Abstract
11q13 amplification is a late-stage event in several cancers that is often associated with poor prognosis. Among 11q13-amplified genes, the actin assembly protein cortactin/CTTN is considered a likely candidate for direct involvement in tumor progression, because of its cell motility-enhancing functions. We modulated cortactin expression in head and neck squamous cell carcinoma (HNSCC) lines. Cortactin expression levels directly correlated with tumor size, vascularization, and cell proliferation in an orthotopic HNSCC in vivo model. In contrast, under normal in vitro culture conditions, cortactin expression levels had no effect on cell proliferation. However, cell lines in which cortactin expression was reduced by knockdown (KD) grew poorly in vitro under harsh conditions of growth-factor deprivation, anchorage independence, and space constraint. Conversely, overexpression of cortactin enhanced in vitro growth under the same harsh conditions. Surprisingly, defects in growth factor-independent proliferation of cortactin-KD cells were rescued by co-culture with cortactin-expressing cells. Since the co-cultured cells are separated by permeable filters, cortactin-expressing cells must secrete growth-supporting autocrine factors to rescue the cortactin-KD cells. Overall, cortactin expression modulates multiple cellular traits that may allow survival in a tumor environment, suggesting that the frequent overexpression of cortactin in tumors is not an epiphenomenon but rather promotes tumor aggressiveness.
Introduction
Genetic alterations are a frequent event in cancer. Recurrent chromosomal aberrations, such as amplifications or deletions, often harbor genes that participate in tumor initiation or progression. 11q13 amplification occurs frequently as a late event that correlates with poor prognosis in various cancer types (Hui et al., 1998; Myllykangas et al., 2007; Schuuring, 1995). In head and neck squamous carcinoma (HNSCC), 11q13 amplification occurs in 30-40% of tumors and correlates with an increase in tumor grade, lymph node metastases, recurrence, and decreased survival (Akervall et al., 1995; Meredith et al., 1995; Rodrigo et al., 2000; Takes et al., 1997; Williams et al., 1993). Within the 11q13 amplicon, cyclin D1 and CTTN/cortactin (formerly EMS1) are thought to be the two best candidate genes responsible for amplicon-associated poor prognosis due to the consistent correlation of cyclinD1 and cortactin gene amplification with protein overexpression (Ormandy et al., 2003; Schuuring, 1995; Schuuring et al., 1992). Although many investigators have assumed that cyclin D1 is the major gene responsible for the 11q13-associated tumor aggressiveness, Rodrigo et al. examined the rare instances of independent amplifications of cyclinD1 or cortactin in HNSCC and found that decreased survival and other measures of poor prognosis correlated with cortactin amplification but not with that of cyclinD1 (Rodrigo et al., 2000). Furthermore, cortactin expression levels were recently found to correlate with poor outcomes in HNSCC (Gibcus et al., 2008; Hofman et al., 2008). However, other genes within the 11q13 amplicon are overexpressed and could account for the associated poor prognosis (Freier et al., 2006; Gibcus et al., 2007).
Cortactin is a prominent src kinase substrate (Wu & Parsons, 1993; Wu et al., 1991) that promotes Arp2/3 complex-mediated branched actin assembly by multiple mechanisms, including stabilization of branched actin networks, augmenting actin nucleation, and serving as a scaffold for cytoskeletal molecules (Uruno et al., 2001; Weaver, 2008; Weaver et al., 2001). Relevant to tumor progression, cortactin promotes cell motility and invasion and is required for proper functioning of invadopodia, subcellular organelles associated with extracellular matrix (ECM) degradation (Weaver, 2008). Recently, we identified regulation of protease secretion as a critical function for cortactin in invadopodia (Clark & Weaver, 2008; Clark et al., 2007). A general role for cortactin in autocrine secretion is suggested by our concurrent finding that cortactin is also essential for secretion of a non-invadopodia protein, ApoA1 (Clark et al., 2007). Consistent with an important role for cortactin in cellular membrane trafficking, data from other laboratories implicates cortactin in endocytosis and trafficking of model proteins from the Golgi apparatus (Cao et al., 2003; Cao et al., 2005; Merrifield et al., 2005; Zhu et al., 2005).
In xenograft tumor studies, cortactin was found to enhance metastasis of breast cancer cells to bone (Li et al., 2001), of esophageal squamous cell carcinomas to the lung (Luo et al., 2006), and intrahepatic metastasis of hepatocellular carcinoma (Chuma et al., 2004). In the same studies, divergent results were found for effects on primary tumor growth, with a significant inhibition of subcutaneous growth of injected esophageal tumors by cortactin siRNA but no effect of cortactin overexpression on orthotopic breast or hepatic tumor growth (Chuma et al., 2004; Li et al., 2001; Luo et al., 2006). Based on these results and the well-established cellular role of cortactin in motility and invasion, the general focus in the field has been on the role of cortactin in tumor invasion and metastasis.
The goal of this study was to define the importance and role of cortactin in HNSCC tumor progression. Using a semi-orthotopic xenograft model (Shores & Yarbrough, 1998), tumors were grown from HNSCC cancer cells with altered cortactin expression levels. Surprisingly, for three independent HNSCC lines, cortactin overexpression increases while cortactin knockdown decreases tumor size. Expression of cortactin enhances in vivo proliferation and vascularization, in vitro anchorage- and serum-independent proliferation, and growth in embedded 3-dimensional Matrigel culture. Coculture experiments implicate autocrine secretion as a likely control point for cortactin expression in tuning tumor phenotypes, since secreted factors from control and cortactin-overexpressing cells rescue the serum-free growth of cortactin-KD cells. Similar to previous studies, we confirm a role for cortactin in tumor invasiveness. Overall, cortactin regulates the general aggressiveness of HNSCC tumors.
Results
Cortactin promotes HNSCC tumor growth
Cortactin expression was manipulated in tumorigenic non-11q13-amplified (SCC61, SCC25) and 11q13-amplified (FaDu) HNSCC cell lines using retroviral expression of shRNA or mouse cortactin cDNA to respectively downregulate or upregulate cortactin levels (Figs 1A, 2A, Supp Fig 1A). Tumors were grown using a semi-orthotopic rat trachea model in which HNSCC cells are placed in denuded rat tracheas and then inserted into the flanks of nude mice. Epithelial morphology of oral squamous cells grown in this model was previously shown to be consistent with known biologic attributes of the implanted cells, e.g. normal, dysplastic, tumorigenic, or invasive (Shores & Yarbrough, 1998). After four weeks, tracheas implanted with tumor cell lines were removed from the mice and processed. Two tumor-specific stains were used: a human-specific Ku70 antibody to identify human tumor cells and a pan-cytokeratin antibody that labels cells of epithelial origin (Figs 1C, 2C, Supp Fig 1D). Tracheal area positive for both Ku70 and cytokeratin was measured from microscopic images to determine tumor area. This histological measurement correlates well with both tumor weight and caliper measurements of tumor diameter (data not shown) but avoids measuring the confounding contribution of normal tissue. Surprisingly, cortactin expression has a major effect on tumor size. For all three cell lines, cortactin-KD (pRS-KD1 and pRS-KD2) tumors are greatly decreased in size while cortactin-overexpressing (LZRS-CortFL) tumors grow larger than control tumors (Figs 1B,C, 2B,C, Supp Fig 1B,D). Of the three cell lines, the 11q13-amplified FaDu cell line is the most aggressive in vivo, forming large tumors that invade through and degrade the tracheal rings by the end of the 4-week growth period. FaDu cells express high levels of cortactin, approximately 6-fold higher than the SCC61 cell line (data not shown) and knockdown of cortactin is never complete (∼40% the cortactin level of control cells). Nonetheless, there is a ∼60% decrease in the size of tumors expressing cortactin shRNA, compared with scrambled control shRNA-expressing tumors (Fig 2B,C). Exogenous expression of mouse cortactin in FaDu results in only a slight increase in cortactin protein expression (Fig 2A) and likewise there is a non-statistically significant increase in tumor size in FaDu cells expressing mouse cortactin (Fig 2B). For both SCC61 and SCC25 tumors, cortactin-KD and -overexpression (OE: LZRS-FL) leads to statistically significant respective decreases and increases in tumor size (Fig 1B, Supp Fig 1B). For SCC61 cells, two additional cell lines were engineered to verify that we could rescue cortactin-KD phenotypes. Indeed, the decrease in tumor size with cortactin knockdown is rescued to control levels by reexpression of mouse cortactin in the human-specific shRNA background (pRS-KD1/LZRS-CortFL) (Fig 1). To facilitate subsequent in-depth studies, the majority of further analyses were performed using only the SCC61 and FaDu cell lines as models.
