Abstract
Background and objectives: The peritoneal equilibration test (PET) was developed some 25 yr ago and has been used to help prescribe peritoneal dialysis. However, PET is affected by several factors, including diabetes and inflammation. It was speculated that extracellular fluid overload would increase PET ultrafiltration volumes, and therefore the usefulness of the PET in routine clinical practice was audited.
Design, setting, participants, & measurements: Data from 211 consecutive patients attending a university teaching hospital for a standard PET who had multifrequency bioimpedance performance were analyzed to determine which factors affected net PET ultrafiltration volumes.
Results: Net PET ultrafiltration volume was independent of gender, age, diabetes, residual renal function, peritoneal dialysis prescriptions (modes and dialysates), extracellular fluid volume, or C-reactive protein (CRP). There was an inverse regression with serum albumin and sodium on multiple logistical regression analysis (F = 13.4, P < 0.001 and F = 10.1, P = 0.001, respectively) and a positive regression with 24-h net peritoneal ultrafiltration volumes (F = 15.5, P < 0.001). As expected, there was a strong correlation with net sodium losses (r = 0.99, P < 0001).
Conclusions: It was found that PET test ultrafiltration volume in routine clinical practice was not affected by CRP, hyperglycemia, or extracellular fluid volume overload. Ultrafiltration volumes were increased in those patients with reduced serum sodium and albumin, most likely because of inflammation and protein malnutrition.
Worldwide many patients with ESRD are treated with peritoneal and hemodialysis. Patients treated with hemodialysis are typically assessed by small solute clearances (Kt/V) (1) and interdialytic weight gains (2). Although peritoneal dialysis patients are also assessed by small solute clearances (3), failure to control volume overload, particularly because of ultrafiltration failure, is more likely to lead to treatment failure, and fluid overload is also associated with an increased risk of patient mortality (4). Sodium removal during peritoneal dialysis is primarily by convective transport and is therefore dependent on effective ultrafiltration (5). To assess ultrafiltration and small solute transport, the peritoneal equilibration test (PET) was introduced some 25 yr ago (6) and has been used to help provide guidance in prescribing peritoneal dialysis for patients.
In their original report on the PET, Twardowski and colleagues commented on greater dialysate urea and creatinine values in diabetic compared with nondiabetic subjects (6). To determine whether other factors (e.g., fluid overload) affect PET ultrafiltration volumes, we audited the results of PET in patients who had multifrequency bioimpedance measurements on the day of PET testing.
Materials and Methods
Patients
Two-hundred eleven (27.8% diabetic) stable peritoneal dialysis patients under the care of a tertiary university teaching hospital (Table 1) underwent a standard PET. Patients were requested to attend the PET early in the morning, either before the first exchange of the day, or, if they had 7.5% icodextrin overnight, to drain out and then attend the hospital with a 13.6% glucose dialysate instilled. After draining out, 2 L of 22.7-g/L glucose was instilled, allowed to dwell for 4 h, and then drained out. Dialysate bags were weighed before and after the PET by regularly calibrated scales (MPPS-250, Marsden, Henley-on-Thames, UK). Height was measured by a standard wall-mounted measure (Sigmeas 1, Doherty signature range, Edward Doherty, Beverley, UK), and body surface area was estimated by standard formula (7). A blood sample was taken at the midpoint of the dwell (8). Dialysate creatinine was measured using a kinetic enzymatic method to prevent glucose interference (P module analyzer, Roche Integra, Roche diagnostics, Lewes, UK). Serum and dialysate sodium were measured using a two-point ion potentiometer (P module analyzer, Roche Integra, Roche diagnostics, Lewes, UK). Dialysis adequacy was calculated according to standard formula (9) using total body water according to the Watson formula (10). Blood pressure (BP) was recorded in the supine position after patients had rested before PET test (Dinamap compact TS, Critikon, Tampa, FL, USA). Multifrequency bioimpedance was measured after drainage at the end of the PET test (Biospace in body 720, Derwent Healthcare, Newcastle, UK) (11). No patient had suffered with peritonitis within the previous 3 mo before bioimpedance measurement.
Table 1.
