Abstract
Background
Because white matter hyperintensities (WMHs) on magnetic resonance imaging (MRI) may be linked to geriatric syndromes involving mobility, cognition, and affect, we postulated that involvement of areas critical to bladder control could influence urinary incontinence (UI).
Methods
One hundred community-dwelling individuals (75–89 years) were recruited into three groups stratified by age and gender reflecting normal and mildly and moderately impaired mobility. Baseline incontinence status and related symptoms were evaluated in 97 individuals using validated instruments (3IQ, Urinary Incontinence Severity Index, Urogenital Distress Inventory, Incontinence Impact Questionnaire). Regional WMH was measured using an MRI brain imaging segmentation pipeline and WM tract–based parcellation atlas.
Results
Sixty-two (64%) of the participants were incontinent, mostly with urgency (37; 60%) and moderate–severe symptoms (36; 58%). Incontinent individuals were more likely to be women with worse scores for depression and mobility. WMH located in right inferior frontal regions predicted UI severity, with no significant relationship with incontinence, incontinence type, bother, or functional impact. As regards WM tracts, WMH within regions normally occupied by the anterior corona radiata predicted severity and degree of bother, cingulate gyrus predicted incontinence and severity, whereas cingulate (hippocampal portion) and superior fronto-occipital fasciculus predicted severity.
Conclusions
Presence of WMH in right inferior frontal regions and selected WM tracts predicts incontinence, incontinence severity, and degree of bother. Our observations support the findings of recent functional MRI studies indicating a critical role for the cingulum in bladder control, while also suggesting potential involvement of other nearby WM tracts such as anterior corona radiata and superior fronto-occipital fasciculus.
Keywords: Leukoaraiosis, White matter hyperintensities, Magnetic resonance imaging, Urinary incontinence, Frailty, Geriatric syndrome
THE introduction of brain magnetic resonance imaging (MRI) into clinical practice brought with it the sensitivity to visualize white matter (WM) structural abnormalities, termed white matter hyperintensities (WMHs). WMHs are common in older persons and have been provisionally linked to WM demyelination, spongiosis, and glial proliferation. Because global WMH has been linked to impairments of mobility, cognition, affect, and continence (1–3), we postulated that focal involvement of selected regions felt to be critical to bladder control could influence voiding and urinary incontinence (UI) in elderly adults (4).
We chose anterior brain regions representing potential overlap between areas of WMH accrual on MRI (5) and WM circuits linked to control of voiding on functional brain imaging (4,6). Areas of interest chosen in this hypothesis-driven manner included frontal regions, as well as seven different WM tracts. To assess continence-related symptoms, four established UI assessment tools were administered to 97 individuals at baseline as part of a 4-year longitudinal study evaluating the impact of WMH and its accrual on gait and mobility performance in 100 community-dwelling older adults. These instruments assess UI status (7), type of UI (7), UI severity (8), degree of bother (9), and impact on function (10).
In frail older adults, UI is viewed as a geriatric syndrome because multiple risk factors contribute, with many unrelated to the genitourinary tract (11). We propose that WMH within critical periventricular and subcortical regions may represent risk factors for UI and related problems, thus providing potential targets for future diagnostic and therapeutic strategies (11,12).
METHODS
Participants
One hundred individuals 75 years or older were recruited from the community for a 4-year prospective study conducted at the University of Connecticut Health Center defining the relationship between WMH accrual and mobility impairments. Participants were recruited using a balanced 3 × 3 matrix, which was stratified by age (75–79, 80–84, and ≥85 years) and mobility performance in terms of Short Physical Performance Battery (SPPB) scores (11–12, 9–10, and <9). Exclusion criteria included medications, systemic conditions (eg, arthritis, peripheral vascular disease), and neurological diseases (eg, neuropathy, myelopathy, Parkinson’s disease, stroke), which could compromise mobility. Additional exclusion factors included cognitive impairment (Mini-Mental State Examination [MMSE] <24), corrected distance vision greater than 20/70, unstable cardiovascular disease (eg, myocardial infarction within 6 months, unstable angina), pulmonary disease requiring oxygen, inability to walk 10 m independently in less than 50 seconds, lower extremity amputation, weight greater than 250 lbs, claustrophobia, presence of a pacemaker or other metallic devices/implants, excessive alcohol intake, and expected life span less than 4 years. After initial screening, participants underwent clinical assessment plus MRI brain imaging. Functional status was evaluated in terms of instrumental activities of daily living (IADLs), SPPB, Center for Epidemiological Studies-Depression scale (CESD), and MMSE. Subsequently, 97 participants completed four validated UI assessment questionnaires. Participants were fully informed, provided consent before enrollment, and were blinded to the results of clinical, mobility, and imaging assessments. The protocol was approved by the institutional review board.
