Abstract
In this case report we describe the blood metabolic profile (“metabolomics”) by nuclear magnetic resonance (NMR) spectroscopy and principle component analysis (PCA) from a patient who underwent two consecutive liver transplantations. The first graft from a living-related donor failed and was followed by a second successful transplant from a deceased donor. Using quantitative high-resolution 1H-NMR spectroscopy, 48 endogenous metabolites were analyzed in whole blood samples at baseline and different time points after each transplantation. From 48 analyzed metabolites, six metabolites were identified by PCA as metabolic markers consistent with a non-functional liver after first transplantation. Importantly, this distinctive metabolic profile was present as early as two hours after first transplant surgery when no other variable or conventional laboratory tests indicated poor graft function. This article reports the potential usefulness of quantitative 1H-NMR based metabolomics to diagnose early graft dysfunction in liver transplantation.
Keywords: Primary graft dysfunction, Living-related liver transplantation, Metabolomics, 1H-NMR spectroscopy
Failure of donor graft function remains a significant cause of morbidity and mortality (1). To date, the distinction between graft injury and graft dysfunction depends on the observation of clinical and laboratory findings and is often difficult. Liver injury is usually defined by elevation of serum aminotransferase levels, however it is not a good surrogate for impaired liver function. Total or conjugated bilirubin, albumin, and protein synthesis of the coagulation pathways are often used to assess liver function. However, a lack of reliable biomarkers remains a problem in the hyperacute setting.
Simultaneous quantitative analysis on numerous blood biochemicals can be performed using nuclear magnetic resonance (NMR) spectroscopy (2–4). Combined with advanced statistical approaches, 1H-NMR spectroscopy measures metabolic profiles that characterize changes of specific organ functions. Referred to as “metabolomics” or “metabonomics” (5, 6), this analytic technique has identified a number of novel metabolic biomarkers for the early detection of renal donor graft dysfunction (6–9), but have yet to be systematically tested in hepatic injury that results in graft failure (10, 11).
In this case report, NMR analysis was applied to identify metabolic markers of graft dysfunction in a patient who suffered graft failure after the first orthotopic liver transplantation (OLTx) from a living-related donor followed by a second successful OLTx from a deceased donor. Hence, the patient served as his own control for comparing metabolic profiles before and after transplantation. This patient was part of a larger ongoing study which was approved by the Institutional Review Board (Human Research of the University of Colorado).
A 67-year-old caucasian male patient with hepatitis B viral infection complicated by hepatocellular carcinoma and a Model of End-Stage Liver Disease (MELD) score of 22 received a right lobe, living liver transplant from his daughter. A preoperative blood sample (T1-pre) was obtained as required per study protocol. One milliliter of blood was sent for NMR analysis. After reperfusion of the graft, good bile flow was noted in the surgical field. At the conclusion of surgery, excellent hemostasis was obtained and the donor graft looked normal, without swelling. Palpation and Doppler examination demonstrated good flow in the hepatic artery and the portal vein. Intraoperative blood product usage included four units of fresh frozen plasma and six units of red blood cells. Coagulation profiles and platelet count were adequate upon admission to the intensive care unit (international normalized ratio 1.4, platelets 127×109/L). Blood samples for NMR analysis were taken two hours after admission to the intensive care unit (ICU; T1-2hr), followed by 24 hr (T1-24hr), and 48 hr (T1-48hr) after transplantation. Routine laboratories values for the same time points of the first transplantation are presented in Table 1.
TABLE 1.
Conventional clinical laboratory results for the first live donor liver transplantation (failed) and second deceased donor liver transplantation (succeeded) at the different time point prior and after surgery
| Orthotopic liver transplantation 1 |
Orthotopic liver transplantation 2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| PreTx | 2 hours postTx | 24 hours postTx | 48 hours postTx | PreTx | 2 hours postTx | 24 hours postTx | 48 hours postTx | |
| Albumin (g/dL) | 3.2L | 3.2L | 3.1L | 3.5 | 4.6 | 3.4 | 3.0L | 3.5 |
| ALP (U/L) | 71 | 45 | 61 | 97 | 57 | 100 | 53 | 67 |
| ALT (U/L) | 11 | 120 | 4300H | 3140H | 128H | 2225H | 1749H | 860H |
| AST (U/L) | 28 | 157 | 3360H | 2830H | 90H | 3850H | 2149H | 788H |
| Bilirubin (mg/dL) | 1.4 | 3.7 | 5.8 | 7.6 | 26.2 | 22.1 | 23.4 | 18.2 |
ALP, alkaline phosphatase; ALT, alanine transferase; ASP, aspartate transferase; H, high level; L, low level; Tx, transplantation.
