Abstract
Background and objectives: Anemia and hemoglobin (Hb) variability are associated with mortality in hemodialysis patients who are on erythropoiesis-stimulating agents (ESA). Our aim was to describe the degree of Hb variability present in nondialysis patients with chronic kidney disease (CKD), including those who were not receiving ESA, and to investigate the association between Hb variability and mortality.
Design, setting, participants, & measurements: Hb variability was determined using 6 mo of “baseline” data between January 1, 2003, and October 31, 2005. A variety of definitions for Hb variability were examined to ensure consistency and robustness.
Results: A total of 6165 patients from 22 centers in seven countries were followed for a mean of 34.0 ± 15.8 mo; 49% were prescribed an ESA. There was increased Hb variability with ESA use; the residual SD of Hb was 4.9 ± 4.4 g/L in patients who were not receiving an ESA, compared with 6.8 ± 4.8 g/L. Hb variability was associated with a small but significantly increased risk for death per g/L residual SD, irrespective of ESA use. Multivariate linear regression model explained only 11% of the total variance of Hb variability.
Conclusions: Hb variability is increased in patients who have CKD and are receiving ESA and is associated with an increased risk for death (even in those who are not receiving ESAs). This analysis cannot determine whether Hb variability causally affects mortality. Thus, the concept of targeting Hb variability with specific agents needs to be examined within the context of factors that affect both Hb variability and mortality.
Substantial numbers of epidemiologic studies have described the association of anemia with worse outcomes in all populations, including chronic kidney disease (CKD) populations. More recently, the identified phenomenon of hemoglobin (Hb) variability (the oscillations or fluctuations of an individual dialysis patient's Hb over time) (1–5) has also been associated with adverse outcomes and has received increasing attention as a therapeutic target (4,6–9). It remains unclear what the best method is to define or measure Hb variability and whether its association with adverse outcomes is simply an epiphenomenon or a causal relationship. Furthermore, CKD studies to date have been primarily restricted almost exclusively to hemodialysis patients who were receiving erythropoiesis-stimulating agents (ESA) (1–8)
Debate exists on which factors may influence the severity and frequency of Hb variability. Identification of these factors is necessary if manipulation of Hb variability in experimental or clinical settings is a goal (3,4,8,10,11,12). There are some data to suggest that physician prescribing patterns of ESA and the type of ESA affect variability; however, because both may be confounded by other factors, the specific contribution of these and other factors to Hb variability remains uncertain (3,4,10,11,12).
The goal of this analysis was to assess the presence of anemia and Hb variability within a culturally diverse, nondialysis CKD population that included patients who were not receiving ESA therapy. We assessed the association of Hb variability with mortality and explored factors that are associated with Hb variability.
Materials and Methods
This was an international, multicenter cohort study involving 22 sites from Australia, Canada, Spain, France, Italy, Israel, and the United Kingdom. Eligible participants were patients who were enrolled at a particular participating center between January 1, 2003, and October 31, 2005, and had stages 3 through 5 CKD (estimated GFR [eGFR] <60 ml/min per 1.73 m2). To be included, patients required a minimum of three Hb measurements within a 6-mo period, and they were excluded if they required dialysis or received a kidney transplant during this period. All subsequent laboratory data were collected, and patients were followed up until April 30, 2007, providing a minimum of 18 mo of potential follow-up. Those who were not receiving an ESA were also included in this study. Approval was obtained from each individual participating center's ethics committee as required.
Definitions of Hb Variability
Because the preferred definition of Hb variability remains to be elucidated, we elected to examine our data set using a variety of the current published methods to ensure consistency and robustness of the results. The intraindividual Hb variability was defined using the earliest 6-mo baseline period that included at least three Hb measurements for each patient. Hb measurements included in the analysis were a minimum of 25 d apart, to avoid or minimize the potential for multiple measurements as a result of intercurrent illness.
