Abstract
Background and objectives: The study aim was to establish the incidence and characterize all encapsulating peritoneal sclerosis (EPS) cases in patients treated by peritoneal dialysis (PD).
Design, setting, participants, & measurements: The patient cohort, which started PD from January 1, 2000, to December 31, 2007, was identified from the Scottish Renal Registry (n = 1238). Possible EPS cases were identified by the ten adult Scottish renal units. Patient records were examined to ensure cases met diagnostic criteria.
Results: Forty-six cases were identified; 19 had their first PD exposure after January 1, 2000. The rate was 1.5%, an incidence of 4.9 per 1000 person-years. The incidence increased with PD duration, with rates of 0, 0.6, 2.0, 3.5, 8.1, 8.8 and 5% at <1, 1 to 2, >2 to 3, >3 to 4, >4 to 5, >5 to 6 and >6 yr PD exposure, respectively. The median PD duration of EPS cases was 5.1 yr (interquartile range [IQR] 3.4 to 6.1 yr). At diagnosis, 12 (26%) were on PD and 33 (72%) were diagnosed <2 yr after PD stopped. The cases had a median of 3.3 episodes of peritonitis (range 0 to 20, IQR 1 to 4.5). Thirty (65%) had used 3.86% dextrose dialysate and 45 (98%) had used Extraneal. The mortality was 42% at 1 yr postdiagnosis with a median survival of 149 d (IQR 61 to 408 d).
Conclusions: The incidence reported in this study may be used to inform patients of the minimum risk of developing EPS on PD.
Encapsulating peritoneal sclerosis (EPS) is a devastating complication of peritoneal dialysis (PD), first described in 1980 (1). EPS is thought to result from chronic intra-abdominal inflammation that is multifactorial in origin. Prolonged PD represents the most consistent “risk factor” identified (2–6).
EPS is uncommon, but the incidence is unknown. A UK series identified 27 EPS cases, indicating a rate of 3.3% over 7 yr (7). An Australian study identified 54 cases in 14 yr, giving a rate of 0.7% (4). Japanese and Korean studies describe rates of 0.8 to 2.5% (2,3,5,6). Kawanishi et al. published a 2 yr follow-up of a PD cohort in 2001, suggesting an incidence of 0.8% (2). They published a further analysis in 2004 reporting an incidence of 2.5%, which may reflect the longer follow-up inherent to the study design rather than a true incidence increase (3). These studies include incident and prevalent patients on PD, which does not allow accurate calculation of incidence (2–7). To calculate a true incidence of EPS, a cohort of patients must be followed from the start of PD to identify all cases diagnosed thereafter.
The clinical features of EPS have been described previously (2–9). Onset may occur on PD, but most cases become apparent after stopping PD, including after renal transplantation (2–8). Clinical, radiologic and pathologic criteria for EPS diagnosis were defined by the International Society for Peritoneal Dialysis (ISPD) in 2000 (10).
There is little evidence to guide management of EPS patients. In Japan, enterolysis (surgical division of adhesions) is recommended but there are few European reports on EPS surgery (11–15). Case series have assessed total parenteral nutrition, immunosuppression and tamoxifen, and some have shown encouraging results (3,7,16- 26). There are no reliable biochemical or radiologic screening tests to identify patients at risk or in the early stages of EPS (27,28).
The primary aim of this study was to report the EPS incidence in patients using PD for end-stage renal failure (ESRF) in Scotland between January 1, 2000, and December 31, 2007. A secondary aim was to characterize these cases.
Materials and Methods
The cohort of adult patients who started PD between January 1, 2000, to December 31, 2007, in Scotland (n = 1238) was identified from the Scottish Renal Registry (SRR). The ten adult renal units in Scotland identified potential EPS cases diagnosed on or after January 1, 2000. Medical records were examined to ensure all cases met ISPD diagnostic criteria including clinical features and either radiologic and/or histopathological confirmation (10). Seven cases were excluded because there was another potential cause for their presentation (n = 5) or they lacked radiologic or pathologic confirmation of EPS (n = 2). Peritonitis was defined as a PD effluent white cell count above 100 per mm3.
We searched the SRR database (for International Classification of Disease codes; ICD-9/ICD-10) reported in hospital discharge statistics, but no additional cases were found. All cases have been used to describe the clinical presentation. Only incident cases who started PD after January 1, 2000, are used to calculate the EPS incidence. For clarity, we refer to the 46 period-prevalent cases as Group A and the subgroup of 19 incident cases as Group B.
Statistical analyses were performed using SPSS®. The incidence of EPS was calculated as number of EPS cases divided by number of patients at risk, taking into account the person-time during which events were observed and time elapsed before EPS diagnosis (29). Logistic analysis was undertaken and odds ratios calculated comparing PD duration and probability of developing EPS. Cumulative risks were calculated using the Kaplan-Meier method to plot time from first PD to EPS diagnosis. Peritonitis rates were calculated as the number of patient months on PD divided by number of infections and expressed as number of months between episodes. The rates were converted to events per person-years for Poisson regression analysis to test the difference between the groups (relative risk).