Incontinence Assessment Tools
Four validated UI assessment tools were administered. Incontinence was defined as a positive answer to the question, “During the last 3 months, have you leaked urine (even a small amount)?” (7). Type of UI was evaluated by the 3IQ, a questionnaire that screens for and classifies the type of UI using three items: presence of leakage, circumstances of leakage (urge with inability to reach toilet, with physical activity, neither with urge nor with physical activity), and the predominant type of UI (urge—urge UI, activity—stress UI, both urge and activity—mixed UI, neither urge or activity—other (7)). UI severity was measured using the Urinary Incontinence Severity Index (8) in which leakage is characterized as none, slight, moderate, or severe based on the multiple of the scores for UI frequency (“never” to “every day and/or night”) and amount (“drops or little” or “more”). A short version of the Urogenital Distress Inventory (UDI-6; 9) measured the bother associated with lower urinary tract symptoms and UI using a 4-point scale (0 [not at all] to 3 [greatly]). IIQ-7, the abbreviated version of the Incontinence Impact Questionnaire (10), evaluated UI impact on function (eg, social activities, physical recreation) using a 4-point scale (0 [not at all] to 3 [greatly]); the sum of the scores for all items are then converted to a 100-point scale. To ensure consistency of responses, we compared individuals’ answers as they pertained to UI, UI type, and degree of bother with leakage, obtaining consistent answers 92%–100% of the time among incontinent individuals.
Brain MRI
A 3-Tesla Siemens Allegra (Siemens, Erlangen, Germany) was used to acquire the following MR brain scans: T1-weighted magnetization-prepared rapid gradient echo (MPRAGE), T2-weighted T2 three-dimensional fast-spin echo (T2), and T2-weighted fluid-attenuated inversion recovery (FLAIR). Acquisition parameters and image preprocessing have been as described by Moscufo and colleagues (N. Moscufo, PhD, C.R. Guttmann, MD, D. Meier, PhD, I. Csapo, BS, P.G. Hildenbrand, MD, J.A. Schmidt, BS, L. Wolfson, MD, unpublished data, 2009).
T1-Weighted Probabilistic Brain Atlas (International Consortium on Brain Mapping)
A reference brain atlas (International Consortium on Brain Mapping [ICBM]) was obtained from the Laboratory of NeuroImaging (University of California, Los Angeles; 13). The ICBM provided brain WM, gray matter (GM), and cerebrospinal fluid (CSF) probabilistic maps, which were then used for calibrating the segmentation method.
Brain Tissues Segmentation
MPRAGE and FLAIR images from individual participants were used in a semiautomated segmentation method to classify brain compartments into WM, GM, CSF, and T2 hyperintense WM (WMH). We obtained the final output by combining segmentation maps using 3D-Slicer (14) and Freesurfer (15) applications. To correct for differences in head size, WMH volumes (mL) were normalized by the intracranial cavity (ICC) and expressed as fractions. ICC maps were obtained by using in-house software that identified nonbrain tissues (skull, skin) from the T2 image set of each participant.
Regional WMH Burden
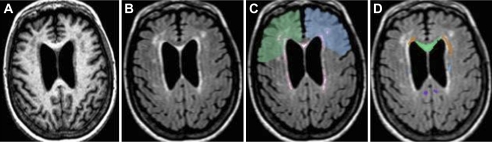
For regional analysis, we used a WM parcellation atlas (WMPA), the ICBM diffusor tensor imaging (DTI) 81 (16). WMPA combines DTI-based WM and anatomical information to provide a functional map of approximately one third (32%) of total brain WM. WMPA was first coaligned to the WMH segmentation map of each participant (13) and then used as a mask to measure WMH burden within selected regions of interest. WMH burdens were calculated by dividing the regional WMH volume (mL) by volume (mL) of the region and expressed as fractions. Frontal regions (Figure 1C) were manually outlined on the ICBM atlas brain to include frontal WM anterior to the precentral gyrus comprising association areas as well as parts of premotor areas. Frontal regions were divided into superior and inferior regions based on the outmost anterior tip of the lateral ventricles in axial view of the ICBM brain. These maps were subsequently warped to align them to each individual brain and used as mask to determine the frontal regional WMH volumes. These volumes were normalized and expressed as fraction of the subject ICC. Minimal or no WMH was observed in the brainstem and cerebellum upon independent review by a neuroradiologist, and thus, subtentorial regions were excluded from further analysis.
Figure 1.
Example of magnetic resonance series and regions of interest (ROIs) used in the study: (A) MPRAGE, (B) FLAIR, and (C) frontal right (blue) and left (green) ROIs overlaid to a FLAIR image on which the areas of white matter (WM) hyperintensity are outlined in red (output of the segmentation method); (D) a FLAIR image overlaid with some of the selected WM tracts–based ROIs, namely, genu of the corpus callosum (green), anterior corona radiata (orange-brown), cingulum/cingulate gyrus (purple), superior fronto-occipital fasciculus (light blue). Note: View is from top of the brain. FLAIR = fluid-attenuated inversion recovery; MPRAGE = magnetization-prepared rapid gradient echo.
To establish a hypothesis-driven approach, focal WMH was measured within 11 brain regions based on published literature. We selected four frontal regions (right superior frontal, right inferior frontal, left superior frontal, and left inferior frontal) based on functional MRI (fMRI) studies demonstrating a failure of orbitofrontal activation in individuals with limited ability to suppress urgency during bladder filling (17). We also included seven brain regions representing the location of relevant WM tracts: anterior corona radiata, inferior fronto-occipital fasciculus, cingulate gyrus, cingulate hippocampal portion, superior fronto-occipital fasciculus, uncinate fasciculus, and genu of the corpus callosum (Table 2). These anterior regions were chosen because they represented anticipated locations of WM tracts that play a potential role in bladder control (18,19) and overlapped with regions at risk for WMH (5). Furthermore, the anterior corona radiata contains both descending prefrontal corticopontine projections and ascending thalamocortical (frontal/prefrontal) projections. These pathways normally exert inhibitory bladder effects (19) and if compromised could contribute to urge UI as suggested for normal pressure hydrocephalus (20).
