Abstract
ERdj3 was identified as a soluble, lumenal DnaJ family member that binds to unassembled immunoglobulin heavy chains (HC) along with the BiP chaperone complex in the endoplasmic reticulum of mammalian cells. Here we demonstrated that ERdj3 binds directly to unfolded substrates. Secondary structure predictions suggested that the substrate binding domain of ERdj3 was likely to closely resemble Ydj1, a yeast cytosolic DnaJ family member, which was previously crystallized with a peptide bound to the Cterminal fragment composed of domains I, II, and III. Mutation of conserved residues in domain I, which formed the peptide binding site in Ydj1, affected ERdj3’s substrate binding ability in mammalian cells and in vitro binding studies. Somewhat unexpectedly, we found that domain II, which is highly conserved among ERdj3 homologues, but very different from domain II of Ydj1, was also critical for substrate binding. In addition, we demonstrated that ERdj3 forms multimers in cells and found that the conserved carboxyterminal residue phenylalanine 326 played a critical role in self-assembly. In vitro binding assays revealed that mutation of this residue to alanine diminished ERdj3’s substrate binding ability, arguing that multimerization is important for substrate binding. Together these studies demonstrate that the Ydj1 structure is conserved in another family member and reveal that among this group of DnaJ proteins domain II, which is not resent in the closely related type II family members, also plays an essential role in ubstrate binding.
Hsp70 proteins are a family of molecular chaperones found in all organisms and all organelles. BiP is the mammalian ER Hsp70 homologue and was first identified as an immunoglobulin heavy chain binding protein (1–3). Like other Hsp70s, BiP contains an N-terminal nucleotide binding domain that can interact with ATP or ADP (4) and a C-terminal substrate binding domain (5;6). The nucleotide bound state of Hsp70 regulates substrate binding. In the ATP-bound state, the substrate binding domain (SBD) is open, which results in both a fast on and off rate for interaction with unfolded proteins. In the ADP-bound state, SBD is closed and binds to substrates slowly but tightly (7). Binding of unfolded proteins to the SBD stimulates Hsp70’s ATPase activity and induces the hydrolysis of ATP to ADP in vitro (8). During this process, Hsp70 protein undergoes a conformational change, which induces closure of a lid over the SBD and stabilizes the interaction between Hsp70 and the substrate. The release of ADP and rebinding of ATP resets the Hsp70 to the “open” form, which allows the substrate to be released and to fold (3;7;9).
The ATPase cycle of Hsp70 proteins is regulated by co-factors that either induce ATP hydrolysis or regulate nucleotide exchange. DnaJ proteins interact with the ATP bound form of Hsp70s and induce ATP hydrolysis (10). To date, a large number of proteins have been designated as DnaJ-like proteins due to the presence of a highly conserved ~70 amino acid domain that has been termed the “J domain”. The J domain contains the signature His-Pro-Asp (HPD) tri-peptide motif, which plays a critical role in the interaction with Hsp70 (11;12). Mutation of HPD to either QPD or HPN abolishes the interaction between the mutant J domain and its Hsp70 partners. DnaJ proteins can be divided into three subgroups according to their domain conservation with E. coli DnaJ (10;13). Like E. coli DnaJ, Type I DnaJ proteins contain four domains: an N terminal J domain, a Gly/Phe rich flexible linker domain, followed by a Cys-rich region that forms two Zn2+ binding sites, and a C terminal domain that appears to contribute to substrate binding. Type II DnaJ proteins are similar to type I proteins, except that they lack the Cys-rich region. Type III DnaJs possess only the J domain, which can be present anywhere in the molecule. Like Hsp70s, DnaJ proteins exist in all organelles and organisms, but often far out number the Hsp70s present. Type I and type II DnaJ proteins can interact directly with unfolded substrates and inhibit protein aggregation in vitro. Studies on E. coli DnaJ (14;15) and the yeast cytosolic DnaJ proteins, Ydj1 (16;17) and Sis1 (18) have further defined the peptide binding region of type I and II DnaJ proteins. Although some type III DnaJs bind to substrate directly, like auxilin, which binds to clathrin and assists in the uncoating of clathrin-coated vesicles (19), NMR data demonstrate that the protein binding domain does not resemble that of type I and II proteins.
Six mammalian ER localized DnaJ-like proteins have been identified, which we have referred to as ERdj1-6. ERdj3 was identified as a soluble, lumenal DnaJ family member that contains an N-terminal J domain and binds to shiga toxin (20) and unassembled immunoglobulin heavy chains (γHC) along with the BiP chaperone complex in the ER (21). Subsequently it was shown that ERdj3 associates with a number of other unfolded proteins that are BiP substrates (22). The demonstration that the ERdj3 J domain mutant (HPD→QPD), which abolished the interaction with BiP, did not inhibit their ability to bind to substrate (22) led us to hypothesize that ERdj3 might bind directly to unfolded substrates. Since the interaction between ERdj3 and unassembled heavy chains is unusually stable in vivo, it was a particularly good model to investigate the structural features of ERdj3 that are important for substrate binding. Using secondary structure predictions and tertiary structure modeling, we found that the C-terminus of ERdj3 is very similar to that of Ydj1 (16). The domain I sequence is interrupted with a domain II, although it is much smaller and has an entirely different sequence than that of Ydj1. Like Ydj1, domain I of ERdj3 was found to be important for substrate binding. Mutation of conserved hydrophobic residues in domain I significantly reduced interaction with substrates in vitro. In addition, we demonstrated that ERdj3 exists as a dimer in cells and found that phenylalanine 326 played a critical role in dimerization, which also affected substrate binding. Finally, our data revealed that domain II was essential for association of ERdj3 with substrates.
EXPERIMENTAL PROCEDURES
Production of ERdj3 mutants
ERdj3 mutants were made using a Quick Change Site– Directed Mutagenesis PCR Kit (Stratagene) with wild type HA-tagged ERdj3 (3HA-DSL-ERdj3) and QE-ERdj3 serving as the templates. The QPD mutant was generated previously in our lab (22). Other mutants were generated by PCR using the indicated primer pairs.