Results
Incidence
Between January 1, 2000, to December 31, 2007, 46 patients met ISPD criteria for EPS diagnosis (Group A), of which 19 were first exposed to PD on or after January 1, 2000 (Group B). Of the 1238 patients exposed to PD after January 1, 2000, the overall rate is 1.5% in the 8 yr to end December 2007 (19 of 1238). This represents an incidence of 4.9 per 1000 person- years or 8.7 per 1000 person-years of PD.
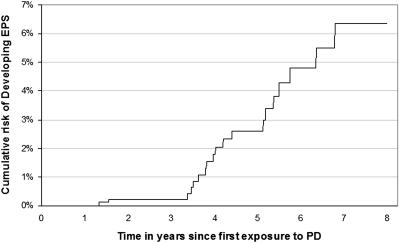
The rates according to PD exposure are shown in Table 1. Beyond 4 yr of PD there is a marked increase in the proportion developing EPS (one in 12 patients at risk). The odds ratio of developing EPS with a PD exposure of >2 to 4 yr versus ≤2 yr is 10.4 (2.2 to 49.4) and for >4 yr versus >3 to 4 yr exposure is 3.2 (1.2 to 8.6). The cumulative risk of developing EPS after starting PD is shown in Figure 1.
Table 1.
Incidence rates of EPS related to total duration of PD exposure (not necessarily continuous)
| PD Exposure (years) | PD Patients at Risk (n) | EPS Cases (n) | Incidence (%) | 95% Confidence Intervals (%) |
|---|---|---|---|---|
| <1 | 480 | 0 | 0 | – |
| 1–2 | 326 | 2 | 0.6 | 0.2–2.1 |
| >2–3 | 202 | 4 | 2.0 | 0.8–5.0 |
| >3–4 | 114 | 4 | 3.5 | 1.4–8.7 |
| >4–5 | 62 | 5 | 8.1 | 3.6–17.6 |
| >5–6 | 34 | 3 | 8.8 | 3.2–23.1 |
| >6 | 20 | 1 | 5.0 | 1.2–23.8 |
| Total | 1238 | 19 |
EPS, encapsulating peritoneal sclerosis; PD, peritoneal dialysis.
Figure 1.
Cumulative risk function of developing EPS according to days since first PD exposure between January 1, 2000, and December 31, 2007.
Comparison with Previous Studies
To allow comparison, in Table 2 (last column) we present our data using the same calculation of EPS rate as previous studies (number of EPS cases/incident and prevalent PD patients during study period).
Table 2.
Comparison of previous and current epidemiological studies of EPS
| Study
|
|||||||
|---|---|---|---|---|---|---|---|
| Nomoto et al. Japan 1996 | Rigby et al. Australia 1998 | Lee et al. Korea 2003 | Kawanishi et al. Japan 2001 | Kawanishi et al. Japan 2004 | Summers et al. UK 2005 | Brown et al. UK (current study) | |
| Number of EPS Casesa | 62 | 54 (46) | 31 | 17 | 48 | 27 (23) | 46 |
| Dates of Study | 1980–1994 | 1980–1994 | 1981–2002 | 1999–2001 | 1999–2003 | 1998–2003 | 2000–2007 |
| Study Design | Retrospective Multi-center | Retrospective Multi-center | Retrospective Multi-center | Prospective Multi-center | Prospective Multi-center | Retrospective Single-center | Retrospective Multi-center |
| Denominator Populationb | 6923 | 7374 | 3888 | 2216 | 1958 | 810 | 1638 |
| Overall Rate | 0.9% | 0.7% | 0.8% | 0.8% | 2.5% | 3.3% | 2.8% |
| Mean PD Exposure (yrs) | 5.1 | 4.3 | 5.8 | 10 | 4.3 | 6.1 | 5.4 |
| Mortality (over study period) | 43.5% | 56% | 25.8% | 35% | 37.5% | 29.6% | 56.5% |
EPS, encapsulating peritoneal sclerosis; PD, peritoneal dialysis.
Those meeting ISPD 2000 criteria in brackets.
Prevalent + incident PD patients.
