Abstract
Schizophrenics show P3 amplitude reduction and topographic asymmetries. It is unclear whether the underlying cause of these deficits is primarily functional or structural. This study examined the effect of stimulus discriminability and task instruction on behavioral performance and P3 in schizophrenics and normal control subjects. Stimulus discriminability was manipulated by varying the overall loudness and pitch disparity of the two tones in an auditory oddball paradigm. Instructions emphasized either speed or accuracy of response. Instructions had no significant effects on reaction time, perceptual sensitivity, response bias, or P3. With increased discriminability, however, both groups improved in mean reaction time to targets and perceptual sensitivity. In controls, P3 became earlier and larger with increased stimulus discriminability and was consistently larger over left temporal areas than over right temporal areas. In schizophrenics, P3 latency was related to stimulus discriminability, but amplitude was not; P3 amplitude did not increase with improvement of perceptual sensitivity and reaction time. Unlike normal controls, schizophrenics had a P3 asymmetry at temporal sites, with reduced left-sided voltages. The results are not consistent with a primarily functional cause of P3 aberrations in schizophrenia and are compatible with the hypothesis that P3 amplitude deficits in schizophrenia are related to underlying pathophysiology of temporal lobe generator sites.
Descriptors: Event-related potentials, P3, Scalp asymmetry, Schizophrenia, Stimulus parameters
The association of the P3 event-related potential (ERP) with selective attention and processing of novel stimuli is well established (e.g., Donchin, Karis, Bashore, Coles, & Gratton, 1986; Hillyard, Hink, Schwent, & Picton, 1973; Isreal, Wickens, & Donchin, 1979; Squires, Squires, & Hillyard, 1975). The problems in selective attention, veridical perception of novel stimuli, and disturbed thought processes characteristic of schizophrenia make the P3 a useful probe of information processing in schizophrenics. The demonstration that schizophrenics show an overall decrement in auditory-evoked P3 amplitude relative to controls remains one of the most replicable ERP findings in psychiatry (see Begleiter & Porjesz, 1986; Holzman, 1987; Pritchard, 1986; Roth, 1977, for review).
The relative contributions of functional and structural deficits to the voltage reduction in P3 amplitude in schizophrenics remains unclear. Functionally, auditory P3 amplitude varies with task difficulty in normal subjects (e.g., Polich, 1987). The decrement observed in schizophrenics may be due to lowered sensitivity and performance in comparison with control subjects, with little involvement of the neural substrate of P3. That is, schizophrenics’ P3 responses to stimuli in standard oddball tasks may be morphologically similar to those of controls in conditions where stimuli are not easily discriminable. A greater degree of equivocation in stimulus analysis could lead to smaller P3 amplitudes (Ruchkin & Sutton, 1978). In this case, increases in stimulus discriminability should result in P3 amplitudes in the schizophrenics more in the normal range. Likewise, lack of attention to task leads to P3 reduction (e.g., Hillyard et al., 1973; Johnson, 1986). The P3 reduction demonstrated by schizophrenics may reflect inattention without neuropathology. Monitoring sensitivity and response bias in task performance and analyzing only correct responses allows assessment of the role of poor sensitivity and attention in P3 reduction in schizophrenics.
Alternatively, the voltage decrement in schizophrenics may be more directly attributable to neuropathology in the medial and lateral temporal lobe generator sites of P3 (Halgren et al., 1980; Knight, Scabini, Woods, & Clayworth, 1987; McCarley et al., 1993; McCarley, Shenton, O’Donnell, Holinger, & Nestor, 1992; Okada, Kaufman, & Williamson, 1983; Rogers et al., 1991). In this case, increases in stimulus discriminability would have little systematic effect on P3 voltage in schizophrenics, even if performance and sensitivity improve. If schizophrenics demonstrate sensitivity, attention to task, and performance similar to that of controls, yet P3 amplitude remains reduced, then neuropathology in P3 generators is likely.
Schizophrenics also show left-sided temporal area electro-physiological abnormalities, both in electroencephalograms (e.g., alpha coherence [Michelogiannis, Paritsis, & Trikas, 1991]) and P3 voltage (e.g., Morstyn, Duffy, & McCarley, 1983). Recent experiments in our lab have replicated this left temporal scalp area deficit in P3 in schizophrenics, with smaller amplitudes at electrode sites overlying left temporal lobe relative to those on the right (Faux, Shenton, McCarley, Torello, & Duffy, 1988a; Faux, Torello, McCarley, Shenton, & Duffy, 1988b; Faux et al., 1990). However, control subjects show symmetric or larger left-sided amplitudes. This left temporal scalp deficit in P3 amplitude in schizophrenics has been interpreted as consistent with involvement of left temporal lobe structures in the pathogenesis of schizophrenia (see McCarley, Faux, Shenton, Nestor, & Adams, 1991). Although unequivocal judgements of source location cannot be made from scalp topography alone, converging evidence from dipole modeling, lesion, MEG, and structural/imaging studies has elucidated neural generators of scalp potentials. For example, Knight, Scabini, Woods, and Clayworth (1988) demonstrated quite clearly that unilateral lesions of the superior temporal gyrus (STG) in humans led to lateralized ERP reductions, and our group has recently demonstrated high correlations between lateralized P3 reductions and reduced left STG magnetic resonance imaging (MRI) volume in schizophrenics (McCarley et al., 1993).
The presence of left-sided P3 amplitude deficits in schizophrenia was replicated by Kraft, Schwarzkopf, Torello, Olson, and Nasrallah (1991) in 29 male schizophrenic outpatients (handedness not reported), Sieg, Willsie, Preston, and Gaffney (1991) in a case report of a right-handed female schizophrenic, and Bruder et al. (1992) in a group of 8 right-handed psychotic patients (4 schizophrenic, 2 schizoaffective, 1 paranoid delusional, and 1 schizotype; sex not reported). However, Maurer and Dierks (1987) reported a right-sided P3 deficit in 10 paranoid schizophrenics (handedness not reported), and Pfefferbaum, Ford, White, and Roth (1989) reported fairly symmetrical P3s in schizophrenics for an auditory reaction time task (30 schizophrenics, mixed handedness, 18 medication free).
