Abstract
In the absence of the methyl donor S-adenosyl methionine and under certain permissive reaction conditions, EcoPI shows non-specific endonuclease activity. We show here that the cofactor analogue S-adenosyl homocysteine promotes this promiscuous DNA cleavage. Additionally, an extensive exonuclease-like processing of the DNA is also observed that can even result in digestion of non-specific DNA in trans. We suggest a model for how DNA communication events initiating from non-specific sites, and in particular free DNA ends, could produce the observed cleavage patterns.
INTRODUCTION
Restriction-modification (RM) enzymes are important systems involved in protecting bacteria and archaea from infection by bacteriophage DNA. They are widespread in nature, with more than 85% of sequenced genomes containing one or more confirmed and/or putative RM system, and with over 20% having more than five (1). For the three major classes, Types I, II and III, the basal RM enzyme activities required are a modification methyltransferase (MTase) and an endonuclease (ENase), both targeting the same DNA sequence. The MTase covalently modifies one or both strands of every recognition site in the host genome, so rendering them resistant to cleavage by the cognate ENase. This is of paramount importance given the lethality of dsDNA breaks (2). For the Type I RM systems and the majority of Type II RM systems [with the exception of MmeI and closely related Type IIG enzymes—ref. (3)], methylation occurs on both DNA strands. Following semi-conservative replication, each site is still methylated in one strand (hemimethylated) and thus protected from cleavage. Maintenance methylation then reinstates the full methylation pattern before the next round of replication. For the Type III RM enzymes, however, only one strand of the asymmetric recognition site is ever methylated (4–8). Semi-conservative replication of hemimethylated DNA produces sites that are completely unmethylated, and potentially unprotected (9,10). Type III restriction endonucleases overcome this potential threat to the host genome by only cleaving DNA following interaction with two indirectly repeated sequences (9–12). In this arrangement, one site of each pair will always be hemimethylated following replication, so preventing cleavage (10)—directly repeated sites would not offer the same protection. We show here for the Type III RM enzyme EcoPI that this regulation of cleavage activity can be completely turned off by the cofactor product S-adenosyl homocysteine (AdoHcy) and that EcoPI can initiate illegitimate cleavage from DNA ends.
EcoPI is a prototypical Type III enzyme (13,14). It comprises a hetero-tetrameric complex between MTase (Mod) and helicase-nuclease (Res) subunits with the stoichiometry Res2Mod2 (15). The complex recognises the DNA sequence 5′-AGACC-3′: the MTase activity modifies the exocyclic N7 of the second adenine (AGmACC), the nuclease activity cleaving the DNA 25–27 nucleotides 3′ to the site (4). The in vitro DNA cleavage properties of EcoPI are dependent upon both buffer conditions and the presence or absence of the MTase cofactor S-adenosyl methionine (AdoMet) (11). AdoMet plays three roles: as a methyl donor for DNA methylation (16); as an allosteric activator of DNA binding (17,18) and as a specificity factor, preventing unregulated cleavage of DNA substrates (11). Under non-permissive conditions (the presence of Na+ ions in the reaction buffers), DNA cleavage only occurs on DNA with two indirectly repeated recognition sites and AdoMet significantly stimulates this cleavage (11). Under permissive cleavage conditions (the presence of K+ ions in the reaction buffers), EcoPI will also cleave DNA with two directly repeated sites and even DNA with a single site (11). This ‘secondary’ cleavage is an order of magnitude slower than the primary cleavage, requires a sizeable molar excess of enzyme over sites (>3-fold—see below and Figure 1) and is inefficient if the DNA is not cut first by a head-to-head (HtH) directed cleavage event. Under these conditions, AdoMet actually enhances the specificity of EcoPI, by turning off the cleavage activity on the non-specific substrates whilst maintaining cleavage of specific DNA.
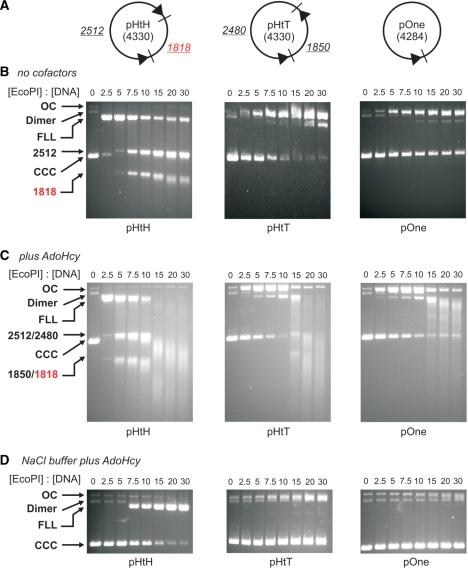
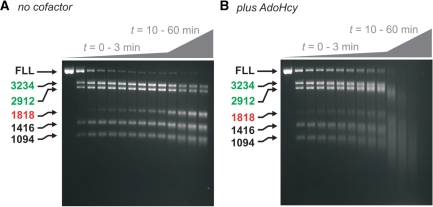
Figure 1.
Effect of S-adenosyl homocysteine on plasmid DNA cleavage by EcoPI. (A) One and two-site plasmids used (11). The EcoPI-recognition site is indicated by an arrowhead (the direction defined by the AGACC strand) and the downstream cleavage site by an adjacent tick mark. The distances underlined are in base pairs and represent the length of the DNA resulting from cleavage at both sites of the two-site DNA. (B) Cleavage of 10 nM DNA after incubation for 1 h at different concentrations of EcoPI in K-based buffer. (C) Cleavage reactions as in B except with the addition of 100 μM AdoHcy in K-based buffer. (D) Cleavage reactions as in (B) except with the addition of 100 μM AdoHcy and in a Na-based buffer (see ‘Materials and Methods’ section). The ratios of EcoPI to DNA shown are the number of Res2Mod2 complexes per DNA molecule. Linear DNA is indicated by their sizes in bp or as FLL (full length linear). Other species are indicated as CCC (covalently closed circular DNA), OC (open circle/nicked DNA) or Dimer (a minor contaminant of our plasmid preparations). The DNA smearing is not dependent on the presence of protein in the gel (i.e. because of a reverse bandshift) as the same patterns were observed following phenol–chloroform extraction of the samples (e.g. see Figure 7). Note, the non-specific 1818-bp product fragment highlighted in red is also processed by EcoPI to produce smeared DNA.
To address how cofactor binding modulates the nuclease activity of EcoPI, we examined DNA cleavage in the presence of AdoHcy, the cofactor product that results from the transfer of the methyl group from AdoMet to DNA by the methyltransferase. AdoHcy has been shown to have a similar overall allosteric effect on DNA binding by EcoPI as AdoMet (18) and generally acts as a competitive inhibitor of methyltransferases (19). Here, we found that AdoHcy supports and promotes DNA cleavage without the requirement for two indirectly repeated sites. This secondary cleavage was particularly enhanced by the presence of free DNA ends. Following the primary- and secondary-cleavage events, EcoPI could also become activated to cleave non-specific DNA via an exonuclease-like route, causing a processive shortening of linear DNA substrates. Our data suggest that there is a fine balance between enhancing DNA binding whilst preventing undesirable DNA-cleavage events.