ΔJ (deleting amino acids 17 – 87): 5’primer: GGGGCGGTGATTGCCAAAGATGGTCATCAG and 3 ’primer: CTGATGACCATCTTTGGCAATCACCGCCCC
ΔG/F (deleting amino acids 88 – 128): 5’primer GGTGAAGAAGGATTACCTCGTCAGCAAGAC and 3’primer GTCTTGCTGACGAGGTAATCCTTCTTCACC
ΔIa (deleting amino acids 129 – 159): 5’primer CATGTTTGGAGGAACCAAGTGCAATTGTCGGC and 3’primer GCCGACAATTGCACTTGGTTCCTCCAAACATG
ΔG/FΔIa (deleting amino acids 88 – 159): 5’primer GGTGAAGAAGGATTAAAGTGCAATTGTCGGC and 3’primer GCCGACAATTGCACTTTAATCCTTCTTCACC
ΔII (deleting amino acids 160 – 200): 5’primer GTTAGAAACAAACCTGTGGCACTAGTGAATGAAGAACGAACG and 3’primer CGTTCGTTCTTCATTCACTAGTGCCACAGGTTTGTTTCTAAC
ΔIIGSGG (deleting amino acids 160 – 200):5’primer GTTAGAAACAAACCTGTGGCAGGCAGCGGGGGCCTAGTGAATGAAGAACGAACG and 3’primer CGTTCGTTCTTCATTCACTAGGCCCCCGCTGCCTGCCAAGGTTTGTTTCTAAC
I113A: 5’primer CCAAGAGGAAGTGATGCTATTGTAGATCTAGAAGTC and 3’ primer GACTTCTAGATCTACAATACCATCACTTCCTCTTGG
V132A: 5’ primer GCAGGAAATTTTGTGGAAGCAGTTAGAAACAAACCTGTGG and 3’ primer CCACAGGTTTGTTTCTAACTCCTTCCACAAAATTTCCTGC
L226A: 5’ primer GTGAATGAAGAACGAACGGCGGAAGTAGAAATAGAGCCTG and 3’ primer CAGGCTCTATTTCTACTTCCCCCGTTCGTTCTTCATTCAC
F241A: 5’ primer CCTGGAGATTTACGGGCCCGAATCAAAGTTGTC and 3’ primer GACAACTTTGATTCGGGCCCGTAAATCTCCAGG
F326A: 5’ primer GGGCTCTTTGATAATCACTTTTGATGTGGATGCTCCAAAAGAACAG and 3’ primer CTGTTCTTTTGGAGCATCCACATCAAAAGTGATTATCAAAGAGCCC
F326D: 5’primer GGGCTCTTTGATAATCACTTTTGATGTGGATGATCCAAAAGAACAG and 3’primer CTGTTCTTTTGGATCATCCACATCAAAAGTGATTATCAAAGAGCCC
To generate the ΔIII+C-aa mutant, a PCR reaction was performed using 3HA-DSLERdj3 as the template with 5’EcoRI-CGGAATTCGGACCCGGGAC and 3’NotIATAAGAATGCGGCCGCCAGTGCTTGACAACTTTGATTCGG as primer pairs, which removed amino acids 251 to the C-terminus at 358. After cutting the PCR product with EcoRI and NotI, it was ligated into the 3HA-DSL vector in place of the corresponding EcoRI – NotI fragment present in full length ERdj3. This mutant removes all of domain III as well as the remaining 30 C-terminal amino acids.
Vectors for eukaryotic and bacterial expression
Untagged versions of WT ERdj3 as well as two dimerization mutants, F326A and F326D were inserted into the pSG vector for expression in mammalian cells. Epitope tagged versions of constructs containing one HA sequence at the C-terminus were constructed in the HA-DSL vector. These include WT ERdj3, all of the domain deletion mutants described above, the point mutations in domain I, and two dimerization mutants, F326A and F326D. Finally, a wild-type ERdj3 construct encoding three HA sequences at the C-terminus was inserted into 3XHA-DSL. A humanized immunoglobulin γ heavy chain (23) in the pSVL vector. To produce recombinant proteins in bacteria, ERdj3 constructs were introduced into the pQE vector. These include wild type ERdj3, ΔIa, ΔIIGSGG, I113A, V132A, L226A, F241A, I113A/V132A, IVLF-A, and F326A/D.
Cell culture, transfection, and immunoprecipitation
COS-1 monkey kidney fibroblast cells were maintained in DMEM media supplemented with 10% fetal bovine serum, 2mM L-glutamine, and a 1% antibiotic solution and cultured in 3% CO2 at 37°C. Cells were transfected with the indicated ERdj3 constructs and where indicated, an Ig heavy chain, using the Fugene 6 transfection reagent. Forty-eight hours post-transfection cells were labeled with 35S methionine and cysteine for 3h. Cells were incubated with 3,3’-dithio-bis (propionic acid N-hydroxysuccinimide ester) (DSP), a membrane permeable cross-linking reagent as described previously (21) to stabilize the interaction of ERdj3 with γHC. Cell lysates were prepared using an NP40 lysing buffer (24) and immunoprecipitated with the indicated antisera followed by binding to Protein A Sepharose beads. The rabbit polyclonal ERdj3 antibody (22) was affinity purified on recombinant mouse ERdj3 protein for our studies. All the ERdj3 deletion constructs were engineered with an HA-epitope tag at their C terminus, allowing them to be recognized by an anti-HA monoclonal antibody (a kind gift of Dr. Al Reynolds, Vanderbilt University). Immunoprecipitated complexes were analyzed by SDS-PAGE under reducing conditions, and the signal was detected using Amplify (Amersham Bioscience) for autoradiographic visualization. To assess whether these various deletions grossly affected the overall structure of the ERdj3 mutants, we performed pulse-chase experiments. All of the indicated mutants were transiently expressed in COS-1 cells. Twenty-four hours post-transfection cells were labeled with 35S methionine and cysteine for 45 min and then chased in complete media for indicated times. Cell lysates were prepared and immunoprecipitated with an anti-HA monoclonal antibody and analyzed by 10% SDS-PAGE. The signal was detected by autoradiography.
Protein Purification
The expression of His-tagged wild-type and mutant ERdj3 proteins were induced in E. coli M15 cells with 0.1 mM isopropyl β-D-thiogalactoside (Sigma) followed by growth for 18 h at 18°C. The recombinant proteins were purified on Ni2+-agarose columns under non-denaturing conditions and dialyzed against ATPase buffer (20 mM HEPES (pH 7.2), 50 mM KCl, 5 mM MgCl2) A protease inhibitor cocktail was added to the proteins, and they were made to 50% glycerol for storage at - 20°C. To assess affects on global protein folding, the recombinant proteins were examined for sensitivity to Proteinase K. Briefly, 5 µg of recombinant ERdj3 proteins were incubated with 1.0 µg of protease K at 37°C in ATPase buffer (total reaction mixture is 30 µl for the indicated times. The reaction was stopped by adding 1.5 µl of 10 mg/ml PMSF and incubating on ice for 30 min. The protein products were analyzed on 15% SDS polyacrylamide gels and visualized by Coomassie Blue staining.
