Abstract
American Indians have a higher prevalence of albuminuria than the general population, likely resulting from a combination of environmental and genetic risk factors. To localize gene regions influencing variation in urinary albumin-to-creatinine ratio, we performed a linkage analysis and explored gene-by-diabetes, -hypertension, and -obesity interactions in a large cohort of American Indian families. We recruited >3600 individuals from 13 American Indian tribes from three centers (Arizona, North and South Dakota, and Oklahoma). We performed multipoint variance component linkage analysis in each center as well as in the entire cohort after controlling for center effects. We used two modeling strategies: Model 1 incorporated age, gender, and interaction terms; model 2 also controlled for diabetes, BP, body mass index, HDL, LDL, triglycerides, and smoking status. We evaluated interactions with diabetes, hypertension, and obesity using additive, interaction-specific linkage and stratified analyses. Loci suggestive for linkage to urinary albumin-to-creatinine ratio included 1q, 6p, 9q, 18q, and 20p. Gene-by-diabetes interaction was present with a quantitative trait locus specific to the diabetic stratum in the Dakotas isolated on 18q21.2 to 21.3 using model 1 (logarithm of odds = 3.3). Gene-by-hypertension interaction was present with quantitative trait loci specific to the hypertensive stratum in the Dakotas on 7q21.11 using model 1 (logarithm of odds = 3.4) and 10q25.1 using model 2 (logarithm of odds = 3.3). These loci replicate findings from multiple other genome scans of kidney disease phenotypes with distinct populations and are worthy of further study.
Albuminuria is a well-established risk factor for cardiovascular disease (CVD) and the development and progression of chronic kidney disease.1–4 For every 0.4-mg/mmol increase in urinary albumin-to-creatinine ratio (UACR), the risk for a major cardiovascular event increases by 6%.3 Individuals who have diabetes without proteinuria have a negligible annual risk for developing chronic renal insufficiency, whereas those with macroalbuminuria have a risk of 2.3% per year.5
The prevalence of albuminuria in the American population is 12%.6 American Indians are at higher risk, with prevalence estimates in this population ranging between 21 and 36%.7–9 Risk factors for albuminuria, such as diabetes, hypertension, and obesity, are overrepresented in the American Indian population.9 Nonetheless, it is likely that genetic risk factors also contribute to the high prevalence of albuminuria in this population. An increase in the prevalence of albuminuria with increasing percentage of self-identified American Indian heritage has been reported.8,10
The heritability for albuminuria ranges from approximately 0.17 to 0.20 in general populations,11,12 0.20 in families with diabetes,13 and 0.12 to 0.49 in families with hypertension,14,15 depending on ethnicity. Genome-wide scans for albuminuria have mostly yielded regions with suggestive evidence for linkage. Evidence of linkage for UACR to 20q12 was isolated by one study of Mexican Americans (logarithm of odds [LOD] = 3.5).12 Suggestive evidence of linkage of UACR to 18q22 has been replicated by four studies of distinct populations, three with diabetes and another with hypertension.16–19 It has been suggested that the genes controlling urinary albumin excretion are the same in relatives both with and without diabetes.13 In individuals with diabetes, however, the degree of albuminuria is an order of magnitude higher, suggesting gene–gene or gene–environment interaction.20
The goal of this study was two-fold. First, we aimed to isolate chromosomal regions influencing the phenotypic variation in urinary albumin excretion in a large, diverse cohort of American Indian families. Second, we aimed to identify evidence for gene-by-diabetes, -hypertension, or -obesity interaction on albuminuria.
RESULTS
A total of 3497 individuals were available for genetic analysis (Arizona 1161; Dakotas 1153; Oklahoma 1183). Descriptive characteristics of the entire Strong Heart Family Study (SHFS) participants (n = 3665) are summarized in Table 1. The average age ± SD of participants in each center was approximately 39 ± 15, 41 ± 17, and 44 ± 17 yr in Arizona, North and South Dakota, and Oklahoma, respectively. Diabetes, hypertension, and obesity were highly prevalent, especially in the Arizona center. Kidney disease was common, with albuminuria (UACR >30 μg/mg) present in approximately 19% and depressed GFR (<60 ml/min per 1.73 m2) present in approximately 7% of individuals in all centers (center-specific data not shown). There was wide variation in albuminuria and estimated GFR with means of 106 ± 598 μg/mg and 99 ± 27 ml/min per 1.73 m2 in all centers, respectively. For the analyses of those not on antihypertensive medications, 738 participants were excluded from analysis and 486 of these had diabetes and/or proteinuria.
Table 1.
Descriptive statistics of phase IV SHFS participants (2001 through 2003)
| Characteristics | All Centers (n = 3665) | Arizona (n = 1235) | Dakotas (n = 1220) | Oklahoma (n = 1210) |
|---|---|---|---|---|
| Age (yr; mean [SD]) | 40 (17) | 37 (16) | 39 (17) | 44 (17) |
| Female gender (n [%]) | 2197 (60) | 769 (62) | 717 (59) | 711 (59) |
| UACR (mg/g; mean [SD]) | 97 (574) | 152 (728) | 76 (508) | 64 (439) |
| Estimated GFR (ml/min per 1.73 m2; mean [SD])a | 100 (29) | 112 (33) | 96 (25) | 93 (24) |
| Body mass index (kg/m2; mean [SD]) | 32 (8) | 35 (9) | 30 (7) | 31 (7) |
| SBP (mmHg; mean [SD]) | 123 (17) | 121 (17) | 120 (16) | 127 (17) |
| DBP (mmHg; mean [SD]) | 76 (11) | 77 (12) | 75 (11) | 77 (11) |
| LDL cholesterol (mg/dl; mean [SD]) | 98 (29) | 94 (26) | 101 (31) | 100 (30) |
| HDL cholesterol (mg/dl; mean [SD]) | 51 (15) | 49 (14) | 51 (14) | 53 (15) |
| TG (mgl/dl; mean [SD]) | 168 (171) | 170 (134) | 161 (201) | 173 (171) |
| Hemoglobin A1c (%; mean [SD]) | 8.4 (2.1) | 8.7 (2.2) | 7.8 (1.9) | 8.2 (2.0) |
| Hypertension (n [%]) | 1153 (31) | 420 (34) | 285 (23) | 448 (37) |
| Diabetes (n [%]) | 830 (23) | 410 (33) | 172 (14) | 248 (21) |
| Smoking (n [%]) | ||||
| current | 1230 (34) | 311 (25) | 518 (42) | 401 (33) |
| previous | 885 (24) | 303 (25) | 284 (23) | 298 (25) |
| never | 132 (42) | 606 (49) | 416 (34) | 510 (42) |
Calculated using simplified Modification of Diet in Renal Disease (MDRD) equation.
Genetic data were available for >59,000 relative pairs in the full data set and >18,000, 22,000, and 18,000 relative pairs in Arizona, the Dakotas, and Oklahoma, respectively. Using the fully adjusted model, the heritability with standard error of UACR was approximately 0.17 (SE 0.03), 0.24 (SE 0.06), 0.15 (SE 0.05), and 0.10 (SE 0.06) in the full sample, Arizona, the Dakotas, and Oklahoma, respectively. Analyses yielding LOD scores ≥1.8 (suggestive evidence for linkage) included 1q32.2, 6p12.2, 9q22.2, 18q21.2, and 20p11.21 (Table 2).21 After removing individuals on antihypertensive medication, only the regions on chromosomes 9 and 20 were not attenuated (data not shown). Two additional loci were implicated: 2p16.2 at 76 cM in Arizona using both models (LOD = 2.6) and 8p23.2 at 3 cM in Oklahoma using model 1 (LOD = 2.1).
