Abstract
The purpose of this study was to compare 2-[18F]fluoro-2-deoxy-d-glucose positron emission tomography (FDG-PET), hippocampal volumetry (HV), T2 relaxometry, and proton magnetic resonance spectroscopic imaging (1H-MRSI) in the presurgical neuroimaging lateralization of patients with nonlesional, electroencephalogram (EEG)-defined unilateral temporal lobe epilepsy (TLE). Twenty-five patients were prospectively studied, along with age-matched controls. T2 relaxometry examinations were performed in 13 patients. Comparison of FDG-PET, HV, and 1H-MRSI was possible in 23 patients. FDG-PET lateralized 87% of patients, HV 65%, N-acetyl aspartate (NAA)/(choline [Cho] + creatine [Cr]) 61%, and [NAA] 57%. Combined HV and NAA/(Cho + Cr) results lateralized 83% of the patients, a value similar to PET. Of 10 patients with normal magnetic resonance imaging (MRI) scans, 2 were lateralized with HV, 6 with FDG-PET, 4 with NAA/(Cho + Cr), and 3 with [NAA]. T2 relaxometry lateralized no patients without hippocampal atrophy. Bilateral abnormality was present in 29 to 33% of patients with 1H-MRSI measures and 17% with HV. Only hippocampal atrophy correlated with postoperative seizure-free outcome. FDG-PET remains the most sensitive imaging method to correlate with EEG-lateralized TLE. Both FDG-PET and 1H-MRSI can lateralize patients with normal MRI, but only the presence of relative unilateral hippocampal atrophy is predictive of seizure-free outcome. Bilaterally abnormal MRI and 1H-MRSI measures do not preclude good surgical outcome.
Multiple imaging methods are available to help lateralize temporal lobe epilepsy (TLE). To minimize the requirement for intracranial electrodes and to localize the epileptogenic zone with increased confidence, 2-[18F]fluoro-2-deoxy-d-glucose positron emission tomography (FDG-PET) and magnetic resonance imaging (MRI) are used to provide confirmatory localizing information. Relative temporal lobe (TL) hypometabolism on FDG-PET and hippocampal atrophy or prolonged T2 signal on MRI predict reliably lateralization of the epileptogenic temporal lobe [1-5]. Localized TL hypometabolism on FDG-PET is reported to be more sensitive than the detection of lateralized abnormality on MRI [3]; however, little has been published on the direct comparison of FDG-PET to advanced MRI and magnetic resonance spectroscopic imaging (MRSI) techniques. Gaillard and colleagues [6] compared FDG-PET with MRI-based hippocampal volumetry (HV) and showed that although FDG-PET was more sensitive than HV, it did not provide any additional clinical value when hippocampal atrophy was present. Several groups have reported the ability of 1H-MRSI to detect unilateral and bilateral metabolite abnormalities in TLE [7-17]. No studies have compared 1H-MRSI lateralization results directly with FDG-PET and quantitative MRI techniques in patients with and without hippocampal atrophy. This study compares FDG-PET, HV, T2 relaxometry, and 1H-MRSI acquired prospectively in patients with nonlesional TLE. The overall purpose was to determine whether advanced MR methodologies provide the same or additional clinical information as FDG-PET. Our first aim was to compare the performance of high-resolution FDG-PET with quantitative MRI techniques and 1H-MRSI in the lateralization of electroencephalogram (EEG)-defined TLE. The second was to compare the sensitivity of MR methods in the detection of bilatetal hippocampal abnormalities. The third was to correlate lateralization and detection of bilateral imaging abnormalities with surgical outcome.
Patients and Methods
Patients and Epilepsy Evaluation
Patient selection commenced in April 1994 as part of a prospective imaging project with PET and MRSI at University of California, San Francisco (UCSF). Informed consent was obtained from all subjects according to guidelines of the UCSF Committee on Human Research. All surgical candidates suspected to have seizures arising from the TLs between April 1994 and June 1995 were screened for selection. After scalp-sphenoidal EEG/video telemetry, 36 patients were suspected to have TLE. Subdural strip EEG (n = 7) and stereotactic implanted depth electrode EEG (SEEG) (n = 3) was performed in patients with nonlocalizing scalp-sphenoidal EEG. The approach to investigation with scalp and intracranial electrodes used at UCSF has been previously reported [18]. Patients were considered to have nonlesional unilateral TLE based on the following criteria: (1) no lesion on diagnostic MRI, excluding evidence for mesial temporal sclerosis (MTS); (2) initial or delayed focal ictal TL patterns recorded with scalp-sphenoidal EEG [19], or mesial temporal seizure onset in patients requiring subdural or SEEG ictal recordings; (3) EEG recording of at least three spontaneous seizures and no independent contralateral seizure onsets; and (4) clinical features consistent with seizures of TL origin. Amobarbital (Wada) and neuropsychometric examinations were performed in all patients.
At UCSF, patients are selected for standard anterior–medial TL resection based on ictal electrophysiological localization. Imaging, psychometric, and Wada examinations are only used to provide outcome and neurological risk information to patients before they make a decision to proceed with surgery. Intraoperative electrocorticography and language mapping tailor the extent of resection minimally. Although we cannot exclude all bias that comes from information provided by imaging studies, our presurgical approach allows the best opportunity to assess neuroimaging lateralization in the typical patients who undergo anterior–medial TL resection.
Of the 36 patients screened for this study, 8 had foreign tissue lesions (including vascular malformations, glial tumors, hamartomas, and dysembryoplastic lesions) and were excluded from further consideration. Twenty-eight patients were defined as having nonlesional unilateral TLE. One patient was excluded because both MRSI and FDG-PET data sets were obscured severely by motion artifact. Two additional patients were excluded because they did not complete protocol examinations for either MRSI or HV. Ultimately, 25 patients (12 males and 13 females; age range, 14–56 years; mean, 38 years) were included. Because examination with T2 relaxometry did not begin at the start of this project, only 13 patients (6 males and 7 females; age range, 14–56 years; mean, 37 years) were studied with all four modalities.
