Summary
To colonize the human small intestine, Giardia lamblia monitors a dynamic environment. Trophozoites attach to enterocytes that mature and die. The parasites must “decide” whether to re-attach or differentiate into cysts that survive in the environment and re-activate when ingested. Other intestinal parasites face similar challenges. Study of these parasites is limited because they do not encyst in vitro. Giardia trophozoites were persuaded to encyst in vitro by mimicking physiologic stimuli.
Cysts are dormant, yet “spring-loaded for action” to excyst upon ingestion. Giardial encystation has been studied from morphological, cell-biological, biochemical and molecular viewpoints. Yet important gaps remain and the mechanisms that co-ordinate responses to external signals remain enigmatic.
Introduction
As a major cause of waterborne diarrheal disease, Giardia contributes to the burden of malnutrition worldwide [1••]. Giardia’s simple, two-stage life cycle is central to its success as a parasite. G. lamblia cysts can survive in cold fresh water for months and fewer than 10 cysts are needed for human infection. Exposure of ingested cysts to gastric acid triggers excystation, a rapid and dramatic differentiation. After entry into the small intestine, the cyst wall opens and the parasite emerges. Trophozoites colonize below the entry of the common bile duct and can cause disease, although they do not invade. If they are carried downstream, trophozoites must encyst to survive outside the host. In vitro, Giardia encysts in response to the physiologic stimuli of increased bile and slightly alkaline pH [2]. The “gold standard” for successful encystation is the ability of cysts to excyst.
Other important intestinal parasites, including Entamoeba, Toxoplasma, Cryptosporidium, several tapeworms and nematodes, are transmitted as cysts or oocysts. However, study of these organisms is limited by the inability to generate mature cysts in vitro.
The giardial encystation pathway is a key virulence mechanism whose “biological goal” is differentiation into a form that can survive in the environment and infect a new host. Encystation also promotes immune evasion and is a target for vaccine and drug development [3-5]. The construction of the extracellular cyst wall (CW) is of primary importance as it allows the parasite to persist in fresh water, resist disinfectants, pass through the new host’s stomach and infect in the small intestine. This 300 nm thick fibrous structure excludes small molecules such as water, but transmits the physiological stimuli that regulate excystation. It is a model extracellular matrix with both protective and signaling functions.
Encystation is a gradual transformation of the motile, flagellated binucleate (4N), half-pear-shaped trophozoite (Figure 1). Trophozoites lose the ability to attach; the attachment disk fragments [6••] and the flagella are internalized. Metabolism also decreases as cells round up and enter dormancy. The oval, immotile, quadrinucleate (16N) cyst is encased in the refractile CW that contains protein (CWP) and glycopolymer (CWG) in insoluble fibrils [7]. Synthesis of CWP begins early in encystation, and leads to formation of novel large encystation secretory vesicles (ESV), which export CWP. Several excellent reviews have focused on the ESV pathway [8-11•].
Figure 1. Giardia lamblia encystation: from trophozoite to cyst.
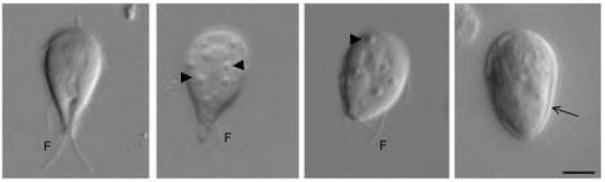
Images from left to right show a vegetative trophozoite, trophozoites after 21 and 42 hours of encystation and a water-resistant cyst. Encysting trophozoites gradually round up and develop numerous ESV (arrowheads) that export CW (arrow) components. F, flagella. Bar: 5μm
This review presents a global overview of major events in giardial encystation, emphasizing recent progress and important areas where further research is needed.
