Abstract
Aims
Highly proliferative, CD34+/CD45+ fibroblasts derived from monocytic, blood-borne precursor cells play a critical role in the development of fibrosis in a murine ischaemic/reperfusion cardiomyopathy (I/RC) model. The differentiation of human monocytes into fibroblasts in vitro occurs after transendothelial migration (TEM) induced by monocyte chemoattractant protein 1 (MCP-1). Because Rho-associated kinase-1 (ROCK-1) has been implicated in fibrosis and leukocyte TEM, we investigated its involvement in I/RC.
Methods and results
We subjected mice with genetic deletion of ROCK-1 to I/RC. We found that ROCK-1−/− mice did not develop the fibrosis and cardiac dysfunction characteristic for I/RC: compared with wild-type, ROCK-1−/− hearts showed markedly lower numbers of I/RC-induced α-smooth muscle actin+ fibroblasts and CD34+/CD45+ fibroblast precursors. Isolated cardiac fibroblasts from ROCK-1−/− mice undergoing I/RC were large and slowly proliferating, similar to fibroblasts isolated from sham-treated hearts. We also performed in vitro assays in which human peripheral blood mononuclear cells (PBMC) migrated through endothelial cells in response to MCP-1. Prior to migration, PBMC were incubated with ROCK-1-targeting small interfering RNA to silence ROCK-1 expression. We found that an 80% reduction of ROCK-1 protein did not inhibit TEM, but significantly reduced the amount of mononuclear cells that differentiated into fibroblasts by >20-fold.
Conclusion
Our data implicate an important role for ROCK-1 in the differentiation, but not in the TEM of monocytes that mature into cardiac fibroblasts. These cells mediate non-adaptive fibrosis.
KEYWORDS: Cardiac fibroblasts, Monocytes, Rho-associated kinase-1, Endothelial transmigration, Fibrosis
1. Introduction
Non-adaptive or augmented interstitial fibrosis in the heart invariably accompanies ventricular remodelling and failure and is therefore considered an important pathophysiological factor in progressive cardiac dysfunction.1 The development of fibrotic cardiomyopathies is often associated with inflammation.2 However, although several inflammatory cytokines and chemokines have been implicated in adverse remodelling, the cellular and molecular mechanisms by which inflammation contributes to fibrosis are still unclear.
In our previous work, we established a murine, closed-chest myocardial ischaemia and reperfusion model that consisted of multiple 15 min occlusions separated by 24 h reperfusion periods [ischaemic/reperfusion cardiomyopathy (I/RC)].3 Characteristic for this model was the upregulation of monocyte chemoattractant protein 1 (MCP-1), the development of interstitial fibrosis, and myocardial dysfunction in the absence of myocyte death. Genetic deletion of MCP-1 inhibited the development of cardiac fibrosis and preserved cardiac dysfunction after I/RC.4 We further showed that I/RC resulted in the uptake of a blood-borne, CD34+/CD45+, monocytic fibroblast precursor population in the heart.5 Daily administration of serum amyloid P (SAP) to mice undergoing I/RC reduced the appearance of this fibroblast population, virtually eliminated interstitial fibrosis, and preserved cardiac structure and function.5 Since mice deficient in Fcγ receptor signalling were not protected from I/RC by SAP administration,6 we proposed that the Fcγ activation of circulating precursor cells may represent a molecular mechanism modulating monocyte-to-fibroblast differentiation. This view was further supported by our in vitro findings using endothelial transmigration assays in response to MCP-1. We showed that human monocytes were required to migrate through endothelial cells to mature into fibroblasts, and that SAP had to be present before endothelial transmigration to inhibit this maturation.6
The serine/threonine Rho-associated kinase (ROCK) isoforms 1 and 2 are critical downstream mediators of the small GTP-binding protein RhoA,7 and are believed to mediate the organization of the actin cytoskeleton, stress fibre formation, cell adhesion, and cell motility.7–9 Many studies have implicated the RhoA/ROCK pathway in the pathophysiology of diverse cardiovascular diseases,10–12 including myocardial hypertrophy,13,14 hypertension,15 atherosclerosis,16,17 and ischaemia/reperfusion injury.18–20 Since mice deficient in ROCK-2 die embryonically,21 while mice deficient in ROCK-1 (ROCK-1−/−) survive,22,23 distinct tissue distribution and downstream targets of each were suggested despite their 92% identical kinase domains.24 Recently, we demonstrated a critical role for ROCK-1 in fibrosis in the heart in response to pressure overload.22 We showed that, after 3 weeks of aortic banding, non-adaptive fibrosis was reduced in ROCK-1−/− mice compared with wild-type (WT) mice, and the induction of profibrotic gene expression, such as collagens and fibrogenic cytokines including transforming growth factor β2, was abrogated in these animals. Another study using haploinsufficient ROCK-1+/− mice described substantially less cardiac fibrosis in hypertensive and infarcted ROCK-1+/− mice when compared with WT mice,19 thus, also concluding that ROCK-1 may be an important mediator of fibrotic heart diseases. Similar studies implicated a role for ROCK-1 in monocyte-mediated neointima formation following vascular injury and atherosclerosis.16,25 In the current study, we provide new evidence for a potential molecular mechanism linking the requirement for ROCK-1 for monocyte-to-fibroblast differentiation to the pathogenesis of I/RC.
2. Methods
2.1. Generation of ROCK-1−/− mice
ROCK-1−/− mice were generated as previously described.22 Due to the low breeding efficiency of homozygous ROCK-1−/− mice, the colony was maintained via intercrossing heterozygous ROCK-1+/− mice (FvB background). The resulting offspring were genotyped by PCR on tail DNA to distinguish heterozygous ROCK-1+/−, homozygous ROCK-1−/−, and WT ROCK-1+/+ mice, but only the latter two were used for this study. The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). All animals were treated in accordance with the guidelines of the Baylor College of Medicine Animal Care and Research Advisory Committee (AN-124).