Measurement of complex formation between ERdj3 proteins and denatured luciferase
Wild type or mutant ERdj3 recombinant proteins (0.5µg in 100 µl of PBS containing 0.05% BSA) were added in triplicate to each well of a 96-well microtiter plate and allowed to bind overnight at 4°C. Wells were washed with PBS to remove unbound ERdj3 and blocked with 200µl PBS containing 1% BSA for 1h at room temperature. Firefly luciferase (Promega) (15 µg/µl) was denatured in buffer containing 7M urea, 25mM HEPES (pH 7.5), 50mM KCl, 5mM EDTA, 5mM MgCl2, and 5mM dithiothreitol (1:41 v:v) at room temperature for 40 min as described previously (25) and then diluted into PBS containing 0.05% BSA (final concentration of luciferase is 5 µg/ml). 100 µl of this solution was added to the wells containing wild type or mutant ERdj3 and allowed to bind for 1hr at room temperature followed by washing with PBS to remove unbound luciferase. The amount of denatured luciferase that remained bound to ERdj3 was detected with a polyclonal anti-luciferase antiserum followed by donkey anti-rabbit Ig conjugated to alkaline phosphatase. The substrate, 4-Nitrophenyl phosphate disodium salt hexahydrate, was added to the wells for approximately 10 min, and then 0.75 M NaOH was added to stop the reaction. The plates were read on a spectrophotometer (BioRAD) at wavelength 405nM. Negative controls were included for each plate in wells that did not contain either luciferase, ERdj3, or each of the antibodies, but which included all the other steps of the reaction. The amount of luciferase that bound to wild-type ERdj3 was set as 100% and that binding to the mutants was expressed as a percent of wild-type.
RESULTS
Modeling ERdj3’s secondary structure
We recently demonstrated that ERdj3 can bind directly to free HC and denatured luciferase in vitro (Jin et al, submitted). To identify the regions of ERdj3 that contribute to substrate binding, we first used a computer program (Predictproteins) to generate a secondary structure prediction for ERdj3 and compared it with those of two yeast cytosolic DnaJ proteins for which structural data are available for the protein binding domain, Ydj1 (type I) and Sis1 (type II) (17;18). Although the amino acid sequences of these two proteins are not highly conserved, their overall secondary (Figure 1A) and tertiary structures are very similar except that Sis1 lacks the cysteinerich domain II, which is a hallmark domain of type I DnaJ proteins (Figure 1B). Comparison of the secondary structure predictions revealed that ERdj3 is more similar to Ydj1, in that it appears to have an additional sequence (domain II) inserted within domain I (Figure 1A). However, the sequence and size of this domain is quite different from that of Ydj1 (Figure 1C). The corresponding domain in Ydj1 is 65 amino acids in length, with 8 cysteine residues that form two zinc binding centers (CXXCXG motif), whereas the predicted domain in ERdj3 is only 44 amino acids in length and possesses only four cysteine residues that form two intradomain disulfide bonds (26). Using PyMol (DeLano Scientific LLC) to model ERdj3’s structure, we found that domains I and III appeared to be almost identical to those of Ydj1, whereas domain II could not be modeled on the Ydj1 structure (data not shown).
Figure 1. Comparison of ERdj3 with Ydj1 and Sis1.
(A) Secondary structure predictions for ERdj3, Sis1 and Ydj1. Colors represent the different domains: Light grey: ER targeting sequence, grey: domain J, bold and italics: domain I, bold and underlined: domain II, black: domain III, H: α helix, and E: β sheet. (B) The schematic representation domain structures of Ydj1, Sis1 and ERdj3 showing that Sis1does not contain a cysteine-rich domain II and that ERdj3 is more similar to Ydj1, although it contains an atypical, smaller domain II. (C) Domain II sequences of ERdj3 from various species were aligned and compared to domain II of Ydj1. Clear boxes indicate amino acid identities, whereas grey shading indicates amino acid similarity. Cysteine residues (CysXXCys motif) are underlined.
Mapping ERdj3’s substrate binding domain in vivo
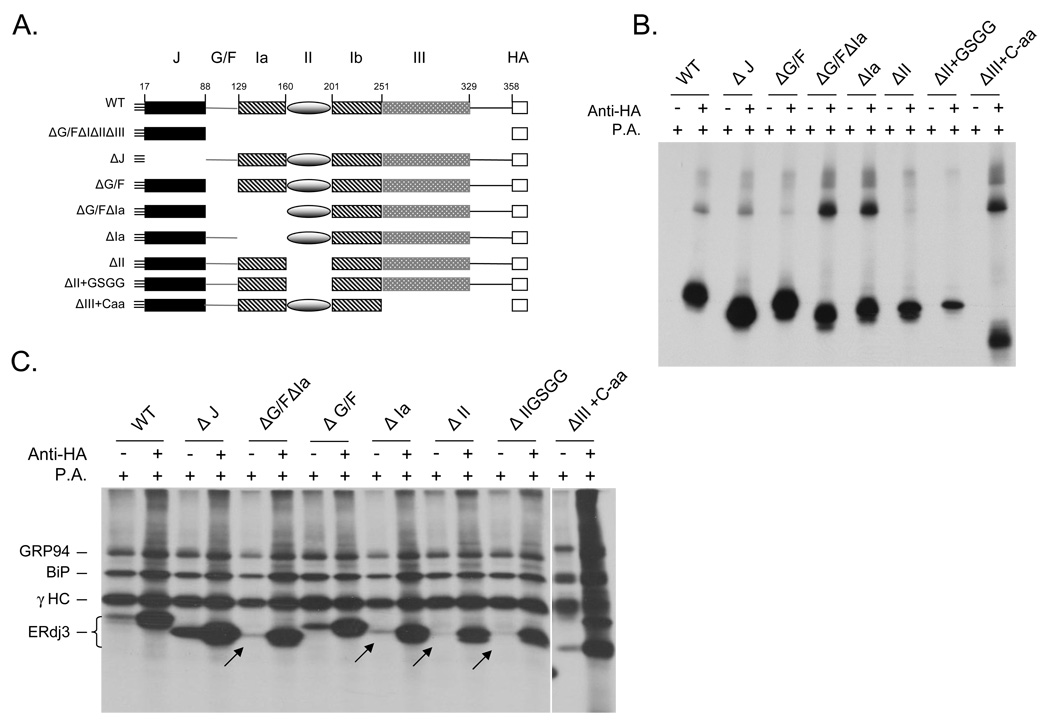
Based on the structures of Ydj1 and Sis1 and our ability to model ERdj3 on the Ydj1 structure, we defined domain boundaries for ERdj3, referring to them as domain I (Ia and Ib), II and III, and made corresponding deletion mutants (Figure 2A). In addition, we deleted the J domain and the Gly/Phe-rich domain both alone and along with domain Ia to investigate the role of each of these domains in substrate binding. An HA-epitope tag was engineered at the carboxyl terminus of each of these mutants for immunoprecipitation purposes. In the case of the domain III mutant, we removed the sequence that was predicted to form this domain, as well as the remaining 30 amino acids at the carboxyterminus. Domain Ia and Ib were deleted separately, even though the structural predictions suggest that they should fold together to form a intact domain I. In the case of domain II, two deletion mutants were made, one that removed only the sequence encoding this domain (ΔII) and a second one in which domain II was replaced by a four amino acid flexible linker, GSGG, to increase the possibility that the two halves of domain I would fold correctly in the absence of domain II (Figure 2A). Each of the ERdj3 constructs was first expressed in COS cells alone. SDS-PAGE analysis revealed that all of the proteins were expressed and could be immunoprecipitated with the anti-HA antibody (Figure 2B). Importantly none of these proteins bound non-specifically to Protein A Sepharose beads (Figure 2B).
