Abstract
Background and objectives: The relationship of contrast-induced nephropathy (CIN) to long-term adverse events (AEs) is controversial. Although an association with AEs has been previously reported, it is unclear whether CIN is causally related to these AEs.
Design, setting, participants, & measurements: We obtained long-term (≥1 yr) follow-up on 294 patients who participated in a randomized, double-blind comparison of two prevention strategies for CIN (iopamidol versus iodixanol). A difference in the incidence of AEs between patients who had developed CIN and those who had not was performed using a χ2 test and Poisson regression analysis. A similar statistical approach was used for the differences in AEs between those who received iopamidol or iodixanol. Multiple definitions of CIN were used to strengthen and validate the results and conclusions.
Results: The rate of long-term AEs was higher in individuals with CIN (all definitions of CIN). After adjustment for baseline comorbidities and risk factors, the adjusted incidence rate ratio for AEs was twice as high in those with CIN. Randomization to iopamidol reduced both the incidence of CIN and AEs.
Conclusions: The parallel decrease in the incidence of CIN and AEs in one arm of this randomized trial supports a causal role for CIN.
Contrast-induced nephropathy (CIN), a form of acute kidney injury (AKI), has received increasing attention in the past few years as a result of new knowledge regarding its pathogenesis, the proliferation of innovative approaches to its prevention, and recognition that CIN is associated with long-term adverse events (AEs) (1–5). The increased incidence of AEs after CIN is derived primarily from retrospective analyses of large databases (2,4,5) or observational studies (3) of patients who have undergone coronary angiography and/or percutaneous coronary intervention. A cause-and-effect relationship cannot be determined from such data. Patients with an increased burden of cardiovascular risk factors before contrast medium exposure may be more likely to develop CIN and independent of the occurrence of CIN have more long-term AEs. Alternatively, the occurrence of CIN may in some as-yet-undefined manner alter the future likelihood of AEs (i.e., CIN is on a pathophysiologic pathway that leads to AEs).
Randomized, prospective trial designs provide an opportunity to explore causal relationships. If CIN is causally related to long-term AEs, then a strategy that prevents CIN should reduce long-term AEs, as long as the strategy itself does not alter any other risk factors for those AEs. In a randomized trial of two different treatments, the assumption is that the baseline risk factors for long-term AEs will be equally distributed between the two treatments being tested. Differences in the incidence of CIN between treatments, if paralleled by differences in long-term AEs, would suggest that CIN is on a pathophysiologic pathway that leads to those AEs.
The Cardiac Angiography in Renally Impaired Patients (CARE) Study was a large, multicenter, prospective, double-blind, randomized clinical trial of patients who had moderate to severe chronic kidney disease and were undergoing cardiac angiography (6). The primary end point was the incidence of CIN. Patients were randomly assigned to two different treatments represented by two different contrast media: The low-osmolar, nonionic monomer iopamidol (Isovue; Bracco Diagnostics Inc., Princeton, NJ) and the iso-osmolar, nonionic dimer iodixanol (Visipaque; GE Healthcare, Princeton, NJ). Neither contrast medium has any known effect on the baseline risk factors that might contribute to long-term AEs. In this report, follow-up data were collected on 294 of the original participants of the CARE trial approximately 12 mo after entry into the trial. To explore whether a causal link exists between CIN and long-term AEs, we studied the differences in the incidence of CIN and long-term AEs between the two treatments (contrast media). Since the results of the CARE study were published, new definitions of AKI and thus CIN have been suggested (7). These new definitions increase the incidence of CIN and thus enhance the statistical power to detect associations with long-term outcomes.
