Abstract
Aims
The presence and potential function of transient receptor potential melastatin 7 (TRPM7), a Ca2+-permeable non-selective cation channel of the TRP channel superfamily in human vascular endothelial cells, were examined.
Methods and results
Whole-cell patch-clamp recordings showed outward-rectifying currents in human umbilical vein endothelial cells (HUVECs), which was potentiated by removing the extracellular Ca2+ and Mg2+, but inhibited by non-specific TRPM7 blocker Gd3+ or 2-aminoethoxydiphenyl borate (2-APB). TRPM7 mRNA was detected in HUVECs by RT–PCR, but TRPM6, its closest homologue, was not. Silencing TRPM7 by small interfering RNA (siRNA) decreased the level of TRPM7 mRNA and the TRPM7-like current. Interestingly, knockdown of TRPM7 with siRNA or inhibition of TRPM7 function with 2-APB increased the phosphorylation of extracellular signal-regulated kinase (ERK) and enhanced growth/proliferation of HUVECs. This enhanced cell growth/proliferation was abolished by an inhibitor of the ERK signalling pathway. In addition to cell growth/proliferation, silencing TRPM7 also increased expression of nitric oxide synthase and nitric oxide production in an ERK pathway-dependent manner.
Conclusion
These observations suggest that TRPM7 channels may play an important role in the function of vascular endothelial cells.
KEYWORDS: TRPM7, Vascular endothelial cells, MAPK, Growth/proliferation
1. Introduction
Transient receptor potential melastatin 7 (TRPM7) is a member of the TRP superfamily.1,2 TRP family channels are ubiquitously expressed and can be subdivided into three major subfamilies: TRPC, TRPV, and TRPM.1 TRPM7, along with its closest member TRPM6, possesses a unique kinase domain that is absent in other TRP channels. The functional role of this kinase remains elusive although considerable effort has been made.3–6 TRPM7 channels are non-selective cation channels with predominant permeability for Ca2+ and Mg2+. Activation of these channels is implicated in diverse physiological/pathological processes such as Mg2+ homeostasis,4,7,8 hypoxic neuronal injury,9 and tumour cell growth/proliferation.10
Increasing evidence suggests that activation of TRP channels might also contribute to the physiology/pathophysiology of the vascular system, whose main components includes endothelium and vascular smooth muscle cells (VSMCs).2,9–13 Activation of TRPM7 channels in VSMCs is implicated in Mg2+ homeostasis, cell growth, and in the pathphysiology of cardiovascular diseases such as hypertension.14,15 Although not directly involved in contraction/relaxation, endothelial cells are involved in the regulation of vascular contractility, release of cytokines, and control of material transport between blood and tissues. In addition, they play an important role in angiogenesis and vascular repair.16 Following the injury of vascular tissues, the endothelial cells may be activated, which leads to cell proliferation. Growth and proliferation of endothelial cells, followed by maturation and resultant angiogenesis, is one of the most important steps in the process of vascular development and repair in both physiological and pathophysiological conditions.16
Although the presence and potential function of TRPM7 channels have been studied in cardiovascular tissues including cardiac myocytes and VSMCs,13–15 their potential function has not been explored in vascular endothelial cells. This study explores the role of TRPM7 channels in human umbilical vein endothelial cells (HUVECs). Our results indicate that functional TRPM7 channels are expressed in HUVECs and that inhibition of TRPM7 accelerates the growth/proliferation of these cells. This effect is mediated, at least in part, by the extracellular signal-regulated kinase (ERK) signalling pathway.
2. Methods
2.1. Reagents and antibodies
The following reagents/antibodies were used: TRIzol (Invitrogen); protease inhibitor cocktail (Roche Diagnostics); phosphatase inhibitor, TNFα, 2-aminoethoxydiphenyl borate (2-APB), NG-nitro-l-arginine methyl ester (l-NAME), 2,3-diaminonaphthalene, tetracycline (Sigma-Aldrich); rabbit polyclonal antibody against β-actin (Abcam); mouse monoclonal antibody against endothelial nitric oxide synthase (eNOS) (BD Biosciences); rabbit polyclonal antibodies against phospho-mitogen-activated protein kinase kinase (MEK1/2), phospho-ERK1/2, phospho-c-Jun N-terminal kinase (JNK), and phospho-p38 mitogen-activated protein kinase (MAPK), MEK1/2 specific inhibitors, U0126 (Cell Signaling).
2.2. Cell culture
HUVECs were cultured as described.17 Cells were purchased from Cambrex, and grown in EGM-2 medium containing 2% fetal bovine serum (FBS) and trophic factors (Lonza). Adult human skin microvascular endothelial cells (HMVECs) were grown in EGM-2 MV medium (Lonza). Subconfluent cultures were passaged according to a standard trypsinization protocol. They were used for experiments at passages three and six. HEK293 cells were cultured in MEM with 10% FBS and antibiotics (Invitrogen).
2.3. Reverse transcription–polymerase chain reaction (RT-PCR)
Total RNAs were extracted with a TRIzol reagent (Invitrogen). cDNAs were synthesized from 0.2 µg total RNA in 20-μL reactions using oligo(dT)15 and Superscript™ II reverse transcriptase (Invitrogen).
PCR reactions were performed using 0.25 µL of cDNAs as templates in 1× PCR reaction buffer, 0.2 mmol/L each dNTP, 0.2 µL DNA polymerase (Advantage™ cDNA Polymerase Mix, BD Biosciences), and 333 nmol/L each primer in 10-μL reactions. For multiplex PCR, additional primer pairs were involved in reactions. The PCR amplification cycles consisted of denaturation at 94°C for 3 min, 29 (Figure 2B) or 35 (Figure 2A) cycles of denaturation at 94°C for 30 s, annealing at 57–61°C for 15 s, and extension at 72°C for 30 s. PCR products were separated by electrophoresis on a 1.5% agarose gel, detected using ethidium bromide, and subsequently sequenced.
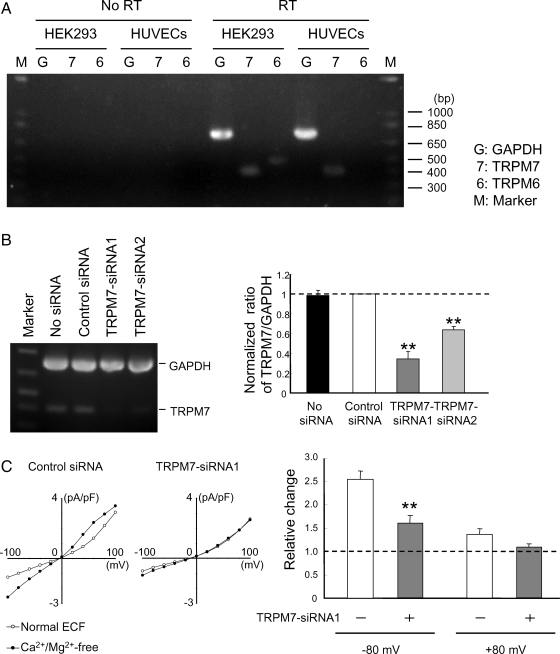
Figure 2.
