Abstract
The Drosophila tumor suppressors fat and discs overgrown (dco) function within an intercellular signaling pathway that controls growth and polarity. fat encodes a transmembrane receptor, but post-translational regulation of Fat has not been described. We show here that Fat is subject to a constitutive proteolytic processing, such that most or all cell surface Fat comprises a heterodimer of stably associated N- and C-terminal fragments. The cytoplasmic domain of Fat is phosphorylated, and this phosphorylation is promoted by the Fat ligand Dachsous. dco encodes a kinase that influences Fat signaling, and Dco is able to promote the phosphorylation of the Fat intracellular domain in cultured cells and in vivo. Evaluation of dco mutants indicates that they affect Fat's influence on growth and gene expression but not its influence on planar cell polarity. Our observations identify processing and phosphorylation as post-translational modifications of Fat, correlate the phosphorylation of Fat with its activation by Dachsous in the Fat-Warts pathway, and enhance our understanding of the requirement for Dco in Fat signaling.
Keywords: Drosophila, growth, signaling, Hippo, kinase
Studies in Drosophila have identified several genes that function to regulate the growth of developing tissues and linked them into 2 interconnected signaling pathways, the Fat and Warts/Hippo pathways (reviewed in ref. 1). Homologues of these genes exist in mammals, and they have been implicated in growth control and tumorigenesis in a wide range of organs. Fat is an atypical cadherin that acts as a receptor for intercellular signaling pathways that influence gene expression and planar cell polarity (PCP) (1). Another atypical cadherin, Dachsous (Ds), appears to act as a ligand for Fat. However, molecular mechanisms associated with activation of Fat by Ds have not been described. The influence of Ds on Fat appears to be modulated by Four-jointed, a Golgi kinase that phosphorylates cadherin domains of Fat and Ds (2).
Fat influences transcription through a kinase, Warts (Wts) (reviewed in ref. 1). Wts is made in an inactive form, and upstream components of the Warts/Hippo pathway activate Wts. Fat acts in parallel to this Hippo-dependent regulation of Wts activity, influencing the levels of Wts protein through the unconventional myosin Dachs, but may also act through Hippo signaling via an effect on Expanded to influence Wts activation. Activated Wts phosphorylates and thereby inactivates a transcriptional coactivator protein, Yorkie. When upstream tumor suppressors are mutant, expression of downstream genes that promote growth and inhibit apoptosis becomes elevated. Fat influences PCP via a distinct pathway or process (reviewed in ref. 1). The separation of Fat PCP and tumor suppressor pathways is illustrated most clearly by the observation that the overgrowth phenotype of fat mutants, but not the PCP phenotypes, can be rescued by Wts overexpression (3). Because Fat itself, and upstream regulators of Fat (Ds and Fj) influence both PCP and growth, distinct PCP and transcriptional pathways must branch at or below Fat.
Discs overgrown (dco) encodes the Drosophila homologue of casein kinase I δ and ε (CKIδ/ε), and was identified as a Drosophila tumor suppressor based on the overgrowth phenotype of a particular recessive allele, dco3 (4). Dco also participates in other processes, including circadian rhythyms (5), apoptosis (6), Hedgehog signaling (7, 8), and Wnt signaling (9, 10). dco was genetically linked to Fat signaling based on the observations that dco3 mutant clones influence the expression of Fat pathway target genes, that the overgrowth and transcriptional phenotypes of dco3 are suppressed by mutation of dachs, and that dco3 influences Wts protein levels in imaginal discs (11). These last 2 features of dco3 are shared by fat, but not by components of the Hippo pathway, linking dco specifically to the Fat pathway, but the biochemical basis for this link is unknown.
Results and Discussion
Proteolytic Processing of Fat.
Activation of transmembrane receptors often involves post-translational modifications, such as phosphorylation or cleavage. To investigate potential modifications, Fat was examined by Western blot analysis. In lysates of wing discs, antisera raised against the Fat intracellular domain (anti-Fat ICD) detected a prominent band with a mobility of ≈95 kDa (Ft-95), and a faint band with a mobility corresponding to a much larger polypeptide (Ft-565) (Fig. 1A). fat is predicted to encode a 5,147 amino acid protein, with a calculated mass of 565 kDa. Thus, Ft-95 is too small to correspond to full length Fat. Nonetheless, examination of lysates from fat mutant discs confirmed that both Ft-95 and Ft-565 are fat-dependent (Fig. 1A).
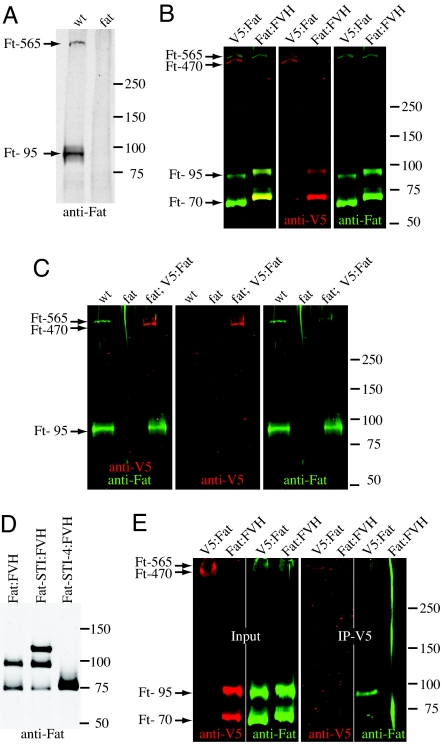
Fig. 1.