Biogenesis of the cyst wall
The CW composition, formation, and supramolecular architecture are incompletely understood. Currently, only four structural CWP are known. Three CWP are related leucine-rich repeat-containing proteins, while the fourth resembles trophozoite variant surface proteins (VSP) [12-15••]. All are sorted, concentrated within and exported to the nascent CW by ESV, the earliest cellular manifestation of encystation [16]. Recent studies focus on complementary aspects of ESV biogenesis. The Lujan lab [17] proposed that CWP aggregate and interact with specific membranes and drive ESV formation. Maturation requires complex interactions between ESV contents and membrane receptors. Using CWP chimeras, they reported that CWP2 is a key regulator of ESV formation and acts as an aggregation factor for CWP1 and CWP3, and as a ligand for sorting via its C-terminal basic extension. They postulate that the CWP2 extension must be removed for transport to the CW. However, we found CWP2 with its tagged C-terminus in the mature CW [14]. They propose that the necessary sorting receptors are lipid molecules [18], which bud off the ESV in a specialized ER or Golgi-like compartment. The CWP have 14 positionally conserved cysteine residues [14] and form extensive disulfide bonded complexes [14]. The importance of the cargo is supported by our finding that reducing these complexes in situ with DTT reversibly disrupted the ESV [19], transforming them to flattened ER-like cisternae [20••].
The Hehl laboratory emphasizes peripheral secretory system proteins (Table 1) and Golgi-like properties of the ESV [9]. Their limited proteomic analysis implicated several cytoplasmic and luminal ER quality control factors [20••], including the ER chaperone HSP70-BiP that cycles between the ESV and ER. Several proteasome subunits relocalize near ESV, suggesting possible cytoplasmic quality control.
Table 1.
Proteins and/or mRNA upregulated in encystation, and/or present in the ESV or CW
| Protein name | Protein ID* | Upregulated in |
Localization to encystation specific structures | Ref. | |
|---|---|---|---|---|---|
| Encysting cultures | Cysts | ||||
| Signaling proteins | |||||
| PP2A-C | gi|29246139 | - | mRNA / protein | CW | [28••] |
| PKB | gi|6274495 | mRNA | ND | ND | [43] |
| Enzymes | |||||
| GNP (UDP-galNAC synthesis) | gi|6090573 | mRNA / protein | ND | ND | [37] |
| GNA “ | gi|28261215 | mRNA / protein | ND | ND | [45] |
| AGM “ | gi|28261217 | mRNA / protein | ND | ND | [45] |
| UAP “ | gi|28396137 | mRNA / protein | ND | ND | [45] |
| UAE “ | gi|28396140 | mRNA / protein | ND | ND | [45] |
| Ubiquitin activating enzyme | |||||
| E1 | gi|29251145 | mRNA | ND | ND | [46] |
| “ | gi|29246853 | mRNA | ND | ND | [46] |
| Transcription factors | |||||
| Myb2 | gi|27979558 | mRNA | ND | - | [34] |
| GARP glp1 | gi|56407639 | mRNA | ND | - | [35] |
| High cysteine membrane proteins | |||||
| HCMp Group 1 | orf:25816 | mRNA | ND | ND | [15••] |
| HCMp EGF-like | orf:113213 | mRNA | ND | ND | [15••] |
| HCNCp | gi|75678095 | mRNA / protein | protein | ESV / CW | [15••] |
| Cyst wall proteins | |||||
| CWP1 | gi|606009 | mRNA / protein | mRNA / protein | ESV / CW | [12] |
| CWP2 | gi|903940 | mRNA / protein | mRNA / protein | ESV / CW | [13] |
| CWP3 | gi|19068147 | mRNA / protein | mRNA / protein | ESV / CW | [14] |
| Secretory proteins | |||||
| Rab11 | gi|28628539 | mRNA | ND | ESV | [47] |
| b’ COP | gi|19401686 | mRNA | ND | ESV | [47] |
| Yip | gi|28974725 | ND | ND | ESV | [47] |
| DLP | gi|19401683 | ND | ND | ESV | [47] |
| CLH | gi|22035407 | - | ND | ESV | [47] |
| [39] | |||||
| GSP | gi|15419593 | mRNA | ND | ESV | [27] |
| APβa | gi|20530732 | mRNA | ND | ND | [47] |
| APβb | gi|20530734 | mRNA | ND | ND | [47] |
| Sec24a | gi|29249050 | mRNA | ND | ND | [47] |
| Rab2a | gi|10047433 | mRNA | ND | ND | [47] |
| RabD | gi|19387539 | mRNA | ND | ND | [47] |
| RabA | gi|19387545 | mRNA | ND | ND | [47] |
| Sar1p | gi|22035409 | mRNA | ND | ND | [47] |
| VPS33 | gi|29248400 | mRNA | ND | ND | [47] |