TRPM7-siRNAs suppress the expression of TRPM7 and TRPM7-like current. (A) Total RNAs were isolated from indicated cells. Equal amounts of total RNA were reverse-transcribed and PCR-amplified using specific primers for TRPM6, TRPM7, or GAPDH. Expected molecular sizes of the fragments are 478, 399, and 723 bp, respectively. (B) RT–PCR analysis shows reduced expression of TRPM7 mRNA by TRPM7-siRNAs. HUVECs were untreated or treated with either control or TRPM7-siRNAs for 48 h. RNAs were extracted and then multiplex RT–PCR was performed. Bar chart shows densitometrically quantified relative ratio of TRPM7/GAPDH in HUVECs transfected with or without siRNAs (n = 3–4, **P < 0.01). (C) I–V relationship of TRPM7-like current in HUVECs transfected with control (left) or TRPM7-siRNA1 (right). Silencing TRPM7 inhibits TRPM7-like current and their potentiation by Ca2+/Mg2+ removal. Bar graph shows relative increase in the amplitude of TRPM7-like currents induced by Ca2+/Mg2+ removal in HUVECs transfected with either control (−) or TRPM7-siRNA1 (+). n = 8–9. **P < 0.01, control vs. TRPM7-siRNA1-treated cells.
The primers used for PCR were described in Supplementary material online, Table S1. Primer pairs were designed to bracket at least an intron for each gene to rule out amplification of genomic DNA.
The detected signals were quantified by laser densitometry. Relative ratios were calculated by dividing the density of the TRPM7 band by that of the glyceraldehyde-3-phosphate dehydrogenase band (GAPDH).
2.4. Immunoblotting
Immunoblotting was performed as described.18 Cells cultured on 35 mm dishes or 6-well plates were lysed in lysis buffer (50 mmol/L Tris–HCl, pH 7.5, 100 mmol/L NaCl, 1% Triton X-100, protease inhibitor, and phosphatase inhibitor). After centrifugation at 12 000g at 4°C for 30 min, the lysates were collected. Protein concentration was assessed using Bradford reagent (Bio-Rad). The aliquots were then mixed with Laemmli sample buffer and boiled at 95°C for 15 min. The samples were resolved by 10% SDS–PAGE, followed by electrotransfer to polyvinylidene difluoride membranes. For visualization, blots were probed with antibodies against phospho-ERK (1:1000), phospho-p38 MAPK (1:500), phospho-JNK (1:1000), eNOS (1:2500), or β-actin (1:2000), and detected using horseradish peroxidase-conjugated secondary antibodies (1:1000; Cell Signaling) and an ECL kit (Amersham Pharmacia Biotech).
2.5. siRNA transfection
Two human TPRM7-silencing small interfering RNA (siRNA) duplexes, TRPM7-siRNA1 and TRPM7-siRNA2 which target nucleotides 406–426 and 455–475 of human TRPM7, respectively (GenBank Accession Number NM017672), were synthesized by Ambion. The TRPM7-siRNA1 was previously reported to down-regulate the TRPM7 channels.10 Transfection was performed with 30 nmol/L siRNA using Amine siRNA transfection reagent (Ambion). A negative control siRNA (Ambion) was used in parallel. Cells were used 2–4 days later for experiments.
2.6. LDH assay
Lactate dehydrogenase (LDH) measurement was performed as described.10,19 Cells grown on 24-well plates were washed with phosphate-buffered saline. 50 µL medium was taken from each well and placed into 96-well plate for background LDH measurement. Cells were then incubated with Triton X-100 (final concentration 0.5%) for 30 min at 37°C. 50 µL of supernatants were withdrawn from each well for maximal LDH measurement. 50 µL of assay reagent from cytotoxicity detection kit (Roche Diagnostics) was added to each sample and mixed. 30 min later, the absorbance at 492 and 620 nm was examined by spectrometer (SpectraMax Plus, Molecular devices), and the values of the absorbance at 492 nm were subtracted by those at 620 nm to yield the value of LDH release.
2.7. Electrophysiology
Whole-cell voltage-clamp recordings were performed as described.9,19 Three to four days after transfection, cells were set on the stage of an inverted microscope (TE2000-U; Nikon) and superfused at room temperature with an extracellular solution containing (in mmol/L) 140 NaCl, 5.4 KCl, 2 CaCl2, 1 MgCl2, 33 glucose, and 20 HEPES (pH 7.4 with NaOH, 320–335 mOsm). Patch electrodes were fabricated from borosilicate capillary tubing of 1.5 mm diameter (WPI) using a vertical puller (PP-83, Narishige). The electrode resistance ranged from 3 to 4 MΩ when filled with the intracellular solution (see below). For current–voltage (I–V) relationship, voltage steps ranged between −100 and +100 mV from a holding potential of −60 mV were applied at 1 s interval. Unless stated otherwise, all recordings were performed at least 10 min after establishing the whole-cell configuration. Membrane currents were recorded using an Axopatch 200B amplifier. Data were filtered at 2 kHz and digitized at 5 kHz by using a Digidata 1322A data-acquisition system (Axon Instruments). Unless described otherwise, pipette solution contained (in mmol/L) 145 Cs-methanesulfonate, 8 NaCl, 4.1 CaCl2, 10 ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid, 5 tetraethyl-ammonium chloride, and 10 HEPES (pH 7.3, with CsOH).
2.8. Measurement of NO metabolite
The production of nitric oxide (NO) was determined by measurement of nitrite, a stable product of NO, using fluorometric reagent, 2,3-diaminonaphthalene.20 100 µL of samples were transferred to 96-well plate, and incubated with 10 µL fresh 2,3-diaminonaphthalene solution (500 µg/mL in 0.62 N HCl) for 10 min at room temperature. The reactions were terminated with 5 µL of 2.8 N NaOH. Formation of 2,3-diaminonaphthotriazole was measured using fluorescent multi-well plate reader (SpectraMax Gemini, Molecular Devices) with excitation/emission at 365/450 nm. Nitrite concentration was calculated from a NaNO2 standard curve. The fluorescence signal was digitized and analysed using SoftMax Pro software.
2.9. Statistical analysis
Data are expressed as means ± SEM. Groups were compared using one-way ANOVA followed by Dunnett's test, or Student's t-test as appropriate. P < 0.05 was regarded as statistically significant.