Materials and Methods
Study Design
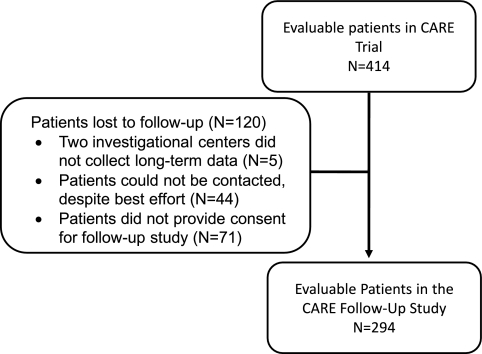
Follow-up data were obtained at least 1 yr after contrast exposure on 294 patients (Figure 1) of the original 414 participants in the CARE study. The 120 patients who were lost to follow-up were not different from the 294 patients in this report in any of the clinical or demographic characteristics (Table 1). Follow-up data were recorded in specifically predesigned case report forms and always validated against hospital records. All of the investigators continued to be blinded as to which patients experienced CIN and which contrast agent had been used in the individual patients. The collection, validation, and analysis of the data went from July 2006 until February 2008. The study was conducted according to good clinical practice standards. The protocol was approved by each participating center's institutional review board. All patients gave written informed consent before enrollment in the follow-up study.
Figure 1.
Flow diagrams of follow-up patients derived from the CARE trial.
Table 1.
Comparison of baseline demographic and clinical characteristics between patients with follow-up information and patients who were lost to follow-upa
| Baseline Characteristic | Patients with Follow-up Information (n = 294) | Patients Who Were Lost to Follow-up (n = 120) | Pb |
|---|---|---|---|
| Age (yr; n [%]) | 0.72 | ||
| 18 to 64 | 66 (22) | 25 (21) | |
| ≥65 | 228 (78) | 95 (79) | |
| Gender (n [%]) | 0.62 | ||
| male | 186 (63) | 79 (66) | |
| female | 108 (37) | 41 (34) | |
| Baseline eGFR (ml/min per 1.73 m2) | |||
| ≤30 (n [%]) | 19 (6) | 13 (11) | 0.13 |
| >30 (n [%]) | 275 (94) | 107 (89) | |
| mean ± SD | 50.0 ± 12.0 | 49.0 ± 13.3 | 0.19 |
| Hypertension (n [%]) | 240 (82) | 100 (83) | 0.68 |
| Diabetes (n [%]) | 114 (39) | 56 (47) | 0.14 |
| Duration of diabetes (yr; mean ± SD) | 13.1 ± 8.7 | 13.2 ± 8.3 | 0.91 |
| Change in SCr (mean ± SD) | 0.09 ± 0.22 | 0.11 ± 0.24 | 0.43 |
| CIN rates (%) | |||
| SCr increase ≥0.3 mg/dl | 17.3 | 21.7 | 0.31 |
| ScysC increase ≥25% | 16.1 | 21.6 | 0.23 |
| ScysC increase ≥20% | 19.4 | 27.8 | 0.09 |
| ScysC increase ≥15% | 24.8 | 33.0 | 0.13 |
CIN, contrast-induced nephropathy; eGFR, estimated GFR; SCr, serum creatinine; ScysC, cystatin C.
P value is from χ2 test or t test, as appropriate.
Adverse Events
Prospectively defined AEs (all events) included death, stroke, myocardial infarction, end-stage kidney disease, percutaneous coronary revascularization, coronary artery bypass graft surgery, other revascularization procedures (e.g., carotid, runoff vessels), and other (e.g., cardiac arrest, development of congestive heart failure or pulmonary edema, need for permanent pacing). Four events were considered major (major events): Death, stroke, myocardial infarction, and ESRD that required dialysis. All of the analyses considered both all events and major events. When more than one AE occurred in the same patient, only the first event was used for analysis.