MATERIALS AND METHODS
Proteins
EcoPI and Tn21 resolvase were purified as described previously (11,12,15). Protein concentrations were determined from the absorption at 280 nm using extinction coefficients derived from the aromatic amino-acid composition in the predicted amino-acid sequences. Concentrations for EcoPI were calculated in terms of a Res2Mod2 heterotetramer. All other enzymes were obtained from either New England Biolabs (MA, USA) or Promega (WI, USA) and were used as recommended by the manufacturer.
DNA
Plasmids constructed previously (11) were used to transform E. coli HB101. Transformants were grown in LB medium plus the appropriate antibiotic and the covalently closed circular form of the DNA purified by either density gradient centrifugation in CsCl–ethidium bromide or Qiagen Midiprep. We have not noted any difference in enzyme activity between the different preparation methods. Catenanes were prepared by treatment of supercoiled plasmid DNA with Tn21 resolvase (12). DNA concentrations were determined from absorbance at 260 nm, assuming an extinction coefficient of 0.02 ml/μg cm and a DNA molecular weight of 6.6 × 105 Da/kbp. End labelling of the DNA with biotin was carried out using Klenow (3′–5′ exo-) polymerase and biotin–dUTP (20). Where the DNA was linearised prior to the addition of EcoPI, the Type II restriction enzymes used were inactivated either by heating at 67°C for >20 min or by phenol–chloroform extraction. dsDNA oligoduplexes were annealed using synthetic oligodeoxyribonucleotides supplied by either Cruachem (Glasgow, Scotland) or MWG (Germany). DNA concentrations were determined from absorbance at 260 nm using the extinction coefficients provided by the suppliers.
DNA-cleavage assays
Cleavage reactions contained DNA (concentrations in figure legends) in either NEB4+ [20 mM Tris–acetate, pH 7.9, 10 mM Mg acetate, 50 mM K acetate, 1 mM DTT, 4 mM ATP], KGlu+ [50 mM Tris–Cl, pH 8.0, 10 mM MgCl2, 20 mM K glutamate, 4 mM ATP] or NaCl+ [50 mM Tris–Cl, pH 8.0, 10 mM MgCl2, 50 mM NaCl, 4 mM ATP] supplemented with 100 μM AdoMet or AdoHcy as indicated in the text. The K-based buffers produced the same effects in these experiments and were interchangeable. Where linear ends were to be capped, streptavidin was added to 100 nM. Reactions were started by addition of EcoPI to reduce the effect of co-methylation and incubated at 37°C. Reaction aliquots were quenched after the required incubation times by addition of one-half volume of STEB [0.1 M Tris–Cl, pH 7.5, 0.2 M EDTA, 40% (w/v) sucrose, 0.4 mg/ml bromophenol blue]. The DNA substrates and products were separated by agarose gel electrophoresis. To avoid the presence of shifted bands, streptavidin was removed by phenol–chloroform extraction. Digital images of the agarose gels were captured on either a Kodak Image Station 440CF or a UVP GelDoc-It Imaging System using a linear intensity scale.
RESULTS
AdoHcy promotes secondary DNA cleavage and additional exonucleolytic processing
To assess the effect of AdoHcy on DNA cleavage, we monitored the extent of DNA cleavage at different EcoPI concentrations. The AdoHcy concentration was fixed at 100 μM. The DNA substrates chosen (Figure 1A), were a two-site indirectly repeated plasmid (pMDS36a, referred to here as pHtH for clarity), which should be a substrate for both primary (specific) and secondary (non-specific) DNA cleavage, as well as a two-site directly repeated plasmid (pMDS36b, referred to here as pHtT) and a one-site plasmid (pMDS33, referred to here as pOne) which are only substrates for secondary cleavage (11). Based on our previous studies of EcoPI (11), K+ ion-based buffers were chosen which support both primary and secondary cleavage. We also tested a NaCl buffer that does not support secondary cleavage and inhibits primary cleavage (11). The EcoPI concentration range was chosen to be in excess of the DNA, as this is the condition under which secondary cleavage is observed. Reactions were initiated by mixing DNA with enzyme and ATP and quenching the reaction after 60 min. DNA species were then separated by agarose gel electrophoresis (Figure 1B, C and D).
In the absence of exogenously added cofactor and the presence of K+ ions, we were able to observe the same secondary DNA cleavage as previously described [ref. (11)—shown for comparison; Figure 1B]. On pHtH, low EcoPI concentrations result in cleavage at only one of the two sites (distributed 50:50). This is the primary cleavage event, which is specific to indirectly repeated pairs of sites. The equal distribution of cleavage between the sites reflects the fact that the sequence surrounding the EcoPI site is identical from 10 bp 5′ of the site to 30 bp 3′ of the site (11,12). As the enzyme concentration was increased, cleavage occurred at both sites indicative of secondary cleavage. We note that these gels reveal linear DNA products, which appear diffuse and of a smaller apparent size than expected, the effect becoming more pronounced as the EcoPI concentration is raised (see below). On pHtT, low EcoPI concentrations did not result in DNA cleavage, as expected, as primary cleavage cannot occur between directly repeated sites. At elevated enzyme concentrations, secondary cleavage resulted in DNA nicking and the production of some linear DNA cut at one or other site (FLL). On pOne, low EcoPI concentrations do not result in DNA cleavage, as expected, whilst elevated enzyme concentrations resulted in DNA nicking and a trace amount of FLL DNA.
When these reactions were repeated in the presence of AdoHcy in the K+ buffer, the efficiency of non-specific DNA cleavage was increased substantially (Figure 1C). First, on all three substrates, secondary cleavage occurred at a lower concentration than with buffer alone. Secondly, dsDNA cleavage at every site was observed on pHtT and pOne. Thirdly, in all cases the linear products were further processed in an EcoPI concentration-dependent manner resulting in smeared DNA indicative of end-specific processing. For pOne, trace amount of linear DNA of a defined size can also be observed within the smear, which may indicate precise cleavage at non-specific or star sites. Note that smearing of the 1818-bp fragment is also observed despite the absence of an EcoPI site on this DNA (fragments without an EcoPI are shown in red in each figure). With the entire DNA tested, trace amounts of substrate and nicked DNA remained, even at the highest EcoPI concentrations. This may result from methylation of the DNA coincident with DNA cleavage due to trace amounts of tightly bound AdoMet that co-purify with EcoPI and cannot be competed out by the excess AdoHcy [see ‘Materials and Methods’ section, ref. (11)].