These dissimilar results may reflect subject sampling differences. Shenton et al. (1989) suggested that right-sided deficits may characterize a subgroup of schizophrenics, namely those with poorer response to neuroleptics, poorer past and current functioning, more thought disorder, positive symptoms, and diffuse neuropsychological deficits. Additionally, Holinger et al. (1992) demonstrated a right-sided reduction in P3 amplitude in left-handed schizophrenics and confirmed the left-sided reduction in right-handed schizophrenics. However, the role that different stimulus parameters and task demands play in both overall and relative P3 amplitude in schizophrenics has not been assessed.
To distinguish between performance deficit (functional) and neuropathological (structural) hypotheses regarding P3 amplitude reduction in schizophrenia, we examined the influence of Stimulus discriminability and response instructions on behavioral and ERP responses of controls and schizophrenics to low-probability target tones in an auditory oddball task. Stimulus discriminability was manipulated by varying the overall loudness (intensity) and pitch (frequency) disparity of the target and standard tones. Presentation of white noise allowed for precise control of the baseline activity level in the auditory system; consequently, signal detection measures of sensitivity and response bias were used to monitor performance while stimulus parameters changed (Green & Swets, 1966). Response demands were manipulated by emphasizing either speed or accuracy of response.
Whereas our previous research has compared voltages from single electrode sites overlying the temporal lobe (e.g., T3 vs. T4), computerized tomography (CT) (Homan, Herman, & Purdy, 1987) and MRI (Steinmetz, Fürst, & Meyer, 1989) studies of electrode localization have demonstrated that six electrode sites in our montage consistently overlie temporal lobe structures. T3 and T4 overlie middle and superior temporal gyri medially, T5 and T6 overlie middle and superior gyri caudally near the temporal-occipital junction, and TCPl (temporal-central-parietal, located in the middle of the grid formed by C3, T3, T5, and P3) and TCP2 overlie the caudal termination of the Sylvian fissure at the junction with inferior parietal lobule. Voltage measures incorporating information from multiple leads may be less susceptible to local transient noise (particularly muscle artifact at T3 and T4) and therefore might provide a more stable measure of left and right temporal voltages. Accordingly, in this study left temporal voltage was assessed by taking the mean of voltages at T3, T5, and TCPl, and right temporal voltage was assessed by taking the mean of voltages at T4, T6, and TCP2.
Method
Subjects
Two groups were recruited for this study. The schizophrenic group consisted of eight right-handed male chronic schizophrenics (mean duration of illness = 19 [±5] years) from the Brockton VAMC, who met DSM-III-R criteria (American Psychiatric Association, 1987) and research diagnostic criteria (Spitzer, Endicott, & Robins, 1978) for classification as schizophrenic. All patients were administered the Schedule for Affective Disorders and Schizophrenia (SADS; Spitzer & Endicott, 1978), the Scale for the Assessment of Positive Symptoms (SAPS; Andreasen, 1984), the Scale for the Assessment of Negative Symptoms (SANS; Andreasen, 1981), and the Thought Disorder Index (TDI; Solovay et al., 1986). This information, in conjunction with chart review and clinical interviews, was used to confirm diagnoses. Subjects showed predominately positive symptoms (MSAPS = 12 [±2]; MSANS = 9 [±3]), as well as a high degree of thought disorder (M TDI = 84.12 [±67.19]). All schizophrenic subjects were medicated, with a mean dose equivalent to 956.9 mg/day of chlorpromazine. The control group consisted of eight right-handed male subjects, recruited from the staff of the Brockton VAMC and through advertisements in local newspapers. No subject had a history of alcohol or drug abuse, Axis II diagnosis, neurological illness, or head trauma. The mean age of the schizophrenic group was 39 (±5.37) years, and of the control group was 39.75 (±10.47) years. Groups did not differ significantly on age (t[14] = 0.18, p = .86). All subjects received detailed information about the study protocol and gave informed consent.
Recording System
EEG activity was recorded from the scalp through 28 tin electrodes in preconfigured caps (ElectroCap International). These electrodes included all sites of the International 10–20 system (Jasper, 1958) as well as Oz, TCP1 and 2, FTCl and 2 (located in the center of the grid formed by F3/4, F7/8, T3/4, and C3/4), CP1 and 2 (located between C3/4 and P3/4), and POl and 2 (located between Pz and O1/2). Linked earlobes were used as the reference, the forehead as ground. Two electrodes located medially and supra- or infraorbitally to the right eye were used to monitor vertical eye movements. Electrodes placed at the outer canthi of the eyes were used to monitor horizontal eye movements. All electrode impedances were below 3 kohms. The EEG amplifier bandpass was 0.15 (6 dB/octave slope) to 40 Hz (36 dB/octave slope). Single trial epochs were digitized at 3.125 ms/sample and stored in an IBM PS/2 microcomputer. Each epoch was of 900-ms duration, including a 100-ms prestimulus baseline. Averaging and artifact rejection were done off-line.
Stimuii
Pure tones were generated by a Dell 386 microcomputer through the Stim package of Neurosoft, Inc., and delivered to the subject via Etymotic insert earphones. Three tones were generated: 1 kHz, 1.4 kHz, and 1.5 kHz. All tones were 50 ms in duration, including 20-ms rise/fall times, with an interstimulus interval of 1.3 s. White noise was presented at 80 dB SPL to each ear in all conditions. Stimulus discriminability was manipulated on both the dimension of loudness (loud, soft), and pitch (near, far). The 1.5-kHz tone was always the target, with a probability of 0.15. Binaural auditory stimuli were presented in a total of four configurations: loud, far 1-kHz standard and 1.5-kHz target at 97 dB; loud, near 1.4-kHz standard and 1.5-kHz target at 97 dB; soft, far 1-kHz standard and 1.5-kHz target at 80 dB; and soft, near 1.4-kHz standard and 1.5-kHz target at 80 dB. Blocks consisted of 400 stimuli. Within each block of stimuli, tones were presented randomly so that no two blocks contained the same sequence.
Procedure
ERPs were recorded in two sessions to maintain patient cooperation and minimize practice/familiarity effects on identical stimulus configurations. Each session lasted 1.5–2.5 hr, including set-up time. There was a 3–7-day interval between sessions. The four blocks of stimuli were presented once each in a counterbalanced fashion. Half of the subjects received instructions at the first session to respond as quickly as possible to the target tone (1.5 kHz) by making a key press with the dominant hand. Reaction times were stored along with the stimulus type. The remaining subjects received instructions to respond as accurately as possible to the target tone. Instructions were reversed at the second session. All subjects therefore responded to each block twice, once with speed instructions and once with accuracy instructions. Subjects received practice runs of each configuration to familiarize them with the different tones.