Processing of Fat. Western blot analysis of Fat and Fat constructs, the approximate positions of markers, and inferred identity of Fat bands, are indicated. (A) Lysates of wild-type and fatG-rv wing discs. (B) Lysates of S2 cells expressing V5:Fat or Fat:FVH. V5 and Fat were detected simultaneously by immunofluorescense, and are shown together (Left) and separately (Center and Right). (C) Lysates of wild-type, fatG-rv, and fatG-rv/fat8 attB-P[acmanV5:fat+] wing discs. (D) Lysates of S2 cells expressing Fat:FVH or truncated isoforms. (E) Left 2 gels show lysates of S2 cells expressing V5:Fat or Fat:FVH whereas the right 2 gels show material immunoprecipitated after anti-V5 antibodies were incubated with intact cells.
To investigate this apparent cleavage of Fat, a C-terminally tagged Fat protein (Fat:FVH) was created. When Fat:FVH was transfected into cultured Drosophila S2 cells, a band with a high apparent molecular weight, consistent with full length Fat, was observed (Fig. 1B). However, most Fat was detected in lower molecular weight bands. One correlates with the 95-kDa fragment of endogenous Fat (after accounting for the C-terminal tags), but the other appears smaller, ≈70 kDa (Ft-70)(Fig. 1B). Although Ft-70 was not detected when endogenous Fat was examined in imaginal discs, it could be detected in discs when Fat:FVH was overexpressed from UAS transgenes. Expression of Fat:FVH under tub-Gal4 control also confirmed that Fat:FVH is functional, because it rescued fat mutant animals. The detection of Ft-95 and Ft-70 with C-terminal epitope tags supports the conclusion that Fat is proteolytically processed. Based on their mobility, the cleavage leading to Ft-95 occurs in or near the 2 extracellular laminin G-like domains, whereas the cleavage leading to Ft-70 occurs near the transmembrane domain (Fig. S1). A Fat construct that excludes the cadherin and EGF domains but includes most of the laminin G domain region (Fat-STI:FVH, Fig. S1) appears to be processed to the same cleavage products as is full-length Fat, whereas a smaller Fat construct that also lacks the laminin G domains (Fat-STI-4:FVH) yields a single major band, suggesting that it is not processed (Fig. 1D).
To further characterize Fat processing, an N-terminally tagged Fat (V5:Fat) was constructed. Examination of V5:Fat by Western blotting lysates of S2 cells identified 2 bands of high apparent molecular weight, and did not detect Ft-70 or Ft-95 (Fig. 1B). Although the resolving power of the gel and the lack of suitable markers precluded precise determination of the size of these large bands, their mobility is consistent with the expected detection of both full-length Fat (Ft-565) and an approximate 470-kDa N-terminal product of proteolytic processing in the Laminin G domain region (Ft-470). Double staining V5:Fat with anti-Fat ICD and anti-V5 supported the conclusion that slowest mobility isoform is full-length Fat, whereas Ft-470 lacks the Fat ICD (Fig. 1B). To characterize cleavage of V5:Fat in vivo at endogenous expression levels, the V5 tag was incorporated into a fat+ genomic clone (Fig. S1), and then phiC31-mediated recombination was used to insert this into the Drosophila genome (12). This genomic V5:fat+ construct rescued fat mutants. Western blotting lysates of imaginal discs revealed that Ft-470 is more abundant than Ft-565 (Fig. 1C). Because these proteins are similar in size, this differential detection is unlikely to be due to differences in blotting transfer efficiency. Hence, we conclude that the majority of Fat protein in vivo is processed.
To investigate the nature of Fat displayed on the cell surface, biochemical experiments were performed on cultured cells. S2 cells expressing V5:Fat were incubated with anti-V5 in the absence of detergent, and then cell surface Fat bound by anti-V5 antibodies was immunoprecipitated. As a control, we expressed Fat:FVH, which includes a cytoplasmic V5 tag that should not be accessible in intact cells. Western blot analysis of the immunoprecipitated material with anti-Fat ICD antibodies confirmed that cell surface V5:Fat is processed (Fig. 1E). In addition, these experiments demonstrate that Ft-470 and Ft-95 remain stably associated after processing. By contrast, Ft-70 was not detected, indicating that it is not associated with Ft-470. Because coimmunoprecipitation of Ft-470 and Ft-95 could be observed under reducing conditions, the association between them does not require disulfide bonds.
Because Fat processing can occur in S2 cells, which do not express detectable levels of Ds and grow as isolated cells, and processing can occur on a truncated Fat polypeptide that lacks the cadherin and EGF domains (Fat-STI:FVH), it appears that Fat processing is part of its normal maturation, rather than a regulated event. In this regard, it appears analogous to the S1 cleavage that is involved in maturation of the Notch receptor (reviewed in ref. 13), or to the apparent processing of the Starry night/Flamingo cadherin (14).
Ds-Dependent Phosphorylation of Fat.
