Summary
C. albicans yeast forms deleted for ALS5, ALS6 or ALS7 are more adherent than a relevant control strain to human vascular endothelial cell monolayers and buccal epithelial cells. In the buccal and vaginal reconstituted human epithelium (RHE) disease models, however, mutant and control strains caused a similar degree of tissue destruction. Deletion of ALS5 or ALS6 significantly slowed growth of the mutant strain; this phenotype was not affected by addition of excess uridine to the culture medium. These studies demonstrate similar phenotypic characteristics for the als5Δ/als5Δ, als6Δ/als6Δ and als7Δ/als7Δ strains that are not observed in any of the other C. albicans alsΔ/alsΔ strains.
Keywords: Candida albicans, ALS gene family, adhesion, host-pathogen interaction
Introduction
The Candida albicans ALS family includes eight genes that encode large cell-surface glycoproteins [1]. Initially, it was hypothesized that the Als proteins function in adhesion of C. albicans to host surfaces [1]. Deletion of single ALS genes showed contributions to adhesion for Als1p, Als2p, Als3p, Als4p and Als9p [2-6]. This work focuses on the remaining ALS genes: ALS5, ALS6 and ALS7. The phenotype of als5Δ/als5Δ, als6Δ/als6Δ, and als7Δ/als7Δ strains was examined in two different adhesion assays and an in vitro model of epithelial tissue destruction.
Materials and methods
Strains and construction of alsΔ/alsΔ mutants
Strains and methods for mutant construction and validation were published previously [3]. Table 1 details the construction of C. albicans strains deleted for ALS5, ALS6 or ALS7. Strain CAI12 (iro1-ura3Δ::λimm434/IRO1 URA3) [11] was used as a control in all phenotypic assays.
Table 1.
Summary of methods for disruption of ALS5, ALS6 or ALS7 in C. albicansa
| PCR Deletion Cassetteb | hisG-URA3-hisG Deletion Cassettec | Reintegration of Wild-Type Alleled | Southern Blot Probe |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| alsΔ/ALS | alsΔ/alsΔ | alsΔ/alsΔ::□□□ | ||||||||||
| Gene | Allelee | Primer Namesf |
Strain Name |
Allele | Upstream Primersf |
Downstream Primersf |
Strain Name |
Allele | Upstream and Coding Region Primersf |
Downstream Primersf |
Strain Name |
Primer Namesf |
| ALS5 | N/Ag | ALS5SA | ALS5upF ALS5upR |
ALS5dnF ALS5dnR |
1447 | ALS5gfpF ALS5gfpR |
||||||
| ALS5LA | 2373 | ALS5SA | ALS5upF ALS5R |
ALS5dnF ALS5dnF |
2407 | |||||||
| ALS6 | ALS6-1 | ALS6-5DR ALS6-3DR |
1268 | ALS6-2 | ALS6upF ALS6upR |
ALS6dnF ALS6dnR |
1420 | ALS6 | ALS6upF ALS6R |
ALS6dnF ALS6dnR |
1522 | ALS6gfpF ALS6gfpR |
| ALS7 | ALS7-1 | ALS7-5DR ALS7-3DR |
1275 | ALS7-2 | ALS7upF ALS7upR |
ALS7dnF ALS7dnR |
1429 | ALS7 | ALS7upF ALS7R |
ALS7dnF ALS7dnR |
1964 | ALS7gfpF ALS7gfpR |
All deletions were constructed in strain CAI4 (iro1-ura3Δ::λimm434/ iro1-ura3Δ::λimm434) [7], which was a generous gift from William Fonzi (Georgetown University).
Amplification of a deletion cassette using plasmid pDDB57 as template was described by Wilson et al. [8]. Plasmid pBBD57, which contains a PCR-amplifiable copy of a deletion cassette that encodes a URA3 selectable marker and direct-repeat flanking sequences to promote excision of the cassette, was provided by Aaron Mitchell (Columbia University).
This method for gene deletion was described by Fonzi and Irwin [6]. Deletion of ALS genes uses plasmid pHUL [3], which is a modified version of pMB7 [7] that contains HindIII-AvrII-XhoI-SpeI restriction sites 5′ of the hisG-URA3-hisG cassette, and KpnI-SstII-NgoMIV-SstI sites downstream of the cassette. To make a disruption cassette, sequences upstream and downstream of the target gene are cloned into pHUL using the AvrII-XhoI and SstII-NgoMIV restriction sites, respectively. The final cassette is excised by AvrII-NgoMIV digestion prior to transformation into a Uri- strain.