To control for time-locked button-press artifact, each subject responded to 100 consecutive trials of the 1.5-kHz tone with a button press, and ERPs were recorded. Two blocks of this simple reaction time task were presented, one at each loudness level. These control blocks were presented to half the subjects at the beginning of the first session and to the remainder at the conclusion of the second session.
Analysis
ERPs
Within each block of single trial ERP responses, epochs from each electrode site were baseline corrected by subtraction of the average prestimulus voltage and corrected for eye movement artifact using regression-based weighting coefficients (Semlitsch, Anderer, Schuster, & Presslich, 1986). Epochs that contained voltage exceeding ±50 µV at any scalp electrode were excluded. Averages were computed in the four blocks separately for speed and accuracy instructions according to the responses made. ERP responses to correctly detected targets were averaged. Averages were constructed similarly for hits on the simple reaction time task.
Averages were digitally low-pass filtered at 15 Hz with a 24-dB/octave slope. Button-press simple reaction time task ERP averages were subtracted from the target averages, allowing for measurement of the long-latency potentials without overlapping sensory and motor contamination. Due to variable reaction times on each trial within each block, motor potentials were manifest primarily as a broad frontocentral voltage shift, relatively more positive on the right than the left side of the head. This subtraction would tend to reduce any left-sided amplitude deficit. Peak latency of P3 was measured automatically by the computer at the Pz site for the largest positive deflection in the time range from 250 to 600 ms. Voltage measures were derived at each electrode site by calculating the mean voltage over a 50-ms time window centered on the Pz peak latency of each subject.
Perceptual sensitivity
Hit and false alarm rates were calculated for each subject for each block of stimuli. These rates were used to compute nonparametric measures of sensitivity (A′) and response bias (B′) (Boice & Gardner, 1988; Hodos, 1970; Pollack & Norman, 1964). A′ values are between 0.5 and 1; 0.5 reflects chance performance and 1 reflects perfect discrimination. B′ values are between —1 and 1; a negative value indicates a liberal response bias, with —1 most liberal, and positive value indicates a conservative bias, with 1 most conservative.
Reaction time
Reaction times were sorted according to the type of response (hit, false alarm). Only the reaction times of correctly detected targets were included in analyses. A response of <100 ms or >1,200 ms led to rejection of that trial from all analyses, including ERP averaging.
Results
Perceptual Sensitivity
Mixed model analyses of variance (ANOVAs), with instructions, loudness, and pitch as within-subjects variables and group as the between-subjects variable, revealed that sensitivity (A′) was significantly increased by greater overall loudness (F[1,14] = 12.41, p = .003) and pitch separation of the tones (F[l,14] = 16.0, p = .00l). No interactions were significant. Behavioral and performance measures, as well as P3 latencies, are summarized in Table 1.
Table 1.
Behavioral and Performance Measures for Schizophrenics and Controls
| Conditions | ||||
|---|---|---|---|---|
| Group | Loud, far | Loud, near | Soft, far | Soft, near |
| Perceptual sensitivitya | ||||
| Control | ||||
| A′ | 0.996 (0.002) | 0.972 (0.029) | 0.940 (0.056) | 0.867 (0.107) |
| B′ | 0.459 (0.469) | 0.699 (0.310) | 0.793 (0.150) | 0.739 (0.216) |
| Schizophrenic | ||||
| A′ | 0.969 (0.056) | 0.869 (0.125) | 0.896 (0.147) | 0.800 (0.242) |
| B′ | 0.621 (0.496) | 0.595 (0.324) | 0.503 (0.594) | 0.390 (0.565) |
| Reaction time (ms) | ||||
| Control | 0.394 (0.055) | 0.470 (0.052) | 0.488 (0.089) | 0.534 (0.085) |
| Schizophrenic | 0.570 (0.129) | 0.631 (0.137) | 0.622 (0.138) | 0.624 (0.128) |
| Peak P3 latency at Pz (ms) | ||||
| Control | 335.72 (27.97) | 394.38 (39.60) | 385.16 (37.04) | 448.37 (78.09) |
| Schizophrenic | 349.65 (58.76) | 454.25 (96.98) | 417.38 (85.13) | 431.46 (57.52) |
Note: Values are mean (standard deviation).
A′ = nonparametric measure of sensitivity; 0.5 = chance performance, 1.0 = perfect descrimination. B′ = nonparametric measure of response bias; negative values indicate liberal bias, positive values indicate conservative bias.
Response Bias
Mixed model ANOVAs, with instructions, loudness, and pitch as within-subjects variables and group as the between-subjects variable, indicated that response bias (B′) was not significantly affected by any factor. The interaction between group and loudness was significant (F[1,14] = 6.88, p = .02). Separate within-group analyses revealed that control subjects tended to adopt a more conservative response bias at lower loudness, whereas schizophrenics adopted a more liberal response bias, making significantly more errors (F[l,7] = 5.85, p = .046).
Reaction Time
Mixed model ANOVAs, with instructions, loudness, and pitch as within-subjects variables and group as the between-subjects variable, revealed that reaction times were significantly slower in the schizophrenic group across all conditions (F[1,14] = 10.84, p = .005). Larger pitch separation led to shorter reaction times (F[l,14] = 9.36, p = .008), as did increased loudness (F[1,14] = 19.33, p = .001). The interaction between group and loudness was significant (F[l,14] = 5.94, p = .029). Separate within-group analyses revealed that controls tended to respond more quickly to louder stimuli (75 ms faster, F[l,7] = 16.02, p = .005), whereas schizophrenics showed only moderate, statistically nonsignificant response time gains (22 ms).