Dialysis History and Timing of Diagnosis
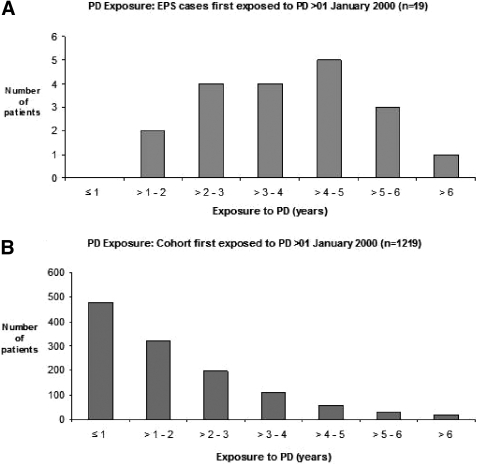
There is a significant association between duration of PD and EPS (P < 0.001). The median PD duration before EPS diagnosis (not necessarily continuous) was 5.1 yr (range 1.1 to 12.2, IQR 3.4 to 6.1 yr). For 1219 patients unaffected by EPS, the median PD duration was 1.3 yr (range 1 d to 7.9 yr, IQR 0.6 to 2.5 yr) while for Group B the median PD duration was 3.6 yr (range 1.1 to 6.1, IQR 2.9 to 4.9 yr) (see Figure 2). Twenty-eight (61%) had used automated peritoneal dialysis.
Figure 2.
(A) PD Duration: Non-EPS cohort first exposed to PD after January 1, 2000 (n = 1219). (B) PD Duration: EPS cases first exposed to PD after January 1, 2000 (n = 19).
Twenty-two (50%) cases had been transplanted before EPS diagnosis and in six the transplant was still functioning. The transplant patients received calcineurin inhibitor-based (cyclosporine or tacrolimus) immunosuppression. At diagnosis 12 of 46 (26%) were on PD, 33 of 46 (72%) were diagnosed within 2 yr and 29 of 46 (63%) within a year of stopping PD. Fourteen (30%) were diagnosed within 3 mo of peritonitis, and in 11 (24%) this episode was severe enough to merit PD catheter removal.
Demographics
There is no significant association with age or sex and EPS. Twenty-five (54%) were male, 43 (94%) Caucasian and median age at diagnosis was 50 yr (range 23 to 82, IQR 42 to 61 yr). The primary renal diagnoses are listed in Table 3.
Table 3.
Underlying cause of renal failure of the EPS cases and incident PD population 2000-2007 (GN = glomerulonephritis)
| Cause of Renal Failure | PD Cohort (N) | PD Cohort (%) | EPS Group A (N) | EPS Group A (%) | EPS Group B (N) | EPS Group B (%) |
|---|---|---|---|---|---|---|
| CRF, uncertain etiology | 219 | 18.0 | 8 | 17.4 | 4 | 21.1 |
| Diabetes Type I (insulin dependent) | 186 | 15.3 | 1 | 2.2 | 1 | 5.3 |
| Autosomal Dominant Polycystic Kidney Disease | 131 | 10.7 | 5 | 10.9 | 3 | 15.8 |
| Pyelonephritis with Urinary Tract Disease | 128 | 10.5 | 7 | 15.2 | 1 | 5.3 |
| Renovascular Disease | 126 | 10.3 | 1 | 2.2 | ||
| Chronic GN (unspecified) | 67 | 5.5 | 4 | 8.7 | 2 | 10.5 |
| Diabetes Type II (non insulin dependent) | 66 | 5.4 | 1 | 2.2 | 1 | 5.3 |
| IgA Nephropathy | 63 | 5.2 | 3 | 6.5 | 2 | 10.5 |
| Other | 56 | 4.6 | 4 | 8.7 | 1 | 5.3 |
| Multisystem Disorders | 52 | 4.3 | 1 | 2.2 | ||
| Membranous Nephropathy | 24 | 2.0 | 2 | 4.3 | ||
| Myeloma/Light Chain Deposition Disease | 22 | 1.8 | ||||
| Focal Segment Glomerulosclerosis | 15 | 1.2 | 5 | 10.9 | 3 | 15.8 |
| Drug-induced Nephropathy | 17 | 1.4 | ||||
| Amyloidosis | 15 | 1.2 | ||||
| Herediatry Nephropahy (inc. Alport's) | 12 | 1.0 | 2 | 4.3 | 1 | 5.3 |
| Rapidly Progressive/Crescentic GN | 11 | 0.9 | 1 | 2.2 | ||
| Membranoproliferative GN | 9 | 0.7 | 1 | 2.2 | ||
| Total | 1219 | 100 | 46 | 100 | 19 | 100 |
EPS, encapsulating peritoneal sclerosis; PD, peritoneal dialysis.
Clinical Presentation
The most common features are shown in Table 4. All patients had at least one of these three symptoms: abdominal pain, vomiting and abdominal distension (with ascites or with bowel obstruction). Forty-two cases (91%) had diagnostic imaging (Table 4) and 29 (63%) had a laparotomy or laparoscopy.
Table 4.