Table 2.
LOD scores suggestive of linkage (LOD ≥1.8) using two modeling strategies for multipoint quantitative trait linkage analyses of ranked UACR in phase IV participants of the SHFS (2001 through 2003)a
| Center (Modelb) | Chromosome | Location (cM) | Chromosomal Region | Nearest Marker | 1-LOD Drop Support Interval | LOD Score |
|---|---|---|---|---|---|---|
| All centers (model 1) | 1 | 227 | 1q32.2 | D1S249 | 1q31.3 to 1q41 | 2.0 |
| Arizona (model 1) | 2.5 | |||||
| All Centers (model 1) | 6 | 75 | 6p12.2 | D6S257 | 6p21.1 to 6q15 | 1.8 |
| Dakotas (models 1, 2) | 9 | 96 | 9q22.2 | D9S283 | 9q21.33 to 9q22.32 | 2.2, 2.4 |
| Dakotas (model 1) | 18 | 71 | 18q21.2 | D18S474 | 18q12.2 to 18q21.32 | 1.8 |
| Oklahoma (models 1, 2) | 20 | 49 | 20p11.21 | D20S195 | 20p12.1 to 20q12 | 1.9, 1.8 |
Linkage significance criteria were as suggested by Rao and Gu.21
Model 1 was adjusted for age, gender, age2, and age–gender interactions. Model 2 was additionally adjusted for diabetes status, HDL cholesterol, LDL cholesterol, TG, SBP, DBP, and smoking status.
There was no evidence of additive interaction with obesity. Additive interaction was demonstrated for diabetes and hypertension. P values for differential genetic effects (ρg ≠ 1 ) and differences in the magnitude of the genetic effect are displayed for gene-by-diabetes in Table 3 and gene-by-hypertension in Table 4.
Table 3.
Genotype-by-diabetes interaction for UACR in phase IV participants of the SHFS (2001 through 2003)
| Center | Model | Genetic Correlation (ρg) among Those with and without Diabetes (P) | Genetic SD
|
||
|---|---|---|---|---|---|
| With Diabetes | Without Diabetes | P | |||
| All centers | 1 | −0.02 (1.9 × 10−5) | 0.95 | 0.48 | 9.3 × 10−4 |
| 2 | 0.07 (8.4 × 10−4) | 0.92 | 0.43 | 5.5 × 10−4 | |
| Arizona | 1 | −0.06 (2.1 × 10−3) | 0.84 | 0.62 | 0.12 |
| 2 | 0.12 (7.6 × 10−3) | 0.81 | 0.59 | 0.12 | |
| Dakotas | 1 | 0.43 (0.02) | 1.12 | 0.38 | 1.8 × 10−5 |
| 2 | 0.27 (4.5 × 10−3) | 1.07 | 0.38 | 8.9 × 10−5 | |
| Oklahoma | 1 | −0.37 (7.2 × 10−3) | 0.94 | 0.48 | 1.9 × 10−3 |
| 2 | −0.27 (0.02) | 0.95 | 0.37 | 2.0 × 10−4 | |
Table 4.
Genotype-by-hypertension interaction for UACR in phase IV participants of the SHFS (2001 through 2003)
| Center | Model | Genetic Correlation (ρg) among Those with and without Hypertension (P) | Genetic SD
|
||
|---|---|---|---|---|---|
| With Hypertension | Without Hypertension | P | |||
| All centers | 1 | 0.52 (3.9 × 10−4) | 0.77 | 0.43 | 3.9 × 10−5 |
| 2 | 0.51 (3.9 × 10−3) | 0.68 | 0.39 | 1.4 × 10−3 | |
| Arizona | 1 | 0.61 (0.04) | 0.74 | 0.52 | 0.12 |
| 2 | 0.58 (0.07) | 0.70 | 0.46 | 0.11 | |
| Dakotas | 1 | 0.44 (8.4 × 10−3) | 0.90 | 0.40 | 9.7 × 10−4 |
| 2 | 0.58 (0.07) | 0.71 | 0.37 | 0.05 | |
| Oklahoma | 1 | 0.56 (0.09) | 0.71 | 0.38 | 0.03 |
| 2 | 0.31 (0.11) | 0.64 | 0.32 | 0.06 | |
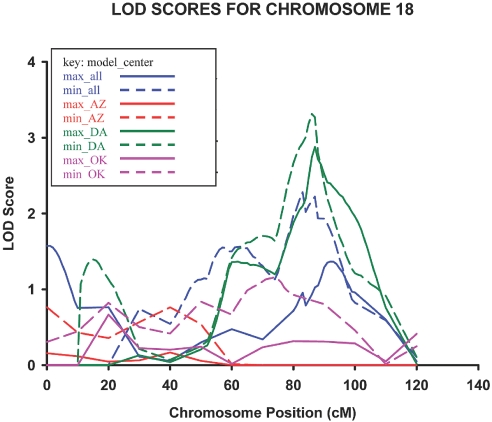
Genotype-by-diabetes interaction–specific analyses yielded a quantitative trait locus for albuminuria in the Dakotas center on 18q21.2 to 21.3 at 78 cM nearest marker D18S474 (LOD = 3.3 and 2.9 using models 1 and 2, respectively) with a 25-cM 1-LOD unit support interval spanning the regions 18q21.1 to 18q21.32 (Figure 1). Other loci with suggestive evidence for gene-by-diabetes interaction on linkage to albuminuria are listed in Table 5. The gene-by-diabetes quantitative trait locus (QTL) on 18q was not isolated with analyses stratified by diabetes status. Stratified analysis indicated the region on 7q34 be specific to relative pairs with diabetes, with the diabetes stratum in all centers having LOD scores of 3.8 and 2.8 using models 1 and 2, respectively. In addition, there was a QTL on 7q21.3 in the relative pairs with diabetes of the Dakotas with LOD scores of 3.0 and 3.1, using models 1 and 2, respectively.
Figure 1.
Gene-by-diabetes cumulative multipoint LOD scores for ranked estimated UACR on chromosome 18 in phase IV participants of the SHFS (2001 through 2003).
Table 5.