2.2. Ischaemia/reperfusion cardiomyopathy (I/RC)
Ten- to 12-week-old ROCK-1−/− and WT mice were subjected to closed chest surgery as previously described.3 One week after suture implantation, 15 min occlusions of the left anterior descending artery were performed for the number of indicated days, allowing a 24 h reperfusion period in between. Mice were euthanized 5 h after the last ischaemic episode. Myocardial ROCK-1 and ROCK-2 protein levels were determined after 5-day I/RC by SDS–PAGE as described in Section 2.7.
2.3. Cardiac dysfunction
Echocardiography was performed before the first ischaemic episode and after 7-day I/RC. Data were obtained by 2D-directed M-mode echocardiography; fractional shortening and anterior wall thickening were calculated as previously described.3
2.4. Cardiac fibrosis
After 7-day I/RC, hearts were embedded in paraffin and sectioned as described earlier.3 To measure collagen deposition, sections below the suture were stained with picrosirius red (Poly Scientific). Collagen stained areas in the ischaemic wall and in the non-ischaemic posterior septum (control) were separately calculated as percentages of the total myocardial area using Zeiss IMAGE analysis software (MicroImaging Inc.). Alpha-smooth muscle actin (α-SMA) positive myofibroblast density (antibody from Sigma-Aldrich) was determined as described previously, as was macrophage density (Mac-2+ cells; antibody from Cedarlane).3,5
2.5. Chemokine levels
After 3-day I/RC, levels of chemokine mRNA expression were determined by ribonuclease protection assay (RiboQuant; Pharmingen) as described previously.3
2.6. Identification of fibroblast populations
After 5-day I/RC, cardiac fibroblasts were isolated and cultured as described previously.5 For flow cytometric analysis, 1 × 105 freshly isolated cells were incubated with 50 nM calcein AM (Invitrogen Molecular Probes) to measure cell viability (only live cells metabolize calcein to an intracellular, green-fluorescent permanent cell dye), 0.5 µg PE-conjugated CD34 or biotin-conjugated CD45 antibodies, followed by PE/Cy-5-conjugated streptavidin (all from BD Biosciences). Fluorescence intensities were measured on a Beckman Coulter Epics XL.MCL. Proliferation of cultured cardiac fibroblasts was determined by BrdU incorporation as described earlier.5 To normalize data from different experiments, enhanced proliferation in response to serum was expressed as the fold increase compared with cells maintained in serum-free medium.
2.7. RNA interference
Blood was obtained from human volunteers under a protocol approved by the Institutional Review Board of Baylor College of Medicine (HA-556). The investigation conformed with the principles outlined in the Declaration of Helsinki. Peripheral blood mononuclear cells (PBMC) were isolated as described previously.6
PBMC were transfected with either of the two small interfering RNAs (siRNAs) specifically targeting ROCK-1 (ID#s 680 and 846, Ambion), or with a non-targeting siRNA using Lipofectamine 2000 (Invitrogen) as per the manufacturer's protocol. Briefly, 4 µL Lipofectamine 2000 was complexed with 250 pmoles siRNA for 20 min before adding 2 × 106 PBMC in 2.25 mL serum-free RPMI 1640 (Invitrogen). The cell suspension was incubated at 37°C in Teflon jars to avoid cell adherence. Serial dilutions were made of 250 µL foetal bovine serum (FBS; Hyclone) at 2 h, 1.8 mL RPMI 1640 and 450 µL FBS at 8 h, and 5.5 mL RPMI 1640 supplemented with 10% FBS at 24 h. After 48 h, cell viability was measured by flow cytometry using 50 nM calcein AM on a Beckman Coulter Quanta using Cell Lab Quanta SC MPL software (0.5 µg PE-conjugated CD14 antibody [Beckman Coulter] was used to determine the percentage of CD14+ cells within the PBMC suspension), then cells were used for experiments.
To confirm the efficiency of siRNA knockdown of ROCK-1 protein expression, western blotting was performed. After 72 h, PBMC were lysed in RIPA buffer (Pierce) and proteins isolated. Fifty microgram protein was resolved on a 10% SDS–PAGE, transferred to nitrocellulose membranes, and probed with monoclonal anti-ROCK-1, anti-ROCK-2 (both from BD Biosciences), and anti-GAPDH (Santa Cruz Biotech). Band intensities were evaluated using Scion Image v1.62 (Scion Corp.) and normalized to GAPDH.
2.8. Transendothelial migration (TEM)
Human cardiac microvascular endothelial cells (HCMEC) were obtained from ScienCell. transendothelial migration (TEM) assays were performed exactly as previously described,6 using siRNA-treated PBMC. At days 4 and 11, the number of CellTracker Green+ (Invitrogen), fibroblast-shaped cells, and total cells were counted in the bottom well. Each donor's cells were measured in triplicate. Due to differences in absolute numbers between PBMC donors, the number of cells in the experimental well was divided by the number in the corresponding control well (diluent) to normalize the data.
Alternatively, cells that migrated through HCMEC were collected in a collagen pad as described previously.6 To distinguish monocytes from lymphocytes and residual endothelial cells, cells were analysed by flow cytometry using 0.5 µg FITC-conjugated CD45 and 0.5 µg PE-conjugated CD14 antibody (both from Beckman Coulter) as described earlier.
To determine the chemotactic response of siRNA-treated PBMC to MCP-1, cell migration under agarose in the absence of endothelial cells were performed as previously described.6 The number of cells migrating towards MCP-1 was divided by the number of cells migrating towards diluent (0.1% human serum albumin; Mediatech).