Main clinical features and radiological abnormalities found in the 46 cases of EPS diagnosed in Scotland 2000-2007
| Clinical Features (Most cases had >1) | N | Imaging Findings (US or CT scan) (some cases had >1) | N |
|---|---|---|---|
| Abdominal Pain | 30 | Ascites | 33 |
| Vomiting | 28 | Septate/loculated ascites | 22 |
| Weight Loss | 24 | Peritoneal thickening | 14 |
| Ascites | 15 | Peritoneal calcification | 11 |
| Elevated Inflammatory Markers | 14 | Bowel obstruction | 3 |
| Bowel Obstruction | 11 | Matted/tethered bowel | 10 |
| Hypoalbuminaemia | 10 | Dilated Small Bowel | 4 |
| Unexplained Anaemia | 10 | ||
| Bloody Ascites/Dialysate | 4 | ||
| Abdominal Mass | 4 | ||
| Diarrhoea | 4 |
EPS, encapsulating peritoneal sclerosis.
Possible Risk Factors
Four patients had no history of peritonitis. For Group A, the median number of peritonitis episodes was three (range 0 to 20, IQR 1 to 4.5 episodes), equating to one episode every 20 mo PD, and for Group B, one episode every 17 mo. The rate in all PD patients in Scotland between 2000 and 2007 was one episode every 20 mo. The differences in these rates were NS (Relative Risk = 1.18, 95% confidence interval 0.9 to 1.57).
In Group A, 16 (35%) had documented episodes of Staphylococcus aureus peritonitis (1 MRSA), 5 (11%) fungal peritonitis and 1 (2%) Pseudomonas peritonitis. The two patients who developed EPS despite under 18 mo PD had one peritonitis episode each, caused by coagulase negative Staphylococci in both cases.
Thirty patients (65%) had used high-strength dextrose (3.86%) and 45 (98%) had used Extraneal. No patients were treated solely with “bio-compatible” dialysate. Various brands of dextrose-based dialysate were used by the EPS cases. The last total creatinine clearance result measured within 6 mo of stopping PD for EPS cases and the Scottish PD population is shown in Table 5. Compared with the remainder of the Scottish PD population, a significantly larger proportion of EPS cases had stopped PD because of ultrafiltration failure or inadequate dialysis (Table 6).
Table 5.
Total creatinine clearance (renal and PD clearance combined) for EPS cases within 6 mo before stopping PD compared to Scottish PD patients’ achieved clearance from Scottish Renal Registry data 2000-2007
| Total Clearance (L/wk) | EPS Cases (N) | EPS Cases (%) | PD Population 2000–2007 (%) |
|---|---|---|---|
| <50 | 5 | 10.9 | 5.7 |
| >50-60 | 12 | 26.1 | 11.0 |
| >60-70 | 14 | 30.4 | 16.0 |
| >70 | 14 | 30.4 | 67.0 |
| Unknown | 1 | 2.2 | 0.0 |
| Total | 46 | 100.0 | 100.0 |
EPS, encapsulating peritoneal sclerosis; PD, peritoneal dialysis.
Table 6.
Reasons for stopping PD in the EPS cases and the Scottish PD population from Scottish Renal Registry data 2000-2007
| Reason for Stopping PD | EPS Cases (N) | EPS Cases (%) | PD Population 2000-2007 (%) | P value |
|---|---|---|---|---|
| Peritonitis | 15 | 32.6 | 24.3 | 0.2 |
| Ultrafiltration Failure | 6 | 13.0 | 2.3 | <0.001 |
| Inadequate Dialysis | 9 | 19.6 | 11.7 | 0.03 |
| Failed Access | — | — | 5.3 | — |
| High Intra—abdominal Pressure | — | — | 4.5 | — |
| Transplant | 7 | 15.2 | 20.1 | 0.4 |
| Patient Choice | 1 | 2.2 | 7.5 | 0.2 |
| Death | — | — | 24.3 | <0.001 |
| EPS | 8 | 17.4 | — | — |
| Total | 46.0 | 100.0 | 100.0 |
EPS, encapsulating peritoneal sclerosis; PD, peritoneal dialysis.
Thirty-one cases (67%) were prescribed β-blockers. We have ACE-inhibitor and angiotensin receptor blocker (ARB) prescription details for two units: 11 of 18 (61%) were not prescribed either drug while on PD, 4 (22%) were prescribed an ACE or ARB for the duration of PD and 3 were prescribed one or other for <50% of PD. We had access to complete radiology records for 34 cases; 6 (18%) had an MRI scan before EPS diagnosis.
Treatment
Table 7 details the drugs prescribed to treat the EPS cases. Only three patients had elective surgical intervention. It was beyond the scope of this retrospective study to assess response to treatment, particularly with respect to mortality.
Table 7.