LOD scores suggestive of linkage (LOD ≥1.8) using two modeling strategies for gene-by-diabetes interaction–specific multipoint quantitative trait linkage analyses of ranked UACR in phase IV participants of the SHFS (2001 through 2003)a
| Center (Modelb) | Chromosome | Location (cM) | Chromosomal Region | Nearest Marker | 1-LOD Drop Support Interval | LOD Score |
|---|---|---|---|---|---|---|
| All centers (model 2) | 1 | 155 | 1q21.1 | D1S498 | 151 to 166 | 1.8 |
| Dakotas (model 1) | 2 | 153 | 2q23.3 | D2S151 | 144 to 172 | 1.9 |
| All centers (model 2) | 3 | 46 to 62 | 3p22.2 to 3p24.3 | D3S1277, D3S3659 | 34 to 71 | 2.6 |
| Dakotas (model 2) | 2.0 | |||||
| Dakotas (model 2) | 183 | 3q26.31 | D3S1565 | 167 to 192 | 1.8 | |
| All centers (model 1) | 6 | 86 | 6q13 | D6S460 | 62 to 104 | 2.3 |
| Oklahoma (model 1) | 2.9 | |||||
| Dakotas (models 1, 2) | 7 | 108 | 7q21.3 | D7S515 | 88 to 124 | 2.4, 2.3 |
| All centers (models 1, 2) | 151 to 168 | 7q34 to 7q36.2 | D7S661, D7S798 | 144 to 172 | 2.7, 2.0 | |
| Arizona (model 1) | 2.0 | |||||
| Dakotas (model 1) | 2.0 | |||||
| Dakotas (models 1, 2) | 9 | 99 | 9q22.31 | D9S283 | 89 to 108 | 2.2, 2.6 |
| Dakotas (model 1) | 12 | 131 | 12q24.22 | D12S86 | 119 to 138 | 2.1 |
| Dakotas (models 1, 2) | 14 | 50 | 14q21.3 | D14S276 | 46 to 85 | 2.7, 1.9 |
| All centers (model 1) | 18 | 78 | 18q21.2 to 21.3 | D18S474 | 69 to 94 | 2.2 |
| Dakotas (models 1, 2) | 3.3, 2.9 | |||||
| Dakotas (model 1) | 19 | 58 | 19q13.11 | D19S414 | 24 to 89 | 1.9 |
| Oklahoma (models 1, 2) | 20 | 49 | 20p11.21 | D20S195 | 39 to 62 | 2.1, 2.7 |
Linkage significance criteria were as suggested by Rao and Gu.21
Model 1 was adjusted for age, gender, age2, and age–gender interactions. Model 2 was additionally adjusted for diabetes status, HDL cholesterol, LDL cholesterol, TG, SBP, DBP, and smoking status.
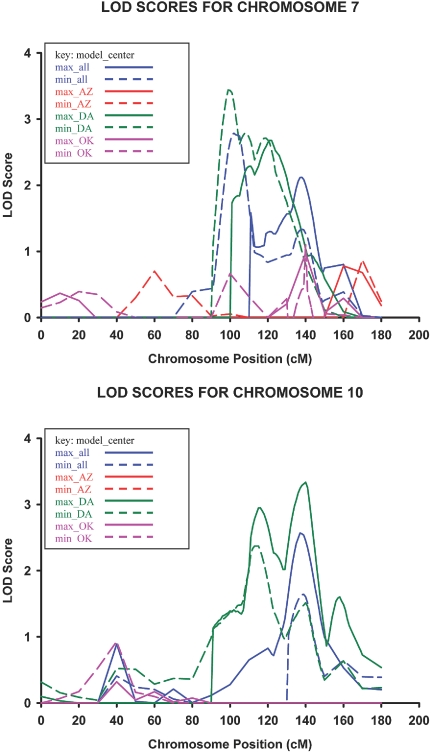
Genotype-by-hypertension interaction–specific analyses yielded a QTL for albuminuria in the Dakotas center on 7q21.11 at 91 cM nearest marker D7S669 (LOD = 3.4 using model 1) with a 19-cM 1-LOD unit support interval spanning the regions 7q11.23 to 7q21.3. Evidence for gene-by-hypertension linkage to albuminuria was also isolated in the Dakotas on 10q25.1 at 127 cM nearest marker D10S597 (LOD = 3.3 using model 2) with a 16-cM 1-LOD unit support interval spanning the regions 10q23.33 to 10q25.3 (Figure 2). Additional loci with suggestive evidence for gene-by-hypertension interaction on linkage to albuminuria are listed in Table 6. Stratified analyses demonstrated the locus on 7q21.11 (LOD = 3.7) and 10q25.1 (LOD = 2.5) to be specific to relative pairs with hypertension. The loci on 1q36.21 to 36.22 and 16q23.3 using model 1 in all centers also became significant using stratified analyses and was specific to relative pairs with hypertension with LOD scores of 3.2 and 3.1, respectively.
Figure 2.
Gene-by-hypertension cumulative multipoint LOD scores for ranked estimated UACR on chromosomes 7 and 10 in phase IV participants of the SHFS (2001 through 2003).
Table 6.
LOD scores suggestive of linkage (LOD ≥1.8) using two modeling strategies for gene-by-hypertension interaction–specific multipoint quantitative trait linkage analyses of ranked UACR in phase IV participants of the SHFS (2001 through 2003)a
| Center (Modelb) | Chromosome | Location (cM) | Chromosomal Region | Nearest Marker | 1-LOD Drop Support Interval | LOD Score |
|---|---|---|---|---|---|---|
| All centers (model 1) | 1 | 35 | 1p36.21 to 36.22 | D1S2697 | 27 to 44 | 2.8 |
| All centers (models 1, 2) | 221 | 1q32.1 | D1S249 | 212 to 234 | 2.9, 2.0 | |
| Arizona (model 1) | 2.9 | |||||
| Dakotas (model 2) | 2 | 104 | 2p12 | D2S2333 | 91 to 110 | 2.5 |
| Dakotas (model 1) | 153 | 2q23.3 | D2S151 | 140 to 163 | 2.1 | |
| Oklahoma (model 2) | 3 | 139 | 3q21.2 | D3S1267 | 120 to 148 | 2.0 |
| All centers (model 1) | 4 | 187 | 4p15.31 | D4S415 | 179 to end | 1.9 |
| All centers (model 1) | 6 | 63 to 81 | 6p12.1 to 6p21.1 | D6S257 | 51 to 91 | 2.5 |
| Arizona (model 1) | 2.1 | |||||
| Dakotas (models 1, 2) | 1.9, 2.0 | |||||
| All centers (model 2) | 7 | 91 to 127 | 7q21.11 to 7q31.32 | D7S669, D7S515 | 86 to 135 | 2.1 |
| All centers (model 1) | 2.8 | |||||
| Dakotas (models 1, 2) | 3.4, 2.7 | |||||
| Dakotas (model 2) | 9 | 99 | 9q22.31 | D9S283 | 88 to 104 | 2.1 |
| All centers (model 2) | 10 | 103 to 127 | 10q23.1 to 10q25.1 | D10S1686, D10S597 | 89 to 135 | 2.6 |
| Dakotas (models 1, 2) | 2.4, 3.3 | |||||
| Arizona (model 1) | 12 | 49 | 12p11.23 | D12S345 | 38 to 58 | 1.8 |
| Dakotas (model 1) | 133 | 12q24.22 to 24.33 | D12S286 | 119 to 142 | 2.6 | |
| Dakotas (model 1) | 14 | 52 | 14q22.1 | D14S276 | 45 to 63 | 1.8 |
| All centers (model 1) | 16 | 116 | 16q23.3 | D16S3091 | 109 to end | 2.5 |
| Dakotas (models 1, 2) | 2.9, 2.1 | |||||
| All centers (model 2) | 18 | 74 | 18q21.2 | D18S474 | 59 to 99 | 1.9 |
| Dakotas (model 1) | 2.2 | |||||
| All centers (model 1) | 20 | 36 to 49 | 20p11.21 to 20p12.1 | D20S195, D20S112 | 27 to 60 | 1.8 |
| Oklahoma (models 1, 2) | 2.3, 1.9 |
Linkage significance criteria were as suggested by Rao and Gu.21
Model 1 was adjusted for age, gender, age2, and age–gender interactions. Model 2 was additionally adjusted for diabetes status, HDL cholesterol, LDL cholesterol, TG, SBP, DBP, and smoking status.