Figure 2. Binding of wild-type and mutant ERdj3 to γHC.
(A) Schematic representation of the domain composition of various ERdj3 mutants. (B) COS cells were transfected with the indicated ERdj3 constructs in the HA-DSL vector, metabolically labeled and immunoprecipitated with anti-ERdj3 or Protein A Sepharose alone. (C) COS cells were cotransfected with γHC and either wild-type ERdj3 or the indicated ERdj3 mutants. Cells were metabolically labeled, treated with DSP before lysing and immunoprecipitated with Protein A Sepharose to isolate γHC or with a monoclonal anti-HA antibody to isolate ERdj3 proteins. Samples were analyzed by reducing SDS-PAGE, and mutants that show diminished binding are indicated with arrows.
To determine the ability of the various ERdj3 proteins to associate with γHC, cells were treated with DSP, which is required to stabilize ERdj3’s association with γHC (21), and then co-immunoprecipitation assays were performed with the indicated antibodies (Figure 2C). The presence of γHC, BiP, and GRP94 in the anti-HA lanes is not meaningful, as the γHC bind directly to Protein A Sepharose and co-precipitate these two chaperones (22). When each of the lysates was incubated with Protein A beads alone to isolate HC, we found that wild-type ERdj3 was co-precipitated as expected (Figure 2C). Examination of each of the mutants revealed that deletion of the J domain (ΔJ) actually caused the mutant to bind to γHC better than wild-type ERdj3, which is consistent with our previous finding that mutation of the HPD motif in this domain resulted in better binding of mutant ERdj3 with several different substrate proteins in cells (22) and that a functional interaction with BiP is required to release ERdj3 from substrates (25). Deletion of the Gly/Phe rich region (ΔG/F) did not obviously affect binding to γHC, and removing domain III along with C-terminal 30 amino acids had only a very modest effect on binding. When domain Ia was deleted alone or along with the Gly/Phe rich region, we observed a slight decrease in the ability of the mutant proteins to associate with the substrate. Structural data on Ydj1 revealed that domain I is in direct contact with a peptide that was co-crystallized with Ydj1 and is therefore is very likely to form at least part of the substrate binding site (17). Domain I of Sis1 also contains a similar substrate binding structure based on crystallographic data (18). Somewhat unexpectedly, we found that deletion of domain II (ΔII), which bears little homology to the corresponding domain in Ydj1 and is missing in Sis1, had the most dramatic effect on ΔHC binding. Pulse-chase experiments were performed on the ΔII-GSGG mutant to assess the overall affect of the deletion on the remaining protein structure (Supplemental data, Figure S1). We found that this mutant had a somewhat reduced half-life suggesting that deletion of this domain even with the addition of a linker affected another region of the protein.
Binding of ERdj3 to purified denatured luciferase in vitro
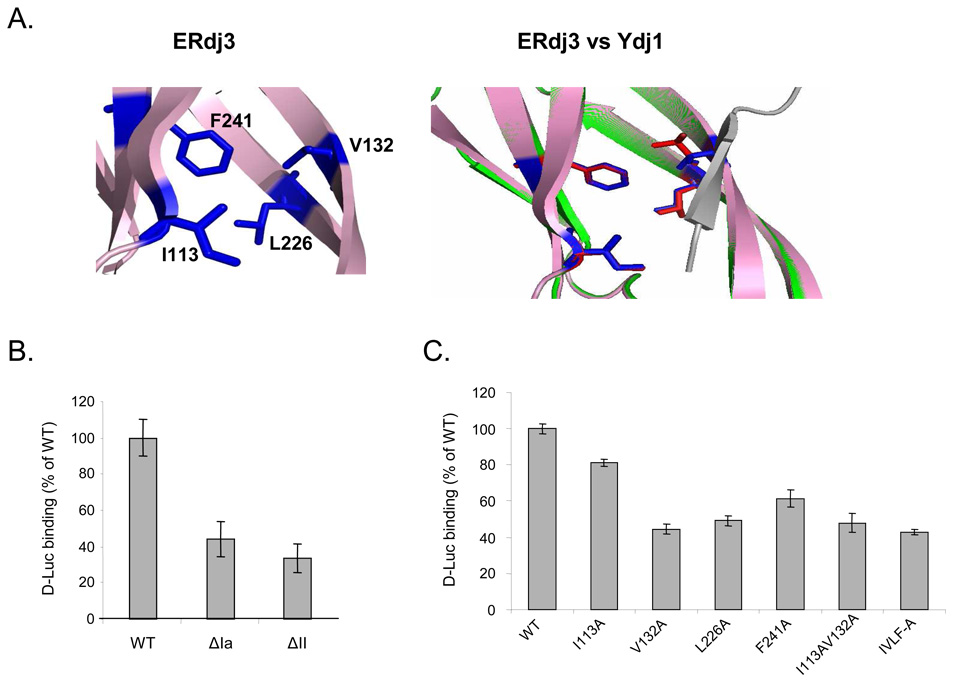
A caveat to these data is that binding of even wild-type ERdj3 to γHC in cells can only be detected with a chemical cross-linker, due to the detergent sensitive nature of its association with substrates, and the effects of most of the deletions are quite modest. In addition, the presence of endogenous ERdj3 and other ER chaperones with which ERdj3 can interact further complicated the analysis. Since we recently demonstrated that purified recombinant ERdj3 could bind directly to chemically denatured luciferase in solution (25), we used this assay to further probe the regions of ERdj3 required for substrate binding. Recombinant proteins corresponding to wild-type ERdj3 and two of the mutants tested above were produced and examined for binding to luciferase. Similar to data obtained for the in vivo binding of these mutants to γHC, we found that deletion either domain Ia or II significantly diminished ERdj3’s ability to bind to this substrate (Figure 3B). However, deletion of domain Ia is likely to distort the overall structure of this domain, since it cooperates with domain Ib to form the intact domain I. Thus we felt that data obtained with this mutant could not be clearly interpreted and wished to make more conservative point mutations in this domain.
Figure 3. Both domain I and domain II of ERdj3 contribute to its ability to bind to denatured luciferase.
