Abstract
Goals of the work
To develop, validate, and assess the reliability of a clinical index for assessing post-radiation dentition breakdown.
Materials and methods
An expert panel of four dentists with expertise in post-radiation patient care, oral radiology and mineralized tissues reviewed a series of clinical photographs (n=60) depicting a wide range of post-radiation lesions varying in size, severity and location. Based on panel input related to lesion severity rankings and cut-points along a continuum of destruction, a semi-quantitative, ordinal lesion scale was developed. A companion scale was developed to account for existing restorations. The index was then reviewed by a separate panel of dental clinician/researchers for confirmation of face and content validity and was refined based on their input. Following index approval by the expert and confirmatory panels, the index was evaluated for test-retest reliability by two educator/clinicians. After a brief calibration session, examiners reviewed and independently scored a second series of lesion images (n=60). One week later, the same examiners independently scored the same images displayed in a different order. Inter and intra-rater reliability and agreement were assessed (Spearman r and Kappa statistic).
Main results
Respective to session 1 and 2, inter-rater reliability values were r=.97 and r=.98, with Kappa values of K=.93 and K=.95. Respective intra-rater reliability and agreement values were .99 and .98 (rater 1), and .98 and .95 (rater 2).
Conclusions
A new index was developed and subsequently demonstrated face validity and excellent inter and intra-rater reliability for potentially evaluating the severity of post-radiation dentition breakdown.
Keywords: Post-radiation dental caries, post-radiation dental index, reliability, oral cancer
Introduction
The American Cancer Society and Oral Cancer Foundation estimate that approximately 35,000 new cases of oral cavity and oropharyngeal cancer are diagnosed in the U.S. annually with nearly 60% of those patients surviving for at least 5 years [1, 2, 25]. Even though head and neck radiotherapy will save the lives of many patients, the quality of life for these patients is drastically diminished due to numerous radiation-induced complications such as hyposalivation, severe debilitating destruction of tooth structure, and associated loss of masticatory function [4, 20, 21, 23, 30]. Although complete dentures might appear to be the answer to this problem, a removable prosthesis is not well-tolerated by irradiated oral mucosa. Patients tend to suffer from severe chronic denture irritations. With little or no saliva, there is a decreased adhesion between denture and tissue [5]. The current approach to dental care is to maintain as many healthy teeth as possible [6, 26]; therefore, a better understanding of post-irradiation dental disease is necessary.
The post-radiation breakdown of the dentition tends to start within the first year following radiotherapy and becomes more severe with increasing time [30]. Post-radiation dental lesions differ considerably in clinical appearance, development and progression from dental caries in non-irradiated patients. Post-radiation lesions develop in a distinct manner with initial shear fracture of enamel followed by rapid decay of the exposed underlying dentin [17, 18]. Post-radiation lesions tend to occur at the gingival margins, cusp tips, and incisal surfaces in contrast to typical caries, which develop in pits, fissures, and proximal areas. A brown-black discoloration of the tooth surface that occurs prior to surface breakdown is sometimes associated with teeth exposed to therapeutic radiation, again dissimilar from non-irradiated, carious teeth [15, 20, 30].
While the danger of developing ravaging dental destruction as a sequelae to head and neck radiation therapy has been well-documented, radiation-induced hyposalivation or xerostomia has to date been considered the only significant etiological factor [4, 20, 21, 23, 30]. However, anecdotal clinical observations suggest that post-radiation dental lesions tend to start earlier and be more severe in teeth within the radiation field. Thus, it has been proposed that the direct effect of therapeutic radiation on mineralized tooth structure along with xerostomia may be a significant causal factor [24]. To determine if there is a direct link between post-radiation lesions and factors such as radiation dose at the tooth level, tooth destruction/lesion severity must be accurately measured. Current measures of typical dental caries (DMFT and DMFS) are computed based on describing decayed, missing, or filled teeth or tooth surfaces. These indices are commonly used for epidemiologic field studies to assess the prevalence of dental disease and need for treatment [32]. Because post-radiation dental lesions develop and progress in a manner dissimilar from typical caries, traditional caries measures may not be valid for capturing the unique pattern and extent of tooth destruction seen in patients following therapeutic radiation. Therefore, this project was undertaken to develop, validate, and assess the reliability of a clinical index for assessing post-radiation dentition breakdown in order to determine whether there is a correlation between tooth destruction severity and tooth-level radiation dose.