DISCUSSION
Using interaction-specific linkage analysis, we isolated one locus with evidence for gene-by-diabetes interaction on linkage to urinary albumin excretion on 18q21.2 to 21.3 and two loci with evidence for gene-by-hypertension interaction on 7q21.11 to 7q31.32 and 10q23.1 to 25.1. In addition, stratified analyses implicated 7q21.3 and 7q34 to 36.2 to be linked to UACR in individuals with diabetes and 1p36.21 to 36.22 and 16q23.3 to be linked to UACR in individuals with hypertension. The majority of these loci, along with several loci with suggestive evidence for linkage to UACR, replicate findings from previous genome scans of human kidney disease phenotypes (Table 7).
Table 7.
Supporting evidence of linkage from other genome-wide scans of kidney disease phenotypes with LOD score of ≥1.8 or P < 0.05a
| SHFS Findings
|
Supporting Evidence
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Center (Modelb) | Interaction Analysisc | Chromosome | 1-LOD Unit Support Interval (cM) | LOD Score | Phenotype | Location (cM) | LOD Score or P | Reference |
| All centers (models 1, 2) | Hypertension | 1 | 212 to 234 | 2.9, 2.0 | Gene-by-age of diagnosis for nondiabetic ESRD | 232 | 0.016 | Freedman 2004 (22) |
| Dakotas (model 1) | Diabetes | 2 | 140 to 172 | 1.9 | CrCl in families with CVD | 145 | 3.1 | Hunt 2004 (23) |
| Hypertension | 2.1 | |||||||
| All centers (model 2) | Diabetes | 3 | 34 to 71 | 2.6 | CrCl in siblings with hypertension | 66 | 4.7 | DeWan 2002 (24) |
| Dakotas (model 2) | Diabetes | 2.0 | GFR in siblings with hypertension | 68 | 2.3 | Turner 2006 (15) | ||
| Oklahoma (model 2) | Hypertension | 3 | 120 to 149 | 2.0 | Nephropathy in siblings with type 1 diabetes | 149 | 2.2 | Osterholm 2007 (25) |
| Dakotas (model 2) | Diabetes | 3 | 167 to 192 | 1.8 | Nephropathy in siblings with type 2 diabetes | 181 | 2.0 | Imperatore 1998 (26) |
| Dakotas (models 1, 2) | Diabetes | 7 | 86 to 135 | 2.4, 2.3 | Nephropathy in siblings with type 1 or 2 diabetes | 104 | 6 × 10−5 | Iyengar 2007 (18) |
| Dakotas (models 1, 2) | Hypertension | 3.4, 2.7 | Albuminuria in siblings with hypertension | 112 | 2.9 | Turner 2006 (15) | ||
| GFR/albuminuria in siblings with hypertension | 133 | 3.4 | Leon 2006 (27) | |||||
| All centers (models 1, 2) | Diabetes | 7 | 144 to 172 | 2.7, 2.0 | Nephropathy in siblings with type 2 diabetes | 144 | 2.7 | Imperatore 1998 (26) |
| Arizona (model 1) | Diabetes | 2.0 | Albuminuria in siblings with type 2 diabetes | 172 | 3.1 | Krolewski 2006 (13) | ||
| Dakotas (model 1) | Diabetes | 2.0 | ||||||
| Dakotas (models 1, 2) | Diabetes | 9 | 88 to 108 | 2.2, 2.6 | Nephropathy in siblings with type 2 diabetes | 93 | 0.0001 | Bowden 2004 (16) |
| Dakotas (model 1) | Hypertension | 2.1 | ||||||
| Dakotas (models 1, 2) | Hypertension | 10 | 89 to 133 | 2.4, 3.3 | GFR in relatives with type 2 diabetes, creatinine in siblings with type 2 diabetes, CrCl in families with CVD, siblings with ESRD | 114 | 3.6 | Placha 2006 (28) |
| 94 | 2.5 | Chen 2007 (29) | ||||||
| 113 | 2.1 | Hunt 2002 (30) | ||||||
| 117 | 2.5 | Freedman 2002 (31) | ||||||
| Arizona (model 1) | Hypertension | 12 | 38 to 58 | 1.8 | Gene-by-age of diagnosis for ESRD | 49 | 0.02 | Freedman 2005 (32) |
| Dakotas (model 1) | Diabetes | 12 | 119 to 142 | 2.1 | Creatinine in general population | 125 | 1.8 | Fox 2004 (33) |
| Dakotas (model 1) | Hypertension | 2.6 | ||||||
| Dakotas (models 1, 2) | Diabetes | 14 | 45 to 85 | 2.7, 1.9 | Nephropathy in siblings with type 1 or 2 diabetes | 55 | 2 × 10−5 | Iyengar 2007 (18) |
| Dakotas (model 1) | Hypertension | 1.8 | ||||||
| Dakotas (models 1, 2) | Hypertension | 16 | 111 to end | 2.9, 2.1 | Albuminuria in siblings with hypertension, CrCl in siblings with type 2 diabetes | 129 | 2.2 | Leon 2006 (27) |
| 125 | 3.6 | Chen 2007 (29) | ||||||
| Dakotas (models 1, 2) | Diabetes | 18 | 59 to 94 | 3.3, 2.9 | Nephropathy in families with type 2 diabetes, CrCl in families with CVD | 75 | 0.005 | Bowden 2004 (16) |
| Dakotas (model 1) | Hypertension | 2.2 | 89 | 2.6 | Hunt 2004 (23) | |||
| Dakotas (model 1) | Diabetes | 19 | 39 to 62 | 1.9 | Albuminuria in siblings with hypertension | 49 | 2.2 | Leon 2006 (27) |
CrCl, creatinine clearance.
Model 1 was adjusted for age, gender, age2, and age–gender interactions. Model 2 was additionally adjusted for diabetes status, HDL cholesterol, LDL cholesterol, TG, SBP, DBP, and smoking status.
Interaction-specific analyses performed on diabetes or hypertension status.
To our knowledge, this is the first genome scan to investigate gene-by-hypertension and only the second to investigate gene-by-diabetes interaction on albuminuria.13 Contrary to our findings, Krolewski et al.13 did not find evidence of gene-by-diabetes interaction, suggesting that the QTLs for urinary albumin excretion were the same in individuals with and without diabetes. Because their population was primarily white, it may be that the interaction with diabetes in our population is population specific. The prevalence of diabetes in our population was lower than that in the study by Krolewski et al. (23 versus 50%); therefore, the overrepresentation of diabetes in their study may have masked the effect of interaction. In addition, the SHFS is a much larger population (>59,000 versus >5600 relative pairs) and hence had greater power to detect such interaction.
It should be noted that linkage analyses are solely hypothesis generating; however, replicability and the presence of plausible candidate genes within a QTL are important factors to consider. Four linkage studies have implicated regions within the 1-LOD support interval of our peak on 10q25.28–31 The human homolog for the renal failure 1 gene (Rf1) in the fawn-hooded rat animal model of ESRD has been suggested as a potential candidate gene for this region.34 Fawn-hooded rats develop severe hypertension, proteinuria, and rapid progression to chronic kidney disease, although the functional role of this gene has yet to be elucidated.