Under optimal conditions, Ft-95 from wing discs runs as doublet, with a prominent lower band, a weaker upper band, and a faint smear in between (Fig. 2). Treatment of lysates with calf intestinal alkaline phosphatase (CIP) resulted in a single sharp band ≈95 kDa, with a mobility similar to the fastest of the 95-kDa mobility isoforms in untreated samples (Fig. 2B). Thus, a fraction of Ft-95 in vivo is phosphorylated. Because Ft-95 is too C-terminal to include the cadherin domains (Fig. S1), the phosphorylation detected presumably reflects a phosphorylation of the intracellular domain, rather than Fj-mediated phosphorylation of cadherin domains (2). To investigate the relationship between Ft-95 phosphorylation and Fat signaling, Fat was examined in lysates of wing imaginal discs in which its putative ligand, ds, was either mutant or overexpressed. Proteolytic processing of Fat was not Ds-dependent, because Ft-95 was observed at similar levels in all cases. Mutation of ds results in enlarged wings and wing discs, and lower levels of Wts protein, a phenotype similar to, although weaker than, that of fat (Fig. 2 and Fig. S2) (15). Western blot analysis of Fat from ds mutant wing discs revealed that levels of the faster mobility Ft-95 band are elevated, whereas the slower mobility band (Ft-95-P) is reduced (Fig. 2). Ds overexpression reduces wing size (Fig. S2) (16, 17). When we overexpressed Ds under tub-Gal4 control, quantitative Western blot analysis of wing disc lysates identified an average increase in Ds levels of 10-fold. Strikingly, this overexpression of Ds increased the relative amount of Ft-95-P (Fig. 2). These observations imply that the presence or absence of Ds modulates Fat phosphorylation. This was confirmed by the observation that phosphatase treatment of lysates from Ds-expressing discs collapsed the Ft-95 doublets into a single band (Fig. 2B). The visual impression that the presence of the slower mobility (Ft-95-P) isoform(s) was promoted by Ds was confirmed by quantitative line scanning of Western blot analyses (Fig. S2).
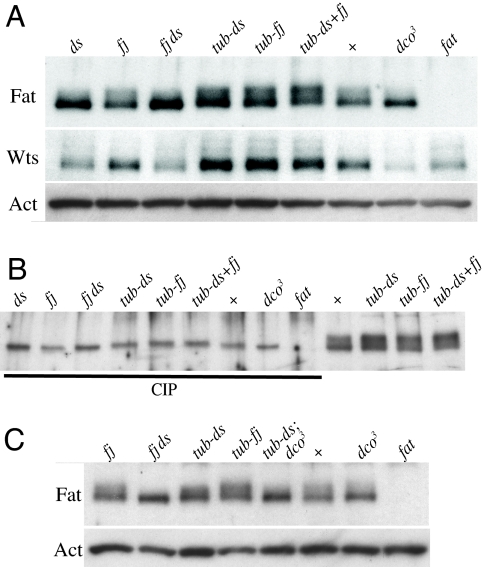
Fig. 2.
Ds-promoted phosphorylation of Fat. Western blot analyses of lysates of wing discs from ds36D/Df(2L) ds, fjd1, fjd1 ds36D/fjd1 Df(2L) ds, tub-Gal4 UAS-ds, tub-Gal4 UAS-fj, tub-Gal4 UAS-ds UAS-fj, wild-type, dco3, and ftG-rv as indicated. (A) Ft-95 mobility in different genotypes; Wts levels indicate relative Fat activity and Act (actin) is a loading control. (B) Comparison of lysates treated with CIP versus untreated lysates. (C) Lysates from wing discs of the indicated genotypes shows that dco3 reverses the promotion of Ft-95 phosphorylation by ds.
Both mutation of fj and fj overexpression are associated with modest reductions in wing and leg size (Fig. S2). When we overexpressed fj under tub-Gal4 control, quantitative Western blot analysis of wing disc lysates identified an average increase in Fj levels of 100-fold. This overexpression of fj was associated with an increase in the relative amount of phosphorylated Fat, and when coexpressed with ds, the increase in phosphorylated Fat appeared even greater, consistent with the reductions in wing size (Fig. 2 and Fig. S2). Mutation of fj had only subtle affects (Fig. 2).
Altogether, these observations identify a correlation between the presence of the Fat ligand Ds, the level of signaling through Fat to regulate Warts levels and wing growth, and the phosphorylation of the Fat cytoplasmic domain. Thus, they suggest that activation of Fat by its ligand Ds is associated with Fat phosphorylation. We can infer from the relative levels of different mobility isoforms that in the absence of Ds overexpression, a majority of Fat is in a hypophosphorylated form, whereas overexpression of Ds promotes the production of a hyperphosphorylated form. This identification of a posttranslational modification of Fat that is promoted by Ds is consistent with the hypothesis that Fat and Ds act as receptor and ligand in a signal transduction pathway, and identifies a molecular process that appears correlated with Fat activation. Constructs that lack most of the extracellular domain, and presumably can not interact with Ds, can rescue fat mutants (15). However, this rescue is only partial, and has only been observed when intracellular domain constructs are overexpressed. One possibility is that interaction with ligand triggers clustering of Fat, and that overexpression of the intracellular domain allows ligand-independent clustering. This could be analogous to other signaling pathways (e.g., TGF-ß, receptor tyrosine kinase), in which ligand-mediated clustering promotes phosphorylation of the cytoplasmic domain of the receptor, and for which the requirement for ligand can sometimes be bypassed by receptor overexpression.