Patient demographicsa
| Demographic | Value | Range |
|---|---|---|
| Age (yr) | 54.5 ± 16.0 | 17 to 89 |
| Gender | 47.4% male | |
| Weight (kg) | 70.1 ± 15.5 | 42.5 to 122.7 |
| Months on peritoneal dialysis | 29.9 ± 29.6 | 1 to 203 |
| Urine output (ml/d) | 941 ± 734 | 0 to 2925 |
| Systolic BP (mmHg) | 138 ± 27.4 | 63 to 219 |
| Diastolic BP (mmHg) | 81.3 ± 15.6 | 43 to 137 |
| Weekly peritoneal Kt/V | 1.47 ± 0.61 | 0.34 to 3.6 |
| Total weekly Kt/V | 2.61 ± 1.06 | 1.32 to 11.1 |
| Intracellular water l | 21.4 ± 5.4 | 12 to 37.4 |
| Extracellular water l | 13.7 ± 3.4 | 7.8 to 24.3 |
| ECW/ht l/m | 8.29 ± 1.58 | 5.2 to 12.9 |
| ECW/1.73m2 | 10.1 ± 0.61 | 4.5 to 14.0 |
| ECW/TBW | 0.39 ± 0.01 | 0.366 to 0.43 |
| Albumin (g/L) | 38.7 ± 4.3 | 21 to 50 |
| CRP (g/L) | 9.1 ± 2.3 | 0.1 to 233 |
| Glucose (mmol/L) | 6.59 ± 4.3 | 1.1 to 34 |
| HbA1c (%) | 6.06 ± 1.32 | 3.7 to 11.0 |
| Sodium (mmol/L) | 137.6 ± 4.6 | 121 to 150 |
| D4/P creatinine | 0.72 ± 0.13 | 0.32 to 1.06 |
| D4/P sodium | 0.929 ± 0.039 | 0.814 to 1.02 |
| D4/D0 glucose | 0.33 ± 0.07 | 0.08 to 0.59 |
| Sodium removed (mmol) | 36.2 ± 26.6 | −40 to 128. |
| Net ultrafiltrate (ml) | 281.3 ± 212.6 | −300 to 1000 |
| Net ultrafiltrate (ml/1.73 m2) | 358.1 ± 55.4 | −319 to 397 |
Extracellular water adjusted for height (ECW/ht), and ratio to total body water (ECW/TBW). BP, blood pressure; CRP, C-reactive protein; D4-h/P sodium, 4-h PET dialysate to plasma sodium ratio; D4/P creatinine, 4-h PET dialysate to plasma creatinine; D4/D0 glucose, 4-h PET dialysate glucose to initial dialysate.
Because multifrequency bioimpedance is a relatively simple technique (12) and has become part of routine patient management, this study was performed as part of an approved internal audit; as such, formal informed consent was waived by the local ethics committee. To standardize measurements, bioimpedance was performed when peritoneal dialysate had been drained out at the end of a PET (8). Patients with pacemakers and/or implantable defibrillators were excluded.
Statistical Analyses
Statistical analyses were by simple correlation analysis (GraphPad Prism version 3.0, San Diego, CA, USA), then multiple logistical linear regression analyses were undertaken with SPSS software for Windows version 15.0 (SPSS Inc., Chicago, IL, USA). The regression model was created based on those variables that had a simple correlation of P < 0.05 to PET ultrafiltration and estimates of extracellular fluid volume, then analyzed in a step-backward fashion. Data are expressed as mean ± SD, median and interquartile range, or percentages. Statistical significance was taken at or below the 5% level.
Results
Two-hundred eleven individual patients attending for a PET were studied (Table 1). Patients were treated by different modes of peritoneal dialysis: 32% continuous ambulatory peritoneal dialysis (CAPD), 17% automated peritoneal dialysis with dry day (APD), 45% automated peritoneal dialysis with one daytime long dwell (CCPD), and 6% automated peritoneal dialysis with two daytime exchanges (OCPD). Most patients (77.7%) used 7.5% icodextrin and 27.8% used 22.7-g/L glucose containing dialysates, but no patient was treated with any higher glucose dialysate concentration. Few patients (15.2%) used neutral pH bicarbonate-based dialysates. Of the patients, 27.5% were diabetic and 68.2% were prescribed antihypertensive medication; the mean number of antihypertensive medications prescribed was 1.23 (range 0 to 6), and 65.8% were prescribed diuretics, typically frusemide.