Materials and methods
An expert panel comprised of four dentists with expertise in post-radiation patient care, oral radiology and mineralized tissues was convened to review existing criteria for measuring dental caries and identify factors important in assessing characteristics typical of dental destruction seen in the post-radiation patient population. The expert panel was then asked to review a series of clinical photographs depicting a wide range of post-radiation lesions that varied in size, severity and location. The images were made from teeth extracted from patients previously exposed to therapeutic radiation. Tooth collection procedures were approved by UMKC Adult Health Science IRB. In order to preserve the teeth for subsequent evaluation, oral surgeons treating the patients were informed as to the purpose of the study and used care to prevent forceps damage as the teeth were extracted.
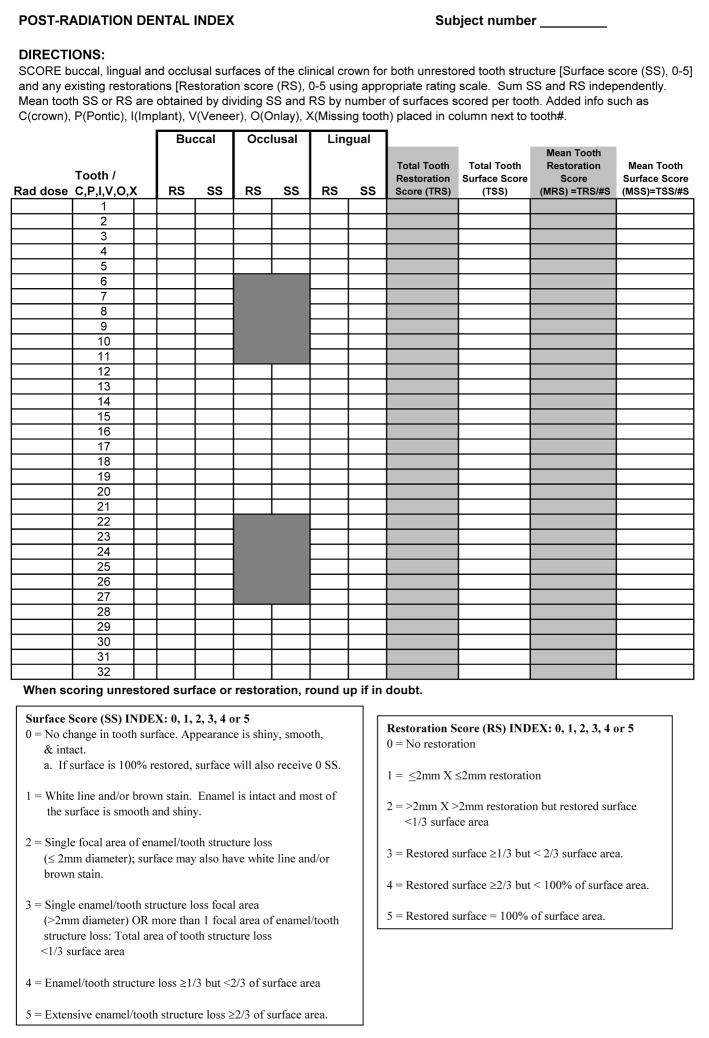
The series of clinical photographs was compiled into two sets of 60 slides. The expert panel members first independently reviewed one of the two sets of images and characterized lesion severity with respect to appearance, defining attributes, and extent of tooth surface destruction. Panel members then discussed how the attributes collectively spread lesion severity along the continuum of destruction, and identified meaningful cut-points in severity ranking along this continuum. Once the group reached consensus on these rankings and cut points, a semi-quantitative, ordinal lesion scale was developed. Category descriptors for each scale point were drafted by the principal investigators and re-reviewed by the panel for content validity. The index was then reviewed by a separate panel of dental clinician/researchers for confirmation of face and content validity and subsequently refined based on their input. In addition, a companion scale was developed to account for existing restorations and potential associated repair over time. Based on input from the expert and confirmatory panels, the principal investigators developed the rubric for computing the mean surface score (MSS) and mean restoration score (MRS) for each tooth present in the mouth. The resultant post-radiation dental index scale incorporating separate scores for surface lesions (MSS) and restorations (MRS) and the directions for application are displayed in Figure 1.
