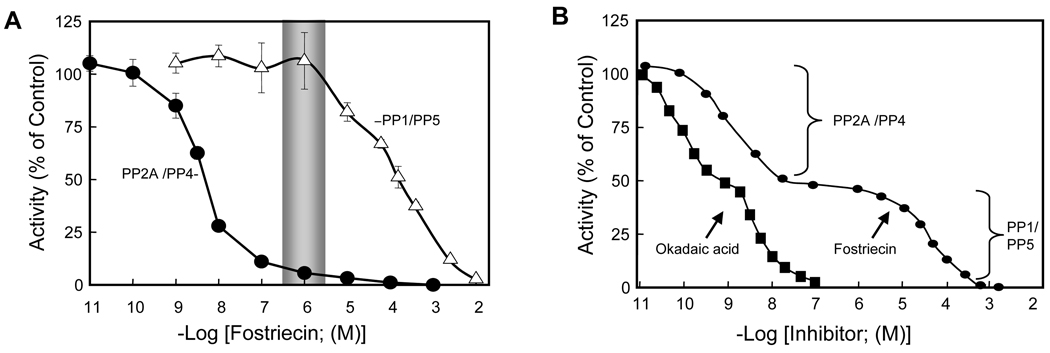
Typical dose-response inhibition curves that can be obtained using purified PP2A (similar results are expected for PP4) or PP1/PP5 are show in Figure 2A. Figure 2B is a theoretical example illustrating the inhibiton of fostriecin in appropriately dilute samples containing a mixture of PP1, PP2A, PP4 and PP5, such as that present in crude cell homogenate.
Figure 2.
Dose-response inhibition curves with fostriecin and okadaic acid.
As illustrated in Figure 2A by a shaded bar and in Figure 2B the plateau in the inhibition curve, ~1 µM fostriecin is sufficient to completely inhibit the activity of PP2A/PP4 while having little, if any, effect on PP1 or PP5. Thus, the addition of ~1µM fostriecin to a sufficiently dilute crude cell homogenate is sufficient to completely inhibit the activity of these enzymes and can be used to implicate the role of PP2A/PP4 in a given event. With great care, okadaic acid can be employed in a similar fashion, and 1–5 nM okadaic acid can be used to inactivate PP2A/PP4 in dilute crude cell homogenates following mixing. However, when compared to fostriecin, okadaic acid has higher affinity for PP2A/PP4 and is not nearly as selective. Thus, studies with okadaic acid are much more likely to be influenced by “titrations artifacts”. Still both of these inhibitors can be used in a wide variety of biological studies, and such dose-response studies can be employed with immunoprecipitation studies to characterize a phosphatase that co-immunoprecipitates with other proteins. Similarly, ~1 µM fostriecin should be sufficient to inhibit the actions of PP2A and PP4 in living cells, assuming the cells have a functioning reduced folate carrier system and sufficient volume is used during treatment. However, it should be noted that at this time it is not clear if the reduced folate carrier system can concentrate fostriecin intracellularly, which may be accomplished with folate (See Note 10).
4. Notes
To date the sensitivity of PP6 to the inhibitors listed has not been reported. However, due to the extreme conservation of the amino acids in the toxin-binding domain of PP6 with that of PP2A and PP4, it is predicted that PP2A, PP4 and PP6 will demonstrate comparable sensitivity to most, if not all, of the inhibitors discussed.
When added to cell cultures, for hydrophobic inhibitors such as calyculin A and okadaic acid, it may take 24–48 hours before equilibrium conditions are archived inside a cell. This equilibrium process is dependent on the amount of inhibitor added, the water solubility of the inhibitor, and the ability of the inhibitor to cross the membrane. As a result, adding higher amounts of inhibitor to cells in culture will help saturate the media, but ultimately the water solubility becomes limiting. Once the culture media becomes saturated, the inhibitors must still cross the membrane to have access to the PPases. Assuming that the process is passive, the inhibitor has to first partition into the lipid bilayer (a rapid process) and then partition back into an aqueous environment (a slower process). So again water solubility becomes an issue. However, inside the cell, the PPases have very high affinity binding sites, which is reflected by the Ki for a given compound. As a result, free cytostoic inhibitor rapidly binds to sensitive PPases, and there is a net flux of inhibitor into the cell until all of the high affinity binding sties are occupied. Due to the very low water solubility of the hydrophobic inhibitors, the entire process is relatively slow.
When diluted in solutions containing ascorbic acid the solution should be clear (it may have a tinge of yellow if the ascorbic acid is getting old). When the solution turns yellow it is very likely that the fostriecin has been oxidized and will no longer be useful.
Phosphohistone is a very poor substrate for PP2B. In addition, because they require divalent cations for activity, EDTA and EGTA can be added to assays using this substrate to inhibit the activity of PP2B and the PPM-family phosphatases (e.g. PP2C) that may be present.