Table 2.
Regions of Interest (ROIs) Studied and Their Potential Relevance to UI-Related Symptoms
| ROI | Potential Relevance to Incontinence |
| Frontal | Poor bladder control is associated with inadequate activation of orbitofrontal regions (17). |
| Anterior corona radiata | Descending prefrontal corticopontine projections and ascending thalamocortical (frontal/prefrontal) projections may normally exert inhibitory control on micturition (4,19,20). |
| Cingulate gyrus | Cingulate gyrus is involved in monitoring of sensations from the bladder and in suppressing micturition during bladder filling, providing cortical control of voiding responses (4,6,19). |
| Cingulate hippocampal portion | The hippocampal portion of the cingulum may play a role in bladder control by integrating inputs from autonomic and emotional nervous systems with frontal cortical control (19). |
| Genu of corpus callosum | Corpus callosum connects the two hemispheres and the genu (anterior) portion contains frontal pathways that could be part of a network activated during bladder control (17). |
| Inferior fronto-occipital fasciculus | These connections between frontal cortex, peristriate, and temporal lobe may be activated during normal bladder control (6,17). |
| Superior fronto-occipital fasciculus | This tract connects occipital, parietal, and temporal regions to prefrontal and frontal cortex, with a potential role in mediating voluntary control of micturition (6,19). |
| Uncinate fasciculus | Uncinate fasciculus is part of a network with temporal–orbitofrontal connections, with a potential role in bladder control (17,19). |
Statistical Analysis
SAS version 9.1 (SAS Inc, Cary, NC) was used for the statistical analysis. Descriptive statistics are expressed as mean (standard deviation) or frequency (percent). Several UI measures were used as dependent variables: wet versus dry, urge versus other, UI severity, degree of bother, and functional impact. All 97 participants were included in the analysis of wet versus dry and incontinence severity (with dry participants given a severity of “none”). All other analyses included only the 62 incontinent participants. Multivariate logistic regression analysis (both logit and multinomial, depending on outcome variable) was performed to evaluate MRI variables that significantly contributed to prediction of the UI measure (wet vs dry, urge vs other, incontinence severity). Linear regression analysis was used for continuous measures (degree of bother and functional impact). Each model had one MRI variable, as well as age and gender. Finally, for each outcome, any significant MRI variables, SPPB, CESD, age and gender were entered into a stepwise regression. A two-tailed level of α ≤ .05 was the threshold for statistical significance.
RESULTS
Participant Characteristics
At baseline, the cohort was elderly (mean = 82.1 ± 4.1 years, range 75–89 years; Table 1). All participants were non-Hispanic whites. Enrollment was stratified by age, gender (female, 60%), and mobility creating three mobility groups—normal mobility (SPPB = 11–12), mild impairment (SPPB = 9–10), and moderate impairment (SPPB <9) of comparable age and gender. Fifty-eight participants were woman, 49 (51%) were married, and 47 (48%) lived alone: the majority 74 (76%) in their own house or apartment. The average participant took 5.3 ± 2.9 prescribed medications, including anticholinergics in 7 (11%). Mean IADL (23.5 ± 1.1%), CESD (8.2 ± 6.7), and MMSE (28.4 ± 1.3) scores indicated that most were independent with normal affect and cognitive function.
Table 1.