(A) Model of ERdj3’s putative substrate binding domain (left) shows amino acid residues that could form a structure similar to that found on Ydj1. An overlap of the peptide binding site of Ydj1 with the model of the corresponding region of ERdj3 is shown on right. Grey: substrate peptide, green: Ydj1, pink: ERdj3 model, red: peptide interacting residues on Ydj1, and blue: corresponding residues on ERdj3. (B) Measurement of complex formation between wild-type ERdj3 and the ΔIa and ΔII +GSGG mutants and denatured luciferase was performed as described in the Materials and Methods section. The quantity of luciferase bound to wild-type ERdj3 was set to 100%, and the values for the various mutants were expressed as a percent of this value. Standard errors are indicated. (C) Measurement of complex formation between the indicated ERdj3 point mutants and luciferase.
According to structural data for Ydj1, Sis1, and most recently Hdj1, domain I contains a hydrophobic pocket that forms the substrate binding site (17;18;27) Using PyMol (DeLano Scientific LLC) to model this region of ERdj3 on the structure of Ydj1, we found that four out of five amino acids in Ydj1’s domain I that form the hydrophobic pocket (28) were conserved in ERdj3 (Figure 3A). Ile113 and Val132 are in domain Ia and Leu226 and Phe241 are in domain Ib, which is the same as the location of the corresponding pocket residues in Ydj1. To examine the roles of these amino acids in ERdj3’s substrate binding, we mutated each of these four amino acids alone (I113A, V132A, L226A, F241A), the first two amino acids together (I113A-V132A), or all four of them to alanine (IVLF-A) and tested their ability to bind to luciferase in vitro. We found that except for I113A, all of the point mutations or combinations of them affected ERdj3’s ability to bind to luciferase (Figure 3C). It is unlikely that these mutations grossly affected the overall structure of the mutant ERdj3 proteins as both of these proteins were as stable as wild-type ERdj3 as determined by pulse-chase and protease sensitivity experiments (Supplemental data, Figure S1 and S2). These data suggested that these hydrophobic amino acids are likely to also form the substrate binding site in ERdj3, which agrees with the structure model (Figure 3A).
ERdj3 is dimeric, which is important for substrate binding
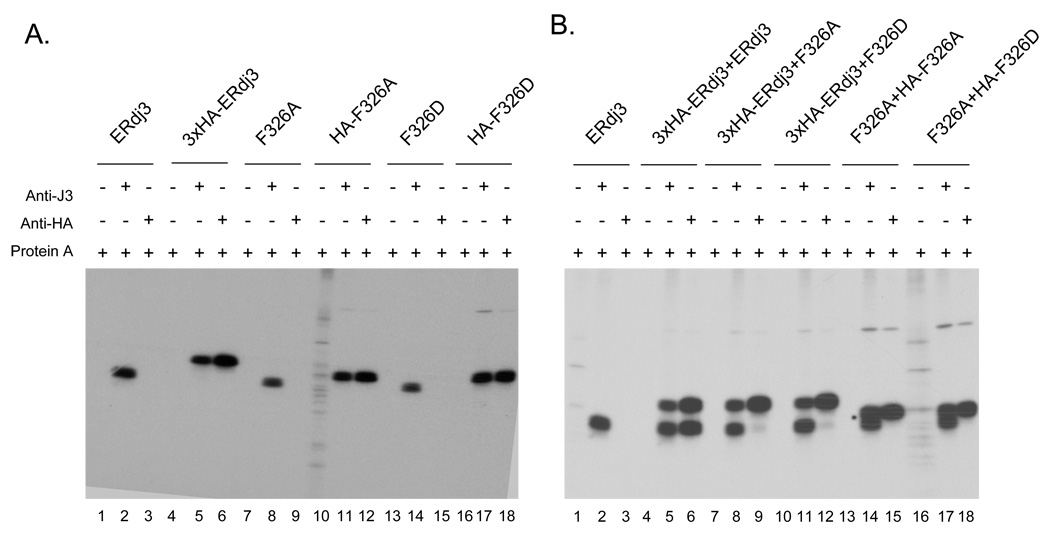
The yeast cytoplasmic DnaJ homologues, Ydj1 and Sis1, form dimers via sequences located near their C terminus, which are essential for substrate binding (18;29). Since ERdj3 is similar to Ydj1 in overall structure, we tested whether ERdj3 forms dimers in cells. First, we made a 3×HA-ERdj3 construct, which contains three HA tags at the C terminus of ERdj3 in order to clearly separate it from the untagged versions of ERdj3. Each of the tagged and untagged constructs were first expressed in COS cells alone. Cell lysates were prepared and immunoprecipitated with Protein A Sepharose beads alone, the anti-ERdj3 serum, or a monoclonal anti-HA antibody. The monoclonal anti-HA antibody interacted only with HA-tagged form of ERdj3, whereas the polyclonal anti-ERdj3 recognized both 3X HAERdj3 and ERdj3 (Figure 4A). The 3X HA-tagged form of ERdj3 was readily distinguished from ERdj3 on 10% SDS-PAGE gels. Next, the 3X HA-tagged form of ERdj3 was co-expressed with untagged wild-type ERdj3. We observed that the anti-HA antibody precipitated both 3X HA-ERdj3 and ERdj3 at an approximately 1:1 ratio when they were co-expressed (Figure 4B, lane 6), suggesting that 3X HA-ERdj3 and ERdj3 are associated with each other in cells as either dimers or multimers. Unlike ERdj3’s association with substrate, ERdj3 oligomers are stable to detergents and can be detected in the absence of crosslinker.
Figure 4. ERdj3 forms dimers in cells.
(A) COS cells were transfected with Cdna encoding untagged wild-type ERdj3 (lanes1–3), 3×HA-ERdj3 (lanes 4–6), untagged ERdj3 F326A (lanes 7–9) and F326D (lanes 13–15), HA-ERdj3 F326A (lanes10–12) or HA-ERdj3 F326D (lane 16–18) alone. (B) COS cells were cotransfected with ×HAERdj3 and untagged ERdj3 (lanes 4–6), with 3×HA-ERdj3 and untagged F326A (lanes 7–9), with 3×HA-ERdj3 and untagged F326D (lane 10–12), with untagged F326A and HA-F326A (lane 13–15), or with untagged F326D and HA-F326D (lane 16–18). The cell lysates were divided to three parts equally and incubated with indicated antibodies. Immune complexes were precipitated with Protein A Sepharose, and samples were analyzed by reducing SDS-PAGE.