Our standard temporal lobectomy procedure uses the subpial resection technique along with intraoperative electrocorticography and awake language mapping (language-dominant TL only). Resection of the anterior–medial TL, including the amygdala and hippocampus, is performed in all cases. Most specimens from the subpial resection technique did not allow grading of hippocampal neuronal loss; in 9 specimens, moderate to severe pyramidal neuron loss was unequivocal (all with MRI evidence for mesial temporal sclerosis [MTS]); in the others no reliable comment about neuron loss was possible.
2-[18F]Fluoro-2-Deoxy-d-Glucose Positron Emission Tomography
PET scans were performed before implanted electrode recordings with a CTI/Siemens 961 HR EXACT scanner with full width half-maximum resolution of 3.5 mm in plane and 4.0 mm transaxially. A total of 47 slices were acquired simultaneously over a 15-cm field of view. Subjects were studied in a resting condition with eyes open and ears unoccluded in a dimly illuminated room. Emission data were accumulated 45 minutes after intravenous injection of approximately 10 mCi FDG. Before obtaining emission data, a transmission scan was performed for attenuation correction. For visual interpretation, axial, coronal, and sagittal planes were reconstructed.
Diagnostic Magnetic Resonance Imaging
All patients underwent diagnostic MRI studies independent of research MRI and MRSI examinations. Diagnostic MRI examinations were performed at 1.5 T (Signa, GE, Milwaukee, WI) according to a standard protocol, including 3D-SPGR (repetition time [TR] 50 msec, echo time [TE] minutes; flip angle, 40°; slice thickness, 1.5 mm), coronal T2* gradient echo (TR 500 msec, TE 15–34 msec; flip angle, 20°), and high-resolution (512 × 512 matrix) coronal T2 fast spin echo sequences.
Hippocampal Volumetry
All 25 patients and 19 healthy controls (5 females and 14 males; age range, 23–56 years) had research MR imaging performed at 1.5 T (Magnetom Vision, Siemens, Erlangen, Germany). For hippocampal volumes an MPRAGE sequence (TR/TE 10/4 msec; flip angle, 15°; slice thickness, 1.5 mm) was acquired in the coronal plane orthogonal to the long axis of the hippocampus in the same sitting as T2 and 1H-MRSI examinations. Volume measurements of the hippocampus were performed according to the method of Watson and associates [20], using contiguous 3-mm slices. Processing of images and calculation of volumes was performed on an SPARC LX computer (Sun Microsystems, Palo Alto, CA) with a volumetric image display and analysis package (VIDA, Division of Physiologic Imaging, University of Iowa, Iowa City, IA).
T2 Relaxometry
Thirteen of the 25 patients and 11 healthy controls (2 females and 8 males; age range, 23–56 years) had hippocampal T2 measured using a procedure similar to that reported by Jackson and co-workers [21] and Grunewald and associates [22]. A multi–spin echo sequence (16 echoes) with TR 2,000 msec and TE 22.5, 45 ⋯ 360 msec from an 8-mm-thick slice of the anterior hippocampus was performed. Two circular regions of interest (ROIs) were placed within the margins of the hippocampal body by two independent operators. The average of the T2 relaxation times for each pixel within the ROIs was used as the final measurement for T2 of the corresponding hippocampus.
Proton Magnetic Resonance Spectroscopic Imaging
Twenty-four of the 25 patients and 16 controls (5 females and 11 males; age range, 12–56 years) were studied with 1H-MRSI. Sixteen patients were included in a previous 1H-MRSI study [7]. Nuclear magnetic resonance spectra were acquired from the hippocampus proper according to a previously reported method [7]. Quantitation of the metabolites was based on use of the unsuppressed water signal obtained in a second MRSI examination with otherwise identical measurement parameters. Details of MRSI after processing and the quantitation method have been previously reported [23].
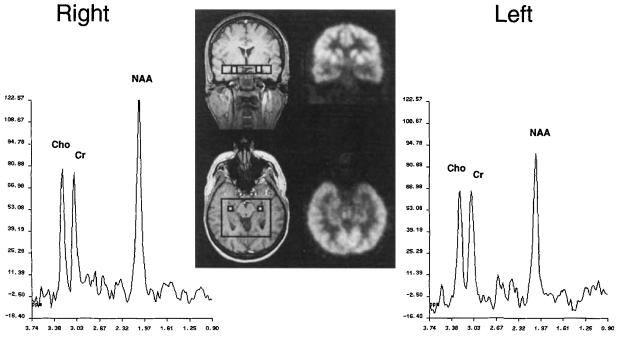
Only voxels including hippocampal gray matter were selected for signal analysis of N-acetyl aspartate (NAA), creatine (Cr), and choline (Cho). The first two voxels from the anterior and posterior borders of the PRESS volume were avoided because the profile of the 180° selective pulse in this direction was suboptimal in these voxels. An average of five voxels (range from two to seven) was selected from each hippocampal region. Figure 1 shows an example of spectra obtained from single homotopic voxels within the hippocampus; the patient's coregistered FDG-PET is included for comparison.
Fig 1.
Proton magnetic resonance spectroscopic imaging spectra from right and left homotopic voxels (one of five hippocampal voxels included for analysis). Inset shows the voxel locations on magnetic resonance imaging with coregistered 2-[18F]fluoro-2-deoxy-d-glucose positron emission tomography. This patient had left temporal lobe epilepsy with concordant hippocampal atrophy. All modalities provided correct lateralization, and the patient became seizure free after surgery. Cho = choline; Cr = creatine; NAA = N-acetyl aspartate.
