Abstract
During development of the adult olfactory system of the moth Manduca sexta, olfactory receptor neurons extend axons from the olfactory epithelium in the antenna into the brain. As they arrive at the brain, interactions with centrally-derived glial cells cause axons to sort and fasciculate with other axons destined to innervate the same glomeruli. Here we report studies that indicate that activation of the epidermal growth factor receptor (EGFR) is involved in axon ingrowth and targeting. Blocking the EGFR kinase domain pharmacologically leads to stalling of many axons in the sorting zone and nerve layer, as well as abnormal axonal fasciculation in the sorting zone. We also find that neuroglian, an IgCAM known to activate the EGFR through homophilic interactions in other systems, is transiently present on olfactory receptor neuron axons and on glia during the critical stages of the sorting process. The neuroglian is resistant to extraction with Triton X-100 in the sorting zone and nerve layer, possibly indicating its stabilization by homophilic binding in these regions. Our results suggest a mechanism whereby neuroglian molecules on axons and possibly sorting zone glia bind homophilically, leading to activation of EGFRs with subsequent effects on axon sorting, pathfinding, and extension, and glomerulus development.
Indexing Terms: glial cells, olfactory receptor neuron, neuroglian, cell adhesion molecule, antennal system
INTRODUCTION
During development, olfactory receptor neurons (ORNs) of different odor specificities arise in the olfactory epithelium and extend axons into the brain in such a way that axons of a given specificity converge onto particular glomeruli, creating “odotopically” organized odor-processing neuropils (Ressler et al., 1994; Vasser et al., 1994; Mombaerts et al., 1996; Hildebrand and Shepherd, 1997; Vosshall et al., 2000). Axons of receptor neurons of differing specificities are intermixed as they grow toward the brain; they must therefore undergo a sorting process before innervating their particular target glomeruli. The sorting and targeting of ORNs has been shown to depend on the integration of numerous chemical signals provided by extracellular matrix molecules (Treloar et al., 1996; Crandall et al., 2000; Raabe et al., 1997), cell adhesion molecules (Alenius and Bohm, 1997; 2003; Treloar et al., 1997; Yoshihara et al., 1997), glycosylation patterns (Schwarting et al., 1992; Puche et al., 1996; Lipscomb et al., 2003), receptor kinases (St. John et al., 2002; Kaneko and Nighorn, 2003), semaphorins (Pasterkamp et al., 1999), and individual odorant receptor proteins (Mombaerts et al., 1996; Wang et al., 1998; Barnea et al., 2004).
In other, non-olfactory systems, direct cell-to-cell interactions have been shown to involve many of these molecules. Most relevant to the current study are reports of critical involvement of cell adhesion molecules belonging to the Ig superfamily (IgCAMs) (Hortsch and Goodman, 1991; Walsh and Doherty, 1997; Kamiguchi and Lemmon, 2000), and receptor tyrosine kinase-mediated signaling, which can be activated both by diffusible ligands (e.g. epidermal growth factor, EGF, and fibroblast growth factor, FGF) and directly by IgCAMs (Williams et al., 1994; Doherty and Walsh, 1996; Saffell et al., 1995; 1997; Islam et al., 2004).
Numerous studies have established that glial cells play major roles in instructing axons as they make their pathfinding and sorting choices (see Auld, 1999; Lemke, 2001; Hidalgo, 2003; Oland and Tolbert, 2003; Chotard and Salecker, 2004; Tolbert et al., 2004 for reviews). In most cases the importance of neuron-glia interaction has been demonstrated without identifying the signals involved. Notable exceptions include the netrin-slit-roundabout-commissureless system (Seeger et al., 1993; Chisholm and Tessier-Lavigne, 1999; Long et al., 2004) and gliolectin (Tiemeyer and Goodman, 1996; Sharrow and Tiemeyer, 2001).
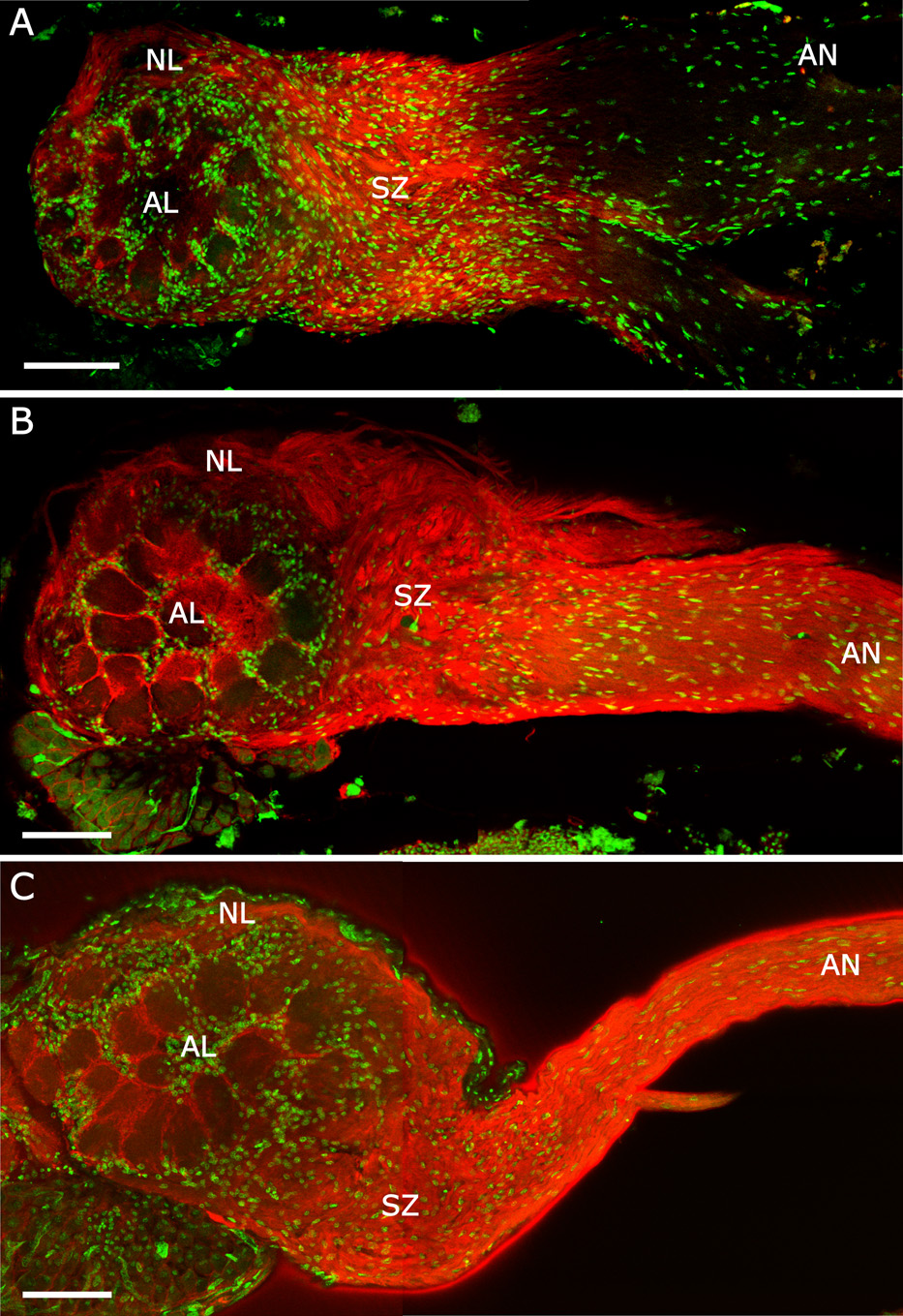
Previous work by our group in olfactory development in the moth Manduca sexta has revealed important interactions between glial cells and ORN axons, which extend from cell bodies in the olfactory epithelium of the antennae into the antennal lobes of the brain (Fig. 1; Oland and Tolbert, 1996; 2003). Axons encounter three types of glial cells as they grow into the antennal lobes: (1) antennal nerve (AN) glial cells, which arise in the periphery and migrate along ORN axons growing toward the brain; (2) sorting-zone (SZ) glial cells, which are centrally derived and migrate out from the nascent antennal lobe to populate the base of the antennal nerve, where ingrowing ORN axons sort according to glomerular target; and (3) neuropil-associated (NP) glia, also centrally derived, which migrate to surround protoglomeruli, the precursors of glomeruli (Oland et al., 1990). Using methods of reducing glial numbers and of removing antennal input, we have found that the earliest ORN axons induce the SZ glia to form the sorting zone, and those glia, in turn, are essential for the sorting and possibly the targeting of subsequently ingrowing ORN axons (Rössler et al., 1999). Similarly, ORN axons induce NP glia to surround the protoglomeruli formed by their axon terminals (Oland and Tolbert, 1987), and those glia, in turn, are essential to stabilize the developing glomerular structures (Oland et al., 1988; Oland and Tolbert, 1988; Baumann et al., 1996). Neuron-glia interactions have also been shown to be important in development of the mammalian olfactory bulb (Bailey et al., 1999; Treloar et al., 1999). Understanding the signaling mechanisms by which these neurons and glial cells communicate and influence each other’s behavior is important for understanding olfactory development and is a primary goal of the current study.
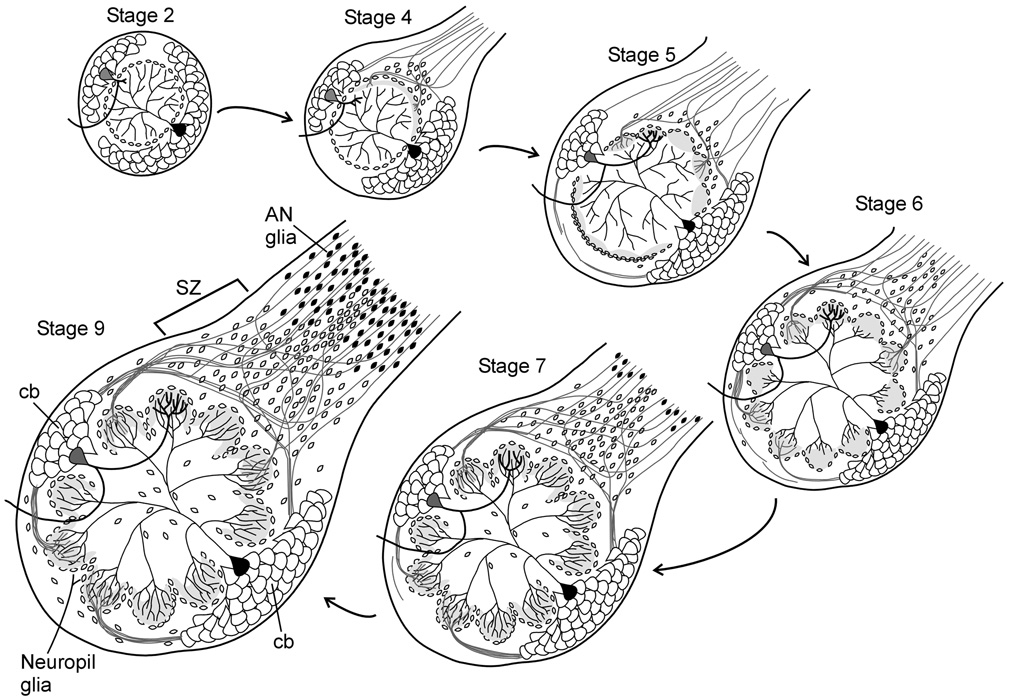
Figure 1. Development of the antennal lobe (AL) during metamorphic adult development of Manduca sexta.
ORN axons arrive at the nascent antennal lobe at stage 3 (Oland et al., 1996; not shown), inducing the proliferation and migration of central glia (St 4, open cell bodies) to form the sorting zone. Subsequently arriving axons change course in the sorting zone, defasciculating and refasciculating to join other axons targeting common glomeruli. ORN axon terminals form protoglomeruli at stage 5, inducing neuropil glia (open cell bodies) to migrate to surround and stabilize the glomeruli. Antennal nerve glia (small gray cell bodies) migrate along the ORN axons from the antennae toward the antennal lobes, stopping at the outer edge of the sorting zone at stage 7. By stage 9, the basic organizational structure of the antennal lobe is complete. (Reprinted from Tolbert et al., 2004, with permission from Elsevier.)
Previous experiments in our laboratory have suggested roles for fasciclin II (an IgCAM with homology to vertebrate NCAM and OCAM) and nitric oxide in neuron-glia interactions during development of the olfactory pathway of Manduca (Gibson et al., 2001; Higgins et al., 2002). Here we describe experimental results that indicate a role for EGF receptor (EGFR, a receptor tyrosine kinase), possibly activated by neuroglian (another invertebrate IgCAM, homologous to vertebrate L1; Bieber et al., 1989; Nardi, 1994) or fasciclin II, in extension, fasciculation, and targeting of ORN axons.
MATERIALS AND METHODS
Animals
Manduca sexta (Lepidoptera: Sphingidae) were reared from eggs on an artificial diet in a laboratory colony as previously described (Sanes and Hildebrand, 1976a). The animals were reared at 26° C and 50–60% relative humidity, under a long-day photoperiod regimen (17 hours light, 7 hours dark). Metamorphic development, when the adult antennal system develops, can be divided into 18 stages, each lasting 1–4 days, that span the time from pupation to eclosion of the adult moth. Animals were staged according to features, such as eye pigmentation and leg development, visible through the cuticle under fiber-optic illumination as described by Tolbert et al. (1983) and Oland and Tolbert (1987).
Removal of antennal input
In some animals, the antennal lobe on one side was deprived of ORN axon input throughout development, using surgical methods described previously (Sanes et al., 1977; Oland and Tolbert, 1987). Briefly, animals at stage 1 of adult development were anaesthetized by exposure to CO2. The cuticle covering the base of one antenna was removed and the underlying part of the antennal anlage removed with forceps. The opening was then filled with melted wax to prevent ORN axons from surviving distal receptor neurons from extending toward the brain, and the animals were returned to the rearing facility and allowed to develop under standard conditions. Because ORN axons do not project contralaterally, the antennal lobe on the operated side received no input from ORNs (Sanes et al., 1977; Kent, 1985), but did receive the normal small input from the receptor neurons in the labial palp pit organ, which terminate in a single, readily identified glomerulus in the ventromedial part of the antennal lobe (Kent et al., 1986; 1999).