Routine ultrasound evaluation performed approximately three hours after ICU admission failed to show portal venous flow. The patient was emergently returned to the operating room for re-exploration. The portal vein was not thrombosed but had minimal flow most likely due to a competing splenorenal shunt. The portal venous anastomosis was redone to access the recipient’s portal vein just distal to the confluence of the splenic and superior mesenteric veins. Doppler examination confirmed the return of brisk portal venous flow. However, two days postoperatively, hepatic artery and portal vein thrombosis was diagnosed by ultrasound examination and ultrasound appearance of the liver was consistent with ischemia and infarction. The patient was listed as a status 1 and received a graft from a donor after cardiac death (DCD donor), eight days following the first transplant. Blood samples were taken again at the same specified time points as for the first surgery (T2-pre, T2-2hr, T2-24hr, T2-48hr). The second transplant was successful and the patient was discharged from the hospital approximately three weeks after the second transplant.
Routine laboratory values after admission to the ICU did not demonstrate any unexpected major abnormalities after the first unsuccessful liver transplant (Table 1). Aminotransferase levels increased only 24 hr after the first transplantation when clinical diagnosis of failed graft had already been made based upon ultrasound findings and an increasing need for vasoactive drugs to support blood pressure. Thus, we were interested whether NMR based metabolomics of the blood may provide additional information for prediction of graft dysfunction at the two-hour sampling point.
To identify and compare putative metabolic biomarkers of graft dysfunction in this patient, blood samples from four healthy male subjects served as a baseline control.
After dual chloroform/methanol extraction (2, 9), both water-soluble and lipid-containing blood extracts were analyzed by 1H-NMR spectroscopy with a total run time of 10 min per sample. Seventy-five endogenous metabolites were identified from each of the blood samples (initial two-dimensional structural NMR was applied to confirm metabolite assignment from the NMR metabolic data base), with 47 metabolites being quantifiable (the lower limit of quantification is 10 nmol/mL). Uric acid was metabolite 48, which was quantified by enzyme-linked immunosorbent assay (9). The NMR reproducibility for metabolite concentrations was 93% for high-abundant and over 88% for low-abundant metabolites.
The two-step principle component analysis (PCA) was applied to the established metabolic profiles in order to: i) cluster the samples among first and second transplant versus healthy controls (scores ti) and ii) identify markers responsible for this group clustering (plots pi), followed by Z-test for metabolite fold changes (12, 13).
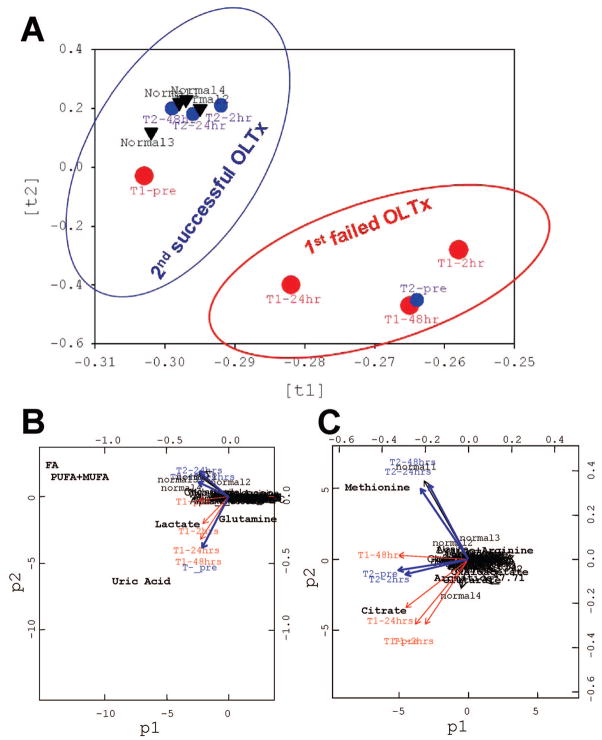
The PCA scores showed that the first failed transplant did not cluster with our healthy controls (Fig. 1A). At the two-hour time point, our analysis demonstrated distinct metabolic abnormalities after the first OLTx, which were not reflected in conventional laboratory values. By contrast, NMR analysis at the same time after the second transplant showed an entirely different profile. The profile after the second OLTx was similar to our healthy control group at all time points (Fig. 1A).