Subject Characteristics
| Study Variable (n) | All Subjects (97) | All Incontinence (62) | Urge UI (37) | Stress UI (8) | Other UI (11) | Mixed UI (6) | Dry/Slight Severity (26) | Moderate Severity (19) | Severe Severity (17) |
| Demographics: | |||||||||
| Agea | 82.1 (4.1) | 82.0 (4.1) | 82.8 (4.0) | 79.4 (4.3) | 81.6 (4.1) | 81.0 (3.8) | 82.0 (4.5) | 81.8 (4.1) | 82.1 (3.8) |
| Femaleb | 58 (60) | 45 (73) | 26 (70) | 7 (88) | 6 (55) | 6 (100) | 15 (58) | 14 (74) | 16 (94) |
| Marriedb | 49 (51) | 29 (47) | 16 (43) | 4 (50) | 7 (64) | 2 (33) | 14 (54) | 8 (42) | 7 (41) |
| House/Apartmb | 74 (76) | 45 (73) | 28 (76) | 6 (75) | 7 (64) | 4 (67) | 19 (73) | 11 (58) | 15 (88) |
| Lives Aloneb | 47 (48) | 32 (52) | 20 (54) | 4 (50) | 5 (45) | 3 (50) | 14 (54) | 10 (53) | 8 (47) |
| Total Medsa | 5.3 (2.9) | 5.3 (2.6) | 5.8 (2.6) | 4.8 (3.3) | 4.5 (1.9) | 4.5 (2.4) | 5.5 (2.4) | 5.1 (2.4) | 5.2 (3.1) |
| Anticholinergic Medsb | 7 (11) | 7 (11) | 5 (14) | 1 (13) | 1 (9) | 0 (0) | 2 (8) | 2 (11) | 3 (18) |
| IADLa | 23.5 (1.1) | 23.4 (1.1) | 23.4 (1.2) | 23.6 (0.7) | 23.2 (1.2) | 23.8 (0.4) | 23.3 (1.3) | 23.7 (0.6) | 23.3 (1.0) |
| Mobility: SPPBa | 9.2 (2.1) | 8.9 (2.4) | 8.3 (2.6) | 10.4 (1.2) | 9.6 (1.7) | 9.2 (2.3) | 9.0 (2.4) | 9.4 (2.2) | 8.0 (2.4) |
| Depression: CESDa | 8.2 (6.7) | 9.5 (6.9) | 10.8 (6.9) | 10.0 (8.0) | 6.8 (6.0) | 5.8 (6.1) | 9.4 (6.6) | 7.3 (6.4) | 12.2 (7.5) |
| Cognitive: MMSEa | 28.4 (1.3) | 28.6 (1.2) | 28.8 (1.2) | 28.4 (1.6) | 28.5 (1.3) | 28.2 (0.8) | 28.7 (1.1) | 28.9 (1.0) | 28.4 (1.5) |
| Incontinence Measures: | |||||||||
| Severity:b | |||||||||
| Continent | 35 (36) | n/a | n/a | n/a | n/a | n/a | |||
| Dry/Slight | 26 (27) | 26 (42) | 16 (43) | 3 (37.5) | 6 (55) | 1 (17) | |||
| Moderate | 19 (20) | 19 (31) | 11 (30) | 3 (37.5) | 3 (27) | 2 (33) | |||
| Severe | 17 (18) | 17 (27) | 10 (27) | 2 (25) | 2 (18) | 3 (50) | |||
| Degree of Bothera | n/a | 27.9 (17.4) | 29.1 (14.6) | 34.7 (20.7) | 14.6 (18.6) | 35.2 (18.1) | 17.1 (12.0) | 28.1 (13.5) | 44.1 (16.0) |
| Functional Impacta | n/a | 10.4 (14.7) | 9.0 (10.9) | 9.5 (14.8) | 5.6 (7.9) | 28.6 (30.0) | 4.9 (12.4) | 10.8 (14.1) | 18.2 (15.9) |
Notes: CESD = Center for Epidemiological Studies-Depression scale; IADL = instrumental activities of daily living; MMSE = Mini-Mental State Examination; n/a = not applicable; severity = Urinary Incontinence Severity Index; SPPB = Short Physical Performance Battery; UI = urinary incontinence. Degree of bother, Urogenital Distress Inventory (UDI-6); functional impact, Incontinence Impact Questionnaire (IIQ-7).
Mean (standard deviation).
Frequency (%).
Incontinence Prevalence and Characteristics
Almost two thirds, 62 of 97 (64%), reported UI. Urgency was the only or predominant type of UI in 37 of 62 (59.7%) and stress in 8 of 62 (12.9%). Other categories of UI were described by 11 of 62 (17.7%) and mixed UI by 6 of 62 (9.7%). Overall, 36 of 62 participants (58%) reported moderate–severe UI. Symptoms directly related to UI were especially important contributors to a sense of bother. For example, although urgency-related leakage was described as a moderate–severe contributor to bother by 28 of 62 (45%) of the incontinent participants, pelvic pain/discomfort was described in this manner by only 5 of 62 (8%). Only 18 of 62 (29%) of incontinent participants noted a moderate–severe impact on quality of life based on their responses to the IIQ. Finally, incontinent participants were 4.5 times more likely to be female (73% vs 27%; p < .001; unadjusted odds ratio 4.48, 95% confidence interval [CI] 1.85–10.84), have lower SPPB scores (8.9 ± 2.4 vs 9.8 ± 1.5; p = .02), and higher values on CESD scores (9.5 ± 6.9 vs 6.0 ± 5.7; p = .01). Trend analysis showed that the proportion of women increased with UI severity (58% mild, 74% moderate, and 94% severe; p <.01).
Global WMH
Global WMH expressed as fraction of brain volume predicted UI severity (χ2 = 7.0; p = .008) and degree of bother (t = 1.99; p = .052) when controlling for age and gender.
Frontal WMH
WMH was measured within superior, inferior, and total right and left frontal regions (Tables 2 and 3). None of these regional WMH burdens predicted presence of UI, category of UI, degree of bother, or impact on function (Table 3). In multivariate logistic regression models, which controlled for gender and age, UI severity was predicted by WMH located within the right frontal (1.05; 95% CI 1.00–1.10) and right inferior frontal (1.33; 95% CI 1.00–1.77) regions (Table 3). Similar relationships, but with a borderline significance, were observed with WMH located in left frontal and left superior frontal regions (Table 3). Because these regions had near-significance values, all were entered into a stepwise regression along with SPPB score, CESD score, age, and gender. In the final model, the total portion of WMH in the right frontal region (p = .045), SPPB score (p = .036), and gender (p = .0002) remained.