Figure 1. Cortactin promotes tumor growth (SCC61 cells).
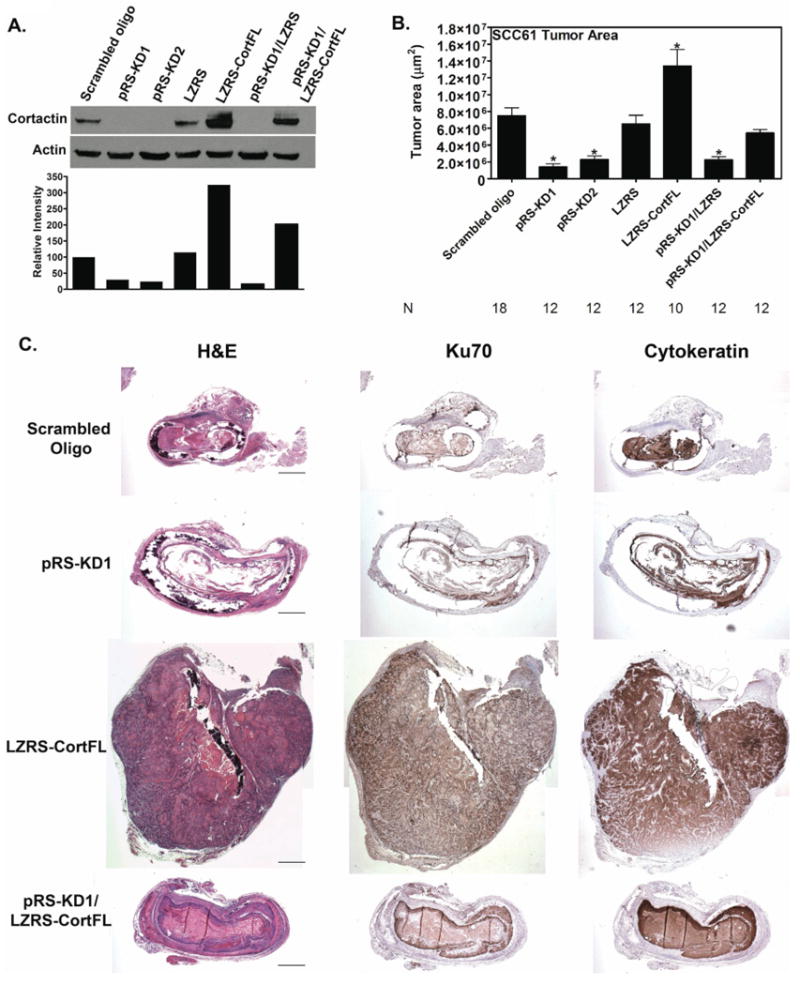
Immunohistochemistry analysis of tumor size indicates that cortactin expression levels have a major effect on the size of SCC61 non-11q13 amplified HNSCC tumors grown in rat tracheas. A. Representative Western blot and densitometry of cortactin expression in SCC61 cells expressing either scrambled cortactin siRNA oligo (scrambled oligo), cortactin knockdown cells (pRS-KD1 and pRS-KD2), overexpression vector control (LZRS), cortactin overexpressing cells (LZRS-CortFL), knockdown cells rescued with vector only (pRS-KD1/LZRS) and knockdown cells rescued with full length mouse cortactin (pRS-KD1/LZRS-CortFL). Protein lysates were prepared at the time of trachea implantation in mice. B. Tumor area measured from IHC-stained tumor sections. Data are combined from at least two separate trials for each cell line with three mice used for each cell line in each trial. N=number of trachea implanted for each cell line and is shown for each cell line. Data are represented as mean ± SEM. Asterisks indicate p<0.05 compared with scrambled oligo control. C. Representative images from tumors derived from cortactin-manipulated SCC61 cells. Tumor cells were grown in rat tracheas implanted into the flanks of nude mice. Tracheas were left for one month, removed, fixed and sagitally sectioned and stained. Shown are the hematoxylin and eosin (H&E), Ku70 (identifies human cells) and cytokeratin (identifies cells of epithelial origin). Positive staining for Ku70 and cytokeratin is brown. Note that Cort-KD tumors are small or absent with a “ragged” morphology, whereas Cort-OE increases the size of tumors compared to scrambled oligo or vector only (LZRS) controls. Scale bar = 1 mm.
Figure 2. Cortactin promotes tumor growth (FaDu cells).
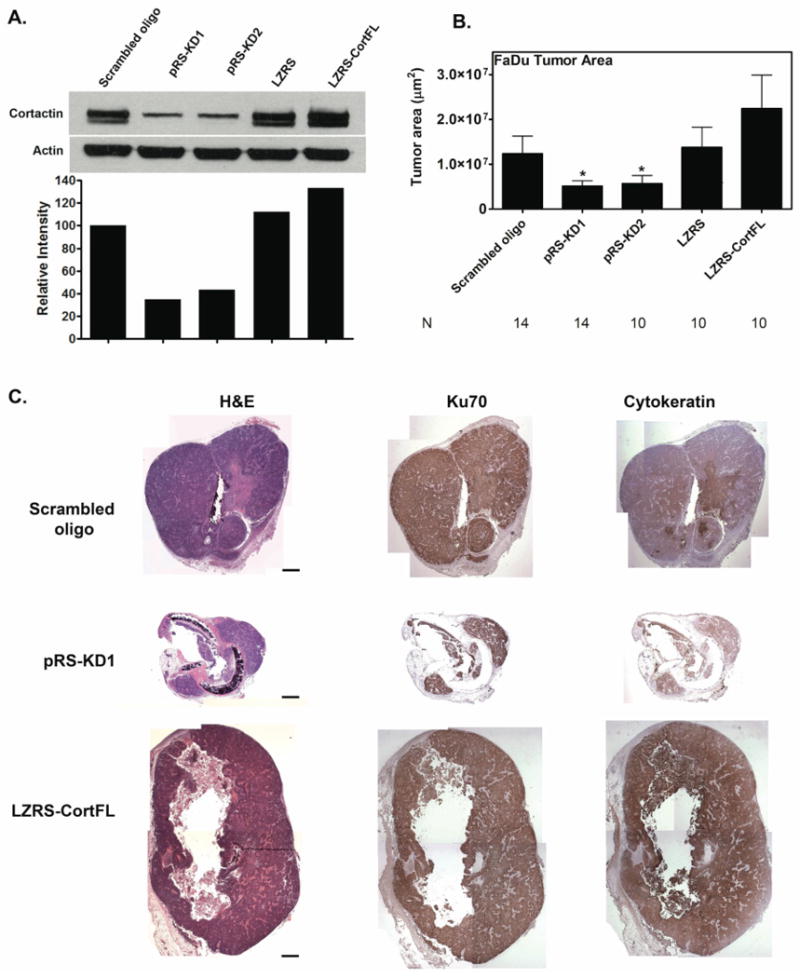
Immunohistochemistry analysis of tumor size indicates that cortactin expression levels regulate the size of FaDu 11q13 amplified HNSCC tumors grown in rat tracheas. A. Representative Western blot and densitometry of cortactin expression in FaDu cells expressing either scrambled cortactin siRNA oligo (scrambled oligo), cortactin knockdown cells (pRS-KD1 and pRS-KD2), overexpression vector control (LZRS) and cortactin overexpressing cells (LZRS-CortFL). Protein lysates were prepared at the time of trachea implantation in mice. B. Tumor area measured from IHC-stained tumor sections. Data are combined from at least two separate trials for each cell line with three mice used for each cell line in each trial. N=number of trachea implanted for each cell line and is shown for each cell line. Data are represented as mean ± SEM. Asterisks indicate p<0.05 compared with scrambled oligo control. Note that cortactin expression levels correlate well with tumor size (compare cortactin levels in Western blot densitometry in A with tumor sizes in B). C. Representative images from tumors derived from cortactin-manipulated FaDu cells. Tumor cells were grown in rat tracheas implanted into the flanks of nude mice. Tracheas were left for one month, removed, fixed and sagitally sectioned and stained. Shown are the hematoxylin and eosin (H&E), Ku70 (identifies human cells) and cytokeratin (identifies cells of epithelial origin). Positive staining for Ku70 and cytokeratin is brown. Scale bar = 1 mm.