Reintegration of a wild-type ALS allele was accomplished using plasmid pUL [3]. Plasmid pUL differs from pHUL in that the hisG-URA3-hisG cassette in pHUL is replaced by URA3 in pUL.
In SC5314, the sequence of the ALS5 alleles is nearly identical except that the small allele (ALS5SA) has 4 tandem copies of the repeated sequence within the central domain while the large allele (ALS5LA) has 5 tandem copies [8]. ALS6 alleles each encode 4 tandem repeat copies and are likely to have only minor sequence differences [9]. SC5314 ALS7 alleles were described by Zhang et al. [10] and have the same number of tandem repeat copies and same configuration of repeated sequences within the 3′ domain.
Primer names correspond to sequences shown in Table 2.
N/A = not applicable. This method was not used to disrupt this gene.
Culture conditions
Strains for phenotypic testing were streaked from -80°C glycerol stocks to YPD plates (1% yeast extract, 2% peptone, 2% glucose with 2% agar for plates). Plates were incubated 24 h at 37°C and stored at 4°C for no more than one week. A single C. albicans colony was resuspended in 1 ml YPD and 10 μl used to inoculate a 10 ml YPD culture. The culture was incubated 16 h at 37°C with 200 rpm shaking. Typical cell counts following this procedure are approximately 2 to 3 × 108 cells ml-1 with approximately 20% budding cells.
Growth rate and cellular aggregation measurements
Growth rate measurement methods were published previously [3]. Cellular aggregation measurements were made using a published method [3]. C. albicans cells were grown in YPD as described above and washed in DPBS (Cambrex catalog number 17-512Q) prior to the assay. The number of yeast cells in an aggregate was recorded for 100 independent cellular units. Budding cells were counted as one cell, since bud attachment is not due to aggregation. Differences in cellular aggregation were judged by mixed model analysis of variance (PROC MIXED in SAS; SAS Institute) of the means of duplicate measurements from three separate days [3].
Adhesion assays
Yeast forms for adhesion assays were grown in YPD and washed in DPBS as described above. Human umbilical vein endothelial cell assays were conducted in a 6-well plate format [3]. Buccal epithelial cell adhesion assays also followed a published method [5].
Reconstituted human epithelium (RHE) model
Methods for assessing C. albicans destruction of buccal and vaginal RHE are published [3, 12, 13].
Results
Deletion of ALS5, ALS6 or ALS7 resulted in significantly increased adhesion of the mutant strain to human vascular endothelial cell monolayers and buccal epithelial cells (Fig. 1A and B). This observation is opposite of the result expected from deletion of a putative adhesin and suggests the potential for an anti-adhesive role for Als5p, Als6p and Als7p. C. albicans mutant strains cultured in the presence of exogenous uridine also showed increased adhesion relative to a control strain suggesting that the observed phenotype is not due to placement of the URA3 marker (Fig. 1C). Reintegration of a single wild-type ALS allele did not restore wild-type adhesion levels except for the ALS6 strains in the endothelial cell assay (Fig. 1A). Further investigation is required to determine if these results reflect allelic functional differences or a requirement for the presence of both alleles for wild-type function. The need to reintegrate both wild-type alleles to restore wild-type function was reported for another C. albicans cell wall protein-encoding gene, ECM33 [14]. Cellular aggregation was evaluated for the mutant strains since this property can affect adhesion assay results. ALS5 or ALS6 deletion produced a slight, but statistically significant, increase in cellular aggregation (Table 3). However, this aggregation increase is not sufficient to account for the adhesion assay results (Fig. 1) suggesting that an alternative explanation is required. Potential explanations for the increased adhesion of the mutant strains include up-regulation of another adhesin-encoding gene in response to ALS deletion or alteration of cell wall structure that exposes other adhesive moieties on the C. albicans surface. Additional investigation is necessary to distinguish between these possibilities.
Fig. 1.
