Abstract
Objective:
To examine the degree to which neonatal illness severity, post-neonatal health problems, child characteristics, parenting quality as measured by the HOME Inventory, and maternal characteristics related to the development of wheezing in prematurely born children over the first 27 months after term.
Design:
Longitudinal predictive study.
Setting:
Infants were recruited from two NICUs, one in southeast and one in Midwest.
Participants:
One hundred thirteen (113) preterm infants who weighed less than 1500 gm or required mechanical ventilation and their mothers.
Main outcome measures:
The presence of wheezing was obtained from maternal report at 2, 6, 9, 13, 18, 22, and 27 months. Wheezing was considered to be medically significant if the child was using bronchodilators or pulmonary anti-inflammatory medications.
Results:
Sixty eight (68) percent of the children had wheezing at least one age; 47% of the children were also taking bronchodilators or pulmonary anti-inflammatory medications and thus had medically significant wheezing.
Conclusion:
Post-neonatal health problems and the social environment appear to be more important in developing wheezing in prematurely born children than neonatal medical complications.
Keywords: Wheezing, Premature Infants, Risk, Overweight
Asthma is the most common chronic illness of children (Yawn, Wollan, Kurland, & Scanlon, 2002). One group of children at particular risk for wheezing and asthma is premature infants (Berz et al., 2007; Gessner & Chimonas, 2007; Greenough et al., 2005; Milner, Stein, McCarter, & Moon, 2004; Siltanen, Savilahti, Pohjavuori, & Kajosaari, 2004), but only limited prospective research has examined how neonatal and post-neonatal factors relate to the development of wheezing in prematures. According to the developmental science perspective (Cairns, Elder, & Costello, 1996; Miles & Holditch-Davis, 2003), children's health and development are outcomes of their continuous, reciprocal interactions with their environment. The child and environment form a complex system, made up of elements that are also systems, such as the mother and child (Cairns et al., 1996; Miles & Holditch-Davis, 2003). Thus, the development of wheezing is due to interactions among characteristics of the infant, such as age and other illnesses, and factors in the infant's environment including parenting quality and can be best understood by exploring the effects of multiple aspects of the child and environment at the same time. The purpose of this study, therefore, was to examine environmental and medical factors related to the development of wheezing in prematurely born children over the first 27 months after term. Although this paper focuses on wheezing, definitions of wheezing and asthma used in previous studies overlap, and the predictors of wheezing and asthma were quite similar so literature on both wheezing and asthma will be reviewed.
Predictors of Wheezing and Asthma
The incidence of wheezing and asthma is related to neonatal illness severity. Birthweight of less than 2500 grams has been related to transient wheezing occurring before 4 years of age but not to persistent wheezing or wheezing beginning at later ages (Kurukulaaratchy, Waterhouse, Matthews, & Arshad, 2005), and birth weight less than 1500 grams was related to an increased rate of asthma diagnoses (Brooks, Byrd, Weitzman, Auinger, & McBride, 2001; Gessner & Chimonas, 2007). Premature infants with oxygen dependence at 36 weeks or longer mechanical ventilation had a higher incidence of asthma by 12 months corrected age than other premature infants (Greenough et al., 2005; Grischkan et al., 2004; Koivisto et al., 2005). Some investigators have found that small for gestational age (SGA) premature infants had lower rates of asthma (Grischkan et al., 2004), whereas others have found no differences in the asthma rates of SGA and average-sized prematures (Bardin, Piuze, & Papageorgiou, 2004; Gessner & Chimonas, 2007).
The incidence of wheezing and asthma are also related to post-neonatal health problems. Children with allergies are more likely to develop asthma than other children (Kocabas et al., 2005; Kurukulaaratchy, Matthews, & Arshad, 2004; Nafstad, Brunekreef, Skrondal, & Nystad, 2005), but prematurely born children with wheezing were less likely to have allergies than fullterms (Siltanen et al., 2004). Children with more respiratory infections in the first year of life, including lower respiratory tract infections, respiratory syncytial virus (RSV) infections, or croup, were at higher risk of asthma at 3 to 10 years of age than other children (Gessner & Chimonas, 2007; Korppi, Piippo-Savolainen, Korhonen, & Remes, 2004; Kurukulaaratchy et al., 2004; Lee et al., 2007; Nafstad et al., 2005). Obesity increased asthma risk in school-aged children and adolescents (Flaherman & Rutherford, 2006; Grischkan et al., 2004; Mai, Gaddlin, Nilsson, & Leijon, 2005; Saha, Riner, & Liu, 2005). Some investigators have found that being large for gestational age at birth increased asthma risk in fullterms (Flaherman & Rutherford, 2006; Sin et al., 2004) although other investigators did not (Taveras et al., 2006). No investigators have examined the impact of excess weight gain on wheezing risk in premature infants or toddlers.
Wheezing and asthma also are related to child characteristics. In particular, in studies of both fullterm and premature infants before 10 years of age, boys have been found to be at greater risk of wheezing and asthma than girls (Berz et al., 2007; Gessner & Chimonas, 2007; Greenough et al., 2005; Grischkan et al., 2004; Higgins, Wakefield, & Cloutier, 2005; Koivisto et al., 2005; Kurukulaaratchy et al., 2005; Milner et al., 2004; Saha, Riner, & Liu, 2005; Wright, Stern, Kauffmann, & Martinez, 2006). Minority children, particularly African Americans, have been found to have higher rates of asthma in population-based studies and studies of prematurely born children (Akinbami, Rodes, & Lara, 2005; Arif, Borders, Patterson, Rohrer, & Xu, 2004; Berz et al., 2007; Grischkan et al., 2004; Higgins et al., 2005; McDaniel, Paxson, & Waldfogel, 2006; Milner et al., 2004; Ramsey, Celedon, Sredl, Weiss, & Cloutier, 2005; Saha et al., 2005). Being born by cesarean birth was also associated with an increased risk of asthma, particularly in premature infants (Debley, Smith, Redding, & Critchlow, 2005; Gessner & Chimonas, 2007; Kero et al., 2002), because of lower exposure to maternal vaginal and fecal flora during delivery.
Finally, developing wheezing and asthma has been related to maternal characteristics and parenting. Mothers with low socioeconomic status (SES), as indicated by low education, low income, living in rented housing, or being unmarried, are usually found to be more likely to have children with asthma (Berz et al., 2007; Gessner & Chimonas, 2007; Greenough et al., 2005; Higgins et al., 2005; Kurukulaaratchy et al., 2004; Milner et al., 2004), but Yawn et al. (2002) found that higher SES children were more likely to be diagnosed with asthma. Health problems and asthma in particular are more common in infants experiencing less positive parenting and greater punitiveness, possibly because of the stress (Klinnert et al., 2001; Mantymaa et al., 2003).