Figure 1.
Post-radiation dental index clinical research form.
In order to calculate the mean surface score for each tooth, the buccal, lingual and occlusal surfaces of posterior teeth, or buccal and lingual surface of anterior teeth are individually scored according to the 0–5 surface scale using a mouth mirror and direct light only. A tooth mean surface score is calculated as the sum of each tooth surface score divided by the number of evaluated surfaces (3 for posterior teeth and 2 for anterior teeth). Incisal surfaces on anterior teeth are not scored to avoid artificially inflating the tooth destruction score as a result of wear (attrition or abrasion). It is important to note that proximal surfaces are not scored to avoid the necessity of diagnostic radiographs for index use. This decision was discussed at length by the expert panel, and it was based on two factors: 1) current radiographs are often not available in this patient population; and 2) proximal lesions have not been considered a typical characteristic of post-radiation dental lesions [15, 20, 30]. A similar strategy is used to compute mean restoration scores (MRS).
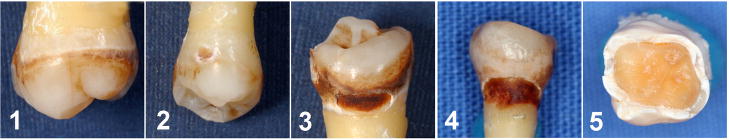
Once the index was developed and approved by the expert and confirmatory panels, assessment of psychometric characteristics was undertaken. Specifically, surface score index stability (test-retest reliability) was assessed by two independent, well-experienced educator/clinicians who scored teeth with lesions on two separate occasions, separated by one week. The examiners were initially trained and calibrated on the surface score index. A few days later, the examiners independently scored the second set of coded lesion images (n=60) using the 0–5 surface score index (example lesions presented in Figure 2). One week later, the same examiners independently scored the same images; however, the order for the second display was randomized to reduce bias in scoring. Inter- and intra-rater reliability and agreement were assessed using Spearman rank order correlation coefficient and the Kappa statistic. In contrast to the surface score index which is based on the unique pattern of post-radiation tooth destruction, the restoration score index is based on the typical dental exam charting evaluation, i.e. how much of the tooth surface is restored and thus, was not included in the test-retest reliability.
Figure 2.
Representative post-radiation surface lesions scored 1–5.
Results
Representative photos of post-radiation lesions scored 1 through 5 during the surface score/lesion index reliability testing phase are displayed in Figure 2. Inter-rater reliability for the first and second rating sessions were r = 0.97 and r = 0.98, respectively. In order to control for chance agreement, the Kappa statistic was also computed with values of K = 0.93 and K = 0.95 for the first and second rating sessions, respectively. A similar high level of intra-rater reliability (test-retest) for the two examiners was determined for rater 1 (r= 0.98; K = 0.99) and rater 2 (r = 0.95; K = 0.95). Collectively, the surface score index was shown to have very high level of inter-and intra-rater reliability.
Discussion
Tooth destruction following therapeutic radiation is an important concern for treating cancer patients. To date, assessment of post-radiation tooth destruction has been relatively limited due to a lack of distinction between traditional dental caries and the unusual pattern of post radiation tooth destruction. In addition, no scales have been developed that accurately capture these patterns in a meaningful way. The primary purpose of this project was to develop a valid index and subsequently test the usability and stability of the index for assessing post-radiation tooth destruction. This study provides preliminary evidence that the scale has utility and meaningfulness for its intended use.