Image Interpretation and Analysis
The PET images were interpreted for evidence of relative focal hypometabolism by two investigators, including one (R.A.H.) blinded to clinical and EEG data. Five TL regions, ie, temporal pole, anterior medial, posterior medial, anterior lateral, and posterior lateral cortex, were scored for presence of relative hypometabolism. The sum of the hypometabolic regions was used to provide a qualitative measure of the degree of TL hypometabolism.
Diagnostic MRI scans were read by a neuroradiologist (H.A.R.) blinded to clinical, EEG, and other imaging data. Interpretation of mesial temporal abnormalities was based on volume and signal changes.
With all quantitative MR measures, lateralization criteria were investigated with measurement of the percent concordance with ictal EEG as a function of the degree of asymmetry and absolute difference from control means. The following asymmetry index (AI) was used to measure degree of asymmetry for HV, T2 mapping, and 1H-MRSI:
Criteria for best lateralization (maximal percent concordance and minimal percent discordance with ictal EEG) were determined for each modality. Mean values and standard deviations (SD) were calculated for right and left hippocampi of control subjects with each quantitative MR measure. Student's t test for unmatched pairs was used to test the difference between right and left mean values in control studies. Fisher's exact test was used to test correlation of lateralization results with surgical outcome classification defined by Engel [24].
Results
Controls
No significant differences were present in control subjects between right and left hippocampi for any measure. The means of combined right and left hippocampal measures are as follows: HV (38 hippocampi) = 3,452 mm3 (± 342), T2 relaxation time (22 hippocampi) = 113.9 msec (± 4.6 msec), concentration of NAA ([NAA]) (32 hippocampi) = 11.61 mmol/L (± 1.30 mmol/L), and NAA/(Cho + Cr) (32 hippocampi) = 0.81 (± 0.06). The mean AI plus 2 SD for the respective controls are HV = 8.4%, T2 = 9.1%, [NAA] = 20.9%, and NAA/(Cho + Cr) = 25.6%.
Patients
All but 5 patients had unequivocal unilateral focal temporal ictal patterns on scalp EEG. Intracranial ictal recordings confirmed unilateral mesial onset T2 seizures in each of the 5 patients (3 with and 2 without MRI evidence of MTS).
Twenty-four patients had surgery. The one exception was a patient with normal MRI. All but 1 of the patients with evidence for MTS on diagnostic MRI (n = 15) became seizure free. Of the 9 patients with normal MRI, outcome was class I in 3 (including 2 with relative hippocampal atrophy defined by HV), class II in 3, class III in 1, and class IV in 2. Thus, only 1 of 7 patients without either HV detected hippocampal atrophy had a class I outcome.
Lateralization
FDG-PET
Twenty-four of 25 patients had FDG-PET examinations. Because of the subjective nature of determining relative hypometabolism and the risk of false interpretation in the presence of asymmetric partial volume effects, several asymmetry thresholds were evaluated. With low asymmetry thresholds (only one or two TL regions with relative hypometabolism required to define the examination lateralized), a sensitivity as high as 92 to 96% could be obtained. However, two interobserver discrepancies were encountered. PET to MRI coregistration was necessary to resolve lateralization in 1 patient. Requiring three or more regions of relative hypometabolism was a conservative but consistently reliable threshold for lateralization that resulted in 21 of 24 (87.5%) patients lateralized by FDG-PET and no discordance.
DIAGNOSTIC MRI
MRI performed in all 25 patients revealed easily discernible relative hippocampal atrophy in 15 patients (60%) and unilateral increase in hippocampal T2 signal in 9 patients (36%). In no case was T2 hyperintensity seen without hippocampal atrophy. MRI-identified hippocampal atrophy was concordant with ictal EEG in all cases.
HIPPOCAMPAL VOLUMETRY
All 25 patients had HV examinations. An AI of 8% or more was required to eliminate all discordance. With this threshold, 17 of 25 patients (68%) were lateralized. Sixteen of these 17 had concordant hippocampal atrophy more than 2 SD below the mean of controls. To determine if the yield for accurate lateralization could be improved, a requirement was added that HV be lateralizing only if hippocampal volumes were more than 2.0 SD below the mean of controls. This decreased the percent discordance for all AI thresholds of less than 8%, but percent concordance was also lower. Thus, the optimum criterion for lateralization with HV was an AI of 8% or more without any additional requirement for absolute abnormality.
T2 RELAXOMETRY
To eliminate all discordance, an AI of 4% or more was required. With this threshold, 9 of 13 patients (69%) were lateralized. If the requirement was added that T2 be lateralizing only if it was more than 2.0 SD above the mean of controls, discordance was eliminated at all AI values, but sensitivity was decreased to 54%. Thus, the optimum criterion for T2 lateralization was an AI of 4% or more without any additional requirement for absolute abnormality.
1H-MRSI
Both NAA/(Cho + Cr) and [NAA] included some discordant patients with relatively high asymmetry. An AI of 16% or more was required to eliminate all discordance with NAA/(Cho + Cr). This threshold resulted in 10 of 24 patients (42%) lateralized. Adding the requirement that NAA/(Cho + Cr) be lateralizing only if it was more than 2.0 SD below the mean of controls did not reduce the AI that was required to eliminate discordance. With [NAA] lateralization, an AI of 24% or more was required to eliminate all discordance, a threshold that resulted in a sensitivity of only 21%. As with NAA/(Cho + Cr), adding the requirement that [NAA] be lateralizing only if it was more than 2.0 SD below the mean of controls did not reduce the AI that was required to eliminate discordance. These data suggest that no optimum criteria for 1H-MRSI lateralization exist without including one or more cases discordant with ictal EEG.