Immunosuppressive drug therapy prescribed to EPS cases
| Treatment/Transplant Status | No. of Cases |
|---|---|
| Tamoxifen only | 6 |
| Sirolimus | 3a |
| Prednisolone + Tamoxifen | 4 |
| Tamoxifen + Azathioprine | 1 |
| Prednisolone + Azathioprine | 1 |
| Functioning transplant (Tamoxifen added) | 6 |
| Transplanted <4 months post-diagnosis | 4 |
| Total treated with Tamoxifen | 13 |
| Total Treated with immunosuppression | 24 |
EPS, encapsulating peritoneal sclerosis.
For one of the three treated with Sirolimus it was commenced as part of posttransplant immunosuppressive therapy.
Survival
By the study end, on December 31, 2007, 26 of the patients with EPS (57%) had died. The mortality rate was 42% one year after diagnosis. The median survival from diagnosis was 180 d (range 1 to 1075, IQR 61–408 d).
Discussion
Incidence and Cumulative Risk of EPS
The aim of this study was to report the incidence and establish the risk of EPS for patients starting PD in Scotland. Our data support previous reports, with 98% of our cases (45 of 46) developing EPS either on PD or within 2 yr of stopping PD (3–7). More of our cohort may still develop EPS; therefore, we report the minimum risk of developing EPS when treated with PD in Scotland.
Previous studies suggest that PD duration should not exceed 5 yr, to try to avoid EPS (6,7). However, by 5 yr of PD, 22 of 46 (48%) of our cases had developed EPS. Our figures suggest that if PD is continued beyond 4 yr, at least 8.1% will develop EPS. Previous studies reported rates at 4 yr PD exposure of 5% (Australia) and <1% (Japan) (3,4). We do not know whether the rates in our study represent an increase in the incidence and/or clinical awareness of EPS, or if it reflects differences in study design. This makes direct comparison with other studies difficult. To allow a crude comparison of the EPS rate, we used the same methodology as in previous studies (Table 2), which suggests the overall rate in Scotland is comparable to recent UK and Japanese studies (3,7). Given the higher incidence of EPS with shorter PD duration in our and Rigby's series from Australia compared with Japanese studies, future prospective studies should investigate the differences between our countries’ practice: namely population characteristics, PD prescription/technique and peritonitis details.
Patients should be informed of risks associated with treatments. Two questions need to be answered: 1) Should we continue PD for patients established on treatment (i.e., what is the risk of EPS if a patient continues on PD treatment having survived on PD for a given time period)? and 2) should we start a patient on PD given the risk of EPS (i.e., what is the overall risk of developing EPS for a patient before starting PD)?
With respect to the first question, our incidence data allows more accurate quantification of the risk of developing EPS for patients already on PD (Table 1). After 1 yr of PD, the EPS risk is almost 0, but after 4 yr it is at least one in 12. The risk must be interpreted in context; for a patient awaiting cadaveric transplantation after several years of PD, the risk of EPS may be considered to be too great to remain on PD, whereas the same risk may be acceptable for an elderly patient with no option of transplantation.
The second question is more difficult to answer. When a patient starts PD, it is not known how long he or she will continue PD, or how long the patient will survive. Although the risk of EPS is significant beyond 4 yr, the chance of a patient remaining on PD for 4 yr is small, and so the overall risk of EPS is small. The cumulative risk calculates the percentage of patients who have developed EPS by year of follow-up after starting PD, regardless of whether they remained on PD or not (Figure 1). This allows quantification of the predicted risk of developing EPS.
Diagnosis
The main presenting symptoms were abdominal pain, vomiting and/or abdominal distension. In the early case series (1980 to1994), 92% of patients had bowel obstruction at some point (4). Only 26% of our cases had evidence of bowel obstruction, which is comparable with Summers’ study (22%) (7). These data suggest we are diagnosing EPS earlier than in historical studies.
There appear to be two presentations of EPS. An “acute” presentation following severe peritonitis, with rapid development of clinical features (observed in 24%), would correspond with Japanese data where 25% of cases appeared to be triggered by peritonitis (3). Other cases followed a “subacute” course, with grumbling symptoms. We believe EPS develops as a result of peritoneal irritation, which may come in a variety or combination of forms. If the insult is intense, the fibrotic process may be more aggressive and the clinical course more acute. If the insult is less intense, but recurrent or persistent, the fibrosis and clinical course may be more insidious.
Risk Factors
Previous studies suggest that PD peritonitis may predispose to EPS, particularly if caused by Staphylococcus aureus, fungi and/or Pseudomonas (6,30). The peritonitis rates and causative organisms in our EPS cases were comparable to concurrent rates in the Scottish PD population.
All except one case had used Extraneal dialysate, and the majority required treatment with high-strength dextrose dialysis fluid (30 of 46). It is difficult to untangle whether this reflects ultrafiltration failure in the early stages of EPS, or whether these fluids somehow promote EPS. Unfortunately, consecutive peritoneal equilibration test results were not available to help determine the relationship of ultrafiltration failure to use of these products. Despite speculation that Extraneal could contribute to the pathogenesis of EPS, the data published are inconclusive (31,32).