An individual's Hb variability was subsequently quantified according to a number of definitions:
Using regression-based parameters with the Hb intercept defining the absolute Hb level, the Hb slope representing the change of Hb levels over time, and the residual SD representing Hb variability (8,9)
The intraindividual mean change and SD of the change in consecutive Hb measurements (1)
The change in consecutive Hb measurements categorized as (a) all changes ≤5 g/L, (b) Hb decrease >5 g/L, (c) Hb increase >5 g/L, and (d) both Hb decrease and increase >5 g/L (7)
The Hb amplitude, defined as the difference between the highest and lowest Hb levels during the baseline period (3)
Classification of all patients into one of six groups (4): Group I: All Hb measurements >130 g/L; group II: At least one Hb measurement >130 g/L and at least one other Hb measurement 110 to 130 g/L with no Hb <110 g/L; group III: All Hb measurements between 110 and 130 g/L; group IV: At least one Hb measurement between 110 and 130 g/L and at least one other Hb measurement <110 g/L with no Hb measurement >130 g/L; group V: All Hb measurements <110 g/L; or group VI: At least one Hb measurement >130 g/L and 110 to 130 and <110 g/L (4,6). By this definition, groups I, III, and V have the smallest degree of variability, followed by groups II and IV, with group VI having the greatest degree of variability.
We considered mortality from any cause as the primary outcome, because this was the only outcome that was easily validated and comparable. Patient demographics, full blood count, biochemistry, iron studies, CKD status, and commencement of dialysis were recorded. Patients were grouped into the following categories: (1) No use of ESA therapy throughout the baseline period, (2) receiving ESA throughout the baseline period, or (3) commencement of ESA therapy during the baseline period. Data were obtained in some centers through local clinical and administrative databases, whereas others used detailed chart reviews.
Statistical Analysis
Descriptive statistics are presented as mean with SD or median with range, depending on the underlying distribution. Continuous and categorical variables were compared across groups on the basis of ESA use during baseline period using ANOVA or Kruskal-Wallis and χ2 tests, respectively. Patient survival was estimated using the Kaplan-Meier method. Survival curves, by Hb categories described, were compared using the log-rank test. Only the patients who were either on ESA throughout the baseline period or not on ESA throughout were included in the survival analysis. Commencement of ESA resulted in an intentional increase in Hb variability for a period of time that would make it inappropriate to include this group in the outcome analysis. The Cox proportional hazards model was used to identify factors that were associated with mortality. All models included Hb variability parameters using definitions described. In addition, age, gender, diabetes, baseline eGFR, dialysis initiation, Hb intercept, and Hb trend were eligible covariates, with selection guided by a backward elimination procedure. Dialysis initiation was considered as a time-dependent covariate.
Potential predictors of Hb variability (i.e., factors that were observed before 6-mo baseline period) as well as factors that were associated with Hb variability (i.e., factors that were observed during the 6-mo baseline period) were explored by fitting multivariate linear regression models and logistic regression models. The high level of Hb variability in logistic regression model was defined using the fourth quartile of residual SD as the cutoff point (i.e., >7 g/L). P < 0.05 from two-sided tests was considered to indicate statistical significance. All statistical analyses were performed using SAS 9.1 (SAS Institute, Cary, NC).
Results
Demographics
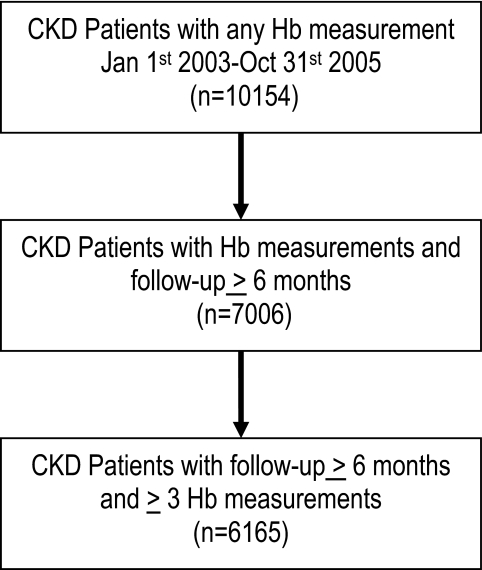
Data were received on 10,154 nondialysis and nontransplant patients with CKD during the baseline period (equating to >90% of the patients with CKD in the nephrology programs of the recruiting centers), 7006 had 6 mo of baseline data, and 6165 of these had three or more Hb values at least 25 d apart during this baseline period and were considered in these analyses (Figure 1). Participants represented 22 centers from seven countries and were followed for a median of 32.3 mo, with a lower and upper quartile of 21.5 and 47.1 mo. Table 1 describes the demographic characteristics of this sample in detail and as a function of ESA use, nonuse, or commencement during the 6-mo baseline period. Patients who were on ESA throughout had significantly worse kidney function, were older, were more likely to be white, had lower Hb, and had less diabetes than those who were not prescribed an ESA during the baseline period.