ERPs
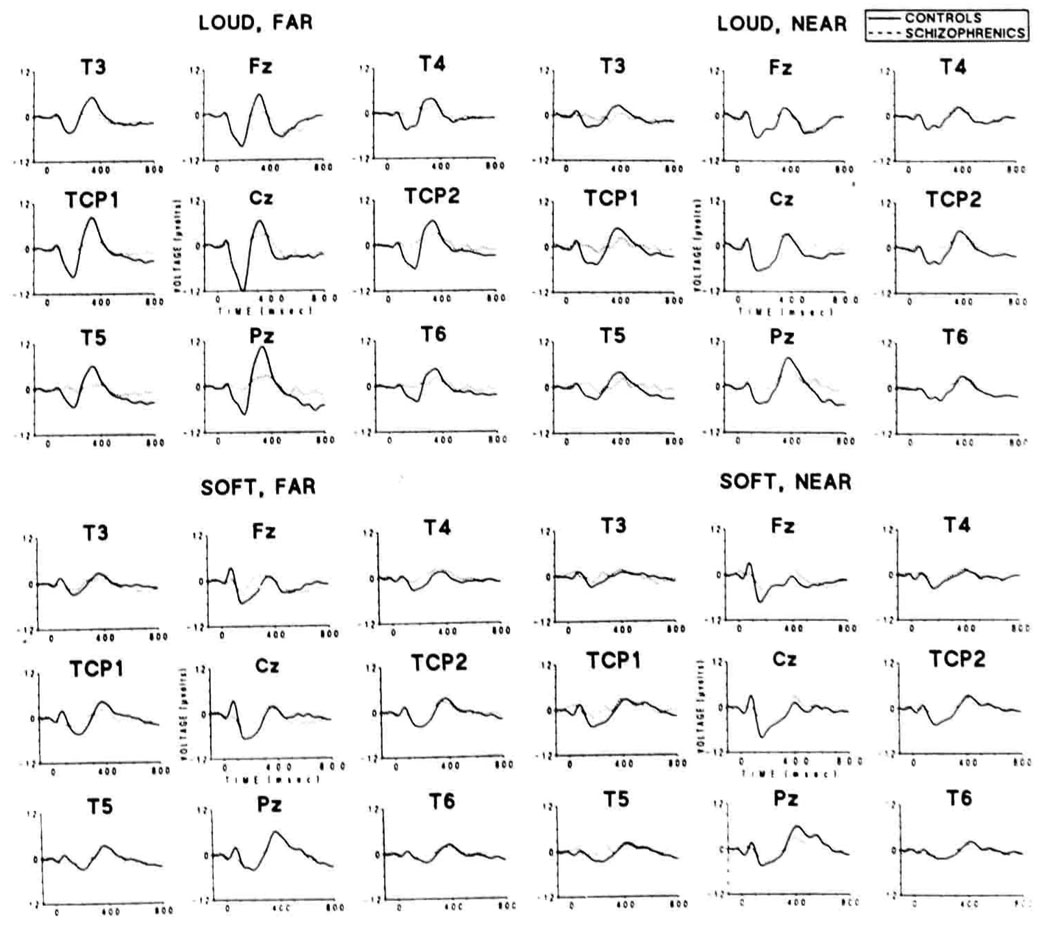
ERP data are presented in Figure 1 and Figure 2. Grand average waveforms for each condition and electrode site included in analyses are presented in Figure 1. (Instructions had no significant effects on behavioral or ERP data, and data in all figures and tables have been collapsed across this variable.) The amplitude of P3 in control subjects changed systematically as stimulus parameters were varied, but the P3 amplitude in the schizophrenic group remained largely unaffected. Left temporal area and Pz P3 amplitudes in the schizophrenic group were consistently smaller than those of controls in the loud, far; loud, near; and soft, far conditions. On the left side, a positive potential preceded P3 at roughly 275 ms, and there was a prolonged slow positive shift on the descending phase of P3 in the schizophrenic group for the soft, near condition, where the left temporal area P3 amplitude deficit was not present.
Figure 1.
Grand averaged ERP responses of each group to correctly detected target tones. Loud, far: 1-kHz standard, 1.5-kHz target at 97 dB. Loud, near: 1.4-kHz standard, 1.5-kHz target at 97 dB. Soft, far: 1-kHz standard, 1.5-kHz target at 80 dB. Soft, near: 1.4-kHz standard, 1.5-kHz target at 80 dB.
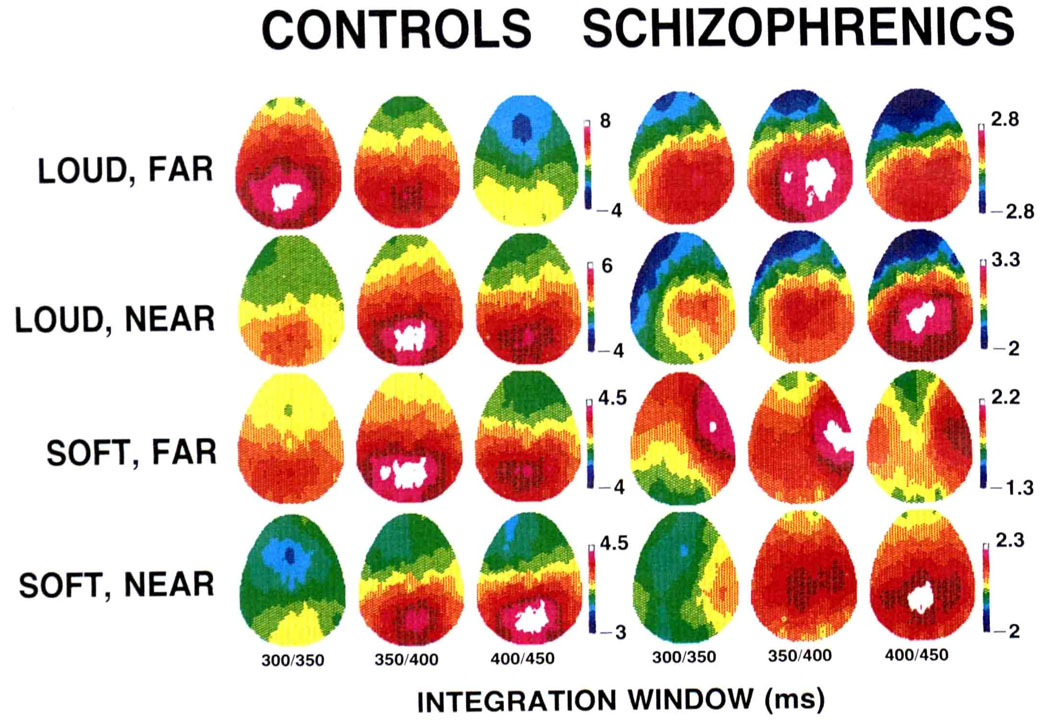
Figure 2.