CIN Definitions
In the original CARE trial, CIN was defined as a serum creatinine (SCr) increase of ≥0.5 mg/dl or an increase of ≥25%. Combining the two end points, CIN occurred in 11.1% of the 414 patients (9.8% with iopamidol versus 12.4% with iodixanol; P = 0.39). Of the original 414 participants, 350 had serum cystatin C measured at the same time as the SCr measurements. Cystatin C has been suggested as a more sensitive marker of AKI in intensive care unit patients, after renal transplantation, and after contrast exposure (8–10). In an exploratory analysis, a relative increase in cystatin C of ≥25% identified a greater number of patients with CIN compared with a SCr increase of ≥25% (17.4 versus 11.1%, respectively). An even higher incidence of CIN resulted from cystatin C increases of 15 and 20% (Table 2). Significant differences also emerged between the two treatment arms. We chose these cystatin C definitions of CIN in addition to a SCr increase of ≥0.3 mg/dl as suggested by the Acute Kidney Injury Network (AKIN) group (7) to investigate whether they were predictive of AEs. In the CARE Follow-Up study, all 294 patients had SCr measurements but only 242 had cystatin C measurements.
Table 2.
Cystatin C definition of CIN from the 350 patients with cystatin C measurements in the original CARE trial
| CIN Definition | Total (%) | Iopamidol (%) | Iodixanol (%) | P |
|---|---|---|---|---|
| ≥15% increase | 93 (26.6) | 35 (20.5) | 58 (32.4) | 0.01 |
| ≥20% increase | 75 (21.4) | 27 (15.8) | 48 (26.8) | 0.01 |
| ≥25% increase | 61 (17.4) | 22 (12.9) | 39 (21.8) | 0.03 |
Statistical Analysis
Definition of Study Groups.
For each of the CIN definitions, two groups of patients were defined: Those who experienced CIN (CIN group) and those who did not develop CIN (non-CIN group). For the assessment of the effects of the two contrast agents on long-term events, two additional study groups were defined: Patients who had received iopamidol (iopamidol group) and those who had received iodixanol (iodixanol group).
Comparability of Study Groups.
The baseline demographic and clinical characteristics of the CIN and non-CIN groups and of the iopamidol and iodixanol groups were compared using χ2 test, Fisher exact test, or t test, as appropriate. The following baseline variables were tested: Age, gender, renal function (estimated GFR), diabetes (presence, type, and duration), hypertension (presence/absence), presence and severity of coronary artery disease (single-vessel, two-vessel, or three-vessel disease), left ventricular function (left ventricular ejection fraction), and use of concomitant medications that are known possibly to affect events (including β blockers, angiotensin-converting enzyme inhibitors, calcium-channel blockers, and diuretics).
CIN versus Non-CIN Study Groups.
The comparison of the incidence of AEs was performed for the various CIN versus non-CIN groups using a χ2 test. Poisson regression analysis was performed for the CIN versus non-CIN groups using count of AEs as the response variable; the log of person-days of follow-up as an offset in the model; and adjustment for age, gender, baseline estimated GFR, hypertension, severity of coronary artery disease, type and duration of diabetes, and CIN rates to compute the adjusted incidence rate ratios (all events analysis). All of the analyses were then repeated taking into consideration just the count of the four major events: Death, stroke, myocardial infarction, and ESRD that required dialysis (major events analysis).
Iopamidol versus Iodixanol Study Groups.
The difference in the incidence of CIN rates between the iopamidol and iodixanol groups was calculated for CIN end points using the χ2 test. The Poisson regression analyses performed for the CIN versus non-CIN groups was repeated for the comparison of the iopamidol and the iodixanol groups. To explore further the interaction between contrast agent and the development of CIN, we estimated the adjusted incidence rate ratio using the same covariables used in the adjusted Poisson regression model and the outcome rate in those who received iopamidol and had CIN as the reference. Goodness of fit and selection of the model was based on scaled deviance and log likelihood estimates.
P < 0.05 was considered to be significant. Statistical analyses were performed using SAS 8.2 (SAS Institute, Cary, NC).
Results
CIN Is Associated with Long-Term AEs
Ninety-two (31%) of the 294 patients experienced 120 AEs. Thirty-eight (13%) of the 294 patients experienced a major event (death, stroke, myocardial infarction, or ESRD that required dialysis) during the period of follow-up. For all definitions of CIN, the incidence of AEs was statistically higher in the CIN groups than in the non-CIN groups (Table 3).
Table 3.