Figure 1.
Flowchart of cohort derivation.
Table 1.
Demographics based on ESA use during the baseline perioda
| Variable | No ESA Use (n = 3143) | On ESA Throughout (n = 1823) | Commenced ESA (n = 1199) | Total (n = 6165) | P |
|---|---|---|---|---|---|
| Age (yr; mean ± SD) | 68.5 ± 13.2 | 70.0 ± 14.3 | 68.9 ± 13.7 | 69.0 ± 13.7 | 0.0008 |
| Male (%) | 61.6 | 51.1 | 53.5 | 56.9 | <0.0001 |
| Race (%) | <0.0001 | ||||
| white | 78.8 | 66.7 | 69.7 | 73.3 | |
| Asian | 15.8 | 28.0 | 23.5 | 21.1 | |
| other | 5.4 | 5.3 | 6.8 | 5.6 | |
| GFR (ml/min per 1.73 m2; mean ± SD) | 27.7 ± 11.3 | 23.8 ± 10.8 | 21.9 ± 10.1 | 25.4 ± 11.2 | <0.0001 |
| 30 to 60 (%) | 40.2 | 27.5 | 20.6 | 32.6 | |
| 15 to 30 (%) | 47.4 | 49.8 | 52.5 | 49.1 | |
| <15 (%) | 12.4 | 22.8 | 27.0 | 18.3 | |
| Hemoglobin (g/L; mean ± SD) | 125.0 ± 15.7 | 110.9 ± 15.5 | 103.0 ± 12.6 | 116.5 ± 17.6 | <0.0001 |
| Diabetes (%) | 36.5 | 34.1 | 38.7 | 36.2 | 0.038 |
| Follow-up (mo; mean ± SD) | 34.6 ± 15.0 | 34.6 ± 17.4 | 31.3 ± 15.0 | 34.0 ± 15.8 | <0.0001 |
| Hb measurements during baseline period | |||||
| n (range) | 4 (3 to 7) | 4 (3 to 7) | 5 (3 to 7) | 5 (3 to 7) | |
| mean ± SD | 4.52 ± 1.37 | 4.09 ± 1.23 | 4.83 ± 1.34 | 5.05 ± 1.38 | <0.0001 |
ESA, erythropoiesis-stimulating agent; Hb, hemoglobin.
ESA Use and Hb Variability
There were differences in Hb variability among patients who had CKD and were receiving and not receiving ESA (Table 2). Those who were not using ESA during the entire baseline period had higher Hb intercepts, lower slopes, and a higher frequency of lower amplitude changes in Hb. Greater variability was apparent in patients who had CKD and were receiving ESA therapy using the residual SD as compared with those who were not receiving ESA therapy: 6.8 ± 4.8 versus 4.9 ± 4.4 g/L. The most stable Hb categories (I, III, and V) accounted for 52.8% of patients who were not receiving ESA therapy throughout, compared with 26.8% for patients who were receiving ESA throughout (P < 0.0001).
Table 2.