Color-coded voltage maps derived from the full set of 28 electrodes for each group and each condition. Voltages are scaled relatively for each group and condition such that maximum voltage is white and minimum is blue so as to preserve topographic Information (refer to Figure 1 for absolute amplitudes).
Color-coded relative-voltage maps derived from grand average ERPs at all scalp electrode sites in each condition are presented in Figure 2. The P3 amplitudes for controls were generally symmetrical or slightly left shifted, both during the ascending phase and at peak voltage. Schizophrenics, however, had right-shifted P3 amplitudes during the ascending phase and at peak voltage, with the exception of the soft, near condition, where peak voltage appeared fairly symmetrical.
Latency
Latency to peak of P3 at the Pz site was submitted to a mixed model ANOVA, with instructions, loudness, and pitch as within-subjects variables and group as the between-subjects variable. The ANOVA revealed significant main effects for loudness (F[1,14] =7.01, p= .019) and pitch (F[1,14] = 20.36, p < .001). Of primary interest was a significant interaction among group, loudness, and pitch (F[1,14] = 9.85, p = .007). Separate within-group analyses revealed that increased loudness led to significantly earlier P3 latencies in the controls by approximately 50 ms (F[l,7] = 25.96, p = .001). In contrast, for the schizophrenic group, increased loudness was associated with shorter P3 latency in the far pitch condition (70 ms) but not in the near pitch condition, which is reflected by a nonsignificant loudness main effect and a significant Pitch × Loudness interaction (F[l,7] = 12.59, p = .009).
Amplitude
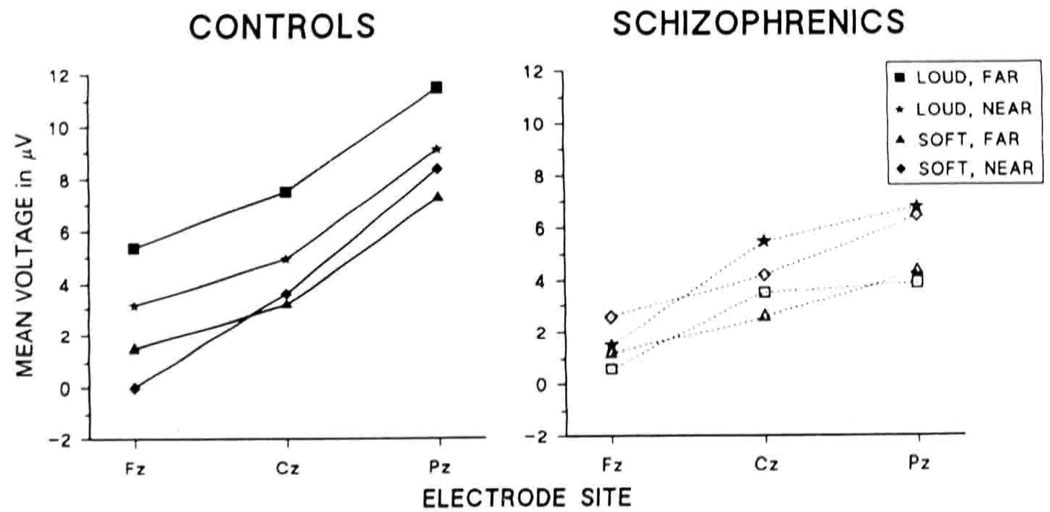
Mean P3 amplitudes among the mid-sagittal chain of electrodes (Fz, Cz, and Pz) are presented in Figure 3. Mixed model ANOVAs with instructions, loudness, pitch, and electrode site as the within-subjects variables and group as the between-subjects variable were used, with the Huynh-Feldt epsilon used to adjust degrees of freedom for electrode site. Analyses revealed a main effect on P3 amplitude for loudness (F[1,14] = 5.62, p = .033) and electrode site (F[2,28] = 30.48, p < .001, ε = 0.60). The Group × Pitch interaction was significant (F[1,14] = 6.90, p = .02). Separate within-group analyses revealed that the interaction was due to increased P3 amplitude with increased pitch separation in controls (F[l,7] = 7.9, p = .026) but not in schizophrenics. As evident in Figure 1–Figure 3, P3 amplitudes showed a centroposterior distribution. However, P3 amplitudes of schizophrenics tended to be somewhat more centrally distributed than those of controls, as is reflected in the main ANOVA by a significant Group × Loudness × Electrode site interaction (F[2,28] = 5.02, p = .017, ε = 0.87). Separate within-group analyses revealed that schizophrenics tended to show a more centrally distributed P3, particularly for the louder stimuli, as reflected in a trend toward a Loudness × Electrode interaction (F[2,14] = 3.82, p = .08, ε = 0.54) (Figure 3).
Figure 3.
Mean P3 voltages at the midline chain of electrodes. Both groups show larger amplitudes at posterior sites, although schizophrenics tend to show a more centrally distributed peak than controls.