FIGURE 1.
Principle component analysis (PCA): (A) PCA scores (ti) on global metabolic pattern to visualize group clustering between the first failed and second successful OLTx compared to the normal blood profile; (B) PCA plots (pi) on absolute metabolite concentrations and (C) on normalized metabolite concentrations to distinguish single putative biomarkers responsible for cloistering pattern on the loading plot in (A). In the first step, the PCA scores (ti,) described the variation in the sample direction, i.e. similarity/dissimilarity between samples. This step allowed for pattern recognition (normal/abnormal group assignment in A). Each point (in circles or triangles) represent a metabolic profile from each samples. In the next step, the PCA loading plots (pi) described the variation in the variable direction, i.e. similarity/dissimilarity between variables. This step explained the patterns in previous scores (ti) and identified metabolites which were responsible for the group clustering (B, C). The metabolites condensed in the center do not contribute to group clustering in (A). The “outliner” metabolites differ among the group. All mathematical models were built with R package (2.00). First OLTx (graft failure, big red circles) time points: T1-pretransplant; T1-2hr after first transplantation; T1-24hr; and T1-48-hr; and second OLTx (functional graft, blue small circles) time points: T2-pre; T2-2hr after second transplantation; T2-24hr; and T2-48hr. Four blood profiles from healthy volunteers (normal 1, 2, 3, and 4, black triangles) are clustering with the data points from the second transplant. All data are based on PCA of blood metabolic profiles.
The PCA plots on NMR metabolic profiles demonstrated that the absolute concentrations of uric acid, lactate, total fatty acids, as well as glutamine differed among two transplantations and contributed to the group clustering (Fig. 1B). In addition, the normalized concentrations of low-range metabolites methionine and citrate were also identified as possible metabolic markers of hepatic dysfunction.
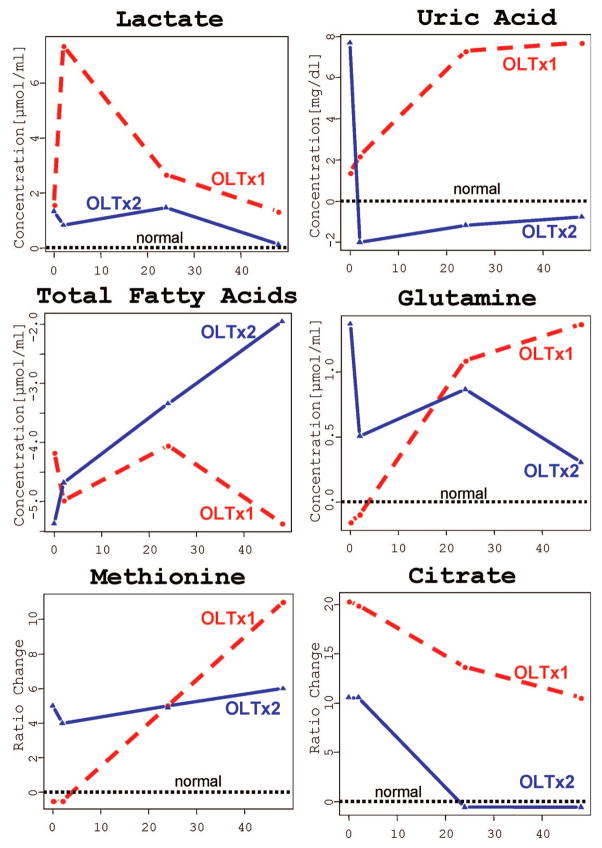
Finally, Figure 2 demonstrates metabolic trajectories for the concentration fold-changes of identified metabolic markers at the different time points after transplantations. The absolute (lactate, uric acid, glutamine, total fatty acids) and normalized (methionine and citrate) concentration profiles of putative metabolic markers as compared to the normal blood range (set to 0) showed distinct quantitative differences between the first and second transplant as early as at the two-hour time point. The increased concentrations of lactate (8.59 μmol/mL first OLT versus 2.06 μmol/mL second OLT), uric acid (5.71 vs. 1.53 mg/dL) and citrate (0.47 vs. 0.26 μmol/mL) demonstrated severely impaired metabolic graft function at a time point when liver enzymes were noncontributory to clinical decision making. The metabolic trajectories for glutamine, total fatty acids and methionine were indicative at 24 and 48 hr after transplantation.