Dco-Mediated Phosphorylation of the Fat Intracellular Domain.
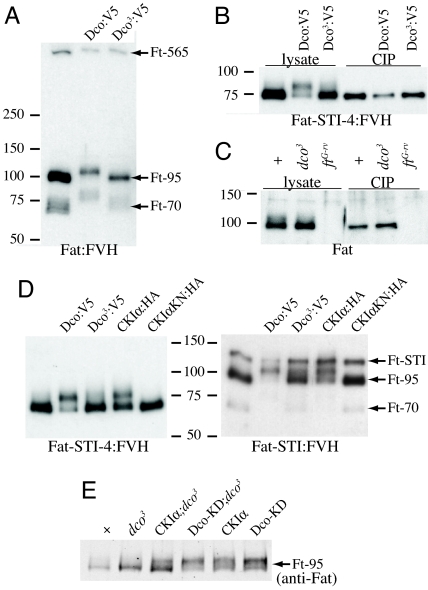
In considering kinases that might contribute to the Ds-promoted phosphorylation of Fat, the CKIδ/ε family member Dco was a logical candidate. Genetic epistasis tests positioned dco within the Fat pathway, upstream of dachs (11). At the same time, dco3 exerts cell-autonomous affects on the expression of Fat target genes, which implies that it acts within receiving cells (11). These observations suggested Dachs or Fat as potential substrates. Initial assessment of the ability of Dco to phosphorylate them was conducted by assaying for mobility shifts in S2 cells. Dco had no effect on Dachs. By contrast, when Dco was cotransfected together with Fat, a shift in the mobility of the C-terminal cleavage products was observed (Fig. 3A). A Dco-dependent mobility shift was also observed for both the Fat-STI:FVH and Fat-STI-4:FVH constructs (Fig. 3D). Confirmation that this mobility shift was due to phosphorylation of Fat was provided by the observation that it could be reversed by phosphatase (Fig. 3B). Overexpression of a Dco construct under UAS-Gal4 control could also increase phosphorylation of endogenous Fat in vivo (Fig. 3E).
Fig. 3.
Dco-mediated phosphorylation of Fat. Western blot analysis of Fat and Fat constructs (detected using anti-FLAG). Where indicated by labelling above gels, Fat constructs were cotransfected with Dco or CKI constructs (tagged with V5 or HA epitopes), or treated with phosphatase (CIP). (A) Fat:FVH in S2 cells. (B) Fat-STI-4:FVH in S2 cells. (C) wild-type (+), dco3, and fatG-rv wing disc lysates. (D) Fat-STI:FVH and Fat-STI-4:FVH in S2 cells. CKIαKN:HA is a mutant form of CKIα that lacks kinase activity, and serves as a negative control. (E) Lysates from wild-type (+), dco3, tub-Gal4 UAS-CKIα; dco3, tub-Gal4 UAS-Dco-KD (kinase domain), dco3, tub-Gal4 UAS-CKIα, and tub-Gal4 UAS-Dco-KD wing discs as indicated.
If phosphorylation of Fat by Dco is relevant to the participation of Dco in Fat signaling, then the dco3 mutation, which causes loss of Fat signaling, should impair Fat phosphorylation. Sequencing of dco3 identified 2 distinct amino acid substitutions (4); these were introduced into a Dco:V5 expression construct. Dco3:V5 resulted in much less shift in the mobility of Fat in S2 cells than did wild-type Dco:V5 (Fig. 3 A, B, and D). Thus, the same amino acid changes that cause overgrowth in vivo impair Dco-dependent phosphorylation of Fat in cultured cells. To investigate whether endogenous phosphorylation of Fat could also be influenced by mutation of dco, we examined the mobility of Fat in lysates from dco3 mutant wing discs. Unphosphorylated Fat (Ft-95) appeared slightly elevated, and a distinct Ft-95-P band was no longer visible, but rather a faint smear was detected (Figs. 2 and 3). This change in Fat mobility was confirmed by line scanning (Fig. S2). Thus, dco3 reduces levels of phosphorylated Fat in vivo.
To explore the relationship between the Ds-promoted phosphorylation of Fat, and the Dco-dependent phosphorylation of Fat, the mobility of Fat isolated from discs simultaneously overexpressing Ds and mutant for dco3 was examined. Direct examination of Western blots, as well as line scanning, revealed that Fat mobility in these lysates was similar to that in dco3 mutants (Fig. 2C and Fig. S2). Thus, Ds-mediated phosphorylation can be influenced by Dco. dco3 mutant clones have no obvious effect on Fat protein staining in wing imaginal discs (Fig. S3), suggesting that they do not affect its overall levels or distribution. Nor did dco3 noticeably affect processing of Fat.
The simplest explanation for Dco-promoted Fat phosphorylation, and for dco-dependent effects on Fat signaling, would be that Dco directly phosphorylates Fat. A purified mammalian homologue of Dco (CKIδ) phosphorylated the Fat intracellular domain in vitro, but with reduced specificity, because even greater mobility shifts than those observed in vivo could be induced (Fig. S4). CKI's are Ser/Thr kinases, and the 538 amino acid Fat ICD includes 109 Ser or Thr residues. Three different kinase site prediction programs individually predict 7, 15, or 36 CKI sites, and cumulatively identify 46 potential CKI sites (Fig. S5). This variation emphasizes the limited accuracy of kinase site predictions. We also note that distinct CKI sites could act redundantly, and that among the many potential CKI sites within the Fat ICD, phosphorylation sites responsible for the evident mobility shift on SDS-PAGE gels could be distinct from sites responsible for the influence of ds or dco3 on Fat activity. Thus, the identification of specific phosphorylation sites within the Fat ICD that are required for its biological activity will ultimately be essential for confirming the importance of Dco- and Ds-promoted phosphorylation of Fat to Fat signaling.