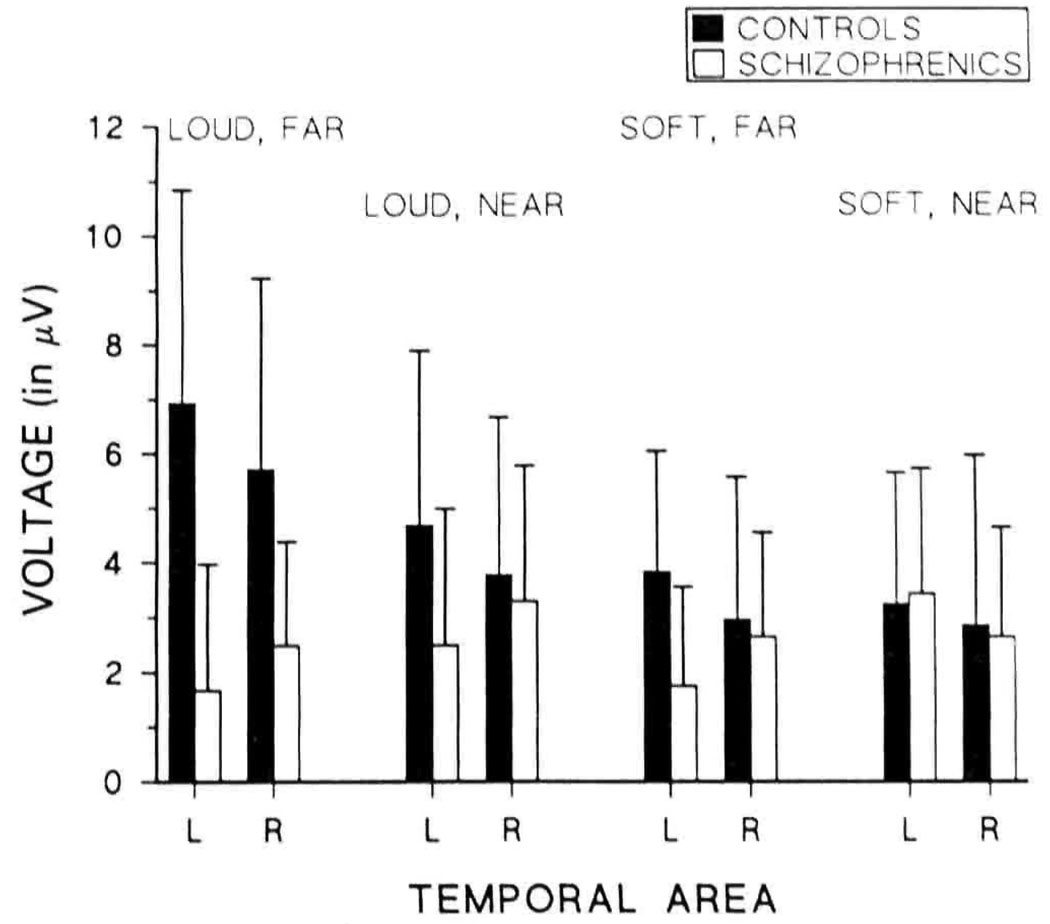
Mean left and right temporal area P3 amplitudes are presented in Figure 4. Mixed model ANOVAs with instructions, loudness, pitch, and side as the within-subjects variables and group as the between-subjects variable revealed a main effect on temporal voltage for loudness (F[1,14] = 6.15, p = .026). The Group × Loudness interaction was significant (F[1,14] = 8.02, P = .013), as was the Group × Pitch interaction (F[l,14] = 15.29, p = .002). Separate within-group analyses revealed that the first interaction was due to significant augmentation of temporal voltage with increased loudness in controls (F[1,7] = 8.88, p = .021) but not in schizophrenics. The second interaction was due to significantly greater temporal voltages with increased pitch separation in controls (F[1,7] = 5.93, p = .045) but significantly smaller temporal voltages with increased pitch separation in the schizophrenic group (F[l,7] = 28.33, p = .001). As indicated in Figure 2 and Figure 4, schizophrenics had a left-sided temporal P3 asymmetry, shown by a significant Group × Side interaction (F[1,14] = 4.6, p = .05). Controls had larger temporal amplitudes on the left than on the right, and schizophrenic amplitudes were smaller on the left than the right, although the main effect of side was not significant in either group. Schizophrenics’ left temporal voltages were smaller than right temporal voltages in all cases except the soft, near condition, as reflected in a significant Group × Pitch × Side interaction (F[l,14] = 5.28, p = .037). Separate within-group analyses revealed a Pitch × Side trend in schizophrenics (F[1,7] = 4.26, p = .078) but not in controls.
Figure 4.
Mean P3 voltages over left and right temporal areas.
Discussion
The ERPs and behavioral responses of normal control subjects showed systematic effects with increases in stimulus discriminability. As pitch separation and overall loudness were increased, P3 became larger and earlier, and response time and perceptual sensitivity improved. Schizophrenics did not differ significantly from the controls in perceptual sensitivity (A′), suggesting non-degraded auditory signals and attention to task. They responded more quickly with greater pitch separation and marginally more quickly with increased loudness. P3 latency in the schizophrenics slowed as task difficulty increased, although it was roughly the same in the soft conditions. P3 amplitude, however, was largely insensitive to stimulus parameters. Mean voltages at all sites were smaller with increased pitch separation, and the effect of increased loudness was minimal. The decrease in P3 amplitude from the left temporal area was present despite increasingly good behavioral performance in the schizophrenic group (.969 in the loud, far condition). The cause of the P3 amplitude deficits in schizophrenics observed in this study thus cannot be attributed to low stimulus discriminability, lack of attention, or other functional factors: P3 amplitude was dissociated in this regard from other measures.
The ERP responses of the schizophrenics to the least discriminable condition (soft, near) are intriguing. In this condition, the relative voltage distribution shows an asymmetry similar to that of controls. This one condition suggests that stimulus parameters might be important in evoking left-sided asymmetries in schizophrenics. However, it remains unclear whether P3 aberrations in schizophrenia are alleviated by making stimuli less discriminable, or whether the responses in this case are different due to low reliability of P3 in this single condition. This particular condition shows an unusual positive deflection on the left side of schizophrenics. We suspect the “normal” topography in this condition is due to a combination of reduced signal to noise ratio in the average, and a floor effect in P3; it is probably not due to reduced number of trials in the averages of schizophrenics, because t tests revealed no significant differences in the numbers of trials in each condition. (For comparative purposes, amplitudes at T3 and T4 were compared across all conditions in the main ANOVA, with virtually identical results as the comparisons of left and right temporal area voltages, which suggests that the more global measure of right and left temporal area voltage is not somehow causing the unexpected result.) Further experimental investigation of P3 responses in schizophrenics should indicate whether weak signals with concomitant decreases in behavioral performance lead to a paradoxical improvement in P3 lateral distribution but not in overall amplitude or latency.
Although each group tended to be conservative in regard to response bias, they employed different strategies as discriminability changed. Controls were fairly conservative at low levels of discriminability and became progressively more liberal at higher levels. In contrast, schizophrenics were relatively liberal in response bias at low levels of discriminability and became more conservative at higher levels. This result, although preliminary, suggests a difference in response strategies in changing environments in the two groups.
Both groups responded more quickly with larger pitch separation and increased loudness, although in the latter case the gains in schizophrenics were small. Overall reaction times were longer in the schizophrenics, but the observation that peak P3 latencies did not differ significantly between groups indicates that the slowed reaction times are due to psychomotor/output slowing rather than slowed perceptual/input processing, which further suggests that the differences in P3 amplitude between groups in this study are not due to peripheral, sensory deficits in the schizophrenic group. P3 amplitudes in schizophrenics appear to have a restricted range independent of sensory processes.