Comparison of incidence of all events between patients (n = 294) with postcontrast CIN and patients without CINa
| Definition of CIN | Overall CIN Incidence (%) | All AEs
|
Pb | |
|---|---|---|---|---|
| CIN Group | Non-CIN Group | |||
| SCysC increase ≥15% | 24.8 | 25/60 (42%) | 47/182 (26%) | 0.02 |
| ScysC increase ≥20% | 19.4 | 20/47 (43%) | 52/195 (27%) | 0.03 |
| ScysC increase ≥25% | 16.1 | 18/39 (46%) | 54/203 (27%) | 0.01 |
| SCr increase ≥0.3 mg/dl | 17.3 | 22/51 (43%) | 70/243 (29%) | 0.04 |
AE, adverse event.
P value from χ2 test.
Baseline demographic variables were similar between the CIN and non-CIN groups for all definitions of CIN (data not shown); however, to explore further the potential of confounding by baseline risk factors, we used Poisson regression modeling. Table 4 presents the adjusted incidence rate ratio for all events and major events as the response variable and comparing the various CIN and non-CIN groups, adjusting for contrast agent, age, gender, diabetes, hypertension, congestive heart failure, coronary artery disease severity, and left ventricular ejection fraction. The results confirmed that CIN was associated with a higher probability of all events. There were fewer events for major events and thus a loss of statistical power. The adjusted incidence rate ratios were nevertheless similar for all comparisons. This analysis confirmed the association between CIN and long-term AEs even after adjustment for potential confounding baseline risk factors. The analysis validates the use of these newer definitions of CIN because they predict long-term AEs.
Table 4.
Comparison of the incidence of AEs in CIN versus non-CIN groups by various CIN definitions (n = 294)a
| CIN Definition | ScysC Increase
|
SCR Increase ≥0.3 mg/dl | ||
|---|---|---|---|---|
| ≥15% | ≥20% | ≥25% | ||
| All AEs | ||||
| adjusted IRR | 2.0 | 1.7 | 2.0 | 2.2 |
| 95% CI | 1.1 to 3.6 | 0.9 to 3.3 | 1.0 to 3.9 | 1.3 to 3.8 |
| Pb | 0.0291 | 0.0935 | 0.0356 | 0.0029 |
| Major AEsc | ||||
| adjusted IRR | 2.2 | 1.9 | 1.2 | 3.2 |
| 95% CI | 0.9 to 5.1 | 0.8 to 4.5 | 0.3 to 3.2 | 1.1 to 8.7 |
| Pb | 0.0632 | 0.1437 | 0.7591 | 0.0213 |
Incidence rate ratio (IRR) comparing those with CIN and those without CIN from Poisson regression model. CI, confidence interval.
P value from Poisson regression analysis.
Major events: Death, stroke, myocardial infarction, and ESRD requiring dialysis.
Incidence of CIN Differs between Treatment Groups
The CARE trial was designed to compare the incidence of CIN between two treatments, as reflected in the randomization to two different contrast media. Neither contrast medium is known to influence any potential risk factor for long-term AEs that was present before contrast medium exposure. In the follow-up study, the two contrast medium groups were comparable for most of the variables tested, with the exception of the gender distribution and a significantly lower mean value of left ventricular ejection fraction in the iopamidol group (Table 5).
Table 5.