Dco3 Is Antimorphic and Specifically Deficient in Fat Phosphorylation.
In contrast to the overgrowth associated with dco3 mutants, dco null mutants lack discs, and dco null mutant clones grow poorly (4). This could reflect the participation of dco in other processes. However, targets of Fat signaling, including Wingless (WG) in the proximal wing, and Diap1, are up-regulated in dco3 mutant clones (11), but not in dco null (dcole88) mutant clones (6). The apparent absence of fat phenotypes in dco null alleles suggests that dco3 is an unusual allele.
Dco is also known as double time, because viable alleles were independently isolated as circadian rhythm mutants (5). This circadian phenotype reflects a role for Dco in phosphorylating, and thereby promoting the turnover, of the circadian protein Period (18, 19). This activity of Dco can be reproduced in S2 cells. Notably, Dco3:V5 was as effective as wild-type Dco:V5 at promoting Period turnover in S2 cells, whereas a circadian rhythm mutant isoform, DcoDbt-AR, was less effective (Fig. 4). Thus, dco3 is impaired in promoting Fat phosphorylation, but active on another substrate.
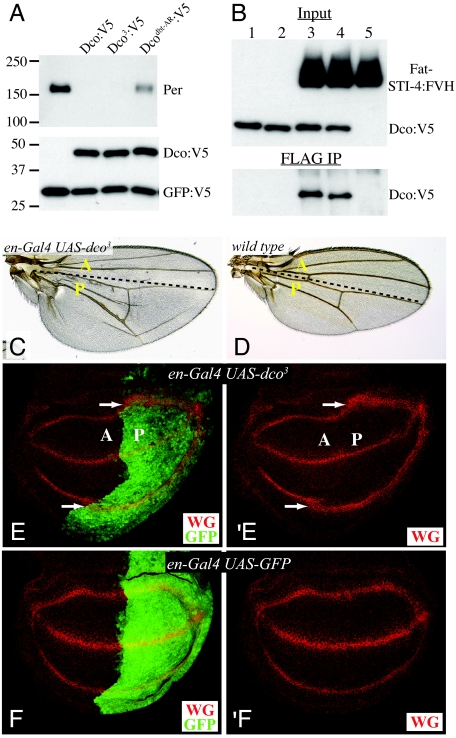
Fig. 4.
Dominant negative activity of Dco3. (A) Dco-dependent destabilization of Per. (Upper) Western blot analysis of Per in lysates of transfected S2 cells shows that Dco and Dco3 promote Per degradation. (Lower) Western blot analysis of Dco:V5 shows that similar amounts of each isoform were expressed; GFP:V5 is a transfection and loading control. (B) Western blot analyses (anti-V5) on S2 cells cotransfected with plasmids expressing (lane 1) Dco:V5, (2) Dco3:V5, (3)Dco:V5 + Fat-STI-4:FVH, (4) Dco3:V5 + Fat-STI-4:FVH, and (5) Fat-STI-4:FVH. (Upper) Shown are blot of lysates (Input). (Lower) Shown are blots of material precipitated with anti-FLAG beads. (C) Adult wing showing that overexpression of dco3 under en-Gal4 control induces overgrowth of the posterior compartment. (D) wild-type wing. (E) Wing imaginal disc expressing of dco3 under en-Gal4. The posterior compartment (marked by GFP, green) is enlarged and WG expression (red) is broadened. (F) WG expression in a disc without dco3 expression.
Analysis of the Dco-Period interaction revealed that Dco and Period can be stably associated, as assayed by their ability to be coprecipitated from cultured cells (5, 18). Similarly, Dco and the Fat-ICD can be coprecipitated, and this association was not impaired by the Dco3 mutations (Fig. 4). Because Dco3 can associate with Fat, but does not efficiently phosphorylate it, Dco3 might act as an antimorphic (dominant-negative) protein by competing with wild-type kinase. Indeed, although dco3 is recessive at endogenous expression levels, when dco3 was overexpressed, aspects of the dco3 phenotype, including wing overgrowth (Fig. 4C) and the induction of a Fat pathway target gene (Fig. 4E) could be reproduced. By contrast, overexpression of wild-type forms of Dco does not cause detectable overgrowth phenotypes (5–10). Instead overexpression of Dco modestly decreased wing growth and slightly reduced transcription of diap1 (Fig. S6), suggesting that Fat pathway activity might be increased.