3. Results
3.1. Functional expression of TRPM7 channels in HUVECs
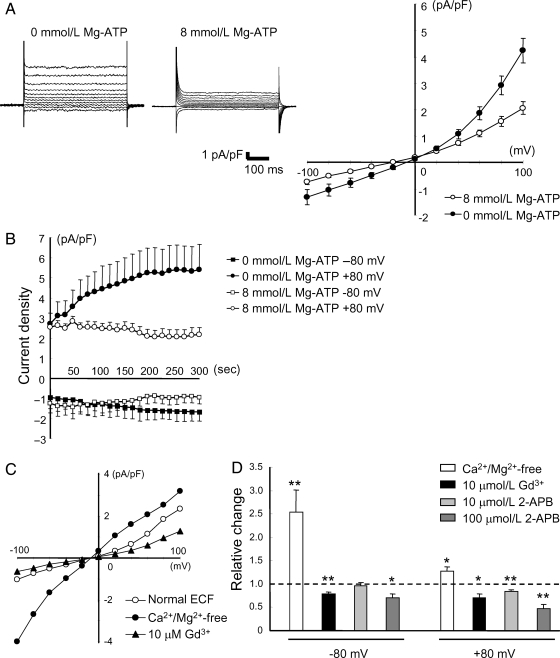
Previous studies showed conflicting results on TRPM7 expression in human vascular endothelial cells, with one report showing little evidence of TRPM7-like current while others showed clear detection of TRPM7 gene expression.15,21 Therefore, our first experiment was to examine the existence of functional TRPM7 channels in HUVECs by whole-cell patch-clamp recordings. It has been demonstrated previously that TPRM7 channels exhibit outward-rectifying I–V relationship when activated in the absence of divalent cations4,8 and that the current is enhanced when Mg2+ was omitted from the intracellular solution.8,22,23 Consistent with these properties, HUVECs recorded without Mg-ATP in the pipette solution showed progressive increases in the amplitude of TRPM7-like currents (Figure 1A and B). In addition, currents were potentiated by removing the extracellular Ca2+/Mg2+ (to 253.7 ± 47.2% at −80 mV, and 128.1 ± 8.3% at +80 mV, n = 8; P < 0.05 at both voltages). Replacement of Na+ with choline in the extracellular solution caused a significant reduction in the amplitude of inward currents and a significant shift in the reversal potential by about −20 mV (n = 5, see Supplementary material online, Figure S1A). This finding is consistent with previous reports on the TRPM7 current.24 In contrast, replacing the extracellular Cl− with non-permeable anion gluconate had little effect on the current (n = 4, see Supplementary material online, Figure S1B), suggesting that volume-regulated Cl− channels were not involved.25 Furthermore, currents in HUVECs were suppressed by Gd3+ and 2-APB (Figure 1C and D), two commonly used non-specific blockers for TRPM7 channels.9,10,22,26–29 Together, these results strongly suggest that functional TRPM7 are expressed in HUVECs.
Figure 1.
TRPM7-like currents in HUVECs. (A) Representative currents elicited by voltage steps ranging from −100 to +100 mV with or without 8 mmol/L Mg-ATP in the pipette solution (n = 10 for 0 mmol/L Mg-ATP; n = 4 for 8 mmol/L Mg-ATP). (B) Time-dependent changes in the amplitude of TRPM7-like currents recorded at −80 and +80 mV in the absence or presence of Mg-ATP in the pipette solution (n = 7 for 0 mmol/L Mg-ATP; n = 4 for 8 mmol/L Mg-ATP). (C) Representative I–V relationship in the presence or absence of extracellular Ca2+/Mg2+, or following bath application of 10 µmol/L Gd3+. (D) Relative changes in the amplitude of TRPM7-like currents by Ca2+/Mg2+ removal, or by bath application of Gd3+ or 2-APB. n = 3–8. *P < 0.05, **P < 0.01.
3.2. Silencing TRPM7 reduced TRPM7 mRNA and the amplitude of TRPM7-like current
When RT–PCR was carried out, a band of the expected size for TRPM7 was observed (Figure 2A). In contrast, expression of TRPM6, which has highest structural homology and similar electrophysiological properties to TRPM7, was not detected.
To further demonstrate the presence of TRPM7 in HUVECs, we employed siRNAs specific for human TRPM7 (TRPM7-siRNA1 and TRPM7-siRNA2) to knockdown the expression of TRPM7 gene.10,22 Two to four days following the transfection with TRPM7-siRNAs, the level of TRPM7 mRNA was reduced by ∼70% as compared with the cells transfected with control siRNA (Figure 2B and Supplementary material online, Figure S2A). In contrast to TRPM7 channels, transfection with TRPM7-siRNA had no effect on the expression level of other TRP channels such as TRPM6 and TRPC1 (see Supplementary material online, Figure S2B). Consistent with reduced TRPM7 expression, HUVECs transfected with TRPM7-siRNA exhibited a smaller potentiation of the TRPM7-like currents in response to the removal of extracellular Ca2+/Mg2+. The amplitude of TRPM7-like current at −80 mV was increased to 256.0 ± 17.6% in cells transfected with control siRNA (n = 9), however, it was only increased to 160.8 ± 16.9% in cells transfected with TRPM7-siRNA1 (n = 8, P < 0.01, Figure 2C). These results provide additional evidence that functional TRPM7 channels are present in HUVECs.
3.3. Effect of TRPM7 silencing on the growth/proliferation of HUVECs
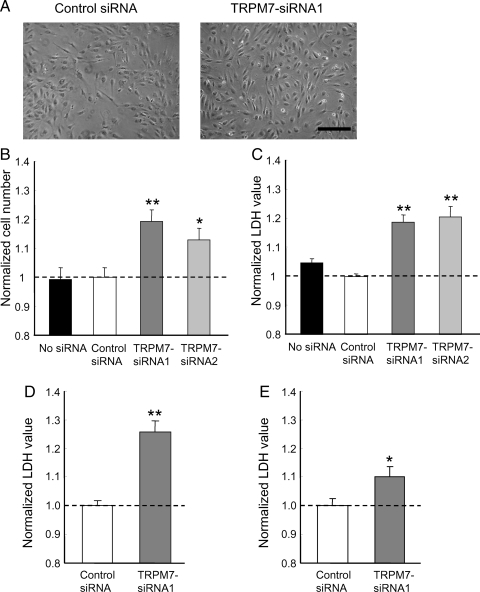
TRPM7 has been reported to affect the growth/proliferation of various cell types.10,11,14,22 Our next experiment was to determine whether TRPM7 also affects the growth/proliferation of HUVECs. 72 h following the transfection with TRPM7-siRNA, cells in each well were inspected under a microscope. As shown in Figure 3A, the total number of HUVECs in culture wells transfected with TRPM7-siRNAs appeared to be greater than the wells transfected with control siRNA (Figure 3A). When the cell numbers were counted, those transfected with TRPM7-siRNAs were significantly larger than that transfected with control siRNA (TRPM7-siRNA1: 119.2 ± 3.9%, P < 0.01; TRPM7-siRNA2: 113.0 ± 3.9%, P < 0.05, compared with control siRNA, n = 24, Figure 3B). To further quantify the changes in cell number, LDH release in each well was determined. Previous studies have indicated that the total amount of LDH is proportional to the total number of cells available in the culture.10,30 As shown in Figure 3C, 72 h after transfection, HUVECs transfected with TRPM7-siRNAs showed higher values of LDH than the cells transfected with control siRNA (TRPM7-siRNA1: 118.7 ± 2.5%, P < 0.01; TRPM7-siRNA2: 120.5 ± 3.6%, P < 0.01, compared with control siRNA, n = 18). Similar difference was obtained when total protein amounts were used as an indication of total number of cells (TRPM7-siRNA1: 118.6 ± 5.1%, compared with control siRNA, n = 9, P < 0.05, data not shown). Thus, silencing TRPM7 leads to enhanced growth/proliferation of HUVECs.