2.9. Immunofluorescence
Staining for type I collagen in cells cultured in vitro after TEM was performed as described previously,6 using an affinity-purified, type I collagen rabbit antibody (Rockland). Microscopy was performed on a Delta Vision Spectris (Applied Precision) from which z-stack images were deconvolved using soft-WoRx.
2.10. Statistical analysis
All data are expressed as mean ± SEM. Two-tailed, unpaired Student's t-test was used to determine a significant difference between two groups. One-way ANOVA was used to evaluate differences between three or more groups. Post hoc testing (Tukey–Kramer Method) was performed when appropriate. A P-value <0.05 was considered statistically significant.
3. Results
3.1. Genetic deletion of ROCK-1 inhibited the development of the I/RC-induced fibrotic cardiomyopathy
3.1.1. ROCK-1−/− mice were protected from I/RC-induced cardiac dysfunction
As described previously, I/RC induced global ventricular dysfunction in WT mice.3,5,6 We now found that ROCK-1−/− mice were protected from the development of I/RC-mediated myocardial dysfunction as manifested by higher fractional shortening (38 ± 2 vs. 47 ± 2%; Figure 1A), and that their cardiac structure was preserved as indicated by higher anterior wall thickening (38 ± 3 vs. 59 ± 3%; Figure 1B) compared with WT mice, and did not differ from sham animals.
Figure 1.
ROCK-1−/− mice were protected from ischaemic/reperfusion cardiomyopathy (I/RC)-induced cardiac dysfunction. Wild-type (WT) and ROCK-1−/− mice underwent 7-day I/RC. In contrast to WT mice, deletion of ROCK-1 preserved (A) fractional shortening and (B) anterior wall thickening (n = 5/8 per group; #P < 0.01 between sham and WT groups; NS = not significant).
3.1.2. ROCK-1−/− mice were protected from I/RC-induced cardiac fibrosis
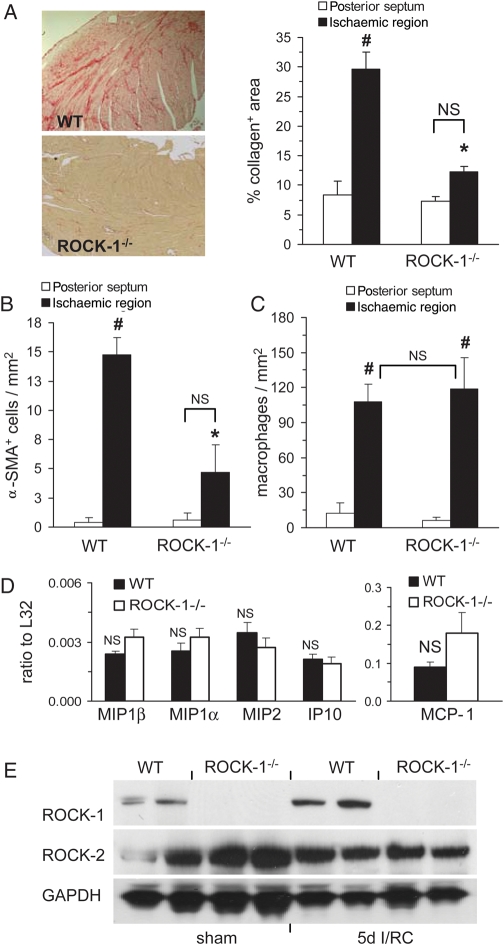
As shown in Figure 2A, after I/RC, interstitial collagen deposition in WT hearts was significantly greater than in ROCK-1−/− hearts (30 ± 3 vs. 12 ± 1% area; Figure 2A). Similarly, the amount of I/RC-induced α-SMA+ cells was markedly lower in ROCK-1−/− mouse hearts compared with WT hearts (14.8 ± 1.4 vs. 4.6 ± 2.3 cells/mm2; Figure 2B). However, the number of macrophages (108 ± 15 vs. 119 ± 27 cells/mm2; Figure 2C) measured within the ischaemic cardiac regions was not different between ROCK-1−/− and WT mice, which was consistent with previous studies showing that interventions that inhibited fibrosis in I/RC did not inhibit the influx of macrophages.5
Figure 2.
ROCK-1−/− mice were protected from ischaemic/reperfusion cardiomyopathy (I/RC)-induced cardiac fibrosis. Wild-type (WT) and ROCK-1−/− mice underwent 7-day I/RC. Tissue sections were stained for (A) collagen (image magnification: ×100), (B) alpha-smooth muscle actin (α-SMA), and (C) macrophages. Ischaemic and non-ischaemic (posterior septum) regions were analysed; the latter served as an interior control because this region should not be affected by I/RC. In contrast to WT mice, ROCK-1−/− mice were protected: after I/RC, interstitial deposition of collagen and amount of α-SMA+ cells were lower than in WT mice. Deletion of ROCK-1 did not reduce the number of macrophages. In sham-treated WT and ROCK-1−/− mice, no differences between ischaemic and non-ischaemic regions were observed for collagen, α-SMA+ cells, and macrophages (data not shown) (n = 5/7 per group; #P < 0.01 between ischaemic and non-ischaemic regions within the same group; *P < 0.01 between ischaemic ROCK-1−/− and WT groups; NS = not significant). (D) After 3-day I/RC, expression of monocyte chemoattractant protein 1 (MCP-1) mRNA was not reduced in ROCK-1−/− mice compared with WT mice (n = 4/4 per group; NS = not significant); [macrophage inflammatory protein (MIP)-1β, -1α, -2; interferon-γ induced protein (IP)-10; data were normalized to a ribosomal housekeeping gene L32]. (E) Representative western blots showing myocardial levels of ROCK-1 and ROCK-2 after 5-day I/RC. GAPDH served as loading normalization.