Legend: CW, cyst wall; ESV, encystation secretory vesicle; ND, not determined; -, negative Proteins: AGM, phosphoacetylglucosamine mutase; AP, adaptor protein; CLH, clathrin heavy chain; COP, coat protein; CWP, cyst wall protein; DLP, dynamin like protein; GNA, glucosamine 6-phosphate N-acetyltransferase; GNP, glucosamine 6-phosphate deaminase; GSP, granule specific protein; HCMp, high cysteine membrane protein; HCNCp, high cysteine non-variant cyst protein; PKB, protein kinase B; PP2A-C, protein phosphatase 2A catalytic domain; UAE, UDP-N-acetylglucosamine 4-epimerase; UAP, UDP-N-acetylglucosamine pyrophosphorylase; VPS, vacuolar protein sorting; Yip, Yip like protein
NCBI numbers, except for 2 HCMp, where orf numbers (http://gmod.mbl.edu/perl/site/giardia14) were used because no NCBI numbers have been assigned yet.
In contrast to CWP1-3, whose exclusive destination is the CW, the high cysteine non-variant cyst protein (HCNCp) differs [15••]. HCNCp is detected in trophozoites and it co-localizes with CWP to the ESV during encystation. Although HCNCp is in the wall of mature cysts, much of it remains in the cell body. HCNCp is much larger than CWP and resembles VSP. HCNCp lacks LRR and has ~14% cysteines with many “CxxC” or “CxC” motifs and a divergent, VSP-like C-terminal transmembrane domain. The roles of HCNCp and the 60 other non-VSP high cysteine proteins [15••] in the genome remain enigmatic.
The ESV contents must attain their insoluble architecture after secretion [10]. Several enzymatic activities have been implicated in post-translational processing in the ESV pathway (Table 1):
The major known post-translational modification of the three CWP is formation of extensive intermolecular disulfide bonds by protein disulfide isomerases (PDI) [12-14,19]. Giardia has five protein disulfide isomerases [21] and the three that are characterized localize to ESV matrix but not CW [22].
PDI 1-3 also have transglutaminase activity which forms isopeptide protein crosslinks that are resistant to degradation [23,24]. Isopeptide bonds increase during encystation and transglutaminase inhibition decreases cyst formation. However, the cross-linked proteins remain to be identified.
A lysosomal cysteine proteinase was implicated in cleavage of the C-terminal extension of CWP2, suggesting cross-talk between the lysosomal compartment and ESV [25]. HCNCp is cleaved [15••] by a yet unknown protease.
CWP 1 and 2 are phosphorylated [26], but no kinase has been implicated.
The Giardia granule-specific protein (gGSP) has a calsequentrin domain, binds calcium, is upregulated in encystation, and localizes to the ESV [27]. Knockdown of gGSP inhibits ESV release, suggesting a calcium-dependent process [27].
Thus, a number of independent studies show that the ESV are central to CW biogenesis as any genetic or chemical manipulation that interferes with the ESV pathway blocks all downstream events [19,23,27,28••].
Many cells and organisms have extracellular walls that permit them to survive in the environment. Sugar polymers are key components of these walls and are often composed of repeating hexose units. Although the monomers are closely related, the polymers have distinct physical properties. Beta 1-3 polyhexoses do not associate as strongly as beta (1-4)-linked polysaccharides. Chitin, (beta 1-4 linked N-acetyl glucosamine) of arthropod and insect exoskeleton and fungal cell walls, is widespread in evolution [29,30]. Pioneering studies from the Jarroll group showed beta 1-3 polymer of galNAc as the major CWG [31]. Their insoluble material was purified by extensive enzymatic and chemical extractions that might have removed other important CW components. They defined an enzymatic pathway for synthesis of UDP-galNAc from glucose by an encystation-specific cytosolic pathway (Table 1) [12-15••]. An activity in crude cyst wall particles, termed “cyst wall synthase” (CWS), specifically incorporates galNAc from UDP-galNAc into insoluble material. However, no CWS gene has been identified. Based on the complexity of chitin synthase systems [32], “CWS” activity may require more than one protein.