FIGURE 2.
Metabolic trajectories for fold-changes of identified biomarkers (lactate, uric acid, fatty acids, glutamine, methionine and citrate) in the time course of the first OLTx1 (failed live donor graft, red circles and red dash lines) and second OLTx2 (functional deceased donor graft, blue triangles and blue solid lines) liver transplantations. Normal levels are referred to 0 (“normal”, black dash lines). Z-test was performed to validate identified markers and find significant fold-changes in their concentrations between patient and healthy controls. Due to a large concentration range of endogenous metabolites in the blood detected by NMR (from 10 μM to 10 mM and above), the absolute concentrations of high-expressed metabolites (lactate, uric acid, fatty acids, and glutamine) were used for the statistical analysis. For low-abounded metabolites (citrate and methionine), normalized concentrations (as a percentage of healthy subject concentrations [change = ([patient − normal]/normal)]) were used.
The end product of glycolysis, lactate, is increased in ischemic conditions as seen in our patient after first failed transplantation. The end product of xanthine pathway, uric acid, accumulates under ischemia/reperfusion conditions (9), and was increased in our patient following the first transplant. Being the major organ for fatty acid metabolism and synthesis, liver graft failure results in lipid disturbance and a low circulation concentrations of fatty acids similar to thats observed in our patient. The liver is also the primary site of amino acid catabolism: an increase in circulating amino acids (e.g., tyrosine, glutamine, leucine) is reflective of hepatocyte injury and death (14, 15). In our study, blood glutamine and methionine, two of the key compounds in liver nitrogen metabolism (11, 15), as well as citrate were highly increased in the failed graft.
Currently only few tests are capable of rapidly assessing the function of a donor graft in the immediate postoperative period. Conventional laboratory tests are limited by relative non-specificity and they are not time sensitive. These tests generally tend to measure cell membrane integrity and this information is extrapolated as a measure of liver integrity and thus function. Tests that measure hepatic function such as indocyanine green (ICG) (16) and lidocaine metabolism (15, 17) are influenced by multiple factors and results are inconsistent. Blood metabolomics is a functional test that is able to monitor several metabolic pathways simultaneously (6). It does not rely on blood flow and is independent of drug metabolism, both shortcomings of the ICG and lidocaine test. The NMR assay is also a high-throughput method: the total time for multiple sample preparation, NMR run and NMR analysis can be reduced to less than two hours.
In summary, we demonstrated distinctively different metabolic profiles from a failed and well functioning graft in the same patient. This metabolic clustering pattern and the changes in absolute concentrations of distinguished metabolites were evident as early as two hours after transplant surgery when no prediction could be made from conventionally employed clinical parameters. Although our results present a single case and cannot be extrapolated directly to the heterogeneous population of liver recipients without additional validation, the data suggest that 1H-NMR analysis can be a valuable additional tool in clinical decision making.