Cortactin promotes HNSCC tumor invasion
The finding that cortactin profoundly increases HNSCC tumor size in the xenograft model was unexpected. A more accepted biologic role for cortactin is promotion of cancer cell invasion (Weaver, 2008). To determine whether cortactin promotes invasion in this system, both in vitro and in vivo analyses were performed. Compared to control SCC61 and FaDu cells, cortactin overexpression promotes whereas cortactin knockdown greatly decreases invasion through transwells coated with a thick gel layer of Matrigel (Supp Fig 2A). It is more difficult to quantify invasion in vivo; however, because the trachea provides a recognizable physical barrier, across which tumor cells must invade in order to escape from the lumen, the tumor area outside of the trachea was used as an in vivo measure of invasion. Control SCC61 and SCC25 cells filled the tracheal lumen but were usually contained within the ring, while cortactin-KD tumors were greatly reduced in size and never identified outside of the tracheal lumen (Supp Fig 1C, Supp Fig 2B). In contrast, overexpression of cortactin in SCC61 and SCC25 tumor cells (LZRS-FL) resulted in invasion beyond the tracheal ring (Supp Fig 1C, Supp Fig 2B). We could not perform this calculation for FaDu-derived tumors, as this cell line is more aggressive than the other two cell lines resulting in destruction of the tracheal architecture by the end of the four week incubation in control cells (Fig 2C).
Cortactin expression levels affect tumor proliferation, apoptosis and vascularization
To identify potential mechanisms for the observed differences in tumor size with cortactin expression, immunohistochemical stains for proliferation, apoptosis and vascularization were performed. Staining of tumor sections with an antibody against Ki67, a marker of proliferation, demonstrated that cortactin knockdown significantly decreases (∼2-fold) the proportion of Ki67-positive tumor cells in both FaDu and SCC61 cells (Fig 3). Interestingly, in SCC61 Cort-KD tumors, Ki67-positive cells are found primarily at the periphery of the tumors, suggesting that positive interactions with the tracheal cartilage, associated connective tissue, or other infiltrating host cells may maintain proliferation in those cells (Fig 3B). Despite rescue of tumor size, expression of mouse cortactin in KD cells (pRSKD1/LZRS-FL) does not rescue Ki67 staining percentage or restore the more homogeneous pattern of Ki67 observed in control cells (Fig 3A and data not shown). In addition, unlike for tumor size, Cort-OE (LZRS-CortFL) cells have no increase in Ki67 staining compared with controls. Since expression of two separate shRNA sequences result in identical downregulation of Ki67 staining, it is unlikely that the decrease in tumor proliferation with Cort-KD is an artifact of shRNA expression; however, the lack of rescue by the CortFL construct suggests some complexity in cortactin-mediated phenotypes or a potential difference in function of mouse and human cortactin. As a complicating factor, there are multiple isoforms of cortactin. Our rescue construct utilizes isoform A, which contains all of the actin-binding repeats (Katsube et al., 2004; Ohoka & Takai, 1998); however other isoforms might have different biological effects. For a subset of the tumor studies, we also performed BrdU incorporation as a second marker of proliferation and found the same trend as Ki67 staining, with decreased proliferation in KD SCC61 and FaDu cells (data not shown).
Figure 3. Cortactin expression levels correlate with in vivo proliferation and FaDu apoptosis.
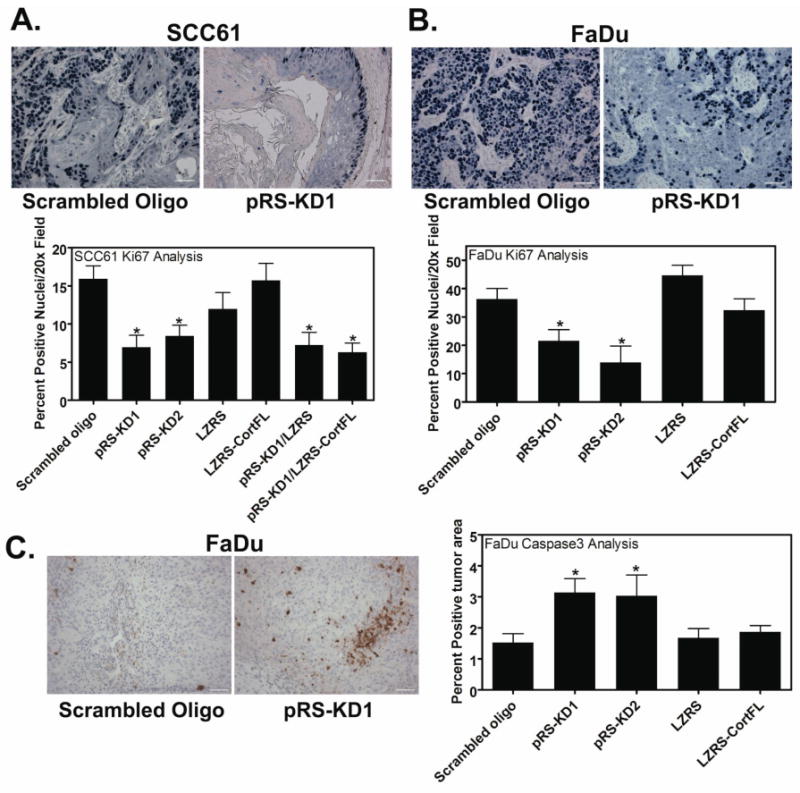
Staining of tumor sections with Ki67 as a marker of proliferating cells indicates that cortactin expression regulates tumor cell proliferation. A. Representative 20× images from control (scrambled oligo) and Cort-KD (pRS-KD1) SCC61-derived tumors showing positive staining for Ki67 (dark blue nuclei), a marker of proliferating cells. Quantification of Ki67-positive nuclei/20× field for all cell lines is shown below the images. B. Representative images from FaDu-derived tumor sections stained for Ki67 along with quantification of Ki67-positive nuclei/20× field. Scale bar = 100 μm. Note that in Cort-KD SCC61 cells, Ki67-positivity is most frequently evident in cells that are in direct contact with the trachea, whereas in Cort-KD FaDu cells, Ki67-positivity is reduced but present throughout the tumor. Data are represented as mean ± SEM. C. Identification of apoptotic cells within tumors by staining with an antibody against cleaved caspase 3 indicates that cort-KD FaDu tumors have twice the number of apoptotic cells as control FaDu tumors. By contrast, SCC61 tumors had very little apoptotic staining and no correlation with cortactin expression (data not shown). Shown are representative 20× images of FaDu scrambled oligo control and pRS-KD1-derived tumors stained for cleaved caspase 3. Positive staining is brown and all nuclei are counterstained light blue. Scale bar = 100 μm. The bar graph shows quantification of percent of tumor area that is caspase 3 positive for all FaDu cell lines. Quantification was performed using the Metamorph color threshold tool. Data are represented as mean ± SEM. Asterisks indicate p<0.05 compared with scrambled oligo control.
Tumor apoptosis was examined by staining for the presence of cleaved caspase 3. FaDu-derived cortactin-KD tumors show a ∼2-fold increase in the percent tumor area that is positively stained compared with control tumors (Fig 3C). The increased staining in KD tumors is patchy, suggesting that local microenvironmental conditions may regulate induction of apoptosis. It might be expected that slower tumor growth may result in less tumor apoptosis due to better supply of growth factors and nutrients; therefore it is interesting that apoptosis was increased in the cortactin-KD tumors despite ∼50% smaller size compared with control tumors. Conversely, there is very little positive staining for cleaved caspase 3 staining in SCC61 tumors and it does not correlate with cortactin expression (data not shown). Because SCC61 KD tumors are extremely small or absent (Fig 1C), it is possible that any apoptotic cell death is likely to have occurred during early tumor growth before analyses at 4 weeks.