The same AdoHcy titrations in the presence of Na+ ions produced quite different effects (Figure 1D). Whilst the extent of pHtH was enhanced compared to in the absence of cofactor (11), complete DNA cleavage was only observed with a large excess of enzyme over DNA and no secondary cleavage was observed. On pHtT, a small amount of nicked DNA was produced with a trace of linear DNA. This suggests that some secondary cleavage is occurring but only of one DNA strand—this would not be observed in the linear products on pHtH. On pOne, a trace amount of nicked DNA was observed at the highest enzyme concentrations. These results are similar to those presented previously (11), in that Na+ ions tend to disfavour endonuclease activity to such an extent that secondary cleavage is not observed.
The results obtained suggest that, in the presence of K+ ions, AdoHcy does not prevent secondary cleavage but actually enhances the non-specific cleavage. The smearing seen is much more pronounced overall. However, as seen in the absence of cofactor, the secondary cleavage is still more efficient when there are two indirectly repeated sites present. All subsequent experiments were carried out using the K-based buffers.
AdoHcy-dependent DNA processing requires an EcoPI site, ATP hydrolysis and a wild-type nuclease active site
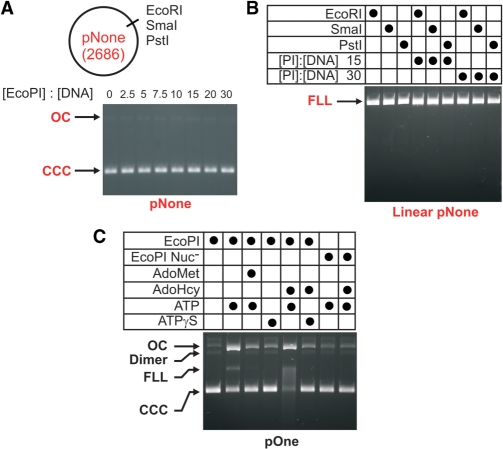
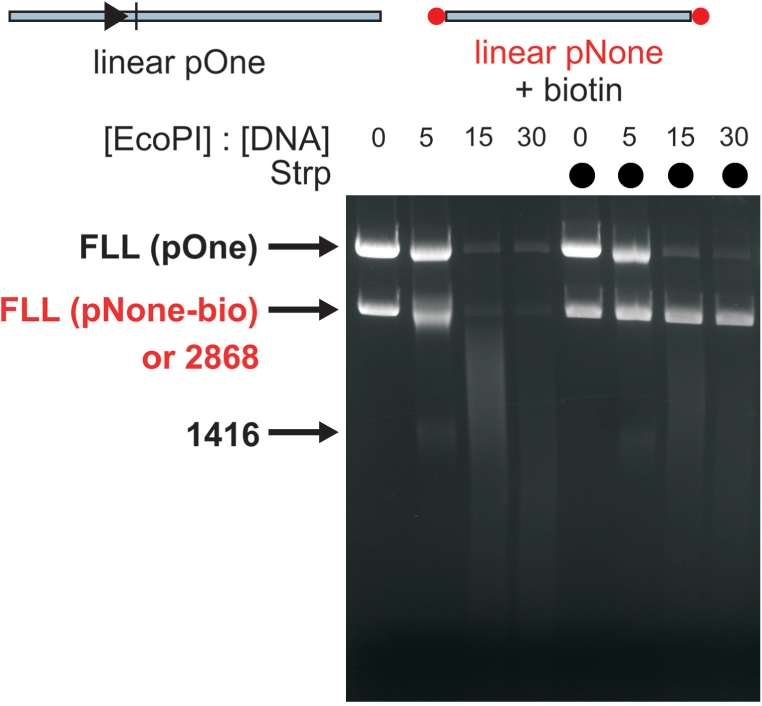
One interpretation of the above results is that AdoHcy promotes random DNA cleavage independent of the interaction with an EcoPI-recognition site. Another interpretation is that a contaminant in our protein preparations caused the additional processing. To explore these possibilities, the AdoHcy-dependent secondary cleavage of DNA was tested at high EcoPI concentrations on circular DNA and linear DNA lacking an EcoPI site (based on pLJP10, referred to here as pNone; Figure 2A and B). No cleavage was observed on either circular or linear DNA (regardless of whether the end was blunt, 5′ overhang or 3′ overhang), indicating that the cleavage seen in Figure 1 is specific to the presence of at least one EcoPI site. Using the pOne substrate we also showed that secondary cleavage (i.e. DNA nicking) in the presence of AdoHcy was dependent upon both ATP hydrolysis and the presence of a wild-type nuclease motif (Figure 2C). These results are completely consistent with an EcoPI-dependent effect.
Figure 2.
Secondary DNA cleavage is dependent on the presence of an EcoPI site, ATP and a wild-type P(D/E)xK nuclease active site. (A) The requirement for an EcoPI site on circular DNA. Ten nanomolar non-specific DNA substrate pNone was treated with varying concentrations of EcoPI for 1 h with 100 μM AdoHcy. (B) The requirement for an EcoPI site on linear DNA. Non-specific linear DNA substrates were generated by cleavage of pNone with different Type II restriction enzymes, as indicated. 10 nM linear DNA was treated with EcoPI at one of two concentrations for 1 h with 100 μM AdoHcy. (C) The effect of ATP on secondary cleavage. Ten nanomolar pOne (Figure 1A) was treated with 300 nM wild-type EcoPI or nuclease mutant EcoPI(K918A) (15) for 1 h. Reactions were supplemented with 4 mM ATP, 4 mM ATPγS, 100 μM AdoMet and/or 100 μM AdoHcy, as indicated. For details of the gel labelling, see Figure 1.
AdoHcy-dependent non-specific DNA cleavage is slower than specific DNA cleavage
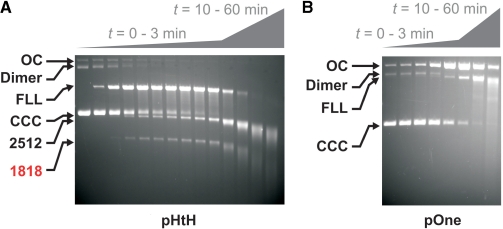
Previously, we showed that secondary DNA cleavage in the absence of AdoMet is more than 10-fold slower than the primary cleavage (11). In a similar manner, we measured the time-dependence of DNA cleavage in the presence of AdoHcy at a fixed, elevated concentration of EcoPI. On pHtH (Figure 1A), the first DNA cleavage intermediate that accumulated within 3 min was the FLL DNA cleaved at one or other EcoPI site (Figure 3A). Very little nicked DNA intermediate is liberated. These kinetics match those observed in the absence of cofactor and represent the rate of the site-orientation specific, primary cleavage reaction. Subsequently, cleavage at the remaining intact site occurs, albeit on a much slower timescale. This DNA is then further processed to give a smear of DNA of different lengths. The shortening of the 2512 and 1818 bp fragments and the increasing length of the smear with time are indicative of either an exonuclease activity (i.e. removal of 1 bp at a time from a DNA end) or a processive, end-specific endonuclease activity (i.e. removal of >1 bp at a time from a DNA end). This additional processing occurs on a timescale similar to or slower than the secondary cleavage. We have been unable to determine the exact nature of the end processing and so cannot distinguish between these two models at this time.