Demographic and clinical characteristics at baseline between the two treatments (iopamidol versus iodixanol; n = 294)a
| Characteristic | Iopamidol Group (n = 145) | Iodixanol Group (n = 149) | Pb |
|---|---|---|---|
| Age (yr; %) | |||
| 18 to 64 | 19 | 26 | 0.12 |
| ≥65 | 81 | 74 | |
| Gender (%) | |||
| male | 71 | 56 | 0.01 |
| female | 29 | 44 | |
| Baseline eGFR | |||
| severity of CKD (ml/min per 1.73 m2; %) | |||
| <30 | 6 | 7 | 0.86 |
| 30 to 59 | 94 | 93 | |
| eGFR (mean ± SD) | 50 ± 11 | 51 ± 13 | 0.61 |
| Severity of CAD (%) | |||
| no disease | 14 | 17 | 0.12 |
| 1-vessel disease | 21 | 20 | |
| 2-vessel disease | 34 | 22 | |
| 3-vessel disease | 31 | 41 | |
| Congestive heart failure (%) | 12 | 9 | 0.40 |
| Left ventricular ejection fraction (%) | |||
| mean ± SD | 49 ± 13 | 54 ± 12 | 0.01 |
| <50% (%) | 34.7 | 20.4 | 0.02 |
| Hypertension (%) | 81 | 83 | 0.68 |
| Diabetes (%) | 38 | 40 | 0.77 |
| insulin dependent | 15 | 17 | |
| non–insulin dependent | 23 | 23 | |
| Duration of diabetes (yr; mean ± SD) | 11.3 ± 9.0 | 12.4 ± 8.3 | 0.05 |
| Concomitant medications (%) | |||
| β blockers | 82.1 | 79.2 | 0.53 |
| ACEIs | 47.6 | 49.7 | 0.72 |
| calcium channel blockers | 42.1 | 41.6 | 0.94 |
| diuretics | 54.5 | 55.7 | 0.83 |
ACE, angiotensin-converting enzyme inhibitor; CAD, coronary artery disease.
P value from Fisher exact test or t test, as appropriate.
Incidence of AEs Differs between Treatment Groups
To adjust for possible confounding risk factors present at baseline, we repeated the Poisson regression analyses comparing the two treatments for all events and major events. Table 6 shows the results of this analysis. Although there was no difference in follow-up time between the agents, there was a significant difference in the incidence of AEs between the two treatments.
Table 6.
Comparison of the incidence of all and major AEs between treatments (iopamidol versus iodixanol)a
| AEs | Total (n = 294) | Iopamidol Group (n = 145) | Iodixanol Group (n = 149) | IRR (95% CI) | Pb |
|---|---|---|---|---|---|
| All events | 1.8 (1.1 to 3.0) | 0.016 | |||
| patients with events (n [%]) | 92 (31%) | 39 (27%) | 53 (36%) | ||
| no. of events | 120 | 48 | 72 | ||
| person-time of follow-up (yr) | 387 | 198 | 189 | ||
| Major eventsc | 3.2 (1.2 to 8.9) | 0.024 | |||
| patients with events (n [%]) | 38 (13%) | 16 (11%) | 22 (15%) | ||
| no. of major events | 45 | 17 | 28 | ||
| person-time of follow-up (yr) | 387 | 198 | 189 |
IRR comparing iodixanol and iopamidol from Poisson regression model.
P value from Poisson regression analysis.
Major events: Death, stroke, myocardial infarction, and ESRD requiring dialysis.
Interaction between CIN and AEs
To explore further the interaction between treatment groups, the incidence of CIN, and AEs, we estimated the adjusted incidence rate ratio using the same covariables in the adjusted Poisson regression model and using the adverse event rate in those who received one of the treatments (iopamidol) and with CIN as the reference. Table 7 shows the results of this adjusted analysis. A significant interaction between CIN and type of treatment was confirmed. In the absence of CIN (by any definition), there is no significant increased risk of AEs. A higher incidence of CIN was observed with iodixanol, and there was a two- to four-fold increase in risk for AEs in those patients. Thus, the interaction is largely explained by the higher number of AEs in patients who had CIN after randomization to a less effective treatment for CIN (iodixanol).
Table 7.