Despite its central importance and the accessibility of the giardial life cycle, many gaps remain in our knowledge of the CW composition, formation, and architecture.
Transcriptional regulation of encystation
The molecular control of encystation is not well understood. RNA expression of the three CWP and the CWG biosynthetic enzymes, is largely upregulated transcriptionally (Table 1). In addition, several other proteins, whose roles in encystation are yet to be discovered, are upregulated at the transcriptional level (Table 1).
To date, three giardial DNA-binding transcription factors have been described. Only GARP glp1 and Myb2 are upregulated in encystation [33-35]. Myb2 binds to target sequences in the proximal upstream regions of the CWP genes and of g6PI-B, the first enzyme in the galNAc biosynthetic pathway, and of Myb2 itself [33]. Transcripts of most giardial genes initiate in A/T-rich initiator-like sequences near the start of translation [2]. This and several upstream sequences have been implicated in transcription of encystation genes [33,36-39]. A downstream region was reported to affect transcript stability [38,39].
Signal transduction in encystation
Trophozoites in the small intestine constantly monitor and respond to their environment. The lumenal composition varies with location and host nutrition. Trophozoites that are attached to enterocytes are beneath a mucus blanket and bathed in a serum-like microfiltrate, near neutral pH and at low bile concentration. As enterocytes mature, they are sloughed off and trophozoites must swim upstream to re-attach. If they remain in the lumen, trophozoites are exposed to the slightly alkaline pH and increased bile that lead to encystation.
During encystation, morphological modifications are coordinated with cell cycle exit and decreased metabolism. However, the proteins and pathways involved in transducing the physiological signals into effective responses are only beginning to be understood. Certain intracellular signaling proteins have been implicated in encystation based on their increased mRNA or protein expression and/or their localization to ESV and CW (Table 1). ERK1/2, PKAc, PKAr, PP2A-C and a PKCβ were reported to play a role in Giardia encystation [28••,40••-43]. PKA and ERK1/2 activities and ERK1/2 phosphorylation increase during encystation [28••,40••-43]. Importantly, inhibition of PP2A-C and of PKCβ decreases encystation [28••,42••].
These signaling proteins are all universal regulators of growth and differentiation in other organisms. Their specific functions in Giardia, however, are dependent on their cellular geography. All of these signaling proteins (except ERK2 and PKCβ) localize constitutively to the Giardia basal bodies/centrosomes. They also localize to cytoskeletal structures unique to Giardia, such as characteristic paraflagellar rods and the attachment disk. Their diverse targeting suggests that each signaling protein has a distinct role in encystation. The localization of PKAc/r, PP2A-C, PKCβ and ERK1/2 changes in response to the physiologic stimuli that induce encystation [28••,40••-42••,44]. Much additional research is needed to elucidate the complex cell signaling pathways that regulate encystation. Individual signaling proteins are regulated, often in cascades, by addition and removal of phosphates. Giardia has few transmembrane kinases (H.G. Morrison et al., in press) and the surface receptors for detecting and transmitting the extracellular encystation signals have not been defined.
Conclusions and perspectives
We have summarized progress in understanding giardial encystation from molecular and cell biological points of view. What emerges is the need for additional research to unmask the complexities of this important differentiation. In addition to being a model for other parasites, Giardia may provide useful hypotheses and paradigms for the entry into and exit from dormancy of a wide variety of cell types.
Acknowledgments
Research in our laboratory is supported by grants RO1 AI 42488, RO1 AI 51687, and RO1 GM 61896 from the National Institutes of Health awarded to Frances Gillin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Tineke Lauwaet, Email: tlauwaet@ucsd.edu.
Barbara J. Davids, Email: bdavids@ucsd.edu.
David S. Reiner, Email: dreiner@ucsd.edu.