We previously demonstrated that in I/RC, MCP-1 mRNA was markedly induced for several days while the expression of other chemokines was only modestly elevated.3 We now show that the deletion of ROCK-1 did not reduce the I/RC-typical upregulation of MCP-1 expression (Figure 2D).
Figure 2E indicates that ROCK-1 protein levels in WT hearts were upregulated after 5-day I/RC compared with sham WT, but were not present in ROCK-1−/− mice. ROCK-2 protein levels were not affected by I/RC indicating that ROCK-2 was neither upregulated by I/RC nor by compensatory mechanisms due to genetic deletion of ROCK-1.
3.1.3. The fibroblast population found in I/RC was absent in ROCK-1−/− mice
Comparing ROCK-1−/− and WT mice undergoing I/RC, we found significantly reduced percentages of CD34 (5.7 ± 0.6 vs. 1.8 ± 0.6%), CD45 (13.0 ± 1.1 vs. 8.2 ± 1.3%), and CD34/CD45 (2.7 ± 0.5 vs. 0.2 ± 0.1%) positive cells of all viable, non-cardiomyocyte cells (Figure 3A). When cultured in vitro, cells from I/RC-treated ROCK-1−/− hearts consisted mainly of large, flat cells that differed from the small and spindle-shaped population obtained from I/RC-treated WT hearts (Figure 3B). Moreover, the in vitro proliferation rate of cultured cardiac fibroblasts from I/RC-treated ROCK-1−/− mice was lower than that of fibroblasts from I/RC-treated WT mice (5.5 ± 1.2 vs. 2.5 ± 0.5; Figure 3C) and did not differ from fibroblasts from sham mice. Therefore, when ROCK-1 was deleted, the monocyte-derived, spindle-shaped, fast proliferating cardiac fibroblasts characteristic for I/RC were absent.
Figure 3.
The ischaemic/reperfusion cardiomyopathy (I/RC)-characteristic fibroblast population was absent in ROCK-1−/− mice. Wild-type (WT) and ROCK-1−/− mice underwent 5-day I/RC. Hearts were removed and cardiac fibroblasts isolated. (A) Dispersed cells were analysed for CD34 and CD45 expression on viable (calcein+) cells by flow cytometry. Deletion of ROCK-1 reduced the number of CD34+, CD45+, and CD34+/CD45+ cells compared with WT (n = 4 per group). (B) The same cell suspensions were cultured in vitro and morphology was determined by phase contrast microscopy (magnification: ×200). Cell cultures from ROCK-1−/− mice undergoing I/RC showed similar morphologic characteristics (large, flat) as cultures from sham-treated hearts. Cultures from WT hearts were small and spindle-shaped as described earlier.5 (C) Cell isolations from ROCK-1−/− mice after I/RC proliferated (BrdU incorporation) at similar rates as isolations from sham-treated mice, whereas isolations from WT mice proliferated two-fold faster as described earlier,5 (n = 3/5 per group; #P < 0.05 between sham and I/RC groups, *P < 0.05 between ROCK-1−/− and WT groups). For the described parameters, there was no difference between sham WT and sham ROCK-1−/− mice; thus animals of both groups were pooled.
3.2. Reduction of ROCK-1 protein interfered with monocyte-to-fibroblast differentiation
3.2.1. ROCK-1-targeting siRNA reduced ROCK-1 protein expression in human peripheral blood mononuclear cells
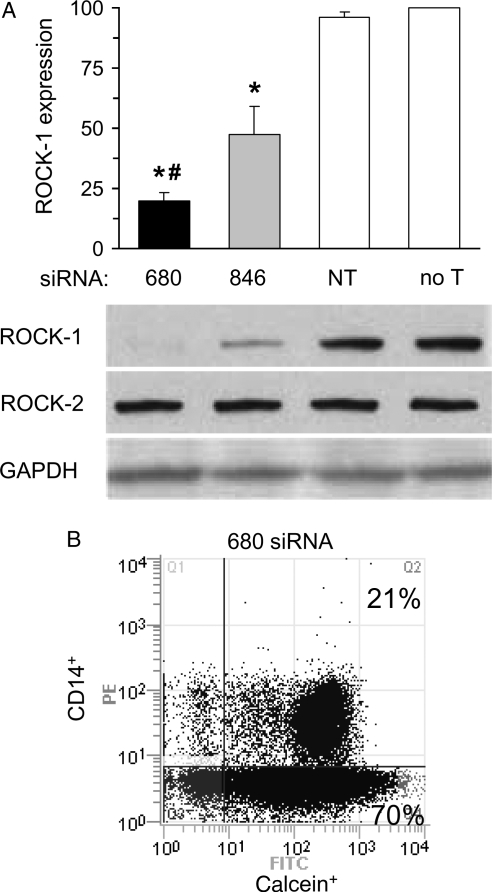
As shown in Figure 4A, ROCK-1 protein was equally expressed in human PBMC treated with non-targeting siRNA and untreated PBMC. Treatment of PBMC with ROCK-1-targeting siRNA 680 and siRNA 846 significantly reduced the ROCK-1 protein level by 80 and 53%, respectively, without affecting the expression levels of the closely related ROCK-2 protein. We further demonstrated by flow cytometry that >90% of all cells were viable after successful siRNA treatment (Figure 4B); in particular the amount of viable, monocytic CD14+ cells, that have been shown to contain the cell population that matures into fibroblasts,6 was not reduced by siRNA treatment.
Figure 4.