Table 3.
UI and WMH Burden in Frontal Regions
| Right Frontal |
Left Frontal |
|||||
| Incontinence Measures | Superior | Inferior | Total | Superior | Inferior | Total |
| Wet vs dry | 1.05 (0.98 to 1.12), p = .170 | 1.08 (0.75 to 1.56), p = .676 | 1.04 (0.98 to 1.11), p = .185 | 1.04 (0.96 to 1.13), p = .339 | 1.08 (0.73 to 1.61), p = .694 | 1.03 (0.96 to 1.11), p = .357 |
| Predominant urge vs other categories | 0.98 (0.93 to 1.05), p = .600 | 0.89 (0.65 to 1.23), p = .485 | 0.98 (0.93 to 1.04), p = .555 | 0.97 (0.90 to 1.05), p = .471 | 1.27 (0.78 to 2.07), p = .330 | 0.99 (0.92 to 1.05), p = .671 |
| Severity | 1.05 (1.00 to 1.10), p = .051 | 1.33 (1.00 to 1.77), p = .053 | 1.05 (1.00 to 1.10), p = .037 | 1.05 (0.99 to 1.11), p = .098 | 1.19 (0.89 to 1.59), p = .236 | 1.05 (0.99 to 1.10), p = .092 |
| Degree of bother (UDI-6) | 0.43 (−0.06 to 0.92), p = .087 | 1.87 (−0.86 to 4.59), p = .175 | 0.40 (−0.04 to 0.84), p = .076 | 0.42 (−0.21 to 1.06), p = .190 | 0.35 (−2.46 to 3.16), p = .805 | 0.33 (−0.22 to 0.88), p = .238 |
| Impact on function (IIQ-7) | 0.14 (−0.30 to 0.58), p = .519 | 1.27 (−1.12 to 3.66), p = .293 | 0.15 (−0.25 to 0.55), p = .448 | 0.14 (−0.42 to 0.71), p = .607 | 0.30 (−2.15 to 2.75), p = .807 | 0.12 (−0.37 to 0.61), p = .622 |
Note: UI = urinary incontinence; WM = white matter hyperintensity. Values shown represent odds ratio (95% confidence interval) or estimate (95% confidence interval) controlling for age and gender. Independent variable: incontinence measure. Urogenital Distress Inventory (UDI)-6 and Incontinence Impact Questionnaire (IIQ)-7 analyzed using linear regression; all others with logistic regression. Values with p value ≤.05 are shown in bold (bold indicates statistical significance).
Anterior WM Tracts
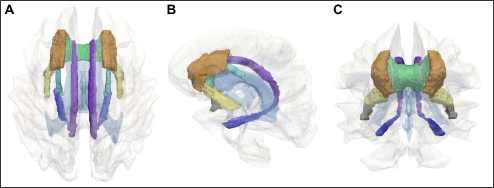
Using a mechanism-guided and hypothesis-driven approach, we explored relationships between UI and amount of WMH within seven WM tracts (Table 2; Figure 2). In none of these tracts did WMH predict type of UI present or its impact on functional status (Table 4).
Figure 2.
Three-dimensional (3D) models of the seven white matter (WM) tracts selected for analysis: Top (A), side (B), and frontal (C) views of a 3D reconstruction of the human brain are presented with ventricles (faint blue) and total brain WM (white/gray) shown in transparency. Seven WM tracts were selected for analysis. A WM parcellation atlas was used to generate images of the following regions of interest: anterior corona radiata (orange-brown), inferior fronto-occipital fasciculus (yellow), cingulum/cingulate gyrus (purple), cingulum/hippocampus (blue), superior fronto-occipital fasciculus (light blue), uncinate fasciculus (gray), and genu of the corpus callosum (green).
Table 4.
UI and WMH Burden in Selected White Matter Tracts
| White Matter Tract | Wet vs Dry | Urge vs Other Categories | Severity | Degree of Bother (UDI-6) | Impact on Function (IIQ-7) |
| Anterior corona radiata | 1.04 (0.99 to 1.09), p = .132 | 1.00 (0.96 to 1.04), p = .953 | 1.05 (1.01 to 1.09), p = .008 | 0.37 (0.04, 0.70), p = .028 | 16.4 (−13.4, 46.2), p = .275 |
| Inferior fronto-occipital fasciculus | 1.13 (0.87, 1.49), p = .362 | 0.90 (0.71, 1.14), p = .407 | 1.13 (0.94, 1.35), p = .182 | 0.49 (−1.48, 2.46), p = .619 | −57.4 (−228.7, 113.8), p = .504 |
| Cingulate gyrus | 1.52 (1.01, 2.3), p = .047 | 1.01 (0.79, 1.29), p = .962 | 1.26 (1.02, 1.55), p = .033 | −0.13 (−2.26, 2.00), p = .901 | −48.1 (−233.6, 137.4), p = .606 |
| Cingulate hippocampal portion | 1.21 (0.99, 1.48), p = .068 | 1.05 (0.91, 1.20), p = .541 | 1.13 (1.01, 1.27), p = .038 | 0.14 (−1.05, 1.33), p = .820 | −10.1 (−113.7, 93.6), p = .847 |
| Superior fronto-occipital fasciculus | 1.10 (0.99, 1.22), p = .085 | 0.98 (0.92, 1.04), p = .521 | 1.07 (1.01, 1.14), p = .034 | 0.48 (−0.04, 1.00), p = .070 | 4.23 (−42.2, 50.7), p = .856 |
| Uncinate fasciculus | 1.07 (0.98, 1.16), p = .142 | 1.02 (0.95, 1.09), p = .636 | 1.04 (0.98, 1.10), p = .221 | −0.09 (−0.66, 0.48), p = .748 | −30.1 (−79.1, 19.0), p = .224 |
| Genu of corpus callosum | 1.04 (0.93, 1.17), p = .490 | 0.96 (0.85, 1.08), p = .474 | 1.03 (0.95, 1.12), p = .477 | 0.59 (−0.36, 1.54), p = .218 | −0.16 (−84.3, 83.9), p = .997 |
Note: UI = urinary incontinence; WM = white matter hyperintensity. Values shown represent odds ratio (95% confidence interval) or estimate (95% confidence interval) controlling for age and gender. Independent variable: incontinence measure. Urogenital Distress Inventory (UDI)-6 and Incontinence Impact Questionnaire (IIQ)-7 analyzed using linear regression; all others with logistic regression. Values with p value ≤.05 are shown in bold (bold indicates statistical significance).