Because discordance is not necessarily an indication of false lateralization, we examined 1H-MRSI lateralization results in patients completely free of seizures (including auras or simple partial seizures) after surgery. In this subgroup of 15 patients, NAA/(Cho + Cr) discordance was present in 4 patients (with AIs of 11 % in 2, and 15% and 1% in the others). None of the 15 patients had discordant [NAA]. The mean AI of normal controls ± 2 SD was approximately 20% for both 1H-MRSI measures. If the asymmetry threshold was based on this value, lateralization sensitivity was prohibitively low, ie, 27% with NAA/(Cho + Cr) and 47% with [NAA] (n = 15). Forced lateralization (choosing the side with the lowest value, regardless of degree of asymmetry) is unreasonable, even if only considering patients with abnormal values. Thus, with either 1H-MRSI measure, the decision of an optimized lateralization criterion remained arbitrary. We chose an AI of 12% or more as a reasonable threshold for 1H-MRSI lateralization, because NAA/(Cho + Cr) lateralization is simply unreliable with an AI of less than 12% (regardless of whether only patients with abnormal values are included). With this threshold applied to all 24 patients studied with NAA/(Cho + Cr), sensitivity was 58%, with discordant lateralization in 1 patient. The sensitivity for [NAA] was 63%, with discordant lateralization in 1 (different) patient.
Comparison of Lateralization
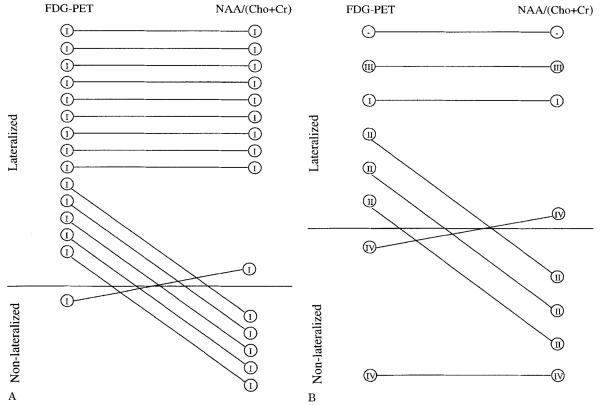
Comparison of lateralization can only be performed in those patients who completed all examinations included in the evaluation. Table 1 lists the lateralization for the 23 patients who completed FDG-PET, HV, and 1H-MRSI studies (from the total of 25 patients, 1 did not have PET, and in another the 1H-MRSI PRESS volume selection was displaced above the plane of the hippocampi). Using the criteria described above, lateralization sensitivity was 87% with FDG-PET, 65% with HV, 61% with NAA/(Cho + Cr), and 57% with [NAA]. Combining the HV and NAA/(Cho + Cr) results together lateralized 83% of patients, a sensitivity similar to PET. No discordance was found with FDG-PET or HV. Two patients were discordant with 1H-MRSI measures, 1 each with NAA/(Cho + Cr) and [NAA]. Table 2 lists the lateralization in patients grouped according to the presence or absence of hip-pocampal atrophy defined by HV. FDG-PET lateralized all but 1 (93%) of the 15 patients with hippocampal atrophy; each of the 1H-MRSI measures lateralized 10 patients (75%) of this subgroup (Fig 2A). NAA/(Cho + Cr) lateralization was discordant in 1 patient who became seizure free with surgery. Of the 8 patients without hippocampal atrophy, FDG-PET lateralized 6 (75%), NAA/(Cho + Cr) 4 (50%), and [NAA] 3 (38%) (Fig 2B). The [NAA] lateralization was discordant in 1 patient who had no worthwhile improvement in seizures since surgery. This patient was nonlateralized by FDG-PET and NAA/(Cho + Cr).
Table 1.
Comparison of Lateralization (n = 23)
| Concordant | Discordant | Non- lateralized |
|
|---|---|---|---|
| FDG-PET | |||
| Asymmetry score ≥ 3 | 20 | 0 | 3 |
| HV | |||
| AI ≥ 8% | 15 | 0 | 8 |
| NAA/(Cho + Cr) | |||
| AI ≥ 12% | 14 | 1 | 8 |
| [NAA] | |||
| AI ≥ 12% | 15 | 1 | 7 |
FDG-PET = 2-[18F]fluoro-2-deoxy-d-glucose positron emission tomography; HV = hippocampal volumetry; AI = asymmetry index; NAA = N-acetyl aspartate; Cho = choline; Cr = creatine; [NAA] = NAA concentration.
Table 2.
Lateralization Sensitivity in Patients Based on HV Defined Hippocampal Atrophy
| With Atrophy (n = 15) |
Without Atrophy (n = 8) |
|
|---|---|---|
| FDG-PET | 14 (93%) | 6 (75%) |
| NAA/(Cho + Cr) | 10 (75%)a | 4 (50%) |
| [NAA] | 10 (75%) | 3 (38%)a |
Includes 1 patient with discordant lateralization.
FDG-PET = 2-[18F]fluoro-2-deoxy-d-glucose positron emission tomography; NAA = N-acetyl aspartate; Cho = choline; Cr = creatine; [NAA] = NAA concentration.
Fig 2.
Lateralization achieved with positron emission tomography (PET) and N-acetyl aspartate (NAA)/(choline [Cho] + creatine [Cr]) is compared in patients with (A) and without (B) hippocampal volumetry–defined hippocampal atrophy. The roman numerals within the circles designate each patient's surgical outcome classification defined by Engel [24]. (A) As can be seen, PET lateralized 14 of 15 patients with hippocampal atrophy, compared with 10 of 15 by NAA/(Cho + Cr). In patients without atrophy (B), PET lateralized 6 of 8, compared with 4 of 8 by NAA/(Cho + Cr). Note: Two patients, 1 without 2-[18F]fluoro-2-deoxy-D-glucose (FDG)-PET data (class III outcome) and another without proton magnetic resonance spectroscopic imaging data (class I outcome), are not shown in A.