Inhibition of EGFR activity
The highly selective, cell-permeable EGFR inhibitor PD168393 acts by inserting into the ATP-binding pocket and alkylating human EGFR at Cys-773, irreversibly inactivating the kinase function of the EGFR but not other protein kinases (Fry et al., 1998). Fifty animals at stages 3, 4 and 5 were injected with 5–20 µl of PD168393 (# 513033, Calbiochem, La Jolla, CA; IC50 = 700 pM) at concentrations of 1 mM or 10 mM in 100% DMSO (see Table I). Injections were made into the headspace just anterior to the brain or into the left optic lobe of the brain in order to minimize the likelihood of the drug being carried away by circulating hemolymph. Injection sites were sealed with dental wax and the animals allowed to develop as above until they reached stage 7–8 by external criteria. Vehicle-control animals were injected with equivalent amounts of DMSO without PD168393.
Table 1.
Effects of PD168393 Injection on Antennal Lobe Development
| PD168393 Injected (nmol) | Stage at injection | ||
|---|---|---|---|
| Headspace | 3 | 4 | e5 |
| 10 | Small Irreg Glomeruli (1/3) | Small Irreg Glomeruli (1/1) | Small Irreg Glomeruli (1/1) Enlarged SZ/NL (1/1) |
| 100 | Enlarged SZ/NL (2/2) Small Irreg Glomeruli (1/2) |
-- | Small Irreg Glomeruli (1/3) |
| 200 | Enlarged SZ/NL (2/2) Small Irreg Glomeruli (1/2) |
-- | Small AL (2/2) |
| 500 | Stalled Axons (4/4) Small Irreg Glomeruli (3/4) Enlarged SZ/NL (2/4) Small AL (4/4) Thin AN (2/4) |
Stalled Axons (2/2) Small Irreg Glomeruli (2/2) Small AL (2/2) Enlarged SZ/NL (1/2) |
|
| Optic Lobe | |||
| 50 | Stalled Axons (4/6) Small AL (2/6) Thin AN (2/6) Enlarged SZ/NL (1/6) |
Small AL (5/5) Stalled Axons (3/5) Small Irreg Glomeruli (2/5) Enlarged SZ/NL (2/5) |
Small AL (3/4) Thin AN (3/4) Enlarged SZ/NL (1/4) |
| 100 | Small Irreg Glomeruli (2/2) Enlarged SZ/NL (2/2) |
Small Irreg Glomeruli (3/3) Enlarged SZ/NL (3/3) Small AL (1/3) Thin AN (1/3) |
|
Animals were injected at the stages indicated, then allowed to develop until stage 7–8. Numbers in parentheses denote the number of animals exhibiting a particular phenotype out of the total number receiving the treatment. For each cell, abnormal phenotypes are ordered by descending frequency of occurance. Abbreviations: AL, antennal lobe; AN, antennal nerve; NL, nerve layer of the antennal lobe; SZ, sorting zone.
Primary Antibodies for Immunocytochemistry
Neuroglian
Mouse monoclonal antibody 3B11 against Manduca sexta neuroglian (Nardi, 1993; Chen et al., 1997) was the generous gift of Dr. James Nardi, University of Illinois, Urbana, IL. In Western blots it recognizes a single band of 156 kDa (Nardi, 1993; Fig. 2).
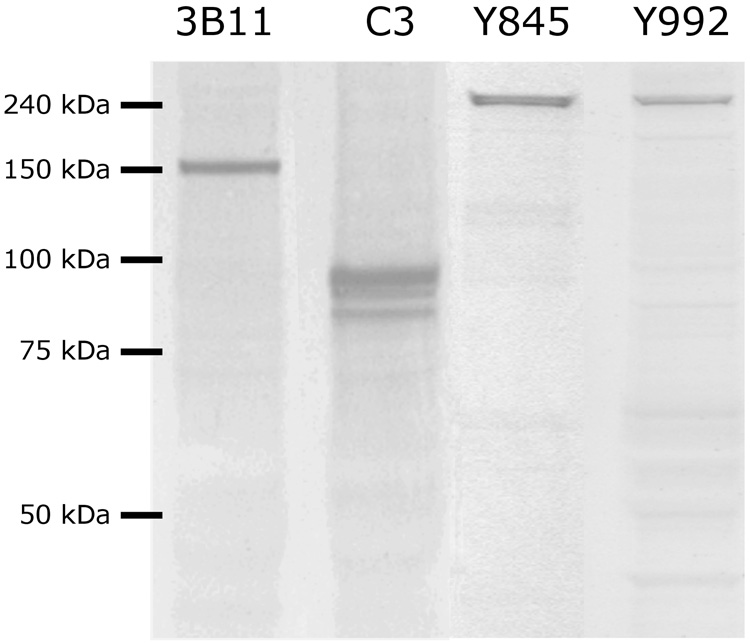
Figure 2. Western blots of tissue from desheathed stage 7 antennal lobes demonstrate the specificity of the 3B11 (neuroglian), C3 (fasciclin II) and pEGFR (Y845 and Y992) antibodies.
The 3B11 antibody recognizes a single band at 156 kDa; the C3 antibody recognizes multiple bands, representing transmembrane and GPI-linked isoforms, at 85 – 95 kDa; both pEGFR antibodies recognize a band at 250 kDa.
Fasciclin II
Mouse monoclonal antibody P1E1-1C3 (“C3,” Wright et al., 1999; Higgins et al., 2002), developed against the extracellular domain common to all isoforms of Manduca sexta fasciclin II (MfasII), was a generous gift of Dr. Philip Copenhaver, Oregon Health Sciences University, Portland, OR. It recognizes 3 bands of approximately 85, 90 and 95 kDa (Fig. 2, and as reported by us previously: Higgins et al., 2001).
EGFR
Absent availability of an antibody raised against a Manduca EGFR, we used an antibody (#ab62 from Abcam, Cambridge, MA), developed against a highly conserved portion of the mammalian EGFR. Ab62 is a mouse monoclonal antibody raised against a peptide corresponding to amino acids 985–996 (DVVDADEYLIPQ) of the human EGFR protein (ErbB-1). This sequence is very close to the corresponding EGFR sequence from another moth, Bombyx mori (SLVDADEYLQPK; from the Bombyx mori sequence published by the Beijing Genomics Institute at http://silkworm.genomics.org.cn; see also Xia et al., 2004; Wang et al., 2005). In addition, a translated BLAST (Altschul et al., 1997) search of the Bombyx mori sequence against Manduca expressed sequence tags produced a good match to gi|14860778|gb|BI262654.1| (SLVEADEYLQPK; Zhu et al., 2003), with 11 of the 12 amino acids being identical between the two moth species and 7 amino acids identical between the Manduca sequence and that of human EGFR. This provided a reasonable expectation that the anti-human EGFR antibody would work well in Manduca. This antibody produced localized labeling in immunocytochemistry, but did not work for Western blots (not shown), so we tested for specificity of labeling using preabsorption of the antibody (see below).
To better understand the time course and location of EGFR activation, it was important to differentiate between activated EGFRs (those phosphorylated at particular tyrosine residues) and non-activated EGFRs. The Bombyx sequence reveals two regions, with high homology to those in humans, containing tyrosine residues that are phosphorylated as the result of activation in human EGFRs. We obtained two antibodies (#2231 and 2235, Cell Signaling Technology, Beverly, MA), which are specific for human EGFR phosphorylated at tyrosine residues #845 and 992, respectively (pEGFR-Y845 and pEGFR-Y992). We performed Western blots with these antibodies and found that both produced a strong band at approximately 250 kDa (Fig 2). The Bombyx sequence has a predicted molecular weight of 120 kDa; the actual value would be expected to be somewhat greater due to glycosylation. Therefore the observed value of 250 kDa is in line with the predicted value for EGFR dimers.
Immunocytochemistry Protocol
Pupal animals were anaesthetized by cooling on ice. Brains were dissected under insect saline solution (150 mM NaCl, 4 mM KCl, 6 mM CaCl2, 10 mM HEPES, 5 mM glucose, pH 7.0, adjusted to 360 mOsm with mannitol; Hayashi and Hildebrand, 1990). The perineurial sheaths covering the brains were removed to aid in fixative and antibody penetration. For 3B11 and C3 immunocytochemistry, brains were fixed on a shaker overnight at 4° C in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. For EGFR immunocytochemistry, the fixation protocol of Sinakevitch et al. (2001) was used. Desheathed brains were dissected into 2.5% paraformaldehyde, 1% glutaraldehyde, 1% sodium metabisulfite in 0.1M cacodylate buffer, pH 7.2, microwaved as described, and fixed overnight on a shaker at 4° C. This second fixation technique was followed by a wash for 30 min in freshly prepared 0.01M NaBH4, 0.5% sodium metabisulfite, in 0.05M Tris-HCl, pH7.5. Tissue fixed by any of these methods was washed in Tris-buffered saline (20 mM Tris-HCl, 150 mM NaCl, pH 7.2), embedded in 5% agarose (Sigma, #A-0169), and cut into 100 µm slices using a vibrating microtome (Vibratome, Technical Products International, St. Louis, MO).
For all but the phospho-EGFR antibodies, sections were incubated in phosphate-buffered saline (PBS), pH 7.4 + 0.5% Triton X-100 + 0.1% sodium azide (PBSAT) with 5% normal goat serum (3B11, and C3 antibodies), in PBSAT + 2% bovine serum albumin (Sigma # A4378) (Abcam anti-EGFR antibody) or in PBS + 0.1% sodium azide (PBSA) with 5% normal goat serum (3B11) for 1 hour at room temperature. Primary antibodies were added as follows: Abcam anti-EGFR at 1:500; 3B11 at 1:60,000; and C3 at 1:10,000. The sections were incubated overnight at room temperature on a shaker, then washed 4X in PBS + 0.5% Triton X-100 (PBST) or PBS, and incubated in PBSAT or PBS containing Cy3-conjugated goat anti-rabbit or goat anti-mouse IgG+IgM antibodies (Jackson ImmunoResearch, West Grove, PA) at 1:300 for 2 hours at room temperature. Sections were then washed 3X in PBST and 2X in PBS and mounted in 20% PBS:80% glycerol.
Immunocytochemistry with the pEGFR-Y845 and pEGFR-Y992 antibodies was carried out as described for the Abcam EGFR antibody, except that Tris buffer, pH 7.5, was substituted for phosphate buffer in all solutions. The antibodies were added at 1:5,000.
Controls for nonspecific immunolabeling by the secondary antibodies consisted of brains prepared as described above but with the primary antibody omitted. Control sections imaged using microscope settings identical to those used to produce the images reported in this work displayed no visible labeling (not shown).
Preabsorption of EGFR Antibody
The peptide EGPESLVDADEYLQPK, corresponding to the region of the Bombyx mori EGFR that we expected the antibodies to recognize in Manduca (given the sequence similarity described above), was produced for us by Biomer Technology, Concord, CA. Five mg of a crude preparation was dissolved in 1 ml PBSAT. A single brain, fixed in the 1% glutaraldehyde fixative described above, was bisected down the midline, and the two halves embedded and sectioned as described above. Both brain halves were incubated in PBSAT + 2% BSA for one hour at room temperature. During this time, BSA was added to give a final concentration of 2% in 0.5 ml of the peptide solution, and 0.5 µl of the Abcam anti-EGFR antibody added. The mixture was rotated continuously at room temperature for one hour. One set of brain sections was then incubated overnight at 4° C in 0.5 ml PBSAT containing 2% BSA + 0.5 µl antibody; the other set was incubated overnight at 4° C in the preabsorption mixture. Sections were then washed, incubated with secondary antibodies, and mounted as described above.
Labeling of Cell Nuclei
To render glial cells visible, all cell nuclei were labeled with a DNA-specific tag. Brain sections were washed two times in Tris-HCl, pH 7.2 to remove phosphate ions and salt, then incubated 15 min at room temperature in Syto 59 (Molecular Probes, #S-11341) diluted 1:5,000, or Syto 13 (Molecular Probes, #S-7575) diluted 1:7500 in Tris-HCl. Sections were then washed 3X in Tris-HCl and 1X in 60% glycerol in water, and mounted in 80% glycerol in distilled water.
Dye-Labeling of ORN Axons
Brains were fixed overnight at 4° C in 4% paraformaldehyde + 0.15% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4. Extent of growth and arborization of ORN axons was studied by mass-labeling of the axons with the lipophilic dye 1,1'-dioctadecyl-3,3,3',3'-tetramethylindodicarbocyanine, 4-chlorobenzene-sulfonate salt (DiD, Molecular Probes #D-7757), as described previously (Gibson et al., 2001). Brains were dissected with long antennal nerves attached and were fixed overnight at 4° C in 4% paraformaldehyde plus 0.15% glutaraldehyde. Small amounts of the dye (which has a tar-like consistency) then were inserted into the antennal nerves using fine insect pins. The brains were returned to the fixative solution and stored at room temperature for seven days to allow the dye to diffuse along the axons. The brains then were washed, sectioned, labeled with Syto 13 as described above, and mounted in 80% glycerol in distilled water.
Western Blots
Antennal lobes of animals at stage 7 of development were quick-frozen in liquid nitrogen and stored overnight at −80° C. The tissue was homogenized in SDS sample buffer containing protease inhibitor cocktail (Sigma, # P2714). Samples to be probed with the C3 and 3B11 antibodies were run under non-reducing conditions (Nardi, 1993), those probed with EGFR antibodies were run under both reducing and non-reducing conditions, which produced similar results. Proteins were separated using the Novex electrophoresis system (InVitrogen, Carlsbad, CA) with a NuPage 4–12% Bis-Tris polyacrylamide gradient gel, transferred to PVDF membrane (Immobilon-P, Millipore, Bedford, MA), and probed with the primary antibodies in TBS+0.1%Tween 20 (TBS-Tween) followed by HRP-conjugated secondary antibodies (Jackson Immunoresearch). The blots were developed using the Opti-4CN kit (4-chloro-1-naphthol substrate; BioRad, Hercules, CA).