The net volume of ultrafiltration drained during the PET was inversely related to the serum sodium and albumin concentrations (Table 2), but not associated with patient demographics (age, gender, diabetes, body mass index), dialysis modality (APD, CAPD, CCPD, or OCPD), dialysates (icodextrin with 22.7-g/L exchanges or bicarbonate-based neutral pH solutions), residual renal function, BP, BP medications, blood glucose, hemoglobin A1c, C-reactive protein (CRP), or hydration status as assessed by bioimpedance (r = −0.12, P = 0.096 for extracellular fluid adjusted for height, and r = −0.13, P = 0.068 when adjusted for normalized body surface area). There was no correlation between the ratio of extracellular water to total body water and PET ultrafiltration volumes (r = −0.06, P = 0.39). Multiple linear logistical regression models, based on the variables in Table 2, confirmed the results of the simple correlation analysis (Table 3). In addition, a further series of models were created based on extracellular fluid volumes and factors that have previously been suggested to affect peritoneal transport status, including blood glucose, glycated hemoglobin, CRP, residual renal function, and Caucasian versus non-Caucasian race (13,14), but no significant correlations were found with PET ultrafiltration volumes. The PET ultrafiltration volumes corrected for body size did not statistically differ between men and women [median 232 (123 to 388) ml versus 316 (177 to 477) ml, respectively], diabetics versus nondiabetics [median 254 (142 to 371) ml versus median 261 (125 to 475) ml, respectively], those treated by CAPD compared with those using overnight cyclers [median 319 (171 to 442) ml versus 226 (86 to 421) ml, respectively], and those using icodextrin compared with patients only treated with glucose dialysates [median 261 (138 to 416) ml versus 277 (117 to 470) ml, respectively].
Table 2.
Factors associated with peritoneal equilibration test (PET) ultrafiltration volumesa
| Factor | r Value | P Value |
|---|---|---|
| Sodium in ultrafiltrate | 0.9972 | <0.0001 |
| D4-h/P sodium | −0.2575 | 0.0002 |
| 24-h peritoneal dialysis ultrafiltration | 0.2399 | 0.0005 |
| D4/D0 glucose | 0.2313 | 0.0008 |
| Serum albumin | −0.2128 | 0.0020 |
| D4/P creatinine | −0.1966 | 0.0043 |
| D2/P sodium | −0.1608 | 0.0206 |
| Serum sodium | −0.1513 | 0.0292 |
Dialysate (D) and plasma (P) values. Extracellular water (ECW) and total body water (TBW) were corrected to 1.73 m2. The sodium in the ultrafiltrate was the difference between the sodium instilled and that recovered at the end of the PET.
D4-h/P sodium, 4-h PET dialysate to plasma sodium ratio; D4/P creatinine, 4-h PET dialysate to plasma creatinine; D4/D0 glucose, 4-h PET dialysate glucose to initial dialysate.
Table 3.
Factors associated with PET ultrafiltration volumesa
| Variable | F Value | P Value |
|---|---|---|
| Serum albumin | 13.38 | 0.000 |
| Serum sodium | 10.065 | 0.001 |
| Net sodium removed | >50 | 0.000 |
| D4/P sodium | 14.053 | 0.000 |
| 24-h peritoneal ultrafiltrate | 15.516 | 0.000 |
Multiple linear logistical regression model. Net sodium removed refers to the PET test. D4/P sodium, 4-h PET dialysate to plasma sodium ratio.