However, most studies of wheezing or asthma risk have been conducted in fullterm infants or older children. Studies of risk for wheezing in prematures have been focused primarily on neonatal illnesses or child characteristics (Debley et al., 2005; Greenough et al., 2005; Grischkan et al., 2004; Koivisto et al., 2005). Post-neonatal health problems, maternal characteristics, and parenting have been examined mainly in population-based studies or studies of fullterms. Yet according to the developmental science perspective, these factors need to be examined along with more infant-focused factors. Environmental factors and post-neonatal health problems may be more accessible to nursing interventions than neonatal illnesses or child characteristics. In order for nurses to better target preventive efforts, identifying the multitude of factors related to the development of wheezing in premature infants is needed. Thus, the purpose of this study was to examine environmental and medical factors related to developing wheezing in prematurely born children over the first 27 months after term. Specifically, we determined the degree to which neonatal illness severity (birthweight, being small for gestational, length of mechanical ventilation), post-neonatal health problems (allergies, upper respiratory tract infections, pneumonia, weight for height), child characteristics (gender, race, cesarean delivery), parenting quality, and maternal characteristics (marital status, education) were related to wheezing.
Methods
Participants
The participants in this study were 113 infants and mothers who were enrolled in a larger study of biological and social risks of prematures (Holditch-Davis, Scher, Schwartz, & Hudson-Barr, 2004; Holditch-Davis, Schwartz, Black, & Scher, 2007). All infants were born before 35 weeks gestation and were high risk for developmental problems due to either a birthweight less than 1500 grams or a requirement for mechanical ventilation or continuous positive airway pressure. The infants were recruited from two tertiary hospitals: 60 from a southeastern perinatal center with a rural/small town population and 53 from an urban midwestern children's hospital. Infants with congenital problems affecting development (such as Down Syndrome) were excluded, but infants with postnatal neurological insults were eligible. To allow time for infants to manifest wheezing not directly due to neonatal respiratory problems, only infants from the larger study with data for at least 6 months after term were included in this report. The demographic characteristics of the sample are given in Table 1. Infants from the two hospitals and their mothers did not differ on any demographic variable except that more multiple births and first births were recruited from the southeastern hospital (Holditch-Davis et al., 2004). The 21 infants and mothers who were lost to follow-up before 6 months differed significantly from the 113 infant-mother dyads in this report only in that the mothers of drop-outs were younger (mean of 23.7 years versus 28.8 years).
Table 1.
Demographic Characteristics of the 113 Premature Infants and Their Mothers.
| Mean | (SD) | Percent | |
|---|---|---|---|
| Gestational Age (weeks) | 28.8 | (2.7) | |
| Birth Weight in Grams | 1221 | (430) | |
| Small for Gestational Age | 13.3% | ||
| Sex of Child: Male | 52.2% | ||
| Female | 47.8% | ||
| Mechanical Ventilation (days) | 11.0 | (18.4) | |
| Neurological Insults | 2.8 | (3.0) | |
| IVH | 22.1% | ||
| Percent: Singletons | 69.9% | ||
| Twins | 24.8% | ||
| Triplets | 5.3% | ||
| Maternal Age (years) | 28.5 | (6.5) | |
| Married Mothers | 57.5% | ||
| Maternal Education (years) | 13.7 | (2.3) | |
| First-Time Mothers | 55.8% | ||
| Race: African American | 46.0% | ||
| White | 53.1% | ||
| Asian | 0.9% |
Note: The two hospitals differed significantly in multiple births and percent of first births.
Variables for Analysis
All data were collected using ages corrected for prematurity.
Presence of wheezing
The presence of wheezing was obtained from a child health history. At 2, 6, 9, 13, 18, 22, and 27 months, the mother reported whether her child had experienced any of 10 listed health problems, including wheezing or asthma, since the previous contact. She also listed any medications the child was currently receiving. The investigators considered wheezing to be medically significant if the mother reported that the child was using any medications that the investigators later classified as pulmonary anti-inflammatory medications or bronchodilators. Otherwise, the child was considered to have mild wheezing. Children could change back and forth from no wheezing to mild wheezing or medically significant wheezing.
Child characteristics and illness severity
Days of mechanical ventilation, gestational age, birthweight, whether the infant was SGA, cesarean delivery, and gender were obtained from the neonatal medical record. The length of mechanical ventilation was skewed (mean 11.0, SD 18.4), so infants with 1 day or less of ventilation were scored as receiving 1 day, and the logarithm (base 10) of each subject's days of ventilation was used in analyses. Forty-six infants had chronic lung disease, but chronic lung disease was correlated (Spearman correlation = .65) with longer mechanical ventilation so ventilation length was used instead of chronic lung disease in analyses.
Child race was scored as either white or minority from the demographic questionnaire that the mothers completed at each contact. (One minority infant was Asian; the rest were African American.) Allergies, upper respiratory tract infections (listed as colds, croup, or cough), and pneumonia or RSV infection--were three health problems listed on the child health history. Infant post-neonatal health problems were obtained by maternal report on this history. Seven dichotomous allergy and seven dichotomous upper respiratory infection variables (presence of allergies or upper respiratory infections from hospital discharge to 2 months, between 2 and 6 months, between 6 and 9 months, between 9 and 13 months, between 13 and 18 months, between 18 and 22 months, and between 22 and 27 months) and one dichotomous pneumonia variable (occurrence of pneumonia or RSV any time in the first 13 months) were used in analyses. The pneumonia and RSV variable was limited to the first 13 months because previous investigators indicated that having these illnesses in the first year increased asthma risk (Korppi et al., 2004), and these illnesses occurred too infrequently in this sample to be sampled at 3-6 month intervals.
Height and weight were obtained at each in-person contact (2, 6, 9, 18, and 27 months). Infants were weighed on a battery-operated, electronic scale with a capacity of 20 kilograms and accuracy within 10 gm. Length was measured on a height measuring board, which was accurate to the nearest 0.1 cm. The equipment was portable and was taken into the home. Weight in kilograms divided by length (cm2) was calculated, and the mean values at early ages (2, 6, 9 months) and at later ages (18, 27 months) were used in analyses. Forty-nine of the 107 infants with measurements were above the 90th percentile on weight-for-length ratio for premature infants and toddlers (Guo, Wholihan, Roche, Chumlea, & Casey, 1996) at the early ages, and 22 of 72 were above the 90th percentile at older ages.
HOME
The HOME inventory (0-3 version) consists of 45 items in six sub-scales and is designed to identify children who are at risk for developmental delay due to a lack of appropriate stimulation in the home environment (Caldwell & Bradley, 1980). Each item is scored as present or absent; the score equals the number of present items. The HOME is administered using maternal interview and observation. The first author trained research assistants at both sites until they achieved 90% inter-rater reliability. Test-retest reliability for the total scale over 6-months was .76-.77 (Holditch-Davis, Tesh, Goldman, Miles, & D'Auria, 2000). Two HOME sub-scales were used in this report: sub-scale I, Maternal Emotional and Verbal Responsivity, which measures the mother's sensitivity to child cues, and sub-scale II, Acceptance of the Child's Behavior, which measures the mother's avoidance of use of punishment and restriction. Internal consistency of sub-scale I for this sample was .90 at 6 months and .69 at 18 months; sub-scale II was .77 at 6 months and .58 at 18 months. These sub-scales were correlated with other measures of parenting quality (Holditch-Davis et al., 2000; Tesh & Holditch-Davis, 1997).
Maternal characteristics
Maternal characteristics of years of education and marital status were recorded on the demographic questionnaire that the mothers completed at enrollment.