To examine tumor angiogenesis, major tumor vessels were identified by the presence of von Willebrand factor (vWF), a coagulation factor that is synthesized by endothelial cells and secreted into the subendothelial matrix. Interestingly, cortactin expression correlates with vessel area/tumor area resulting in a 3-fold increase in SCC61 Cort-OE (LZRS-FL) tumors and a ∼4-fold decrease in SCC61 Cort-KD tumors compared with control tumors (Fig 4A,B). In FaDu tumors, there is no correlation between cortactin expression and the presence of vWF staining. However, there is only minimal and peripheral vWF staining (data not shown), suggesting that FaDu tumors may induce a different type of vasculature that is not vWF-positive. We therefore stained FaDu tumor sections with an antibody recognizing CD31, an endothelial cell-specific marker that is frequently used to identify small vessels and capillaries. In this case, FaDu tumors derived from Cort-KD cells have a greatly decreased density of CD31-positive structures compared with control tumors, consistent with an important role for cortactin in promoting tumor angiogenesis (Fig 4C,D).
Figure 4. Cortactin promotes tumor vascularization.
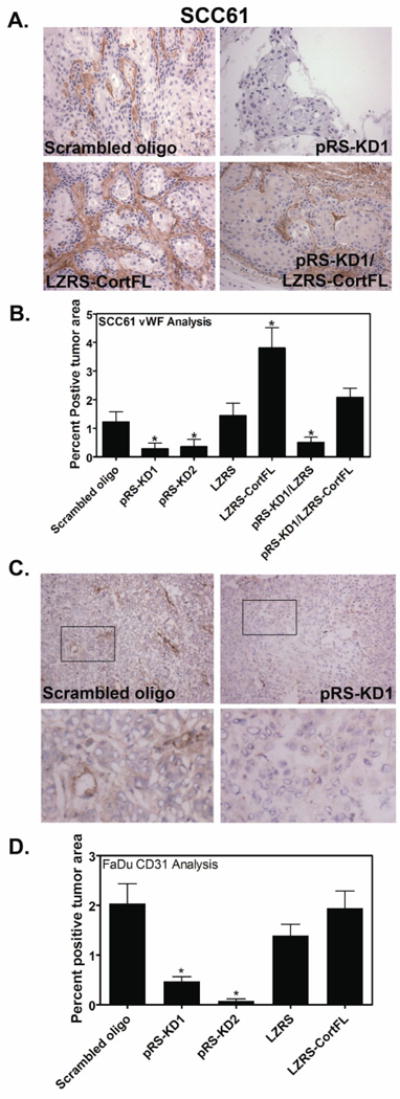
Tumor vascularization was assessed by staining with an antibody against for von Willebrand Factor (vWF), a coagulation factor that underlies endothelial cells in large vessels or with an antibody recognizing the endothelial cell marker CD31 to identify small vessels. Cortactin expression strongly correlates with the presence of large vessels in SCC61 cells (A and B) and small vessels in FaDu cells (C and D). A. Representative images of SCC61-derived tumors stained for von Willebrand Factor (vWF). Positive staining is brown and all nuclei are counterstained light blue. 20× images are shown. Scale bar = 100 μm. B. Quantification of vWF stained tumors for all cell lines is shown as percent positive tumor area as determined by color thresholding in Metamorph. Data are represented as mean ± SEM. C. Representative images of FaDu-derived tumors stained for CD31. Positive staining is brown and all nuclei are counterstained light blue. 20× images are shown. Scale bar = 100 μm. D. Quantification of CD31-stained tumors for all cell lines is shown as percent positive tumor area as determined by color thresholding in Metamorph. Data are represented as mean ± SEM. Asterisks indicate p<0.05 compared to scrambled cortactin oligo control.
Cortactin expression regulates growth in growth factor-deprived, ECM-deprived, or space-constrained environments
Immunohistochemical staining of tumors demonstrated a close correlation between cortactin expression levels and the proliferation of tumor cells in vivo. Since proliferative index within the xenograft tumor could be an indirect effect related to altered vascularization, the effect of cortactin on intrinsic growth rates of HNSCC cells in vitro was tested. Cortactin-manipulated SCC61 and FaDu cells were cultured in complete growth medium and counted each day for five days. In the presence of serum, cortactin expression does not alter cell counts for either SCC61 or FaDu (Fig 5A,B). However, in the absence of serum, overexpression of cortactin in SCC61 and FaDu cells results in increased cell numbers apparent within 3 days of serum removal compared to both control and cortactin-KD cells (Fig 5C, D). In contrast, cell numbers in serum-free culture are decreased in cortactin-KD cells relative to both control cells and cells overexpressing cortactin. Remarkably, decreased cortactin expression resulted in diminished absolute cell numbers over the 6 day time course of the experiment, suggesting that cortactin may be essential for survival as well as proliferation in the absence of exogenously provided growth factors.
Figure 5. Cortactin promotes serum-independent and anchorage-independent growth.
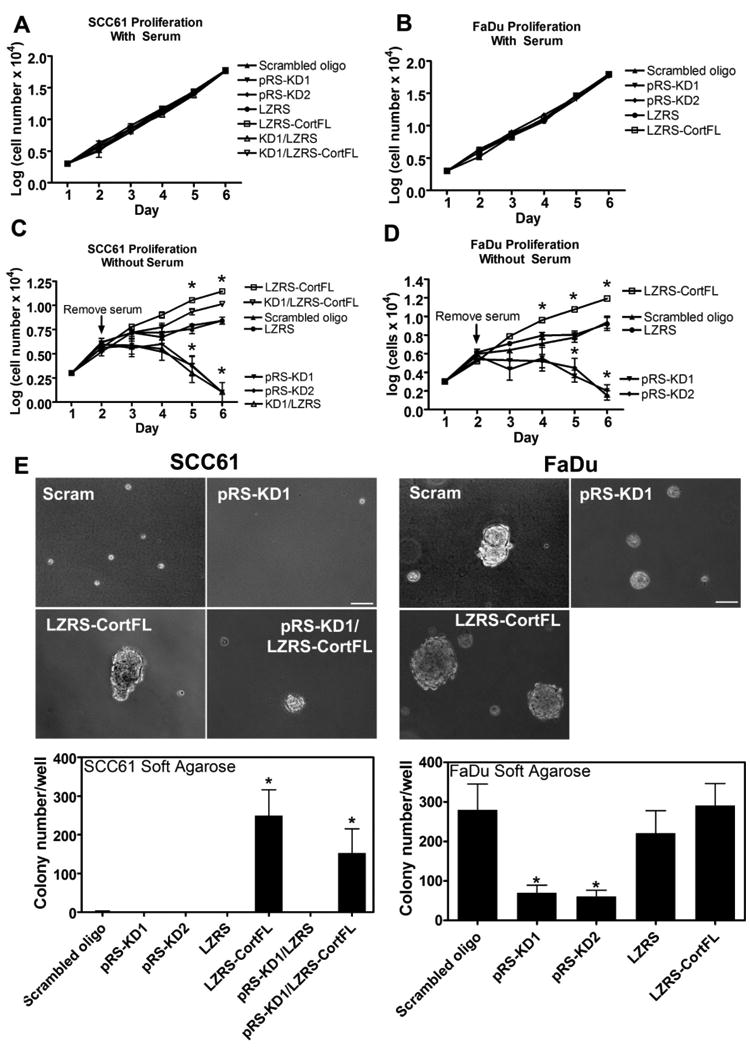
Culture of cortactin-manipulated SCC61 and FaDu cells demonstrates that cortactin has no effect on the growth of these cells in their full growth media. However, removal of serum leads to exquisite sensitivity of cell growth to the level of cortactin expression. SCC61 (A and C) or FaDu (B and D) cells were cultured in the presence (A and B) or absence (C and D) of 20% serum in DMEM in triplicate wells of a 24-well plate before trypsinization and counting on the indicated days. Note that for C and D serum was removed and cells were washed into DMEM on day 2. Shown is the combined data from three independent experiments. Each cortactin-manipulated cell line is indicated in the legend to the right of each graph. Data are represented as mean ± SEM of the log of the cell number. E. To test the role of cortactin in anchorage-independent growth, cortactin-manipulated SCC61 and FaDu cells were cultured for four weeks in 0.35% (soft) agarose in full growth media. Upper panel: Representative images from 4-week cultures. Note that for the less aggressive SCC61 cells, control cells did not form colonies but persisted as single cells. Cort-KD cells (pRS-KD1) were mostly absent from the cultures, suggesting apoptotic death. Cort-OE (LZRS-CortFL) and KD/rescue cells (pRS-KD1/LZRS-CortFL) both formed colonies. For the FaDu cells, all cell lines formed colonies but the size and number correlates with cortactin expressin levels. Scale bar = 100 μm. Graphs: Quantitation of colony number per well. Colonies were defined as cellular structures that were ≥100 μm in diameter. Data are represented as mean ± SEM and are from ≥3 independent trials for each cell line. Asterisks indicate p<0.05 compared to scrambled cortactin oligo control.