Table 3 shows the lateralization results of only the 13 patients who underwent T2 relaxometry. None of the patients with normal hippocampal volumes had lateralized or abnormal absolute T2 relaxometry values. Of the 4 patients with nonlateralized T2 (AI < 4%), all 4 had lateralized FDG-PET and NAA/(Cho + Cr); 1 was lateralized with HV and [NAA].
Table 3.
Comparison of Lateralization (n = 13)
| Concordant | Discordant | Non- lateralized |
|
|---|---|---|---|
| FDG-PET | |||
| Asymmetry score ≥ 3 | 12 | 0 | 1 |
| HV | |||
| AI ≥ 8% | 10 | 0 | 3 |
| T2 | |||
| AI ≥ 4% | 9 | 0 | 4 |
| NAA/(Cho + Cr) | |||
| AI ≥ 12% | 7 | 1 | 5 |
| [NAA] | |||
| AI ≥ 12% | 7 | 0 | 6 |
FDG-PET = 2-[18F]fluoro-2-deoxy-d-glucose positron emission tomography; HV = hippocampal volumetry; AI = asymmetry index; NAA = N-acetyl aspartate; Cho = choline; Cr = creatine; [NAA] = NAA concentration.
Detection of Bilateral Abnormalities
Abnormality was defined as 2.0 SD from the mean of controls (lower values for HV and 1H-MRSI, higher values for T2). Of the 24 patients available for comparison with HV and 1H-MRSI, bilateral abnormalities were detected in 4 with HV (17%), 8 with NAA/(Cho + Cr) (33%), and 6 with [NAA] (29%). Of the 13 patients available to compare T2 relaxometry, bilateral abnormalities were detected in 3 with HV (23%), 6 with NAA/(Cho + Cr) (46%), 4 with [NAA] (31%), and 1 with T2 (8%).
Correlation with Outcome
The mean follow-up for the 24 patients who had surgery was 23 months (range, 18–31 months). Lateralized hippocampal atrophy detected by HV and T2 relaxometry correlated strongly (p < 0.0001) with seizure-free outcome. FDG-PET (three or more regions of relative hypometabolism) correlated (p = 0.04) with good outcome (classes I and II). Lateralized 1H-MRSI did not correlate with outcome.
Patients with bilateral MR abnormalities were not associated with poor outcome. Three patients had bilateral hippocampal atrophy. Two became seizure free and 1 had a class III outcome. Five of 7 patients with bilaterally abnormal NAA/(Cho + Cr) and 5 of 8 patients with bilaterally abnormal [NAA] became seizure free. The 1 patient with bilaterally abnormal T2 relax-ometry became seizure free.
Discussion
Historically, FDG-PET has provided evidence of lateralized metabolic abnormality for nonlesional TLE patients. However, the role of FDG-PET in the presurgical evaluation among presently available advanced MR techniques is unclear. In particular, which imaging techniques reliably lateralize TLE in patients without MRI evidence of MTS is unanswered.
Histopathological studies of TLE reveal that 30 to 40% of hippocampal specimens do not have classic MTS (at least 30–50% neuronal cell loss in hippocampal subfields CA1, CA3, and CA4) [24-26]. These specimens have either mild or limited neuronal loss and variable gliosis, or even no pathological change. MRI detects classic MTS (93% sensitivity and 100% specificity) [26]. Yet, approximately 50% of patients without hippocampal atrophy on MRI have good surgical outcomes [5, 27]. It is this subgroup of cryptogenic TLE patients that need additional lateralizing data to improve selection for surgery.
Lateralization
FDG-PET
Even with conservative lateralization criteria, FDG-PET was the most sensitive imaging modality and lateralized 87% of the patients correctly (based on class I or II outcome). This included 75% of patients without hippocampal atrophy. These data confirm other evidence that recent generation FDG-PET cameras reliably detect lateralized metabolic abnormalities in unilateral TLE patients with normal MRI [6]. The absence of en bloc hippocampal surgical specimens precludes correlating our PET and other imaging findings with quantitatively defined MTS. We recognize this as an important issue that must be addressed in future studies, to further understand the basis of multimodality imaging findings in true cryptogenic EEG-defined TLE.
MRI (DIAGNOSTIC AND HV)
HV lateralized 2 additional patients compared with visual analysis of MRI, an improvement in lateralization of 8%, which is similar to that previously reported [2]. These results emphasize that clinically significant relative hippocampal atrophy is obvious on optimized images, ie, gradient echo or inversion recovery Tl-weighted sequences that provide high resolution and good contrast in a well-aligned plane, orthogonal to the long axis of the hippocampus. Conversely, risk for false lateralization is present with subtle asymmetry and such data sets should not be interpreted to demonstrate relative hippocampal atrophy unless they are confirmed with whole hippocampal volume measurements using conservative lateralization criteria.
T2 RELAXOMETRY
T2 relaxometry provided no additional lateralizing information to that of visual analysis or HV. The interpretation of results with T2 is limited because the number of patients was small. However, it is still noteworthy that 2 patients with obvious atrophy had normal absolute T2 values and that none of the 3 without atrophy had lateralized asymmetry or abnormal T2 values. Similar results with T2 relaxometry and HV were reported by Achten and co-workers [28]. Yet, a few rare patients with MTS have been reported who have prolonged hippocampal T2 in the absence of hippocampal atrophy [29]. The value of T2 remains for both clinical and research purposes because it is simple to perform, is highly reliable (low variance in normals), and can be performed in any ROI.