Procedures
The institutional committee for protection of human subjects at each institution approved the study. Infants were enrolled when their medical conditions were no longer critical (not mechanically ventilated or in an immediate life-threatening situation) if an additional hospital stay of at least 1 week was anticipated and informed consent was obtained from the mothers. Immediately after enrollment, mothers completed questionnaires on demographic characteristics. Infant medical records were reviewed weekly until hospital discharge.
Follow-up at home was conducted at 6 and 18 months corrected for prematurity, and the HOME Inventory was administered. The mothers also completed questionnaires (demographics, health history). Questionnaires were also administered by mail (13 and 22 months) or during hospital visits (2, 9, and 27 months) using the same procedures. Height and weight measurements were obtained during home and hospital visits. Mothers were paid $10 each time they completed questionnaires. The infant was given a small gift at the end of each home visit.
Data Analyses
Alpha was set at .05 for two-tailed tests. The likelihood of having medically significant wheezing, mild wheezing, or neither at each age was analyzed using generalized estimating equations (GEE; Zeger, Liang, & Albert, 1988), an extension to the proportional odds model (Stokes, Davis, & Koch, 2000). The GEE approach is a flexible procedure capable of analyzing ordinal longitudinal categorical data and easily accommodates missing and mistimed values, as occurred in this study. As a repeated measures regression model, it can assess the relationship between an ordinal categorical outcome measure and continuous and categorical covariates. Parameterization of GEE includes population (fixed) effects (the effect of age and static and time-varying covariates). Time was modeled as a continuous explanatory variable, and the repeated measures within a subject were treated as being correlated. Our GEE models tested for the linear and quadratic effects of post-menstrual age, the effects of the covariates, and any pairwise interactions among the environmental and medical variables as well as between these variables and age. In the GEE approach, missing data are assumed to be missing completely at random, and the predicted trends are not affected. An odds ratio was estimated for each predictor.
Because the ages of the children at the contacts varied slightly, the actual age of the child at the time of each contact was used in analyses. Age was adjusted so that the intercepts equaled the cumulative log odds of having medically significant or mild wheezing at 29 weeks post-menstrual age, roughly the mean gestational age of the sample. The groupings of neonatal illness severity, post-neonatal health problems, child characteristics, parenting quality, and maternal characteristics were candidate covariates. Two variables were measured at all ages: the presence of allergies and the presence of upper respiratory infections. For all other covariates, a single value was used for each infant. The model selection strategy was performed in multiple stages. First, models of the relation of the wheezing variable within each grouping of predictors were fitted and reduced using backward selection at p = .15. Then, we tested a combined model that included all individual variables significant at p < .15. Interactions of these variables with age, and pairwise interactions among these variables were tested separately. A group-wise test of the interactions was performed at p < .10, and individual interactions were only tested if the group met this criterion. Backward elimination was used for the remaining variables until a final mixed model with each variable having p < .05 was obtained. (Higher p-levels were used in the preliminary analyses to avoid prematurely eliminating a variable, resulting in a Type II error.) Overall, our procedure simplified the model and led to inferences that some effects were either zero or not large enough to be detected.
Results
Table 2 shows the percent of infants reported to have wheezing at each age. About 47% of the children were reported to have medically significant wheezing by 27 months corrected age. The mean age of the children at the first report of significant wheezing was 53 weeks or about 12 months. There were three patterns of medically significant wheezing: 10.6% of children had a report of medically significant wheezing at one age and no other report of wheezing, 11.5% had significant wheezing at one age along with mild wheezing at one or more ages, and 24.8% of children had recurrent medically significant wheezing (10.6% at two ages, 7.1% at three, 5.3% at four, 0.9% at five, 0.9% at six). In addition, 21.2% of the children had mild wheezing at one or more ages but no medically significant wheezing. Only 31.9% of the children had no reports of wheezing at any age. Only eight children had medically significant wheezing at 2 months (one with significant wheezing at only that age and the rest with wheezing at other ages) so very few children showed transient medically significant wheezing that was likely to be due exclusively to neonatal respiratory problems.
Table 2.
Percent of Infants Reported to Have Each Wheezing Variable at Each Age
| Na | No Wheezing |
Mild Wheezing |
Medically Significant Wheezing |
|
|---|---|---|---|---|
| 2 months | 98 | 83.7% | 8.2% | 8.2% |
| 6 months | 98 | 69.4% | 17.4% | 13.3% |
| 9 months | 90 | 63.3% | 22.2% | 14.4% |
| 13 months | 88 | 60.2% | 17.1% | 22.7% |
| 18 months | 94 | 54.3% | 20.2% | 25.5% |
| 22 months | 81 | 64.2% | 17.3% | 18.5% |
| 27 months | 57 | 59.7% | 14.0% | 26.3% |
The N varies over time because of missing contacts, study withdrawals, and incomplete questionnaires.
Medical and Environmental Effects on Wheezing
Tables 1 and 3 present the descriptive statistics for the medical and environmental variables. The parenting variables and maternal characteristics were inter-correlated (r = .25 to .40) but uncorrelated with the child variables except for race (correlated with all maternal variables, r = −.35 to −.56) and cesarean delivery (r = .23 with education). Child variables showed only isolated correlations (birthweight with mechanical ventilation, early and late weight/length2, and cesarean delivery; mechanical ventilation with pneumonia; SGA with URIs and cesarean delivery; allergies with URIs; and early weight/length2 with pneumonia, cesarean delivery, and late weight/length2).
Table 3.
Descriptive Characteristics of the Medical and Environmental Variables
| Na | Mean | (SD) | Percent | |
|---|---|---|---|---|
| Allergies: 2 months | 96 | 3.1% | ||
| 6 months | 97 | 7.2% | ||
| 9 months | 88 | 14.8% | ||
| 13 months | 86 | 8.1% | ||
| 18 months | 91 | 14.3% | ||
| 22 months | 83 | 14.5% | ||
| 27 months | 55 | 21.8% | ||
| Upper respiratory tract infections: 2 months | 98 | 36.7% | ||
| 6 months | 98 | 78.6% | ||
| 9 months | 90 | 64.4% | ||
| 13 months | 88 | 77.3% | ||
| 18 months | 94 | 83.0% | ||
| 22 months | 81 | 80.3% | ||
| 27 months | 57 | 86.0% | ||
| Pneumonia in first 13 months | 111 | 9.9% | ||
| Weight for Length2: Early Ages | 107 | 1.7 | (0.2) | |
| Later Ages | 72 | 1.6 | (0.2) | |
| Cesarean Delivery | 113 | 54.0% | ||
| HOME Sub-Scale I | 109 | 10.0 | (0.9) | |
| Home Sub-Scale II | 109 | 6.5 | (1.3) |
The N varies over time because of missing contacts, study withdrawals, and incomplete questionnaires.
In preliminary GEEs, we examined the relationship of the variables within each group of predictors with wheezing (see Table 4). For the backward elimination model with the neonatal illness variables, mechanical ventilation was related to the development of wheezing although at p > .05. Three of the post-neonatal health problems--URIs, allergies, and weight to length2 ratio at early ages--were associated with a greater probability of having wheezing. Race was the only child characteristic related to wheezing; minority children were more likely to develop wheezing. One parenting variable (HOME sub-scale II) and one maternal characteristic (marital status) were also related to the development of wheezing. Lower scores on HOME subscale II (greater punitiveness) and unmarried mothers were associated with a greater risk for wheezing.