Frances D. Gillin, Email: fgillin@ucsd.edu.
References
- 1••.Huang DB, White AC. An updated review on Cryptosporidium and Giardia. Gastroenterol Clin North Am. 2006;35:291–314. viii. doi: 10.1016/j.gtc.2006.03.006.. This recent review summarizes the pathogenesis, clinical symptoms and epidemiology of giardiasis compared with cryptosporidiosis.
- 2.Adam RD. Biology of Giardia lamblia. Clin Microbiol Rev. 2001;14:447–475. doi: 10.1128/CMR.14.3.447-475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee P, Faubert GM. Oral immunization of BALB/c mice by intragastric delivery of Streptococcus gordonii-expressing Giardia cyst wall protein 2 decreases cyst shedding in challenged mice. FEMS Microbiol Lett. 2006;265:225–236. doi: 10.1111/j.1574-6968.2006.00490.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee P, Faubert GM. Expression of the Giardia lamblia cyst wall protein 2 in Lactococcus lactis. Microbiology. 2006;152:1981–1990. doi: 10.1099/mic.0.28877-0. [DOI] [PubMed] [Google Scholar]
- 5.Larocque R, Nakagaki K, Lee P, Abdul-Wahid A, Faubert GM. Oral immunization of BALB/c mice with Giardia duodenalis recombinant cyst wall protein inhibits shedding of cysts. Infect Immun. 2003;71:5662–5669. doi: 10.1128/IAI.71.10.5662-5669.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6••.Palm D, Weiland M, McArthur AG, Winiecka-Krusnell J, Cipriano MJ, Birkeland SR, Pacocha SE, Davids B, Gillin F, Linder E, et al. Developmental changes in the adhesive disk during Giardia differentiation. Mol Biochem Parasitol. 2005;141:199–207. doi: 10.1016/j.molbiopara.2005.03.005.. In this paper, the authors describe the structure and protein composition of the attachment disk in the Giardia life cycle. In addition, they characterize the new disk protein SALP-1. This is one of the few papers showing immunofluorescence images of excysting cells.
- 7.Svard SG, Hagblom P, Palm JE. Giardia lamblia—a model organism for eukaryotic cell differentiation. FEMS Microbiol Lett. 2003;218:3–7. doi: 10.1111/j.1574-6968.2003.tb11490.x. [DOI] [PubMed] [Google Scholar]
- 8.Marti M, Hehl AB. Encystation-specific vesicles in Giardia: a primordial Golgi or just another secretory compartment? Trends Parasitol. 2003;19:440–446. doi: 10.1016/s1471-4922(03)00201-0. [DOI] [PubMed] [Google Scholar]
- 9.Hehl AB, Marti M. Secretory protein trafficking in Giardia intestinalis. Mol Microbiol. 2004;53:19–28. doi: 10.1111/j.1365-2958.2004.04115.x. [DOI] [PubMed] [Google Scholar]
- 10.Lujan HD, Touz MC. Protein trafficking in Giardia lamblia. Cell Microbiol. 2003;5:427–434. doi: 10.1046/j.1462-5822.2003.00284.x. [DOI] [PubMed] [Google Scholar]
- 11•.Chavez-Munguia B, Omana-Molina M, Gonzalez-Lazaro M, Gonzalez-Robles A, Cedillo-Rivera R, Bonilla P, Martinez-Palomo A. Ultrastructure of cyst differentiation in parasitic protozoa. Parasitol Res. 2007;100:1169–1175. doi: 10.1007/s00436-006-0447-x.. This review illustrates and compares the ultrastructure of E. invadens, A. castellanii and G. lamblia ESV and cyst walls.