Given that dental caries and post-radiation tooth destruction have quite different characteristics of enamel and dentin loss, this scale may allow clinical researchers to capture the extent of destruction observed in the post-radiation patient population with higher validity. Although the proposed post-radiation dental index is primarily focused on capturing the severity of dentition breakdown via the surface score aspect of the index (MSS), the addition of the restoration score component (MRS) allows for the assessment of post-radiation lesions that might be restored over time.
Historically, post-radiation lesions were presumed to be associated with salivary gland damage and the resultant hyposalivation; thus, the saliva glands, the parotid gland in particular, are considered in any radiation-sparing treatment using intensity modulated radiation therapy (IMRT) with or without the cytoprotectant amifostine [16, 19, 27, 28]. Despite the salivary gland protection techniques, most patients remain susceptible to post-radiation dentition breakdown. This could be related to a number of factors. For instance, IMRT techniques typically spare one or both of the parotid glands [8, 12–14] without sparing the submandibular glands, which produce the majority of unstimulated saliva [7]. Moreover, amifostine might not be as efficacious for preventing xerostomia as initially reported [3, 9, 10, 22, 29, 31] as indicated by a recent double-blinded, placebo-controlled phase III trial in which amifostine did not significantly reduce xerostomia [11]. Beyond these factors, the post-radiation tooth destruction might also be related to a potential direct link between tooth breakdown and radiation dose [24]. Currently, the developed index is being applied to a population of post-radiation patients to determine if cumulative tooth-level radiation dose is related to the severity of the post-radiation dental index score. In addition, the authors are currently evaluating the degree to which various scoring strategies and rubrics best capture the pattern of tooth surface destruction change over time. Because tooth surface destruction is cumulative, and restoration or extraction of teeth may confound interpretation of change over time, it is critical that validity and reliability of cumulative surface change over time be empirically evaluated.
Based on the results of the current study, the proposed post-radiation dental lesion index demonstrates the potential for assessing post-radiation dentition breakdown. Preliminary evidence suggests that the index, when used by experienced clinicians, has excellent inter-rater and intra-rater reliability and agreement in relation to assessing post-radiation dental lesion severity. However, it must be noted that these conclusions were based on viewing clinical photos of extracted teeth. As part of the current clinical study, we are evaluating the degree to which application of the index in a live patient population demonstrates similar consistency as that reported here.
In conclusion, the findings of this study provide encouraging indications about the validity and reproducibility of the post-radiation dental index. This would suggest the index will be applicable for elucidating whether there is a correlation between tooth destruction severity and tooth-level radiation dose and potentially for measuring dentition breakdown over time.
Acknowledgments
This study was supported by the National Institutes of Health/National Institute of Dental Craniofacial Research Grant K23 DE01623. The authors thank Paulette Spencer, Brenda Bohaty, Christos Angelopoulos, Nadeem Dallal, Chris Rice, Mary Lynn Froeschle, Kathy Dockter, and Susan McMillen for their assistance with this project.
Contributor Information
Mary P. Walker, Co-Director of Graduate Studies and Research, Departments of Oral Biology and Restorative Dentistry, University of Missouri-Kansas City School of Dentistry, Kansas City, MO
Karen B. Williams, Director of Clinical Research Center, Department of Behavioral Science, UMKC School of Dentistry
Brian Wichman, Chief Medical Physicist, Kansas City Cancer Centers, Overland Park, KS.
References
- 1.American Cancer Society. What are the key statistics about oral cavity and oropharyngeal cancer? 2007 Available at http://www.cancer.org/docroot/CRI/content/CRI 2 4 1X What are the key statistics for oral cavity and oropharyngeal cancer 60.asp?rnav=cri.
- 2.American Cancer Society. Oral Cancer. 2007 Available at www.cancer.org/downloads/PRO/OralCancer.pdf.