Located 4 cM from our peak on 10q25 lies the gene transcription factor 7-like 2 (TCF7L2), a member of the canonical WNT signaling pathway. Common polymorphisms within TCF7L2 have been associated with an increased risk for kidney disease in three population-based studies,35 diabetes in multiple, ethnically diverse populations36–40 as well as glucose control within individuals with diabetes.41 WNT signaling is reduced under high glucose conditions and in the kidney has been demonstrated to result in apoptosis of mesangial cells.42 Our analysis isolated this locus with the incorporation of gene-by-hypertension rather than diabetes interaction; however, it is biologically plausible for TCF7L2 to affect kidney function under conditions other than hyperglycemia. The canonical WNT pathway has a multitude of effects, including cell growth, proliferation, differentiation, and maturation.43 It is crucial to tubular formation in nephrogenesis,44 and several components have been shown to be upregulated in renal injury45 and proteinuric nephropathies.46
Our locus on 7q has been isolated by three other genome scans.15,18,19 The paraoxonase gene cluster (PON1 through 3) lies on 7q21.3. Paraoxonase is an HDL-associated enzyme that decreases oxidation of lipids and is a plausible candidate gene influencing the progression of kidney disease. Serum paraoxonase levels have been associated with diabetes and its complications.47 Several polymorphisms within PON1 and PON2 have been associated with gene expression and proteinuria in populations with diabetes.48,49 Another plausible candidate gene in this region is plasminogen activator inhibitor 1 (SERPINE1), which has also been cited as a potential mediator of diabetic nephropathy and glomerulosclerosis.50
No obvious candidate genes lie within our locus on 18q21.2 to 21.3; however, proximal to this location is the carnosinase 1 (CNDP1) gene located on 18q22.3 to 23, a region isolated by several other genome scans of diabetic nephropathy.16–19 The shortest form of a leucine trinucleotide repeat in CNDP1 (Mannheim variant) has been associated with decreased carnosinase levels51 and risk for diabetic kidney disease in two separate populations.51,52 Additional evidence for linkage of kidney disease to the region on 18q comes from the Framingham Heart Study, which found a genome-wide association between cystatin C and the single-nucleotide polymorphism rs563754 on 18q22.1.53
There are several caveats to our findings. The Strong Heart Study (SHS) is a general population, and, hence, a diversity of kidney diseases is represented. This would likely bias our results toward the null, however. In addition, it has been suggested that predisposition to albuminuria is genetically based, and inciting environmental factors, such as diabetes, simply shift the albumin excretion curve to the right.13
It is possible that our results are confounded by population stratification. Because percentage of American Indian ancestry was self-reported in the SHS and the reliability of such information is unknown, we chose not to use these data. Moreover, although population stratification may be a concern in linkage studies, the extent of this problem has not been officially interrogated and it is likely much smaller than what is observed in association studies, because we are following the co-segregation of disease susceptibility loci and genetic markers in families.
We did not possess information regarding treatment with specific antiproteinuric medications such as angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers. We attempted to address this issue by repeating our analysis after excluding those on antihypertensive medications and obtained only minor differences. This indirect method is admittedly flawed; however, others possessing information regarding ACE inhibition have found it did not contribute significantly to the model and excluded it from adjustment in their linkage analyses.13
We used a spot urine sample to calculate UACR, which has the potential to induce misclassification as opposed to a 24-h urine sample; however, a 24-h urine sample is limited by its completeness, and spot urine samples have been accepted as adequate measures of albuminuria.54 We had single time point estimates for BP, lipids, and glycosylated hemoglobin that may not have reliably captured the severity of these comorbid physiologic states. It is likely, however, that this possibility is offset by the large size of the SHS population. We also did not have reliable information regarding duration of diabetes, which is often difficult to assess but has the potential to alter the magnitude and/or precision of our results.
It is difficult to gauge the LOD score evidence reported herein in the face of the large number of comparisons made in conducting genome scans stratifying by possible effect modifiers. In total, we conducted 14 genome scans for each center (six for diabetes interaction, six for hypertension interaction, and two for albuminuria models 1 and 2) using a Bonferroni correction of P = 0.05/14 * 380 markers = 0.0000094 that was too conservative. Clearly, none of our results would reach this statistical significance threshold; however, given the correlation and nonindependence of these multiple genome scans of albuminuria, a systematic correction would be extremely difficult. We also recognize that reporting false-positive results is both undesirable and misleading and that concerns about false-negative results (i.e., missing true signals) are at least as important, as argued by several authors who have suggested different ways to achieve a better “balance” between these two types of error.21 It is imperative that we look to the internal and external consistency of the study findings to inform our overall interpretation.
In summary, we isolated on 7q, 10q, and 18q several loci that have significant linkage to UACR and also have plausible candidate genes.21 Our findings are strengthened by the large size of the SHS population with >59,000 relative pairs. In addition, the robustness of our results is evidenced by the fact that all loci are replications from genome scans of other populations with kidney disease; therefore, although the SHS population is American Indian, results are likely to be generalizable to other populations as well. We investigated interaction for three comorbid conditions that have a high likelihood of influencing linkage to proteinuria: Diabetes, hypertension, and obesity. Such interaction is critical to the isolation of genetic loci linked to common, complex traits such as albuminuria, as evidenced by our analysis. Further exploration of the candidate genes underlying the regions implicated in our study is warranted.
CONCISE METHODS
Study Population
The SHS began in 1988 to investigate CVD and its risk factors in a geographically diverse group of resident American Indian tribal members at three study centers in Arizona, Oklahoma, and North and South Dakota. The SHFS, a component of the SHS, was initiated in 1996 with the goal of localizing genes that influence CVD and its risk factors. The data utilized for the current analysis were gathered during phase IV of the SHFS, which occurred between 2001 and 2003. More than 3600 men and women aged 14 to 93 were recruited from >90 extended families originating from 13 separate American Indian tribes. All protocols were approved by the Indian Health Service institutional review board, by the institutional review boards of the participating institutions, and by the 13 American Indian tribes participating in these studies.
Phenotypes
Detailed descriptions of the SHS and SHFS design and laboratory protocols have been published previously.55,56 BP and body morphometrics were measured during the physical examination. After 5 min of rest, upper arm seated BP was measured three times by a trained technician using a mercury column sphygmomanometer (WA Baum Co., New York, NY) and size-adjusted cuffs. The first and fifth Korotkoff sounds were recorded. The average of the last two measures was used for all analyses. Hypertension was defined by a systolic BP (SBP) ≥140 mmHg or diastolic BP (DBP) ≥90 mmHg or use of antihypertensive drugs.57 Body mass index was calculated as weight (kg)/height (m2), and obesity was defined as a body mass index ≥30 kg/m2. Type 2 diabetes was determined according to the American Diabetes Association criteria.58
Fasting blood samples were assayed at MedStar Research Institute (Washington, DC) using standard laboratory methods as described previously.59 Triglycerides (TG), total cholesterol, and HDL cholesterol were measured using enzymatic reagents and the Hitachi 717 (Roche Diagnostics, Indianapolis, IN). LDL cholesterol was derived using the Friedewald equation; it was directly measured in individuals with TG values of >400 mg/dl.55 Urine albumin content was measured by a sensitive, nephelometric technique.60 Urine creatinine was measured by the picric acid method.61 Information regarding smoking status was obtained during a personal interview and was defined as having smoked at least 100 cigarettes.
Genotypes
The SHFS genotyping procedures have been described previously.62 In brief, DNA was isolated from fasting blood samples using organic solvents and then amplified in separate PCRs with primers specific for short tandem repeat markers using the ABI PRISM Linkage Mapping Set-MD10 2.5 (Applied Biosystems, Foster City, CA). PCR products were loaded into an ABI PRISM 377 DNA sequencer for laser-based automated genotyping. Analyses and assignment of the marker alleles were done using computerized algorithms (Applied Biosystems). The 400 short tandem repeats were chosen at 10-cM intervals, and average heterozygosity was 0.69, 0.76, and 0.74 in Arizona, North and South Dakota, and Oklahoma, respectively.