Table 4.
Relationship of Wheezing to Variables Within Each Group of Medical and Environmental Predictors (Initial and Reduced Models Within Each Group of Variables).
| Initial Model | Reduced Model | ||||
|---|---|---|---|---|---|
| Group | Predictors | Estimatea | (SE) | Estimatea | (SE) |
| Neonatal Illness | Birthweight | 0.00 | (0.00) | ||
| Mechanical Ventilation | 0.34 | (0.28) | 0.35# | (0.21) | |
| Size (Being SGA) | −0.41 | (0.50) | |||
| Post-Neonatal Health | Allergies | 0.78# | (0.36) | 0.93** | (0.32) |
| Problems | URIs | 0.92** | (0.35) | 1.05*** | (0.28) |
| Pneumonia | 0.18 | (0.46) | |||
| Early Weight/Length2 | 2.58* | (0.88) | 2.10** | (0.73) | |
| Later Weight/Length2 | 0.14 | (0.91) | |||
| Child Characteristics | Gender (Being Female) | 0.12 | (0.28) | ||
| Race (Being Minority) | 0.98*** | (0.29) | 1.02*** | (0.28) | |
| Cesarean Delivery | −0.17 | (0.29) | |||
| Parenting | HOME--I (Responsivity) | 0.03 | (0.13) | ||
| HOME--II (Acceptance) | −0.39*** | (0.11) | −0.39*** | (0.10) | |
| Maternal Characteristics | Marital Status | −1.11*** | (0.32) | −1.18*** | (0.28) |
| Maternal Education | −0.03 | (0.07) | |||
Estimates are the natural log of the cumulative odds ratios.
p < .05.
p < .01.
p < .001.
p < .15.
Final Model Predicting Wheezing from Medical and Environmental Factors
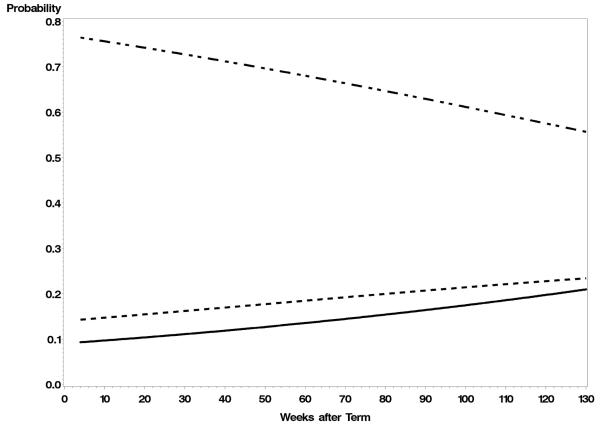
We then tested a combined GEE model that included all individual variables with p < .15 on the preliminary analyses; pairwise interactions among these variables and of each variable with age were also tested. Figure 1 shows the results with significant covariates held constant at their means. The probability of medically significant wheezing increased from term age through 27 months (from about a 10% probability to slightly less than 20% probability), while the probability that the children would not have wheezing decreased. The likelihood of having mild wheezing also increased but at a slower rate from about 14% to 22%.
Figure 1.
The model-predicted probabilities of a prematurely born children having medically significant and mild wheezing over age. The covariates are held constant at their means. The solid line shows the predicted probability for medically significant wheezing, the dotted line is mild wheezing, and the line with short and long dashes is no wheezing.
Table 5 shows the results of the final reduced model. In addition to the age effect, mechanical ventilation, allergies, URIs, weight to length2 ratio at early ages, sub-scale II of the HOME Inventory (avoidance of punitiveness), and marital status were related to wheezing. Children with longer mechanical ventilation, with allergies, with URIs, greater weight for length in the first year, lower scores on HOME sub-scale II, and unmarried mothers were more likely to have wheezing. The odds ratios indicated that gaining 52 weeks in age increased the odds of having wheezing by about 50%. An increase in 1 in log mechanical ventilation (the difference between 1 and 10 days or between 10 and 100 days) was associated with 1.6 times the odds of wheezing. Children with allergies had 2.8 times the odds of wheezing as children without allergies, and children with URIs had 2.9 times the odds of wheezing. An increase in 1 in the weight to length2 ratio (roughly the difference between the smallest and the heaviest infant) was associated with four times the risk of wheezing. A decrease in 1 point in the HOME sub-scale II score was associated with 1.3 times the odds of wheezing. Having an unmarried mother was associated with 2.4 times the odds of wheezing. There were no significant interactions.
Table 5.
Final Model of Predictors of Wheezing by Medical and Environmental Variables
| Cumulative Odds Ratiosa |
||||
|---|---|---|---|---|
| Estimate | SE | Estimate | Confidence Limits | |
| Intercept1b | −3.918** | 1.437 | ||
| Intercept2c | −2.817* | 1.414 | ||
| Age (weeks) | 0.007* | 0.003 | 1.007 | 1.001 - 1.013 |
| Mechanical Ventilation | 0.461* | 0.199 | 1.586 | 1.075 – 2.342 |
| Allergies | 1.027** | 0.321 | 2.792 | 1.487 – 5.241 |
| URIs | 1.056*** | 0.264 | 2.875 | 1.714 – 4.823 |
| Early Weight/Length2 | 1.483* | 0.660 | 4.404 | 1.209 – 16.049 |
| HOME Sub-Scale II | −0.245* | 0.110 | 0.782 | 0.631 – 0.970 |
| Marital Status | −0.868** | 0.320 | 0.420 | 0.224 - 0.785 |
The odds ratio both for medically significant wheezing versus mild wheezing or no wheezing and for any wheezing versus no wheezing.
Intercept for the cumulative odds of medically significant wheezing versus either mild wheezing or no wheezing.
Intercept for the cumulative odds of any wheezing (medically significant or mild) versus no wheezing.
p < .05.
p < .01.
p < .001.
Discussion
Similar to the results of other studies (Gessner & Chimonas, 2007; Greenough et al., 2005; Milner et al., 2004; Siltanen et al., 2004), we found that mothers reported that a high percentage of prematurely born children had medically significant wheezing (47%) or mild wheezing (21%) in the first 27 months after term. Children who experienced longer mechanical ventilation, were older, had allergies or more frequent upper respiratory tract infections, had greater weight for length in the first year, or had mothers who were more punitive or unmarried were more likely to be reported to have wheezing. Studies of fullterms and population-based studies have had similar results (e.g., Berz et al., 2007; Grischkan et al., 2004; Korppi et al., 2004; Kurukulaaratchy et al., 2004; Nafstad et al., 2005; Saha et al., 2005), but this is one of the very few studies in which multiple factors were examined in prematures (Gessner & Chimonas, 2007; Siltanen et al., 2004).
The incidence of recurrent medically significant wheezing in this study, 24.8%, was similar to rates reported by other investigators. In studies using parental report, 19%-33% of prematures had asthma by 8-15 years (Nixon, Washburn, Schechter, & O'Shea, 2007; Palta et al., 2001; Saigal et al., 2001), and 16-34% of fullterms had wheezing by 3 years (Berz et al., 2007; Traveras et al., 2006). Using physician diagnoses, investigators found 20%-43% of prematures had wheezing or asthma (Brooks et al., 2001; Greenough et al., 2005; Siltanen et al., 2004). In our study, another 43% of infants had medically significant wheezing at one age or mild wheezing.