Intraindividual variability in Hb levels dependent on ESA usage, defined by residual SD of Hb, intraindividual change in Hb in consecutive measurements, Hb amplitude, and Hb category
| Hb Variability Definitions | No ESA Use (n = 3143) | On ESA Throughout (n = 1823) | Commenced ESA (n = 1199) | P |
|---|---|---|---|---|
| Hb intercept (g/L; mean ± SD) | 124.9 ± 15.3 | 112.6 ± 15.4 | 103.1 ± 11.8 | <0.0001 |
| Hb slope (g/L per mo; mean ± SD) | −0.1 ± 2.6 | 1.4 ± 4.1 | 2.7 ± 4.0 | <0.0001 |
| Residual SD (g/L; mean ± SD) | 4.9 ± 4.4 | 6.8 ± 4.8 | 7.3 ± 4.9 | <0.0001 |
| Intraindividual change in Hb (g/L; mean ± SD) | 6.6 ± 5.3 | 8.6 ± 5.5 | 9.7 ± 5.6 | <0.0001 |
| SD of intraindividual change in Hb (g/L; mean ± SD) | 8.0 ± 7.4 | 10.0 ± 7.2 | 11.1 ± 7.5 | <0.0001 |
| Hb change (%) | ||||
| all consecutive Hb change ≤5 g/L | 25.1 | 7.4 | 4.7 | <0.0001 |
| consecutive Hb decrease >5 g/L | 19.9 | 31.0 | 38.8 | |
| consecutive Hb increase >5 g/L | 24.1 | 11.8 | 7.3 | |
| both consecutive Hb decrease and increase >5 g/L | 30.9 | 49.8 | 49.2 | |
| Hb amplitude (g/L; %) | ||||
| 0 to 10 | 50.0 | 18.2 | 12.9 | <0.0001 |
| 11 to 20 | 33.7 | 37.0 | 33.3 | |
| >20 | 16.3 | 44.8 | 53.8 | |
| Classification of Hb variability (%) | <0.0001 | |||
| group I (Hb >130 g/L) | 22.8 | 2.9 | 0.3 | |
| group II (Hb >130 and 110 to 130 g/L) | 24.1 | 22.3 | 4.6 | |
| group III (Hb 110 to 130 g/L) | 24.6 | 12.8 | 5.1 | |
| group IV (Hb 110 to 130 and <110 g/L) | 18.4 | 33.9 | 49.6 | |
| group V (Hb <110 g/L) | 5.4 | 11.1 | 17.3 | |
| group VI (Hb >130 and 110 to 130 and <110 g/L) | 4.7 | 17.0 | 23.1 |
During the follow-up period, 29.6% (1824 of 6165) of patients initiated dialysis. The patients who started dialysis were significantly more likely to have either commenced or to have already received ESA therapy throughout the baseline period (37.3 and 35.9%) versus 23.0% who were not receiving ESAs (P < 0.0001). Dialysis initiation was included in further multivariate analysis that examined the relationship between Hb variability and mortality as a time-dependent covariate.
Hb Variability and Mortality
By the completion of follow-up, 26.9% (1235 of 4966) of patients who were receiving ESA or not receiving ESA throughout the baseline period had died. A greater proportion (31.7% [578 of 1823]) of patients who were receiving ESA throughout the baseline period died as compared with those who did not receive ESA (20.4% [640 of 3143]; P < 0.0001).
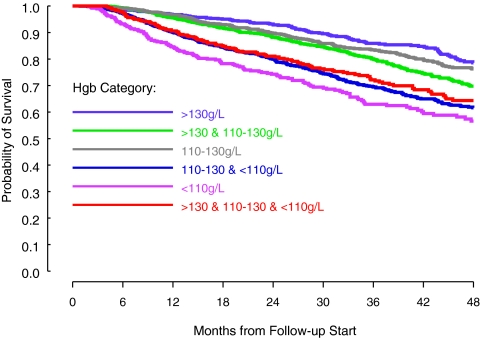
Categories with the greatest association with mortality were groups V and IV, with a first quartile of survival of 23 mo (95% confidence interval [CI] 18 to 28 mo) and 30 mo (95% CI 27 to 32 mo), respectively (Figure 2). This was even greater than the group with the greatest degree of variability (VI), with a first quartile of survival of 32 mo (95% CI 27 to 88 mo; P < 0.001).
Figure 2.
Survival curves for Hb categories. Groups with anemia—especially persistent—had the greatest risk for death, even more than the group with the greatest degree of variability (P < 0.0001).
The factors that were associated with mortality in patients who were not receiving ESA were qualitatively and quantitatively similar to those who were receiving ESA, with comparable hazard ratios (HR), excluding those who commenced ESA at some point during the baseline period (Table 3). Specifically, lower Hb, decreasing Hb trend, and greater Hb variability were associated with higher mortality irrespective of ESA use during the baseline period.
Table 3.