Genetic distances were obtained using gender-averaged chromosomal maps from the Marshfield Center for Medical Genetics (http://research.marshfieldclinic.org/genetics) and are reported in Haldane centiMorgans. Pedigree relationships were verified using the PREST (pedigree relationship statistical tests) package, which uses likelihood-based inference statistics for genome-wide identity-by-descent (IBD) allele sharing.63 Mendelian inconsistencies and spurious double recombinants were detected using the SimWalk2 package.64 The overall blanking rate for both types of errors was <1% of the total number of genotypes for Arizona, North and South Dakota, and Oklahoma. The cytogenetic locations of markers were determined using the Web resources of the University of California Santa Cruz (http://genome/ucsc.edu) and the Marshfield Linkage maps (http://research.marshfieldclinic.org/genetics/MarkerSearch/buildMap.asp).
Quantitative Genetic Analyses
UACR was rank-transformed to normalize the distribution and attain a kurtosis of <1.0. SAS 9.1 (SAS Institute, Cary, NC) was used to calculate the residual variability in UACR after covariate adjustment. To maximize the power to detect genetic effects, we considered two different models of covariate adjustment in each center and the full sample. In model 1, adjustments were made for age, gender, and age2 as well as age-by-gender interactions. Model 2 incorporated these covariates as well as those supported by current literature as potential confounders: BMI, SBP, DBP, TG, HDL cholesterol, LDL cholesterol, diabetes status, and smoking status (current versus former versus never). TG levels were log-transformed, and outliers from the distribution of SBP were set to 200 mmHg (winsorization) to maintain normal distributions. Some antihypertensive medications, such as ACEIs and angiotensin receptor blockers, could potentially confound or modify the genetic influence over UACR; therefore, we additionally ran models 1 and 2 excluding individuals on any antihypertensive medication, because the specific medications used were unknown. All models were stratified by center.
SOLAR 2.1.2 (Southwest Foundation for Biomedical Research, San Antonio, TX) was used to perform multipoint variance component linkage analysis of the residuals. Details of this model have been described previously.65,66 The use of the variance component approach requires an estimate of the IBD matrix. We used the Loki package, which uses a Markov chain Monte Carlo stochastic procedure to compute the IBD allele sharing at points throughout the genome conditional on the genotype information available at neighboring markers.67
Interaction Analyses
Genotype-by-diabetes, -hypertension, and -obesity interactions on UACR were explored using a three-step strategy. We initially tested for evidence of additive interaction by accounting for the genetic covariance differences according to diabetes, hypertension, or obesity status in relative pairs. In these analyses, the likelihood of a model including genotype-by-diabetes, -hypertension, or -obesity interaction is compared with the likelihood of restricted models in which such interactions are excluded. We tested for differential additive genetic effects among diabetic versus nondiabetic, hypertensive versus normotensive, or obese versus nonobese participants (genetic correlation [ρg] ≠1); for differences in the magnitude of the genetic effects among diabetic versus nondiabetic, hypertensive versus normotensive, and obese versus nonobese participants (genetic variance [σg] ≠ among two groups); and for differences in residual environmental interaction with diabetes, hypertension, or obesity status (environmental variance [σe] ≠ among two groups). Significant interaction was defined as P < 0.006, which accounts for multiple testing.
When additive interaction was present, we performed variance component linkage analysis with a customized model to include diabetes-, hypertension-, or obese-specific QTL effects. Interaction-specific linkage analysis is underpowered; therefore, when additive interaction was significant in at least one center, linkage analysis using the customized model was performed in each center and all centers combined. The customized variance component linkage model incorporates an additional QTL variance component as compared with the standard linkage model. Corrected LOD scores assume that the QTL variances of the two groups (σQe) are independent under the null, producing a test statistic distribution of ¼χ22, ½χ21, ¼ point mass at 0. This assumption may be overly conservative. Last, we performed standard linkage analyses within each stratified subset at the loci in which interaction was identified to determine in which stratum linkage was present.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
This research was funded by a cooperative agreement that includes grants U01 HL65520, U01 HL41642, U01 HL41652, U01 HL41654, and U01 HL65521 from the National Heart, Lung, and Blood Institute. Development of SOLAR and the methods implemented in it are supported by US Public Health Service grant MH059490 from the National Institutes of Health.
We first thank the SHFS participants. Without their participation, this project would not have been possible. In addition, the cooperation of the Indian Health Service hospitals and clinics and the directors of the SHS clinics and the many collaborators and staff of the SHS have made this project possible.
Published online ahead of print. Publication date available at www.jasn.org.
The views expressed in this article are those of the authors and do not necessarily reflect those of the Indian Health Service.
REFERENCES
- 1.Verhave JC, Gansevoort RT, Hillege HL, Bakker SJ, De Zeeuw D, de Jong PE: An elevated urinary albumin excretion predicts de novo development of renal function impairment in the general population. Kidney Int Suppl S18–S21, 2004 [DOI] [PubMed]
- 2.Hillege HL, Fidler V, Diercks GF, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, Gans RO, Janssen WM, Grobbee DE, de Jong PE: Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 106: 1777–1782, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Halle JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S: Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 286: 421–426, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Lemley KV, Boothroyd DB, Blouch KL, Nelson RG, Jones LI, Olshen RA, Myers BD: Modeling GFR trajectories in diabetic nephropathy. Am J Physiol Renal Physiol 289: F863–F870, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR: Development and progression of nephropathy in type 2 diabetes: The United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 63: 225–232, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Coresh J, Byrd-Holt D, Astor BC, Briggs JP, Eggers PW, Lacher DA, Hostetter TH: Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc Nephrol 16: 180–188, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Scavini M, Shah VO, Stidley CA, Tentori F, Paine SS, Harford AM, Narva AS, Kessler DS, Bobelu A, Albert CP, Bobelu J, Jamon E, Natachu K, Neha D, Welty TK, MacCluer JW, Zager PG: Kidney disease among the Zuni Indians: The Zuni Kidney Project. Kidney Int Suppl S126–S131, 2005 [DOI] [PubMed]