ROCK-1-targeting siRNA reduced ROCK-1 protein expression in human PBMC. Human PBMC were treated with either ROCK-1-targeting siRNA 680, ROCK-1-targeting siRNA 846, non-targeting siRNA (NT), or were untreated (no T). (A) Representative western blots and corresponding group data showing ROCK-1 and ROCK-2 protein levels. Compared with no treatment levels, both siRNA 680 and 846 abrogated ROCK-1 without affecting ROCK-2 protein expression (n = 6 per group; #P < 0.05 between siRNA 680 and 846 treated groups, *P < 0.001 between ROCK-1-targeting siRNA treated and untreated groups). (B) Representative cytometric diagram showing that >90% of the total cells were positive for calcein, and thus were viable, and >20% of the viable cells were CD14+ after ROCK-1-targeting siRNA 680 treatment which was not different than in untreated cells (data not shown).
3.2.2. ROCK-1 expression was not necessary for transendothelial migration
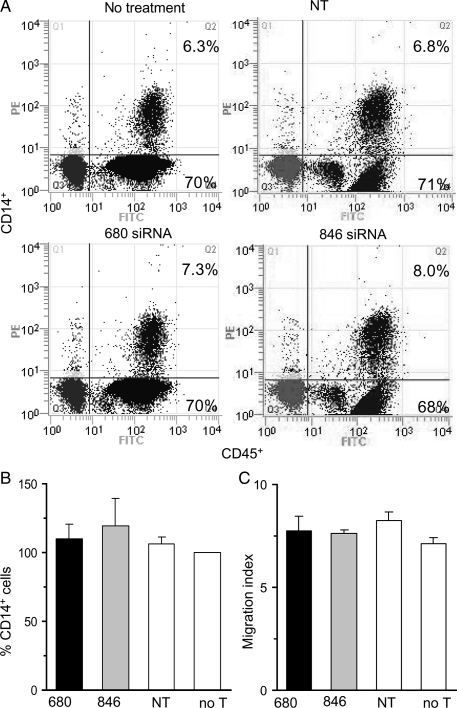
We performed TEM with siRNA-treated and untreated PBMC, and counted the total number of cells that successfully migrated through HCMEC in response to MCP-1. We found that the total number of cells that migrated was not different between PBMC treated with ROCK-1-targeting siRNA 680 (160 ± 13 × 103), ROCK-1-targeting siRNA 846 (156 ± 23 × 103), or untreated PBMC (145 ± 17 × 103). Importantly, the amount of monocytic CD14+ cells (∼7% of all CD45+ cells) was not different between groups (Figures 5A and B). We then tested whether siRNA treatment altered the PBMCs' chemotactic response to MCP-1. We found that the chemotactic index to MCP-1, as defined by the ratio of the directed migration to the spontaneous migration, did not differ between treated and untreated groups (Figure 5C). These data indicate that ROCK-1 was not necessary for monocytic CD14+ cells to migrate though endothelial cells in response to MCP-1.
Figure 5.
ROCK-1 expression was not necessary for TEM. Human peripheral blood mononuclear cells, pre-treated with either ROCK-1-targeting siRNA 680, ROCK-1-targeting siRNA 846, non-targeting siRNA (NT), or untreated (no T), were allowed to migrate through human cardiac microvascular endothelial cells (HCMEC) in response to MCP-1, but were collected in collagen pads between the endothelial cell layer and the filter (pore size: 0.4 µm). The collagen pad was digested and the total number of CD45+/CD14+ monocytes was counted by flow cytometry. (A) Representative cytometric diagrams showing that the amount of CD14+ mononuclear cells (PE-labelled) that migrated through HCMEC represented ∼7% of all CD45+ cells (FITC-labelled). (B) Corresponding group data showing no difference between groups when experimental data were expressed as percentage of ‘no treatment’ data (n = 3 per group). (C) The same groups were allowed to migrate towards MCP-1 in agarose pads in the absence of endothelial cells. The net migration in response to MCP-1 was determined and the migration index calculated. There was no difference in the chemotactic behaviour between groups (n = 3 per group).
3.2.3. ROCK-1 expression was necessary for fibroblast formation
We have previously shown that following TEM of untreated PBMC, 10% of all successfully migrated, adherent mononuclear cells mature into fibroblasts.6 We now found that the amount of cells that mature into fibroblasts following TEM of ROCK-1-targeting siRNA-treated PBMC was 23-fold decreased compared with untreated PBMC. As seen in Figure 6A and B, after 4 days of culture, compared with untreated PBMC, only 4.3 ± 0.3% of PBMC treated with ROCK-1-targeting siRNA 680 matured into fibroblasts. Treatment with ROCK-1-targeting siRNA 846, that knocked down ROCK-1 protein levels by only half (see Figure 4A), reduced the number of fibroblasts following TEM by 3.5-fold compared with untreated PBMC (29 ± 12%). Similarly, after 11 days of culture, only 6.7 ± 0.1% of the amount seen in untreated PBMC matured into fibroblasts (18.1 ± 2.6% for siRNA 846). Fibroblasts were identified by an elongated, spindle-shaped morphology and positive stain for type I collagen. These data indicate that ROCK-1 was required for monocyte-to-fibroblast maturation.
Figure 6.