Figure 3.
Secondary cleavage of plasmid DNA is slower than primary cleavage. (A) Ten nanomolar pHtH was mixed with 150 nM EcoPI and reaction aliquots quenched at the following times (in s): 5, 10, 15, 20, 25, 30, 60, 90, 180, 600, 1200, 2400 and 3600. (B) Ten nanomolar pOne was mixed with 150 nM EcoPI and reaction aliquots quenched at the following times (in s): 30, 60, 180, 600, 1200, 2400 and 3600. Plasmid maps and gel labelling as in Figure 1. Because the DNA smearing overlapped with linear DNA products, we could not accurately quantify these results.
On pOne (Figure 1A), the first DNA cleavage intermediate that accumulated was nicked DNA (Figure 3B). In contrast to pHtH, this appeared only after hundreds of seconds, indicative of slow, secondary cleavage. The linear DNA and smeared products accumulated thereafter, also with a slow rate. This nicked intermediate produced during secondary cleavage was not observed in Figure 3A as the DNA undergoing secondary cleavage is linear and the agarose gels cannot resolve nicked from intact forms. An accumulation of nicked DNA could indicate that either: (i) two separate DNA-communication and nicking events produce a dsDNA break or (ii) a single communication event results in fast first strand cleavage followed by a slow second strand cleavage. We cannot distinguish between these models from our data.
AdoHcy enhances cleavage of uncapped linear DNA but has a lesser effect on capped linear DNA
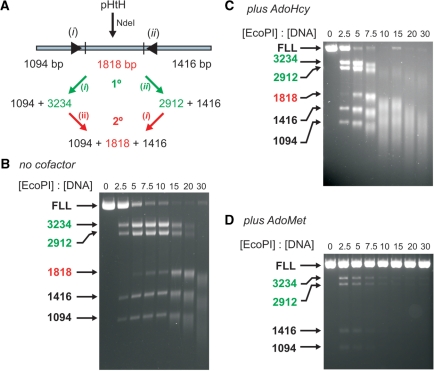
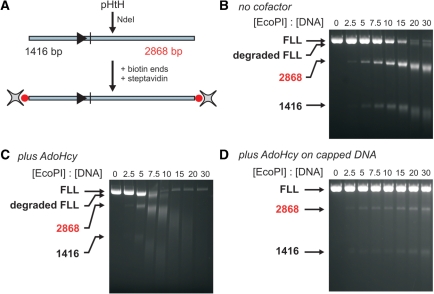
Whilst cleavage of the circular form of pHtH is efficient even in the absence of cofactor (Figure 1B), cleavage of a linear form in which the two sites are in the HtH orientation is surprisingly inefficient; while the circular DNA is fully cleaved at the lowest enzyme concentration, 3-fold more enzyme is required to achieve full cleavage of the linear DNA (Figure 4A). DNA topology has no obvious effect on Type III RM enzymes (12). Instead, we recently showed that the poor cleavage of linear DNA was due to the inhibitory effect of DNA ends on the long-range communication process [ref. (20), see ‘Discussion’ section]. This can be overcome by circularising the DNA [i.e. as in a plasmid form—ref. (11)] or by capping the ends of the DNA using a bulky molecule such as streptavidin (20). However, whilst primary cleavage appears less efficient, secondary cleavage of the uncapped linear DNA at elevated EcoPI concentrations appears to be more efficient than on plasmid DNA, with a higher proportion of DNA smearing observed (Figure 4B). In the presence of AdoHcy, the efficiency of the secondary cleavage was enhanced further while primary cleavage was still poor (Figure 4C). In the presence of AdoMet very little primary or secondary DNA cleavage was observed (Figure 4D). In contrast to the profiles above, increasing the EcoPI concentration actually resulted in less nuclease activity with virtually no DNA cleavage observed above a molar excess of 10 enzymes per DNA. The time-dependence of linear DNA cleavage with and without AdoHcy was measured at a fixed EcoPI concentration (Figure 5). In both cases a rapid primary cleavage was followed by a more than 10-fold slower secondary cleavage. In the presence of AdoHcy, the proportion of initial primary cleavage product was lower than in its absence. We demonstrate below that the interaction between specifically bound enzymes and enzymes initiating from a free DNA end produces secondary cleavage and this can in fact inhibit the primary events.
Figure 4.
The effect of different cofactors on EcoPI cleavage of a linear head-to-head DNA. (A) pHtH (Figure 1A) was treated with NdeI to generate the head-to-head substrate shown. The DNA intermediates and products resulting from primary cleavage at sites i and ii are indicated. Cleavage of 10 nM linear DNA (FLL) after incubation for 1 h at different concentrations of EcoPI is shown in the absence of cofactor (B), the presence of 100 μM AdoHcy (C) and the presence of 100 μM AdoMet (D). Note, the non-specific 1818 bp product fragment highlighted in red is also processed by EcoPI to produce smeared DNA.
Figure 5.
Secondary cleavage of linear DNA is slower than primary cleavage. Ten nanomolar linear head-to-head DNA (Figure 4A) was mixed with 150 nM EcoPI in the absence (A) or presence (B) of 100 μM AdoHcy, and reaction aliquots quenched at the following times (in s): 5, 10, 15, 20, 25, 30, 60, 90, 180, 600, 1200, 2400 and 3600. For details of the gel labelling, see Figure 4.
To examine further the effects of DNA ends on secondary cleavage, we tested a linear form of pOne (Figure 6A). In the absence of cofactor, complete DNA cleavage and additional processing of the product DNA was observed at elevated EcoPI concentrations. This contrasts with the corresponding data for the plasmid DNA where very little full-length linear product is observed even at the highest EcoPI concentrations (compare Figures 1B and 6B). In the presence of AdoHcy, the cleavage was even more efficient, with complete cleavage at lower EcoPI concentrations and extensive processing of the DNA products (Figure 6C). Again, the extent of cleavage was far greater than that seen with the parental plasmid DNA plus AdoHcy.
Figure 6.
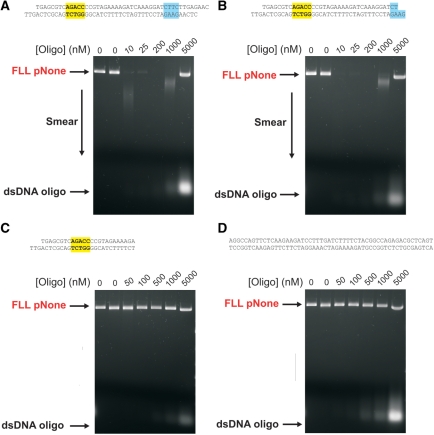
The effect of AdoHcy on EcoPI cleavage of capped and uncapped linear one-site DNA. (A) pOne (Figure 1A) was treated with NdeI to generate the linear substrate shown. This DNA was then labelled at both ends with biotin. Addition of streptavidin then capped the ends (20). Distances shown are the lengths of the DNA products following cleavage at the single EcoPI site. Cleavage of 10 nM linear DNA (FLL) after incubation for 1 h at different concentrations of EcoPI is shown in the absence of cofactor (B), the presence of 100 μM AdoHcy (C) and the presence of both 100 μM AdoHcy and strepavidin (D). Note, the non-specific 2868-bp product fragment highlighted in red is also processed by EcoPI to produce smeared DNA in B and C.