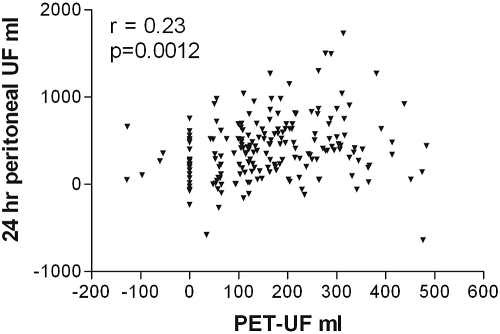
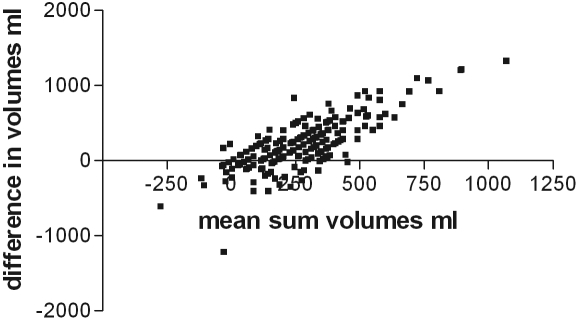
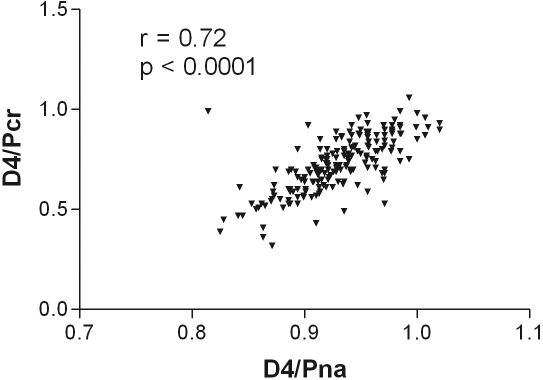
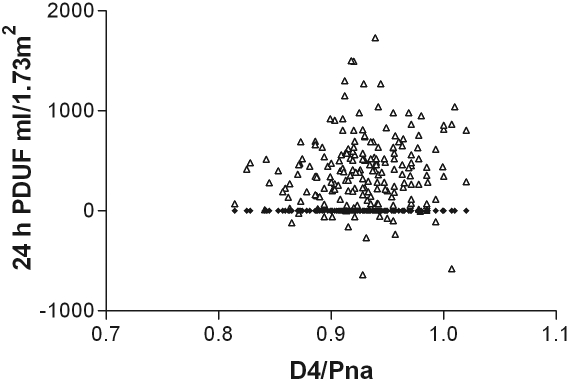
As expected, there was a correlation between the net ultrafiltration volume and the amount of sodium removed during the PET, between the dialysate-to-plasma ratios for sodium at 2 h and at the end of the PET test, and with the initial dialysate to final dialysate glucose concentration (Table 2). On further analysis, only the relationships to sodium were maintained (Table 3). There was also a relatively weak correlation between the PET net ultrafiltration volume, and that of the 24-h peritoneal net ultrafiltration volume (Figure 1), which was sustained on further analysis (Table 3). To investigate this further, a Bland–Altman plot was performed (Figure 2), which showed that there was limited agreement with a scatter of values. Although there was a positive correlation between the PET 4-h dialysate-to-plasma ratio for sodium and the dialysate to plasma ratio for creatinine (Figure 3), and also the PET ultrafiltrate corrected for body size (r = −0.25, P = 0.05), but, there was no correlation between the 4-h PET dialysate to plasma sodium ratio and total net 24-h peritoneal ultrafiltration volume (Figure 4). The 4-h PET dialysate to plasma creatinine ratio was greater in the diabetic patients [median 0.77 (0.69 to 0.86)] compared with the nondiabetic patients [0.70 (0.61 to 0.82), P = 0.0089].
Figure 1.
There was a positive correlation between the net PET ultrafiltrate volume and the 24-h net peritoneal dialysate ultrafiltrate corrected for body surface area (1.73 m2).
Figure 2.
Bland–Altman plot of the difference between the net PET and 24-h peritoneal dialysate volumes corrected for body surface area (1.73 m2) and compared with the mean of the sum of values.
Figure 3.
There was a positive correlation between the PET 4-h dialysate to plasma creatinine (D/PCr) and the 4-h PET dialysate to plasma sodium (D/PNa) ratio.
Figure 4.
There was no correlation between the 4-h dialysate to plasma sodium (D4/PNa) and the 24-h net peritoneal dialysate ultrafiltrate corrected for body surface area (1.73 m2). r = 0.09, P = 0.18.