ROCK-1 expression was necessary for fibroblast formation. Human peripheral blood mononuclear cells (PBMC), pre-treated with either ROCK-1-targeting siRNA 680, ROCK-1-targeting siRNA 846, non-targeting siRNA (NT), or untreated (no T), were allowed to migrate through human cardiac microvascular endothelial cells in response to MCP-1. (A) Representative images of adherent cells in the bottom well after 4 and 11 days of culture. Fibroblasts were spindle-shaped and elongated (phase contrast insert in bottom left image, magnification: ×200) and were positive for type I collagen (green; blue represents DAPI stain for nucleus identification; magnification: ×200). Macrophages were round and lacked type I collagen expression. (B) Corresponding group data comparing the total number of fibroblasts in the entire bottom well (no T: 269 ± 42; NT: 264 ± 40; siRNA 846: 66 ± 39, siRNA 680: 11 ± 2) when experimental data were expressed as a percentage of ‘no treatment’ data. Compared with the untreated PBMC group, fibroblast formation was significantly reduced in ROCK-1-targeting siRNA treated PBMC groups, with siRNA 680 being more effective than siRNA 846 (n = 3 per group; *P < 0.001 between siRNA treated and untreated groups, #P < 0.05 between siRNA 680 and siRNA 846).
4. Discussion
Previously, we developed a murine I/RC model in which, in the absence of cardiomyocyte death, the development of non-adaptive cardiac fibrosis was preceded by an MCP-1-driven uptake of a fibroblast precursor population of monocyte origin.3,5,6 This population mainly consisted of spindle-shaped, fast proliferating, CD34 (primitive cell marker) and CD45 (haematopoietic marker) expressing cells.5 The I/RC-mediated, fibrotic cardiomyopathy as well as the appearance of the fibroblast precursor population in the heart was prevented by genetic deletion of MCP-1 or daily administration of SAP.4,5 Further in vivo studies revealed that SAP acted via Fcγ receptors expressed on monocytes,6 and in vitro that CD14+ monocytes must be exposed to circulating SAP before or during their migration through endothelium.6 Exposure to SAP after successful endothelial transmigration did not inhibit fibroblast differentiation. In the current article, we describe signalling through ROCK-1 as a novel critical molecular control point of fibroblast differentiation from myeloid cells resulting in non-adaptive fibrosis in repetitive ischaemia/reperfusion.
The involvement of the RhoA/ROCK pathways in the pathogenesis and development of diverse cardiovascular diseases have been the focus of several studies.13–20 However, most of these studies used the pharmacologic ROCK inhibitors Y27632 or fasudil, which do not distinguish between the two isoforms ROCK-1 and ROCK-2 and, in higher doses, possibly also inhibit other enzymes such as protein kinases A and C.26 Thus, the generation of mice with targeted deletion of ROCK-1 has been crucial in determining the critical involvement of ROCK-1 specifically in the development of fibrotic heart diseases. Indeed, we and others demonstrated that genetic deletion of ROCK-1 inhibited the development of cardiac fibrosis in a murine pressure overload model without affecting the development of pathological hypertrophy.22
Our results from the current study validate previous observations linking ROCK-1 to non-adaptive fibrosis,13,14,19,22,27,28 and further address a potential mechanism by which deletion of ROCK-1 abrogates fibrosis. We show that myocardial ROCK-1, but not ROCK-2, was upregulated in a repetitive ischaemia/reperfusion injury model, and that the presence of ROCK-1 was obligate in I/RC-mediated development of non-adaptive fibrosis. The ROCK-1−/− mice were also protected from the deleterious effects of I/RC on cardiac function. In contrast, we found that the deletion of ROCK-1 did not alter the increased levels of MCP-1 and the consequential tissue migration of macrophages seen in WT I/RC. Despite this, there was a marked reduction in CD34+/CD45+ fibroblast precursors found in the ROCK-1−/− heart in comparison with WT mice and virtual elimination of the collagen deposition. This suggested a role for ROCK-1 in the maturation of fibroblasts from myeloid precursors identified by our laboratory.5,6
To further pursue this issue, we evaluated the role of ROCK-1 in an in vitro model of monocyte-to-fibroblast transition requiring TEM that was developed in our laboratory.6 This model, using human monocytes and endothelial cells, has mimicked I/RC in regard to the importance of Fcγ receptor activation and MCP-1-mediated TEM. Despite the fact that MCP-1-mediated TEM was not reduced with siRNA silencing of ROCK-1, an 80% depletion of ROCK-1 protein inhibited the maturation of a monocytic cell population into fibroblasts after successful TEM.
The in vitro model of TEM allowed us to investigate the discrete steps in monocyte-to-fibroblast maturation; we could assess ROCK-1 requirements for the transmigration step separately from the subsequent maturation into fibroblasts. ROCK-1 knockdown did not affect the ability of monocytes to migrate through endothelium, which was consistent with our in vivo data that there was no diminution of macrophages infiltrating the injured hearts of ROCK-1−/− mice compared with WT. Previous studies demonstrating a failure of ROCK-deficient monocytes to complete endothelial transmigration by releasing their trailing edges used inhibition of both ROCK isoforms;29 subsequent studies using isoform-specific siRNA knockdown in embryonic fibroblasts demonstrated that ROCK-2, not ROCK-1, was responsible for trailing edge retraction.30 Assuming that ROCK-1 subserves a similar role in monocytes and embryonic fibroblasts, the latter study was compatible with our data that ROCK-1 was not necessary for monocytes to complete TEM.
In contrast, ROCK-1 knockdown in monocytes resulted in a profound defect in their maturation to a fibroblast phenotype subsequent to TEM. This defect included the failure of the cells to polarize into a spindle-shape as well as a failure to produce type I collagen, both of which are diagnostic for identifying monocyte-derived cells as fibroblasts. In particular, in our study, ROCK-1-depleted cells had a more rounded morphology compared with ROCK-1 expressing cells; similar to other studies showing that ROCK-1 was necessary for the assembly of microfilament bundles and focal adhesions, and that its absence lead to altered cell morphology and contractility.31 In addition, ROCK-1-depleted cells failed to express type I collagen, indicating that the maturation pathway leading to matrix production was also affected directly or indirectly by ROCK-1, by cell shape, or both. We therefore propose that ROCK-1 is crucial in regulating the switch of cells from a monocytic to a fibroblast cell type due, at least in part, to its effects on cytoskeletal function. ROCK-regulated cell shape has been reported to determine the lineage commitment of mesenchymal stem cells,32 and so the differentiation of monocytes to fibroblasts may have similar requirements.