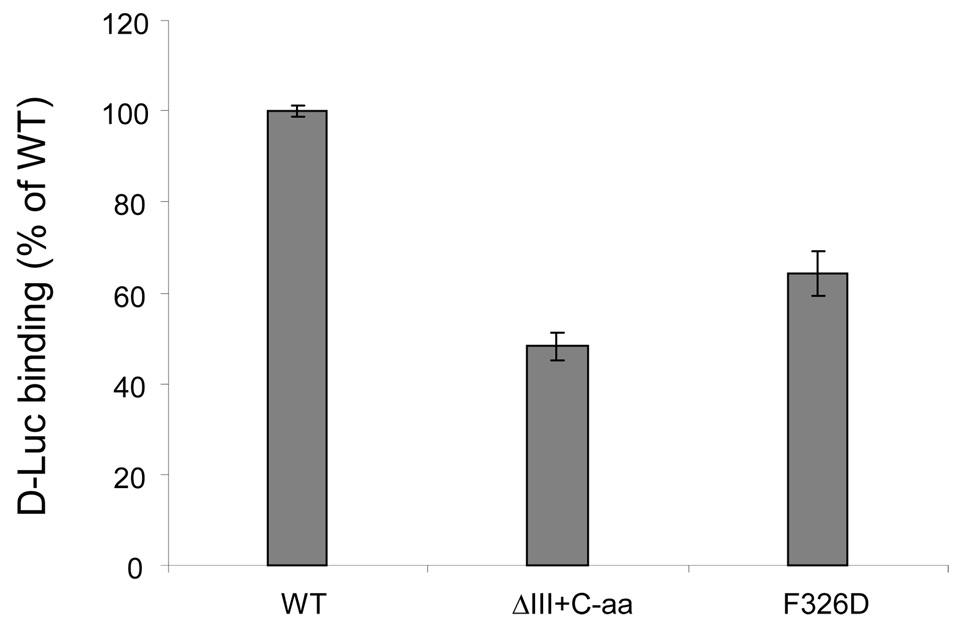
It has been reported that tyrosine 336 on Sis1 plays an important role in dimerization (18). Phenylalanine 335 of Ydj1 is the counterpart of Y336 of Sis1 and is also critical for Ydj1 dimerization (29). Sequence alignment of Sis1, Ydj1 and ERdj3 identified phenylalanine 326 on ERdj3 as the likely counterpart of F335 of Ydj1 and Y336 of Sis1, and similar to these two proteins, this residue is very near the C-terminus of domain III, which occurs at amino acid 326. Thus, we mutated F326 to either alanine (A) or aspartic (D) amino acid on both an untagged or singly tagged background. The various mutants were first expressed in COS cells and immunoprecipitated with the indicated reagents. To examine whether the ERdj3 F3326A/D mutants associated with wild type ERdj3, HA-tagged wild type ERdj3 was co-expressed with the untagged F326A or F326D ERdj3 mutants and immunoprecipitation experiments were performed (Figure 4B, lanes 7–12). We found that untagged F326A/D was not co-precipitated along with ERdj3HA-tagged wild type ERdj3 hen it was precipitated with the anti-HA antibody, demonstrating that the F326A/D mutations affected the ability of ERdj3 to self assemble. We also co-expressed untagged F326A/D ERdj3 along with HA-tagged F326A/D ERdj3 (Figure 4B, lanes 13-18). Again, we found that the mutation of F326 to either alanine or aspartic acid abolished ERdj3’s ability to forms dimers/oligomers. The ability of F326D ERdj3 to bind to luciferase was next examined. We found that this mutation decreased the binding of ERdj3 to substrate, as did deletion of domain III along with C-terminal 30 amino acids (ΔIII+ C-aa), which lacks F326 (Figure 5). This argues that self assembly is also important for ERdj3’s ability to bind substrate.
Figure 5. Deletion of domain III or mutation of phenylalanine 326 to aspartic acid affects ERdj3’s substrate binding ability.
Plates were coated with he indicated ERdj3 proteins and denatured luciferase was allowed to bind. ELISA assays were performed and quantified as described in Figure 3B.
DISCUSSION
According to their domain conservation with E. coli DnaJ, DnaJ proteins have been divided into three subgroups. Type I and II DnaJ proteins appear to bind directly and promiscuously to the unfolded regions of multiple substrates through their substrate binding domain, whereas type III proteins either do not bind to unfolded proteins or have a very limited client repertoire. A crystal structure has been obtained for the C terminal fragment of Ydj1 with a bound peptide (16). This fragment lacks both the N terminal J domain and G/F rich flexible linker and contains a mutation of phenylalanine 335 to aspartic acid, which disrupts its ability to form dimers. The peptide bound to a hydrophobic pocket on domain I and made contact with five different polar residues in this pocket. The importance of these residues in binding to peptide was confirmed with mutagenesis studies (17). The structure of the corresponding C terminal region of Sis1 had been solved previously, but in this case there was no substrate bound (18). Although the amino acid sequence homology is not high between these two proteins in this region, their tertiary structures are nearly identical, except that Sis1 lacks the Cys-rich domain II (18), suggesting that domain I of Sis1 is likely to contain the substrate binding site. This possibility is supported by mutagenesis studies in which mutations in the hydrophobic pocket affected Sis1’s ability to bind substrate (28). Our secondary structure predictions argue that ERdj3 should be considered to be a type I DnaJ protein even though its domain II is quite different from that of Ydj1. We found that most of hydrophobic amino acids that form the substrate binding site on Ydj1 are conserved in ERdj3. Mutations of these amino acids alone or in combination affected ERdj3’s ability to bind to luciferase in vitro. Overall these studies suggest that this substrate binding structure may be common to all type I and II DnaJ proteins. This is supported by earlier studies showing that DnaJ preferred binding to hydrophobic peptides of ~ 8 residues in length, which is compatible with it also having a hydrophobic substrate binding site (30). This study revealed that E. coli DnaJ preferred to bind peptides with hydrophobic features, which is compatible with the substrate binding site on DnaJ also possessing a hydrophobic pocket.
Both type I and type II DnaJ proteins form homodimers and the critical residues for dimerization are located in the C terminus of these proteins. Ydj1 and Sis1 dimerize via a number of hydrophobic residues at their C-terminus that form a hydrophobic patch. Mutation of F335 of Ydj1 to aspartic acid yielded monomers (16). Disruption of dimmer formation for both Ydj1 and Sis1 results in severe defects in their ability to bind to substrates and to facilitate Hsp70’s ability to refold substrates (18;29). We demonstrated that ERdj3 forms dimers or multimers in cells and that F326 is critical for this self-association and for ERdj3’s ability to interact with substrates. This finding supports a model where the dimerization of both type I and II DnaJ proteins allows the two substrate binding domains to form a clamp around unfolded substrates (31). Although type III DnaJ proteins are much less conserved, some of them have been shown to have substrate proteins (19). Although it has not been examined, it is possible that this group of proteins also dimerizes or conversely that dimerization is more important for the ability to interact with a broader range of substrates, which is a hallmark of the type I and II DnaJ proteins.