Like serum-independence, the ability of cells to grow in an anchorage-independent manner (without ECM) is a hallmark of malignancy (Hanahan & Weinberg, 2000). The role of cortactin in anchorage independence was tested by culturing cortactin-manipulated SCC61 and FaDu cells embedded in soft agarose with full growth media. Colony size and number were manually counted using phase contrast microscopy. For the less aggressive non-11q13-amplified SCC61 cells, only cortactin-overexpressing cells (LZRS-CortFL and pRS-KD1/LZRS-CortFL rescue) form colonies (Fig 5). In contrast, by the end of the 4-week incubation time, control cells are only present as single cells and very few cortactin-KD cells can be observed (Fig 5). Regardless of cortactin status, FaDu cells are able to form colonies; however, cortactin-KD cells form smaller and fewer colonies compared to control cells (Fig 5).
We also examined whether cortactin affects the growth or morphology of colonies in 3-dimensional Matrigel culture, since aberrant behavior in this environment is frequently associated with transformation and/or tumor progression (Debnath & Brugge, 2005). HNSCC cells were cultured for 16 days in complete medium either in a “fluid” environment in which the cells were grown on top of a bed of Matrigel or in a “solid” environment fully embedded in a final concentration of 90% Matrigel. Interestingly, there is no effect of cortactin on growth in the “fluid” Matrigel environment for either cell line (Supp Fig 3). However, cortactin-KD profoundly affects the size of colonies grown in the space-constrained embedded Matrigel environment (Fig 6). As with the tumor studies, overexpression of cortactin only slightly increases the size of FaDu colonies but significantly increases the size of SCC61 Cort-overexpressing colonies at days 8-16 (Fig 6B). In addition, Cort-overexpressing SCC61 colonies have aberrant morphologies compared with controls (Fig 6A).
Figure 6. Cortactin promotes growth in a “solid” 3D Matrigel culture environment.
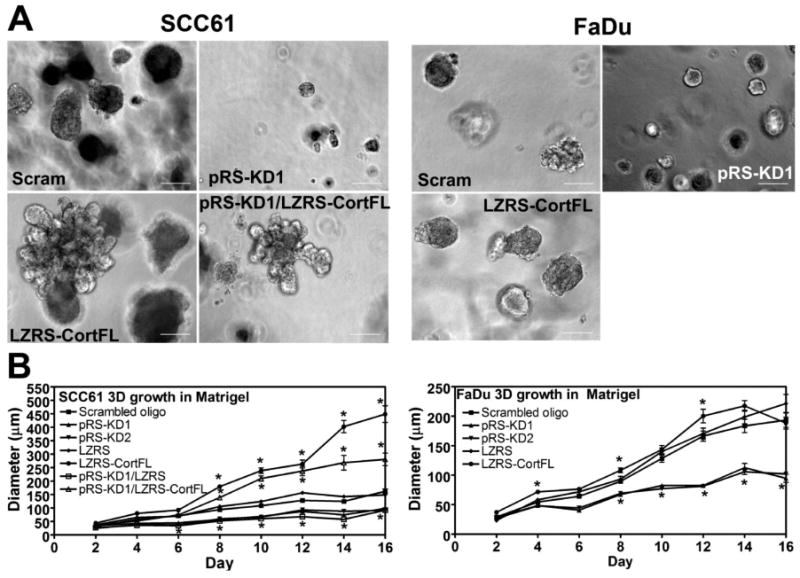
To test the role of cortactin in a space-constrained environment, SCC61 and FaDu cortactin-manipulated cell lines were cultured for 16 days fully embedded in 90% Matrigel. Duplicate wells were used for each cell line and the experiment was performed twice. Cells were imaged every two days. For each, eight randomly chosen 10× fields were imaged and the diameter of the “in-focus” structures were measured using Metamorph software. A. Representative images from Day 12. Scale bar = 100 μm. B. Combined data from two independent experiments for SCC61 and FaDu cells. Note that cortactin expression (both increased and decreased) correlates with the size of colonies growing in this “solid” embedded Matrigel environment. By contrast, cortactin levels do not affect the size of cells grown in a “fluid” Matrigel environment (Supp Fig 2), suggesting that the key factor may be the ability of cortactin to remodel ECM in the solid environment. Data are represented as mean ± SEM. Asterisks indicate p<0.05 compared with scrambled oligo control.
Autocrine secretion of growth-promoting factors is controlled by cortactin
Our in vitro analyses indicate that cortactin affects at least three separate properties that relate to tumor growth: serum- and anchorage-independence, and the ability to grow in a space-constrained environment (Figs 5,6). In addition, cortactin affects tumor invasiveness (Supp Fig 2) and the ability of tumors to recruit vasculature (Fig 4). We hypothesized that a single cellular function, regulated by cortactin, might account for the apparent overall change in tumor cell aggressiveness. Based on our recent discovery that cortactin is essential for secretion of both matrix metalloproteinases (MMPs) and an unrelated protein, ApoA1, in HNSCC cells (Clark et al., 2007), it seems likely that cortactin may promote the secretion of additional tumor regulatory factors such as growth factors, extracellular matrix molecules, or angiogenic factors. Such a general alteration in autocrine secretion might lead to a “tuning” of multiple phenotypes, similar to the general effect on tumor aggressiveness that we observe with cortactin expression. To test this putative mechanism, we performed coculture experiments in which SCC61 or FaDu cortactin-KD cells were cultured in serum-free media in the presence of Transwell inserts containing scrambled control, cort-KD (pRS-KD1), cort-OE (LZRS-CortFL), or cort-KD/Rescue (pRS-KD1/LZRS-CortFL) cells or media alone. The Transwell insert filters have 0.4 μm pores; thus only secreted factors can pass through the pores. We find that inserts containing cort-KD cells are not able to increase serum-independent growth of cort-KD cells in the wells above that observed with serum-free medium alone. However, inserts with cortactin-expressing cells do increase the growth of cort-KD cells in all cases (Fig 7). Interestingly, the level of cortactin expressed by insert cells affects the growth of cort-KD cells in the wells. Thus, cortactin-OE SCC61 cells (LZRS-CortFL) induce a larger increase in the cell numbers of cort-KD cells than do scrambled control or cort-KD/Rescue SCC61 cells (2.5-fold increase for LZRS-CortFL compared with a 1.7-fold increase for scrambled oligo or cort-KD/rescue cells, Fig 7A). Similar results were found with FaDu cells, in that scrambled oligo- and cort-OE-expressing cells express similar levels of cortactin protein (Fig 2A) and increase cort-KD growth under serum-free conditions to a similar extent (∼1.5-fold, Fig 7B). The sensitivity of secreted factors to the cortactin expression level is consistent with our previous finding that the level of cortactin correlates with the level of secreted MMPs (Clark & Weaver, 2008; Clark et al., 2007). Because those studies were only performed with SCC61 cells, we tested whether cortactin also affects trafficking of MMPs in FaDu cells (Supp Fig 4). Consistent with a defect in membrane trafficking, Cort-KD FaDu and SCC61 cells have a defect in MT1-MMP surface localization that cannot be rescued by overexpression of MT1-MMP-GFP (Supp Fig 4). Unlike SCC61 cells (Clark & Weaver, 2008; Clark et al., 2007), FaDu cells express extremely low levels of MMP2 and MMP9 (data not shown). Thus, it is unlikely that the growth-promoting factor is MMP2 or MMP9 but rather another diffusible growth-promoting factor.
Figure 7. Rescue of Cort-KD cell growth under serum-free conditions by coculture with cortactin-expressing cells.