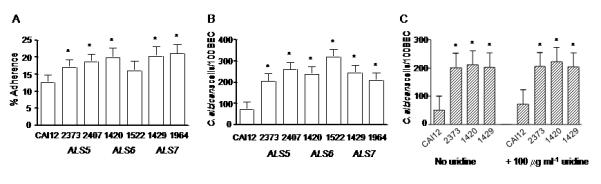
Adhesion assay data. Histogram showing the adherence of C. albicans strains to monolayers of human umbilical vascular endothelial cells (A), and buccal epithelial cells (B and C). Asterisks mark results that are significantly different from the control strain CAI12 (P < 0.05). Deletion of ALS5, ALS6 or ALS7 results in increased adhesion of C. albicans yeast forms to both cell types. Reintegration of a wild-type allele complemented the als6Δ/als6Δ strain in the endothelial cell assay. In general, however, reintegration of a single ALS allele did not complement the mutant phenotype suggesting the potential for allelic effects or a gene dosage requirement for full activity. Increased adhesive effects were also observed for C. albicans yeast forms cultured in the presence of excess uridine (C), suggesting that the results observed in (A) and (B) are not due to placement of the URA3 marker.
Table 3.
Cellular aggregation and growth rate dataa
| Strain | ALS Gene Genotype |
Cellular Aggregation (cells/aggregate) |
Doubling Time (h) |
|---|---|---|---|
| CAI12 | Control | 1.002 ± 0.005 | 1.67 ± 0.051, 1.75 ± 0.062 1.85 ± 0.063 |
| 2373 | als5Δ/als5Δ | 1.032 ± 0.005* | 1.60 ± 0.051* |
| 2407 | als5Δ/als5Δ::ALS5SA | 1.013 ± 0.005 | 1.68 ± 0.081 |
| 1420 | als6Δ/als6Δ | 1.023 ± 0.005* | 1.66 ± 0.072* |
| 1522 | als6Δ/als6Δ::ALS6 | 1.017 ± 0.005* | 1.69 ± 0.032* |
| 1429 | als7Δ/als7Δ | 1.010 ± 0.005 | 1.67 ± 0.073 |
| 1964 | als7Δ/als7Δ::ALS7 | 1.013 ± 0.005 | 1.64 ± 0.083 |
Means and standard errors are reported. Asterisks indicate means that are significantly different from the CAI12 control (P < 0.05).
Growth rate was measured separately for ALS5-, ALS6- and ALS7-related strains. The superscript 1, 2 or 3 for the CAI12 data matches the superscripts for the different experimental groups in the growth rate column.
Despite the large increase in adhesion associated with deletion of ALS5, ALS6 or ALS7, destruction of epithelial cells in the buccal RHE disease model showed no difference between a control strain and the als5Δ/als5Δ, als6Δ/als6Δ and als7Δ/als7Δ mutant strains (Fig. 2). Testing of vaginal RHE produced similar results (data not shown). Inoculation of the vaginal RHE model with other previously published alsΔ/alsΔ strains [3-5] produced results similar to those observed for buccal RHE (data not shown). The greatest decrease in epithelial layer damage was observed for the als3Δ/als3Δ strain with lesser reductions observed for the als1Δ/als1Δ and als2Δ/PMAL2-ALS2 strains. No changes from control strain activity were observed for the als4Δ/als4Δ and als9Δ/als9Δ strains.
Fig. 2.
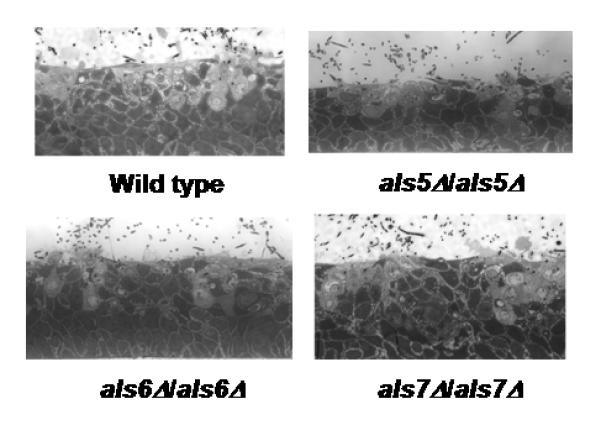
Light micrographs of buccal RHE inoculated with various C. albicans strains for 8 h. The ability of strains lacking ALS5, ALS6 or ALS7 to destroy epithelial cells was similar to that of the control strain CAI12.
Another notable phenotype of the C. albicans strains in which ALS5, ALS6 or ALS7 was deleted was decreased growth rate compared to the control strain (Table 3). While all three mutant strains consistently grew more slowly than the control, only the growth rate for strains lacking ALS5 or ALS6 was statistically significantly slower (Table 3). Reintegration of the ALS5SA allele (Table 1) restored the growth rate, but reintegration of a single ALS6 allele did not (Table 3). Addition of 100 μg ml-1 uridine to the culture medium did not change the growth kinetics of the mutant strains suggesting that placement of the URA3 marker is not responsible for this phenotype (data not shown). The slowed growth rate phenotype prompted repeated construction of the als5Δ/als5Δ strain. Each independently constructed als5Δ/als5Δ strain had a slowed growth rate. It is formally possible that these ALS genes play a role in maintaining wild-type growth rate in C. albicans.