- 12.Mowatt MR, Lujan HD, Cotten DB, Bowers B, Yee J, Nash TE, Stibbs HH. Developmentally regulated expression of a Giardia lamblia cyst wall protein gene. Mol Microbiol. 1995;15:955–963. doi: 10.1111/j.1365-2958.1995.tb02364.x. [DOI] [PubMed] [Google Scholar]
- 13.Lujan HD, Mowatt MR, Conrad JT, Bowers B, Nash TE. Identification of a novel Giardia lamblia cyst wall protein with leucine-rich repeats. Implications for secretory granule formation and protein assembly into the cyst wall. J Biol Chem. 1995;270:29307–29313. doi: 10.1074/jbc.270.49.29307. [DOI] [PubMed] [Google Scholar]
- 14.Sun CH, McCaffery JM, Reiner DS, Gillin FD. Mining the Giardia lamblia genome for new cyst wall proteins. J Biol Chem. 2003;278:21701–21708. doi: 10.1074/jbc.M302023200. [DOI] [PubMed] [Google Scholar]
- 15••.Davids BJ, Reiner DS, Birkeland SR, Preheim SP, Cipriano MJ, McArthur AG, Gillin FD. A New Family of Giardial Cysteine-Rich Non-VSP Protein Genes and a Novel Cyst Protein. PLoS ONE. 2006;1:e44. doi: 10.1371/journal.pone.0000044.. This paper describes a protein, HCNCp, with sequence similarity to Giardia variant surface proteins, which surprisingly does not localize to the Giardia trophozoite surface membrane, but traffics via the ESV to the cyst wall It belongs to a group of 61 unusual cysteine-rich proteins.
- 16.Reiner DS, McCaffery M, Gillin FD. Sorting of cyst wall proteins to a regulated secretory pathway during differentiation of the primitive eukaryote, Giardia lamblia. Eur J Cell Biol. 1990;53:142–153. [PubMed] [Google Scholar]
- 17.Gottig N, Elias EV, Quiroga R, Nores MJ, Solari AJ, Touz MC, Lujan HD. Active and passive mechanisms drive secretory granule biogenesis during differentiation of the intestinal parasite Giardia lamblia. J Biol Chem. 2006;281:18156–18166. doi: 10.1074/jbc.M602081200. [DOI] [PubMed] [Google Scholar]
- 18.Thiele C, Gerdes HH, Huttner WB. Protein secretion: puzzling receptors. Curr Biol. 1997;7:R496–500. doi: 10.1016/s0960-9822(06)00247-8. [DOI] [PubMed] [Google Scholar]
- 19.Reiner DS, McCaffery JM, Gillin FD. Reversible interruption of Giardia lamblia cyst wall protein transport in a novel regulated secretory pathway. Cell Microbiol. 2001;3:459–472. doi: 10.1046/j.1462-5822.2001.00129.x. [DOI] [PubMed] [Google Scholar]
- 20••.Stefanic S, Palm D, Svard SG, Hehl AB. Organelle proteomics reveals cargo maturation mechanisms associated with Golgi-like encystation vesicles in the early-diverged protozoan Giardia lamblia. J Biol Chem. 2006;281:7595–7604. doi: 10.1074/jbc.M510940200.. In this paper, the authors report the first limited proteomic analysis of isolated ESV The ESV were isolated from encysting trophozoites by sucrose density fractionation and proteins in the ESV containing fraction were resolved by 2D-PAGE and identified by mass spectrometry.