Multivariate model of factors associated with mortality, with groups based on ESA use during the baseline perioda
| Variable | No ESA Use (n = 3143)
|
On ESA Throughout (n = 1823)
|
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age (per 10 yr) | 1.57 | 1.46 to 1.70 | <0.001 | 1.53 | 1.41 to 1.65 | <0.001 |
| Male | 1.25 | 1.06 to 1.48 | 0.01 | 1.14 | 0.96 to 1.35 | 0.13 |
| Diabetes | 1.31 | 1.12 to 1.54 | 0.001 | 0.94 | 0.79 to 1.12 | 0.49 |
| GFR (per 5 ml/min) | 0.84 | 0.80 to 0.88 | <0.001 | 0.93 | 0.88 to 0.97 | 0.001 |
| Dialysis initiation | 1.94 | 1.58 to 2.38 | <0.001 | 1.70 | 1.37 to 2.11 | <0.001 |
| Absolute Hb (intercept; g/L) | 0.99 | 0.980 to 0.993 | <0.001 | 0.99 | 0.980 to 0.993 | <0.001 |
| Hb trend (slope; g/L per mo) | 0.96 | 0.93 to 0.99 | 0.013 | 0.94 | 0.92 to 0.97 | <0.001 |
| Residual SD (g/L) | 1.03 | 1.02 to 1.05 | <0.001 | 1.02 | 1.01 to 1.04 | 0.008 |
CI, confidence interval; HR, hazard ratio.
Given that the HR for Hb variability were similar among patients who were and were not receiving ESA, pooling of data across these groups was appropriate and provided more precise estimates (Table 4). In a sensitivity analysis that censored patients upon dialysis initiation, our findings did not alter with regard to the association between Hb variability and mortality (data not shown). A variable for ESA use was also included in the model but did not reach statistical significance (P = 0.13). The second part of Table 4 demonstrates the association of Hb categories with mortality: Group V had the highest HR, whereas groups IV and VI had an approximately 60% higher HR for mortality than patients who remained within the target range (reference group). The inclusion of interaction terms did not significantly change the HR reported in Table 4 (data not shown). When Hb variability was quantified using changes in consecutive intraindividual Hb measurements, the association with mortality was maintained with the mean SD of the change in Hb having an HR of 1.020 per g/L (95% CI 1.002 to 1.040 per g/L) that was independent of age, gender, diabetes, kidney function, ESA use, baseline Hb, and mean change in Hb.
Table 4.
Hb parameters (anemia and variability) associated with mortality in multivariate analysis on all patients except for those who commenced ESA therapy during the baseline period (n = 4966)a
| Variable | HR | 95% CI | P |
|---|---|---|---|
| Model with regression-based Hb parameters | |||
| age (per 10 yr) | 1.55 | 1.47 to 1.64 | <0.001 |
| male | 1.21 | 1.07 to 1.36 | 0.002 |
| GFR (per 5 ml/min) | 0.88 | 0.85 to 0.91 | <0.001 |
| dialysis initiation | 1.76 | 1.52 to 2.05 | <0.001 |
| absolute Hb (intercept; g/L) | 0.99 | 0.98 to 0.99 | <0.001 |
| Hb trend (slope; g/L per mo) | 0.95 | 0.93 to 0.97 | <0.001 |
| residual SD (g/L) | 1.03 | 1.02 to 1.04 | <0.001 |
| Model with regression-based Hb parameters and six-group classification of Hb parameters | |||
| age (per 10 yr) | 1.55 | 1.47 to 1.64 | <0.001 |
| male | 1.20 | 1.07 to 1.36 | 0.002 |
| GFR (per 5 ml/min) | 0.87 | 0.84 to 0.90 | <0.001 |
| dialysis initiation | 1.80 | 1.54 to 2.08 | <0.001 |
| group III (Hb 110 to 130 g/L; reference) | 1.00 | ||
| group I (Hb >130 g/L) | 1.001 | 0.79 to 1.27 | 0.99 |
| group II (Hb >130 and 110 to 130 g/L) | 1.20 | 1.002 to 1.45 | 0.048 |
| group IV (Hb 110 to 130 and <110 g/L) | 1.62 | 1.36 to 1.94 | <0.001 |
| group V (Hb <110 g/L) | 1.85 | 1.47 to 2.32 | <0.001 |
| group VI (Hb >130 and 110 to 130 and <110 g/L) | 1.57 | 1.24 to 1.98 | <0.001 |
ESA use during baseline period was not statistically significantly associated with mortality: Epoetins versus no ESA (HR 1.11; 95% CI 0.97 to 1.27); darbepoetin versus no ESA (HR 1.08; 95% CI 0.91 to 1.28); overall χ2 = 2.39, P = 0.30.