The data above suggest that in the absence of AdoMet, the presence of free ends on linear DNA can enhance the secondary cleavage activity of EcoPI. We tested this by capping the DNA ends with streptavidin (Figure 6D). The amount of DNA cleavage seen with AdoHcy was significantly reduced and the residual cleavage must be the result of a lower level of initiation of secondary cleavage from internal non-specific sites. Note, nicking of the linear DNA cannot be observed in this assay.
End-specific processing does not have a directionality
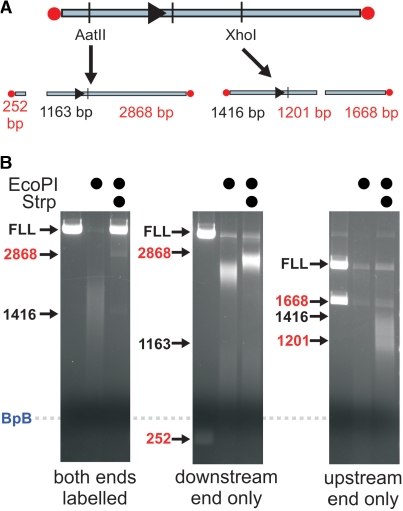
The data above suggest that interaction of EcoPI with a free DNA end can significantly enhance secondary DNA cleavage both in the absence and presence of AdoHcy. But does this interaction have a polarity? In other words, did the secondary cleavage of the one-site linear DNA in Figure 6B and C initiate from either the upstream end or downstream end exclusively? To answer this, we end labelled a one-site linear DNA with biotin and then cut the DNA either up- or downstream of the EcoPI site to generate a single unlabelled DNA end (Figure 7A). We then tested DNA cleavage in the absence or presence of streptavidin, which would block the labelled end only (Figure 7B). When both ends were capped, end-directed DNA cleavage was prevented. A residual level of cleavage remained due either to enzymes initiating internally (as in Figure 6D) or to end capping being less than 100% efficient. When only one end was capped, secondary DNA cleavage still occurred, albeit at a reduced level. No significant difference between the ends was observed, indicating that either: (i) interaction with a DNA end can occur in either polarity or (ii) interaction only occurs in one polarity but that subsequent communication is via a process that doesn't retain the polarity, such as 3D DNA looping.
Figure 7.
Secondary DNA cleavage by EcoPI is enhanced equally well by the presence of an up- or downstream free DNA end. (A) A biotin-labelled one-site linear DNA (Figure 6A) was further treated with either AatII or XhoI to remove either the upstream or downstream biotin, respectively. Cleavage of 10 nM one-site DNA labelled at both ends (left gel), at the downstream end only (middle gel) or at the upstream end only (right gel) was tested in the presence of 100 μM AdoHcy and 150 nM EcoPI. Where indicated, strepavidin was added to cap the biotin ends. Note, the non-specific fragments highlighted in red were also processed by EcoPI to produce smeared DNA.
End-specific DNA processing occurs on both ends of the cut DNA
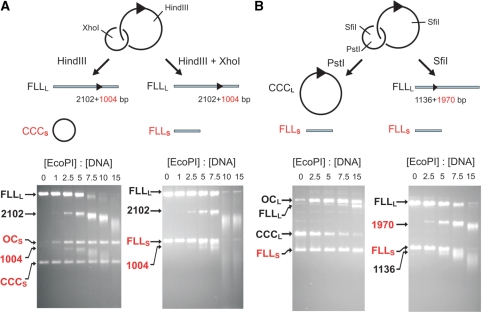
As noted in red in Figures 1, 4, 6 and 7, the products of cleavage at a recognition site are extensively processed regardless of whether or not they retain an EcoPI site. These results might seem surprising given that we showed in Figure 2A that linear DNA without an EcoPI sites is resistant to DNA cleavage. One explanation for this may be that following dsDNA hydrolysis with AdoHcy, EcoPI retains a tight hold on both ends of the cleaved DNA. Consequently, the enzyme could process a linear DNA that does not contain a recognition site. This model would predict that secondary processing would only occur on the products of a site-dependent cleavage reaction and not on any other exogenously added non-specific DNA. We tested this by mixing a linear one-site DNA with either circular or linear non-specific DNA (these substrates were generated from a one-site catenane—Figure 8). In both cases (Figure 8A), the one-site DNA was processed as expected. When the additional non-specific DNA was linear, it was also processed by EcoPI, as efficiently as the products from the site-specific cleavage. However, when the non-specific DNA was circular, only DNA nicking was observed. This indicates that following DNA cleavage of a DNA carrying an EcoPI site, the nuclease can leave that DNA and go on to process other DNA with no requirement for the presence of a site. This reaction is particularly noticeable if the non-specific DNA has a free DNA end. One prediction from this new model is that as the amount of site-dependent cleavage increases, there will also be an increase in site-independent DNA processing. This was tested in Figure 8B by using circular or linear specific one-site DNA with linear non-specific DNA in trans. When the specific DNA was circular, the absence of free DNA ends resulted in low levels of secondary cleavage and correspondingly, very little, if any, processing of the linear non-specific DNA. In contrast, when the specific DNA was linear, end-directed secondary cleavage was more extensive resulting in a correspondingly higher level of processing of the non-specific DNA.
Figure 8.
Processing of non-specific DNA in trans by EcoPI is enhanced by DNA ends and is dependent upon the extent of initial EcoPI cleavage. pOne (Figure 1A) was treated with Tn21 resolvase to produce a singly interlinked catenane as shown. This was further treated with different Type II enzymes to give the combinations of linear and circular DNA shown. The DNA mixtures (10 nM of each species) were incubated for 1 h at the different concentrations of EcoPI indicated. See text for full details. Note, the fragments highlighted in red are also processed by EcoPI to produce smeared DNA.
To further test what is required to activate cleavage of non-specific DNA in trans, we followed the cleavage of a linear non-specific DNA (pNone—Figure 2A) at a fixed concentration of EcoPI in the presence of AdoHcy and various oligoduplex substrates (Figure 9). Consistent with Figure 2, in the absence of an EcoPI site, no cleavage was observed. Extensive in trans processing of the non-specific DNA was observed in the presence of an oligoduplex containing an EcoPI recognition sequence and either, an intact or pre-cut cleavage site (Figure 9A and B). At the highest concentrations of the oligoduplex (5 μM), the trans-activation step was inhibited, presumably because the excess EcoPI sites and/or DNA ends sequestered the entire available enzyme. In contrast, in trans processing of the non-specific DNA was not observed if the oligoduplex lacked a DNA cleavage site (Figure 9C) or lacked an EcoPI-binding site (Figure 9D). These data suggest that EcoPI does not need to have undergone a DNA-cleavage step itself—there merely needs to be the presence of a suitably cleaved EcoPI site.