Our in vivo assay to examine the binding of ERdj3 mutants with HC revealed that the ΔIII+C-aa ERdj3 mutant, which lacked the dimerization domain, bound to HC as well as wild-type ERdj3, whereas the in vitro association of this mutant with luciferase was significantly diminished. We can think of four possibilities to account for this, which are not mutually exclusive. First, in the in vivo assays a crosslinker was used, which could serve to stabilize more transient interactions between mutant ERdj3 and the substrate. Second, there are a number of other chaperones and folding enzymes like BiP present in cells. Since a number of these proteins form a complex in cells (21), it is possible that the substrate is binding to one of these and not to the ΔIII mutant directly. Third, it is conceivable that the use of a crosslinker might stabilize the interaction between the ΔIII mutant and endogenous ERdj3, which would result in the apparent association of this mutant with HC in vivo. Finally, it is possible that the differences we are observing reflect distinct affinities of ERdj3 for these two substrates.
Our results argue that domain II is critical for ERdj3’s substrate binding ability. This Cys-rich region in other type I proteins contributes to their chaperone activities, in some cases by affecting the transfer of substrate to the Hsp70 partner (14;32;33). For other type I DnaJ proteins, the eight cysteines in domain II form two zinc binding sites (14), whereas in ERdj3 the four cysteines present in this domain form two intra-chain disulfide bonds (26). Although domain II of ERdj3 is very different from that of Ydj1, it is very highly conserved among ERdj3s (64% identity and 70% similarity between mouse and C. elegans). Modeling of ERdj3’s domain II, suggests that the two β sheets found in Ydj1 are present, but that the flexible loops, which contain the disulfide bonds in place of zinc binding sites, are likely to be quite different (Supplemental data, Figure S3). In spite of the importance of this domain in the function of type I DnaJ proteins, it is perhaps surprising that Sis1, which possesses a very a similar overall substrate binding structure as Ydj1, does not possess a domain II at all. It is conceivable that the substrate binding repertoire of type I proteins is different than that of type II DnaJ proteins and requires a more complex binding site. In support of this possibility, deletion of domain II from E. coli DnaJ only affected its interaction with some substrates but not others (14).
Alternatively, it possible that domain II could play a role in stabilizing domain I of type I DnaJ proteins in the absence of substrate. In support of this possibility, the structure of the Ydj1 C-terminal fragment could only be obtained with a bound peptide, whereas Sis1’s was crystallized without the presence of a peptide, suggesting a possible difference in the stabilities of these two proteins. Furthermore, we found that the ERdj3 Δ II mutant was somewhat less stable than wild-type ERdj3 or any of the point mutants, which could be interpreted to argue that this domain plays a role in stabilizing another portion of the protein such as domain I (Supplemental data, Figures S1 and S2).
In summary, we have demonstrated that domain I and II of ERdj3 contribute to substrate binding. The hydrophobic residues in domain I of ERdj3 apparently form a substrate binding site that resembles that of Ydj1. The role of domain II is less clear but could be important in maintaining domain I in a configuration that is critical for substrate binding or be required only for the interaction with a subset of unfolded proteins. In addition, we found that ERdj3 exists as a dimer/oligomer in cells, which contributes to substrate binding. Together these data help to define the structural elements required for substrate binding for DnaJ proteins.
Supplementary Material
Three figures are included which examine the half-live of the various ERdj3 proteins when expressed in COS-1 cells, the protease sensitivity of the various recombinant proteins, and a model of the predicted structure of the substrate binding domain of ERdj3. This material is available free of charge via the Internet at http://pubs.acs.org
ACKNOWLEDGEMENTS
We thank Isaac Estrada in our laboratory for technical support and Dr. Stephen White (St. Jude Children's Research Hospital) for helpful scientific discussions.
ABBREVIATIONS
- BiP
Immunoglobulin heavy chain binding protein
- Hsp70
Heat shock protein 70
- SBD
substrate binding domain
- γHC
immunoglobulin heavy chain
Footnotes
This work was supported by NIH Grant GM54068 (LMH), the Cancer Center CORE Grant CA21765, and the American Lebanese Syrian Associated Charities of St. Jude Children's Research Hospital.
REFERENCES
- 1.Haas IG, Wabl M. Immunoglobulin heavy chain binding protein. Nature. 1983;306:387–389. doi: 10.1038/306387a0. [DOI] [PubMed] [Google Scholar]
- 2.Bole DG, Hendershot LM, Kearney JF. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J. Cell. Biol. 1986;102:1558–1566. doi: 10.1083/jcb.102.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munro S, Pelham HR. An Hsp70-like protein in the ER: identity with the 78 kd glucose- regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986;46:291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- 4.Kassenbrock CK, Kelly RB. Interaction of heavy chain binding protein (BiP/GRP78) with adenine nucleotides. EMBO. J. 1989;8:1461–1467. doi: 10.1002/j.1460-2075.1989.tb03529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hendershot LM, Wei J-Y, Gaut JR, Lawson B, Freiden PJ, Murti KG. In vivo expression of mammalian BiP ATPase mutants causes disruption of the endoplasmic reticulum. Mol. Biol. Cell. 1995;6:283–296. doi: 10.1091/mbc.6.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chevalier M, King L, Wang C, Gething MJ, Elguindi E, Blond SY. Substrate binding induces depolymerization of the C-terminal peptide binding domain of murine GRP78/BiP. J. Biol. Chem. 1998;273:26827–26835. doi: 10.1074/jbc.273.41.26827. [DOI] [PubMed] [Google Scholar]
- 7.Liberek K, Skowyra D, Zylicz M, Johnson C, Georgopoulos C. The Escherichia coli DnaK chaperone, the 70-kDa heat shock protein eukaryotic equivalent, changes conformation upon ATP hydrolysis, thus triggering its dissociation from a bound target protein. J. Biol. Chem. 1991;266:14491–14496. [PubMed] [Google Scholar]
- 8.Flynn GC, Chappell TG, Rothman JE. Peptide binding and release by proteins implicated as catalysts of protein assembly. Science. 1989;245:385–390. doi: 10.1126/science.2756425. [DOI] [PubMed] [Google Scholar]