Discussion
Over the last 25 yr the PET has been used to help guide the prescription of peritoneal dialysis and as such has been incorporated into many clinical guidelines and treatment protocols (3,15). Ultrafiltration during the PET depends on the balance between the different forces acting across the peritoneal capillaries and interstitial tissues. On one hand, capillary hydrostatic pressure moves fluid, passing through aquaporin water channels and small pores out from the capillaries because the intraperitoneal hydrostatic pressure is typically much lower. Because elevated systemic BP is not transmitted down to the capillary level, it was not unexpected that there was no relationship between the systemic BP (systolic, diastolic, pulse pressure, or mean arterial pressure) and PET ultrafiltration losses; however, ultrafiltration was sustained even in those patients with marked chronic hypotension. On the other hand, capillary oncotic and osmotic pressures counteract the hydrostatic forces. In peritoneal dialysis, hypertonic glucose dialysates raise the osmolality in excess of the capillary, which creates the main initial driving force of ultrafiltration. Earlier reports have suggested that hyperglycemia in diabetic patients can lead to reduced PET ultrafiltration volumes (16). Despite our diabetic patients having a greater D4/Pcreatinine ratio than the nondiabetic patients, in keeping with other reports (6) we found no association between PET net ultrafiltration volumes and either blood glucose or hemoglobin A1c measurements. Our findings support the observations of previous studies (17).
During hemodialysis, it is easier to remove fluid by ultrafiltration when the patient is hypervolemic, rather than when close to “dry” or target weight (18). However, we found no association between absolute fluid volumes as determined by multifrequency bioimpedance or when patients were assessed for volume overload, either by extracellular fluid volume adjusted for height (19) or normalized body surface area. Icodextrin may better preserve the effective vascular volume and residual renal function than 22.7-g/L glucose exchanges (20), but we found no effect of different dialysates prescribed and PET net ultrafiltration volumes.
Inflammation both within the peritoneum (21) and systemically (22) can affect peritoneal transport. However, we did not find any relationship between CRP or log-transformed CRP and PET ultrafiltration volumes. Previous studies have reported reduced PET ultrafiltration shortly after an episode of peritonitis (16), although more recently the relationship between peritoneal transport and inflammation has been questioned (23). Inflammation typically leads to an increased CRP and fall in albumin. In this study we cannot exclude that any effect of CRP on ultrafiltration may have been obscured by the fall in albumin because of colinearity in the statistical models; however, we did observe a negative correlation with the serum albumin and sodium and net PET ultrafiltration volumes. Although the measurement of serum sodium can be affected by icodextrin and hyperglycemia (5,24), the correlation remained after correcting for a possible glucose effect (r = −0.39, P = <0.001) (24). Thus it is more likely that the lower sodium is associated with relative water retention as found in inflammatory states associated with chronic illness, often associated with loss of fat and poor nutrition (25,26), and this is in keeping with the reduced albumin (27). A relative increase in body water without sodium expands intracellular and extracellular volumes, therefore the overall ratio is not affected (28). In these patients, the osmotic gradient is increased because of the reduced capillary osmotic and oncotic pressure due to the lower sodium and albumin concentrations, resulting in greater PET ultrafiltration volumes.
As expected, those patients who have faster transport characteristics, with higher dialysate creatinine and lower glucose values at the end of the PET, had lower PET ultrafiltration volumes because of the dissipation of the glucose osmotic gradient. Similarly, because sodium removal is dependent on the ultrafiltration volume, as expected, net sodium losses during the PET were positively correlated with the ultrafiltration volume. There was an inverse correlation between the ratio of dialysate-to-serum sodium, even after correcting for a glucose effect (r = −0.286, P = 0.002) (24). This is most likely due to a reduced dialysate sodium concentration in association with increased ultrafiltration water loss, which would suggest that during the PET increasing ultrafiltration volumes resulted in relatively more water removal than sodium. This is because water movement is predominantly through aquaporin channels whereas sodium movement is through small pores and, because it is positively charged, sodium may be retarded by negatively charged matrix glycoproteins.
Although net peritoneal ultrafiltration during the PET test was associated with lower dialysate-to-plasma ratios for creatinine and sodium and final dialysate glucose to initial glucose concentration, there was no association of these variables with measured net 24-h ultrafiltration volumes.