Other studies on wheezing or asthma risk in prematures have been focused primarily on the effects of neonatal illnesses or child characteristics (Debley et al., 2005; Greenough et al., 2005; Grischkan et al., 2004, Koivisto et al., 2005). Yet the only neonatal illness related to wheezing in our analyses was longer mechanical ventilation. Infants experiencing longer mechanical ventilation or chronic lung disease are known to be more likely to have respiratory illnesses in the first 2 years of life (Chien, Tsao, Chou, Tang, & Tsou, 2002; Koivisto et al., 2005).
Most of the predictors of wheezing we identified were similar to those identified in studies of fullterms and in population-based studies. Like other investigators, we found the rate of wheezing increased with age (Akinbami et al., 2005; Arif et al., 2004; Saha et al., 2005). However, we did not follow our children into school-age so we do not know whether this trend will continue. Wheezing diagnosed before 4 years does not necessarily continue to be problem in later childhood and has different predictors than chronic asthma (Kurukulaaratchy et al., 2005; Siltanen et al., 2004), so whether our predictors would be useful for predicting asthma is unknown. Therefore, prospective longitudinal research is needed to examine the course of early wheezing in preterm infants and how it evolves from infancy to school-age.
We also found that the risk of wheezing was increased by post-neonatal health problems particularly allergies, upper respiratory infections, and greater weight for length. Other investigators had similar results for allergies and upper respiratory tract infections (Flaherman & Rutherford, 2006; Grischkan et al., 2004; Kocabas et al., 2005; Kurukulaaratchy et al., 2005; Nafstad et al., 2005; Saha et al., 2005). However, unlike other studies (Korppi et al., 2004; Kurukulaaratchy et al., 2004; Lee et al., 2007; Nafstad et al., 2005), we did not find a relationship between wheezing and pneumonia or RSV, probably due to the low incidence of these illnesses in our sample. Only 11 of our infants experienced pneumonia or RSV by 13 months corrected age.
Ours in the first study to find that greater weight for length in infancy is related to wheezing risk in the first 27 months. Premature infants may be at particular risk for excess weight gain in infancy because they are more likely to be formula fed than fullterms, and formula feeding leads to more rapid weight gain in early infancy than breastfeeding (Kramer et al., 2004). Also, mothers may feel pressured by their families, friends, and even health care providers to “fatten up” their tiny prematures. On the other hand, we did not find a relationship between wheezing and greater weight for length in toddlers. Infants in our sample were more likely to be above the 90th percentile on weight for length in infancy than in toddlerhood. Because we followed the children only until 27 months, children who were normal weight for length in infancy but became overweight at 18 or 27 months may not have had enough time to develop wheezing. Our findings do differ from those of Mai et al. (2005) that very-low-birth-weight infants showing rapid weight gain in the first 6 months after term were less likely to have asthma at 12 years. However, Mai et al. did not examine whether their infants with rapid weight gain ended up with larger weights for length than other infants, nor did they assess wheezing or asthma at earlier ages.
We found that maternal characteristics and parenting were related to wheezing. Like other studies (Berz et al., 2007; Greenough et al., 2005; Higgins et al., 2005; Kurukulaaratchy et al., 2004; Mantymaa et al., 2003; Milner et al., 2004), we found that maternal punitiveness and maternal marital status were related to an increased risk for wheezing. Children of unmarried mothers and children with more punitive mothers were more likely to develop medically significant wheezing. All of the parenting and maternal characteristic variables were inter-correlated, suggesting that there may be an underlying socioeconomic risk for wheezing. The particular variable showing significance may just be an artifact of the particular study. There are two possible explanations for these effects: low SES children are more likely to be exposed to allergens, tobacco smoke, and other environmental triggers of wheezing (Chen, Matthews, & Boyce, 2002; Wood, 2003); or living in poverty and more punitive parenting may be stressors for children that reduce the effectiveness of their immune systems (Chen et al., 2002; Klinnert et al., 2001; Mantymaa et al., 2003). Other investigators have found that the risk of asthma is increased by a chronic, low-grade stress, such as night-time traffic noise (Dulime et al., 1996; Ising, Lange-Asschenfeldt, Lieber, Weinhold, & Eilts, 2003), so the stresses associated with being raised in a low SES household or by a punitive mother might have a similar effect.
Minority children had more wheezing than whites when child characteristics were analyzed separately but not when race was analyzed along with health problems, parenting, and maternal variables. Other investigators found that race is a risk factor for wheezing and asthma (Akinbami et al., 2005; Berz et al., 2007; Grischkan et al., 2004; Higgins et al., 2005; McDaniel et al., 2006; Saha et al., 2005). Our findings suggest that the higher risk of wheezing in prematurely born minorities is due to the increased rate of low SES and higher use of punitive discipline in minority families (McLoyd, 1998), rather than race or culture per se. Population-based studies of fullterms have had opposite findings: the rate of asthma in African Americans was higher than whites even after controlling for SES (Higgins et al., 2005; McDaniel et al., 2006).
On the other hand, we did not find a relationship between wheezing in prematurely born children and many factors associated with wheezing or asthma in the literature. For example, we did not find a relationship between wheezing risk and either being small for gestational age or being born by cesarean delivery. We also did not find a difference in the incidence of wheezing in boys and girls. Most investigators have found that wheezing incidence is higher in boys (Berz et al., 2007; Gessner & Chimonas, 2007; Greenough et al., 2005; Grischkan et al., 2004; Higgins et al., 2005; Koivitso et al., 2005; Kurukulaaratchy et al., 2005; Milner et al., 2004; Saha et al., 2005; Wright et al., 2006; Yawn et al., 2002). However, this finding is not universal (Osman et al., 2007), and different sub-populations of children with wheezing or asthma show different gender distributions. Thus, Webber, Carpiniello, Oruwariye, and Appel (2002) found that boys were more likely to have an asthma diagnosis, but girls were more common in the children with asthma symptoms but without a diagnosis. Clearly additional research is needed in this area.
Finally, several factors may limit the generalizability of this study. The use of a maternal self-report health questionnaire may have affected our findings. In a study by Cane, Ranganatahan, and McKenzie (2000), there was less than 50% agreement between parents' and clinicians' reports of asthma. In our study, maternal reports of wheezing were confirmed by maternal report of child medications, which nurses on our research team classified as bronchodilators or pulmonary anti-inflammatory medications. Because these medications may also be prescribed for transient wheezing and reactive airway disease, we do not know the medical diagnoses of the children whom we classified with medically significant wheezing. Thus, additional research on the health problems of premature infants that uses medical diagnoses is needed.
Another limitation of our study was the failure to obtain information on a common risk factor for wheezing: parental smoking. Parental smoking is known to increase asthma rates (Berz et al., 2007; Higgins et al., 2005; Murray et al., 2004), and smoking rates are higher in low SES families (Chen et al., 2002; Walker et al., 2004). Thus, some of the increased risk of wheezing in low-income families may have been due to parental smoking. Also, mothers of premature infants are more likely to smoke than mothers of fullterms (Burguet et al., 2004; Gennaro, Dunphy, Dowd, Fehder, & Douglas, 2001). On the other hand, one study found no relationship between maternal prenatal smoking and asthma (Gessner & Chimonas, 2007).