Confocal Microscopy
Sections were viewed on a Nikon PCM 2000 laser scanning confocal system (Nikon E800 microscope with argon, green He Ne, and red He Ne lasers) and Simple 32 software (Compix Inc., Cranberry Township, PA). Serial optical sections were imaged at 1- to 10-µm intervals through the depth of the Vibratome sections spanning the antennal lobe and saved as three-dimensional stacks. Different fluorophores were imaged sequentially, in order to optimize image quality, intensity and contrast for each fluorophore in each section.
Image Processing
Confocal image stacks were projected and merged in false color using Confocal Assistant (copyrighted by Todd Brelje, distributed by Bio-Rad, Richmond, CA), and then imported into Corel Photopaint, where image hue, intensity, and contrast were adjusted for maximum clarity. The images were then combined into figures in Corel Draw, where annotations were added.
RESULTS
EGFR-like Immunoreactivity
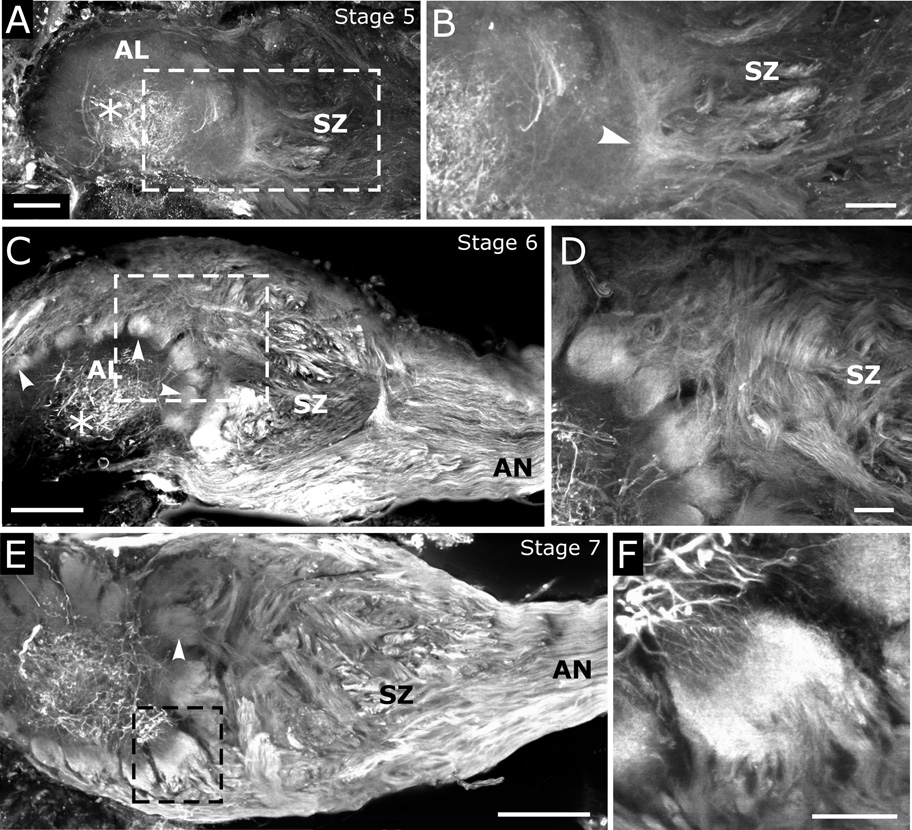
Using an antibody that recognizes a peptide sequence from the human EGFR that is closely homologous to a sequence found in moths, we observed prominent, developmentally regulated immunocytochemical labeling of ORNs and neurites of antennal lobe neurons (Fig. 3). ORN axons first displayed EGFR-like immunoreactivity as they traversed the sorting zone and began to coalesce to form protoglomeruli (the precursors to glomeruli: Oland et al., 1990) at stage 5 (Fig 3A,B). Antennal lobe neurons at stages 5–10 displayed intense labeling of their thick (generally non-synaptic: Tolbert, 1989) neurites in the coarse neuropil at the center of the lobe (Fig. 3A,B). By stage 6, ORN axons and their terminals in the protoglomeruli were intensely labeled (Fig. 3C,D), with greatest intensity of labeling in the sorting zone and protoglomeruli. Neurites of antennal lobe neurons could also be seen entering protoglomeruli (Fig. 3D, lower left). By stage 7, some developing glomeruli were labeled heavily in their middle regions, where ORN axon terminals overlap with terminals of antennal lobe neurons (Fig. 3E,F), whereas others were labeled only lightly (Fig. 3E, arrowhead).
Figure 3. EGFR immunocytochemistry of developing antennal lobes (ALs), using the Abcam antibody to human EGFR.
A) At stage 5, neurites of antennal lobe neurons in the coarse neuropil (*) are intensely labeled. Boxed area is shown at higher magnification in panel B. B) ORN axons are labeled in the sorting zone (SZ). Axons label more intensely as they sort (arrowhead). Labeling of neurites of antennal lobe neurons fades as the neurites extend away from the center of the antennal lobe. C) ORN axons are intensely labeled in the antennal nerve (AN) and SZ and in developing glomeruli (arrowheads) at stage 6. D) Higher magnification view of boxed area in panel C reveals intense labeling of neurites of AL neurons in the coarse neuropil (lower left) but not as they grow into glomeruli. Glomeruli are innervated by EGFR-immunoreactive axon terminals. Axons also can be seen to label in the SZ. E) By stage 7, some glomeruli have lost their intense labeling (arrowhead); others have not (boxed area). F) Higher magnification view of boxed area in panel E reveals intense labeling of coarse neurites of antennal lobe neurons (upper left corner), as well as their fine neurites entering a glomerulus. In addition, ORN axons are labeled. Scale bars = 100 µm (C,E), 50 µm (A), and 25 µm (B,D,F).
As a check for the specificity of the EGFR antibody, we performed preabsorption controls using a peptide corresponding to the region of the Bombyx mori EGFR that closely matched the human EGFR sequences against which the antibodies were raised. Sections incubated with the preabsorbed antibody showed no labeling (Supplemental Fig. 1).
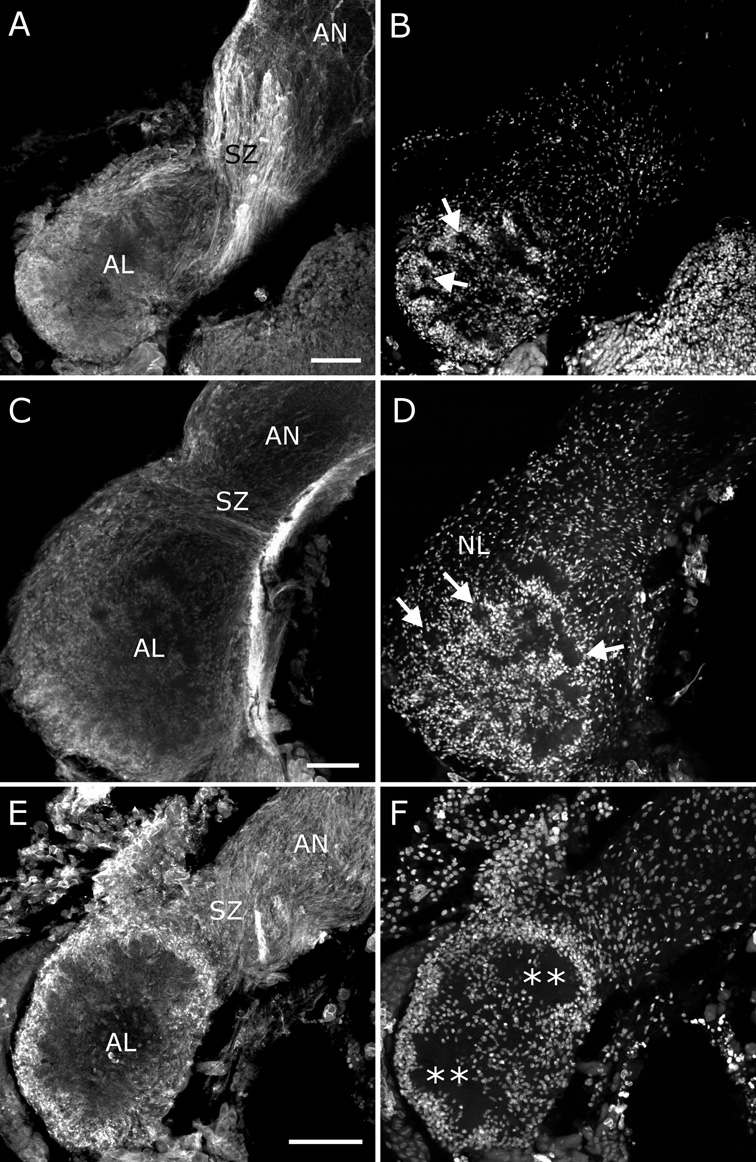
Immunocytochemical results with the EGFR antibody suggested that EGFRs are present on ORN axons and neurites of antennal lobe neurons. It was important to learn which of these receptors are actually activated during establishment of the antennal lobe architecture. We used two antibodies to the activated EGFR (pEGFR-Y845, phosphorylated at the tyrosine corresponding to Y845 of the human EGFR, and pEGFR-Y992, which recognizes the phosphorylated form of the sequence that is recognized by the Abcam EGFR antibody). The antibodies were used not only to determine EGFR activation but also for confirmation of the specificity of the Abcam EGFR antibody. The two pEGFR antibodies indeed labeled a subset of the structures labeled by the EGFR antibody: they labeled ORN axons, especially in glomeruli and the sorting zone (Fig 4). At stage early-5, fascicles of axons leaving the sorting zone were labeled intensely (Fig. 4A, arrowheads), while their terminals, lying beneath the nerve layer around the neuropil, were not labeled. At stage early-6, axon terminals forming the dorsolateral protoglomeruli (including the macroglomerulus in males), which have by now been in place for about a day, labeled intensely (Fig. 4B); the more recently formed glomeruli lying more ventromedially were not labeled (arrowheads in panel 4B). The lack of labeling of recently formed protoglomeruli indicate that EGFR activation declines following passage through the nerve layer and then increases again after protoglomerulus formation is already underway and maturation proceeds. By stage 7 all glomeruli were labeled, and axon labeling in the sorting zone had begun to fade (Fig. 4C,D). Because of the intense labeling of ORN axons in the sorting zone and the close apposition of glial and axonal membranes, we could not determine whether SZ glia labeled during these stages. Due to a decrease in intensity of ORN labeling distal to the sorting zone, AN glia, which arise in the antenna and migrate inward along ORN axons (Rössler et al., 1999), were seen to label intensely for the EGFR at stages 6 and 7 (Fig. 4E), but absence of labeling with the pEGFR antibody (Fig. 4F) suggests that the EGFRs are not activated at these stages.
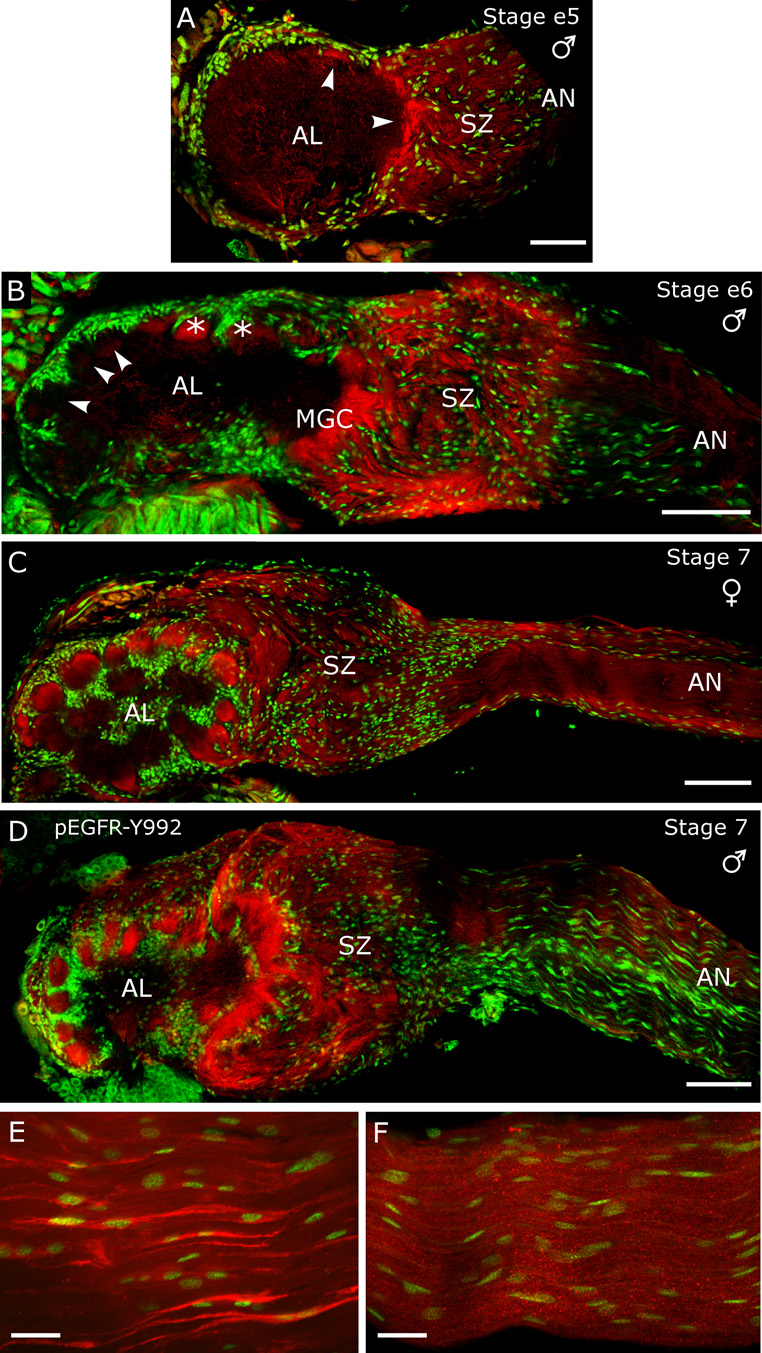
Figure 4. Immunocytochemistry for activated EGFRs in developing antennal lobes labels ORN axons in the sorting zone and glomeruli.