The tube must be able to withstand both acid (TCA) and ether. Polystyrene tubes commonly used in tissue culture will dissolve and are thus not suitable.
For resuspension, it is not necessary that the solution becomes clear, but no visible “chunks” should be present. Failure to adequately resuspend or thoroughly remove the supernatant after centrifugation will result in increased background in the phosphatase assay. If this should occur, the substrate can be precipitated with TCA and the ethanol/ether washes can be repeated to salvage the preparation.
It takes time for the pellet to hydrate and go completely go back into solution. It usually takes ~1 hour with vortexing every 5–10 minutes
The phosphohistone will be concentrated at the interface of the organic (top) and aqueous (bottom) solutions. When removing a sample of the organic phase to count, it is important not to allow the tip to hit the interface. If the tip breaks the interface radioactive histone will stick to the outside of the tip. If this should happen, the tip should be discarded and the sample should be taken with a fresh tip after the sample is centrifuged again.
A common mistake is to conduct serial dilutions in assay buffer. This will not work for hydrophobic inhibitors.
Due to its uptake via the reduced folate carrier system it is possible that fostriecin accumulates inside a cell. However, for folate intracellular accumulation may be due to the prevention of efflux following polyglutamylation.
Safety in handling. Although several studies have shown that fostriecin and cantharidin possess antitumor activity, both okadaic acid and microcystin-LR have been touted to act as tumor promoting agents. For all of the inhibitors discussed the pharmacological and toxicological properties have not been fully investigated. According exercise caution in use and handling. They should be handled as if they were carcinogenic, and they are all toxic. Wear gloves when handling product and mask when dispensing large amounts. Avoid contact by all modes of exposure.
Acknowledgements
This work was supported, in part, by a research grant from the National Institutes of Health, (NCI grant CA 60750) and was conducted in a facility constructed with support from a Research Facilities Improvement Program Grant (CO6 RR11174) from the National Center for Research Resources.
References
- 1.Bialojan C, Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J. 1988;256:283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Honkanen RE, Golden T. Regulators of serine/threonine protein phosphatases at the dawn of a clinical era? Curr Med Chem. 2002;9:2055–2075. doi: 10.2174/0929867023368836. [DOI] [PubMed] [Google Scholar]
- 3.Sakoff JA, McCluskey A. Protein phosphatase inhibition: structure based design. Towards new therapeutic agents. Curr Pharm Des. 2004;10:1139–1159. doi: 10.2174/1381612043452686. [DOI] [PubMed] [Google Scholar]
- 4.Buck SB, Hardouin C, Ichikawa S, Soenen DR, Gauss CM, Hwang I, Swingle MR, Bonness KM, Honkanen RE, Boger DL. Fundamental role of the fostriecin unsaturated lactone and implications for selective protein phosphatase inhibition. J Am Chem Soc. 2003;125:15694–15695. doi: 10.1021/ja038672n. [DOI] [PubMed] [Google Scholar]
- 5.Lewy DS, Gauss CM, Soenen DR, Boger DL. Fostriecin: chemistry and biology. Curr Med Chem. 2002;9:2005–2032. doi: 10.2174/0929867023368809. [DOI] [PubMed] [Google Scholar]
- 6.Ichinose M, Endo S, Critz SD, Shenolikar S, Byrne JH. Microcystin-LR, a potent protein phosphatase inhibitor, prolongs the serotonin- and cAMP-induced currents in sensory neurons of Aplysia californica. Brain Res. 1990;533:137–140. doi: 10.1016/0006-8993(90)91806-r. [DOI] [PubMed] [Google Scholar]
- 7.Fry DW, Besserer JA, Boritzki TJ. Transport of the antitumor antibiotic Cl-920 into L1210 leukemia cells by the reduced folate carrier system. Cancer Res. 1984;44:3366–3370. [PubMed] [Google Scholar]
- 8.Favre B, Turowski P, Hemmings BA. Differential inhibition and posttranslational modification of protein phosphatase 1 and 2A in MCF7 cells treated with calyculin-A, okadaic acid, and tautomycin. J Biol Chem. 1997;272:13856–13863. doi: 10.1074/jbc.272.21.13856. [DOI] [PubMed] [Google Scholar]
- 9.Golden TA, Honkanen RE. Regulating the expression of protein phosphatase type 5. Methods Enzymol. 2003;366:372–390. doi: 10.1016/s0076-6879(03)66028-3. [DOI] [PubMed] [Google Scholar]
- 10.Killilea SD, Mellgren RL, Aylward JH, Lee EY. Inhibition of phosphorylase phosphatase by polyamines. Biochem Biophys Res Commun. 1978;81:1040–1046. doi: 10.1016/0006-291x(78)91455-9. [DOI] [PubMed] [Google Scholar]