Although initial reports implicated β-blockers in the pathogenesis of EPS, recent data, including our own, do not support this theory in relation to β-blockers currently used (4,33). ACE-inhibitor and/or ARB drugs may delay peritoneal fibrosis but some of our EPS cases were treated with these drugs (34,35). Gadolinium contrast is linked to the development of nephrogenic systemic fibrosis, but as only six cases had an MRI scan, it is unlikely that gadolinium causes EPS (36,37).
Treatment
There are no randomized, controlled trials to guide EPS treatment. Case-series suggest that immunosuppression (prednisolone, azathioprine, mycophenolate mofetil) posttransplant or as specific therapy can help (3,7,16–21). Interest has focused on tamoxifen, which has fibrinolytic properties, and encouraging results have been observed (22–26). In Scotland, treatment was inconsistent but tamoxifen was prescribed most often. There is evidence that calcineurin inhibitors may promote peritoneal fibrosis, which may help explain why patients can develop EPS despite immunosuppression following renal transplantation (38).
Surgery (enterolysis) is associated with variable outcomes and high mortality rates (3,9–15). However, with experienced surgeons, Japan reports impressive results and improved survival using corticosteroids and surgery (11–13,39). The rarity of EPS in Europe makes it difficult for surgeons to develop sufficient expertise unless surgical treatment is centralized to a specialist center.
Survival
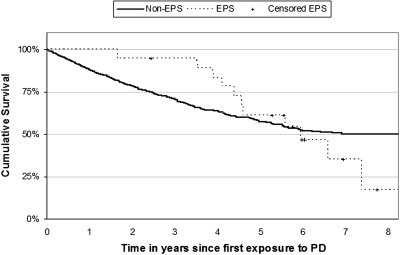
Patient survival on PD is poor even without EPS; the mortality rate for our cohort was 34% by the study end (Figure 3). Patients usually continue PD for several years before EPS develops. Therefore patients who develop EPS are likely to have been among the fitter PD patients and may be expected to live longer than the average PD patient had they not developed EPS. The survival plot illustrates this whereby the survival lines initially diverge and the EPS patients have a better survival rate until 4 yr when the survival curves converge, then cross. The key observation is the high mortality of 42% at 1 yr after EPS diagnosis.
Figure 3.
Kaplan-Meier survival plots of patients from start of PD for EPS cases (n = 19) and unaffected cohort (n = 1219).
In our case series, EPS was listed as a contributing cause of death in only 14 of 25 cases (56%) despite definite premorbid diagnosis. It will be difficult to monitor EPS if it is under-recorded in official statistics.
Limitations
Our study was retrospective until June 2006 and prospective from July 1, 2006, to December 31, 2007. If cases have been missed, the incidence we report is an underestimate. To minimize this we performed a search of the national diagnostic records system but found no additional cases.
Conclusion
Large, prospective, multinational studies are required to address the clinical problems created by a possible rising incidence of EPS. Using our data as a guide, the risk of EPS for patients treated with PD in Scotland is near zero after 1 yr of PD, but the minimum risk after 4 yr of PD is one in 12. However, the cumulative risk is modest at 2.6% by 5 yr, reflecting the reality that few patients continue PD beyond 4 yr. The incidence reported in this study may be used to inform patients of the minimum risk of developing EPS after starting PD.
Disclosures
None.
Acknowledgments
We would like to thank the nurses, doctors and administrative staff of all the individual renal centers for their assistance in identifying the cases of EPS and providing their clinical records. Thanks also to the Scottish Renal Registry staff.