Figure 9.
Processing of non-specific DNA in trans by EcoPI is enhanced by DNA oligoduplexes containing the recognition and cleavage sites. pNone (Figure 2A) was cleaved with NdeI. Five nanomolar of this linear DNA was mixed with 150 nM EcoPI in the presence of AdoHcy. The oligoduplexes shown were added at the concentrations indicated and incubated for 1 h. (A) Oligoduplex containing an EcoPI-recognition sequence (in yellow) and an intact cleavage site (in blue). (B) Oligoduplex containing an EcoPI-recognition sequence and a DNA end that mimics an EcoPI cleavage product. (C) Oligoduplex containing an EcoPI-recognition sequence but no cleavage site. (D) Non-specific oligoduplex. Processing of the non-specific linear DNA in (A) and (B) is so extensive that little-or-no DNA products remain visible.
The data above show that once EcoPI is associated with a cleaved DNA end, it can go on to cleave other DNA in trans, but with a particular preference for molecules with a free end. Consistent with this idea, capping of the ends of a linear non-specific DNA with streptavidin significantly reduced the non-specific processing (Figure 10) (although we cannot rule out nicking of this substrate as was observed with the circular DNA—as in Figure 8B).
Figure 10.
Processing of non-specific linear DNA in trans by EcoPI is blocked by capping the DNA ends with strepavidin. pNone (Figure 2A) was cleaved with NdeI and the ends labelled with biotin. 10 nM of this DNA was mixed with 10 nM linear one-site DNA (Figure 6A) and incubated for 1 h at the different concentrations of EcoPI indicated. Where streptavidin was included in the reaction, so capping the ends of the non-specific DNA, processing in trans was significantly decreased.
DISCUSSION
A model for secondary cleavage and end-specific processing by EcoPI
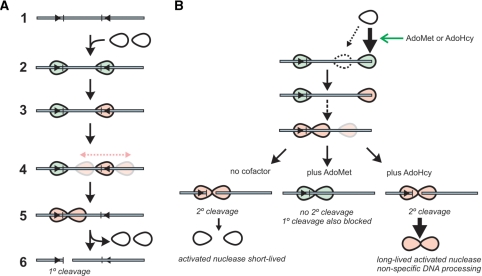
We have recently proposed a 1D sliding model for long-range communication on DNA between Type III enzymes (20), in which DNA cleavage results from collision between a diffusing EcoPI complex and a static EcoPI complex bound at a specific site (Figure 11A). Movement is licensed by an ATP-hydrolysis step that initiates 1D diffusion (ATP hydrolysis thereafter is no longer essential although it could still occur). Based around this model, we propose a scheme, which can explain the observations presented above (Figure 11B). We suggest that the ATP-hydrolysis step, and thus initiation of motion, can occur at any DNA sequence (both specific and non-specific DNA-dependent ATPase activities have been measured for EcoPI—Júlia Tóth and M.D.S., unpublished data). However, because the ATP-hydrolysis step is slow, the binding lifetime at a site will dictate whether motion initiates. Conditions that promote binding to non-specific DNA will therefore lead to an increase in the occurrence of illegitimate licensing events (i.e. initiation of communication without first binding a recognition site) and thus secondary cleavage. Such conditions could be: (i) an increase in protein concentration that, by the law of mass action, would lead to an increased probability of non-specific DNA binding; (ii) an allosteric effector such as AdoMet or AdoHcy that acts to increase DNA-binding lifetime and (iii) ionic conditions that favour DNA association. If EcoPI has a much tighter binding to DNA ends compared to internal sites, then the probability of non-specific interactions with uncapped linear DNA will be greater than on capped or circular DNA.
Figure 11.
The role of diffusion and DNA ends on the primary and secondary DNA cleavage activity of EcoPI. (A) Sliding model for long-range communication by Type III restriction enzymes (20). DNA is shown as a grey line with Type III recognition sequences as arrowheads and cleavage site as tick marks. Type III enzyme complexes are shown as ellipsoids in white (free in solution, inactive), green (DNA and ATP bound) and pink (ATP hydrolysed). Steps in the cleavage mechanism are: (1) Type III enzymes bind to the specific sites; (2) ATP binds; (3) Slow ATP hydrolysis occurs in the right-hand enzyme, which undergoes a conformational change; (4) 1D sliding of the right-hand enzyme occurs on a much faster timescale than ATP hydrolysis. Collision of the diffusing enzyme with the site-bound enzyme; (5) Long-lived collision complex allows second enzyme to hydrolyse ATP resulting in an active nuclease and (6) dsDNA cleavage adjacent to the site. Because the ATP hydrolysis is slow and sliding is presumed to be much faster, collisions (and thus cleavage) principally occur at either one site or the other and not at non-specific sites. (B) Outcome of non-specific initiation of sliding with different cofactors. At elevated enzyme concentration, binding to DNA ends, or to a lesser extent to internal non-specific sites occurs. These binding events are promoted by cofactor binding. ATP hydrolysis and communication leads to collision with a specifically bound enzyme. The resulting outcome then depends on the cofactor present, as shown.
Although the probability of non-specific interaction could be increased by the inclusion of an allosteric effector of DNA binding such as AdoMet or AdoHcy, the relative effects of the two cofactors on subsequent DNA cleavage are quite distinct. In the presence of AdoMet, EcoPI can initiate from a non-specific site/end but subsequent collision with a specifically bound enzyme leads to inhibition of cleavage [Figure 4D, ref. (11)]. In contrast, in the presence of AdoHcy, there is a stimulation of promiscuous DNA cleavage by both endonuclease- and exonuclease-like routes (Figures 1, 4 and 6). Why do AdoMet and AdoHcy have such strikingly different effects on EcoPI? A difference in conformation of the Mod subunit must therefore be transmitted to the Res subunit. However, footprinting experiments on the related EcoP15I enzyme did not demonstrate any change in DNA binding with AdoHcy (21). Unfortunately, we currently do not have any structural information on Type III Mod subunits and a sensible answer to this question awaits structural information on the Res2Mod2 complex.
How could the cleavage of non-specific DNA in trans come about? In our model (Figure 11A), an active endonuclease is only formed following a long-range communication event between two enzymes, one bound to a specific site, the other diffusing on the DNA. Each nuclease contributes to cleavage of one DNA strand and a single EcoPI enzyme cannot produce a dsDNA break (15). Moreover, the lack of cleavage activity on DNA in the complete absence of EcoPI sites (Figures 2 and 9) suggests that the formation of the active endonuclease requires at least one specific recognition site (and cleavage site). Thereafter, under certain conditions—high enzyme concentration and AdoHcy—EcoPI can recruit further protein and stabilise the nuclease state. This hyperactivated enzyme is then capable of hydrolysing any non-specific DNA. The presence of AdoMet prevents formation of the hyperactivated state. This state is distinct to the secondary cleavage form, where at least one recognition site is required, and where cleavage only occurs at a specific site.