- 8.Kasiske BL, Rith-Najarian S, Casper ML, Croft JB: American Indian heritage and risk factors for renal injury. Kidney Int 54: 1305–1310, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Welty TK, Rhoades DA, Yeh F, Lee ET, Cowan LD, Fabsitz RR, Robbins DC, Devereux RB, Henderson JA, Howard BV: Changes in cardiovascular disease risk factors among American Indians. The Strong Heart Study. Ann Epidemiol 12: 97–106, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Robbins DC, Knowler WC, Lee ET, Yeh J, Go OT, Welty T, Fabsitz R, Howard BV: Regional differences in albuminuria among American Indians: An epidemic of renal disease. Kidney Int 49: 557–563, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Fox CS, Yang Q, Guo CY, Cupples LA, Wilson PW, Levy D, Meigs JB: Genome-wide linkage analysis to urinary microalbuminuria in a community-based sample: The Framingham Heart Study. Kidney Int 67: 70–74, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Arar N, Nath S, Thameem F, Bauer R, Voruganti S, Comuzzie A, Cole S, Blangero J, MacCluer J, Abboud H: Genome-wide scans for microalbuminuria in Mexican Americans: The San Antonio Family Heart Study. Genet Med 9: 80–87, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Krolewski AS, Poznik GD, Placha G, Canani L, Dunn J, Walker W, Smiles A, Krolewski B, Fogarty DG, Moczulski D, Araki S, Makita Y, Ng DP, Rogus J, Duggirala R, Rich SS, Warram JH: A genome-wide linkage scan for genes controlling variation in urinary albumin excretion in type II diabetes. Kidney Int 69: 129–136, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Freedman BI, Beck SR, Rich SS, Heiss G, Lewis CE, Turner S, Province MA, Schwander KL, Arnett DK, Mellen BG: A genome-wide scan for urinary albumin excretion in hypertensive families. Hypertension 42: 291–296, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Turner ST, Kardia SL, Mosley TH, Rule AD, Boerwinkle E, de Andrade M: Influence of genomic loci on measures of chronic kidney disease in hypertensive sibships. J Am Soc Nephrol 17: 2048–2055, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Bowden DW, Colicigno CJ, Langefeld CD, Sale MM, Williams A, Anderson PJ, Rich SS, Freedman BI: A genome scan for diabetic nephropathy in African Americans. Kidney Int 66: 1517–1526, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Vardarli I, Baier LJ, Hanson RL, Akkoyun I, Fischer C, Rohmeiss P, Basci A, Bartram CR, Van Der Woude FJ, Janssen B: Gene for susceptibility to diabetic nephropathy in type 2 diabetes maps to 18q22.3–23. Kidney Int 62: 2176–2183, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Iyengar SK, Abboud HE, Goddard KA, Saad MF, Adler SG, Arar NH, Bowden DW, Duggirala R, Elston RC, Hanson RL, Ipp E, Kao WH, Kimmel PL, Klag MJ, Knowler WC, Meoni LA, Nelson RG, Nicholas SB, Pahl MV, Parekh RS, Quade SR, Rich SS, Rotter JI, Scavini M, Schelling JR, Sedor JR, Sehgal AR, Shah VO, Smith MW, Taylor KD, Winkler CA, Zager PG, Freedman BI: Genome-wide scans for diabetic nephropathy and albuminuria in multiethnic populations: The family investigation of nephropathy and diabetes (FIND). Diabetes 56: 1577–1585, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Leon JM, Freedman BI, Miller MB, North KE, Hunt SC, Eckfeldt JH, Lewis CE, Kraja AT, Djousse L, Arnett DK: Genome scan of glomerular filtration rate and albuminuria: The HyperGEN study. Nephrol Dial Transplant 22: 763–771, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Fogarty DG, Rich SS, Hanna L, Warram JH, Krolewski AS: Urinary albumin excretion in families with type 2 diabetes is heritable and genetically correlated to blood pressure. Kidney Int 57: 250–257, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Rao DC, Gu C: False positives and false negatives in genome scans. Adv Genet 42: 487–498, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Freedman BI, Langefeld CD, Rich SS, Valis CJ, Sale MM, Williams AH, Brown WM, Beck SR, Hicks PJ, Bowden DW: A genome scan for ESRD in black families enriched for nondiabetic nephropathy. J Am Soc Nephrol 15: 2710–2727, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Hunt SC, Coon H, Hasstedt SJ, Cawthon RM, Camp NJ, Wu LL, Hopkins PN: Linkage of serum creatinine and glomerular filtration rate to chromosome 2 in Utah pedigrees. Am J Hyptension 17: 511–515, 2004 [DOI] [PubMed] [Google Scholar]
- 24.DeWan AT, Arnett DK, Atwood LD, Province MA, Lewis CE, Hunt SC, Eckfeldt J: A genome scan for renal function among hypertensives: The HyperGEN Study. Am J Hum Genetics 68: 136–144, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osterholm AM, He B, Pitkanicmi J, Albinsson L, Berg T, Tuomilchto J, Tryggvason K: Genome-wide scan for type 1 diabetic nephropathy in the Finnish population reveals suggestive linkage to a single locus on chromosome 3q. Kidney Int 71: 140–145, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Imperatore G, Hanson RL, Pettitt DJ, Kobes S, Bennett PH, Knowler WC, and the Pima Diabetes Genes Group: Sib-pair linkage analysis for susceptibility genes for microvascular complications among Pima Indians with type 2 diabetes. Diabetes 47: 821–830, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Leon JM, Freedman BI, Miller MB, North KE, Hunt SC, Eckfeldt JH, Lewis CF, Kraja AT, Djousse L, Arnett DK: Genome scan of glomerular filtration rate and albuminuria; the HyperGEN study. Nephrol Dial Transplant 22: 763–771, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Placha G, Poznik GD, Dunn J, Smiles A, Krolewski B, Glew T, Puppala S, Schneider J, Rogus JJ, Rich SS, Duggirala R, Warram JH, Krolewski AS: A genome-wide linkage scan for genes controlling variation in renal function estimated by serum cystatin C levels in extended families with type 2 diabetes. Diabetes 55: 3358–3365, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Chen G, Adyemo AA, Zhou J, Chen Y, Doumatey A, Lashley K, Huang H, Amoah A, Agyenim-Boateng K, Eghan BA, Okafor G, Acheampong J, Oli J, Fasanmade O, Johnson T, Rotimi C: A genome-wide search for linkage to renal function phenotypes in West Africans with type 2 diabetes. Am J Kidney Dis 49: 394–400, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Hunt SC, Hasstedt SJ, Coon H, Camp NJ, Cawthon RM, Wu LL, Hopkins PN: Linkage of creatinine clearance to chromosome 10 in Utah pedigrees replicates a locus for end-stage renal disease in humans and renal failure in the fawn-hooded rat. Kidney Int 62: 1143–1148, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Freedman BI, Rich SS, Yu H, Roh BH, Bowden DW: Linkage heterogeneity of end-stage renal disease on human chromosome 10. Kidney Int 62: 770–774, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Freedman BI, Bowden DW, Rich SS, Valis CJ, Sale MM, Hicks PJ, Langefeld CD: A genome scan for all-cause end-stage renal disease in African Americans. Nephrol Dial Transplant 20: 712–718, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Fox CS, Yang Q, Cupples LA, Guo CY, Larson MG, Leip EP, Wilson PW, Levy D: Genomewide linkage analysis to serum creatinine, GFR, and creatinine clearance in a community-based population: The Framingham Heart Study. J Am Soc Nephrol 15: 2457–2461, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Brown DM, Provoost AP, Daly MJ, Lander ES, Jacob HJ: Renal disease susceptibility and hypertension are under independent genetic control in the fawn-hooded rat. Nat Genet 12: 44–51, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Köttgen A, Hwang SJ, Rampersaud E, Coresh J, North KE, Pankow JS, Meigs JB, Florez JC, Parsa A, Levy D, Boerwinkle E, Shuldiner AR, Fox CS, Kao WH: TCF7L2 variants associate with CKD progression and renal function in population-based cohorts. J Am Soc Nephrol 10: 1989–1999, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cauchi S, El Achhab Y, Choquet H, Dina C, Krempler F, Weitgasser R, Nejjari C, Patsch W, Chikri