Discussion
Data presented here complete an initial phenotypic analysis of adhesion of C. albicans mutant strains in which wild-type expression of one of the ALS genes was disrupted. Growth conditions for adhesion assays were selected based on knowledge of ALS gene expression patterns in vitro. Adhesion assays used germ tubes grown in RPMI 1640 medium to assess the adhesive contribution of Als1p, Als2p, Als3p and Als4p [3, 4] while yeast forms grown overnight in YPD were assayed for Als5p, Als6p, and Als7p in this manuscript, and for Als9p [5]. As more is learned about in vitro Als protein production, additional culture conditions can be identified and used to better understand the adhesive contribution of Als proteins.
The phenotypes of increased adhesion and slightly slowed growth rate are common to C. albicans strains lacking ALS5, ALS6 or ALS7. These genes are also consistently transcribed at a lower level than for other ALS genes, even in C. albicans cells recovered from human clinical specimens and animal models of disease [reviewed in 1]. These observations suggest that a sparse presence of Als5p, Als6p and Als7p is sufficient for function. Cellular localization of Als5p, Als6p and Als7p has not been determined, leaving open the possibility that the proteins are membrane-bound, rather than crosslinked to cell wall glucan like other Als proteins [reviewed in 1]. Similar phenotypic data for ALS5, ALS6 and ALS7 could indicate that their encoded proteins have functional redundancy or even that they function in a multiprotein complex. The intriguing preliminary results presented here highlight the numerous features of these genes and proteins that remain to be explored in C. albicans.
Table 2.
Oligonucleotide primers used in this study
| Primer Name |
Primer Orientation |
Primer Sequence (5′–3′) |
|---|---|---|
| ALS5upF | F | CCCCCTAGGACCAGCATTGTCAATCGAACCA |
| ALS5upR | R | CCCCTCGAGTATGAGGCTCCGGCAAAAGC |
| ALS5R | R | CCCCTCGAGTGTCAACGTTTGAGAATGACG |
| ALS5dnF | R | CCCCCGCGGCGTCATTCTCAAACGTTGACA |
| ALS5dnR | R | CCCGCCGGCATATCCACTTATTAGCTGTCA |
| ALS5F13 | F | CCCGGTACCCAACTAAAACTTTATCATCAAATCAC |
| ALS5gfpR | R | CCCCTCGAGCTGGTGTTAGCAGTTGGTAGTTGTTTG |
| ALS6–5DR | F | AAGTTTAAAAGAAAGCATGTTTCCTGTAGGAAATTTCATTCA TTGACTTGAATAAACATCGTTTTCCCAGTCACGACGTT |
| ALS6–3DR | R | TAAATAAACTCTAGAAATTGAAATATCTATAATAACGAAAAT AACAAAGTCAACGTTTGATGTGGAATTGTGAGCGGATA |
| ALS6upF | F | CCCCCTAGGAATCCCTGCGTATTATGGTATGG |
| ALS6upR | R | CCCCTCGAGTGAAATTTCCTACAGGAAACATGC |
| ALS6dnF | F | CCCCCGCGGCAAACGGGATTGTACCAAATC |
| ALS6dnR | R | CCCGCCGGCTGCTTCAGATCCAACACGTAA |
| ALS6R | R | CCCCTCGAGAAAAACAGAACAAAAAAAACGACACC |
| ALS6gfpF | F | CCCGGTACCTGTTTCAATCAATTGCCTATC |
| ALS6gfpR | R | CCCCTCGAGTGTCGGTGAATGGTGATGCTG |
| ALS7–5DR | F | TTTGAAAATAAGAATTTTTCATCAATCTAACAATCTACAATT TTCAACAGTCTAATACCTGTTTTCCCAGTCACGACGTT |
| ALS7–3DR | R | CATATAAATAATACATAAAACCTGGGTTTAAAAAACTGAAA ATTCATAACGAAAATCTTGTGTGGAATTGTGAGCGGATA |
| ALS7upF | F | CCCCCTAGGACCCGCCACAAAGTCACAGAA |
| ALS7—upR | R | CCCCTCGAGGTATAGTAATTGTAAGGTAACC |
| ALS7dnF | F | CCCCCGCGGCAAGATTTTCGTTATGAATTTTCAG |
| ALS7dnR | R | CCCGCCGGCAACCAGTGCTTTAGTATTGTG |
| ALS7R | R | CCCCTCGAGATCTTAGTCTCGATATAGTGTATC |
| ALS7gfpF | F | CCCGGTACCCCCAATAAATAATAATGAACACAAAAA |
| ALS7gfpR | R | CCCCTCGAGAGGTATTAGACTGTTGAAAATTGTAGAT |
Acknowledgements
This research was funded by grant DE14158 from the National Institute of Dental and Craniofacial Research, National Institutes of Health. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06 RR16515-01 from the National Center for Research Resources, National Institutes of Health.