In addition to having a CKIδ/ε homologue, Drosophila also have a CKIα homologue, and in some contexts they can act partially redundantly (20). A partial shift in Fat ICD mobility could be detected when CKIα was expressed in S2 cells or in wing discs (Fig. 3 D and E). Thus, CKIα can promote phosphorylation of Fat, although it appears less effective than Dco. This observation, together with the dco3 phenotypes observed when Dco3 is overexpressed, and the observation that although dco3 is defective in Fat phosphorylation, dco null mutant cells do not appear to be impaired for Fat signaling, suggest that dco3 might act as an antimorphic, or dominant negative, mutation, failing to effectively phosphorylate Fat and at the same time interfering with an ability of CKIα to phosphorylate Fat. By contrast, we hypothesize that in dco-null mutant cells, CKIα or other kinases could phosphorylate Fat without interference. Although we were unable to rescue dco3 with a UAS-CKIα transgene (Fig. S6), different CKI transgenes are inserted in different chromosomal locations, and their specific activities on Fat might be distinct. Thus, it remains possible that Dco and CKIα could be partially redundantly for Fat signaling.
Dco3 Specifically Affects the Fat-Warts Pathway.
Dco also participates in other pathways and processes. To determine whether the tumor suppressor phenotype of dco3 can be accounted for solely by its influence on Fat signaling, we took advantage of the observation that overexpression of Wts under the control of a heterologous promoter (tub-Gal4 UAS-Myc:Wts) could rescue the lethality and tumor suppressor phenotype of fat mutants (3). The lethality and overgrowth phenotypes of dco3 were also rescued by Wts overexpression (tub-Gal4 UAS-Myc:Wts), resulting in animals that, aside from some mild wing vein phenotypes, are indistinguishable from wild-type animals overexpressing Wts (Fig. 5). Because they are rescued simply by elevating Wts expression, dco3 mutant animals are specifically defective in Fat signaling; other essential processes that Dco participates in are not impaired.
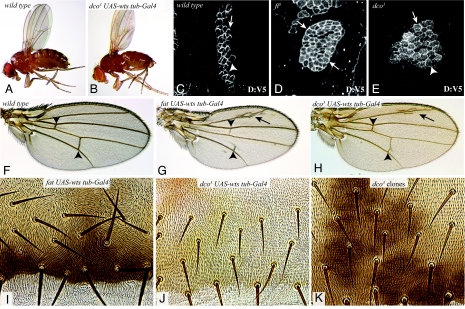
Fig. 5.
dco3 specifically affects the Fat tumor suppressor pathway. (A) Wild-type fly. (B) dco3 fly rescued by overexpression of wts. (C–E) Clones of cells expressing Dachs:V5 (white); in D and E the MARCM technique was used to make these clones mutant for fat8 (D) or dco3 (E). Arrows point to clone edges with accumulation of Dachs:V5, arrowheads point to clone edges where Dachs:V5 is low. (F–H) Wings from wild-type (F), ft8/ftG-rv rescued by overexpression of Wts (G), and dco3 rescued by overexpression of Wts (H). Arrowheads point to the cross-veins, arrow points to extra vein material. cross-vein spacing is rescued for dco3, but not for fat. (I–K) Portions of adult abdomens from ft8/ftG-rv rescued by overexpression of Wts (I), dco3 rescued by overexpression of Wts (J), an animal with dco3 mutant clones, marked by y+ (dark patches). A PCP phenotype is observed in I, but not in J or K.
Dco3 Does Not Affect the Fat Polarity Pathway.
Although Wts overexpression rescued the overgrowth and lethality of fat mutants, these animals have obvious PCP phenotypes in multiple tissues, consistent with the conclusion that Wts functions specifically in a Fat tumor suppressor pathway, and not in a Fat PCP pathway (3) (Fig. 5). By contrast, Wts-rescued dco3 mutants appear to have normal PCP (Fig. 5). The absence of an obvious PCP phenotype also indicates that the influence of Dco and CKIα on PCP through phosphorylation of Dishevelled (9, 10) is not affected by dco3.
To confirm the lack of influence of dco3 on PCP, we also examined dco3 mutant clones. fat mutant clones in the abdomen exhibit obvious disruptions in the normal posterior orientation of hairs and bristles (21, 22), but dco3 mutant clones had no effect (Fig. 5). In addition to affecting the canonical PCP pathway, studies of the relationship between Fat and its downstream effector Dachs revealed a form of PCP in which Fat signaling causes a polarized distribution of Dachs, which can be visualized by mosaic expression of a tagged form of Dachs, Dachs:V5 (17, 21). In the developing wing, Dachs:V5 is present on distal cell membranes, but not on proximal cell membranes. In clones of cells mutant for fat, Dachs:V5 is equally distributed on proximal and distal membranes (Fig. 5). In clones of cells mutants for dco3, Dachs:V5 localization is still polarized (Fig. 5). Thus, the regulation of Dachs localization by Fat does not appear to be affected by dco3, although a weak effect on Dachs localization cannot be excluded. The absence of visible Dachs relocalization in dco3 clones appears to conflict with the hypothesis that the influence of Fat signaling on Warts depends on its ability to polarize Dachs (1, 17), and further studies will be required to resolve this.
Phosphorylation and Fat Signaling Pathways.
The atypical cadherin Fat is a transmembrane receptor for pathways that control PCP and transcription. We have identified 2 posttranslational modifications of Fat. First, Fat is proteolytically processed, resulting in the production of stably associated N- and C-terminal polypeptides. The functional significance of this processing is not known, but its discovery is a necessary precursor to further experiments aimed at this question. Processing appears to be constitutive rather than regulated. Nonetheless, processing may facilitate subsequent events that regulate Fat.