Figure 3.
TRPM7 silencing promotes growth/proliferation of HUVECs. (A) Photos taken at 72 h following transfection with control (left) or TRPM7-siRNA1 (right). Scale bar: 250 µm. (B and C) Normalized cell counting and LDH release for HUVECs treated with control or TRPM7-siRNAs for 72 h. n = 18–24. *P < 0.05, **P < 0.01 between control and siRNA-treated cells. (D) Cells were growth-arrested in 1% serum without supplement for 24 h and then allowed to grow in normal culture media for 72 h, followed by LDH assay. n = 12. **P < 0.01 between control and siRNA-treated cells. (E) Normalized LDH release for HMVECs treated with control or TRPM7-siRNA1 for 72 h. n = 20. *P < 0.05 between control and TRPM7-siRNA-treated cells.
Although commonly used, HUVECs are often referred to as fetal endothelial cells. To know whether the effect of TRPM7 on the growth/proliferation is limited to the fetal phenotype of these cells, we also tested the effect of TRPM7 siRNA on the growth/proliferation of HUVECs following a 24 h growth arrest, as described in previous studies.31 As shown in Figure 3D, knockdown of TRPM7 still facilitated growth/proliferation of HUVECs under this condition. In addition, we tested the effect of TRPM7 siRNA on the growth/proliferation of HMVECs, an adult cell line of microvascular endothelial cells.32 As shown in Figure 3E, a lightly less but still significant enhancement in the growth/proliferation by TRPM7-siRNA was also observed in HMVECs (TRPM7-siRNA1: 110.0 ± 16.8%, compared with control siRNA, n = 20, P < 0.05). Together, these findings suggest that silencing TRPM7 channels promotes the growth/proliferation of both fetal and adult vascular endothelial cells.
3.4. Effect of TRPM7 silencing on MAPK signalling pathways
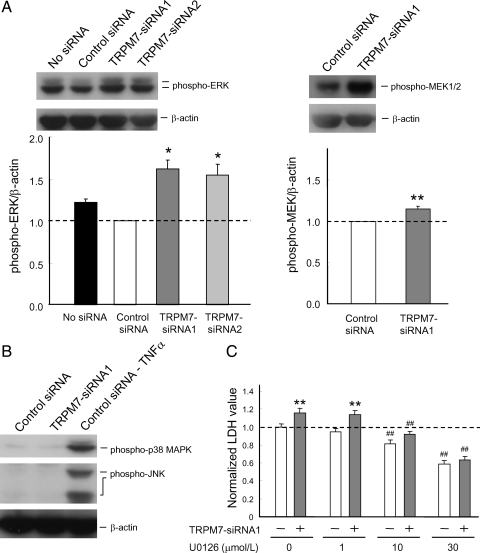
MAPK signalling pathways are important in cellular behaviour including growth/proliferation.17,33–35 To determine if silencing TRPM7 affects the activity of MAPKs, we detected the change of phosphorylated form of ERK, JNK, and p38 MAPK using phospho-specific antibodies. Immunoblotting analysis showed a clear increase in phosphorylated ERKs in HUVECs transfected with TRPM7-siRNAs (Figure 4A). Similarly, increased phosphorylation of MEK1/2, upstream kinases of ERKs, was detected following TRPM7 silencing (Figure 4A). In contrast, silencing TRPM7 did not affect the phosphorylation of p38 MAPK and JNK (Figure 4B).
Figure 4.
ERK activation is involved in enhanced growth/proliferation of HUVECs by silencing TRPM7. (A) Representative immunoblots showing increased phospho-ERK (left) or phospho-MEK1/2 (right) in HUVECs 72 h following the transfection of the indicated siRNAs. Bar graphs show densitometrically quantified ratio of phospho-ERK/β-actin (left) and phospho-MEK1/2/β-actin (right) in HUVECs transfected with or without control or TRPM7-siRNAs (n=3, *P < 0.05, **P < 0.01, control siRNA vs. TRPM7-siRNA1-treated cells. (B) Immonoblotting showing the lack of effect on phospho-p38 MARK and phospho-JNK by TRPM7-siRNA1. Third lane shows control siRNA-treated cells incubated with 20 ng/ml TNFα (for 15 min) as a positive control. (C) Treatment of HUVECs with ERK inhibitor U0126 (10 or 30 µmol/L) attenuated or prevented enhancement of growth/proliferation by TRPM7-siRNA1. n = 7–11. **P < 0.01, control vs. TRPM7-siRNA1-treated cells, ##P < 0.01, U0126 untreated vs. treated cells.
3.5. Effect of ERK pathway on the growth/proliferation of HUVECs
To determine whether activation of ERK signalling pathway is involved in enhanced growth/proliferation of HUVECs by TRPM7-siRNA, the same experiments were performed in the presence of MEK1/2 specific inhibitor, U0126. As shown in Figure 4C, treatment of HUVECs with 10 or 30 µmol/L U0126 alone induced a dose-dependent inhibition of the cell growth/proliferation (Figure 4C). Addition of TRPM7-siRNA did not induce a significant increase of the growth/proliferation of HUVECs in the presence of U0126. In the presence of 30 µmol/L U0126, for example, the relative LDH was 58.9 ± 3.5% in cells transfected with control siRNA and 63.5 ± 3.3% in cells transfected with TRPM7-siRNA1 (n = 7 each, P = 0.36). These data suggest that proliferative effect by silencing TRPM7 may be accomplished, at least in part, through ERK activation.