- 3.Anne PR, Curran WJ., Jr A phase II trial of subcutaneous amifostine and radiation therapy in patients with head and neck cancer. Semin Radiat Oncol. 2002;12:18–19. doi: 10.1053/srao.2002.31358. [DOI] [PubMed] [Google Scholar]
- 4.Anneroth G, Holm LE, Karlsson G. The effect of radiation on teeth. A clinical, histologic and microradiographic study. Int J Oral Surg. 1985;14:269–274. doi: 10.1016/s0300-9785(85)80038-7. [DOI] [PubMed] [Google Scholar]
- 5.Beumer J, Curtis TA, Nishimura R. Prosthodontic Procedures: Complete Dentures. In: Beumer J, Curtis TA, Marunick MT, editors. Maxillofacial Rehabilitation: Prosthodontic and Surgical Considerations. Ishiyaku EuroAmerica, Inc; St. Louis: 1996. pp. 101–102. [Google Scholar]
- 6.Beumer J, Curtis TA, Nishimura R. Preradiation Extractions: Current Philosophies and Literature Review. In: Beumer J, Curtis TA, Marunick MT, editors. Maxillofacial Rehabilitation: Prosthodontic and Surgical Considerations. Ishiyaku EuroAmerica, Inc; St. Louis: 1996. pp. 74–78. [Google Scholar]
- 7.Bhaskar SN. Orban’s Oral Histology and Embryology. 11. Mosby-Year Book, Inc; St Louis: 1991. Salivary Glands; pp. 337–371. [Google Scholar]
- 8.Blanco AI, Chao KS, El Naqa I, et al. Dose-volume modeling of salivary function in patients with head-and-neck cancer receiving radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:1055–1069. doi: 10.1016/j.ijrobp.2004.12.076. [DOI] [PubMed] [Google Scholar]
- 9.Bourhis J, De Crevoisier R, Abdulkarim B, et al. A randomized study of very accelerated radiotherapy with and without amifostine in head and neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2000;46:1105–1108. doi: 10.1016/s0360-3016(99)00532-5. [DOI] [PubMed] [Google Scholar]
- 10.Brizel DM, Wasserman TH, Henke M, et al. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol. 2000;18:3339–3345. doi: 10.1200/JCO.2000.18.19.3339. [DOI] [PubMed] [Google Scholar]
- 11.Buentzel J, Micke O, Adamietz IA, Monnier A, Glatzel M, de Vries A. Intravenous amifostine during chemoradiotherapy for head-and-neck cancer: a randomized placebo-controlled phase III study. Int J Radiat Oncol Biol Phys. 2006;64:684–691. doi: 10.1016/j.ijrobp.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Chao KS, Deasy JO, Markman J, et al. A prospective study of salivary function sparing in patients with head-and-neck cancers receiving intensity-modulated or three-dimensional radiation therapy: initial results. Int J Radiat Oncol Biol Phys. 2001;49:907–916. doi: 10.1016/s0360-3016(00)01441-3. [DOI] [PubMed] [Google Scholar]
- 13.Chao KS. Protection of salivary function by intensity-modulated radiation therapy in patients with head and neck cancer. Semin Radiat Oncol. 2002;12(1 Suppl 1):20–25. doi: 10.1053/srao.2002.31359. [DOI] [PubMed] [Google Scholar]
- 14.Eisbruch A, Ship JA, Dawson LA, et al. Salivary gland sparing and improved target irradiation by conformal and intensity modulated irradiation of head and neck cancer. World J Surg. 2003;27:832–837. doi: 10.1007/s00268-003-7105-6. [DOI] [PubMed] [Google Scholar]
- 15.Frank RM, Herdly J, Philippe E. Acquired dental defects and salivary gland lesions after irradiation for carcinoma. J Am Dent Assoc. 1965;70:868–883. doi: 10.14219/jada.archive.1965.0220. [DOI] [PubMed] [Google Scholar]