Predictors and Associations with Residual SD
Table 5 describes the factors that predicted and those that were associated with the highest quartile of residual SD, demonstrating that they were similar and comparable in the size of their odds ratio. It is interesting that the odds ratio differed between long- and short-acting ESA, with long-acting ESA being more likely to be associated with increased Hb variability. In the linear regression model that included all of the recorded variables, the r2 value was 0.113; that is, factors other than those captured herein accounted for the majority of the Hb variability in this cohort.
Table 5.
OR of the factors that predict the degree of Hb variability (as defined by the highest quartile of residual SD) compared with those that are associated with Hb variabilitya
| Variable | Factors that Predict Hb Variability
|
Factors that Are Associated with Hb Variability
|
||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Age (per 10 yr) | 0.90 (0.85 to 0.96) | <0.001 | 0.89 (0.83 to 0.95) | <0.001 |
| Epoetins | 2.53 (2.00 to 3.19) | <0.001 | 2.59 (1.98 to 3.38) | <0.001 |
| Darbepoetin | 4.12 (3.32 to 5.12) | <0.001 | 3.24 (2.53 to 4.15) | <0.001 |
| Months on ESA (per 3 mo) | 0.95 (0.91 to 0.99) | 0.035 | 0.94 (0.86 to 0.99) | 0.015 |
| Baseline GFR (per 5 ml/min) | Not in model | 0.94 (0.89 to 0.99) | 0.011 | |
| Variability in GFR (ml/min) | 1.03 (1.001 to 1.06) | 0.041 | 1.19 (1.14 to 1.25) | <0.001 |
| Albumin (g/L) | 0.96 (0.94 to 0.97) | <0.001 | 0.95 (0.93 to 0.98) | <0.001 |
| White cell count (×109/L) | 1.020 (0.998 to 1.040) | 0.083 | 1.07 (1.04 to 1.11) | <0.001 |
| Transferrin saturation (%) | Not in model | 3.45 (1.31 to 9.12) | 0.013 | |
The values of factors 6 mo before the 6-mo baseline period were used in the model that examined the prediction of Hb variability; the values of factors during the 6-mo baseline period were used in the model that examined the association with Hb variability. OR, odds ratio.
Discussion
Hb variability has been identified as being associated with morbidity and mortality in hemodialysis patients; however, full understanding of this phenomenon and its significance remain unclear (4,6–9). This analysis represents the largest cohort to date describing Hb variability in an international, nondialysis CKD population; importantly, it includes both those who were and were not receiving ESA therapy. We demonstrated that the average degree of Hb variability was greater in patients who were receiving ESA, but it existed nonetheless in all patients with CKD. As others have seen among dialysis populations, Hb variability was independently associated with mortality in this nondialysis CKD cohort. This association was statistically significant and independent of the absolute Hb level, change in Hb level over time, and ESA use. We were able to assess relative contributions of known factors on Hb variability, but these factors did not explain all of the variation seen.
This analysis importantly displays the value of examining the concept of Hb variability in the various populations. Importantly, the prevalence of Hb variability in association with both ESA use and nonuse, in a CKD population, may direct the nephrology community to consider Hb and its variability as markers of illness and comorbidities. It is unknown whether Hb variability can be manipulated and, if it is possible, then how best to achieve this. It seems unlikely that targeting any of the factors in our multivariate model would contribute greatly, because the model accounted for only 11.3% of the variation in Hb variability.
The definition of Hb variability has been described differently by various authors. The concept remains the same, but the specific mathematical constructs vary. Irrespective of which definition was used in this analysis, Hb variability was consistently associated with poor outcomes. A recent publication provided an alternative definition that allows the initial Hb level, the rate and direction of change in Hb concentration, and the degree of Hb variability to be quantified and therefore examined statistically (8,9). We used this definition predominantly for this analysis, although comparisons were done with all other published definitions, with consistent results.