Activation of ERK pathway may require various factors included in the serum. Culture medium we used contained FBS and other trophic factors, some of which, such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor, are known to facilitate the ERK activation.33,34 To test whether these components are required for TRPM7-mediated changes of cell growth/proliferation, the same experiment was performed with cells incubated in serum containing media without supplement of growth factors. Similar to the media supplemented with various growth factors, media with only serum still sustained the difference in growth/proliferation between control and TRPM7-siRNA transfected cells (control siRNA: 78.4 ± 3.7%, n = 8; TRPM7-siRNA1: 95.6 ± 5.4%, n = 8; P < 0.05, data not shown). However, if cells were cultured in the absence of both serum and growth factor supplement, there was no apparent difference in growth/proliferation between control siRNA and TRPM7-siRNA transfected cells (control siRNA: 69.7 ± 4.6%, n = 11; TRPM7-siRNA1: 68.1 ± 4.8%, n = 12; P = 0.81), regardless of the fact that the expression of TRPM7 channels in these cells was maintained and that transfection of TRPM7 siRNA reduced the level of channel expression (see Supplementary material online, Figure S3A and B). These findings further suggest that a condition for normal activation of ERK pathway is required for TRPM7-mediated change of cell growth/proliferation to take place.
3.6. Effect of TRPM7 silencing on eNOS expression and NO production
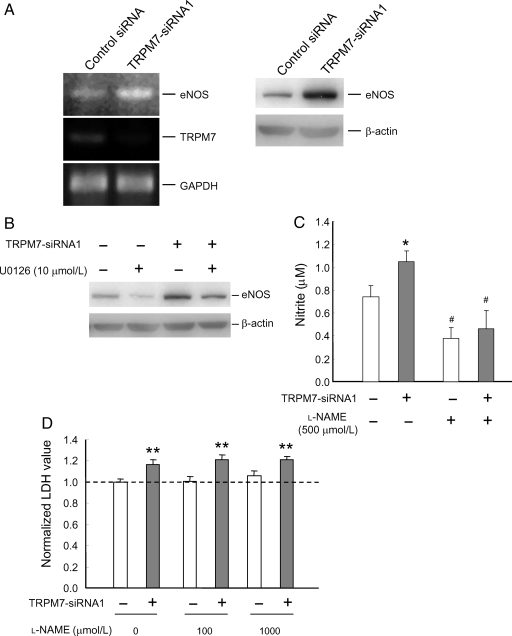
Earlier studies demonstrated that activation of ERK pathway affects endothelial eNOS expression induced by stimuli such as shear stress or change of pressure.36,37 To determine whether changes in TRPM7 expression also affect the level of eNOS, eNOS expression was examined following the transfection of TRPM7-siRNA. As shown in Figure 5A, TRPM7-siRNA transfected cells showed increased eNOS mRNA and protein level (Figure 5A). The increased eNOS expression was attenuated by U0126, suggesting the involvement of ERK pathway (Figure 5B).
Figure 5.
TRPM7 silencing enhances eNOS expression via ERK pathway. (A) RT–PCR (left) or immunoblotting (right) showing the effect of TRPM7-siRNA1 on the level of eNOS mRNA and protein in HUVECs. HUVECs were treated with control or TRPM7-siRNA1. 48 h after transfection, RT–PCR was performed using specific primers for TRPM7, eNOS, and GAPDH. For immunoblotting, eNOS protein level was examined 72 h after the transfection by immunoblotting with anti-eNOS antibody. (B) Treatment with ERK inhibitor U0126 reduced expression of eNOS and its enhancement by TRPM7-siRNA1. (C) TRPM7 silencing increased the production of NO as demonstrated by nitrite measurement. This increased production of nitrite was attenuated by 500 µmol/L l-NAME. n = 12–20. *P < 0.05, control vs. TRPM7-siRNA1-treated cells; #P < 0.05, l-NAME-treated vs. untreated cells. (D) Treatment of cells with l-NAME (100 and 1000 µmol/L) did not affect the enhancement of cell growth/proliferation by TRPM7-siRNA1. n = 9–11. **P < 0.01, control vs. TRPM7-siRNA1-treated cells.
To know whether increased eNOS expression can be translated into increased NO release, NO metabolite (nitrite) was measured in control and TRPM7-siRNA transfected cells. As shown in Figure 5C, transfection of HUVECs with TRPM7-siRNA induced a significant increase in the level of NO metabolite (control siRNA: 0.74 ± 0.1 µmol/L, n = 20; TRPM7-siRNA1: 1.04 ± 0.1 µmol/L, n = 20; P < 0.05, Figure 5C). Consistent with an increased activity of eNOS, the increase of NO metabolite was inhibited by the presence of l-NAME, a commonly used non-specific NOS inhibitor (control siRNA: 0.38 ± 0.1 µmol/L, n = 12; TRPM7-siRNA1: 0.46 ± 0.2 µmol/L, n = 12; P = 0.65, Figure 5C).
The role of NO in endothelial proliferation has been controversial.38–40 To determine whether NO production is involved in the growth/proliferation of HUVECs, we examined whether treatment with NO inhibitor affects the growth/proliferation of these cells. As shown in Figure 5D, treating cells with l-NAME did not affect the baseline growth/proliferation of HUVECs or its potentiation by TRPM7-siRNA. In the presence of 1 mmol/L l-NAME, for example, the relative LDH was 105.8 ± 4.5% in cell transfected with control siRNA (n = 9) but 121.4 ± 2.4% in cells transfected with TRPM7-siRNA1 (n = 9, P < 0.01, Figure 5D). Thus, increased NO production does not seem to be responsible for enhanced growth/proliferation of HUVECs by TRPM7 silencing.
3.7. 2-APB on the growth/proliferation of HUVECs
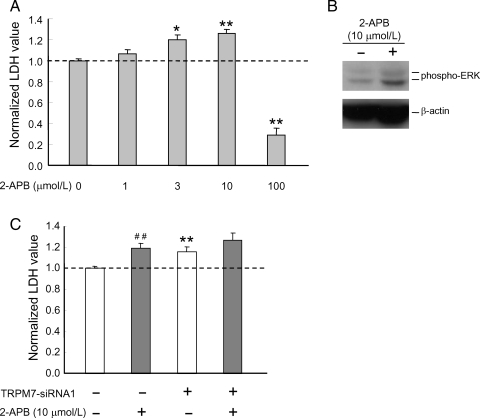
To further establish the role of TRPM7 in the growth/proliferation of HUVECs, the effect of 2-APB, a commonly used non-specific blocker of TRPM7 channels, on the growth/proliferation was examined. Similar to TRPM7-siRNAs, inhibition of TRPM7 with 2-APB (e.g. 10 µmol/L) significantly increased the growth/proliferation of HUVECs as well as the phosphorylation of ERKs (Figure 6A and B). High concentration of 2-APB (e.g. 100 µmol/L), however, inhibited the growth/proliferation of these cells, likely due to the non-specific effect of this agent.41
Figure 6.