A–C) Anti-human pEGFR-Y845 (red) and the nuclear stain Syto 13 (green), D) Anti-human pEGFR-Y992 (red) and the nuclear stain Syto 13 (green). A) At stage early-5, fascicles of ORN axons label intensely as they leave the sorting zone (arrowheads). B) At stage early-6, dorsolateral protoglomeruli, including the male-specific macroglomerular complex (MGC) have been in place for about a day and label intensely (*); more recently formed ventromedial protoglomeruli lack significant labeling (arrowheads). The SZ continues to label intensely as well. C) In a stage-7 female, labeling in the SZ has begun to fade and all glomeruli label intensely. D) The pEGFR-Y992 antibody labeling for a stage-7 male matches that seen with the pEGFR-Y845 antibody in panel C. The intense labeling of neurites of AL neurons seen with the EGFR antibody in Fig. 3 is absent at all stages examined with the pEGFR antibodies. E,F) AN glia exhibit very strong labeling for EGFRs in the antennal nerve, but they are not activated. E) Longitudinal section of a stage-7 antennal nerve labeled with the Abcam EGFR antibody (red) and the nuclear stain Syto 13 (green). AN glia, which at this stage are migrating toward the antennal lobe along ORN axons, exhibit more intense EGFR labeling than the axons. F) Staining of this region with the pEGFR-Y845 antibody (red) produces no labeling of AN glia, indicating a lack of activation. Scale bars = 100 µm (B,C,D), 50 µm (A), 25 µm (E,F).
Inhibition of EGFR Activation
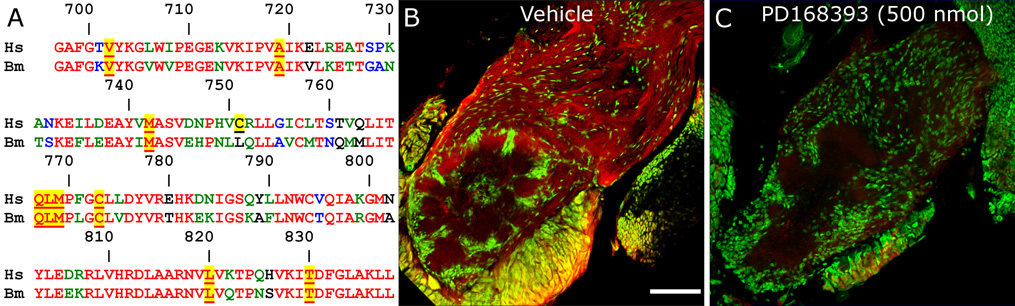
To test the hypothesis that activation of EGFRs plays a role in the events that occur during development of the antennal lobes, the EGFR-specific kinase domain inhibitor PD168393 (Fry et al., 1998) was used at the critical stages 3 (when ORN axons first reach the brain and cause proliferation of glia that will populate the sorting zone (Oland and Tolbert, 1996) through 5 (when ORN axons form protoglomeruli and NP glia begin migrating to surround the protoglomeruli). Comparing the amino acids forming the ATP- and PD168393-binding pocket in human EGFR (Palmer et al., 1997; Fry et al., 1998) with the corresponding sequence in Bombyx mori, we found 69% identity (98/142) and 9 of 10 of the amino acids believed to interact with the drug, including the reactive cysteine (Fig. 5A). The corresponding sequence for Manduca sexta has not been found, nor did a translated BLAST (Altschul et al., 1997) search of this part of the tyrosine kinase domain against Manduca ESTs produce a match. However, alignments of the Bombyx sequence with known insect EGFRs produced 89, 85, and 83% identities with those from Apis melifera, Anopheles gambiae, and Drosophila melanogaster, respectively. The critical amino acids for binding ATP and PD168393 were 100% conserved among the four insects. Thus the likelihood of inactivation of a Manduca EGFR was high. We tested the effectiveness of PD168393 in blocking activation of the Manduca EGFRs by immunolabeling with the pEGFR-Y845 antibody. Comparison of labeling of control (DMSO only, Fig. 5B) with drug-treated (DMSO + PD168393, Fig. 5C) brains removed 24 hours post-injection revealed a significant loss of labeling for activated EGFR in the drug-treated brains.
Figure 5. Treatment with the EGFR inhibitor PD168393 blocks activation of the Manduca EGFR.
A) Alignment of the portion of the human EGFR (Hs= human ErbB1) that binds PD168393 with the homologous sequence found in the Bombyx mori EGFR (Bm). Amino acids participating in binding the drug are underlined and highlighted in yellow. All but one (C-751) are conserved in Bombyx. Numbers apply to the human sequence. B,C) Males at stage early-6 were injected in the headspace with 10 µl DMSO (panel B) or with 10 µl DMSO containing 500 nmol PD168393 (panel C). Brains were removed and fixed 24 hours post-injection and immunolabeled with the pEGFR-Y845 antibody (red) and Syto 13 (green). The absence of significant labeling in panel C indicates blockade of EGFR activation. Scale bar = 100 µm.
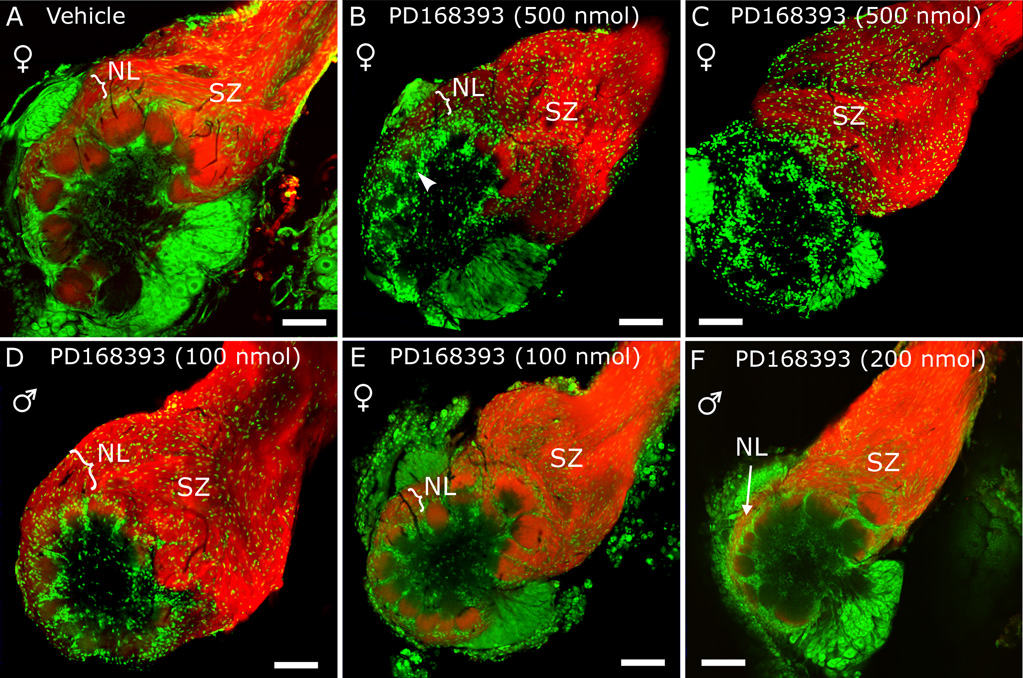
Several days after injection of PD168393 either into the optic lobe of the brain (which lies near the antennal lobe but is not connected to it by direct neural pathways) or into the hemolymph in the headspace anterior to the brain, animals were examined for effects on olfactory system development. In headspace-injected animals examined at stage 7–8, antennal lobe glomeruli were generally smaller than in vehicle-injected controls (Fig. 6 and Table I). Measurements in confocal images revealed that many glomeruli in antennal lobes from drug-treated animals examined at stage 7–8 had diameters of 40–60 µm, compared to 80–100 µm in normal and vehicle-injected control antennal lobes (Fig. 6). Glial borders of glomeruli, normally sharply defined (Fig. 6A), were often less well-delineated in drug-treated animals (Fig. 6B,C).
Figure 6. Treatment with the EGFR inhibitor PD168393 appears to cause ORN axons to stall.
Animals were injected (10 mM PD168393 in DMSO or DMSO without drug) at stage 3 (A–E) or early-5 (F) in the headspace anterior to the brain, then allowed to develop to approximately stage 7. A–F) Antennal lobes in which the antennal nerve was labeled with the lipophilic dye DiD (red). Glial cells and antennal lobe neurons were labeled with the nucleic acid dye, Syto 13 (green). A) Control (DMSO-injected) animal. B–F) Animals treated with PD168393. B,C) Females, injected with 500 nmol PD168393. Most axons failed to grow past the SZ, and DiD labeling is very faint in the small glomeruli (e.g. at arrowhead, surrounded by glia). The SZ in panel C is thickened. D) Male, injected with 100 nmol PD168393. As in panel C, the SZ is thickened and glomeruli are smaller. Here, the nerve layer (NL) around the antennal lobe is also thickened. E) Female, injected with 100 nmol PD168393. Thickening of SZ is even more pronounced, and nerve layer (NL) is very thin. F) Male, injected at stage early-5 with 200 nmol PD168393. Antennal nerve is thin and few axons reached the glomeruli. Scale bars = 100 µm.
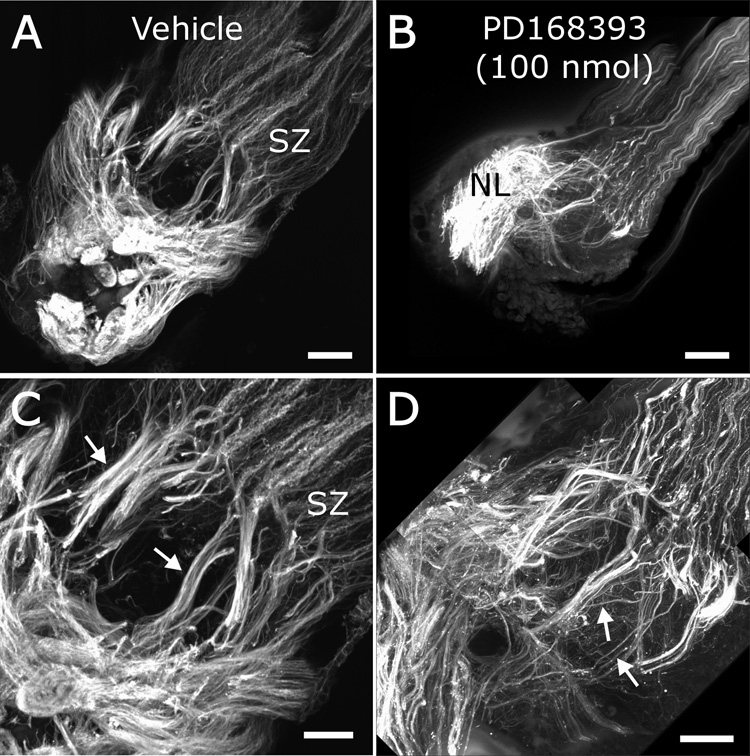
In many drug-treated animals (see Table I), the sorting zone and olfactory nerve layer of the antennal lobe, visualized via DiD labeling of axons, were dramatically thicker than in controls (compare Fig. 6D,E with Fig. 6A). The findings of small glomeruli coupled with thickened sorting zones a nd nerve layers raised the possibility that many axons arrested their outgrowth prematurely following blockade of the EGFR. Effects of the drug varied, even between the antennal lobes of a single animal, suggesting variation in localized concentration due to dispersion by circulating hemolymph. Some animals developed smaller antennal lobes and thinner antennal nerves (Fig. 6F and Table I), most likely indicating that ORN axons stalled distal to the sorting zone as the result of higher drug concentration in the distal antenna. To gain further insight into effects of PD168393 on ORN axon growth, we labeled the sections shown in Figure 6 with an antibody to Manduca fasciclin II (see Higgins et al., 2002). In vehicle-injected control animals, the antibody labeled a subset of ORN axons which were randomly distributed in the antennal nerve distal to the sorting zone, but which became regrouped in the sorting zone into fasciclin II-positive bundles (Fig. 7A,C; similar to labeling in normal animals reported by Higgins et al., 2002). In drug-treated animals, axons entering the sorting zone altered their trajectories as in controls, but exhibited several aberrant phenotypes. Many fasciclin-positive axons were seen to end in the nerve layer rather than turning and extending to form glomeruli (Fig. 7B), and many failed to segregate from fasciclin-negative axons as they would normally (Fig. 7D; note absence of thick, clearly delineated, labeled fascicles compared to Fig. 7C). Thus interference with EGFR function caused many ORN axons to stall prematurely and not to sort properly.
Figure 7. Labeling of ORN axons with an antibody against Fas II reveals errors in fasciculation and pathfinding in PD168393-treated animals.
Treated animal shown here (also shown in Figure 6D) was injected in the headspace with 100 nmol PD168393. After being imaged for DiD and Syto 13 labeling (Fig. 6), sections were immunolabeled with the C3 antibody. A,C) Low and higher magnification images of vehicle-injected control male animal showing fascicles of FasII-positive axons (arrows) leaving sorting zone (SZ). B,D) Low and higher magnification images of PD168393-treated male animal, showing reduced sorting of FasII-positive and FasII-negative axons. B) Rather than terminating in glomeruli, many axons terminate in the nerve layer (NL). D) Many FasII-positive ORN axons emerge from sorting zone having failed to join with other FasII-positive axons (arrows). A) shows a 25 µm stack, while B) shows a 50 µm stack. Scale bars = 100 µm (A,B) and 50 µm (C,D).