Comparison of the incidence of all events between the iopamidol and iodixanol groups with CIN as effect modificationa
| Parameter | SCysC Increase
|
SCr Increase ≥0.3 mg/dl | ||
|---|---|---|---|---|
| ≥15% | ≥20% | ≥25% | ||
| Iodixanol with CIN | ||||
| adjusted IRR | 2.5 | 2.9 | 2.1 | 2.9 |
| 95% CI | 1.2 to 5.9 | 1.3 to 7.3 | 0.9 to 5.5 | 1.1 to 8.5 |
| Pb | 0.0241 | 0.0177 | 0.1096 | 0.0316 |
| Iodixanol without CIN | ||||
| adjusted IRR | 0.9 | 0.9 | 0.7 | 1.1 |
| 95% CI | 0.4 to 2.1 | 0.4 to 2.3 | 0.3 to 1.8 | 0.5 to 2.9 |
| Pb | 0.7461 | 0.8915 | 0.4712 | 0.8816 |
| Iopamidol without CIN | ||||
| adjusted IRR | 0.7 | 0.7 | 0.5 | 0.7 |
| 95% CI | 0.3 to 1.7 | 0.3 to 1.8 | 0.2 to 1.2 | 0.3 to 1.8 |
| Pb | 0.4164 | 0.4556 | 0.0999 | 0.3731 |
| Iopamidol with CIN | Reference | Reference | Reference | Reference |
IRR comparing the patients who had acute kidney injury with iopamidol based on test of interaction between contrast agent and status of CIN in Poisson regression model, adjusted for age, gender, diabetes, hypertension, congestive heart failure, CAD, and left ventricular ejection fraction at the beginning of follow-up period.
P value from interaction term in Poisson regression analysis.
Discussion
Unlike myocardial infarction, in which the acute injury to the myocardium can be quantified by serum markers of injury such as troponin and creatine kinase, AKI has no established marker of injury. Instead, all current definitions of AKI, including CIN, depend on serum markers of function (i.e., GFR). SCr is the most widely used serum marker of function, although serum cystatin C has increasingly been reported to be more specific and sensitive to acute changes in function (9,11) Furthermore, cystatin C is not affected by renal tubule secretion or pharmacologic treatments such as the use of N-acetylcysteine (12,13).
Observations using retrospective data analyses have consistently found that CIN is associated with long-term AEs (1–5). The nature of this association is unclear (14). CIN may be a marker of the burden of comorbidities in these patients. Patients who develop CIN may experience more long-term AEs because of this comorbidity burden. In the case of residual loss of kidney function after CIN, the increase in long-term AEs may reflect the altered risk factor profile seen in patients with chronic kidney disease (15). Although CIN may be a marker of disease burden, there is an increasing awareness that AKI, from any cause, contributes to the pathophysiology of long-term AEs.
A causal role of CIN in the development of AEs is understandably difficult to establish. If CIN is a marker of preexisting comorbidity burden, then this comorbidity burden should distribute equally between the arms of a randomized, prospective trial of two treatments to prevent CIN. If one arm of such a trial reduces the incidence of CIN, then that arm would not be expected to have fewer long-term AEs if these are related to the preexisting comorbidity burden. Conversely, if both the incidence of CIN and long-term AEs were reduced by one of the treatments, then this would strengthen the hypothesis that CIN is causally related to those AEs.
The CARE trial is a large, prospective, randomized trial in high-risk patients who undergo cardiac angiography. The large number of patients gave us the opportunity to explore the association of CIN with long-term AEs. In the original cohort, CIN (defined as a ≥25% increase in creatinine) occurred in 11.1% of the patients. In the subgroup analyzed in this report, CIN occurred in 16.1 to 24.8% of patients using more sensitive creatinine and cystatin C definitions of CIN. The higher incidence rates of CIN using cystatin C are consistent with the observations that cystatin C is more sensitive than creatinine to acute changes in GFR (9,10). Furthermore the higher incidence of CIN obtained using these definitions provides more statistical power to explore the association with long-term AE rates as well as differences between treatment groups. We found that SCr increases of ≥0.3 mg/dl and cystatin C increases of 15, 20, and 25% were associated with a significantly increased incidence of AEs (Table 3). A statistically significant increase in AEs was also found using SCr increases of 0.5 mg/dl and 25% (data not shown). After adjustment for a number of baseline risk factors that might contribute to long-term AEs (age, gender, diabetes, hypertension, congestive heart failure, coronary artery disease severity, left ventricular ejection fraction), CIN was associated with a two-fold increase in the incidence of AEs (Table 4). These results confirm the association between CIN and AEs and suggest that these more sensitive definitions of CIN are clinically relevant because they are predictive of AEs. To explore the possibility that CIN was causally related to the long-term AEs, we took advantage of the randomized design of the CARE trial. After adjustment for known baseline comorbidities, a statistically significant interaction remained between the assigned treatment, incidence of CIN, and AEs. Unknown risk factors are presumed to be equally distributed by the randomization process of the CARE study. This analysis therefore provides support for the hypothesis that CIN is causally related to long-term AEs.