Effect of 2-APB on ERK activity and growth/proliferation of HUVECs. (A) Treatment of HUVECs with 3 or 10 µmol/L 2-APB enhanced the growth/proliferation. n = 8–16. *P < 0.05, **P < 0.01, 2-APB-treated vs. untreated cells. Total LDH was analysed 72 h following the treatment with 2-APB. (B) Treatment of HUVECs with 10 µmol/L 2-APB for 48 h increased level of phospho-ERK protein. (C) Treatment of HUVECs with 2-APB (10 µmol/L) did not induce additional enhancement of the growth/proliferation in the presence of TRPM7-siRNA. n = 12. **P < 0.01, control vs. TRPM7-siRNA1-treated cells; ##P < 0.01, 2-APB-treated vs. untreated cells.
To gain more evidence that the effect of 2-APB on the growth/proliferation of HUVECs was due to its effect on TRPM7, the effect of 2-APB on the growth/proliferation of the HUVECs was further examined with cells transfected with TRPM7-siRNA. As shown in Figure 6C, treatment of HUVECs with TRPM7-siRNA or 2-APB both increased the cell growth/proliferation. However, addition of 2-APB in the presence of TRPM7-siRNA did not induce further increase of the growth/proliferation when compared with the cells treated with TRPM7-siRNA alone (without 2-APB: 115.6 ± 4.7%, n = 12; with 2-APB: 126.7 ± 6.7%, n = 12; P = 0.19), suggesting that proliferative response induced by 2-APB is mediated, at least in part, by TRPM7.
4. Discussion
TRP channels are known to be expressed broadly in various tissues, and have been shown to play a critical role in several physiological and pathophysiological processes.1,2 Using a combination of biochemical, molecular biological, and electrophysiological approaches, we demonstrated the presence of functional TRPM7 channels in HUVECs. Although the currents in these cells are not as large as those reported in other cell types,10,14,15,22 the following evidence strongly suggests that they are mediated by the TRPM7 channels: (i) the currents develop gradually after forming whole-cell configuration in the absence of intracellular Mg-ATP; (ii) outward rectification of the current is reduced in the absence of intracellular Mg-ATP; (iii) the currents are potentiated by removing Ca2+/Mg2+ from the extracellular solution; (iv) currents are inhibited by Gd3+ and 2-APB, two commonly used non-specific blockers for TRPM7 channels; and (v) transfection with siRNA specific for TRPM7 decreases both TRPM7 mRNA and TRPM7-like currents. Although TRPM6 shares various properties with TRPM7,29,42 our RT–PCR did not show any expression of this isoform in HUVECs (Figure 2A).
Previous studies by others and our own have shown that the growth/proliferation of various cell types (e.g. head and neck tumour cells) is, in general, inhibited if the activity/expression of TRPM7 is reduced.10,14,22 In contrast to those general findings, inhibition of TRPM7 enhanced the growth/proliferation of HUVECs. Therefore, HUVECs appear to be the first cell type whose growth/proliferation is promoted, instead of inhibited, by TRPM7 silencing/inhibition. However, it is not the first report that shows different prolifereative responses between vascular endothelial cells from other cell types. For example, the opposite proliferative responses between endothelial cells and other cell types have been recently demonstrated by manipulation of the activity/expression of connexin 43 or PPARβ/δ although the detailed mechanisms remain largely unknown.43–46
Another major finding of the present study is an increase of ERK activity in HUVECs following knockdown of TRPM7, and that this enhanced activation of ERK is likely responsible for the increased growth/proliferation of these cells. This finding is supported by the data that phosphorylated form of ERK is dramatically increased following the silencing of TRPM7 or TRPM7 blockade, and that an inhibition of the ERK activity (e.g. by U0126) attenuated the proliferative effect of TRPM7 silencing. Proliferation of vascular endothelial cells is one of critical biological events underlying angiogenesis and recovery of blood vessels from various insults. Trophic factors such as VEGF, insulin-like growth factor, and epithelial growth factor that normally present in serum,47–49 are known to promote cell growth/proliferation via, at least in part, ERK signalling pathway.34,50 Our finding that TRPM7 silencing lost its effect on ERK activation and HUVECs growth/proliferation in serum and trophic factor-free medium suggests that some of those factors are required for the effect of TRPM7 silencing to take place. Although the precise mechanism by which TRPM7 channel activity influences the ERK signalling pathway requires further investigation, the phospholipase C signalling pathway could have been modulated by reduced activity of TRPM7 channels, resulting in ERK activation as described in a previous report.34 Besides, it may be conceivable that compensatory changes in response to reduced TRPM7 channel activity and the decreased level of intracellular Ca2+/Mg2+ might result in an increased ERK activation. A change in the kinase activity of TRPM7 might also influence the ERK signalling pathway although it is still not clear whether inhibition of TRPM7 channel also affects its kinase activity. In addition to enhanced cell growth/proliferation, up-regulation of eNOS expression was found in HUVECs by silencing TRPM7. This effect is also mediated, at least in part, by the activation of ERK pathway. The increased growth/proliferation of HUVECs by TRPM7 silencing is, however, independent of the increased eNOS activity and the production of NO. eNOS activity is not only critical for NO-mediated vasodilation, but also valuable for the control of angiogenic factors at proangiogenic phases.51–53 The pro-angiogenic function of NO was further supported by the observation that endothelial NO released by VEGF promotes angiogenic activity without affecting proliferation.54 Our current finding that both endothelial cell growth/proliferation and NO production are facilitated by knockdown of TRPM7, combined with our previous report that TRPM7 silencing protects neurons from anoxic injury, raises the possibility that targeting TRPM7 channels may prove to be a novel therapeutic strategy for stroke intervention.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by grants from National Institutes of Health (R01NS42926, R01NS49470) and American Heart Association Established Investigator Award (0840132 N).
Acknowledgements
We thank Ms D. Branigan for technical assistance.
Conflict of interest: none declared.