Neuroglian Immunoreactivity
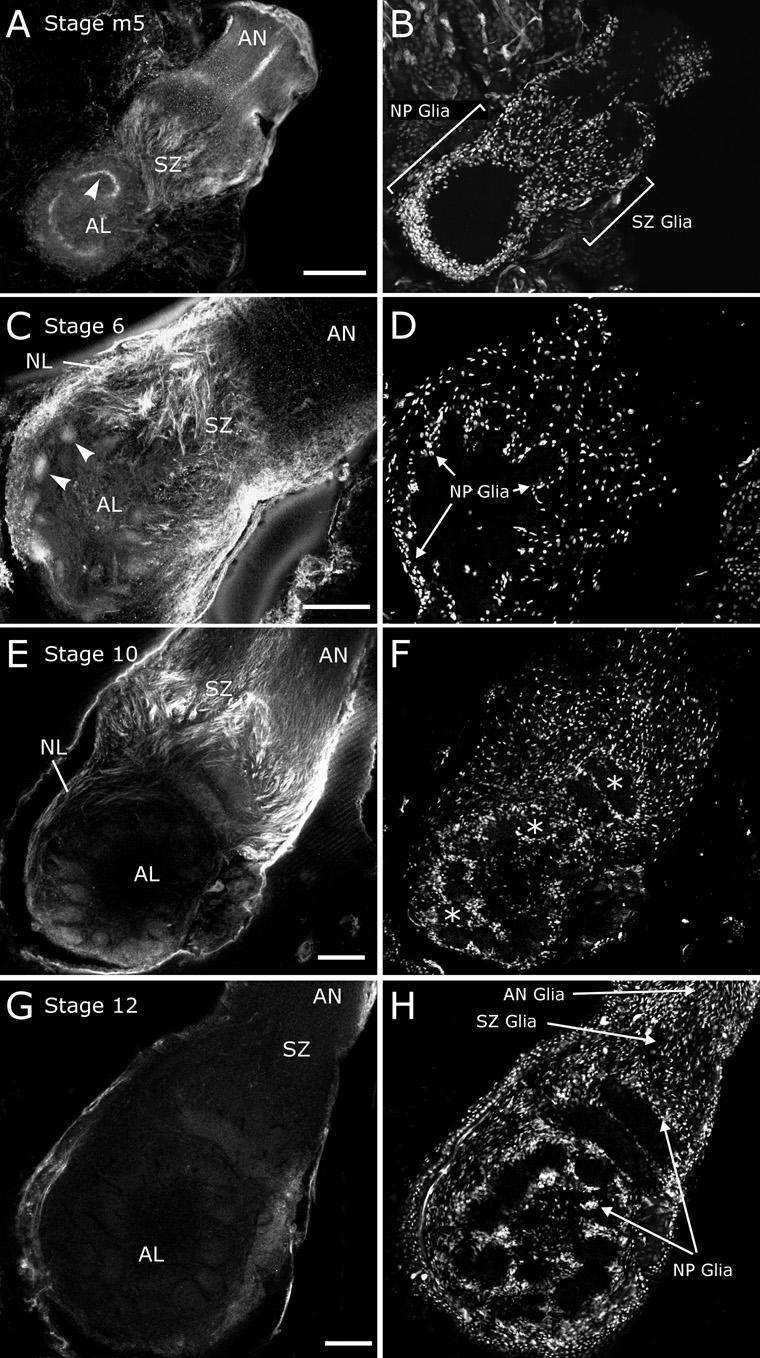
In insects, where no homolog of the vertebrate EGF has been found, EGFRs are known to be activated by TGFα homologs, especially Spitz (see Shilo, 2003 for a review), and by IgCAMS including neuroglian and FasII (Garcia-Alonso et al., 2000; Islam et al., 2004; Kristiansen et al., 2005). Therefore, we also looked at neuroglian expression patterns during development. Immunolabeling of Triton-permeabilized stage-5 brains with the 3B11 antibody against Manduca neuroglian (Nardi, 1994; Chen et al., 1997) revealed low-level labeling along the antennal nerve and in the antennal lobe, with intense labeling of ORN axons in the sorting zone and in a region of the antennal lobe that corresponds to the interface between ORN axon terminals and neurites of antennal lobe neurons (Fig. 8A). At stage 6, as protoglomeruli have formed and NP glia have begun to migrate to surround them, we found a transient intense labeling of the ORN terminals in the protoglomeruli, as well as continued axonal labeling in the sorting zone (Fig. 8C). Several hours later, at mid-stage 6, labeling of protoglomeruli was weak or absent (not shown).
Figure 8. Neuroglian expression is developmentally regulated in the antennal lobe.
A,C,E,G) 3B11 immunocytochemistry of antennal lobes permeabilized with Triton. B,D,F,H) NP and SZ glia in the same sections, visualized via labeling of nuclei with Syto 13. A) At mid-stage 5, ORN axons in the SZ display moderate labeling. Bright labeling in the antennal lobe (arrowhead) corresponds to the interface between ORN axon terminals and neurites of antennal lobe neurons. A strip of subperineurial cells labels brightly in the antennal nerve (AN) distal to the sorting zone. B) SZ glia populate the sorting zone at the base of the nerve. NP glia are in their pre-migration positions around the antennal lobe neuropil. C) By early stage 6, protoglomeruli have begun to form and exhibit neuroglian labeling (arrowheads). ORN axons in the SZ and nerve layer are strongly labeled as well. D) NP glia have now begun to migrate to surround the forming glomeruli. E) By stage 10, neuroglian immunoreactivity has decreased substantially, with axons still labeled in the SZ. F) NP glia now surround all glomeruli (some indicated by “*”). G) By stage 12, all traces of neuroglian immunoreactivity have disappeared, except in the perineurial sheath of the antennal lobe. H) NP glia surround the glomeruli, and AN glia have migrated down the antennal nerve to meet the SZ glia. Scale bars = 100 µm.
The most intense neuroglian labeling was seen at stages 7–8 (Fig. 9A), when labeling of ORN axons was intense in the sorting zone and nerve layer, but very light in axon terminals in the glomeruli and absent in the more distal antennal nerve. Labeling of NP glia, absent or minimal at earlier and later stages, was now intense; the intense labeling of ORN axons made it impossible to determine whether the SZ glia also were labeled. The AN glia, visible distal to the sorting zone by Syto 59 labeling, clearly were not labeled. Labeling at stage 10 was limited to a variable number of axons in the sorting zone (Fig. 8E) and was virtually absent by stage 12 (Fig. 8G).
Figure 9. Immunocytochemistry using antibody 3B11 (red) to Manduca neuroglian reveals intense, Triton-resistant labeling of stage 7 ORN axons as they traverse the sorting zone and nerve layer.
Nuclei were labeled with Syto 59 (green). A) Immunocytochemistry following Triton permeabilization reveals intense labeling of ORN axons in the SZ and nerve layer (NL), as well as of NP glia surrounding the glomeruli. AN glia, seen distal to the sorting zone via Syto 59 labeling, are not labeled by the antibody. B) In the absence of Triton permeabilization, neuroglian labeling is consistently intense along the antennal nerve. NP glia surrounding the glomeruli are also seen to label more intensely than in panel A. C) After fixation in ice-cold methanol/formalin to permeabilize tissue without extracting proteins, the more distal nerve labels intensely, as in panel B. Scale bars = 100 µm.
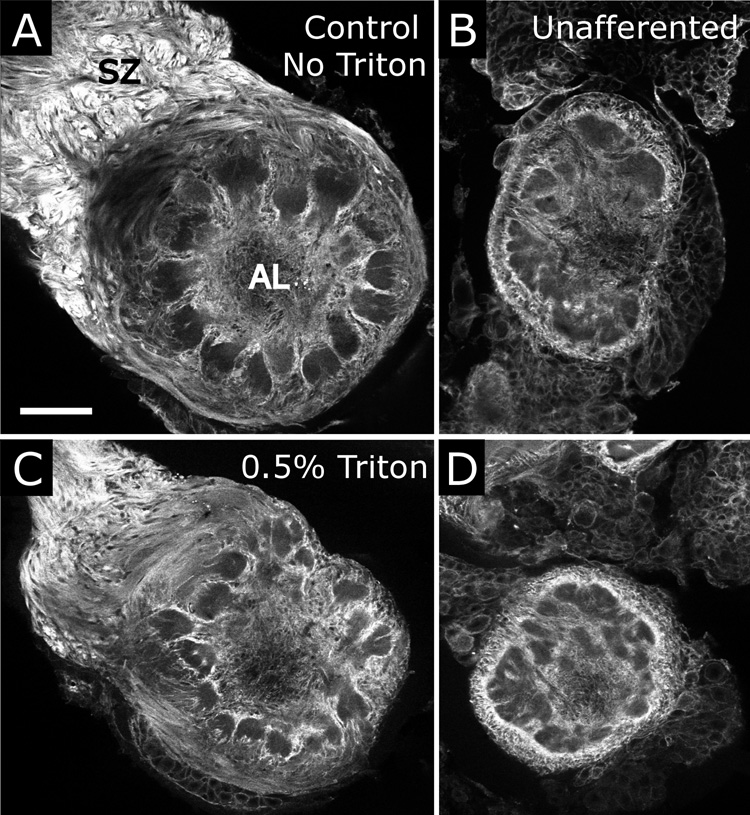
Because several theories exist to explain localized labeling for neuroglian in Triton-permeabilized tissue (Dubreuil et al., 1996; Chen et al., 1997), we explored this issue using animals at stage 7, when labeling is most intense. When Triton X-100 was omitted from the immunocytochemistry protocol, ORN axons in the antennal nerve labeled intensely along the entire length of the nerve and into the olfactory nerve layer outside of the glomeruli (Fig. 9B). Labeling of the NP glia was somewhat stronger than in Figure 9A, suggesting that Triton extracts some neuroglian from the NP glia as well. To test the hypothesis that the loss of axonal labeling in the antennal nerve distal to the sorting zone was due to differential extraction of neuroglian by Triton from membranes, we used a technique, adapted from Ott and Elphick (2002), to simultaneously permeabilize membranes and precipitate and fix proteins via immersion of brains in ice-cold methanol/formalin (9:1). Use of this procedure allowed penetration of antibodies into the tissue without relying on detergent for permeabilization. In methanol-formalin fixed tissue, labeling for neuroglian (Fig. 9C) was intense along the length of the antennal nerve, as was seen in tissue fixed with the standard fixation protocol and not permeabilized with Triton X-100 (Fig. 9B). Thus permeabilization of membranes per se was not responsible for the restricted localization of neuroglian labeling seen with Triton treatment. In light of these results we checked for a similar phenomenon with the EGFR immunolabeling, but found EGFR labeling patterns to be consistent regardless of permeabilization technique used (not shown).
To investigate whether NP glial cells must be exposed to ORN axons in order to express neuroglian, we performed immunolabeling in stage-7 animals in which one antenna had been removed early in development, before ORN axons could reach the brain. On the operated side in these animals, NP glia exhibited labeling as strong as that on the control side, indicating that their expression of neuroglian does not require the presence of ORNs (Fig. 10). Because SZ glial cells are produced only in response to ingrowth of axons (Rössler et al., 1999), in antennal lobes from deantennated animals no SZ glial cells were present for analysis of expression. The presence or absence of Triton had little effect on glial labeling (compare Fig. 10A,B with Fig. 10C,D), indicating that the glial neuroglian was resistant to Triton extraction, even in the chronic absence of ORN axons. Deantennated animals allowed to develop to stage 10 lost all labeling of NP glia (not shown), as was seen in unoperated animals (Fig. 8E,F). Therefore, both up-and down-regulation of neuroglian expression by NP glia appears independent of signals from ORNs.
Figure 10. Glial expression of neuroglian is independent of presence of ORN axons.
A,B) Antennal lobes of a single animal at stage 7 of development. Neuroglian immunocytochemistry with antibody 3B11, without Triton permeabilization/extraction. At stage 2, the animal was surgically deprived of innervation by ORN axons on one side. A) Unoperated side, displaying characteristic intense labeling of NP glia and ORN axons in the SZ. B) Central glia on the side lacking ORN axons exhibit intense neuroglian labeling. C,D) A second animal treated as in (A,B), but in which 3B11 immunocytochemistry included permeabilization with 0.5% Triton. Scale bar = 100 µm.
Sepp and Auld (2003) have reported that expression of a dominant negative EGFR in Drosophila glial cells results in a decrease in expression of several molecules, including neuroglian, by the glia. To test whether pharmacological blockade of the EGFR would produce a similar effect in our system, we labeled brain sections from PD168393-treated animals with the 3B11 antibody. We found that labeling for neuroglian was reduced or virtually eliminated in ORN axons (Fig. 11), supporting the link between EGFR function and neuroglian expression seen in Drosophila. The near absence of axonal labeling (Fig. 11C) allowed us to see that SZ glia do not label for neuroglian under these conditions.
Figure 11. Blocking EGFR activation with PD168393 results in significant but variable reduction of labeling for neuroglian on ORN axons in the SZ and nerve layer.
Male (A–D) and female (E,F) antennal lobes at stage 7–8, following injection with 500 nmol PD168393 in the headspace at early stage 5. A,C,E) 3B11 immunocytochemistry (with Triton). Confocal collection parameters were held constant for all images. B,D,F) Syto 13 labeling of cell nuclei in the same sections. A) 3B11 labeling of ORNs persists in the SZ and nerve layer (NL), while labeling of NP glia is significantly reduced. B) Labeling of cell nuclei in section shown in panel A reveals that NP glia migrated to surround glomeruli (arrows), which are smaller than normal. C) Opposite antennal lobe of the animal shown in panels A and B. Labeling for neuroglian is close to background on ORN axons and glia. D) Labeling of cell nuclei in section shown in panel C reveals normal glial migration, characteristic small glomeruli, and thickened nerve layer. E) In a female animal, the antennal nerve (AN) is lightly labeled and few axons enter the neuropil. F) Labeling of cell nuclei in section shown in panel E reveals that SZ and AN glia migrated normally. NP glia surround an aglomerular ring of neuropil (**); some have migrated into the coarse neuropil. Scale bars = 100 µm.
DISCUSSION
Numerous studies have found EGF receptors to play important roles in insect embryonic and adult development (Perrimon and Perkins, 1997; Garcia-Alonso et al., 2000; Shilo, 2003). Our current results suggest that during development of the adult olfactory system of Manduca sexta, EGFRs play a role in the sorting and extension of receptor axons, perhaps as an effector of neuroglian or fasciclin II signaling among axons and glial cells.