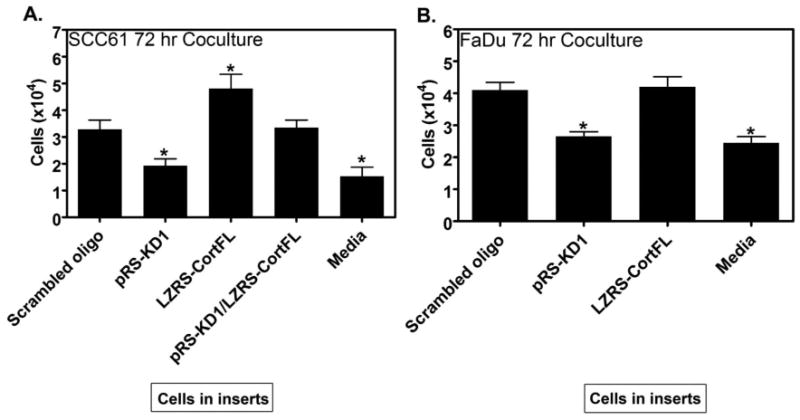
To test whether autocrine secretion can be connected with cortactin-regulated serum-independent growth, cortactin-KD cells were cultured in serum-free media in 12 well plates in the presence of 0.4 μm pore size Transwell inserts containing either scrambled control, pRS-KD1, LZRS-CortFL, pRS-KD1/LZRS-FL (SCC61 only) cells or serum-free media alone (“media”) for 72 h. Quantification of (A) SCC61 or (B) FaDu cort-KD cells is plotted. Note that inserts containing Cort-KD (pRS-KD1) cells cannot rescue the growth of Cort-KD cells growing in the wells below above the level of media alone. By contrast, cortactin-expressing cells grown transwell inserts rescue cort-KD cell growth in wells consistent with the level of cortactin expression (compare LZRS-CortFL SCC61 cells to Scrambled oligo SCC61 cells in inserts in their ability to rescue the growth of Cort-KD cells). Bars represent the combined mean ± SEM from three independent experiments performed in triplicate. Asterisks indicate p<0.05 compared with scrambled oligo control.
Discussion
In HNSCC, 11q13 amplification occurs frequently and has been correlated in numerous studies with poor patient prognosis (Myllykangas et al., 2007; Weaver, 2008). We examined the role of cortactin, a gene in the 11q13 amplicon, in HNSCC aggressiveness. Remarkably, cortactin affects the overall aggressiveness of HNSCC tumors, regardless of 11q13 amplification status, and promotes tumor growth, survival, vascularization, and invasion. In vitro, multiple phenotypic traits associated with tumor aggressiveness are regulated by cortactin, including serum-independent and anchorage-independent growth, as well as invasiveness. Cortactin appears to act as a rheostat, such that the level of cortactin tunes aggressive behavior. Defects in serum-independent growth of cortactin knockdown cells can be rescued by coculture with cortactin-expressing cells, indicating that cortactin controls autocrine secretion of growth-promoting substances.
A few previous studies have examined the role of cortactin in tumorigenesis and progression. Transgenic overexpression of cortactin in the mammary gland under the control of the MMTV promoter did not produce any additional tumors over the spontaneous background level, suggesting that cortactin is unlikely to function as a tumor initiator (van Rossum et al., 2006). More consistent with a role in tumor progression, experimental metastasis studies using tail vein or intracardiac injections of esophageal squamous and breast carcinoma carcinoma cells have found that cortactin promotes extravasation and/or colonization of distant organs (Li et al., 2001; Luo et al., 2006). In addition, cortactin promotes the intrahepatic metastasis of orthotopically injected hepatocellular carcinoma cells (Chuma et al., 2004). Although the breast and hepatocellular studies found no effect of cortactin expression on tumor size (Chuma et al., 2004; Li et al., 2001), subcutaneous growth of esophageal squamous carcinoma tumors was dependent on cortactin expression (Luo et al., 2006). Our results agree with the latter study in that cortactin expression controls the size of tumors grown from three different HNSCC cell lines. We also found that cortactin affects in vivo characteristics associated with tumor growth, including tumor vascularization, proliferation, and apoptosis. Future studies will be required to determine whether cortactin uniquely affects the growth of squamous carcinomas or whether the liver and breast microenvironments differentially regulate tumor growth compared with the subcutaneous or tracheal environments.
For our tumor studies, we used a semiorthotopic model in which cells are implanted in a rat trachea to mimic the physiologic environment of oral squamous cells (Shores & Yarbrough, 1998). Interestingly, we saw similar effects on tumor size to what was observed for subcutaneous growth of esophageal squamous cancer cells (Luo et al., 2006). However, it seems likely that the microenvironmental conditions of our assay will affect the outcomes. For example, the physical constraints of the tracheal ring could lead to alterations in growth patterns, infiltration and angiogenesis compared with other tumor models, such as subcutaneous growth, and spontaneous or experimental metastasis models. It will be important in the future to test our findings in additional tumor models to address the role of HNSCC cortactin in mediating local tissue invasion, regional and distant metastatic spread.
In vitro, we found that the level of cortactin affects many cellular traits that might promote tumor aggressiveness. The coculture experiments, in which cortactin-expressing insert cells rescued the defect in serum-independent growth of cortactin-KD cells, indicate that cortactin regulates the secretion of growth- and/or survival-promoting factors. In a previous study, we found that cortactin is essential for secretion of multiple factors, including MMPs and an unrelated protein, apolipoprotein A1 from HNSCC cells (Clark et al., 2007). Another study found that a dominant negative cortactin protein affects trafficking of the model proteins VSV-G and mannose-6-phosphate from the trans-Golgi apparatus (Cao et al., 2005). Together, these data suggest that cortactin may generally affect autocrine secretion. We speculate that autocrine secretion of multiple factors, such as growth and angiogenic factors, proteases, and ECM molecules, could account for the effect of cortactin expression on diverse cellular traits associated with tumor aggressiveness.
Autocrine secretion in cancer plays an important role in many aspects of tumor progression. For example, recent studies have shown that diffusible substances can result in a variety of outcomes, such as preparation of metastatic niches, recruitment of mesenchymal stem cells, angiogenesis, and tumor progression (Gabrilove, 2001; Hiratsuka et al., 2006; Karnoub et al., 2007; Ostman, 2004). Although serum-independence and other hallmarks of cancer are classically thought to occur through mechanisms such as tyrosine kinase mutations leading to aberrant signaling, these phenotypes are likely to be tuned by autocrine secretion (Ethier et al., 1996; Ethier et al., 1991). Indeed, even in normal cells, growth factor-receptor stimulation is thought to occur primarily through autocrine secretion (Bates et al., 1990; Markowitz et al., 1990; Tsao et al., 1996) an effect that is masked in culture by the abundance of growth factors present in serum. Consistent with that idea, there was no effect of cortactin expression on in vitro proliferation until removal of serum from the media.
Our results indicate that the level of cortactin affects tumor aggressiveness in both 11q13-amplified and non-amplified HNSCC tumors, suggesting that overexpression of cortactin generally promotes tumor progression. This finding is consistent with the finding that cortactin is sometimes overexpressed in cancer independently of 11q13 amplification (Greer et al., 2007; Yuan et al., 2003). Multiple phenotypic traits associated with cancer are altered by the level of cortactin expression, including serum-independence, anchorage-independence, and angiogenesis. We speculate that autocrine secretion is likely to account for many, if not all of these phenotypes. Future work should address the mechanism(s) by which cortactin regulates autocrine secretion and test the connection to diverse aggressive tumor phenotypic traits.
Materials and Methods
Antibodies and Chemicals
The C-90 antibody was previously described (Bryce et al., 2005). Commercial antibodies: anti-β-actin (Sigma (AC-74)), Ku70 (Abcam), and pan-Cytokeratin (Dako Cytomation). H&E and immunohistochemical staining were performed at the Vanderbilt Pathology and Immunohistochemistry (IHC) cores. Antibodies to von Willebrand factor, Ki67, BrdU, caspase 3, and CD31 were provided by IHC core.
Cell Culture
The SCC61, SCC25, and FaDu cell lines have been described (Rangan, 1972; Somers et al., 1990; Weichselbaum et al., 1986). Cells were maintained in DMEM/F12 with 20% FBS with (SCC61, FaDu) or without (SCC25) 0.4 μg/ml hydrocortisone. Construction of shRNA and cDNA expressing cell lines have been described (Bryce et al., 2005; Clark et al., 2007).