Our in vivo and in vitro data contrast with other in vivo studies in which ROCK-1 haploinsufficiency decreased leukocyte recruitment into areas of vascular injury.25 Several reasons may account for this difference. First, Noma et al.25 used C57BL/6 mice with heterozygous deletion of ROCK-1; homozygous deletion of ROCK-1 in this strain resulted in embryonic and perinatal lethality. In our studies, ROCK-1−/− mice were generated in an FvB background. These mice, although underrepresented during breeding, were viable with no detected anatomical abnormalities.22 Therefore, background specific differences may play a role in leukocyte recruitment. Also, Noma et al. identified macrophages by use of the monoclonal antibody MOMA-2.25 This antibody reacts with a subset of macrophages.33 We identified macrophages using the antibody Mac-2, which identifies all mature macrophages. It is possible that, when migrating into tissue, a subset of macrophages may be impaired in its migration when expressing half the normal amount of ROCK-1 and thus would not be detected by MOMA-2. It is also possible that a complete deletion of ROCK-1, as opposed to haploinsufficiency, might activate signalling pathways independent of RhoA leading to migration, as has been described in eosinophils.34 Lastly, global full and/or half deletion of ROCK-1 may have different consequences not only for migrating leukocytes, but also for cells that they must interact with, such as endothelial cells and cardiomyoctes.
In summary, our observations describe a vital role for ROCK-1 in the development of fibrosis and coincident cardiac dysfunction in a mouse model of ischaemic cardiomyopathy. In addition, our in vitro studies with human monocytes and endothelium provide new insight into potential mechanism(s) by which ROCK-1 mediates fibrosis by demonstrating its requirement in the monocyte-to-fibroblast transition of transmigrated monocytes.
Funding
This work was supported by the National Institute of Health [HL42550, R01HL076661, HL089792, T32HL107816]; the Medallion Foundation; and The Methodist Hospital Foundation.
Acknowledgements
We thank Thuy Pham, Geoffrey Bender, and Taiya Williams for expert technical assistance.
Conflict of interest: none declared.
References
- 1.Weber KT, Brilla CG, Janicki JS. Myocardial fibrosis: functional significance and regulatory factors. Cardiovasc Res. 1993;27:341–348. doi: 10.1093/cvr/27.3.341. [DOI] [PubMed] [Google Scholar]
- 2.Frangogiannis NG. Chemokines in the ischemic myocardium: from inflammation to fibrosis. Inflamm Res. 2004;53:585–595. doi: 10.1007/s00011-004-1298-5. [DOI] [PubMed] [Google Scholar]
- 3.Dewald O, Frangogiannis NG, Zoerlein M, Duerr GD, Klemm C, Knuefermann P, et al. Development of murine ischemic cardiomyopathy is associated with a transient inflammatory reaction and depends on reactive oxygen species. Proc Natl Acad Sci USA. 2003;100:2700–2705. doi: 10.1073/pnas.0438035100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frangogiannis NG, Dewald O, Xia Y, Ren G, Haudek S, Leucker T, et al. Critical role of monocyte chemoattractant protein-1/CC chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation. 2007;115:584–592. doi: 10.1161/CIRCULATIONAHA.106.646091. [DOI] [PubMed] [Google Scholar]
- 5.Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, Crawford JR, et al. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci USA. 2006;103:18284–18289. doi: 10.1073/pnas.0608799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haudek SB, Trial J, Xia Y, Gupta D, Pilling D, Entman ML. Fc receptor engagement mediates differentiation of cardiac fibroblast precursor cells. Proc Natl Acad Sci USA. 2008;105:10179–10184. doi: 10.1073/pnas.0804910105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 8.Amano M, Fukata Y, Kaibuchi K. Regulation and functions of Rho-associated kinase. Exp Cell Res. 2000;261:44–51. doi: 10.1006/excr.2000.5046. [DOI] [PubMed] [Google Scholar]
- 9.Honing H, van den Berg TK, van der Pol SM, Dijkstra CD, van der Kammen RA, Collard JG, et al. RhoA activation promotes transendothelial migration of monocytes via ROCK. J Leukoc Biol. 2004;75:523–528. doi: 10.1189/jlb.0203054. [DOI] [PubMed] [Google Scholar]
- 10.Rikitake Y, Liao JK. ROCKs as therapeutic targets in cardiovascular diseases. Expert Rev Cardiovasc Ther. 2005;3:441–451. doi: 10.1586/14779072.3.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noma K, Oyama N, Liao JK. Physiological role of ROCKs in the cardiovascular system. Am J Physiol Cell Physiol. 2006;290:C661–C668. doi: 10.1152/ajpcell.00459.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loirand G, Guerin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res. 2006;98:322–334. doi: 10.1161/01.RES.0000201960.04223.3c. [DOI] [PubMed] [Google Scholar]
- 13.Satoh S, Ueda Y, Koyanagi M, Kadokami T, Sugano M, Yoshikawa Y, et al. Chronic inhibition of Rho kinase blunts the process of left ventricular hypertrophy leading to cardiac contractile dysfunction in hypertension-induced heart failure. J Mol Cell Cardiol. 2003;35:59–70. doi: 10.1016/s0022-2828(02)00278-x. [DOI] [PubMed] [Google Scholar]