Activation of ORN EGFRs is Necessary for Axon Extension and Sorting
Throughout the period of ORN axon ingrowth and formation of glomeruli, EGFRs are present on the ORN axons, on neurites of antennal lobe neurons, and on antennal nerve glia migrating along the axons from the periphery. EGFR labeling is particularly intense on the axons as they traverse the specialized “sorting zone” and emerge in fascicles destined for particular subsets of glomeruli (see Rössler et al., 1999) and, again, as they form protoglomeruli, and is also intense on the neuritis of antennal lobe neurons (Fig. 3). Labeling for activated (phosphorylated) EGFRs confirms that EGFRs on ORNs are activated in the sorting zone and in developing glomeruli (Fig. 4), while also indicating that EGFRs on neurites of antennal lobe neurons and on antennal nerve glia are not activated during this period. To test whether EGFR is important in signaling during olfactory development, we used a blocker of the EGFR kinase domain, PD168393 (Fig. 6,7). Treatment with PD168393 caused a significant loss of EGFR activation (visualized via immunolabeling with the pEGFR antibody, Fig. 5) and produced a significant effect on the extension and sorting of ORN axons. It should be noted that this result does not address the question of specificity; PD168393 blocks EGFRs specifically in humans (Fry et al., 1998) and the high degree of conservation of amino acids important in binding the drug in many insects (Fig. 5A and text) suggests the likelihood of a similar specificity in insects. The only other Manduca RTK sequenced to date, the ephrin receptor (Kaneko and Nighorn, 2003), lacks half of the amino acids critical for binding PD168393, and therefore would appear to be an unlikely target. Nevertheless, future work to characterize other RTKs in Manduca will include examination of their susceptibility to inactivation by PD168393. The most likely explanation for alterations in the antennal nerve (thinning of the nerve near the brain or thickening of the sorting zone) and nerve layer as well as the reduced glomerular diameters in PD168393-treated animals (Fig. 6) is that many ORN axon growth cones had stalled at critical choice points, such as in the sorting zone, where the axons normally change trajectories abruptly and sort into thick fascicles according to target specificity (Rössler et al., 1999), or in the nerve layer of the antennal lobe, where ORN axons normally extend to particular positions, then turn sharply and dive through the layer of NP glia to form particular protoglomeruli (Oland et al., 1990). This result is reminiscent of experiments in Drosophila in which expression of a dominant-negative form of EGFR resulted in stalling of axon extension in ocellar sensory neurons (Garcia-Alonso et al., 2000). Fasciclin II immunocytochemistry in PD168393-treated animals revealed that ORN axon fascicles that form in the sorting zone are abnormal: whereas axons would normally emerge from the sorting zone in large fasciclin II-positive and large fasciclin II-negative bundles destined for different subsets of glomeruli (Higgins et al., 2002), in the treated animals, the axons that emerged from the sorting zone were still intermixed (Fig. 7B,D). In these animals, ORN axons did change their direction of growth as they traversed the sorting zone, suggesting that the signal to change direction does not require significant EGFR function; it is the sorting per se that requires EGFR function. This phenotype is similar to that seen in animals treated to reduce the number of SZ glia (Rössler et al., 1999). In those animals, fasciclin II-positive axons changed trajectories in the sorting zone but failed to sort properly. Many axons then extended past the antennal lobe, suggesting a failure to target correctly. In PD168393-treated animals we did not see axonal overgrowth, perhaps because one effect of blocking EGFRs was axon stalling. Nevertheless, the similar effects of glial reduction and EGFR blockade on axon sorting support the hypothesis that the sorting of axons induced by SZ glia involves EGFR activation.
The immunocytochemical evidence for presence of EGFRs on AN glia is clear (Fig. 4E) but they appear not to be activated in the vicinity of the antennal lobe (Fig. 4F). Blocking EGFRs with PD168393 had no apparent effect on intercellular interactions that cause the migration of the three types of glial cells. In treated animals, AN glia had migrated normally along the ORN axons toward the base of the antennal nerve; SZ glia had migrated normally to form the sorting zone (which occurs in response to the presence of early ORN axons: Rössler et al. 1999); and NP glia generally had migrated to surround the glomeruli (which also occurs in response to axons: Oland and Tolbert, 1987). In cases in which PD168393-treated antennal lobes were aglomerular (Fig. 11E,F), NP glia displayed the arrangement typical of antennal lobes deprived of sufficient ORN innervation: many glia remained in a ring around the antennal lobe while others migrated into the central coarse neuropil of the lobe (Tolbert and Sirianni, 1990). We have shown previously that nitric oxide, released from ORN axons, is likely to be important in stimulating AN and NP glia to migrate (Gibson et al., 2001) and recently we have found evidence for activated FGF receptors on NP and SZ glia (Gibson and Tolbert, unpublished results) suggesting that their behavior may be mediated via FGF, rather than EGF, receptors.
Our results with PD168393 are remarkably similar to those reported for embryonic development in Drosophila mutants in which a dominant negative form of the EGFR was expressed solely in glia (repo∷EgfrDN, Sepp and Auld, 2003). That study demonstrated a significant effect of EGFR activation on sensory axon outgrowth and targeting, suggesting that, in wild-type animals, axon behavior is indirectly affected following activation of the EGFR in glial cells. The authors also reported that expression of this dominant negative EGFR caused loss of expression of gliotactin and neuroglian, considered differentiation markers, in peripheral glial cells. In light of the results presented here, it is possible that the loss of glial neuroglian expression in the repo∷EgfrDN mutant of Sepp and Auld was the cause for the aberrant axon behavior in those experiments.
The high level of expression and activation of EGFRs on ORN axon terminals at stages 6&7, when glomerular architecture is just being established (Tolbert et al., 1983; Oland and Tolbert, 1987; Oland et al., 1990) and synaptic connections with antennal lobe neurons are beginning to develop (Tolbert, 1989; Oland et al., 1990), is particularly intriguing in light of work demonstrating a dependence of excitatory cholinergic synapse formation on EGF-mediated receptor tyrosine kinase signaling in Lymnaea (Hamakawa et al., 1999; Woodin et al., 2002). The ORNs of Manduca have been shown to be cholinergic (Sanes and Hildebrand, 1976b; Sanes et al., 1977); thus EGFR expression and activation on ORN terminals may here, too, be related to cholinergic excitatory synaptogenesis.
Possible Neuroglian-EGFR Interactions During Antennal Lobe Development
L1, the vertebrate homologue of neuroglian (Nardi, 1994), has been shown to mediate cell-cell communication by both homo- and heterophilic interactions (Lemmon et al., 1989; Hortsch, 1996; Walsh and Doherty, 1997; Kiryushko et al., 2004). To date, reported examples of neuroglian-mediated communication between insect cells are restricted to the homophilic type, with the single exception of a heterophilic interaction with the Drosophila IgCam Echinoid in trans (i.e. between cells), leading to inhibition of the EGFR in the Echinoid-expressing cell (Islam et al., 2003). Both L1 and neuroglian have been shown to activate EGF and FGF receptors via homophilic binding, leading to subsequent neurite extension and pathfinding during axon guidance (Williams et al., 1994; Doherty and Walsh, 1996; Kamiguchi and Lemmon, 1997; 2000; Garcia-Alonso et al., 2000; Islam et al., 2004; Forni et al., 2004). Until recently the mechanism by which L1 and neuroglian accomplished receptor tyrosine kinase activation was not known; it now appears that, at least when co-expressed in Drosophila S2 cells in vitro, human L1 directly activates the human EGF receptor kinase function by a combination of cis-interactions between L1 and the EGFR molecules in a given cell and trans-interactions between L1 molecules at cell-cell contact sites (Islam et al., 2004).
Some controversy exists as to whether spatially restricted L1/neuroglian labeling in other immunocytochemical studies resulted from differences in extractability or from a true restriction of surface expression based on developmental status (Dubreuil et al., 1996; Chen et al., 1997). Clustering of neuroglian molecules due to homo- and heterophilic interactions between cells has been shown to lead to anchoring of neuroglian’s cytoplasmic domain to the spectrin-actin cytoskeleton via ankyrin, with one consequence being increased resistance to Triton extraction (Dubreuil et al., 1996; Hortsch et al., 1998; Jenkins and Bennett, 2001). A similar phenomenon has been reported for N- and B-cadherin, which, like neuroglian, become linked to the cytoskeleton following homophilic binding in chick lens formation (Leong et al., 2000; see also Hannah et al., 1998). Our results indicate that, in the developing olfactory pathway of Manduca sexta, neuroglian is present along the entire length of ORN axons, but exists in two states, extractable and non-extractable by Triton. The non-extractable form was limited to the sorting zone and the nerve layer around the antennal lobe neuropil, and was notably absent in the distal antennal nerve and, except briefly, in the glomeruli (which lack glial processes in their centers). Although more work is needed to establish the significance of intense neuroglian labeling in the sorting zone, the co localization of neuroglian and activated EGFRs in the sorting zone suggests a possible mechanism for cell-to-cell communication.
Labeling for neuroglian in the sorting zone may reflect neuroglian interactions between axons and glial cells, but could also arise if interaction with SZ glial cells induces a change in surface expression of molecules (Seeger et al., 1993; Schenk et al., 2003) on the ORN axons that allows them to interact with each other via neuroglian. The possibility that glia affect the ability of ORN axons to interact is supported by the finding of short-lived neuroglian labeling of ORN axon terminals in protoglomeruli at early stage 6 (Fig. 8C), which occurs in a region where the axon terminals first become free from close association with glia (Oland et al., 1990). In the protoglomeruli, neuroglian interactions could only be between axon terminals. The fact that the labeling in the protoglomeruli quickly disappears suggests the possibility that contact with glia is necessary for maintenance of axon-axon interaction via neuroglian. Such an interpretation could be helpful in understanding the defasciculation-refasciculation events that occur in the sorting zone, as ORN axons, bundled with neighbors of dissimilar odor specificity, defasciculate, change course dramatically, and refasciculate with axons targeting common glomeruli (Rössler et al., 1999). It also may be related to the finding of Tucker et al. (2004) that ORN growth cones in culture change their adhesive properties dramatically upon contacting dissociated SZ and NP glial cells.
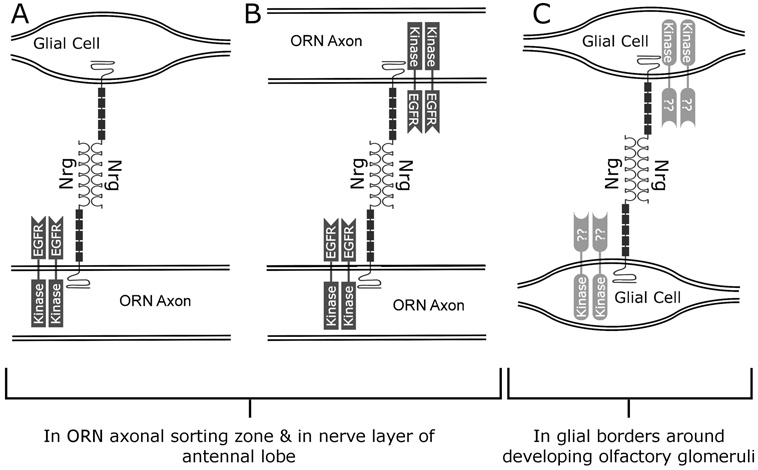
The results presented here suggest a possible scenario depicted in Figure 12. In the sorting zone, EGFRs on ORN axons may be activated via homophilic trans-interactions between neuroglian molecules on axons and glia (Fig. 12A) and between axons (Fig. 12B). The fact that NP glia also express neuroglian, even when they have developed in the absence of ORN axons, (Fig. 9, Fig. 10) suggests that they are capable of interacting among themselves as well (Fig. 12C), possibly via different receptor tyrosine kinases such as FGFRs. The fact that neuroglian labeling of NP glia is restricted to stages 7–8, after glia have migrated to surround glomeruli, suggests that their interactions are aimed at something other than migration, perhaps to stabilization of glomerular borders.
Figure 12. Schematic diagram to illustrate the types of interactions postulated in antennal lobe development.
A,B) In the SZ and nerve layer, EGFRs on ORN axons may be activated via homophilic neuroglian interactions between axons and glia (panel A). This could result first in migration of glia to form the sorting zone, then in effects on subsequently arriving axons resulting in altered extension, fasciculation, and sorting. B) As a result of changes induced by passage through the SZ and nerve layer, ORN axons may become able to interact with each other via homophilic neuroglian binding and activation of EGFRs (panel B). This again could lead to changes in extension, fasciculation and sorting. C) Strong neuroglian labeling of NP glia in normal antennal lobes and lobes deprived of contact with ORN axons suggest that NP glia interact with each other via homophilic neuroglian interactions. The appearance of labeling after these glial cells have migrated may indicate a role for neuroglian in stabilization of glomeruli.
In summary, we have presented evidence that EGFRs are present on ORN axons and peripheral nerve glia, and that neuroglian is present on both ORN axons and central glia of the antennal lobe, at critical times during development of the primary olfactory pathway of Manduca sexta. EGFRs are activated on ORN axons as they navigate the sorting zone and as they form synaptic connections with central neurons; blocking EGFR activation caused axon stalling and defects in sorting, suggesting that EGFRs are essential for these processes. Neuroglian, a known activator of EGFRs in other systems, is present on ORN axons and is resistant to detergent extraction in the sorting zone, one of the two regions of EGFR activation in the antennal lobe (the other being the glomeruli). NP glia also express neuroglian briefly following their migration to surround glomeruli. Blockade of EGFR activation produced no effect on glial migration. Given (1) the co localization of activated EGFRs and neuroglian in the sorting zone, and (2) the growing body of work describing neuroglian-mediated activation of the EGFR in other systems, we hypothesize that ORN axons, on entering the sorting zone, may engage in homophilic neuroglian interactions among themselves and/or with SZ glia. These interactions, expressed through activation of EGFRs, lead to important modulation of axon extension, fasciculation, and/or targeting.