1H-MRSI
Our relatively low sensitivity with 1H-MRSI lateralization contrasts with the nearly 100% sensitivity reported in some earlier studies, including our own [7-9, 14, 30, 31]. Most studies included only a small number of patients, controls, or almost exclusively patients with MRI evidence for MTS. Some studies included patients suspected or proved to have bilateral independent TLE, a finding used to explain discordant or nonlateralized MRSI results [8, 14]. The 1H-MRSI sensitivity (55–65%) reported in the series by Connelly and associates [11] and Cross and colleagues [12], including some discordant lateralization, is nearly identical to our results.
Because of persistent findings of discordant lateralization, we emphasize the need for conservative 1H-MRSI lateralization criteria, even at the expense of sensitivity. This may be less of an issue with [NAA], which we found to be highly reliable in predicting lateralization in patients who became completely seizure free. We could have decreased the [NAA] asymmetry threshold to an AI of 4% or more, which would have yielded a sensitivity of 87.5% (15 of 16 patients with and 6 of 8 patients without atrophy), with only 1 patient presumed falsely lateralized (class II outcome). In contrast, the same asymmetry threshold with NAA/(Cho + Cr) would have yielded a sensitivity of 75%, with 3 patients presumed falsely lateralized (all with class I outcome). Future 1H-MRSI studies should consider this potentially important difference in [NAA] and NAA/(Cho + Cr) measures.
Our method of averaging all voxels containing predominantly hippocampus to provide a single value is a potentially important limitation of our 1H-MRSI lateralization analysis. Single large voxel techniques and summation of smaller voxels can dilute regional hippocampal abnormalities that may be lateralized. Further, our sampling excluded not only extrahippocampal TL regions, but also the most anterior extent of the hippocampus. It may be possible to improve the sensitivity of 1H-MRSI by improving spatial resolution, using MRI-based tissue segmentation, and sampling the entire TL. These issues are presently being addressed with higher field strength scanners [8] and development of multislice and fat-suppression 1H-MRSI techniques [32].
Comparison of Lateralization
The most important finding from our data was the lateralized abnormality, detected by FDG-PET and 1H-MRSI in the absence of HV and other MRI evidence for MTS, including T2 relaxometry. Our results comparing FDG-PET with HV replicate independently those published by Gaillard and colleagues [6]. These results, along with the finding that false lateralizations with FDG-PET are extremely rare in TLE [1, 33], indicate that FDG-PET can be used to confirm lateralization when other imaging or clinical findings are equivocal.
Using 1H-MRSI lateralization data together with HV yielded a total MR lateralization sensitivity comparable with that of FDG-PET. As a result, addition of 1H-MRSI to presurgical MR protocols should add lateralizing data above that of only MRI and eliminate the need for a PET study in additional patients with cryptogenic TLE.
Detection of Bilateral Abnormalities
One reason that 1H-MRSI lateralization may be limited is its relatively high sensitivity in the detection of bilateral abnormalities. Because decreases in 1H-MRSI measures were often found on the contralateral side, these reductions diminished the asymmetry resulting from ipsilateral reductions. Even with a strict definition of abnormality, the detection of bilaterally abnormal 1H-MRSI was twice that of HV.
The significance of bilateral abnormalities detected with 1H-MRSI in patients with unilateral epilepsy is not known. It may be hypothesized that bilaterally abnormal NAA measures reflect the detection of bilateral hippocampal sclerosis, a diagnosis present in 18 to 54% of epilepsy patients with classic hippocampal sclerosis on at least one side [34]. This hypothesis is based on the finding that NAA is almost exclusively found in neurons. Because hippocampal sclerosis is defined by neuron loss, it is presumed to be related to the concentration of NAA measured with 1H-MRSI. Our results demonstrate that NAA measures can be normal in atrophic hippocampi as well as abnormal in normal volume hippocampi (whether ipsilateral or contralateral to ictal EEG). This suggests measurement of NAA does not simply reflect neuronal loss. One possible explanation is that repeated seizure activity impairs mitochondrial metabolism in the neurons of either hippocampi, resulting in diminished NAA concentration [35]. We conclude that the temptation to attribute detection of bilaterally low NAA measures as evidence for hippocampal sclerosis (unilateral or bilateral) should be avoided until the basic pathophysiological cause of NAA abnormalities is better understood.
Correlation with Outcome
The ultimate potential clinical value for detection of lateralized and bilateral abnormalities with neuroimaging is prediction of surgical outcome. In our population, lateralized hippocampal atrophy was strongly correlated with seizure-free outcome (class I), and relative focal TL hypometabolism with FDG-PET correlated with a good outcome (classes I and II). These results for MRI and FDG-PET are consistent with previous studies [5, 36-38]. T2 relaxometry also correlated with good outcome, but it appears to only reflect detection of the same patients as HV. These data support our experience that EEG concordant hippocampal atrophy is the single best predictor for surgical outcome in TLE, and it should be weighted above all other imaging findings with respect to clinical lateralization. Although the number was small, one important finding was revealed in our subgroup of patients with presumed cryptogenic TLE. Two patients had a class IV outcome and both had nonlateralized PET and 1 had severely discordant [NAA]. These results should raise serious concern about lateralization based only on extracranial recordings. In such patients we recommend, regardless of focal scalp ictal EEG, further evaluation to include intracranial ictal recordings.
Our 1H-MRSI lateralization was not correlated with outcome. One reason for this may have been that 2 patients with a class I outcome had bilaterally abnormal [NAA]. Both had lower [NAA] values on the side concordant with atrophy and ictal EEG. The degree of asymmetry, however, was lower than the AI threshold for lateralization. The question remains whether this is a compromise in lateralization sensitivity for MRSI or if long-term outcome will demonstrate that bilaterally abnormal MRSI is ultimately correlated with non-seizure free outcome. Other early surgical outcome results [11, 27] agree with ours that relatively symmetric but bilaterally abnormal HV or MRSI does not preclude seizure-free outcome.