Other observations support the causal relationship between CIN and AEs. For example, Marenzi et al. (16) randomly assigned very high-risk patients to hemofiltration before and after contrast medium exposure during percutaneous coronary intervention. Hemofiltration prevented CIN and reduced mortality at 1 yr from 30% in the control group to 10% in the hemofiltration group (P = 0.01). A similar reduction in both the incidence of CIN and in-hospital mortality was found in a randomized trial of high-dosage N-acetylcysteine (17). A large, multicenter, randomized trial of fenoldopam found no effect on CIN incidence and no effect on 30-d serious AEs or mortality (18). These studies are consistent with the hypothesis that CIN may be causally related to short- and long-term AEs.
The specific pathophysiologic connection between CIN and long-term AEs is unclear. From our data, we cannot determine a biologic gradient (dose-response) that would further strengthen the argument. This undermines the biologic plausibility of this association. The loss of 120 patients of the original cohort could potentially bias the result of this analysis; however, the baseline characteristics of those patients were identical to the 294 included in this analysis. In addition, the incidence of CIN was higher by almost all definitions in those 120 patients. Thus, the main effect of those missed data were to reduce the power of the study to detect significant associations between some of the CIN definitions and AE rates. The loss of 120 patients might also have biased the comparison between contrast agents; however, a lower incidence of CIN was seen in these patients with iopamidol compared with iodixanol, and the differences remained statistically significant for all of the serum cystatin C definitions of CIN. It is likely that the missed data have reduced the power to detect even more significant differences between the two contrast agents.
Conclusions
CIN after exposure to contrast media and defined by changes in SCr of ≥0.3 mg/dl and cystatin C increases of 15, 20, and 25% are associated with long-term AEs. This validates the use of these definitions of CIN. A reduction in the incidence of CIN and AEs was observed in regression analyses to adjust for possible known confounders and randomization to adjust for unknown confounders. This supports the hypothesis that CIN is causally related to AE rates. These more sensitive definitions should be included as primary outcomes in future randomized clinical trials for CIN prevention. In addition, future trials of CIN prevention must include long-term AEs as secondary trial outcomes.
Disclosures
R.J.S. was the principal investigator for this trial and is a consultant to Bracco Diagnostics, Inc. R.M., M.K.N., S.D., R.E.K., C.S.S., S.K.S., M.L., J.L.G., and B.J.B. were investigators in the CARE trial and received research funding for their participation. They have no other relationship with Bracco Diagnostics, Inc.
Supplementary Material
Acknowledgments
This study was supported by a grant from Bracco Diagnostics Inc. (Princeton, NJ). The CARE trial was funded by Bracco Diagnostics, Inc.
Published online ahead of print. Publication date available at www.cjasn.org.
This trial has been registered at www.clinicaltrials.gov (identifier NCT00479024).
See related editorial, “Defining Contrast-Induced Nephropathy,” on pages 1151–1153.