Supplementary Material
Preabsorption of the EGFR antibody with a synthetic peptide representing the Bombyx sequence corresponding to the antigenic peptide from humans eliminates all labeling in the antennal lobe. Two halves of a stage-6 brain were processed for EGFR immunocytochemistry using either the antibody alone (panel A) or the antibody which had been preabsorbed with the Bombyx peptide (panel B). C) Section shown in panel B, but with collection parameters altered to integrate the signal from 16 consecutive scans (instead of the normal single scan), to confirm the presence of antennal lobe tissue. Scale bar = 100 µm.
ACKNOWLEDGMENTS
We thank Drs. Lynne Oland and Norm Davis and Patricia Jansma, M.S., for numerous helpful discussions of ideas and techniques, Dr. James Nardi for sharing the 3B11 antibody and for commenting on an early draft of the manuscript, and Dr. Phillip Copenhaver for sharing the C3 antibody. We also thank Dr. A.A. Osman and Suzanne Mackzum for rearing Manduca sexta and Mark Higgins for much logistical and technical assistance.
Supported by NIH Grant DC004598
LITERATURE CITED
- Alenius M, Bohm S. Differential function of RNCAM isoforms in precise target selection of olfactory sensory neurons. Development. 2003;130:917–927. doi: 10.1242/dev.00317. [DOI] [PubMed] [Google Scholar]
- Alenius M, Bohm S. Identification of a novel neural cell adhesion molecule-related gene with a potential role in selective axonal projection. J Biol Chem. 1997;272:26083–26086. doi: 10.1074/jbc.272.42.26083. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auld V. Glia as mediators of growth cone guidance: studies from insect nervous systems. Cell Mol Life Sci. 1999;55:1377–1385. doi: 10.1007/s000180050378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MS, Puche AC, Shipley MT. Development of the olfactory bulb: evidence for glia-neuron interactions in glomerular formation. J Comp Neurol. 1999;415:423–448. [PubMed] [Google Scholar]
- Barnea G, O'Donnell S, Mancia F, Sun X, Nemes A, Mendelsohn M, Axel R. Odorant receptors on axon termini in the brain. Science. 2004;304:1468. doi: 10.1126/science.1096146. [DOI] [PubMed] [Google Scholar]
- Baumann PM, Oland LA, Tolbert LP. Glial cells stabilize axonal protoglomeruli in the developing olfactory lobe of the moth Manduca sexta. J Comp Neurol. 1996;373:118–128. doi: 10.1002/(SICI)1096-9861(19960909)373:1<118::AID-CNE10>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Bieber AJ, Snow PM, Hortsch M, Patel NH, Jacobs JR, Traquina ZR, Schilling J, Goodman CS. Drosophila neuroglian: a member of the immunoglobulin superfamily with extensive homology to the vertebrate neural adhesion molecule L1. Cell. 1989;59:447–460. doi: 10.1016/0092-8674(89)90029-9. [DOI] [PubMed] [Google Scholar]
- Chen CL, Lampe DJ, Robertson HM, Nardi JB. Neuroglian is expressed on cells destined to form the prothoracic glands of Manduca embryos as they segregate from surrounding cells and rearrange during morphogenesis. Dev Biol. 1997;181:1–13. doi: 10.1006/dbio.1996.8437. [DOI] [PubMed] [Google Scholar]
- Chisholm A, Tessier-Lavigne M. Conservation and divergence of axon guidance mechanisms. Curr Opin Neurobiol. 1999;9:603–615. doi: 10.1016/S0959-4388(99)00021-5. [DOI] [PubMed] [Google Scholar]
- Chotard C, Salecker I. Neurons and glia: team players in axon guidance. Trends Neurosci. 2004;27:655–661. doi: 10.1016/j.tins.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Crandall JE, Dibble C, Butler D, Pays L, Ahmad N, Kostek C, Puschel AW, Schwarting GA. Patterning of olfactory sensory connections is mediated by extracellular matrix proteins in the nerve layer of the olfactory bulb. J Neurobiol. 2000;45:195–206. doi: 10.1002/1097-4695(200012)45:4<195::aid-neu1>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Doherty P, Walsh FS. CAM-FGF receptor interactions: a model for axonal growth. Mol Cell Neurosci. 1996;8:99–111. doi: 10.1006/mcne.1996.0049. [DOI] [PubMed] [Google Scholar]
- Dubreuil RR, MacVicar G, Dissanayake S, Liu C, Homer D, Hortsch M. Neuroglian-mediated cell adhesion induces assembly of the membrane skeleton at cell contact sites. J Cell Biol. 1996;133:647–655. doi: 10.1083/jcb.133.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forni JJ, Romani S, Doherty P, Tear G. Neuroglian and FasciclinII can promote neurite outgrowth via the FGF receptor Heartless. Mol Cell Neurosci. 2004;26:282–291. doi: 10.1016/j.mcn.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Fry DW, Bridges AJ, Denny WA, Doherty A, Greis KD, Hicks JL, Hook KE, Keller PR, Leopold WR, Loo JA, McNamara DJ, Nelson JM, Sherwood V, Smaill JB, Trumpp-Kallmeyer S, Dobrusin EM. Specific, irreversible inactivation of the epidermal growth factor receptor and erbB2, by a new class of tyrosine kinase inhibitor. Proc Natl Acad Sci U S A. 1998;95:12022–12027. doi: 10.1073/pnas.95.20.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alonso L, Romani S, Jimenez F. The EGF and FGF receptors mediate neuroglian function to control growth cone decisions during sensory axon guidance in Drosophila. Neuron. 2000;28:741–752. doi: 10.1016/s0896-6273(00)00150-1. [DOI] [PubMed] [Google Scholar]
- Gibson NJ, Rössler W, Nighorn AJ, Oland LA, Hildebrand JG, Tolbert LP. Neuron-glia communication via nitric oxide is essential in establishing antennal-lobe structure in Manduca sexta. Dev Biol. 2001;240:326–339. doi: 10.1006/dbio.2001.0463. [DOI] [PubMed] [Google Scholar]
- Gibson NJ, Hildebrand JG, Tolbert LP. Glycosylation patterns are sexually dimorphic throughout development of the olfactory system in Manduca sexta. J Comp Neurol. 2004;476:1–18. doi: 10.1002/cne.20178. [DOI] [PubMed] [Google Scholar]
- Hamakawa T, Woodin MA, Bjorgum MC, Painter SD, Takasaki M, Lukowiak K, Nagle GT, Syed NI. Excitatory synaptogenesis between identified Lymnaea neurons requires extrinsic trophic factors and is mediated by receptor tyrosine kinases. J Neurosci. 1999;19:9306–9312. doi: 10.1523/JNEUROSCI.19-21-09306.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah MJ, Weiss U, Huttner WB. Differential extraction of proteins from paraformaldehyde-fixed cells: lessons from synaptophysin and other membrane proteins. Methods. 1998;16:170–181. doi: 10.1006/meth.1998.0664. [DOI] [PubMed] [Google Scholar]
- Hayashi JH, Hildebrand JG. Insect olfactory neurons in vitro: morphological and physiological characterization of cells from the developing antennal lobes of Manduca sexta. J Neurosci. 1990;10:848–859. doi: 10.1523/JNEUROSCI.10-03-00848.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo A. Neuron-glia interactions during axon guidance in Drosophila. Biochem Soc Trans. 2003;31:50–55. doi: 10.1042/bst0310050. [DOI] [PubMed] [Google Scholar]
- Higgins MR, Gibson NJ, Eckholdt PA, Nighorn A, Copenhaver PF, Nardi J, Tolbert LP. Different isoforms of Fasciclin II are expressed by a subset of developing olfactory receptor neurons and by olfactory-nerve glial cells during formation of glomeruli in the moth Manduca sexta. Dev Biol. 2002;244:134–154. doi: 10.1006/dbio.2002.0583. [DOI] [PubMed] [Google Scholar]
- Hildebrand JG, Shepherd GM. Mechanisms of olfactory discrimination: converging evidence for common principles across phyla. Annu Rev Neurosci. 1997;20:595–631. doi: 10.1146/annurev.neuro.20.1.595. [DOI] [PubMed] [Google Scholar]
- Hortsch M. The L1 family of neural cell adhesion molecules: old proteins performing new tricks. Neuron. 1996;17:587–593. doi: 10.1016/s0896-6273(00)80192-0. [DOI] [PubMed] [Google Scholar]
- Hortsch M, Goodman CS. Cell and substrate adhesion molecules in Drosophila. Annu Rev Cell Biol. 1991;7:505–557. doi: 10.1146/annurev.cb.07.110191.002445. [DOI] [PubMed] [Google Scholar]
- Hortsch M, Homer D, Malhotra JD, Chang S, Frankel J, Jefford G, Dubreuil RR. Structural requirements for outside-in and inside-out signaling by Drosophila neuroglian, a member of the L1 family of cell adhesion molecules. J Cell Biol. 1998;142:251–261. doi: 10.1083/jcb.142.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam R, Kristiansen LV, Romani S, Garcia-Alonso L, Hortsch M. Activation of EGF Receptor Kinase by L1-mediated Homophilic Cell Interactions. Mol Biol Cell. 2004;15(4):2003–2012. doi: 10.1091/mbc.E03-05-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam R, Wei SY, Chiu WH, Hortsch M, Hsu JC. Neuroglian activates Echinoid to antagonize the Drosophila EGF receptor signaling pathway. Development. 2003;130:2051–2059. doi: 10.1242/dev.00415. [DOI] [PubMed] [Google Scholar]
- Jenkins SM, Bennett V. Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J Cell Biol. 2001;155:739–746. doi: 10.1083/jcb.200109026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiguchi H, Lemmon V. IgCAMs: bidirectional signals underlying neurite growth. Curr Opin Cell Biol. 2000;12:598–605. doi: 10.1016/s0955-0674(00)00138-1. [DOI] [PubMed] [Google Scholar]
- Kamiguchi H, Lemmon V. Neural cell adhesion molecule L1: signaling pathways and growth cone motility. J Neurosci Res. 1997;49:1–8. doi: 10.1002/(sici)1097-4547(19970701)49:1<1::aid-jnr1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Nighorn A. Interaxonal Eph-ephrin signaling may mediate sorting of olfactory sensory axons in Manduca sexta. J Neurosci. 2003;23:11523–11538. doi: 10.1523/JNEUROSCI.23-37-11523.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent K. PhD dissertation. Harvard University; 1985. Metamorphosis of the antennal center and the influence of sensory innervation on the formation of glomeruli in the hawkmoth Manduca Sexta. [Google Scholar]
- Kent KS, Harrow ID, Quartararo P, Hildebrand JG. An accessory olfactory pathway in Lepidoptera: the labial pit organ and its central projections in Manduca sexta and certain other sphinx moths and silk moths. Cell Tissue Res. 1986;245:237–245. doi: 10.1007/BF00213927. [DOI] [PubMed] [Google Scholar]
- Kent KS, Oland LA, Hildebrand JG. Development of the labial pit organ glomerulus in the antennal lobe of the moth Manduca sexta: the role of afferent projections in the formation of identifiable olfactory glomeruli. J Neurobiol. 1999;40:28–44. doi: 10.1002/(sici)1097-4695(199907)40:1<28::aid-neu3>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Kiryushko D, Berezin V, Bock E. Regulators of neurite outgrowth: role of cell adhesion molecules. Ann N Y Acad Sci. 2004;1014:140–154. doi: 10.1196/annals.1294.015. [DOI] [PubMed] [Google Scholar]
- Kristiansen LV, Velasquez E, Romani S, Baars S, Berezin V, Bock E, Hortsch M, Garcia-Alonso L. Genetic analysis of an overlapping functional requirement for L1- and NCAM-type proteins during sensory axon guidance in Drosophila. Mol Cell Neurosci. 2005;28:141–152. doi: 10.1016/j.mcn.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Lemke G. Glial control of neuronal development. Annu Rev Neurosci. 2001;24:87–105. doi: 10.1146/annurev.neuro.24.1.87. [DOI] [PubMed] [Google Scholar]
- Lemmon V, Farr KL, Lagenaur C. L1-mediated axon outgrowth occurs via a homophilic binding mechanism. Neuron. 1989;2:1597–1603. doi: 10.1016/0896-6273(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Leong L, Menko AS, Grunwald GB. Differential expression of N- and B- cadherin during lens development. Invest Ophthalmol Vis Sci. 2000;41:3503–3510. [PubMed] [Google Scholar]
- Lipscomb BW, Treloar HB, Klenoff J, Greer CA. Cell surface carbohydrates and glomerular targeting of olfactory sensory neuron axons in the mouse. J Comp Neurol. 2003;467:22–31. doi: 10.1002/cne.10910. [DOI] [PubMed] [Google Scholar]
- Long H, Sabatier C, Ma L, Plump A, Yuan W, Ornitz DM, Tamada A, Murakami F, Goodman CS, Tessier-Lavigne M. Conserved roles for Slit and Robo proteins in midline commissural axon guidance. Neuron. 2004;42:213–223. doi: 10.1016/s0896-6273(04)00179-5. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- Nardi JB. Modulated expression of a surface epitope on migrating germ cells of Manduca sexta embryos. Development. 1993;118:967–975. [Google Scholar]
- Nardi JB. Rearrangement of epithelial cell types in an insect wing monolayer is accompanied by differential expression of a cell surface protein. Dev Dyn. 1994;199:315–325. doi: 10.1002/aja.1001990406. [DOI] [PubMed] [Google Scholar]
- Oland LA, Orr G, Tolbert LP. Construction of a protoglomerular template by olfactory axons initiates the formation of olfactory glomeruli in the insect brain. J Neurosci. 1990;10:2096–2112. doi: 10.1523/JNEUROSCI.10-07-02096.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oland LA, Tolbert LP. Effects of hydroxyurea parallel the effects of radiation in developing olfactory glomeruli in insects. J Comp Neurol. 1988;278:377–387. doi: 10.1002/cne.902780307. [DOI] [PubMed] [Google Scholar]