Despite some limitations, our results do provide data for recommendations regarding the selective use of FDG-PET in presurgical evaluation of patients with EEG-defined TLE. For patients with hippocampal atrophy, PET provides essentially no new lateralization or outcome information. In patients without hippocampal atrophy, 1H-MRSI provides lateralization nearly equivalent to PET. For patients with hippocampal atrophy discordant to ictal scalp EEG, we recommend PET and SEEG recording. Use of 1H-MRSI lateralization to resolve discordance between ictal EEG and established imaging methods should wait until the issue of potential false lateralization is further investigated.
Acknowledgments
This study was supported by an Epilepsy Foundation of America Research/Clinical Training Fellowship (R. C. K.) and NIH grant R01-NS31966 (K. D. L.).
References
- 1.Engel JJ, Henry TR, Risinger MW, et al. Presurgical evaluation for partial epilepsy: relative contributions of chronic depth-electrode recordings versus FDG-PET and scalp-sphenoidal ictal EEG. Neurology. 1990;40:1670–1677. doi: 10.1212/wnl.40.11.1670. [DOI] [PubMed] [Google Scholar]
- 2.Jack JR, Sharbrough FW, Twomey CK, et al. Temporal lobe seizures: lateralization with MR volume measurements of the hippocampal formation. Radiology. 1990;175:423–429. doi: 10.1148/radiology.175.2.2183282. [DOI] [PubMed] [Google Scholar]
- 3.Spencer SS, McCarthy G, Spencer DD. Diagnosis of medial temporal lobe seizure onset: relative specificity and sensitivity of quantitative MRI. Neurology. 1993;43:2117–2124. doi: 10.1212/wnl.43.10.2117. [DOI] [PubMed] [Google Scholar]
- 4.Kuzniecky R, Burgard S, Faught E, et al. Predictive value of magnetic resonance imaging in Temporal lobe epilepsy surgery. Arch Neurol. 1993;50:65–69. doi: 10.1001/archneur.1993.00540010059018. [DOI] [PubMed] [Google Scholar]
- 5.Garcia PA, Laxer KD, Barbaro NM, Dillon WP. Prognostic value of qualitative magnetic resonance imaging hippocampal abnormalities in patients undergoing temporal lobectomy for medically refractory seizures. Epilepsia. 1994;35:520–524. doi: 10.1111/j.1528-1157.1994.tb02471.x. [DOI] [PubMed] [Google Scholar]
- 6.Gaillard WD, Bhatia S, Bookheimer SY, et al. FDG-PET and volumetric MRI in the evaluation of patients with partial epilepsy. Neurology. 1995;45:123–126. doi: 10.1212/wnl.45.1.123. [DOI] [PubMed] [Google Scholar]
- 7.Ende GR, Laxer KD, Knowlton RC, et al. Temporal lobe epilepsy: bilateral hippocampal metabolite changes revealed at proton MR spectroscopic imaging. Radiology. 1997;202:809–817. doi: 10.1148/radiology.202.3.9051038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hetherington H, Kuzniecky R, Pan J, et al. Proton nuclear magnetic resonance spectroscopic imaging of human temporal lobe epilepsy at 4.1 T. Ann Neurol. 1995;38:396–404. doi: 10.1002/ana.410380309. [DOI] [PubMed] [Google Scholar]
- 9.Cendes C, Andermann F, Dubeau F, Arnold D. Proton magnetic resonance spectroscopic images and MRI volumetric studies for lateralization of temporal lobe epilepsy. Magn Reson Imaging. 1995;13:1187–1191. doi: 10.1016/0730-725x(95)02031-n. [DOI] [PubMed] [Google Scholar]
- 10.Cendes F, Andermann F, Preul M, Arnold D. Lateralization of temporal lobe epilepsy based on regional metabolic abnormalities in proton magnetic resonance spectroscopic images. Ann Neurol. 1994;35:211–216. doi: 10.1002/ana.410350213. [DOI] [PubMed] [Google Scholar]
- 11.Connelly A, Jackson GD, Duncan JS, et al. Magnetic resonance spectroscopy in temporal lobe epilepsy. Neurology. 1994;44:1411–1417. doi: 10.1212/wnl.44.8.1411. [DOI] [PubMed] [Google Scholar]
- 12.Cross JH, Connelly A, Jackson GD, et al. Proton magnetic resonance spectroscopy in children with temporal lobe epilepsy. Ann Neurol. 1996;39:107–113. doi: 10.1002/ana.410390116. [DOI] [PubMed] [Google Scholar]
- 13.Breiter SN, Arroyo S, Mathews VP, et al. Proton MR spectroscopy in patients with seizure disorders. AJNR Am J Neuroradiol. 1994;15:373–384. [PMC free article] [PubMed] [Google Scholar]
- 14.Ng TC, Comair YG, Xue M, et al. Temporal lobe epilepsy: presurgical localization with proton chemical shift imaging. Radiology. 1994;193:465–472. doi: 10.1148/radiology.193.2.7972764. [DOI] [PubMed] [Google Scholar]
- 15.Gadian DG, Connelly A, Duncan JS, et al. 1H magnetic resonance spectroscopy in the investigation of intractable epilepsy. Acta Neurol Scand Suppl. 1994;152:116–121. doi: 10.1111/j.1600-0404.1994.tb05202.x. [DOI] [PubMed] [Google Scholar]
- 16.Vainio P, Usenius JP, Vapalahti M, et al. Reduced N-acetylaspartate concentration in temporal lobe epilepsy by quantitative 1H MRS in vivo. Neuroreport. 1994;5:1733–1736. doi: 10.1097/00001756-199409080-00011. [DOI] [PubMed] [Google Scholar]