M, Meyre D, Froguel P: TCF7L2 is reproducibly associated with type 2 diabetes in various ethnic groups: A global meta-analysis. J Mol Med 85: 777–782, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Sale MM, Smith SG, Mychaleckyj JC, Keene KL, Langefeld CD, Leak TS, Hicks PJ, Bowden DW, Rich SS, Freedman BI: Variants of the transcription factor 7-like 2 (TCF7L2) gene are associated with type 2 diabetes in an African-American population enriched for nephropathy. Diabetes 56: 2638–2642, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Bodhini D, Radha V, Dhar M, Narayani N, Mohan V: The rs12255372(G/T) and rs7903146(C/T) polymorphisms of the TCF7L2 gene are associated with type 2 diabetes mellitus in Asian Indians. Metabolism 56: 1174–1178, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Chang YC, Chang TJ, Jiang YD, Kuo SS, Lee KC, Chiu KC, Chuang LM: Association study of the genetic polymorphisms of the transcription factor 7-like 2 (TCF7L2) gene and type 2 diabetes in the Chinese population. Diabetes 56: 2631–2637, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Maeda S, Osawa N, Hayashi T, Tsukada S, Kobayashi M, Kikkawa R: Genetic variations associated with diabetic nephropathy and type II diabetes in a Japanese population. Kidney Int Suppl S43–S48, 2007 [DOI] [PubMed]
- 41.Kimber CH, Doney AS, Pearson ER, McCarthy MI, Hattersley AT, Leese GP, Morris AD, Palmer CN: TCF7L2 in the Go-DARTS study: Evidence for a gene dose effect on both diabetes susceptibility and control of glucose levels. Diabetologia 50: 1186–1191, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Lin CL, Wang JY, Huang YT, Kuo YH, Surendran K, Wang FS: Wnt/beta-catenin signaling modulates survival of high glucose-stressed mesangial cells. J Am Soc Nephrol 17: 2812–2820, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Smith U: TCF7L2 and type 2 diabetes-we WNT to know. Diabetologia 50: 5–7, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Iglesias DM, Hueber PA, Chu L, Campbell R, Patenaude AM, Dziarmaga AJ, Quinlan J, Mohamed O, Dufort D, Goodyer PR: Canonical WNT signaling during kidney development. Am J Physiol Renal Physiol 293: F494–F500, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Surendran K, Schiavi S, Hruska KA: Wnt-dependent beta-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J Am Soc Nephrol 16: 2373–2384, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Rudnicki M, Eder S, Perco P, Enrich J, Scheiber K, Koppelstatter C, Schratzberger G, Mayer B, Oberbauer R, Meyer TW, Mayer G: Gene expression profiles of human proximal tubular epithelial cells in proteinuric nephropathies. Kidney Int 71: 325–335, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Ikeda Y, Suehiro T, Inoue M, Nakauchi Y, Morita T, Arii K, Ito H, Kumon Y, Hashimoto K: Serum paraoxonase activity and its relationship to diabetic complications in patients with non-insulin-dependent diabetes mellitus. Metabolism 47: 598–602, 1998 [DOI] [PubMed] [Google Scholar]
- 48.Hofer SE, Bennetts B, Chan AK, Holloway B, Karschimkus C, Jenkins AJ, Silink M, Donaghue KC: Association between PON 1 polymorphisms, PON activity and diabetes complications. J Diabetes Complications 20: 322–328, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Calle R, McCarthy MI, Banerjee P, Zeggini E, Cull CA, Thorne KI, Wiltshire S, Terra S, Meyer D, Richmond J, Mancuso J, Milos P, Fryburg D, Holman RR: Paraoxonase 2 (PON2) polymorphisms and development of renal dysfunction in type 2 diabetes: UKPDS 76. Diabetologia 49: 2892–2899, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Lee EA, Seo JY, Jiang Z, Yu MR, Kwon MK, Ha H, Lee HB: Reactive oxygen species mediate high glucose-induced plasminogen activator inhibitor-1 up-regulation in mesangial cells and in diabetic kidney. Kidney Int 67: 1762–1771, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Janssen B, Hohenadel D, Brinkkoetter P, Peters V, Rind N, Fischer C, Rychlik I, Cerna M, Romzova M, de Heer E, Baelde H, Bakker SJ, Zirie M, Rondeau E, Mathieson P, Saleem MA, Meyer J, Koppel H, Sauerhoefer S, Bartram CR, Nawroth P, Hammes HP, Yard BA, Zschocke J, van der Woude FJ: Carnosine as a protective factor in diabetic nephropathy: Association with a leucine repeat of the carnosinase gene CNDP1. Diabetes 54: 2320–2327, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Freedman BI, Hicks PJ, Sale MM, Pierson ED, Langefeld CD, Rich SS, Xu J, McDonough C, Janssen B, Yard BA, van der Woude FJ, Bowden DW: A leucine repeat in the carnosinase gene CNDP1 is associated with diabetic end-stage renal disease in European Americans. Nephrol Dial Transplant 22: 1131–1135, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Hwang SJ, Yang Q, Meigs JB, Pearce EN, Fox CS: A genome-wide association for kidney function and endocrine-related traits in the NHLBI's Framingham Heart Study. BMC Med Genet 8: S10, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nathan DM, Rosenbaum C, Protasowicki VD: Single-void urine samples can be used to estimate quantitative microalbuminuria. Diabetes Care 10: 414–418, 1987 [DOI] [PubMed] [Google Scholar]
- 55.Lee ET, Welty TK, Fabsitz R, Cowan LD, Le NA, Oopik AJ, Cucchiara AJ, Savage PJ, Howard BV: The Strong Heart Study: A study of cardiovascular disease in American Indians—Design and methods. Am J Epidemiol 132: 1141–1155, 1990 [DOI] [PubMed] [Google Scholar]
- 56.North KE, Howard BV, Welty TK, Best LG, Lee ET, Yeh JL, Fabsitz RR, Roman MJ, MacCluer JW: Genetic and environmental contributions to cardiovascular disease risk in American Indians: The Strong Heart Family Study. Am J Epidemiol 157: 303–314, 2003. 57 [DOI] [PubMed] [Google Scholar]
- 57.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ: Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42: 1206–1252, 2003 [DOI] [PubMed] [Google Scholar]
- 58.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 20: 1183–1197, 1997 [DOI] [PubMed] [Google Scholar]
- 59.Friedewald WT, Levy RI, Fredrickson DS: Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18: 499–502, 1972 [PubMed] [Google Scholar]
- 60.Vasquez B, Flock E, Savage P, Nagulesparan M, Bennion L, Baird H, Bennet P: Sustained reduction in proteinuria in type 2 (non-insulin dependent) diabetes following diet-induced reduction of hyperglycemia. Diabetologia 26: 127–133, 1984 [DOI] [PubMed] [Google Scholar]
- 61.Chasson A, Grady H, Stanley M: Determination of creatinine by means of automatic chemical analysis. Tech Bull Regist Med Tech 30: 207–212, 1957 [PubMed] [Google Scholar]
- 62.North KE, Goring HH, Cole SA, Diego VP, Almasy L, Laston S, Cantu T, Howard BV, Lee ET, Best LG, Fabsitz RR, MacCluer JW: Linkage analysis of LDL cholesterol in American Indian populations: The Strong Heart Family Study. J Lipid Res 47: 59–66, 2006 [DOI] [PubMed] [Google Scholar]
- 63.Sun L, Wilder K, McPeek MS: Enhanced pedigree error detection. Hum Hered 54: 99–110, 2002 [DOI] [PubMed] [Google Scholar]
- 64.Sobel E, Papp JC, Lange K: Detection and integration of genotyping errors in statistical genetics. Am J Hum Genet 70: 496–508, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Almasy L, Blangero J: Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet 62: 1198–1211, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blangero J, Almasy L: Multipoint oligogenic linkage analysis of quantitative traits. Genet Epidemiol 14: 959–964, 1997 [DOI] [PubMed] [Google Scholar]
- 67.Heath SM: Markov chain Monte Carlo segregation and linkage analysis for oligogenic models. Am J Hum Genet 61: 748–760, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.