In general, P3 voltage deficits in schizophrenia are not alleviated by increased stimulus discriminability. When schizophrenic performance is improved functionally, P3 amplitude is not. The greater the discriminability of the stimuli in the odd-ball task, both from each other and background and physiological noise, the greater the separation between control and schizophrenic overall and left-sided P3 responses, regardless of improved behavioral performance on the task. These data are not consistent with the hypothesis that the overall and lateralized P3 voltage deficits seen in schizophrenics reflect functional deficits. The triarchic model of Johnson (1986) primarily accounts for the functional aspects determining P3 amplitude. The parametric manipulations in this study primarily affect the T factor, or information transmission. P3 amplitude in controls followed the model, whereas P3 amplitude in schizophrenics did not, which is also suggestive of a structural cause of the P3 deficit, in view of the dissociation of P3 amplitude and other measures in this group. The demonstration that small overall P3 amplitudes and abnormal P3 asymmetries did not resolve with increased behavioral performance in the schizophrenic group, although latency varied systematically, and the demonstration of left-sided temporal lobe volume reduction in schizophrenia (McCarley et al., 1992, 1993; Barta, Pearlson, Powers, Richards, & Tune, 1990) suggests the presence of an underlying structural deficit in the neural generator sites of P3 in this group that imposes a ceiling on P3 amplitude.
Acknowledgments
This research was supported by NIMH 40,799, the Medical Research Service of the Department of Veterans Affairs, and the Commonwealth of Massachusetts Research Center.
REFERENCES
- American Psychiatric Association, Committee on Nomenclature and Statistics. Diagnostic and statistical manual of mental disorders. rev. 3rd ed. Washington, DC: Author; 1987. [Google Scholar]
- Andreasen N. Scale for the assessment of negative symptoms (SANS) Iowa City: Department of Psychiatry, University of Iowa College of Medicine; 1981. [Google Scholar]
- Andreasen N. Scale for the assessment of positive symptoms (SAPS) Iowa city: Department of Psychiatry, University of Iowa College of Medicine; 1984. [Google Scholar]
- Barta P, Pearlson G, Powers R, Richards S, Tune L. Auditory hallucinations and smaller superior temporal gyral volume in schizophrenia. American Journal of Psychiatry. 1990;147:1457–1462. doi: 10.1176/ajp.147.11.1457. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. The P300 component of the event-related brain potential in psychiatric patients. In: Cracco R, Bodis-Wollender I, editors. Evoked potentials. New York: Alan R. Liss; 1986. [Google Scholar]
- Boice R, Gardner RM. A computer program to generate parametric and non-parametric signal detection parameters. Bulletin of the phychonomic Society. 1988;26:365–367. [Google Scholar]
- Bruder G, Towey J, Malaspina D, Gorman J, Tenke C, Kaufmann C. Brain potentials to complex tones in schizophrenia. New Research Program APA Annual Meeting. 1992:159. [Google Scholar]
- Donchin E, Karis D, Bashore T, Coles MGH, Gratton G. Cognitive psychophysiology and human information processing. In: Coles MGH, Donchin E, Porges SW, editors. Psychophysiology: Systems, processes and applications. New York: Guilford; 1986. [Google Scholar]
- Faux S, Shenton M, McCarley RW, Nestor P, Marcy B, Ludwig A. Preservation of P300 event-related potential asymmetries in schizophrenia with use of either linked-ear or nose reference sites. Electroencephalography and Clinical Neurophysiology. 1990;75:378–391. doi: 10.1016/0013-4694(90)90083-v. [DOI] [PubMed] [Google Scholar]
- Faux S, Shenton M, McCarley RW, Torello M, Duffy F. Differentiation of schizophrenics and normal controls is enhanced by the Goodin subtraction procedure. International Journal of Neuroscience. 1988a;39:117–135. doi: 10.3109/00207458808985697. [DOI] [PubMed] [Google Scholar]
- Faux S, Torello M, McCarley RW, Shenton M, Duffy F. P300 in schizophrenia: Confirmation and statistical validation of temporal region deficit in P300 topography. Biological Psychiatry. 1988b;23:776–790. doi: 10.1016/0006-3223(88)90066-2. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal detection theory and psychophysics. New York: John Wiley and Sons; 1966. [Google Scholar]
- Halgren E, Squires N, Wilson C, Rohrbaugh J, Babb T, Crandall P. Endogenous potentials generated in the human hippocampal formation and amygdala by infrequent events. Science. 1980;210:803–805. doi: 10.1126/science.7434000. [DOI] [PubMed] [Google Scholar]
- Hillyard S, Hink R, Schwent V, Picton T. Electrical signs of selective attention in the human brain. Science. 1973;182:177–180. doi: 10.1126/science.182.4108.177. [DOI] [PubMed] [Google Scholar]
- Hodos W. A non-parametric index of response bias for use in detection and recognition experiments. Psychological Bulletin. 1970;74:351–354. [Google Scholar]
- Hollinger D, Faux S, Shenton M, Sokol N, Seidman L, Green A, McCarley RW. Reversed temporal region asymmetries of P300 topography in left- and right-handed schizophrenic subjects. Electroencephalography and Clinical Neurophysiology. 1992;84:532–537. doi: 10.1016/0168-5597(92)90042-a. [DOI] [PubMed] [Google Scholar]
- Holzman P. Recent studies of psychophysiology in schizophrenia. Schizophrenia Bulletin. 1987;13:49–75. doi: 10.1093/schbul/13.1.49. [DOI] [PubMed] [Google Scholar]
- Homan R, Herman J, Purdy P. Cerebral localization of international 10–20 system electrode placement. Electroencephalography and Clinical Neurophysiology. 1987;66:376–382. doi: 10.1016/0013-4694(87)90206-9. [DOI] [PubMed] [Google Scholar]
- Isreal J, Wickens C, Donchin E. P300 amplitude changes during a tracking task as a function of continuous variations of tracking difficulty. Psychophysiology. 1979;16:175. [Google Scholar]