- 14.Higashi M, Shimokawa H, Hattori T, Hiroki J, Mukai Y, Morikawa K, et al. Long-term inhibition of Rho-kinase suppresses angiotensin II-induced cardiovascular hypertrophy in rats in vivo: effect on endothelial NAD(P)H oxidase system. Circ Res. 2003;93:767–775. doi: 10.1161/01.RES.0000096650.91688.28. [DOI] [PubMed] [Google Scholar]
- 15.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 16.Wang HW, Liu PY, Oyama N, Rikitake Y, Kitamoto S, Gitlin J, et al. Deficiency of ROCK1 in bone marrow-derived cells protects against atherosclerosis in LDLR−/− mice. FASEB J. 2008;22:3561–3570. doi: 10.1096/fj.08-108829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mallat Z, Gojova A, Sauzeau V, Brun V, Silvestre JS, Esposito B, et al. Rho-associated protein kinase contributes to early atherosclerotic lesion formation in mice. Circ Res. 2003;93:884–888. doi: 10.1161/01.RES.0000099062.55042.9A. [DOI] [PubMed] [Google Scholar]
- 18.Hattori T, Shimokawa H, Higashi M, Hiroki J, Mukai Y, Tsutsui H, et al. Long-term inhibition of Rho-kinase suppresses left ventricular remodeling after myocardial infarction in mice. Circulation. 2004;109:2234–2239. doi: 10.1161/01.CIR.0000127939.16111.58. [DOI] [PubMed] [Google Scholar]
- 19.Rikitake Y, Oyama N, Wang CY, Noma K, Satoh M, Kim HH, et al. Decreased perivascular fibrosis but not cardiac hypertrophy in ROCK1+/− haploinsufficient mice. Circulation. 2005;112:2959–2965. doi: 10.1161/CIRCULATIONAHA.105.584623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bao W, Hu E, Tao L, Boyce R, Mirabile R, Thudium DT, et al. Inhibition of Rho-kinase protects the heart against ischemia/reperfusion injury. Cardiovasc Res. 2004;61:548–558. doi: 10.1016/j.cardiores.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Thumkeo D, Keel J, Ishizaki T, Hirose M, Nonomura K, Oshima H, et al. Targeted disruption of the mouse rho-associated kinase 2 gene results in intrauterine growth retardation and fetal death. Mol Cell Biol. 2003;23:5043–5055. doi: 10.1128/MCB.23.14.5043-5055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang YM, Bo J, Taffet GE, Chang J, Shi J, Reddy AK, et al. Targeted deletion of ROCK1 protects the heart against pressure overload by inhibiting reactive fibrosis. FASEB J. 2006;20:916–925. doi: 10.1096/fj.05-5129com. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu Y, Thumkeo D, Keel J, Ishizaki T, Oshima H, Oshima M, et al. ROCK-I regulates closure of the eyelids and ventral body wall by inducing assembly of actomyosin bundles. J Cell Biol. 2005;168:941–953. doi: 10.1083/jcb.200411179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakagawa O, Fujisawa K, Ishizaki T, Saito Y, Nakao K, Narumiya S. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett. 1996;392:189–193. doi: 10.1016/0014-5793(96)00811-3. [DOI] [PubMed] [Google Scholar]
- 25.Noma K, Rikitake Y, Oyama N, Yan G, Alcaide P, Liu PY, et al. ROCK1 mediates leukocyte recruitment and neointima formation following vascular injury. J Clin Invest. 2008;118:1632–1644. doi: 10.1172/JCI29226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rikitake Y, Kim HH, Huang Z, Seto M, Yano K, Asano T, et al. Inhibition of Rho kinase (ROCK) leads to increased cerebral blood flow and stroke protection. Stroke. 2005;36:2251–2257. doi: 10.1161/01.STR.0000181077.84981.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moriyama T, Nagatoya K. The Rho-ROCK system as a new therapeutic target for preventing interstitial fibrosis. Drug News Perspect. 2004;17:29–34. doi: 10.1358/dnp.2004.17.1.829023. [DOI] [PubMed] [Google Scholar]
- 28.Fu P, Liu F, Su S, Wang W, Huang XR, Entman ML, et al. Signaling mechanism of renal fibrosis in unilateral ureteral obstructive kidney disease in ROCK1 knockout mice. J Am Soc Nephrol. 2006;17:3105–3114. doi: 10.1681/ASN.2005121366. [DOI] [PubMed] [Google Scholar]
- 29.Worthylake RA, Lemoine S, Watson JM, Burridge K. RhoA is required for monocyte tail retraction during transendothelial migration. J Cell Biol. 2001;154:147–160. doi: 10.1083/jcb.200103048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwanicki MP, Vomastek T, Tilghman RW, Martin KH, Banerjee J, Wedegaertner PB, et al. FAK, PDZ-RhoGEF and ROCKII cooperate to regulate adhesion movement and trailing-edge retraction in fibroblasts. J Cell Sci. 2008;121:895–905. doi: 10.1242/jcs.020941. [DOI] [PubMed] [Google Scholar]
- 31.Yoneda A, Multhaupt HA, Couchman JR. The Rho kinases I and II regulate different aspects of myosin II activity. J Cell Biol. 2005;170:443–453. doi: 10.1083/jcb.200412043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 33.Leenen PJ, de Bruijn MF, Voerman JS, Campbell PA, van Ewijk W. Markers of mouse macrophage development detected by monoclonal antibodies. J Immunol Methods. 1994;174:5–19. doi: 10.1016/0022-1759(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 34.Muessel MJ, Scott KS, Friedl P, Bradding P, Wardlaw AJ. CCL11 and GM-CSF differentially use the Rho GTPase pathway to regulate motility of human eosinophils in a three-dimensional microenvironment. J Immunol. 2008;180:8354–8360. doi: 10.4049/jimmunol.180.12.8354. [DOI] [PubMed] [Google Scholar]