- 9.Buchberger A, Theyssen H, Schroder H, McCarty JS, Virgallita G, Milkereit P, Reinstein J, Bukau B. Nucleotide-induced conformational changes in the ATPase and substrate binding domains of the DnaK chaperone provide evidence for interdomain communication. J. Biol. Chem. 1995;270:16903–16910. doi: 10.1074/jbc.270.28.16903. [DOI] [PubMed] [Google Scholar]
- 10.Cheetham ME, Caplan AJ. Structure, function and evolution of DnaJ: conservation and adaptation of chaperone function. Cell Stress & Chaperones. 1998;3:28–36. doi: 10.1379/1466-1268(1998)003<0028:sfaeod>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wall D, Zylicz M, Georgopoulos C. The NH2-terminal 108 amino acids of the Escherichia coli DnaJ protein stimulate the ATPase activity of DnaK and are sufficient for lambda replication. J. Biol. Chem. 1994;269:5446–5451. [PubMed] [Google Scholar]
- 12.Suh WC, Burkholder WF, Lu CZ, Zhao X, Gottesman ME, Gross CA. Interaction of the Hsp70 molecular chaperone, DnaK, with its cochaperone DnaJ. Proc. Natl. Acad. Sci. U. S. A. 1998;95:15223–15228. doi: 10.1073/pnas.95.26.15223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caplan AJ, Cyr DM, Douglas MG. Eukaryotic homologues of Escherichia coli dnaJ: a diverse protein family that functions with hsp70 stress proteins. Mol. Biol. Cell. 1993;4:555–563. doi: 10.1091/mbc.4.6.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banecki B, Liberek K, Wall D, Wawrzynow A, Georgopoulos C, Bertoli E, Tanfani F, Zylicz M. Structure-function analysis ofthe zinc finger region of the DnaJ molecular chaperone. J. Biol. Chem. 1996;271:14840–14848. doi: 10.1074/jbc.271.25.14840. [DOI] [PubMed] [Google Scholar]
- 15.Szabo A, Korszun R, Hartl FU, Flanagan J. A zinc finger-like domain of the molecular chaperone DnaJ is involved in binding to denatured protein substrates. EMBO J. 1996;15:408–417. [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Qian X, Sha B. The crystal structure of the yeast Hsp40 Ydj1 complexed with its peptide substrate. Structure. (Camb.) 2003;11:1475–1483. doi: 10.1016/j.str.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Sha B. Structure-based mutagenesis studies of the peptide substrate binding fragment of type I heat-shock protein 40. Biochem. J. 2005;386:453–460. doi: 10.1042/BJ20041050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sha B, Lee S, Cyr DM. The crystal structure of the peptide-binding fragment from the yeast Hsp40 protein Sis1. Structure. 2000;8:799–807. doi: 10.1016/s0969-2126(00)00170-2. [DOI] [PubMed] [Google Scholar]
- 19.Gruschus JM, Han CJ, Greener T, Ferretti JA, Greene LE, Eisenberg E. Structure of the functional fragment of auxilin required for catalytic uncoating of clathrin-coated vesicles. Biochem. 2004;43:3111–3119. doi: 10.1021/bi0354740. [DOI] [PubMed] [Google Scholar]
- 20.Yu M, Haslam RH, Haslam DB. HEDJ, an Hsp40 co-chaperone localized to the endoplasmic reticulum of human cells. J. Biol. Chem. 2000;275:24984–24992. doi: 10.1074/jbc.M000739200. [DOI] [PubMed] [Google Scholar]
- 21.Meunier L, Usherwood YK, Chung KT, Hendershot LM. A subset of chaperones and folding enzymes form multiprotein complexes in endoplasmic reticulum to bind nascent proteins. Mol. Biol. Cell. 2002;13:4456–4469. doi: 10.1091/mbc.E02-05-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen Y, Hendershot LM. ERdj3, a stress-inducible endoplasmic reticulum DnaJ homologue, serves as a cofactor for BiP's interactions with unfolded substrates. Mol. Biol. Cell. 2005;16:40–50. doi: 10.1091/mbc.E04-05-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu AY, Mack PW, Champion CI, Robinson RR. Expression of mouse::human immunoglobulin heavy-chain cDNA in lymphoid cells. Gene. 1987;54:33–40. doi: 10.1016/0378-1119(87)90344-1. [DOI] [PubMed] [Google Scholar]
- 24.Shen Y, Meunier L, Hendershot LM. Identification and characterization of a novel endoplasmic reticulum (ER) DnaJ homologue, which stimulates ATPase activity of BiP in vitro and is induced by ER stress. J. Biol. Chem. 2002;277:15947–15956. doi: 10.1074/jbc.M112214200. [DOI] [PubMed] [Google Scholar]
- 25.Jin Y, Awad W, Petrova K, Hendershot LM. Regulated release of ERdj3 from unfolded proteins by BiP. EMBO J. 2008 doi: 10.1038/emboj.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marcus NY, Marcus RA, Schmidt BZ, Haslam DB. Contribution of the HEDJ/ERdj3 cysteine-rich domain to substrate interactions. Arch. Biochem. Biophys. 2007;468:147–158. doi: 10.1016/j.abb.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu J, Wu Y, Li J, Qian X, Fu Z, Sha B. The crystal structure of the putative peptide-binding fragment from the human Hsp40 protein Hdj1. BMC. Struct. Biol. 2008;8:3. doi: 10.1186/1472-6807-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee S, Fan CY, Younger JM, Ren H, Cyr DM. Identification of essential residues in the type II Hsp40 Sis1 that function in polypeptide binding. J. Biol. Chem. 2002;277:21675–21682. doi: 10.1074/jbc.M111075200. [DOI] [PubMed] [Google Scholar]
- 29.Wu Y, Li J, Jin Z, Fu Z, Sha B. The crystal structure of the C-terminal fragment of yeast Hsp40 Ydj1 reveals novel dimerization motif for Hsp40. J. Mol. Biol. 2005;346:1005–1011. doi: 10.1016/j.jmb.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 30.Rudiger S, Schneider-Mergener J, Bukau B. Its substrate specificity characterizes the DnaJ co-chaperone as a scanning factor for the DnaK chaperone. EMBO J. 2001;20:1042–1050. doi: 10.1093/emboj/20.5.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Landry SJ. Swivels and stators in the Hsp40-Hsp70 chaperone machine. Structure. 2003;11:1465–1466. doi: 10.1016/j.str.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 32.Lu Z, Cyr DM. The conserved carboxyl terminus and zinc finger-like domain of the co-chaperone Ydj1 assist Hsp70 in protein folding. J. Biol. Chem. 1998;273:5970–5978. doi: 10.1074/jbc.273.10.5970. [DOI] [PubMed] [Google Scholar]
- 33.Fan CY, Ren HY, Lee P, Caplan AJ, Cyr DM. The Type I Hsp40 Zinc Finger-like Region Is Required for Hsp70 to Capture Non-native Polypeptides from Ydj1. J. Biol. Chem. 2005;280:695–702. doi: 10.1074/jbc.M410645200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Three figures are included which examine the half-live of the various ERdj3 proteins when expressed in COS-1 cells, the protease sensitivity of the various recombinant proteins, and a model of the predicted structure of the substrate binding domain of ERdj3. This material is available free of charge via the Internet at http://pubs.acs.org