- Jasper H. Report of committee on methods of clinical examination in electroencephalography. Electroencephalography and Clinical Neurophysiology. 1958;10:370–375. [Google Scholar]
- Johnson R. A triarchic model of P300 amplitude. Psychophysiology. 1986;23:367–384. doi: 10.1111/j.1469-8986.1986.tb00649.x. [DOI] [PubMed] [Google Scholar]
- Knight R, Scabini D, Woods D, Clayworth C. Differential effects of parietal or temporo-parietal lesions on human N200 and P300. Neuroscience. 1987;22 Suppl.:521. [Google Scholar]
- Knight R, Scabini D, Woods D, Clayworth C. The effects of lesions of superior temporal gyrus and inferior parietal lobe on temporal and vertex components of the human AEP. Electroencephalography and Clinical Neurophysiology. 1988;70:499–509. doi: 10.1016/0013-4694(88)90148-4. [DOI] [PubMed] [Google Scholar]
- Kraft L, Schwarzkopf S, Torello M, Olson S, Nasrallah H. Auditory P300 changes and third ventricle enlargement in outpatient schizophrenics. Biological Psychiatry. 1991;29:163a. [Google Scholar]
- Maurer K, Dierks T. Functional imaging of the brain in psychiatry—Mapping of EEG and evoked potentials. Neurosurgery Review. 1987;10:275–282. doi: 10.1007/BF01781950. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Faux S, Shenton M, Nestor P, Adams J. Event-related potentials in schizophrenia: Their biological and clinical correlates and a new model of schizophrenic pathophysiology. Schizophrenia Research. 1991;4:209–231. doi: 10.1016/0920-9964(91)90034-o. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Shenton M, O’Donnell B, Faux S, Kikinis R, Nestor P, Jolesz F. Auditory P300 abnormalities and left posterior superior temporal gyrus volume reduction in schizophrenics. Archives of General Psychiatry. 1993;50:190–197. doi: 10.1001/archpsyc.1993.01820150036003. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Shenton M, O’Donnell B, Holinger D, Nestor P. P300 asymmetries and temporal lobe pathology. Scientific Proceedings APA Annual Meeting. 1992:136. [Google Scholar]
- Michelogiannis S, Paritsis N, Trikas P. EEG coherence during hemispheric activation in schizophrenics. European Archives of Psychiatry and Clinical Neuroscience. 1991;241:31–34. doi: 10.1007/BF02193751. [DOI] [PubMed] [Google Scholar]
- Morstyn R, Duffy F, McCarley RW. Altered P300 topography in schizophrenia. Archives of General Psychiatry. 1983;40:729–734. doi: 10.1001/archpsyc.1983.01790060027003. [DOI] [PubMed] [Google Scholar]
- Okada Y, Kaufman L, Williamson S. The hippocampal formation as a source of the slow endogenous potentials. Electroencephalography and Clinical Neurophysiology. 1983;55:417–426. doi: 10.1016/0013-4694(83)90130-x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford J, White P, Roth W. P3 in schizophrenia is affected by stimulus modality, response requirements medication status, and negative symptoms. Archives of General Psychiatry. 1989;46:1035–1044. doi: 10.1001/archpsyc.1989.01810110077011. [DOI] [PubMed] [Google Scholar]
- Polich J. Task difficulty, probability, and inter-stimulus interval as determinants of P300 from auditory stimuli. Electroencephalography and Clinical Neurophysiology. 1987;68:311–320. doi: 10.1016/0168-5597(87)90052-9. [DOI] [PubMed] [Google Scholar]
- Pollack I, Norman DA. A non-parametric analysis of recognition experiments. Psychonomic Science. 1964;1:125–126. [Google Scholar]
- Pritchard WS. Cognitive event-related potential correlates of schizophrenia. Psychological Bulletin. 1986;100:43–66. [PubMed] [Google Scholar]
- Rogers R, Baumann S, Papanicolaou A, Bourbon T, Alagarsamy S, Eisenberg H. Localization of the P3 sources using magnetoencephalography and magnetic resonance imaging. Electroencephalography and Clinical Neurophysiology. 1991;79:308–321. doi: 10.1016/0013-4694(91)90126-o. [DOI] [PubMed] [Google Scholar]
- Roth WT. Late event-related potentials and psychopathology. Schizophrenia Bulletin. 1977;3:105–120. doi: 10.1093/schbul/3.1.105. [DOI] [PubMed] [Google Scholar]
- Ruchkin D, Sutton S. Equivocation and P300 amplitude. In: Otto D, editor. Multidisciplinary perspectives in event-related potential research. Washington, DC: Eastern Psychological Association; 1978. [Google Scholar]
- Seig K, Willsie D, Preston D, Gaffney G. Brain imaging: Evoked potential, quantitative EEG and SPECT abnormalities in schizophrenia. Journal of Psychiatry and Neuroscience. 1991;16:41–44. [PMC free article] [PubMed] [Google Scholar]
- Semlitsch H, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Shenton M, Ballinger R, Marcy B, Faux S, Cane M, Lemay M, Cassens G, Coleman M, Duffy F, McCarley RW. Two syndromes of schizophrenic psychopathology associated with left vs. right temporal deficits in P300 amplitude. Journal of Nervous and Mental Diseases. 1989;177:219–225. doi: 10.1097/00005053-198904000-00005. [DOI] [PubMed] [Google Scholar]
- Solovay M, Shenton M, Gasperetti C, Coleman M, Kestenbaum E, Carpenter T, Holzman P. Scoring manual for the Thought Disorder Index. Schizophrenia Bulletin. (revised edition) 1986;12:483–496. doi: 10.1093/schbul/12.3.483. [DOI] [PubMed] [Google Scholar]
- Spitzer R, Endicott J. Schedule for affective disorders and schizophrenia. New York: Biometrics Research, New York State Psychiatric Institute; 1978. [Google Scholar]
- Spitzer R, Endicott J, Robins E. Research diagnostic criteria (RDC)for a selected group of functional disorders. New York: Biometrics Research, New York State Psychiatric Institute; 1978. [Google Scholar]
- Squires N, Squires K, Hillyard S. Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroencephalography and Clinical Neurophysiology. 1975;38:387–401. doi: 10.1016/0013-4694(75)90263-1. [DOI] [PubMed] [Google Scholar]
- Steinmetz H, Fürst G, Meyer B. Craniocerebral topography within the international 10–20 system. Electroencephatography and Clinical Neurophysiology. 1989;72:499–506. doi: 10.1016/0013-4694(89)90227-7. [DOI] [PubMed] [Google Scholar]