Access to UpToDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1.Dangas G, Iakovou I, Nikolsky E, Aymong ED, Mintz GS, Kipshidze NN, Lansky AJ, Moussa I, Stone GW, Moses JW, Leon MR, Mehran R: Contrast-induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am J Cardiol 95: 13–19, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Gruberg L, Mintz GS, Mehran R, Gangas G, Lansky AJ, Kent KM, Pichard AD, Satler LF, Leon MB: The prognostic implications of further renal function deterioration within 48 h of interventional coronary procedures in patients with pre-existent chronic renal insufficiency. J Am Coll Cardiol 36: 1542–1548, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Gupta R, Gurm HS, Bhatt DL, Chew DP, Ellis SG: Renal failure after percutaneous coronary intervention is associated with high mortality. Catheter Cardiovasc Interv 64: 442–448, 2005 [DOI] [PubMed] [Google Scholar]
- 4.McCullough PA, Wolyn R, Rocher LL, Levin RN, O'Neill WW: Acute renal failure after coronary intervention: Incidence, risk factors, and relationship to mortality. Am J Med 103: 368–375, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, Singh M, Bell MR, Barsness GW, Mathew V, Garratt KN, Holmes DR Jr: Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation 105: 2259–2264, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Solomon R, Natarajan MK, Doucet S, Sharma SK, Staniloae CS, Katholi RE, Gelormini JL, Labinaz M, Moreyra AE: The CARE (Cardiac Angiography in REnally Impaired Patients) Study: A randomized, double-blind trial of contrast-induced nephropathy in high risk patients. Circulation 115: 3189–3196, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A: Acute Kidney Injury Network (AKIN): Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devarajan P: Serum NGAL and cystatin C as predictive biomarkers for acute kidney injury [Abstract]. J Am Soc Nephrol 17: 404A, 2006 [Google Scholar]
- 9.Herget-Rosenthal S, Marggraf G, Husing J, Goring F, Pietruck F, Janssen O, Philipp T, Kribben A: Early detection of acute renal failure by serum cystatin C. Kidney Int 66: 1115–1122, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Herget-Rosenthal S, Pietruck F, Volbracht L, Philipp T, Kribben A: Serum cystatin C: A superior marker of rapidly reduced glomerular filtration after uninephrectomy in kidney donors compared to creatinine. Clin Nephrol 64: 41–46, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Dharnidharka VR, Kwon C, Stevens G: Serum cystatin C is superior to serum creatinine as a marker of kidney function: A meta-analysis. Am J Kidney Dis 40: 221–225, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Rehman T, Fought J, Solomon R: N-acetylcysteine effect on serum creatinine and cystatin C levels in CKD patients. Clin J Am Soc Nephrol 3: 1610–1614, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waikar S, Liu KD, Chertow GM: Diagnosis, epidemiology and outcomes of acute renal failure. Clin J Am Soc Nephrol 3: 844–861, 2008 [DOI] [PubMed] [Google Scholar]
- 14.McCullough P, Jurkovitz CT, Pergola PE, McGill JB, Brown WW, Collins AJ, Chen SC, Li S, Singh A, Norris KC, Klag MJ, Bakris GL, KEEP Investigators: Independent components of chronic kidney disease as a cardiovascular risk state. Arch Intern Med 167: 1122–1129, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Coresh J, Byrd-Holt D, Astor BC, Briggs JP, Eggers PW, Lacher DA, Hostetter TH: Chronic kidney disease awareness, prevalence, and trends among US adults, 1999 to 2000. J Am Soc Nephrol 16: 180–188, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Marenzi G, Marana I, Lauri G, Assanelli E, Grazi M, Campodonico J, Trabattoni D, Fabbiocchi F, Montorsi P, Bartorelli AL: The prevention of radiocontrast-agent-induced nephropathy by hemofiltration. N Engl J Med 349: 1333–1340, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Marenzi G, Assanelli E, Marana I, Lauri G, Campodonico J, Grazi M, De Metrio M, Galli S, Fabbiocchi F, Montorsi P, Veglia F, Bartorelli A: N-Acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med 354: 2773–2782, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Stone GW, McCullough P, Tumlin J, Lepor NE, Madyoon H, Murray P, Wang A, Chu AA, Schaer GL, Stevens M, Wilensky RL, O'Neill WW, CONTRAST Investigators: Fenoldopam mesylate for the prevention of contrast-induced nephropathy: A randomized controlled trial. JAMA 290: 2284–2291, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.