References
- 1.Hoyer LL, Green CB, Oh S-H, Zhao X. Discovering the secrets of the Candida albicans agglutinin-like sequence (ALS) gene family—a sticky pursuit. Med Mycol. 2007 doi: 10.1080/13693780701435317. (Under revision) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fu Y, Ibrahim AS, Sheppard DC, et al. Candida albicans Als1p: an adhesin that is a downstream effector of the EFG1 filamentation pathway. Mol Microbiol. 2002;44:61–72. doi: 10.1046/j.1365-2958.2002.02873.x. [DOI] [PubMed] [Google Scholar]
- 3.Zhao X, Oh S-H, Cheng G, et al. ALS3 and ALS8 represent a single locus that encodes a Candida albicans adhesin; functional comparison between Als3p and Als1p. Microbiology. 2004;150:2415–2428. doi: 10.1099/mic.0.26943-0. [DOI] [PubMed] [Google Scholar]
- 4.Zhao X, Oh S-H, Yeater KM, Hoyer LL. Analysis of the Candida albicans Als2p and Als4p adhesins suggests the potential for compensatory function within the Als family. Microbiology. 2005;151:1619–1630. doi: 10.1099/mic.0.27763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao X, Oh S-H, Hoyer LL. Unequal contribution of ALS9 alleles to adhesion between Candida albicans and vascular endotheial cells. Microbiology. 2007 doi: 10.1099/mic.0.2006/005017-0. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phan QT, Myers CL, Fu Y, Sheppard DC, Yeaman MR, Welch WH, Ibrahim AS, Edwards JE, Filler SG. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol. 5:e64. doi: 10.1371/journal.pbio.0050064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fonzi WA, Irwin MY. Isogenic strain construction and gene mapping in Candida albicans. Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson RB, Davis D, Enloe BM, Mitchell AP. A recyclable Candida albicans URA3 cassette for PCR product-directed gene disruption. Yeast. 2000;16:65–70. doi: 10.1002/(SICI)1097-0061(20000115)16:1<65::AID-YEA508>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 9.Zhao X, Oh S-H, Jajko R, et al. Analysis of ALS5 and ALS6 allelic variability in a geographically diverse collection of Candida albicans isolates. Fungal Genet Biol. 2007 doi: 10.1016/j.fgb.2007.05.004. (Under revision) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang N, Harrex AL, Holland BR, et al. Sixty alleles of the ALS7 open reading frame in Candida albicans: ALS7 is a hypermutable contingency locus. Genome Res. 2003;13:2005–2017. doi: 10.1101/gr.1024903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porta A, Ramon AM, Fonzi WA. PRR1, a homolog of Aspergillus nidulans palF, control pH-dependent gene expression and filamentation in Candida albicans. J Bacteriol. 1999;181:7516–7523. doi: 10.1128/jb.181.24.7516-7523.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green CB, Cheng G, Chandra J, et al. RT-PCR detection of Candida albicans ALS gene expression in the reconstituted human epithelium (RHE) model of oral candidiasis and in model biofilms. Microbiology. 2004;150:267–275. doi: 10.1099/mic.0.26699-0. [DOI] [PubMed] [Google Scholar]
- 13.Cheng G, Wozniak K, Wallig MA, et al. Comparison between Candida albicans agglutinin-like sequence gene expression patterns in human clinical specimens and models of vaginal candidiasis. Infect Immun. 2005;73:1656–1663. doi: 10.1128/IAI.73.3.1656-1663.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez-Lopez RL, Monteoliva R, Diez-Orejas C, Nombela C, Gil C. The GPI-anchored protein CaEcm33p is required for cell wall integrity, morphogenesis and virulence in Candida albicans. Microbiology. 2004;150:3341–3354. doi: 10.1099/mic.0.27320-0. [DOI] [PubMed] [Google Scholar]