References
- 1.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 2.Inoue R, Jensen LJ, Shi J, Morita H, Nishida M, Honda A, et al. Transient receptor potential channels in cardiovascular function and disease. Circ Res. 2006;99:119–131. doi: 10.1161/01.RES.0000233356.10630.8a. [DOI] [PubMed] [Google Scholar]
- 3.Clark K, Langeslag M, van Leeuwen B, Ran L, Ryazanov AG, Figdor CG, et al. TRPM7, a novel regulator of actomyosin contractility and cell adhesion. EMBO J. 2006;25:290–301. doi: 10.1038/sj.emboj.7600931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Runnels LW, Yue L, Clapham DE. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science. 2001;291:1043–1047. doi: 10.1126/science.1058519. [DOI] [PubMed] [Google Scholar]
- 5.Takezawa R, Schmitz C, Demeuse P, Scharenberg AM, Penner R, Fleig A. Receptor-mediated regulation of the TRPM7 channel through its endogenous protein kinase domain. Proc Natl Acad Sci USA. 2004;101:6009–6014. doi: 10.1073/pnas.0307565101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryazanova LV, Dorovkov MV, Ansari A, Ryazanov AG. Characterization of the protein kinase activity of TRPM7/ChaK1, a protein kinase fused to the transient receptor potential ion channel. J Biol Chem. 2004;279:3708–3716. doi: 10.1074/jbc.M308820200. [DOI] [PubMed] [Google Scholar]
- 7.Voets T, Nilius B, Hoefs S, van der Kemp AW, Droogmans G, Bindels RJ, et al. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem. 2004;279:19–25. doi: 10.1074/jbc.M311201200. [DOI] [PubMed] [Google Scholar]
- 8.Nadler MJ, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ, et al. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- 9.Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, et al. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–877. doi: 10.1016/s0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- 10.Jiang J, Li MH, Inoue K, Chu XP, Seeds J, Xiong ZG. Transient receptor potential melastatin 7-like current in human head and neck carcinoma cells: role in cell proliferation. Cancer Res. 2007;67:10929–10938. doi: 10.1158/0008-5472.CAN-07-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, Penner R, et al. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell. 2003;114:191–200. doi: 10.1016/s0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- 12.Wei WL, Sun HS, Olah ME, Sun X, Czerwinska E, Czerwinski W, et al. TRPM7 channels in hippocampal neurons detect levels of extracellular divalent cations. Proc Natl Acad Sci USA. 2007;104:16323–16328. doi: 10.1073/pnas.0701149104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gwanyanya A, Sipido KR, Vereecke J, Mubagwa K. ATP and PIP2 dependence of the magnesium-inhibited, TRPM7-like cation channel in cardiac myocytes. Am J Physiol Cell Physiol. 2006;291:C627–C635. doi: 10.1152/ajpcell.00074.2006. [DOI] [PubMed] [Google Scholar]
- 14.He Y, Yao G, Savoia C, Touyz RM. Transient receptor potential melastatin 7 ion channels regulate magnesium homeostasis in vascular smooth muscle cells: role of angiotensin II. Circ Res. 2005;96:207–215. doi: 10.1161/01.RES.0000152967.88472.3e. [DOI] [PubMed] [Google Scholar]
- 15.Oancea E, Wolfe JT, Clapham DE. Functional TRPM7 channels accumulate at the plasma membrane in response to fluid flow. Circ Res. 2006;98:245–253. doi: 10.1161/01.RES.0000200179.29375.cc. [DOI] [PubMed] [Google Scholar]
- 16.Cleaver O, Melton DA. Endothelial signaling during development. Nat Med. 2003;9:661–668. doi: 10.1038/nm0603-661. [DOI] [PubMed] [Google Scholar]
- 17.Inoue K, Zama T, Kamimoto T, Aoki R, Ikeda Y, Kimura H, et al. TNFalpha-induced ATF3 expression is bidirectionally regulated by the JNK and ERK pathways in vascular endothelial cells. Genes Cells. 2004;9:59–70. doi: 10.1111/j.1356-9597.2004.00707.x. [DOI] [PubMed] [Google Scholar]
- 18.Inoue K, Ueno S, Fukuda A. Interaction of neuron-specific K+-Cl− cotransporter, KCC2, with brain-type creatine kinase. FEBS Lett. 2004;564:131–135. doi: 10.1016/S0014-5793(04)00328-X. [DOI] [PubMed] [Google Scholar]
- 19.Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, et al. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118:687–698. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 20.Misko TP, Schilling RJ, Salvemini D, Moore WM, Currie MG. A fluorometric assay for the measurement of nitrite in biological samples. Anal Biochem. 1993;214:11–16. doi: 10.1006/abio.1993.1449. [DOI] [PubMed] [Google Scholar]
- 21.Hecquet CM, Ahmmed GU, Vogel SM, Malik AB. Role of TRPM2 channel in mediating H2O2-induced Ca2+ entry and endothelial hyperpermeability. Circ Res. 2008;102:347–355. doi: 10.1161/CIRCRESAHA.107.160176. [DOI] [PubMed] [Google Scholar]
- 22.Hanano T, Hara Y, Shi J, Morita H, Umebayashi C, Mori E, et al. Involvement of TRPM7 in cell growth as a spontaneously activated Ca2+ entry pathway in human retinoblastoma cells. J Pharmacol Sci. 2004;95:403–419. doi: 10.1254/jphs.fp0040273. [DOI] [PubMed] [Google Scholar]
- 23.Kozak JA, Cahalan MD. MIC channels are inhibited by internal divalent cations but not ATP. Biophys J. 2003;84:922–927. doi: 10.1016/S0006-3495(03)74909-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang J, Li M, Yue L. Potentiation of TRPM7 inward currents by protons. J Gen Physiol. 2005;126:137–150. doi: 10.1085/jgp.200409185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duan D, Winter C, Cowley S, Hume JR, Horowitz B. Molecular identification of a volume-regulated chloride channel. Nature. 1997;390:417–421. doi: 10.1038/37151. [DOI] [PubMed] [Google Scholar]
- 26.Monteilh-Zoller MK, Hermosura MC, Nadler MJ, Scharenberg AM, Penner R, Fleig A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J Gen Physiol. 2003;121:49–60. doi: 10.1085/jgp.20028740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang X, Newell EW, Schlichter LC. Regulation of a TRPM7-like current in rat brain microglia. J Biol Chem. 2003;278:42867–42876. doi: 10.1074/jbc.M304487200. [DOI] [PubMed] [Google Scholar]
- 28.Li M, Du J, Jiang J, Ratzan W, Su LT, Runnels LW, et al. Molecular determinants of Mg2+ and Ca2+ permeability and pH sensitivity in TRPM6 and TRPM7. J Biol Chem. 2007;282:25817–25830. doi: 10.1074/jbc.M608972200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li M, Jiang J, Yue L. Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J Gen Physiol. 2006;127:525–537. doi: 10.1085/jgp.200609502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koh JY, Choi DW. Quantitative determination of glutamate mediated cortical neuronal injury in cell culture by lactate dehydrogenase efflux assay. J Neurosci Methods. 1987;20:83–90. doi: 10.1016/0165-0270(87)90041-0. [DOI] [PubMed] [Google Scholar]