We also discovered phosphorylation of the Fat cytoplasmic domain. Phosphorylation is promoted by the Fat ligand Ds, is influenced by the Fat pathway kinase Dco, and correlates with Fat pathway activity in ds or dco3 mutant animals, or when Ds or Fj are overexpressed. These observations suggest that phosphorylation of Fat is a key step in Fat receptor activation. When Dco or CKIα are overexpressed, the phenotypic effects appear mild compared with the evident increase in phosphorylation. However, because there could be multiple CKI sites within the Fat ICD, it is possible that the phosphorylation-dependent mobility shift of Fat is a general marker of the extent of Fat phosphorylation, rather than a precise marker of phosphorylation at a site or sites required for Fat activity. Nonetheless, the observation that dco3 can be completely rescued by Warts overexpression, together with the epistasis of dachs to dco3, indicates that the tumor suppressor phenotype of dco3 is due to an impairment of Fat-Warts signaling, which occurs at or upstream of the action of Dachs. Altogether, our observations implicate Fat as the likely target of Dco activity in the Fat pathway.
Materials and Methods
Histology and Imaging.
Imaginal discs were fixed and stained as described in ref. 23, using mouse anti-Wg [1:800, 4D4, Developmental Studies Hybridoma Bank (DSHB)], rat anti-DE-Cadherin (1:40, DCAD2, DSHB), rabbit anti-Gal4 (1:400, preabsorbed, Santa Cruz Biotechnology), mouse anti-Myc (9E10, 1:800, Babco), mouse anti-V5 (1:400 preabsorbed, Invitrogen) and rat anti-Fat (1:1,600). Fluorescent stains were captured on a Leica TCS SP5 confocal microscope. For horizontal sections, maximum projection through multiple sections was used to allow visualization of staining in different focal planes.
For adult tissues, combineZM software was used to allow visualization of features in different focal planes within a single image.
Western Blot Analysis.
Western blot analysis was performed as described in ref. 11, using rabbit anti-Wts (1:5,000, preabsorbed), rabbit anti GFP (1:1,000, Invitrogen), rat anti-Fat-ICD (1:25,000, preabsorbed), rat anti-Fj (1:3,000, D. Strutt), rat anti-Ds (1:3,000, M. Simon), guinea pig anti-Period (1:10,000, I. Edery) mouse anti-Actin JLA20 (1:15,000, Calbiochem), mouse anti-V5-HRP (1:40,000, Invitrogen), goat anti-mouse-HRP (10 ng/mL, Pierce), goat anti-rabbit-HRP (10 ng/mL, Pierce), donkey anti-rat-HRP (1:40,000, Jackson Immunology), donkey anti-guinea pig-HRP (1:40,000, Jackson Immunology). All protein samples were resolved by electrophoresis through 4–15% gradient SDS polyacrylamide gel (Bio-Rad). Chemiluminescent detection was performed with SuperSignal West Femto maximum Sensitivity Substrate (Pierce), and blots stripped by Restore Western Blot Stripping Buffer (Pierce) between antibody blotting. Fluorescent detection was performed on a LiCor Odyssey, using goat anti-mouse IRdye680 (1:10,000, LiCor) and sheep anti-rat IRdye800 (1:10,000, Rockland) secondary antibodies.
For line scanning, blots were scanned and digitized. Ten vertical lines were drawn through each lane, and the intensity values at every pixel were calculated using National Institutes of Health Image J. The average intensity values were then plotted versus position using Microsoft Excel.
Cell Culture and Transfection.
Culturing, transfection and lysis of Drosophila S2 cells was performed as described in ref. 11. pUAS-ft:FVH or pUAST-V5:fat, constructs were cotransfected into S2 cells with pAct-Gal4, pMT-dco:V5, pMT-dco3:V5, pMK-CKIα:HA, pMK-CKIα:KN:HA (kinase dead), or vector control. Dco:V5, Dco3:V5, CKIα:HA, or pMK-CKIα:KN:HA were induced with Cu2+ for 36 h. pAct-Per was cotransfected into S2 cells with pMT-dco or pMT-dco3.
Additional methods are included in the SI Text, and additional information is available in Table S1.
Supplementary Material
Acknowledgments.
We thank S. Blair (University Wisconsin, Madison, WI), I. Edery (Rutgers University, Piscataway, NJ), J. Jiang (UT Southwestern, Dallas, TX), H. McNeill (Samuel Lunenfeld, Toronto), M. Noll (University Zurich, Zurich), the Developmental Studies Hybridoma Bank, and the Bloomington stock center for plasmids, antibodies, and Drosophila stocks; C. Rauskolb for Figs. 5 C and D, and R. Mann and C. Rauskolb for comments on the manuscript. This work was supported by the Howard Hughes Medical Institute and National Institutes of Health Grant GM078620.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811540106/DCSupplemental.