A final limitation was the relatively high number of missing values at some time points. Although 113 infants contributed to the analysis at one or more time points, no time point included more than 98 infants, and as is typical for longitudinal studies, subject attrition increased with time. This tendencey did not seem to be affected by whether the contact was by mail or in person. For this reason we used a statistical technique, the generalized estimating equation (Zeger et al., 1988) in which the predicted trends are not affected missing values and subjects with missing data at one time point can contribute data at a later time.
Implications for Practice
Because prematures have a higher incidence of wheezing and asthma than fullterm infants (Berz et al., 2007; Greenough et al., 2005; Milner et al., 2004; Siltanen et al., 2004), nurses and other health providers need to understand the factors that contribute to developing wheezing and asthma. Our findings provide evidence of the complex processes affecting the development of wheezing in premature infants, although we did not find interactions between variables as expected by the developmental science perspective. Both infant (neonatal and post-neonatal health problems) and environmental factors (maternal marital status) were related to wheezing. Fortunately, many of these factors are potentially amenable to intervention.
Prior to NICU discharge, nurses need to educate parents about the high risk for wheezing in premature infants. Parents need to know what wheezing is (Cane et al., 2000) and the importance of taking a child with wheezing to the pediatrician. Parents should also be instructed to limit the exposure of prematurely born children to respiratory illnesses and potential allergens, not only in the immediate period after discharge but throughout infancy and toddlerhood in order to reduce the incidence of wheezing. In addition, encouraging parents to promote adequate nutrition and growth while avoiding over-feeding may also be beneficial. Weight gain in excess of growth in length in infancy not only leads to increased wheezing in the first few years of life but also is associated with obesity in childhood (Snethen, Hewitt, & Goretzke, 2007).
After discharge, ongoing nursing interventions to promote positive parenting and reduce punitiveness might also reduce the incidence or slow the development of wheezing because this factor was related to wheezing risk. Parents have more difficulties parenting premature infants than fullterms because of their atypical and immature behaviors and parental distress about the NICU experience (Holditch-Davis et al., 2007; Muller-Nix et al., 2004; Singer et al., 2003). These early problems can lead to ongoing problems with discipline (Miles & Holditch-Davis, 1995). Listening to parental concerns about interactions with their premature infants and providing ongoing support should reduce these concerns. In addition, nurses can suggest the use of positive forms of discipline, such as rewarding appropriate behaviors and use of time out. These strategies should lead to better parenting and a reduction in wheezing in this vulnerable group of infants.
Acknowledgments
Supported by NINR NR01894. The authors thank LaTonya Sanders, Sola Park, Lisa Moorehead, Donna Harris, Mary Barkey, Leslie Miller, Tanya Kewson, and Mark Johnson.
Footnotes
The development of wheezing in premature infants is due to the interactions among infant characteristics and factors in the infant's environment including parenting quality.
A high percentage of prematurely born children were reported to have medically significant wheezing (47%) or mild wheezing (21%) in the first 27 months.
Ongoing nursing interventions to promote positive parenting and reduce punitiveness might also reduce the incidence or slow the development of wheezing.
Contributor Information
Diane Holditch-Davis, Nursing and Associate Dean for Research Affairs, School of Nursing, Duke University, Durham, NC, USA.
Piper Merrill, PNP Emergency Department, Children's Medical Center Dallas, TX, USA.
Todd Schwartz, School of Nursing and the Department of Biostatistics, School of Public Health, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Mark Scher, Division of Pediatric Neurology, Department of Pediatrics, Case Western Reserve University School of Medicine and Rainbow Babies and Childrens Hospital Cleveland, OH, USA.
References
- Akinbami LJ, Rhodes JC, Lara M. Racial and ethnic differences in asthma diagnosis among children who wheeze. Pediatrics. 2005;115:1254–12. doi: 10.1542/peds.2004-0897. [DOI] [PubMed] [Google Scholar]
- Arif AA, Borders TF, Patterson PJ, Rohrer JE, Xu KT. Prevalence and correlates of paediatric asthma and wheezing in a largely rural USA population. Journal of Paediatrics and Child Health. 2004;40:189–194. doi: 10.1111/j.1440-1754.2004.00335.x. [DOI] [PubMed] [Google Scholar]
- Bardin C, Piuze G, Papageorgiou A. Outcome at 5 years of age of SGA and AGA infants born less than 28 weeks of gestation. Seminars in Perinatology. 2004;28:288–294. doi: 10.1053/j.semperi.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Berz JB, Carter AS, Wagmiller RL, Horwitz SM, Murdock KK, Briggs-Gowan M. Prevalence and correlates of early onset asthma and wheezing in a healthy birth cohort of 2- to 3-year olds. Journal of Pediatric Psychology. 2007;32:154–166. doi: 10.1093/jpepsy/jsj123. [DOI] [PubMed] [Google Scholar]
- Brooks AM, Byrd RS, Weitzman M, Auinger P, McBride JT. Impact of low birth weight on early childhood asthma in the United States. Archives of Pediatric and Adolescent Medicine. 2001;155:404–406. doi: 10.1001/archpedi.155.3.401. [DOI] [PubMed] [Google Scholar]
- Burguet A, Kaminski M, Abraham-Lerat L, Schall JP, Cambonie G, Fresson J, et al. The complex relationship between smoking in pregnancy and very preterm delivery. Results of the Epipage study. BJOG: An International Journal of Obstetrics and Gynaecology. 2004;111:258–265. doi: 10.1046/j.1471-0528.2003.00037.x. [DOI] [PubMed] [Google Scholar]
- Cairns RB, Elder GH, Costello EJ. Developmental science. Cambridge University Press; Cambridge, Britain: 1996. [Google Scholar]
- Caldwell B, Bradley R. Home Observation for Measurement of the Environment. University of Arkansas at Little Rock; Little Rock: 1980. [Google Scholar]
- Cane RS, Ranganathan SC, McKenzie SA. What do parents of wheezy children understand by “wheeze”? Archives of Disease in Childhood. 2000;82:326–332. doi: 10.1136/adc.82.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Matthews KA, Boyce WT. Socioeconomic differences in children's health: How and why do these relationships change with age? Psychological Bulletin. 2002;128:295–329. doi: 10.1037/0033-2909.128.2.295. [DOI] [PubMed] [Google Scholar]