Although there was a positive correlation between the PET ultrafiltration volume and peritoneal 24-h ultrafiltration volume, this was relatively weak and was shown to have a wide scatter on a Bland–Altman plot. In clinical practice, the choice of peritoneal dialysis modality and prescription depends not only on information obtained from the PET test, in terms of patient transporter status, but is also affected by other factors such as residual renal function, patient choice of peritoneal dialysis modality, constipation, and patient practices in terms of compliance with therapy and timing of exchanges, all of which can affect the net 24-h peritoneal ultrafiltration volume.
The PET ultrafiltration volume has been used to predict daily ultrafiltration losses and the PET dialysate effluent used to predict adequacy. As such, there has been criticism of using the PET to predict ultrafiltration and dialysis prescription on the basis of the results of a single exchange. This has led to the development of other programs based on the results from each peritoneal dialysis exchange (29,30).
The net PET ultrafiltration volume was independent of patient demographics, the choice of dialysate solutions, BP, residual renal function, and hydration status as assessed by extracellular fluid volumes. However, PET ultrafiltration volumes were inversely associated with serum sodium and albumin concentrations, suggesting that inflammation and protein malnutrition may affect ultrafiltration volumes (31).
Disclosures
None.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Spalding EM, Chandna SM, Davenport A, Farrington K: Kt/V underestimates the hemodialysis dose in women and small men. Kidney Int 74: 348–355, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Davenport A, Cox C, Thuraisingham R: The importance of dialysate sodium concentration in determining interdialytic weight gains in chronic hemodialysis patients: The PanThames Renal Audit. Int J Artif Organs 31: 411–417, 2008 [DOI] [PubMed] [Google Scholar]
- 3.II. NKF-K/DOQI Clinical practice guidelines for peritoneal dialysis adequacy: Update 2000. Am J Kidney Dis 37[Suppl 1]: S65–S136, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Chen W, Guo LJ, Wang T: Extracellular water/intracellular water is a strong predictor of patient survival in incident peritoneal dialysis patients. Blood Purif 25: 260–266, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Aanen MC, Venturoli D, Davies SJ: A detailed analysis of sodium removal by peritoneal dialysis: Comparison with predictions from the three-pore model of membrane function. Nephrol Dial Transplant 20: 1192–2000, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Twardowski ZJ, Nolph KD, Khanna R, Khanna R, Prowant BF, Ryan LP, Moore. HL, Neilson MP: Peritoneal equilibrium test. Perit Dial Bull 7: 138–147, 1987 [Google Scholar]
- 7.Du Bois D, Du Bois EF: A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med 17: 863–871, 1916 [PubMed] [Google Scholar]
- 8.Twardowski ZJ: PET—A simple approach for determining prescriptions for adequate dialysis therapy. Adv Perit Dial 6: 186–191, 1990 [PubMed] [Google Scholar]
- 9.Keshaviah PR, Nolph KD, Van Stone JC: The peak concentration hypothesis: A urea kinetic approach to comparing the adequacy of continuous ambulatory peritoneal dialysis (CAPD) and haemodialysis. Perit Dial Int 9: 257–260, 1989 [PubMed] [Google Scholar]
- 10.Watson PE, Watson ID, Batt RD: Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr 33: 27–39, 1980 [DOI] [PubMed] [Google Scholar]
- 11.Thomas EL, Frost G, Harrington T, Bell JD: Validation on “in body” bioelectrical impedance by whole body MRI. Laboratory report, London, United Kingdom, Hammersmith Hospital, 2001
- 12.Bedogni G, Malavolti M, Severi S, Poli M, Mussi C, Fantuzzi AL, Battistini N: Accuracy of an eight-point tactile-electrode impedance method in the assessment of total body water. Eur J Clin Nutr 56: 1143–1148, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Davies SJ: Mitigating peritoneal membrane characteristics in modern peritoneal dialysis therapy. Kidney Int 103: S76–S83, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Cueto-Manzano AM: Rapid solute transport in the peritoneum: Physiologic and clinical consequences. Perit Dial Int 29[Suppl 2]: S90–S95, 2009 [PubMed] [Google Scholar]