- 31.Hanai J, Dhanabal M, Karumanchi SA, Albanese C, Waterman M, Chan B, et al. Endostatin causes G1 arrest of endothelial cells through inhibition of cyclin D1. J Biol Chem. 2002;277:16464–16469. doi: 10.1074/jbc.M112274200. [DOI] [PubMed] [Google Scholar]
- 32.Sharma-Walia N, Krishnan HH, Naranatt PP, Zeng L, Smith MS, Chandran B. ERK1/2 and MEK1/2 induced by Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) early during infection of target cells are essential for expression of viral genes and for establishment of infection. J Virol. 2005;79:10308–10329. doi: 10.1128/JVI.79.16.10308-10329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu Y, Sato JD. MAP kinases, phosphatidylinositol 3-kinase, and p70 S6 kinase mediate the mitogenic response of human endothelial cells to vascular endothelial growth factor. J Cell Physiol. 1999;178:235–246. doi: 10.1002/(SICI)1097-4652(199902)178:2<235::AID-JCP13>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 34.Wu LW, Mayo LD, Dunbar JD, Kessler KM, Baerwald MR, Jaffe EA, et al. Utilization of distinct signaling pathways by receptors for vascular endothelial cell growth factor and other mitogens in the induction of endothelial cell proliferation. J Biol Chem. 2000;275:5096–5103. doi: 10.1074/jbc.275.7.5096. [DOI] [PubMed] [Google Scholar]
- 35.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 36.Cieslik K, Abrams CS, Wu KK. Up-regulation of endothelial nitric-oxide synthase promoter by the phosphatidylinositol 3-kinase gamma/Janus kinase 2/MEK-1-dependent pathway. J Biol Chem. 2001;276:1211–1219. doi: 10.1074/jbc.M005305200. [DOI] [PubMed] [Google Scholar]
- 37.Vouyouka AG, Jiang Y, Rastogi R, Basson MD. Ambient pressure upregulates nitric oxide synthase in a phosphorylated-extracellular regulated kinase- and protein kinase C-dependent manner. J Vasc Surg. 2006;44:1076–1084. doi: 10.1016/j.jvs.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 38.Heller R, Polack T, Grabner R, Till U. Nitric oxide inhibits proliferation of human endothelial cells via a mechanism independent of cGMP. Atherosclerosis. 1999;144:49–57. doi: 10.1016/s0021-9150(99)00041-6. [DOI] [PubMed] [Google Scholar]
- 39.Zanetti M, Katusic ZS, O'Brien T. Expression and function of recombinant endothelial nitric oxide synthase in human endothelial cells. J Vasc Res. 2000;37:449–456. doi: 10.1159/000054077. [DOI] [PubMed] [Google Scholar]
- 40.Ziche M, Morbidelli L, Masini E, Amerini S, Granger HJ, Maggi CA, et al. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest. 1994;94:2036–2044. doi: 10.1172/JCI117557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem. 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- 42.Schmitz C, Dorovkov MV, Zhao X, Davenport BJ, Ryazanov AG, Perraud AL. The channel kinases TRPM6 and TRPM7 are functionally nonredundant. J Biol Chem. 2005;280:37763–37771. doi: 10.1074/jbc.M509175200. [DOI] [PubMed] [Google Scholar]
- 43.Wang HH, Kung CI, Tseng YY, Lin YC, Chen CH, Tsai CH, et al. Activation of endothelial cells to pathological status by down-regulation of connexin43. Cardiovasc Res. 2008;79:509–518. doi: 10.1093/cvr/cvn112. [DOI] [PubMed] [Google Scholar]
- 44.Herrero-Gonzalez S, Valle-Casuso JC, Sanchez-Alvarez R, Giaume C, Medina JM, Tabernero A. Connexin43 is involved in the effect of endothelin-1 on astrocyte proliferation and glucose uptake. Glia. 2009;57:222–233. doi: 10.1002/glia.20748. [DOI] [PubMed] [Google Scholar]
- 45.Piqueras L, Reynolds AR, Hodivala-Dilke KM, Alfranca A, Redondo JM, Hatae T, et al. Activation of PPARbeta/delta induces endothelial cell proliferation and angiogenesis. Arterioscler Thromb Vasc Biol. 2007;27:63–69. doi: 10.1161/01.ATV.0000250972.83623.61. [DOI] [PubMed] [Google Scholar]
- 46.Muller R, Rieck M, Muller-Brusselbach S. Regulation of Cell Proliferation and Differentiation by PPARbeta/delta. PPAR Res. 2008;2008:614852. doi: 10.1155/2008/614852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rommel C, Clarke BA, Zimmermann S, Nunez L, Rossman R, Reid K, et al. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science. 1999;286:1738–1741. doi: 10.1126/science.286.5445.1738. [DOI] [PubMed] [Google Scholar]
- 48.Frost JA, Geppert TD, Cobb MH, Feramisco JR. A requirement for extracellular signal-regulated kinase (ERK) function in the activation of AP-1 by Ha-Ras, phorbol 12-myristate 13-acetate, and serum. Proc Natl Acad Sci USA. 1994;91:3844–3848. doi: 10.1073/pnas.91.9.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cavigelli M, Dolfi F, Claret FX, Karin M. Induction of c-fos expression through JNK-mediated TCF/Elk-1 phosphorylation. EMBO J. 1995;14:5957–5964. doi: 10.1002/j.1460-2075.1995.tb00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu W, Liu Y, Lowe WL., Jr The role of phosphatidylinositol 3-kinase and the mitogen-activated protein kinases in insulin-like growth factor-I-mediated effects in vascular endothelial cells. Endocrinology. 2001;142:1710–1719. doi: 10.1210/endo.142.5.8136. [DOI] [PubMed] [Google Scholar]
- 51.Duda DG, Fukumura D, Jain RK. Role of eNOS in neovascularization: NO for endothelial progenitor cells. Trends Mol Med. 2004;10:143–145. doi: 10.1016/j.molmed.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 52.Gertz K, Priller J, Kronenberg G, Fink KB, Winter B, Schrock H, et al. Physical activity improves long-term stroke outcome via endothelial nitric oxide synthase-dependent augmentation of neovascularization and cerebral blood flow. Circ Res. 2006;99:1132–1140. doi: 10.1161/01.RES.0000250175.14861.77. [DOI] [PubMed] [Google Scholar]
- 53.Han RN, Babaei S, Robb M, Lee T, Ridsdale R, Ackerley C, et al. Defective lung vascular development and fatal respiratory distress in endothelial NO synthase-deficient mice: a model of alveolar capillary dysplasia? Circ Res. 2004;94:1115–1123. doi: 10.1161/01.RES.0000125624.85852.1E. [DOI] [PubMed] [Google Scholar]
- 54.Papapetropoulos A, Garcia-Cardena G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest. 1997;100:3131–3139. doi: 10.1172/JCI119868. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.