- Chien YH, Tsao PN, Chou HC, Tang JR, Tsou KI. Rehospitalization of extremely-low-birth-weight infants in first 2 years of life. Early Human Development. 2002;66:33–40. doi: 10.1016/s0378-3782(01)00233-x. [DOI] [PubMed] [Google Scholar]
- Debley JS, Smith JM, Redding GJ, Critchlow CW. Childhood asthma hospitalization risk after cesarean delivery in former term and premature infants. Annals of Allergy, Asthma, and Immunology. 2005;94:228–233. doi: 10.1016/S1081-1206(10)61300-2. [DOI] [PubMed] [Google Scholar]
- Dulime H, Weiland SK, Keil U, Kraemer B, Schmid M, Stender M, et al. The association between self-reported symptoms of asthma and allergic rhinitis and self-reported traffic density on street of residence in adolescents. Epidemiology. 1996;7:578–582. doi: 10.1097/00001648-199611000-00003. [DOI] [PubMed] [Google Scholar]
- Flaherman V, Rutherford GW. A meta-analysis of the effect of high weight on asthma. Archives of Disease in Childhood. 2006;91:334–339. doi: 10.1136/adc.2005.080390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennaro S, Dunphy P, Dowd M, Fehder W, Douglas SD. Postpartum smoking behaviors and immune response in mothers of term and preterm infants. Research in Nursing and Health. 2001;24:9–17. doi: 10.1002/1098-240x(200102)24:1<9::aid-nur1002>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Gessner BD, Chimonas M-AR. Asthma is associated with preterm birth but not with small for gestational age status among a population-based cohort of Medicaid-enrolled children <10 years of age. Thorax. 2007;62:231–236. doi: 10.1136/thx.2005.053363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough A, Limb E, Marston L, Marlow N, Calvert S, Peacock J. Risk factors for respiratory morbidity in infancy after very premature birth. Archives of Disease in Childhood: Fetal and Neonatal Edition. 2005;90:F320–F323. doi: 10.1136/adc.2004.062018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grischkan J, Storfer-Isser A, Rosen CL, Larkin EK, Kirchner HL, South A, Wilson-Costello DC, et al. Variation in childhood asthma among former preterm infants. Journal of Pediatrics. 2004;144:321–326. doi: 10.1016/j.jpeds.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Guo SS, Wholihan K, Roche A, Chumlea WC, Casey PH. Weight-for-length reference data for preterm, low-birth-weight infants. Archives of Pediatric and Adolescent Medicine. 1996;150:964–970. doi: 10.1001/archpedi.1996.02170340078015. [DOI] [PubMed] [Google Scholar]
- Higgins PS, Wakefield D, Cloutier MM. Risk factors for asthma and asthma severity in nonurban children in Connecticut. Chest. 2005;128:3846–3853. doi: 10.1378/chest.128.6.3846. [DOI] [PubMed] [Google Scholar]
- Holditch-Davis D, Scher M, Schwartz T, Hudson–Barr D. Sleeping and waking state development in preterm infants. Early Human Development. 2004;80:43–64. doi: 10.1016/j.earlhumdev.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Holditch-Davis D, Schwartz T, Black B, Scher M. Correlates of mother-premature infant interactions. Research in Nursing and Health. 2007;30:333–346. doi: 10.1002/nur.20190. [DOI] [PubMed] [Google Scholar]
- Holditch-Davis D, Tesh EM, Goldman BD, Miles MS, D'Auria J. Use of the HOME Inventory with medically fragile infants. Children's Health Care. 2000;29:257–277. [Google Scholar]
- Ising H, Lange-Asschenfeldt H, Lieber GF, Weinhold H, Eilts M. Respiratory and dermatological diseases in children with long-term exposure to road traffic immissions. Noise and Health. 2003;5:41–50. [PubMed] [Google Scholar]
- Kero J, Gissler M, Gronlund MM, Kero P, Koskinen P, Hemminki E, et al. Mode of delivery and asthma -- is there a connection? Pediatric Research. 2002;52:6–11. doi: 10.1203/00006450-200207000-00004. [DOI] [PubMed] [Google Scholar]
- Klinnert MD, Nelson HS, Price MR, Adinoff AD, Leung DY, Mrazek DA. Onset and persistence of childhood asthma: Predictors from infancy. Pediatrics. 2001;108:e69. doi: 10.1542/peds.108.4.e69. Retrieved 9/25/2002 from http://www.pediatrics.org/cgi/content/full/108/4/e69. [DOI] [PubMed]
- Kocabas CN, Civelek E, Sackesen C, Orhan F, Tuncer A, Adalioglu G, et al. Burden of rhinitis in children with asthma. Pediatric Pulmonology. 2005;40:235–240. doi: 10.1002/ppul.20247. [DOI] [PubMed] [Google Scholar]
- Koivisto M, Marttila R, Saarela T, Pokela M-L, Valkama AM, Hallman M. Wheezing illness and re-hospitalization in the first two years of life after neonatal respiratory distress syndrome. Journal of Pediatrics. 2006;147:486–492. doi: 10.1016/j.jpeds.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Korppi M, Piippo-Savolainen E, Korhonen K, Remes S. Respiratory morbidity 20 years after RSV infection in infancy. Pediatric Pulmonology. 2004;38:155–160. doi: 10.1002/ppul.20058. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Guo T, Platt RW, Vanolovich I, Sevkovskaya Z, Michaelsen KF, et al. Feeding effects on growth during infancy. Journal of Pediatrics. 2004;145:600–605. doi: 10.1016/j.jpeds.2004.06.069. [DOI] [PubMed] [Google Scholar]
- Kurukulaaratchy RJ, Matthews S, Arshad SH. Does environment mediate earlier onset of the persistent childhood asthma phenotype? Pediatrics. 2004;113:345–350. doi: 10.1542/peds.113.2.345. [DOI] [PubMed] [Google Scholar]
- Kurukulaaratchy RJ, Waterhouse L, Matthews SM, Arshad SH. Are influences during pregnancy associated with wheezing phenotypes during the first decade of life? Acta Paediatrica. 2005;94:553–558. doi: 10.1111/j.1651-2227.2005.tb01938.x. [DOI] [PubMed] [Google Scholar]
- Lee KK, Hegele RG, Manfreda J, Woolrage K, Becker AB, Ferguson AC, et al. Relationship of early childhood viral exposures to respiratory symptoms, onset of possible asthma and atopy in high risk children: The Canadian Asthma Primary Prevention Study. Pediatric Pulmonology. 2007;42:290–297. doi: 10.1002/ppul.20578. [DOI] [PubMed] [Google Scholar]
- Mai X-M, Gaddlin P-O, Nilsson L, Leijon I. Early rapid weight gain and current overweight in relation to asthma in adolescents born with very low birth weight. Pediatric Allergy and Immunology. 2005;16:380–385. doi: 10.1111/j.1399-3038.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- Mantymaa M, Puura K, Luoma I, Salmelin R, Davis H, Tsiantis J, et al. Infant-mother interaction as a predictor of child's chronic health problems. Child Care, Health, and Development. 2003;29:181–191. doi: 10.1046/j.1365-2214.2003.00330.x. [DOI] [PubMed] [Google Scholar]