- 21.McArthur AG, Knodler LA, Silberman JD, Davids BJ, Gillin FD, Sogin ML. The evolutionary origins of eukaryotic protein disulfide isomerase domains: new evidence from the Amitochondriate protist Giardia lamblia. Mol Biol Evol. 2001;18:1455–1463. doi: 10.1093/oxfordjournals.molbev.a003931. [DOI] [PubMed] [Google Scholar]
- 22.Knodler LA, Noiva R, Mehta K, McCaffery JM, Aley SB, Svard SG, Nystul TG, Reiner DS, Silberman JD, Gillin FD. Novel protein-disulfide isomerases from the early-diverging protist Giardia lamblia. J Biol Chem. 1999;274:29805–29811. doi: 10.1074/jbc.274.42.29805. [DOI] [PubMed] [Google Scholar]
- 23.Davids BJ, Mehta K, Fesus L, McCaffery JM, Gillin FD. Dependence of Giardia lamblia encystation on novel transglutaminase activity. Mol Biochem Parasitol. 2004;136:173–180. doi: 10.1016/j.molbiopara.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Knodler LA, Noiva R, Mehta K, McCaffery JM, Aley SB, Svard SG, Nystul TG, Reiner DS, Silberman JD, Gillin FD. Novel protein-disulfide isomerases from the early-diverging protist giardia lamblia. J Biol Chem. 2000;275:28339. [PubMed] [Google Scholar]
- 25.Touz MC, Nores MJ, Slavin I, Carmona C, Conrad JT, Mowatt MR, Nash TE, Coronel CE, Lujan HD. The activity of a developmentally regulated cysteine proteinase is required for cyst wall formation in the primitive eukaryote Giardia lamblia. J Biol Chem. 2002;277:8474–8481. doi: 10.1074/jbc.M110250200. [DOI] [PubMed] [Google Scholar]
- 26.Slavin I, Saura A, Carranza PG, Touz MC, Nores MJ, Lujan HD. Dephosphorylation of cyst wall proteins by a secreted lysosomal acid phosphatase is essential for excystation of Giardia lamblia. Mol Biochem Parasitol. 2002;122:95–98. doi: 10.1016/s0166-6851(02)00065-8. [DOI] [PubMed] [Google Scholar]
- 27.Touz MC, Gottig N, Nash TE, Lujan HD. Identification and characterization of a novel secretory granule calcium-binding protein from the early branching eukaryote Giardia lamblia. J Biol Chem. 2002;277:50557–50563. doi: 10.1074/jbc.M202558200. [DOI] [PubMed] [Google Scholar]
- 28••.Lauwaet T, Davids BJ, Torres-Escobar A, Birkeland SR, Cipriano MJ, Preheim SP, Palm D, Svard SG, McArthur AG, Gillin FD. Protein phosphatase 2A plays a crucial role in Giardia lamblia differentiation. Mol Biochem Parasitol. 2007;152:80–89. doi: 10.1016/j.molbiopara.2006.12.001.. Using antisense mRNA and immunofluorescence analysis, authors demonstrate a role for PP2A-C in Giardia encystation and excystation and show its localization throughout the life cycle.
- 29.Merzendorfer H. Insect chitin synthases: a review. J Comp Physiol [B] 2006;176:1–15. doi: 10.1007/s00360-005-0005-3. [DOI] [PubMed] [Google Scholar]
- 30.Lesage G, Bussey H. Cell wall assembly in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2006;70:317–343. doi: 10.1128/MMBR.00038-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerwig GJ, van Kuik JA, Leeflang BR, Kamerling JP, Vliegenthart JF, Karr CD, Jarroll EL. The Giardia intestinalis filamentous cyst wall contains a novel beta(1-3)-N-acetyl-D-galactosamine polymer: a structural and conformational study. Glycobiology. 2002;12:499–505. doi: 10.1093/glycob/cwf059. [DOI] [PubMed] [Google Scholar]
- 32.Van Dellen KL, Bulik DA, Specht CA, Robbins PW, Samuelson JC. Heterologous expression of an Entamoeba histolytica chitin synthase in Saccharomyces cerevisiae. Eukaryot Cell. 2006;5:203–206. doi: 10.1128/EC.5.1.203-206.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun CH, Palm D, McArthur AG, Svard SG, Gillin FD. A novel Myb-related protein involved in transcriptional activation of encystation genes in Giardia lamblia. Mol Microbiol. 2002;46:971–984. doi: 10.1046/j.1365-2958.2002.03233.x. [DOI] [PubMed] [Google Scholar]
- 34.Yang H, Chung HJ, Yong T, Lee BH, Park S. Identification of an encystation-specific transcription factor, Myb protein in Giardia lamblia. Mol Biochem Parasitol. 2003;128:167–174. doi: 10.1016/s0166-6851(03)00072-0. [DOI] [PubMed] [Google Scholar]