- 16.Garden AS, Lewin JS, Chambers MS. How to reduce radiation-related toxicity in patients with cancer of the head and neck. Curr Oncol Rep. 2006;8:140–145. doi: 10.1007/s11912-006-0049-x. [DOI] [PubMed] [Google Scholar]
- 17.Jansma J, Vissink A, Jongebloed WL, Retief DH, Johannes’s-Gravenmade E. Natural and induced radiation caries: A SEM study. Am J Dent. 1993;6:130–136. [PubMed] [Google Scholar]
- 18.Jongebloed WL, Gravenmade EJ, Retief DH. Radiation caries. A review and SEM study. Am J Dent. 1988;1:139–146. [PubMed] [Google Scholar]
- 19.Kahn ST, Johnstone PA. Management of xerostomia related to radiotherapy for head and neck cancer. Oncology (Williston Park) 2005;19:1827–1832. discussion 1832-1824, 1837-1829. [PubMed] [Google Scholar]
- 20.Karmiol M, Walsh RF. Dental caries after radiotherapy of the oral regions. J Am Dent Assoc. 1975;91:838–845. doi: 10.14219/jada.archive.1975.0493. [DOI] [PubMed] [Google Scholar]
- 21.Kielbassa AM, Hinkelbein W, Hellwig E, Meyer-Luckel H. Radiation-related damage to dentition. Lancet Oncol. 2006;7:326–335. doi: 10.1016/S1470-2045(06)70658-1. [DOI] [PubMed] [Google Scholar]
- 22.Koukourakis MI, Kyrias G, Kakolyris S, et al. Subcutaneous administration of amifostine during fractionated radiotherapy: a randomized phase II study. J Clin Oncol. 2000;18:2226–2233. doi: 10.1200/JCO.2000.18.11.2226. [DOI] [PubMed] [Google Scholar]
- 23.Lacatusu S, Francu L, Francu D. Clinical and therapeutical aspects of rampant caries in cervico-facial irradiated patients. Rev Med Chir Soc Med Nat Iasi. 1996;100:198–202. [PubMed] [Google Scholar]
- 24.Marx RE, Stern D. Oral and Maxillofacial Pathology: A Rationale for Diagnosis and Treatment. Quintessence Co, Inc; Chicago: 2002. Radiation Caries; pp. 380–381. [Google Scholar]
- 25.Oral Cancer Foundation. Oral Cancer Facts. 2007 Available at http://www.oralcancerfoundation.org/facts/index.htm.
- 26.Ord RA, Blanchaert RH., Jr Current management of oral cancer. A multidisciplinary approach. J Am Dent Assoc. 2001;132(Suppl):19S–23S. doi: 10.14219/jada.archive.2001.0384. [DOI] [PubMed] [Google Scholar]
- 27.Rosenthal DI, Chambers MS, Weber RS, Eisbruch A. A phase II study to assess the efficacy of amifostine for submandibular/sublingual salivary sparing during the treatment of head and neck cancer with intensity modulated radiation therapy for parotid salivary sparing. Semin Oncol. 2004;31:25–28. doi: 10.1053/j.seminoncol.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Ship JA, Hu K. Radiotherapy-induced salivary dysfunction. Semin Oncol. 2004;31:29–36. doi: 10.1053/j.seminoncol.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Vacha P, Fehlauer F, Mahlmann B, et al. Randomized phase III trial of postoperative radiochemotherapy +/− amifostine in head and neck cancer. Is there evidence for radioprotection? Strahlenther Onkol. 2003;179:385–389. doi: 10.1007/s00066-003-1016-1. [DOI] [PubMed] [Google Scholar]
- 30.Vissink A, Jansma J, Spijkervet FK, Burlage FR, Coppes RP. Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14:199–212. doi: 10.1177/154411130301400305. [DOI] [PubMed] [Google Scholar]
- 31.Wagner W, Prott FJ, Schonekas KG. Amifostine: a radioprotector in locally advanced head and neck tumors. Oncol Rep. 1998;5:1255–1257. doi: 10.3892/or.5.5.1255. [DOI] [PubMed] [Google Scholar]
- 32.WorldHealthOrganization. Caries Prevalence: DMFT and DMFS. 2007 Available at http://www.whocollab.od.mah.se/index.html.