UI was predicted by WMH burden within the cingulate gyrus (1.52; 95% CI 1.01–2.3). A similar relationship, but with a borderline statistical significance, was observed both with the cingulate hippocampal portion (1.21; 95% CI 0.99–1.5) and with the superior fronto-occipital fasciculus (1.10; 95% CI 0.99–1.22). When these MRI variables were entered into a stepwise regression along with SPPB, CESD score, age, and gender, only the cingulate gyrus (p = .047) and gender (p = .001) remained.
UI severity was predicted by WMH burdens within the anterior corona radiata (1.05; 95% CI 1.01–1.09), cingulate gyrus (1.26; 95% CI 1.02–1.55), cingulate hippocampal portion (1.13; 95% CI 1.01–1.27), and the superior fronto-occipital fasciculus (1.07; 95% CI 1.01–1.14). UI-related bother was predicted by WMH in the anterior corona radiata (0.37; 95% CI 1.01–1.09) and, with borderline significance, the superior fronto-occipital fasciculus (0.48; 95% CI −0.04 to 1.00). For each of these outcomes, MRI variables were entered into a stepwise regression along with SPPB score, CESD score, age, and gender. Only the anterior corona radiata (p = .005) and gender (p < .0001) remained in the model predicting UI severity and anterior corona radiata (p = .022) in the model predicting UI-related bother.
DISCUSSION
Incontinence Burden
Gender, but not age, emerged as a major risk factor in our study, reflecting the great vulnerability of elderly women to incontinence, plus the fact that only individuals 75 years and older were recruited. Stress UI represents the dominant contributor to UI among younger women; yet, with aging its prevalence rate declines, whereas that for urge UI increases (21). Nearly two thirds of our incontinent participants reported predominant urge UI, whereas only 8 (13%) reported stress UI. These numbers are similar to a study that provided urodynamic evidence of detrusor overactivity among 61% of incontinent elderly nursing home residents and stress UI in 21% (22). Finally, in keeping with our findings, declines in mobility (23,24) and depression (24) have been shown to raise the risk of UI.
Global WMH Measures and Incontinence
Only 2% of the population are completely free of WM lesions on MRI (25). In spite of an interest in establishing WMH as a predictive risk factor in geriatrics (2,3), only two studies have evaluated the relationship between UI and global WMH (1,26), and none have performed a regional analysis. In a study of 63 older women and men, Sakakibara and colleagues (1) provided urodynamic evidence of detrusor overactivity in 82% of individuals with grade 1–4 WM lesions, as compared with 9% of those with grade 0 lesions. Urinary symptoms as well as cognitive and gait deficits increased with higher grades of WM lesions (1). In contrast, Sitoh and colleagues (27) observed no relationship between radiologist-derived impressions of significant WM lesions and UI.
Central Nervous System Control of Micturition and Focal WMH
Reflex voiding is mediated via sacral afferent inputs, the pontine periaqueductal gray (PAG) nucleus, the pontine micturition center, and sacral efferent outputs (4). Several positron-emission tomography (PET) and fMRI studies have highlighted the role played by suprapontine central nervous system (CNS) circuits that inhibit PAG-mediated reflex micturition, thus controlling voiding and ensuring that it takes place in a socially acceptable context (4).
Focal WMH Within Frontal Regions
Right inferior frontal gyrus is active during both urine storage and micturition (4). These and other prefrontal regions appear to be involved in the decision on when and where micturition should occur (4). Individuals with “poor bladder control” and diminished ability to sustain bladder suppression demonstrate weak orbitofrontal activation during bladder filling (17). Our studies demonstrated no relationship between frontal WMH and UI, type of UI, or its impact on function. However, when WMHs are located in right frontal regions, they predict UI severity and degree of bother. Instead of suggesting a role for WMH as a primary risk factor for urgency, they may be indicative of a decreased ability to compensate for and cope with an unexpected detrusor contraction. The absence of a relationship between frontal WMH and functional impact suggests that these individuals are still able to cope and compensate.