- 15.Davies SJ: Module 3b. Peritoneal dialysis. Available online at http://www.renal.org/pages/pages/clinical-affairs/guidelines.php. Accessed February 2009
- 16.Lamb EJ, Worrall J, Buhler R, Harwood S, Cattell WR, Dawnay AB: Effect of diabetes and peritonitis on the peritoneal equilibration test. Kidney Int 47: 1760–1767, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Rubin J, Nolph K, Arfania D, Brown P, Moore H, Rust P: Influence of patient characteristics on peritoneal clearances. Nephron 27: 118–121, 1981 [DOI] [PubMed] [Google Scholar]
- 18.Davenport A: Intradialytic complications during hemodialysis. Hemodial Int 10: 162–167, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Van der Kerkhof J, Hermans M, Beerenhout C, Konings C, Van der Sande FM, Kooman JP: Reference values for multifrequency bioimpedance analysis in dialysis patients. Blood Purif 22: 301–306, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Davies SJ, Woodrow G, Donovan K, Plum J, Williams P, Johansson AC, Bosselmann HP, Heimbürger O, Simonsen O, Davenport A, Tranaeus A, Divino Filho JC: Icodextrin improves the fluid status of peritoneal dialysis patients: Results of a double-blind randomized controlled trial. J Am Soc Nephrol 14: 2338–2344, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Li FK, Davenport A, Robson RL, Loetscher P, Rothlein R, Williams JD, Topley N: Leukocyte migration across human peritoneal mesothelial cells is dependent on directed chemokine secretion and ICAM-1 expression. Kidney Int 54: 2170–2183, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Kim SB, Chang JW, Lee SK, Park JS: Acute systemic inflammation is associated with an increase in peritoneal solute transport rate in chronic peritoneal dialysis patients. Perit Dial Int 24: 597–600, 2004 [PubMed] [Google Scholar]
- 23.Oh KH, Moon JY, Oh J, Kim SG, Hwang YH, Kim S, Lee JS, Ahn C: Baseline peritoneal solute transport rate is not associated with markers of systemic inflammation or comorbidity in incident Korean peritoneal dialysis patients. Nephrol Dial Transplant 23: 2356–2364, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Davenport A: Interdialytic weight gain in diabetic haemodialysis patients and diabetic control. Nephron Clin Pract 2009, in press [DOI] [PubMed]
- 25.Adler SM, Verbalis JG: Disorders of body water homeostasis in critical illness. Endocrinol Metab Clin North Am 35: 873–894, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Davenport A: The brain and the kidney—organ cross talk and interactions. Blood Purif 26: 526–536, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Davies SJ, Garcia Lopez E, Woodrow G, Donovan K, Plum J, Williams P, Johansson AC, Bosselmann HP, Heimburger O, Simonsen O, Davenport A, Lindholm B, Tranaeus A, Divino Filho JC: Longitudinal relationships between fluid status, inflammation, urine volume and plasma metabolites of icodextrin in patients randomized to glucose or icodextrin for the long exchange. Nephrol Dial Transplant 23: 2982–2988, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Jones CH, Wells L, Stoves J, Farquhar F, Woodrow G: Can a reduction in extracellular fluid volume result in increased serum albumin in peritoneal dialysis patients? Am J Kidney Dis 39: 872–875, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Van Biesen W, Van Der Tol A, Veys N, Lameire N, Vanholder R: Evaluation of the peritoneal membrane function by three letter word acronyms: PET, PDC, SPA, PD-Adequest, POL: What to do? Contrib Nephrol 150: 37–41, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Van Biesen W, Van der Tol A, Veys N, Dequidt C, Vijt D, Lameire N, Vanholder R: The personal dialysis capacity test is superior to the peritoneal equilibration test to discriminate inflammation as the cause of fast transport status in peritoneal dialysis patients. Clin J Am Soc Nephrol 1: 269–274, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Rodrigues AS, Almeida M, Fonseca I, Martins M, Carvalho MJ, Silva F, Correia C, Santos MJ, Cabrita A: Peritoneal fast transport in incident peritoneal dialysis patients is not consistently associated with systemic inflammation. Nephrol Dial Transplant 21: 763–769, 2006 [DOI] [PubMed] [Google Scholar]