It has been demonstrated that individual dialysis patients’ Hb concentrations fluctuate greatly over time (1–8). In some studies, only 8 to 18% of dialysis patients maintained stable Hb concentrations throughout a 6- to 12-mo period (1,4,5). In comparing this CKD patient cohort with dialysis cohorts, the degree of Hb variability reported by Ebben et al. (4) was similar in our patients who were on ESA therapy but differed significantly in patients in this cohort who were not receiving ESA. Because Hb variability remained significantly greater in those who were receiving versus not receiving ESA, it is conceivable that the use of ESA contributes to variability. Given that use of ESA is confounded by intention, little more can be said about this finding. The data as gathered and presented cannot ascertain whether prescribing practices, indications, or the agent per se contributes to variation. Given that we do report Hb variability in patients who had CKD and were not receiving ESA, this report may help to define baseline variation that is intrinsic to CKD populations.
The degree of Hb variability that is present in patients with CKD, including those who are not receiving ESA, is greater than would be expected among patients without uremia (13–15). Variation of Hb in healthy individuals does occur (13–16). It is both diurnal and fluctuating over periods of time up to 1 yr (14,15). In a study of 39 healthy individuals, the within-subject coefficient of variation for Hb was 2.8% (15). At a mean Hb of 146 g/L, this would be equivalent to an SD of 4.1 g/L, a value lower than the 8.0 g/L found in our nondialysis patients who had CKD and were not receiving ESA therapy.
The greater Hb variability that is seen in those who are receiving ESA is likely to be multifactorial in cause and includes the half-life and administration practices of the drug, changing patient status, and changes in iron status (2–5). This is best exemplified in our analysis in the subgroup of patients who commenced ESA therapy during the baseline period: They had the lowest Hb intercept yet the greatest mean change in Hb and the greatest SD of change in Hb. Importantly, when all of the variables that are traditionally associated with Hb variability were included, the model accounted for only 11.3% of the Hb variability. Thus, ESA use, ESA type, eGFR, age, gender, white cell count, albumin, and iron studies may not account for a substantial proportion of the variability. This suggests that other inherent factors that were not examined within our nondialysis CKD cohort may induce this phenomenon.
Although there is statistical independence of various factors, including Hb variability, with mortality, given that age, gender, diabetes status, and dialysis initiation also predict Hb variability as well as mortality, the question remains as to whether Hb variability itself causally contributes to mortality or factors that are on the same causal pathway for both Hb variability and mortality are more profound contributors. We demonstrated that there is a significant association between Hb variability and mortality in this nondialysis CKD population, regardless of ESA use. This association is independent of the Hb concentration and change in level over time, both of which were also significantly associated with mortality. This is consistent with a recent publication in hemodialysis patients and was of a similar magnitude: HR (unweighted) was 1.34 per g/dl versus ours of 1.03 per g/L (8).
The external validity of our findings may be limited by the inclusion criteria that we predetermined. A minimum number of Hb measurements, three in this study, are required to be able to define adequately the Hb variability of an individual patient, and ensuring at least 25 d between measurements reduces the likelihood of repeat Hb measurements during an acute illness. This may predispose to bias, however, with our results potentially being applicable only to patients who had CKD and would have occasion for clinical reasons to have at least three Hb measurements during a 6-mo period.
Conclusions
This study describes Hb variability in nondialysis patients with CKD regardless of ESA status, although it is more pronounced in patients who received ESA therapy. Although Hb variability is associated with an increased risk for mortality, it remains unclear whether this is causal in nature or simply an epiphenomenon. Using robust analytical methods, it seems that there are a multiplicity of factors, many as yet unidentified, that may affect both Hb variability and mortality; therefore, the ability to adjust the degree of Hb variability and then potentially effect outcomes remain elusive.
Disclosures
None.
Acknowledgments
Results from this study were presented in abstract form at the 2008 Australian and New Zealand Society of Nephrology Annual Scientific Meeting, New Castle, Australia, September 2 through 10, 2008 and the American Society of Nephrology Renal Week, Philadelphia, PA, November 4 through 9, 2008.