These data have been presented orally at the UK Renal Association meeting in Glasgow, UK, May 13 through 16, 2008 and as a poster at the American Society of Nephrology meeting in Philadelphia, PA, November 4 through 9, 2008.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Gandhi VC, Humayun HM, Ing TS, Daugirdas JT, Jablokow VR, Iwatsuki S, Geis WP, Hano JE: Sclerotic thickening of the peritoneal membrane in maintenance peritoneal dialysis patients. Arch Int Med 140: 1201–1203, 1980 [PubMed] [Google Scholar]
- 2.Kawanishi H: Long-Term Peritoneal Dialysis Study Group: Encapsulating peritoneal sclerosis in Japan: Prospective multicentre controlled study. Perit Dial Int 21(S3): S67–S71, 2001 [PubMed] [Google Scholar]
- 3.Kawanishi H, Kawaguchi Y, Fukui H, Hara S, Imada A, Kubo H, Kin M, Nakamoto M, Ohira S, Shoji T: Encapsulating peritoneal sclerosis in Japan: A prospective. Controlled, Multicentre Study Am J Kidney Dis 44(4): 729–737, 2004 [PubMed] [Google Scholar]
- 4.Rigby RJ, Hawley CM: Sclerosing peritonitis: The experience in Australia. Nephrol Dial Transplant 13: 154–159, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Lee HY, Kim BS, Choi HY, Park HC, Kang SW, Choi KH, Ha SK, Han DS: Sclerosing encapsulating peritonitis as a complication of long-term continuous ambulatory peritoneal dialysis in Korea. Nephrol. (Carlton) 8: S33–S39, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Nomoto Y, Kawaguchi Y, Kubo H, Hirano H, Sakai S, Kurokawa K: Sclerosing encapsulating peritonitis in patients undergoing continuous ambulatory peritoneal dialysis: A report of the Japanese Sclerosing Encapsulating Peritonitis Study Group. Am J Kidney Dis 20(3): 420–427, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Summers AM, Clancy MJ, Syed F, Harwood N, Brenchley PE, Augustine T, Riad H, Hutchison AJ, Taylor P, Pearson R, Gokal R: Single-centre experience of encapsulating peritoneal sclerosis in patients on peritoneal dialysis for end stage renal failure. Kidney Int 68: 2381–2388, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Fieren MW, Betjes MG, Korte MR, Boer WH: Posttransplant encapsulating peritoneal sclerosis: A worrying new trend? Perit Dial Int 27(6): 619–624, 2007 [PubMed] [Google Scholar]
- 9.Perks FJ, Murchison JT, Gibson P, Jackson SHL: Imaging findings in sclerosing encapsulating peritonitis. J R Coll Physicians Edinb 34: 116–119, 2004 [Google Scholar]
- 10.Kawaguchi Y, Kawanishi H, Mujais S, Topley N, Oreopoulos DG: Encapsulating peritoneal sclerosis: Definition. Etiology, Diagnosis and Treatment Perit Dial Int 20: S43–S55, 2000 [PubMed] [Google Scholar]
- 11.Kawanishi H, Moriishi M, Ide K, Dohi K: Recommendation of the surgical option for treatment of encapsulating peritoneal sclerosis. Perit Dial Int 28(S3): S205–S210, 2008 [PubMed] [Google Scholar]
- 12.Kawanishi H: Encapsulating peritoneal sclerosis (review). Nephrology 10: 249–255, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Kawanishi Hideki, Moriishi Misaki, Tsuchiya Shinichiro: Experience of 100 surgical cases of encapsulating peritoneal sclerosis: Investigation of recurrent cases after surgery. Adv Perit Dial 22: 60–64, 2006 [PubMed] [Google Scholar]
- 14.Murakami R, Horigome I, Taguma Y, Sato T, Amada N, Orii T, Kikuchi H, Sasaki S, Ohashi Y: Surgical treatment of encapsulating peritoneal sclerosis in patients with continuous ambulatory peritoneal dialysis—Efficacy of decorticating all sclerosing peritoneum. Jpn J Gastroenterol Surg 38(5): 533–538, 2005 [Google Scholar]
- 15.Célicout B, Levard H, Hay J, Msika S, Fingerhut A, Pelissier E: Sclerosing encapsulating peritonitis: Early and late results of surgical management in 32 cases. French Associations for Surgical Research. Digestive Surgery 15(6): 697–702, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Lafrance JP, Létourneau I, Ouimet D, Bonnardeaux A, Leblanc M, Mathieu N, Pichette V: Successful treatment of encapsulating peritoneal sclerosis with immunosuppressive therapy. Am J Kidney Dis 51(2): 7–10, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Mori Y, Matsuo S, Sutoh H, Toriyama T, Kawahara H, Hotta N: A case of a dialysis patient with sclerosing peritonitis successfully treated with corticosteroid therapy alone. Am J Kidney Dis 30(2): 275–278, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Martins LS, Rodrigues AS, Cabrita AN, Guimaraes S: Sclerosing encapsulating peritonitis: A case successfully treated with immunosuppression. Perit Dial Int 19(5): 478–481, 1999 [PubMed] [Google Scholar]