Secondary cleavage and end-specific effects in other RM enzymes
Whilst the dogma for Type III endonuclease activity requires the presence of two sites in a specific HtH repeat (9,10), cleavage of sites in other orientations or even single sites have been observed (9,21–24). Related to our results in Figures 6 and 7, Raghavendra and Rao showed that the related Type III enzyme EcoP15I could cleave a one-site linear DNA and that this effect was blocked by the binding of Lac repressor between the site and the DNA end (22). However, in contrast to our results in Figure 7, a specific requirement of the EcoP15I reaction was for a free DNA end on the 3′ side of recognition site. Nonetheless, the EcoP15I data could be also explained by our model (Figure 11B): Binding of EcoP15I at a DNA end and motion inwards would eventually result in collision with an enzyme bound at the recognition site and thus cleavage. A difference in the dynamics of DNA binding by EcoP15I compared to EcoPI may favour interactions with enzymes from one particular direction. An internal protein roadblock that prevents 1D communication between the DNA site and DNA end [i.e. Lac in ref. (22)] would block this interaction in the same way as capping of the end by streptavidin as shown here (Figure 6).
Similar to the results from our previous study of EcoPI (11), AdoHcy-dependent secondary cleavage required the presence of K+ ions and a large excess of enzyme over DNA sites. AdoHcy did enhance nuclease activity in the presence of Na+ ions, but not enough to promote significant secondary cleavage. Recently, another group have clearly demonstrated that EcoP15I also exhibits secondary cleavage in the absence of AdoMet (24) and similar results have been inferred by others (22,23). However, the EcoP15I activities are completely independent of the buffer composition (24). Despite the similarity between the Mod subunits of these enzymes (59% identity), buffer cations have completely different influences on the target recognition domains. As we pointed out in our previous study of EcoPI (11), the exact in vitro conditions (buffer composition, cofactor, DNA substrate and enzyme concentration) can have a big effect on the observed DNA cleavage pattern. It is therefore difficult to establish hard-and-fast rules about non-specific cleavage by Type III enzymes. Nevertheless, it is interesting to note that the Type IIS restriction endonuclease BpuJI has a 3′-end-directed endonucleolytic activity that can also cleave non-specific DNA in trans once ‘activated’ by binding to a recognition site (25), reminiscent of the EcoPI activity seen in Figure 9. BpuJI-like nuclease domains can also be found in a subfamily of the Type III R-M systems, suggesting that non-specific end processing may be a more widespread feature.
Does secondary cleavage and additional DNA processing play a role in the cell?
The observations we present here are useful in understanding the dynamic DNA binding of the Type III enzymes, in particular underlining the important influence of DNA ends. But is there a cellular relevance to the additional DNA cleavage observed with AdoHcy? Given that the central role of RM enzymes is to destroy invading foreign DNA, additional end-specific processing following a dsDNA break could be seen as advantageous (26). Typically, end processing of foreign DNA is catalysed by the RecBCD/AddAB pathway (27,28). However, if the foreign DNA contains Chi sequences that can be recognised by the host exonuclease (RecBCD or AddAB), then the fragmented DNA could enter the recombination pathway and restriction activity could indirectly result in the acquisition of foreign DNA (26,29). If Type III enzymes were to both cut and degrade the DNA, this would reduce the chance of this occurring. A functional cooperation between EcoP15I and an exonuclease has also been demonstrated as essential for multiple rounds of DNA cleavage by a single enzyme complex (30). Since we only saw the exonuclease activity of EcoPI at high enzyme concentrations (well above the DNA concentration), it is difficult to assess if this could provide an alternative method to stimulate enzyme turnover following cleavage.
Although the in vitro conditions required for non-specific cleavage activity—a high concentration of AdoHcy and enzyme—may never occur in the cell, in vivo ionic conditions and molecular crowding may promote similar effects at more modest concentrations of enzyme and/or AdoHcy. We also saw some DNA smearing in the absence of cofactor (Figure 1B), showing that end processing by EcoPI is not a special feature of AdoHcy binding per se. However, uninhibited non-specific nuclease activity could be viewed as problematic for a cell as cleavage of isolated sites or non-specific DNA sequences on the host genome would lead to lethal dsDNA breaks. Whilst the 1D communication events necessary for specific cleavage (Figure 11) may be prevented on a protein-coated host genome, the presence of AdoMet may still be important in regulating a potentially dangerous Type III enzyme that would otherwise run amok (11). Even if AdoMet were not available, the lack of DNA ends on the host DNA may make initiation of non-specific events relatively rare.
FUNDING
Wellcome Trust Senior Research Fellowship in Basic Biomedical Sciences (067439 to M.D.S.) and the Wellcome Trust (084086 & 053856). M.D.S. is a Wellcome Trust Senior Research Fellow in Basic Biomedical Sciences. Funding for open access charge: Wellcome Trust value-in-people award.
Conflict of interest statement. None declared.
ACKNOWLEDGEMENTS
We thank members of the DNA–Protein Interactions Unit past and present for advice, and Kara van Aelst, Júlia Tóth and Ralf Seidel for comments on the manuscript. We also thank an anonymous referee for suggesting the oligoduplex-activation experiments.