Microscopy
Phase contrast images were captured using a Nikon Eclipse TE2000-E microscope equipped with a 10× Plan Fluor 0.3NA objective. Paraffin-embedded samples were imaged with a Zeiss Axioplan2 microscope using either 2.5×/0.075NA, 10×/0.3NA, or 20×/0.5NA Plan-Neofluar objectives and an Olympus QColor3 camera. Image analysis was performed using Metamorph.
Semi-orthotopic Rat Trachea Model
The rat tracheal model was described (Shores & Yarbrough, 1998). HNSCC cells (2 × 106) were suspended in 20-40μl of complete media inserted into the tracheal lumen through a small incision that was subsequently sutured shut. Tracheas were then inserted into subcutaneous pockets along the flanks of anesthetized mice (1 trachea per flank) and the skin closed. Four weeks later, mice were sacrificed and tracheas were removed and processed.
Soft Agarose Assay
A 1.5 ml bottom layer of 0.7% low gelling temperature (LGT) agarose in 1× DMEM 10% FBS was added to each well of a 6-well plate. Once solidified, 5 × 103 cells/well were mixed with 0.7% agarose and 2× DMEM 20% FBS to yield 0.35% LGT, 1× DMEM 10% FBS and added on top. Once solidified, 2 ml of growth media was added. The media was changed twice per week for four weeks and the wells were imaged using a 10× objective.
Coculture Assays
Target cells (SCC61 or FaDu cortactin-KD cells) were plated at a density of 1.5 × 104 in a 12-well plate, and the medium-conditioning cells were plated at a density of 5 × 103 in the transwell inserts (Falcon 0.4 μm pore, 12-well format) in another 12-well plate. After 4 hours, the inserts were transferred to the plate containing the target cells and incubated overnight. The following day, the target cells and inserts were washed 2× with PBS then incubated together in 1 ml of DMEM for 72 hours before trypsinization and counting.
3-D Matrigel Culture and analysis
3D cell culture was performed as previously published (Debnath et al., 2003) with slight modifications. Eight-well chamber, tissue culture treated glass slides (BD Falcon CultureSlides) were coated with 45 μl of Matrigel per well. For fluid culture, 6,000 cells were plated in complete medium + 2% Matrigel. For embedded 3D culture, 4,000 cells were plated in 10% complete medium, 90% Matrigel. After 30 minutes, 200 μl of complete medium was added to each well. Medium was replaced every 4 days (+ 2% Matrigel for fluid culture; no Matrigel for embedded culture). Phase contrast images were acquired every 2 days.
Statistical Analyses
Excel was used to calculate means and standard error. Prism was used to perform statistical tests of significance. An unpaired Student's t test was used to compare two groups. Multiple comparisons were performed by ANOVA.
Supplementary Material
Acknowledgments
We thank Julie Maier, Izu Iwueke, and the IHC core for technical support, and Drs. Ambra Pozzi and Vito Quaranta for advice. Funding for this work was provided by 1R21DE018244 to AMW. WGY, BB, ASW, and AK are partially supported through a grant from the Robert J Kleberg, Jr. and Helen C. Kleberg Foundation. An endowment supporting the Barry Baker Laboratory for Head and Neck Oncology as well as the Robert J Kleberg, Jr. and Helen C. Kleberg Foundation and an Ingram Professorship to WGY also provided support for this project.
References
- Akervall JA, Jin Y, Wennerberg JP, Zatterstrom UK, Kjellen E, Mertens F, et al. Chromosomal abnormalities involving 11q13 are associated with poor prognosis in patients with squamous cell carcinoma of the head and neck. Cancer. 1995;76:853–9. doi: 10.1002/1097-0142(19950901)76:5<853::aid-cncr2820760520>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Bates SE, Valverius EM, Ennis BW, Bronzert DA, Sheridan JP, Stampfer MR, et al. Expression of the transforming growth factor-alpha/epidermal growth factor receptor pathway in normal human breast epithelial cells. Endocrinology. 1990;126:596–607. doi: 10.1210/endo-126-1-596. [DOI] [PubMed] [Google Scholar]
- Bryce NS, Clark ES, Leysath JL, Currie JD, Webb DJ, Weaver AM. Cortactin promotes cell motility by enhancing lamellipodial persistence. Curr Biol. 2005;15:1276–85. doi: 10.1016/j.cub.2005.06.043. [DOI] [PubMed] [Google Scholar]
- Cao H, Orth JD, Chen J, Weller SG, Heuser JE, McNiven MA. Cortactin is a component of clathrin-coated pits and participates in receptor-mediated endocytosis. Mol Cell Biol. 2003;23:2162–70. doi: 10.1128/MCB.23.6.2162-2170.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Weller S, Orth JD, Chen J, Huang B, Chen JL, et al. Actin and Arf1-dependent recruitment of a cortactin-dynamin complex to the Golgi regulates post-Golgi transport. Nat Cell Biol. 2005;7:483–92. doi: 10.1038/ncb1246. [DOI] [PubMed] [Google Scholar]
- Chuma M, Sakamoto M, Yasuda J, Fujii G, Nakanishi K, Tsuchiya A, et al. Overexpression of cortactin is involved in motility and metastasis of hepatocellular carcinoma. J Hepatol. 2004;41:629–36. doi: 10.1016/j.jhep.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Clark ES, Weaver AM. A new role for cortactin in invadopodia: Regulation of protease secretion. Eur J Cell Biol. 2008 doi: 10.1016/j.ejcb.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark ES, Whigham AS, Yarbrough WG, Weaver AM. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res. 2007;67:4227–35. doi: 10.1158/0008-5472.CAN-06-3928. [DOI] [PubMed] [Google Scholar]
- Debnath J, Brugge JS. Modelling glandular epithelial cancers in three-dimensional cultures. Nat Rev Cancer. 2005;5:675–88. doi: 10.1038/nrc1695. [DOI] [PubMed] [Google Scholar]
- Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–68. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- Ethier SP, Langton BC, Dilts CA. Growth factor-independent proliferation of rat mammary carcinoma cells by autocrine secretion of neu-differentiation factor/heregulin and transforming growth factor-alpha. Mol Carcinog. 1996;15:134–43. doi: 10.1002/(SICI)1098-2744(199602)15:2<134::AID-MC6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Ethier SP, Moorthy R, Dilts CA. Secretion of an epidermal growth factor-like growth factor by epidermal growth factor-independent rat mammary carcinoma cells. Cell Growth Differ. 1991;2:593–602. [PubMed] [Google Scholar]
- Freier K, Sticht C, Hofele C, Flechtenmacher C, Stange D, Puccio L, et al. Recurrent coamplification of cytoskeleton-associated genes EMS1 and SHANK2 with CCND1 in oral squamous cell carcinoma. Genes Chromosomes Cancer. 2006;45:118–25. doi: 10.1002/gcc.20270. [DOI] [PubMed] [Google Scholar]
- Gabrilove JL. Angiogenic growth factors: autocrine and paracrine regulation of survival in hematologic malignancies. Oncologist. 2001;6(Suppl 5):4–7. doi: 10.1634/theoncologist.6-suppl_5-4. [DOI] [PubMed] [Google Scholar]
- Gibcus JH, Mastik MF, Menkema L, de Bock GH, Kluin PM, Schuuring E, et al. Cortactin expression predicts poor survival in laryngeal carcinoma. Br J Cancer. 2008;98:950–5. doi: 10.1038/sj.bjc.6604246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibcus JH, Menkema L, Mastik MF, Hermsen MA, de Bock GH, van Velthuysen ML, et al. Amplicon mapping and expression profiling identify the Fas-associated death domain gene as a new driver in the 11q13.3 amplicon in laryngeal/pharyngeal cancer. Clin Cancer Res. 2007;13:6257–66. doi: 10.1158/1078-0432.CCR-07-1247. [DOI] [PubMed] [Google Scholar]