- 19.Wong CF, Beshir S, Khalil A, Pai P, Ahmad R: Successful treatment of encapsulating peritoneal sclerosis with azathioprine and prednisolone. Perit Dial Int 25(3): 285–287, 2005 [PubMed] [Google Scholar]
- 20.Junor BJR, McMillan MA: Immunosuppression in sclerosing peritonitis. Adv Perit Dial 9: 187–189, 1993 [PubMed] [Google Scholar]
- 21.Bhandari S: Recovery of gastrointestinal function after renal transplantation in patients with sclerosing peritonitis secondary to continuous ambulatory peritoneal dialysis. Am J Kidney Dis 27(4): 604, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Moustafellos P, Hadjianastassiou V, Roy D, Velzeboer NE, Maniakyn N, Vaidya A, Friend PJ: Tamoxifen therapy in encapsulating sclerosing peritonitis in patients after kidney transplantation. Transplant Proc 38(9): 2913–2914, 2006 [DOI] [PubMed] [Google Scholar]
- 23.del Peso G, Bajo MA, Gil F, Aguilera A, Ros S, Costero O, Castro MJ, Selgas R: Clinical experience with tamoxifen in peritoneal fibrosing syndromes. Adv Perit Dial 19: 32–35, 2003 [PubMed] [Google Scholar]
- 24.Gupta S, Woodrow G: Successful treatment of fulminant encapsulating peritoneal sclerosis following fungal peritonitis with tamoxifen. Clin Nephrol 68(2): 125–129, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Wong CF: Clinical experience with tamoxifen in encapsulating peritoneal sclerosis. Perit Dial Int 26(2): 183–184, 2006 [PubMed] [Google Scholar]
- 26.Allaria PM, Giangrande A, Gandini E, Pisoni IB: Continuous ambulatory peritoneal dialysis and sclerosing encapsulating peritonitis: Tamoxifen as a new therapeutic agent? J of Nephrol 12(6): 395–397 1999 [PubMed] [Google Scholar]
- 27.Tarzi RM, Lim A, Moser S, Ahmad S, George A, Balasubramaniam G, Clutterbuck EJ, Gedroyc W, Brown EA: Assessing the validity of an abdominal CT scoring system in the diagnosis of encapsulating peritoneal sclerosis. Clin J Am Soc Nephrol 3(6): 1702–1710, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yokoyama K, Yoshida H, Matsuo N, Maruyama Y, Kawamura Y, Yamamoto R, Hanaoka K, Ikeda M, Yamamoto H, Nakayama M, Kawaguchi Y, Hosoya T: Serum beta2 microglobulin (beta2MG) level is a potential predictor for encapsulating peritoneal sclerosis (EPS) in peritoneal dialysis patients. Clin Nephrol 69(2): 121–126, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Rothman KJ, Greenland S: Modern Epidemiology. Philadelphia, Lippencott Williams and Wilkins, 1998
- 30.Slingeneyer A: Preliminary report on a cooperative international study on sclerosing encapsulating peritonitis. Nephrol Dial Transplant 3: 66–69, 1988 [DOI] [PubMed] [Google Scholar]
- 31.Moriishi M, Kawanishi H, Tsuchiya S: Impact on peritoneal membrane of use of icodextrin-based dialysis solution in peritoneal dialysis patients. Adv Perit Dial 22: 24–28, 2006 [PubMed] [Google Scholar]
- 32.Moriishi M, Kawanishi H: Icodextrin and intraperitoneal inflammation. Perit Dial Int 28(3): S96–S100, 2008 [PubMed] [Google Scholar]
- 33.Marigold JH, Pounder RE, Pemberton J, Thomson RP: Propranolol, oxprenolol, and sclerosing peritonitis. BMJ 284: 870, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolesnyk I, Dekker FW, Noordzij M, le Cessie S, Struijk DG, Krediet RT: Impact of ACE inhibitors and AII receptor blockers on peritoneal membrane transport characteristics in long-term peritoneal dialysis patients. Perit Dial Int 27(4): 446–453, 2007 [PubMed] [Google Scholar]
- 35.Bozkurt D, Cetin P, Sipahi S, Hur E, Nar H, Ertilav M, Sezak M, Duman S: The effects of renin-angiotensin system inhibition on regression of encapsulating peritoneal sclerosis. Perit Dial Int 28 Suppl 5: S38–S42, 2008 [PubMed] [Google Scholar]
- 36.Collidge TA, Thomson PC, Mark PB, Traynor JP, Jardine AG, Morris ST, Simpson K, Roditi GH: Gadolinium-enhanced MR imaging and nephrogenic systemic fibrosis: Retrospective study of a renal replacement therapy cohort. Radiology 245(1): 168–175, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Edward M, Quinn JA, Mukherjee S, Jensen MB, Jardine AG, Mark PB, Burden AD: Gadodiamide contrast agent ‘activates’ fibroblasts: A possible cause of nephrogenic systemic fibrosis. J Pathol 214(5): 584–593, 2008 [DOI] [PubMed] [Google Scholar]
- 38.van Westrhenen R, Aten J, Hajji N, de Boer OJ, Kunne C, de Waart DR, Krediet RT: Cyclosporin A induces peritoneal fibrosis and angiogenesis during chronic peritoneal exposure to a glucose-based, lactate-buffered dialysis solution in the rat. Blood Purif 25(5–6): 466–472, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Maruyama Y, Nakayama M: Encapsulating peritoneal sclerosis in Japan. Perit Dial Int 28(S3): S201–S204, 2008 [PubMed] [Google Scholar]