REFERENCES
- 1.Roberts RJ, Vincze T, Posfai J, Macelis D. REBASE – enzymes and genes for DNA restriction and modification. Nucleic Acids Res. 2007;35:D269–D270. doi: 10.1093/nar/gkl891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naito T, Kusano K, Kobayashi I. Selfish behavior of restriction-modification systems. Science. 1995;267:897–899. doi: 10.1126/science.7846533. [DOI] [PubMed] [Google Scholar]
- 3.Morgan RD, Bhatia TK, Lovasco L, Davis TB. MmeI: a minimal Type II restriction-modification system that only modifies one DNA strand for host protection. Nucleic Acids Res. 2008;36:6558–6570. doi: 10.1093/nar/gkn711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachi B, Reiser J, Pirrotta V. Methylation and cleavage sequences of the EcoP1 restriction-modification enzyme. J. Mol. Biol. 1979;128:143–163. doi: 10.1016/0022-2836(79)90123-2. [DOI] [PubMed] [Google Scholar]
- 5.Meisel A, Kruger DH, Bickle TA. M.EcoP15I methylates the second adenine in its recognition sequence. Nucleic Acids Res. 1991;19:3997. doi: 10.1093/nar/19.14.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piekarowicz A, Bickle TA, Shepherd JCW, Ineichen K. The DNA sequence recognised by the HinfIII restriction endonuclease. J. Mol. Biol. 1981;146:167–172. doi: 10.1016/0022-2836(81)90372-7. [DOI] [PubMed] [Google Scholar]
- 7.De Backer O, Colson C. Identification of the recognition sequence for the M.StyLTI methyltransferase of Salmonella typhimurium LT7: an asymmetric site typical of type-III enzymes. Gene. 1991;97:103–107. doi: 10.1016/0378-1119(91)90015-4. [DOI] [PubMed] [Google Scholar]
- 8.Sears A, Peakman LJ, Wilson GG, Szczelkun MD. Characterization of the Type III restriction endonuclease PstII from Providencia stuartii. Nucleic Acids Res. 2005;33:4775–4787. doi: 10.1093/nar/gki787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meisel A, Bickle TA, Krüger DH, Schroeder C. Type III restriction enzymes need two inversely oriented recognition sites for DNA cleavage. Nature. 1992;355:467–469. doi: 10.1038/355467a0. [DOI] [PubMed] [Google Scholar]
- 10.Meisel A, Mackeldanz P, Bickle TA, Kruger DH, Schroeder C. Type III restriction endonucleases translocate DNA in a reaction driven by recognition site-specific ATP hydrolysis. EMBO J. 1995;14:2958–2966. doi: 10.1002/j.1460-2075.1995.tb07296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peakman LJ, Antognozzi M, Bickle TA, Janscak P, Szczelkun MD. S-adenosyl methionine prevents promiscuous DNA cleavage by the EcoP1I Type III restriction enzyme. J. Mol. Biol. 2003;333:321–335. doi: 10.1016/j.jmb.2003.08.042. [DOI] [PubMed] [Google Scholar]
- 12.Peakman LJ, Szczelkun MD. DNA communications by Type III restriction endonucleases-confirmation of 1D translocation over 3D looping. Nucleic Acids Res. 2004;32:4166–4174. doi: 10.1093/nar/gkh762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haberman A. The bacteriophage P1 restriction endonuclease. J. Mol. Biol. 1974;89:545–563. doi: 10.1016/0022-2836(74)90035-7. [DOI] [PubMed] [Google Scholar]
- 14.Humbelin M, Suri B, Rao DN, Hornby DP, Eberle H, Pripfl T, Kenel S, Bickle TA. Type III DNA restriction and modification systems EcoP1 and EcoP15. J. Mol. Biol. 1988;200:23–29. doi: 10.1016/0022-2836(88)90330-0. [DOI] [PubMed] [Google Scholar]
- 15.Janscak P, Sandmeier U, Szczelkun MD, Bickle TA. Subunit assembly and mode of DNA cleavage of the Type III restriction endonucleases EcoP1I and EcoP15I. J. Mol. Biol. 2001;306:417–431. doi: 10.1006/jmbi.2000.4411. [DOI] [PubMed] [Google Scholar]
- 16.Hornby DP, Müller M, Bickle TA. High level expression of the EcoP1 modification methylase gene and characterisation of the gene product. Gene. 1987;54:239–245. doi: 10.1016/0378-1119(87)90492-6. [DOI] [PubMed] [Google Scholar]
- 17.Ahmad I, Rao DN. Interaction of EcoP15I DNA methyltransferase with oligonucleotides containing the asymmetric sequence 5′-CAGCAG-3′. J. Mol. Biol. 1994;242:378–388. doi: 10.1006/jmbi.1994.1588. [DOI] [PubMed] [Google Scholar]
- 18.Ahmad I, Krishnamurthy V, Rao DN. DNA recognition by the EcoP15I and EcoPI modification methyltransferases. Gene. 1995;157:143–147. doi: 10.1016/0378-1119(95)00671-r. [DOI] [PubMed] [Google Scholar]
- 19.Kumar R, Srivastava R, Singh RK, Surolia A, Rao DN. Activation and inhibition of DNA methyltransferases by S-adenosyl-L-homocysteine analogues. Bioorg. Med. Chem. 2008;16:2276–2285. doi: 10.1016/j.bmc.2007.11.075. [DOI] [PubMed] [Google Scholar]
- 20.Ramanathan SP, van Aelst K, Sears A, Peakman LJ, Diffin FM, Szczelkun MD, Seidel R. Type III restriction enzymes communicate in 1D without looping between their target sites. Proc. Natl Acad. Sci. USA. 2009;106:1748–1753. doi: 10.1073/pnas.0807193106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mücke M, Reich S, Möncke-Buchner E, Reuter M, Krüger DH. DNA cleavage by Type III restriction-modification enzyme EcoP15I is independent of spacer distance between two head to head oriented recognition sites. J. Mol. Biol. 2001;312:687–698. doi: 10.1006/jmbi.2001.4998. [DOI] [PubMed] [Google Scholar]
- 22.Raghavendra NK, Rao DN. Unidirectional translocation from recognition site and a necessary interaction with DNA end for cleavage by Type III restriction enzyme. Nucleic Acids Res. 2004;32:5703–5711. doi: 10.1093/nar/gkh899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raghavendra NK, Rao DN. Exogenous AdoMet and its analogue sinefungin differentially influence DNA cleavage by R.EcoP15I – usefulness in SAGE. Biochem. Biophys. Res. Commun. 2005;334:803–811. doi: 10.1016/j.bbrc.2005.06.171. [DOI] [PubMed] [Google Scholar]
- 24.Möncke-Buchner E, Rothenberg M, Reich S, Wagenführ K, Matsumura H, Terauchi R, Krüger DH, Reuter M. Functional Characterization and Modulation of the DNA Cleavage Efficiency of Type III Restriction Endonuclease EcoP15I in Its Interaction with Two Sites in the DNA Target. J. Mol. Biol. 2009;387:1309–1319. doi: 10.1016/j.jmb.2009.02.047. [DOI] [PubMed] [Google Scholar]
- 25.Sukackaite R, Lagunavicius A, Stankevicius K, Urbanke C, Venclovas C, Siksnys V. Restriction endonuclease BpuJI specific for the 5′-CCCGT sequence is related to the archaeal Holliday junction resolvase family. Nucleic Acids Res. 2007;35:2377–2389. doi: 10.1093/nar/gkm164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray NE. Fred Griffith review lecture. Immigration control of DNA in bacteria: self versus non-self. Microbiology. 2001;148:3–20. doi: 10.1099/00221287-148-1-3. [DOI] [PubMed] [Google Scholar]
- 27.Simmon VF, Lederberg S. Degradation of bacteriophage lambda deoxyribonucleic acid after restriction by Escherichia coli K-12. J. Bacteriol. 1972;112:161–169. doi: 10.1128/jb.112.1.161-169.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dillingham MS, Kowalczykowski SC. RecBCD enzyme and the repair of double-stranded DNA breaks. Microbiol. Mol. Biol. Rev. 2008;72:642–671. doi: 10.1128/MMBR.00020-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stahl MM, Kobayashi I, Stahl FW, Huntingdon SK. Activation of Chi, a recombinator, by the action of endonuclease at a distant site. Proc. Natl Acad. Sci. USA. 1983;80:2310–2313. doi: 10.1073/pnas.80.8.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raghavendra NK, Rao DN. Functional cooperation between exonucleases and endonucleases-basis for the evolution of restriction enzymes. Nucleic Acids Res. 2003;31:1888–1896. doi: 10.1093/nar/gkg275. [DOI] [PMC free article] [PubMed] [Google Scholar]