References
- 1.Reddy BV, Irvine KD. The Fat and Warts signaling pathways: New insights into their regulation, mechanism, and conservation. Development. 2008;135:2827–2838. doi: 10.1242/dev.020974. [DOI] [PubMed] [Google Scholar]
- 2.Ishikawa HO, Takeuchi H, Haltiwanger RS, Irvine KD. Four-jointed is a Golgi kinase that phosphorylates a subset of cadherin domains. Science. 2008;321:401–404. doi: 10.1126/science.1158159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng Y, Irvine KD. Fat and expanded act in parallel to regulate growth through warts. Proc Natl Acad Sci USA. 2007;104:20362–20367. doi: 10.1073/pnas.0706722105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zilian O, et al. Double-time is identical to discs overgrown, which is required for cell survival, proliferation, and growth arrest in Drosophila imaginal discs. Development. 1999;126:5409–5420. doi: 10.1242/dev.126.23.5409. [DOI] [PubMed] [Google Scholar]
- 5.Kloss B, et al. The Drosophila clock gene double-time encodes a protein closely related to human casein kinase Iepsilon. Cell. 1998;94:97–107. doi: 10.1016/s0092-8674(00)81225-8. [DOI] [PubMed] [Google Scholar]
- 6.Guan J, Li H, Rogulja A, Axelrod JD, Cadigan KM. The Drosophila casein kinase Iepsilon/delta Discs overgrown promotes cell survival via activation of DIAP1 expression. Dev Biol. 2007;303:16–28. doi: 10.1016/j.ydbio.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jia J, et al. Phosphorylation by double-time/CKIepsilon and CKIalpha targets cubitus interruptus for Slimb/beta-TRCP-mediated proteolytic processing. Dev Cell. 2005;9:819–830. doi: 10.1016/j.devcel.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Price MA, Kalderon D. Proteolysis of the Hedgehog signaling effector Cubitus interruptus requires phosphorylation by glycogen synthase kinase 3 and casein kinase 1. Cell. 2002;108:823–835. doi: 10.1016/s0092-8674(02)00664-5. [DOI] [PubMed] [Google Scholar]
- 9.Klein TJ, Jenny A, Djiane A, Mlodzik M. CKIvarepsilon/discs overgrown Promotes Both Wnt-Fz/beta-Catenin and Fz/PCP Signaling in Drosophila. Curr Biol. 2006;16:1337–1343. doi: 10.1016/j.cub.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 10.Strutt H, Price MA, Strutt D. Planar polarity is positively regulated by casein kinase Iepsilon in Drosophila. Curr Biol. 2006;16:1329–1336. doi: 10.1016/j.cub.2006.04.041. [DOI] [PubMed] [Google Scholar]
- 11.Cho E, et al. Delineation of a Fat tumor suppressor pathway. Nat Genet. 2006;38:1142–1150. doi: 10.1038/ng1887. [DOI] [PubMed] [Google Scholar]
- 12.Venken KJ, He Y, Hoskins RA, Bellen HJ. P[acman]: A BAC transgenic platform for targeted insertion of large DNA fragments in D. melanogaster. Science. 2006;314:1747–1751. doi: 10.1126/science.1134426. [DOI] [PubMed] [Google Scholar]
- 13.Kopan R, Cagan R. Notch on the cutting edge. Trends Genet. 1997;13:465–467. doi: 10.1016/s0168-9525(97)01318-8. [DOI] [PubMed] [Google Scholar]
- 14.Usui T, et al. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell. 1999;98:585–595. doi: 10.1016/s0092-8674(00)80046-x. [DOI] [PubMed] [Google Scholar]
- 15.Matakatsu H, Blair SS. Separating the adhesive and signaling functions of the Fat and Dachsous protocadherins. Development. 2006;133:2315–2324. doi: 10.1242/dev.02401. [DOI] [PubMed] [Google Scholar]
- 16.Simon MA. Planar cell polarity in the Drosophila eye is directed by graded Four-jointed and Dachsous expression. Development. 2004;131:6175–6184. doi: 10.1242/dev.01550. [DOI] [PubMed] [Google Scholar]
- 17.Rogulja D, Rauskolb C, Irvine KD. Morphogen control of wing growth through the Fat signaling pathway. Dev Cell. 2008;15:309–321. doi: 10.1016/j.devcel.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ko HW, Jiang J, Edery I. Role for Slimb in the degradation of Drosophila Period protein phosphorylated by Doubletime. Nature. 2002;420:673–678. doi: 10.1038/nature01272. [DOI] [PubMed] [Google Scholar]
- 19.Price JL, et al. Double-time is a novel Drosophila clock gene that regulates PERIOD protein accumulation. Cell. 1998;94:83–95. doi: 10.1016/s0092-8674(00)81224-6. [DOI] [PubMed] [Google Scholar]
- 20.Price MA. CKI, there's more than one: Casein kinase I family members in Wnt and Hedgehog signaling. Genes Dev. 2006;20:399–410. doi: 10.1101/gad.1394306. [DOI] [PubMed] [Google Scholar]
- 21.Mao Y, et al. Dachs: An unconventional myosin that functions downstream of Fat to regulate growth, affinity and gene expression in Drosophila. Development. 2006;133:2539–2551. doi: 10.1242/dev.02427. [DOI] [PubMed] [Google Scholar]
- 22.Casal J, Struhl G, Lawrence P. Developmental compartments and planar polarity in Drosophila. Curr Biol. 2002;12:1189–1198. doi: 10.1016/s0960-9822(02)00974-0. [DOI] [PubMed] [Google Scholar]
- 23.Cho E, Irvine KD. Action of fat, four-jointed, dachsous and dachs in distal-to-proximal wing signaling. Development. 2004;131:4489–4500. doi: 10.1242/dev.01315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.