- Oland LA, Tolbert LP. Glial patterns during early development of antennal lobes of Manduca sexta: a comparison between normal lobes and lobes deprived of antennal axons. J Comp Neurol. 1987;255:196–207. doi: 10.1002/cne.902550204. [DOI] [PubMed] [Google Scholar]
- Oland LA, Tolbert LP. Key interactions between neurons and glial cells during neural development in insects. Annu Rev Entomol. 2003;48:89–110. doi: 10.1146/annurev.ento.48.091801.112654. [DOI] [PubMed] [Google Scholar]
- Oland LA, Tolbert LP. Multiple factors shape development of olfactory glomeruli: insights from an insect model system. J Neurobiol. 1996;30:92–109. doi: 10.1002/(SICI)1097-4695(199605)30:1<92::AID-NEU9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Oland LA, Tolbert LP, Mossman KL. Radiation-induced reduction of the glial population during development disrupts the formation of olfactory glomeruli in an insect. J Neurosci. 1988;8:353–367. doi: 10.1523/JNEUROSCI.08-01-00353.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott SR, Elphick MR. Nitric oxide synthase histochemistry in insect nervous systems: Methanol/formalin fixation reveals the neuroarchitecture of formaldehyde-sensitive NADPH diaphorase in the cockroach Periplaneta americana. J Comp Neurol. 2002;448:165–185. doi: 10.1002/cne.10235. [DOI] [PubMed] [Google Scholar]
- Palmer BD, Trumpp-Kallmeyer S, Fry DW, Nelson JM, Showalter HD, Denny WA. Tyrosine kinase inhibitors. 11. Soluble analogues of pyrrolo- and pyrazoloquinazolines as epidermal growth factor receptor inhibitors: synthesis, biological evaluation, and modeling of the mode of binding. J Med Chem. 1997;40:1519–1529. doi: 10.1021/jm960789h. [DOI] [PubMed] [Google Scholar]
- Pasterkamp RJ, Ruitenberg MJ, Verhaagen J. Semaphorins and their receptors in olfactory axon guidance. Cell Mol Biol (Noisy-le-grand) 1999;45:763–779. [PubMed] [Google Scholar]
- Perrimon N, Perkins LA. There must be 50 ways to rule the signal: the case of the Drosophila EGF receptor. Cell. 1997;89:13–16. doi: 10.1016/s0092-8674(00)80177-4. [DOI] [PubMed] [Google Scholar]
- Puche AC, Poirier F, Hair M, Bartlett PF, Key B. Role of galectin-1 in the developing mouse olfactory system. Dev Biol. 1996;179:274–287. doi: 10.1006/dbio.1996.0257. [DOI] [PubMed] [Google Scholar]
- Raabe EH, Yoshida K, Schwarting GA. Differential laminin isoform expression in the developing rat olfactory system. Brain Res Dev Brain Res. 1997;101:187–196. doi: 10.1016/s0165-3806(97)00064-3. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Sullivan SL, Buck LB. Information coding in the olfactory system: evidence for a stereotyped and highly organized epitope map in the olfactory bulb. Cell. 1994;79:1245–1255. doi: 10.1016/0092-8674(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Rössler W, Oland LA, Higgins MR, Hildebrand JG, Tolbert LP. Development of a glia-rich axon-sorting zone in the olfactory pathway of the moth Manduca sexta. J Neurosci. 1999;19:9865–9877. doi: 10.1523/JNEUROSCI.19-22-09865.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffell JL, Williams EJ, Doherty P, Walsh FW. Axonal growth mediated by cell adhesion molecules requires activation of fibroblast growth factor receptors. Biochem Soc Trans. 1995;23:469–470. doi: 10.1042/bst0230469. [DOI] [PubMed] [Google Scholar]
- Saffell JL, Williams EJ, Mason IJ, Walsh FS, Doherty P. Expression of a dominant negative FGF receptor inhibits axonal growth and FGF receptor phosphorylation stimulated by CAMs. Neuron. 1997;18:231–242. doi: 10.1016/s0896-6273(00)80264-0. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Hildebrand JG. Acetylcholine and its metabolic enzymes in developing antennae of the moth, Manduca sexta. Dev Biol. 1976b;52:105–120. doi: 10.1016/0012-1606(76)90011-7. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Hildebrand JG. Structure and development of antennae in a moth, Manduca sexta. Dev Biol. 1976a;51:280–299. [PubMed] [Google Scholar]
- Sanes JR, Prescott DJ, Hildebrand JG. Cholinergic neurochemical development of normal and deafferented antennal lobes during metamorphosis of the moth, Manduca sexta. Brain Res. 1977;119:389–402. doi: 10.1016/0006-8993(77)90318-3. [DOI] [PubMed] [Google Scholar]
- Schenk S, Hintermann E, Bilban M, Koshikawa N, Hojilla C, Khokha R, Quaranta V. Binding to EGF receptor of a laminin-5 EGF-like fragment liberated during MMP-dependent mammary gland involution. J Cell Biol. 2003;161:197–209. doi: 10.1083/jcb.200208145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarting GA, Deutsch G, Gattey DM, Crandall JE. Glycoconjugates are stage- and position-specific cell surface molecules in the developing olfactory system, 1: The CC1 immunoreactive glycolipid defines a rostrocaudal gradient in the rat vomeronasal system. J Neurobiol. 1992;23:120–129. doi: 10.1002/neu.480230203. [DOI] [PubMed] [Google Scholar]
- Seeger M, Tear G, Ferres-Marco D, Goodman CS. Mutations affecting growth cone guidance in Drosophila: genes necessary for guidance toward or away from the midline. Neuron. 1993;10:409–426. doi: 10.1016/0896-6273(93)90330-t. [DOI] [PubMed] [Google Scholar]
- Sepp KJ, Auld VJ. Reciprocal interactions between neurons and glia are required for Drosophila peripheral nervous system development. J Neurosci. 2003;23:8221–8230. doi: 10.1523/JNEUROSCI.23-23-08221.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrow M, Tiemeyer M. Gliolectin-mediated carbohydrate binding at the Drosophila midline ensures the fidelity of axon pathfinding. Development. 2001;128:4585–4595. doi: 10.1242/dev.128.22.4585. [DOI] [PubMed] [Google Scholar]
- Shilo BZ. Signaling by the Drosophila epidermal growth factor receptor pathway during development. Exp Cell Res. 2003;284:140–149. doi: 10.1016/s0014-4827(02)00094-0. [DOI] [PubMed] [Google Scholar]
- Sinakevitch I, Farris SM, Strausfeld NJ. Taurine-, aspartate- and glutamate-like immunoreactivity identifies chemically distinct subdivisions of Kenyon cells in the cockroach mushroom body. J Comp Neurol. 2001;439:352–367. doi: 10.1002/cne.1355. [DOI] [PubMed] [Google Scholar]
- St John JA, Pasquale EB, Key B. EphA receptors and ephrin-A ligands exhibit highly regulated spatial and temporal expression patterns in the developing olfactory system. Brain Res Dev Brain Res. 2002;138:1–14. doi: 10.1016/s0165-3806(02)00454-6. [DOI] [PubMed] [Google Scholar]
- Tiemeyer M, Goodman CS. Gliolectin is a novel carbohydrate-binding protein expressed by a subset of glia in the embryonic Drosophila nervous system. Development. 1996;122:925–936. doi: 10.1242/dev.122.3.925. [DOI] [PubMed] [Google Scholar]
- Tolbert LP. Afferent axons from the antenna influence the number and placement of intrinsic synapses in the antennal lobes of Manduca sexta. Synapse. 1989;3:83–95. doi: 10.1002/syn.890030112. [DOI] [PubMed] [Google Scholar]
- Tolbert LP, Sirianni PA. Requirement for olfactory axons in the induction and stabilization of olfactory glomeruli in an insect. J Comp Neurol. 1990;298(1):69–82. doi: 10.1002/cne.902980106. [DOI] [PubMed] [Google Scholar]
- Tolbert LP, Matsumoto SG, Hildebrand JG. Development of synapses in the antennal lobes of the moth Manduca sexta during metamorphosis. J Neurosci. 1983;3:1158–1175. doi: 10.1523/JNEUROSCI.03-06-01158.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert LP, Oland LA, Tucker ES, Gibson NJ, Higgins MR, Lipscomb BW. Bidirectional influences between neurons and glial cells in the developing olfactory system. Prog Neurobiol. 2004;73:73–105. doi: 10.1016/j.pneurobio.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Treloar H, Tomasiewicz H, Magnuson T, Key B. The central pathway of primary olfactory axons is abnormal in mice lacking the N-CAM-180 isoform. J Neurobiol. 1997;32:643–658. doi: 10.1002/(sici)1097-4695(19970620)32:7<643::aid-neu1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Treloar HB, Gabeau D, Yoshihara Y, Mori K, Greer CA. Inverse expression of olfactory cell adhesion molecule in a subset of olfactory axons and a subset of mitral/tufted cells in the developing rat main olfactory bulb. J Comp Neurol. 2003;458:389–403. doi: 10.1002/cne.10590. [DOI] [PubMed] [Google Scholar]
- Treloar HB, Nurcombe V, Key B. Expression of extracellular matrix molecules in the embryonic rat olfactory pathway. J Neurobiol. 1996;31:41–55. doi: 10.1002/(SICI)1097-4695(199609)31:1<41::AID-NEU4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Treloar HB, Purcell AL, Greer CA. Glomerular formation in the developing rat olfactory bulb. J Comp Neurol. 1999;413:289–304. [PubMed] [Google Scholar]
- Tucker ES, Oland LA, Tolbert LP. In vitro analyses of interactions between olfactory receptor growth cones and glial cells that mediate axon sorting and glomerulus formation. J Comp Neurol. 2004;472:478–495. doi: 10.1002/cne.20058. [DOI] [PubMed] [Google Scholar]
- Vassar R, Chao SK, Sitcheran R, Nunez JM, Vosshall LB, Axel R. Topographic organization of sensory projections to the olfactory bulb. Cell. 1994;79:981–991. doi: 10.1016/0092-8674(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Wong AM, Axel R. An olfactory sensory map in the fly brain. Cell. 2000;102:147–159. doi: 10.1016/s0092-8674(00)00021-0. [DOI] [PubMed] [Google Scholar]
- Walsh FS, Doherty P. Neural cell adhesion molecules of the immunoglobulin superfamily: role in axon growth and guidance. Annu Rev Cell Dev Biol. 1997;13:425–456. doi: 10.1146/annurev.cellbio.13.1.425. [DOI] [PubMed] [Google Scholar]
- Wang F, Nemes A, Mendelsohn M, Axel R. Odorant receptors govern the formation of a precise topographic map. Cell. 1998;93:47–60. doi: 10.1016/s0092-8674(00)81145-9. [DOI] [PubMed] [Google Scholar]
- Wang J, Xia Q, He X, Dai M, Ruan J, Chen J, Yu G, Yuan H, Hu Y, Li R, Feng T, Ye C, Lu C, Wang J, Li S, Wong GK, Yang H, Wang J, Xiang Z, Zhou Z, Yu J. SilkDB: a knowledgebase for silkworm biology and genomics. Nucleic Acids Res. 2005;33(Database Issue):D399–D402. doi: 10.1093/nar/gki116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EJ, Furness J, Walsh FS, Doherty P. Activation of the FGF receptor underlies neurite outgrowth stimulated by L1, N-CAM, and N-cadherin. Neuron. 1994;13:583–594. doi: 10.1016/0896-6273(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Wright JW, Snyder MA, Schwinof KM, Combes S, Copenhaver PF. A role for fasciclin II in the guidance of neuronal migration. Development. 1999;126:3217–3228. doi: 10.1242/dev.126.14.3217. [DOI] [PubMed] [Google Scholar]
- Woodin MA, Munno DW, Syed NI. Trophic factor-induced excitatory synaptogenesis involves postsynaptic modulation of nicotinic acetylcholine receptors. J Neurosci. 2002;22:505–514. doi: 10.1523/JNEUROSCI.22-02-00505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Q, Zhou Z, Lu C, Cheng D, Dai F, Li B, Zhao P, Zha X, Cheng T, Chai C, Pan G, Xu J, Liu C, Lin Y, Qian J, Hou Y, Wu Z, Li G, Pan M, Li C, Shen Y, Lan X, Yuan L, Li T, Xu H, Yang G, Wan Y, Zhu Y, Yu M, Shen W, Wu D, Xiang Z, Yu J, Wang J, Li R, Shi J, Li H, Li G, Su J, Wang X, Li G, Zhang Z, Wu Q, Li J, Zhang Q, Wei N, Xu J, Sun H, Dong L, Liu D, Zhao S, Zhao X, Meng Q, Lan F, Huang X, Li Y, Fang L, Li C, Li D, Sun Y, Zhang Z, Yang Z, Huang Y, Xi Y, Qi Q, He D, Huang H, Zhang X, Wang Z, Li W, Cao Y, Yu Y, Yu H, Li J, Ye J, Chen H, Zhou Y, Liu B, Wang J, Ye J, Ji H, Li S, Ni P, Zhang J, Zhang Y, Zheng H, Mao B, Wang W, Ye C, Li S, Wang J, Wong GK, Yang H. A draft sequence for the genome of the domesticated silkworm (Bombyx mori) Science. 2004;306:1937–1940. doi: 10.1126/science.1102210. [DOI] [PubMed] [Google Scholar]
- Yoshihara Y, Kawasaki M, Tamada A, Fujita H, Hayashi H, Kagamiyama H, Mori K. OCAM: A new member of the neural cell adhesion molecule family related to zone-to-zone projection of olfactory and vomeronasal axons. J Neurosci. 1997;17:5830–5842. doi: 10.1523/JNEUROSCI.17-15-05830.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Johnson TJ, Myers AA, Kanost MR. Identification by subtractive suppression hybridization of bacteria-induced genes expressed in Manduca sexta fat body. Insect Biochem Mol Biol. 2003;33:541–559. doi: 10.1016/s0965-1748(03)00028-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preabsorption of the EGFR antibody with a synthetic peptide representing the Bombyx sequence corresponding to the antigenic peptide from humans eliminates all labeling in the antennal lobe. Two halves of a stage-6 brain were processed for EGFR immunocytochemistry using either the antibody alone (panel A) or the antibody which had been preabsorbed with the Bombyx peptide (panel B). C) Section shown in panel B, but with collection parameters altered to integrate the signal from 16 consecutive scans (instead of the normal single scan), to confirm the presence of antennal lobe tissue. Scale bar = 100 µm.