- McDaniel M, Paxson C, Waldfogel J. Racial disparities in childhood asthma in the United States: Evidence from the National Health Interview Survey, 1997 to 2003. Pediatrics. 2006;117:e868–e877. doi: 10.1542/peds.2005-1721. Retrieved 5/3/2006 from http://www.pediatrics.org/cgi/content/full/117/5/e868. [DOI] [PubMed]
- McLoyd VC. Socioeconomic disadvantage and child development. American Psychologist. 1998;53:185–204. doi: 10.1037//0003-066x.53.2.185. [DOI] [PubMed] [Google Scholar]
- Miles MS, Holditch-Davis D. Enhancing nursing research with children and families using a developmental science perspective. In: Fitzpatrick JJ, Miles MS, Holditch-Davis D, editors. Annual Review of Nursing Research. Vol. 21. Springer; New York: 2003. pp. 1–20. [PubMed] [Google Scholar]
- Miles MS, Holditch-Davis D. Compensatory parenting: How mothers describe parenting their 3-year-old prematurely born children. Journal of Pediatric Nursing. 1995;10:243–253. doi: 10.1016/s0882-5963(05)80021-1. [DOI] [PubMed] [Google Scholar]
- Milner JD, Stein DM, McCarter R, Moon RY. Early infant multivitamin supplementation is associated with increased risk for food allergy and asthma. Pediatrics. 2004;114:27–32. doi: 10.1542/peds.114.1.27. [DOI] [PubMed] [Google Scholar]
- Muller-Nix C, Forcada-Guex M, Pierrehumbert B, Jaunin L, Borghini A, Ansermet F. Prematurity, maternal stress and mother-child interactions. Early Human Development. 2004;79:145–158. doi: 10.1016/j.earlhumdev.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Murray CS, Woodcock A, Smilie FI, Cain G, Kissen P, Custovic A, et al. Tobacco smoke exposure, wheeze, and atopy. Pediatric Pulmonology. 2004;37:492–498. doi: 10.1002/ppul.20019. [DOI] [PubMed] [Google Scholar]
- Nafstad P, Brunekreef B, Skrondal A, Nystad W. Early respiratory infections, asthma, and allergy: 10-year follow-up of the Oslo Birth Cohort. Pediatrics. 2005;116:e255–262. doi: 10.1542/peds.2004-2785. Retrieved 8/31/2005 from http://www.pediatrics.org/cgi/content/full/116/2/e255. [DOI] [PubMed]
- Nixon PA, Washburn LK, Schechter MS, O'Shea TM. Follow-up study of a randomized controlled trial of postnatal dexamethasone therapy in very low birth weight infants: Effects on pulmonary outcomes at age 8 to 11 years. Journal of Pediatrics. 2007;150:345–350. doi: 10.1016/j.jpeds.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman M, Tagiyeva N, Wassall HJ, Ninan TK, Devenny AM, McNeill G, et al. Changing trends in sex specific prevalence rates for childhood asthma, eczema, and hay fever. Pediatric Pulmonology. 2007;42:60–65. doi: 10.1002/ppul.20545. [DOI] [PubMed] [Google Scholar]
- Palta M, Sadek-Badawl M, Sheehy M, Albanes A, Weinstein M, McGuinness G, et al. Respiratory symptoms at age 8 yrs in a cohort of very low birth weight children. American Journal of Epidemiology. 2001;154:521–529. doi: 10.1093/aje/154.6.521. [DOI] [PubMed] [Google Scholar]
- Ramsey CD, Celedon JC, Sredl DL, Weiss ST, Cloutier MM. Predictors of disease severity in children with asthma in Hartford, Connecticut. Pediatric Pulmonology. 2005;39:268–275. doi: 10.1002/ppul.20177. [DOI] [PubMed] [Google Scholar]
- Saha C, Riner ME, Liu G. Individual and neighborhood-level factors in predicting asthma. Archives of Pediatrics and Adolescent Medicine. 2005;159:759–763. doi: 10.1001/archpedi.159.8.759. [DOI] [PubMed] [Google Scholar]
- Saigal S, Stoskopf BL, Streiner DL, Burrows E. Physical growth and current health status of infants who were of extremely low birth weight and controls at adolescence. Pediatrics. 2001;108:407–415. doi: 10.1542/peds.108.2.407. [DOI] [PubMed] [Google Scholar]
- Siltanen M, Savilahti E, Pohjavuori M, Kajosaari M. Respiratory symptoms and lung function in relation to atopy in children born preterm. Pediatric Pulmonology. 2004;37:43–49. doi: 10.1002/ppul.10402. [DOI] [PubMed] [Google Scholar]
- Sin DD, Spier S, Svenson LW, Schopflocher DP, Senthilselvan A, Cowie LW, et al. The relationship between birth weight and childhood asthma: A population-based cohort study. Archives of Pediatrics and Adolescent Medicine. 2004;158:60–64. doi: 10.1001/archpedi.158.1.60. [DOI] [PubMed] [Google Scholar]
- Singer LT, Fulton S, Davillier M, Koshy D, Salvator A, Baley JE. Effects of infant risk status and maternal psychological distress on maternal-infant interactions during the first year of life. Journal of Developmental and Behavioral Pediatrics. 2003;24:233–241. doi: 10.1097/00004703-200308000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snethen JA, Hewitt JB, Goretzke M. Childhood obesity: The infancy connection. Journal of Obstetric, Gynecologic and Neonatal Nursing. 2007;36(5):501–510. doi: 10.1111/j.1552-6909.2007.00181.x. [DOI] [PubMed] [Google Scholar]
- Stokes ME, Davis CS, Koch GG. Categorical data analysis using the SAS system. 2nd ed. SAS Institute Inc.; Cary, NC: 2000. [Google Scholar]
- Taveras EM, Camargo CA, Jr., Rifas-Shiman SL, Oken E, Gold DR, Weiss ST, et al. Association of birthweight with asthma-related outcomes at age 2 years. Pediatric Pulmonology. 2006;41:643–648. doi: 10.1002/ppul.20427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesh EM, Holditch-Davis D. HOME Inventory and NCATS: Relation to mother and child behaviors during naturalistic observations. Research in Nursing and Health. 1997;20:295–307. doi: 10.1002/(sici)1098-240x(199708)20:4<295::aid-nur3>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Walker L, Freeland-Graves JH, Milani T, George G, Hanss-Nuss H, Kim M, et al. Weight and behavioral and psychosocial factors among ethnically diverse, low-income women after childbirth: II. Trends and correlates. Women Health. 2004;40:19–34. doi: 10.1300/J013v40n02_02. [DOI] [PubMed] [Google Scholar]
- Webber MP, Carpiniello KE, Oruwariye T, Appel D. Prevalence of asthma and asthma-like symptoms in inner-city schoolchildren. Pediatric Pulmonology. 2002;34:105–111. doi: 10.1002/ppul.10146. [DOI] [PubMed] [Google Scholar]
- Wood D. Effect of child and family poverty on child health in the United States. Pediatrics. 2003;112:707–771. [PubMed] [Google Scholar]
- Wright AL, Stern DA, Kauffmann F, Martinez FD. Factors influencing gender differences in the diagnosis and treatment of asthma in childhood: The Tucson Children's Respiratory Study. Pediatric Pulmonology. 2006;41:318–325. doi: 10.1002/ppul.20373. [DOI] [PubMed] [Google Scholar]
- Yawn BP, Wollan P, Kurland M, Scanlon P. A longitudinal study of the prevalence of asthma in a community population of school-age children. Journal of Pediatrics. 2002;140:576–581. doi: 10.1067/mpd.2002.123764. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang K-Y, Albert PS. Models for longitudinal data: A generalized estimating equation approach. Biometrics. 1988;44:1049–1060. [PubMed] [Google Scholar]