References
- 1.Delmonico FL, Sheehy E, Marks WH, et al. Organ donation and utilization in the United States, 2004. Am J Transplant. 2005;5:862. doi: 10.1111/j.1600-6135.2005.00832.x. [DOI] [PubMed] [Google Scholar]
- 2.Serkova NJ, Jackman M, Brown JL, et al. Metabolic profiling of livers and blood from obese Zucker rats. J Hepatol. 2006;44:956. doi: 10.1016/j.jhep.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Kleno TG, Kier B, Baunsgaard D, Sidelmann UG. Combination of “omics” data to investigate the mechanisms of hydrazine-induced hepatotoxicity in rats and to indentify potential biomarkers. Biomarkers. 2004;9:116. doi: 10.1080/13547500410001728408. [DOI] [PubMed] [Google Scholar]
- 4.Mortishire-Smith RJ, Slikes GL, Lawrence JW, et al. Use of metabonomics to identify impaired fatty acid metabolism as the mechanism of a drug-induced toxicity. Chem Res Toxicol. 2004;17:165. doi: 10.1021/tx034123j. [DOI] [PubMed] [Google Scholar]
- 5.Nicholson JK, Lindon JC, Holmes E. “Metabonomics”: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica. 1999;29:1181. doi: 10.1080/004982599238047. [DOI] [PubMed] [Google Scholar]
- 6.Wishart DS. Metabolomics: The principles and potential applications to transplantation. Am J Transplant. 2005;5:2814. doi: 10.1111/j.1600-6143.2005.01119.x. [DOI] [PubMed] [Google Scholar]
- 7.Hauet T, Baumert H, Gibelin H, et al. Noninvasive monitoring of citrate, acetate, lactate, and renal medullary osmolyte excretion in urine as biomarkers of exposure to ischemic reperfusion injury. Cryobiology. 2000;41:280. doi: 10.1006/cryo.2000.2291. [DOI] [PubMed] [Google Scholar]
- 8.Fuller TF, Serkova N, Niemann CU, Freise CE. N-acetylcysteine protects rat kidney graft against ischemia reperfusion injury. J Urol. 2004;1711:1296. doi: 10.1097/01.ju.0000103928.64939.6a. [DOI] [PubMed] [Google Scholar]
- 9.Serkova NJ, Fuller TF, Klawitter J, et al. 1H-NMR based metabolic signatures of mild and severe ischemia/reperfusion injury in rat kidney transplants. Kidney Int. 2005;67:1142. doi: 10.1111/j.1523-1755.2005.00181.x. [DOI] [PubMed] [Google Scholar]
- 10.Paczkowska A, Toczylowska B, Nyckowski P, et al. High-resolution 1H nuclear magnetic resonance spectroscopy analysis of bile samples obtained from a patient after orthotopic liver transplantation: New perspectives. Transplant Proc. 2003;35:2278. doi: 10.1016/s0041-1345(03)00788-7. [DOI] [PubMed] [Google Scholar]
- 11.Singh HK, Yachha SK, Saxena R, et al. A new dimension of 1H-NMR spectroscopy in assessment of liver graft dysfunction. NMR Biomed. 2003;16:185. doi: 10.1002/nbm.829. [DOI] [PubMed] [Google Scholar]
- 12.Holmes E, Nicholls AW, Lindon JC, et al. Chemometric models for toxicity classification based on NMR spectra of biofluids. Chem Res Toxicol. 2000;13:471. doi: 10.1021/tx990210t. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Vincenti MP, Yokota H. Principal component analysis for predicting transcription-factor binding motifs from array-derived data. BMC Bioinformatics. 2005;6:276. doi: 10.1186/1471-2105-6-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shanbhogue RL, Bistrian BR, Lakshman K, et al. Whole body leucine, phenylalanine, and tyrosine kinetics in end-stage liver disease before and after hepatic transplantation. Metabolism. 1987;36:1047. doi: 10.1016/0026-0495(87)90024-2. [DOI] [PubMed] [Google Scholar]
- 15.Saxena V, Gupta A, Nagana Gowda GA, et al. 1H-NMR spectroscopy for the prediction of therapeutic outcome in patients with fulminant hepatic failure. NMR Biomed. 2006;19:521. doi: 10.1002/nbm.1034. [DOI] [PubMed] [Google Scholar]
- 16.Mandell MS, Wachs M, Niemann CU, Henthorn TK. Elimination of indocyanine green in the perioperative evaluation of donor liver function. Anesth Analg. 2002;95:1182. doi: 10.1097/00000539-200211000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Oellrich M, Amstrong VW. The MEGX test: a tool for the real-time assessment of hepatic function. Ther Drug Monit. 2001;23:81. doi: 10.1097/00007691-200104000-00001. [DOI] [PubMed] [Google Scholar]