CNS Connectivity
Retrograde transneuronal labeling animal experiments, combined with human PET and fMRI studies, have provided important insights into the nature of neural circuits involved in bladder control (4). A mathematical physiophysiological connectivity analysis of fMRI studies performed during urodynamic studies supports the hypothesis that losses in CNS connectivity may play a role in urge UI (19). Right insula and anterior cingulate gyrus may normally suppress micturition during bladder filling, and with failure of this mechanism in individuals with detrusor overactivity, there appears to be a diversion toward compensatory pathways within parietotemporal regions (19). DTI represents a powerful imaging tool for defining the nature and integrity of anatomical connections between specific brain regions. To guide future DTI analyses, we used a population-derived brain atlas to explore the hypothesis that presence of WMH in our participants overlapped with anticipated locations of physiologically relevant WM tracts.
Cingulum
Anterior cingulate cortex integrates sensory inputs from the bladder, providing cortical control over attention to bladder signals and types of response (4). Potential responses to such signals range from voiding to recruitment of compensatory mechanisms (eg, urethral sphincter contraction), which allow voiding to be postponed (4). Our observation that presence of WMH in the cingulate gyrus predicts the presence and severity of UI is consistent with the earlier physiological roles. Moreover, none of the other anterior WM tracts selected for study predicted both UI and its severity.
Superior Fronto-Occipital Fasciculus
The superior fronto-occipital (subcallosal) fasciculus connects frontal cortex and insula with occipital, temporal, and superior temporal regions (27,28). Superior fronto-occipital fasciculus and cingulum are separated by the corpus callosum, yet run parallel to each other, joining many of the same structures. These observations may help explain why WMH in both the cingulum and the superior fronto-occipital fasciculus predict UI severity and degree of bother.
Anterior Corona Radiata
Anterior corona radiata axons carry both efferent and afferent neural traffic between the cerebral cortex and the caudal structures within the brainstem and spinal cord. Ventricular dilatation with normal pressure hydrocephalus may contribute to UI and gait disorders associated via tangential shearing forces on these fibers (20). Because DTI studies have failed to identify corticopontine tracts terminating upon the PAG nucleus or pontine micturition center, it is not surprising that WMH within the anterior corona radiata failed to predict UI. However, the ability of anterior corona radiata WMH to predict UI severity and degree of bother may suggest the presence of other, yet to be defined, relationships between these tracts and specific aspects of bladder control.
Inferior Fronto-Occipital and Uncinate Fasciculus, and Genu of Corpus Callosum
The inability of WMH in these regions to predict any UI-related measures offers an important specificity control. While also connecting anterior and posterior structures, the inferior fronto-occipital tract and uncinate fasciculus are more rostral. In contrast, although the genu of the corpus callosum is located in close proximity to both the cingulum and the superior fronto-occipital fasciculus, its fibers connect left and right hemispheres. Thus, the impact of WMH on continence-related symptoms may depend upon the specific location, as well as direction of the WM fibers affected by such lesions.
Study Limitations
Our study was originally powered to address mobility issues. As suggested by the CIs presented in Tables 3 and 4, it is possible that some of our negative findings may represent type II error. Furthermore, our findings must be replicated in other populations, including cohorts that are more diverse and include a substantial minority representation.
Future Directions
Our findings provide the first evidence linking WMH within specific brain regions to UI measures. These observations will now permit the development of a hypothesis-guided analysis of DTI data. Moreover, we will also be able to provide key longitudinal data linking specific categories of WMH to individual clinical outcomes.
FUNDING
This work was supported by the National Institutes of Health through AG022092 (L.W.), training grant AG022092-01A1S1 (N.M.), and AG028657 (G.A.K.), plus the Travelers Chair in Geriatrics and Gerontology (G.A.K.).
Acknowledgments
All authors contributed to the preparation of the manuscript and all meet criteria for authorship. N.M. had equal contribution with the first author.