- 35.Sun CH, Su LH, Gillin FD. Novel plant-GARP-like transcription factors in Giardia lamblia. Mol Biochem Parasitol. 2006;146:45–57. doi: 10.1016/j.molbiopara.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 36.Davis-Hayman SR, Hayman JR, Nash TE. Encystation-specific regulation of the cyst wall protein 2 gene in Giardia lamblia by multiple cis-acting elements. Int J Parasitol. 2003;33:1005–1012. doi: 10.1016/s0020-7519(03)00177-2. [DOI] [PubMed] [Google Scholar]
- 37.Knodler LA, Svard SG, Silberman JD, Davids BJ, Gillin FD. Developmental gene regulation in Giardia lamblia: first evidence for an encystation-specific promoter and differential 5’ mRNA processing. Mol Microbiol. 1999;34:327–340. doi: 10.1046/j.1365-2958.1999.01602.x. [DOI] [PubMed] [Google Scholar]
- 38.Hehl AB, Marti M, Kohler P. Stage-specific expression and targeting of cyst wall protein-green fluorescent protein chimeras in Giardia. Mol Biol Cell. 2000;11:1789–1800. doi: 10.1091/mbc.11.5.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marti M, Li Y, Schraner EM, Wild P, Kohler P, Hehl AB. The secretory apparatus of an ancient eukaryote: protein sorting to separate export pathways occurs before formation of transient Golgi-like compartments. Mol Biol Cell. 2003;14:1433–1447. doi: 10.1091/mbc.E02-08-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40••.Gibson C, Schanen B, Chakrabarti D, Chakrabarti R. Functional characterisation of the regulatory subunit of cyclic AMP-dependent protein kinase A homologue of Giardia lamblia: Differential expression of the regulatory and catalytic subunits during encystation. Int J Parasitol. 2006 doi: 10.1016/j.ijpara.2005.11.008.. The authors report an increased activity of PKAc and describe the expression and localization patterns of PKAc and PKAr in encystation.
- 41.Ellis JGt, Davila M, Chakrabarti R. Potential involvement of extracellular signal-regulated kinase 1 and 2 in encystation of a primitive eukaryote, Giardia lamblia. Stage-specific activation and intracellular localization. J Biol Chem. 2003;278:1936–1945. doi: 10.1074/jbc.M209274200. [DOI] [PubMed] [Google Scholar]
- 42••.Bazan-Tejeda ML, Arguello-Garcia R, Bermudez-Cruz RM, Robles-Flores M, Ortega-Pierres G. Protein kinase C isoforms from Giardia duodenalis: identification and functional characterization of a beta-like molecule during encystment. Arch Microbiol. 2007;187:55–66. doi: 10.1007/s00203-006-0174-9.. In this paper, the presence of PKC-like activity in Giardia is reported for the first time. Inhibition of this activity blocks encystation.
- 43.Kim KT, Mok MT, Edwards MR. Protein kinase B from Giardia intestinalis. Biochem Biophys Res Commun. 2005;334:333–341. doi: 10.1016/j.bbrc.2005.06.106. [DOI] [PubMed] [Google Scholar]
- 44.Abel ES, Davids BJ, Robles LD, Loflin CE, Gillin FD, Chakrabarti R. Possible roles of protein kinase A in cell motility and excystation of the early diverging eukaryote Giardia lamblia. J Biol Chem. 2001;276:10320–10329. doi: 10.1074/jbc.M006589200. [DOI] [PubMed] [Google Scholar]
- 45.Lopez AB, Sener K, Jarroll EL, van Keulen H. Transcription regulation is demonstrated for five key enzymes in Giardia intestinalis cyst wall polysaccharide biosynthesis. Mol Biochem Parasitol. 2003;128:51–57. doi: 10.1016/s0166-6851(03)00049-5. [DOI] [PubMed] [Google Scholar]
- 46.Gallego E, Alvarado M, Wasserman M. Identification and expression of the protein ubiquitination system in Giardia intestinalis. Parasitol Res. 2007;101:1–7. doi: 10.1007/s00436-007-0458-2. [DOI] [PubMed] [Google Scholar]
- 47.Marti M, Regos A, Li Y, Schraner EM, Wild P, Muller N, Knopf LG, Hehl AB. An ancestral secretory apparatus in the protozoan parasite Giardia intestinalis. J Biol Chem. 2003;278:24837–24848. doi: 10.1074/jbc.M302082200. [DOI] [PubMed] [Google Scholar]