- 17.Peeling J, Sutherland G. 1H magnetic resonance spectroscopy of extracts of human epileptic neocortex and hippocampus. Neurology. 1993:589–594. doi: 10.1212/wnl.43.3_part_1.589. [DOI] [PubMed] [Google Scholar]
- 18.Rosenbaum TJ, Laxer KD, Vessely M, Smith WB. Subdural electrodes for seizure focus localization. Neurosurgery. 1986;19:73–81. doi: 10.1227/00006123-198607000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Risinger MW, Engel JJ, Van NP, et al. Ictal localization of temporal lobe seizures with scalp/sphenoidal recordings. Neurology. 1989;39:1288–1293. doi: 10.1212/wnl.39.10.1288. [DOI] [PubMed] [Google Scholar]
- 20.Watson C, Andermann F, Gloor P, et al. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology. 1992;42:1743–1750. doi: 10.1212/wnl.42.9.1743. [DOI] [PubMed] [Google Scholar]
- 21.Jackson GD, Connelly A, Duncan JS, et al. Detection of hippocampal pathology in intractable partial epilepsy: increased sensitivity with quantitative magnetic resonance T2 relaxometry. Neurology. 1993;43:1793–1799. doi: 10.1212/wnl.43.9.1793. [DOI] [PubMed] [Google Scholar]
- 22.Grunewald RA, Jackson GD, Connelly A, Duncan JS. MR detection of hippocampal disease in epilepsy: factors influencing T2 relaxation time. AJNR Am J Neuroradiol. 1994;15:1149–1156. [PMC free article] [PubMed] [Google Scholar]
- 23.Ende G, Laxer K, Knowlton R, et al. Water referenced quantitative 2D 1H SI in the hippocampus of healthy controls and TLE patients. Proc Soc Magn Reson. 1995;3:1962. (Abstract) [Google Scholar]
- 24.Engel JJ. Outcome with respect to epileptic seizures. In: Engel JJ, editor. Surgical treatment of the epilepsies. Raven Press; New York: 1987. pp. 553–571. [Google Scholar]
- 25.Duncan JS, Sagar HJ. Seizure characteristics, pathology, and outcome after temporal lobectomy. Neurology. 1987;37:405–409. doi: 10.1212/wnl.37.3.405. [DOI] [PubMed] [Google Scholar]
- 26.Cascino GD, Jack CR, Parisi JE, et al. Magnetic resonance imaging-based volume studies in temporal lobe epilepsy: pathological correlations. Ann Neurol. 1991;30:31–36. doi: 10.1002/ana.410300107. [DOI] [PubMed] [Google Scholar]
- 27.Jack CJ, Trenerry MR, Cascino GD, et al. Bilarerally symmetric hippocampi and surgical outcome. Neurology. 1995;45:1353–1358. doi: 10.1212/wnl.45.7.1353. [DOI] [PubMed] [Google Scholar]
- 28.Achten E, Boon P, De Poorter J, et al. An MR protocol for presurgical evaluation of patients with complex partial seizures of temporal lobe origin. AJNR Am J Neuroradiol. 1995;16:1201–1213. [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson GD, Kuzniecky RI, Cascino GD. Hippocampal sclerosis without detectable hippocampal atrophy. Neurology. 1994;44:42–46. doi: 10.1212/wnl.44.1.42. [DOI] [PubMed] [Google Scholar]
- 30.Hugg JW, Laxer KD, Matson GB, et al. Neuron loss localizes human temporal lobe epilepsy by in vivo proton magnetic resonance spectroscopic imaging. Ann Neurol. 1993;34:788–794. doi: 10.1002/ana.410340606. [DOI] [PubMed] [Google Scholar]
- 31.Cendes F, Andermann F, Gloor P, et al. MRI volumetric measurement of amygdala and hippocampus in temporal lobe epilepsy. Neurology. 1993;43:719–725. doi: 10.1212/wnl.43.4.719. [DOI] [PubMed] [Google Scholar]
- 32.Haupt C, Schuff N, Weiner M, Maudsley A. Removal of lipid artifacts in 1H spectroscopic imaging by extrapolation. Magn Reson Med. 1996;35:678–687. doi: 10.1002/mrm.1910350509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spencer SS. The relative contributions of MRI, SPECT, and PET imaging in epilepsy. Epilepsia. 1994;(suppl 6):S72–S89. doi: 10.1111/j.1528-1157.1994.tb05990.x. [DOI] [PubMed] [Google Scholar]
- 34.Margerison JH, Corsellis JAN. Epilepsy and the temporal lobes: a clinical, electroencephalographic and neuropathological study of the brain in epilepsy, with particular reference to the temporal lobes. Brain. 1966;89:499–530. doi: 10.1093/brain/89.3.499. [DOI] [PubMed] [Google Scholar]
- 35.De Stefano N, Matthews PM, Arnold DL. Reversible decreases in N-acetylaspartate after acute brain injury. Magn Reson Med. 1995;34:721–727. doi: 10.1002/mrm.1910340511. [DOI] [PubMed] [Google Scholar]
- 36.Jack CJ, Sharbrough FW, Cascino GD, et al. Magnetic resonance image-based hippocampal volumetry: correlation with outcome after temporal lobectomy. Ann Neurol. 1992;31:138–146. doi: 10.1002/ana.410310204. [DOI] [PubMed] [Google Scholar]
- 37.Radtke RA, Hanson MW, Hoffman JM, et al. Temporal lobe hypometabolism on PET: predictor of seizure control after temporal lobectomy. Neurology. 1993;43:1088–1092. doi: 10.1212/wnl.43.6.1088. [DOI] [PubMed] [Google Scholar]
- 38.Manno EM, Sperling MR, Ding X, et al. Predictors of outcome after anterior temporal lobectomy: positron emission tomography. Neurology. 1994;44:2331–2336. doi: 10.1212/wnl.44.12.2321. [DOI] [PubMed] [Google Scholar]