References
- 1.Sakakibara R, Hattori T, Uchiyama T, Yamanishi T. Urinary function in elderly people with and without leukoaraiosis: relation to cognitive and gait function. J Neurol Neurosurg Psychiatry. 1999;67(5):658–660. doi: 10.1136/jnnp.67.5.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pantoni L. Leukoaraiosis: from an ancient term to an actual marker of poor prognosis. Stroke. 2008;39(5):1401–1403. doi: 10.1161/STROKEAHA.107.505602. [DOI] [PubMed] [Google Scholar]
- 3.Pugh KG, Lipsitz LA. The microvascular frontal-subcortical syndrome of aging. Neurobiol Aging. 2002;23(3):421–431. doi: 10.1016/s0197-4580(01)00319-0. [DOI] [PubMed] [Google Scholar]
- 4.Fowler CJ, Griffiths D, De Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9(6):453–466. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolfson L, Wei X, Hall CB, et al. Accrual of MRI white matter abnormalities in elderly with normal and impaired mobility. J Neurol Sci. 2005;232(1–2):23–27. doi: 10.1016/j.jns.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 6.Griffiths D, Tadic SD. Bladder control, urgency, and urge incontinence: evidence from functional brain imaging. Neurourol Urodyn. 2007;27(6):466–474. doi: 10.1002/nau.20549. [DOI] [PubMed] [Google Scholar]
- 7.Brown JS, Bradley CS, Subak LL, et al. The sensitivity and specificity of a simple test to distinguish between urge and stress urinary incontinence. Ann Intern Med. 2006;144(10):715–723. doi: 10.7326/0003-4819-144-10-200605160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanley J, Capewell A, Hagen S. Validity study of the severity index, a simple measure of urinary incontinence in women. BMJ. 2001;322(7294):1096–1097. doi: 10.1136/bmj.322.7294.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uebersax JS, Wyman JF, Shumaker SA, McClish DK, Fantl JA. Short forms to assess life quality and symptom distress for urinary incontinence in women: the Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Continence Program for Women Research Group. Neurourol Urodyn. 1995;14(2):131–139. doi: 10.1002/nau.1930140206. [DOI] [PubMed] [Google Scholar]
- 10.Harvey MA, Kristjansson B, Griffith D, Versi E. The Incontinence Impact Questionnaire and the Urogenital Distress Inventory: a revisit of their validity in women without a urodynamic diagnosis. Am J Obstet Gynecol. 2001;185(1):25–31. doi: 10.1067/mob.2001.116369. [DOI] [PubMed] [Google Scholar]
- 11.Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55(5):780–791. doi: 10.1111/j.1532-5415.2007.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tinetti ME, Inouye SK, Gill TM, Doucette JT. Shared risk factors for falls, incontinence, and functional dependence. Unifying the approach to geriatric syndromes. JAMA. 1995;273(17):1348–1353. [PubMed] [Google Scholar]
- 13.Toga AW, Thompson PM, Mori S, Amunts K, Zilles K. Towards multimodal atlases of the human brain. Nat Rev Neurosci. 2006;7(12):952–966. doi: 10.1038/nrn2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pohl KM, Bouix S, Kikinis R, et al. IEEE International Symposium on Biomedical Imaging: From Nano to Macro. Arlington, VA: Institute of Electrical and Electronics Engineers (IEEE); 2004. Anatomical guided segmentation with non-stationary tissue class distributions in an expectation-maximization framework; pp. 81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 16.Mori S, Oishi K, Jiang H, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40(2):570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffiths D, Derbyshire S, Stenger A, Resnick N. Brain control of normal and overactive bladder. J Urol. 2005;174(5):1862–1867. doi: 10.1097/01.ju.0000177450.34451.97. [DOI] [PubMed] [Google Scholar]
- 18.Griffiths D, Tadic SD, Schaefer W, Resnick NM. Cerebral control of the bladder in normal and urge-incontinent women. Neuroimage. 2007;37(1):1–7. doi: 10.1016/j.neuroimage.2007.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tadic SD, Griffiths D, Schaefer W, Resnick NM. Abnormal connections in the supraspinal bladder control network in women with urge urinary incontinence. Neuroimage. 2008;39(4):1647–1653. doi: 10.1016/j.neuroimage.2007.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hakim S, Venegas JG, Burton JD. The physics of the cranial cavity, hydrocephalus and normal pressure hydrocephalus: mechanical interpretation and mathematical model. Surg Neurol. 1976;5(3):187–210. [PubMed] [Google Scholar]
- 21.Minassian VA, Stewart WF, Wood GC. Urinary incontinence in women: variation in prevalence estimates and risk factors. Obstet Gynecol. 2008;111(2 Pt 1):324–331. doi: 10.1097/01.AOG.0000267220.48987.17. [DOI] [PubMed] [Google Scholar]
- 22.Resnick NM, Yalla SV, Laurino E. The pathophysiology of urinary incontinence among institutionalized elderly persons. N Engl J Med. 1989;320(1):1–7. doi: 10.1056/NEJM198901053200101. [DOI] [PubMed] [Google Scholar]
- 23.Huang AJ, Brown JS, Thom DH, Fink HA, Yaffe K. Urinary incontinence in older community-dwelling women: the role of cognitive and physical function decline. Obstet Gynecol. 2007;109(4):909–916. doi: 10.1097/01.AOG.0000258277.01497.4b. [DOI] [PubMed] [Google Scholar]
- 24.Goode PS, Burgio KL, Redden DT, et al. Population based study of incidence and predictors of urinary incontinence in black and white older adults. J Urol. 2008;179(4):1449–1453. doi: 10.1016/j.juro.2007.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357(18):1821–1828. doi: 10.1056/NEJMoa070972. [DOI] [PubMed] [Google Scholar]
- 26.Sitoh YY, Sitoh YY, Sahadevan S. Clinical significance of cerebral white matter lesions in older Asians with suspected dementia. Age Ageing. 2004;33(1):67–71. doi: 10.1093/ageing/afh005. [DOI] [PubMed] [Google Scholar]
- 27.Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17(1):77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- 28.Aralasmak A, Ulmer JL, Kocak M, Salvan CV, Hillis AE, Yousem DM. Association, commissural, and projection pathways and their functional deficit reported in literature. J Comput Assist Tomogr. 2006;30(5):695–715. doi: 10.1097/01.rct.0000226397.43235.8b. [DOI] [PubMed] [Google Scholar]