We acknowledge that the development of this project was facilitated during a series of educational meetings, the Promoting Excellence for Anemia and the Kidney (PEAK) meetings, which were funded in an unrestricted manner by Roche Pharmaceuticals. No direct financial assistance was provided for the project; neither was Roche involved in any way with the data collection, description, or analyses. The participating centers entered data that happened to be on patients using predominantly non-Roche products; this is by chance and not due to any other reason.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Lacson E Jr, Ofsthun N, Lazarus JM: Effect of variability in anemia management on hemoglobin outcomes in ESRD. Am J Kidney Dis 41: 111–124, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Berns JS, Elzein H, Lynn RI, Fishbane S, Meisels IS, Deoreo PB: Hemoglobin variability in epoetin-treated hemodialysis patients. Kidney Int 64: 1514–1521, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Fishbane S, Berns JS: Hemoglobin cycling in hemodialysis patients treated with recombinant human erythropoietin. Kidney Int 68: 1337–1343, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Ebben JP, Gilbertson DT, Foley RN, Collins AJ: Hemoglobin level variability: Associations with comorbidity, intercurrent events, and hospitalizations. Clin J Am Soc Nephrol 1: 1205–1210, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Collins AJ, Brenner RM, Ofman JJ, Chi EM, Stuccio-White N, Krishnan M, Solid C, Ofsthun NJ, Lazarus JM: Epoetin alfa use in patients with ESRD: An analysis of recent US prescribing patterns and hemoglobin outcomes. Am J Kidney Dis 46: 481–488, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Gilbertson DT, Ebben JP, Foley RN, Weinhandl ED, Bradbury BD, Collins AJ: Hemoglobin level variability: Associations with mortality. Clin J Am Soc Nephrol 3: 133–138, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regidor DL, Kopple JD, Kovesdy CP, Kilpatrick RD, McAllister CJ, Aronovitz J, Greenland S, Kalantar-Zadeh K: Associations between changes in hemoglobin and administered erythropoiesis-stimulating agent and survival in hemodialysis patients. J Am Soc Nephrol 17: 1181–1191, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Yang W, Israni RK, Brunelli SM, Joffe MM, Fishbane S, Feldman HI: Hemoglobin variability and mortality in ESRD. J Am Soc Nephrol 18: 3164–3170, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Brunelli SM, Joffe MM, Israni RK, Yang W, Fishbane S, Berns JS, Feldman, HI: History-adjusted marginal structural analysis of the association between hemoglobin variability and mortality among chronic hemodialysis patients. Clin J Am Soc Nephrol 3: 777–782, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker R, Pussell BA: Fluctuations in haemoglobin levels in haemodialysis patients receiving intravenous epoetin alfa or intravenous darbepoetin alfa. Nephrology (Carlton) 12: 448–451, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Fishbane S: What is needed to achieve a hemoglobin of 11.0–13.0 g/dl in end-stage renal disease. Blood Purif 25: 53–57, 2007 [DOI] [PubMed] [Google Scholar]
- 12.West RM, Harris K, Gilthorpe MS, Tolman C, Will EJ: Functional data analysis applied to a randomized controlled clinical trial in hemodialysis patients describes the variability of patient responses in the control of renal anemia. J Am Soc Nephrol 18: 2371–2376, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Ross DW, Ayscue LH, Watson J, Bentley SA: Stability of hematologic parameters in healthy subjects: Intraindividual versus interindividual variation. Am J Clin Pathol 90: 262–267, 1988 [DOI] [PubMed] [Google Scholar]
- 14.Statland BE, Winkel P, Harris SC, Burdsall MJ, Saunders AM: Evaluation of biologic sources of variation of leukocyte counts and other hematologic quantities using very precise automated analyzers. Am J Clin Pathol 69: 48–54, 1978 [DOI] [PubMed] [Google Scholar]
- 15.Dot D, Miro J, Fuentes-Arderiu X: Within-subject biological variation of hematological quantities and analytical goals. Arch Pathol Lab Med 116: 825–826, 1992 [PubMed] [Google Scholar]
- 16.Brigden ML, Heathcote JC: Problems in interpreting laboratory tests: What do unexpected results mean? Postgrad Med 107: 145–146, 151–152, 155–158 passim, 2000 [DOI] [PubMed] [Google Scholar]