- Greer RO, Jr, Said S, Shroyer KR, Marileila VG, Weed SA. Overexpression of cyclin D1 and cortactin is primarily independent of gene amplification in salivary gland adenoid cystic carcinoma. Oral Oncol. 2007;43:735–41. doi: 10.1016/j.oraloncology.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8:1369–75. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- Hofman P, Butori C, Havet K, Hofman V, Selva E, Guevara N, et al. Prognostic significance of cortactin levels in head and neck squamous cell carcinoma: comparison with epidermal growth factor receptor status. Br J Cancer. 2008;98:956–964. doi: 10.1038/sj.bjc.6604245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui R, Ball JR, Macmillan RD, Kenny FS, Prall OW, Campbell DH, et al. EMS1 gene expression in primary breast cancer: relationship to cyclin D1 and oestrogen receptor expression and patient survival. Oncogene. 1998;17:1053–9. doi: 10.1038/sj.onc.1202023. [DOI] [PubMed] [Google Scholar]
- Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–63. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- Katsube T, Togashi S, Hashimoto N, Ogiu T, Tsuji H. Filamentous actin binding ability of cortactin isoforms is responsible for their cell-cell junctional localization in epithelial cells. Arch Biochem Biophys. 2004;427:79–90. doi: 10.1016/j.abb.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Li Y, Tondravi M, Liu J, Smith E, Haudenschild CC, Kaczmarek M, et al. Cortactin potentiates bone metastasis of breast cancer cells. Cancer Res. 2001;61:6906–11. [PubMed] [Google Scholar]
- Luo ML, Shen XM, Zhang Y, Wei F, Xu X, Cai Y, et al. Amplification and overexpression of CTTN (EMS1) contribute to the metastasis of esophageal squamous cell carcinoma by promoting cell migration and anoikis resistance. Cancer Res. 2006;66:11690–9. doi: 10.1158/0008-5472.CAN-06-1484. [DOI] [PubMed] [Google Scholar]
- Markowitz SD, Molkentin K, Gerbic C, Jackson J, Stellato T, Willson JK. Growth stimulation by coexpression of transforming growth factor-alpha and epidermal growth factor-receptor in normal and adenomatous human colon epithelium. J Clin Invest. 1990;86:356–62. doi: 10.1172/JCI114709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith SD, Levine PA, Burns JA, Gaffey MJ, Boyd JC, Weiss LM, et al. Chromosome 11q13 amplification in head and neck squamous cell carcinoma. Association with poor prognosis. Arch Otolaryngol Head Neck Surg. 1995;121:790–4. doi: 10.1001/archotol.1995.01890070076016. [DOI] [PubMed] [Google Scholar]
- Merrifield CJ, Perrais D, Zenisek D. Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells. Cell. 2005;121:593–606. doi: 10.1016/j.cell.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Myllykangas S, Bohling T, Knuutila S. Specificity, selection and significance of gene amplifications in cancer. Semin Cancer Biol. 2007;17:42–55. doi: 10.1016/j.semcancer.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Ohoka Y, Takai Y. Isolation and characterization of cortactin isoforms and a novel cortactin-binding protein, CBP90. Genes Cells. 1998;3:603–12. doi: 10.1046/j.1365-2443.1998.00216.x. [DOI] [PubMed] [Google Scholar]
- Ormandy CJ, Musgrove EA, Hui R, Daly RJ, Sutherland RL. Cyclin D1, EMS1 and 11q13 amplification in breast cancer. Breast Cancer Res Treat. 2003;78:323–35. doi: 10.1023/a:1023033708204. [DOI] [PubMed] [Google Scholar]
- Ostman A. PDGF receptors-mediators of autocrine tumor growth and regulators of tumor vasculature and stroma. Cytokine Growth Factor Rev. 2004;15:275–86. doi: 10.1016/j.cytogfr.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Rangan SR. A new human cell line (FaDu) from a hypopharyngeal carcinoma. Cancer. 1972;29:117–21. doi: 10.1002/1097-0142(197201)29:1<117::aid-cncr2820290119>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Rodrigo JP, Garcia LA, Ramos S, Lazo PS, Suarez C. EMS1 gene amplification correlates with poor prognosis in squamous cell carcinomas of the head and neck. Clin Cancer Res. 2000;6:3177–82. [PubMed] [Google Scholar]
- Schuuring E. The involvement of the chromosome 11q13 region in human malignancies: cyclin D1 and EMS1 are two new candidate oncogenes--a review. Gene. 1995;159:83–96. doi: 10.1016/0378-1119(94)00562-7. [DOI] [PubMed] [Google Scholar]
- Schuuring E, Verhoeven E, Mooi WJ, Michalides RJ. Identification and cloning of two overexpressed genes, U21B31/PRAD1 and EMS1, within the amplified chromosome 11q13 region in human carcinomas. Oncogene. 1992;7:355–61. [PubMed] [Google Scholar]
- Shores CG, Yarbrough WG. Three-dimensional xenograft model of dysplastic human laryngeal mucosa. Laryngoscope. 1998;108:1358–62. doi: 10.1097/00005537-199809000-00019. [DOI] [PubMed] [Google Scholar]
- Somers KD, Cartwright SL, Schechter GL. Amplification of the int-2 gene in human head and neck squamous cell carcinomas. Oncogene. 1990;5:915–20. [PubMed] [Google Scholar]
- Takes RP, Baatenburg de Jong RJ, Schuuring E, Hermans J, Vis AA, Litvinov SV, et al. Markers for assessment of nodal metastasis in laryngeal carcinoma. Arch Otolaryngol Head Neck Surg. 1997;123:412–9. doi: 10.1001/archotol.1997.01900040048008. [DOI] [PubMed] [Google Scholar]
- Tsao MS, Zhu H, Viallet J. Autocrine growth loop of the epidermal growth factor receptor in normal and immortalized human bronchial epithelial cells. Exp Cell Res. 1996;223:268–73. doi: 10.1006/excr.1996.0081. [DOI] [PubMed] [Google Scholar]
- Uruno T, Liu J, Zhang P, Fan Yx Y, Egile C, Li R, et al. Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nat Cell Biol. 2001;3:259–266. doi: 10.1038/35060051. [DOI] [PubMed] [Google Scholar]
- van Rossum AG, van Bragt MP, Schuuring-Scholtes E, van der Ploeg JC, van Krieken JH, Kluin PM, et al. Transgenic mice with mammary gland targeted expression of human cortactin do not develop (pre-malignant) breast tumors: studies in MMTV-cortactin and MMTV-cortactin/-cyclin D1 bitransgenic mice. BMC Cancer. 2006;6:58. doi: 10.1186/1471-2407-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver AM. Cortactin in tumor invasiveness. Cancer Letters. 2008 doi: 10.1016/j.canlet.2008.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver AM, Karginov AV, Kinley AW, Weed SA, Li Y, Parsons JT, et al. Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Curr Biol. 2001;11:370–4. doi: 10.1016/s0960-9822(01)00098-7. [DOI] [PubMed] [Google Scholar]
- Weichselbaum RR, Dahlberg W, Beckett M, Karrison T, Miller D, Clark J, et al. Radiation-resistant and repair-proficient human tumor cells may be associated with radiotherapy failure in head- and neck-cancer patients. Proc Natl Acad Sci U S A. 1986;83:2684–8. doi: 10.1073/pnas.83.8.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ME, Gaffey MJ, Weiss LM, Wilczynski SP, Schuuring E, Levine PA. Chromosome 11Q13 amplification in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 1993;119:1238–43. doi: 10.1001/archotol.1993.01880230084013. [DOI] [PubMed] [Google Scholar]
- Wu H, Parsons JT. Cortactin, an 80/85-kilodalton pp60src substrate, is a filamentous actin-binding protein enriched in the cell cortex. J Cell Biol. 1993;120:1417–26. doi: 10.1083/jcb.120.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Reynolds AB, Kanner SB, Vines RR, Parsons JT. Identification and characterization of a novel cytoskeleton-associated pp60src substrate. Mol Cell Biol. 1991;11:5113–24. doi: 10.1128/mcb.11.10.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan BZ, Zhou X, Zimonjic DB, Durkin ME, Popescu NC. Amplification and overexpression of the EMS 1 oncogene, a possible prognostic marker, in human hepatocellular carcinoma. J Mol Diagn. 2003;5:48–53. doi: 10.1016/S1525-1578(10)60451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Zhou K, Hao JJ, Liu J, Smith N, Zhan X. Regulation of cortactin/dynamin interaction by actin polymerization during the fission of clathrin-coated pits. J Cell Sci. 2005;118:807–17. doi: 10.1242/jcs.01668. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


