Abstract
The CC-chemokine receptor 5 (CCR5) is a receptor for various proinflammatory chemokines, and a deletion variant of the CCR5 gene (CCR5Δ32) leads to deficiency of the receptor. We hypothesized that CCR5Δ32 modulates inflammation-driven mortality in patients with ESRD. We studied the interaction between CCR5 genotype and levels of high-sensitivity C-reactive protein (hsCRP) in 603 incident dialysis patients from the multicenter, prospective NEtherlands COoperative Study on the Adequacy of Dialysis (NECOSAD) cohort. CCR5 genotype and hsCRP levels were both available for 413 patients. During 5 yr of follow-up, 170 patients died; 87 from cardiovascular causes. Compared with the reference group of patients who had the wild-type CCR5 genotype and hsCRP ≤ 10 mg/L (n = 225), those carrying the deletion allele with hsCRP ≤ 10 mg/L (n = 55) had similar mortality, and those carrying the wild-type genotype with hsCRP > 10 mg/L (n = 108) had an increased risk for mortality (HR: 1.82; 95% CI: 1.29 to 2.58). However, those carrying the deletion allele with hsCRP > 10 mg/L (n = 25) had a mortality rate similar to the reference group; this seemingly protective effect of the CCR5 deletion was even more pronounced for cardiovascular mortality. We replicated these findings in an independent Swedish cohort of 302 ESRD patients. In conclusion, the CCR5Δ32 polymorphism attenuates the adverse effects of inflammation on overall and cardiovascular mortality in ESRD.
Cardiovascular disease (CVD) is a prominent cause of mortality in ESRD patients.1 A persistent inflammatory state has been recognized as a risk factor in this respect. An established marker of systemic inflammation is an elevated serum level of C-reactive protein (CRP).2,3 Although CRP has been strongly associated with overall and cardiovascular mortality in dialysis patients, recent evidence suggests that CRP itself does not have an atherogenic potential.4–9
The inflammatory process in atherosclerosis is characterized by infiltration of monocytes and T lymphocytes in the vascular wall in response to chemokines. Different studies suggest that the chemokines CCL5/RANTES, CCL3/macrophage inflammatory protein-1α (MIP-1α), and CCL4/MIP-1β and their receptor CC-chemokine receptor 5 (CCR5) play a role in the pathogenesis of atherosclerosis.10–14 CCR5 is expressed on the principal cell types implicated in atherogenesis.15–18
In states of inflammation, the CCR5 receptor could contribute to atherogenesis through the binding of its ligands, which in turn mediate the recruitment of inflammatory cells to the endothelium. Interestingly, patients with a dysfunctional CCR5 due to the gene polymorphism CCR5 deletion 32 (CCR5Δ32), a 32-bp deletion in the open reading frame leading to premature termination of the protein and sequestration in the endoplasmic reticulum, have an improved prognosis in atherosclerotic diseases.19–23 Thus, CCR5Δ32 could be a rate-limiting factor in the increased mortality rate associated with systemic inflammation.
We therefore hypothesized that the CCR5Δ32 polymorphism might alter the previously observed association of elevated CRP with mortality in ESRD. To test this hypothesis, we investigated whether the CCR5Δ32 polymorphism modifies the effect of CRP on mortality in a Dutch dialysis cohort, the NEtherlands COoperative Study on the Adequacy of Dialysis (NECOSAD). For independent confirmation, we analyzed the corresponding associations in a Swedish cohort of ESRD patients.
RESULTS
A total of 603 patients were included in the present analysis. In 413 patients (68%), the CCR5 genotype and hsCRP levels were available. Compared with the 190 patients without hsCRP and/or CCR5 data, these patients were slightly older (59.7 versus 57.1 yr, P = 0.05) and included more hemodialysis (HD) patients (67% versus 52%, P < 0.01). Comorbidities such as diabetes mellitus did not differ in both patients groups (35% versus 30%, P = 1.19, and 25% versus 20%, P = 0.11, respectively). No differences were found in serum albumin (35.7 g/l versus 36.3 g/l, P = 0.25). However, a better survival was observed for the 413 patients with both hsCRP and CCR5 data with a median of 1370 d (3.75 yr; 710 to 1826 d) of follow-up, and a mortality rate of 122 per 1000 person years within 5 yr of follow-up, compared with a median of 1006 d of follow-up and a mortality rate of 147 per 1000 person years within 5 yr of follow-up for the 190 patients without hsCRP and/or CCR5 data. Further statistical analyses were performed on the 413 patients.
The CCR5 ins32/del32 polymorphism was distributed as follows: ins/ins, 333 (80.6%); ins/del, 73 (17.7%); and del/del, 7 (1.7%). The genotype distribution did not deviate significantly from Hardy-Weinberg equilibrium (P = 0.21).
Baseline characteristics are shown in the first column of Table 1. The patient characteristics for the different genotype groups were similar at the start of dialysis, except antihypertensive medication use. Patients homo- or heterozygous for the deletion allele used more antihypertensive medications (P = 0.01). The median CRP levels in the two genotype groups were as follows: in the CCR5 ins/ins group: 4.7 mg/L (1.8 to 13.4 mg/L) and in the CCR5 del group: 6.9 mg/L (2.4 to 14.1 mg/L) (P = 0.22).
Table 1.
Baseline characteristics of the Dutch NECOSAD and Swedish cohorts
| NECOSAD n = 413 | Swedish Cohort n = 302 | ||
|---|---|---|---|
| Gender: male | 253 (61.3) | 185 (61.3) | |
| Age (year) | 62 (50 to 71) | 55 (44 to 64) | |
| Caucasian | 379 (91.8) | ||
| Hemodialysis | 277 (67.1) | 142 (47.0) | |
| Peritoneal dialysis | 136 (32.9) | 160 (53.0) | |
| Primary kidney disease | |||
| Diabetes mellitus | 75 (18.2) | 88 (29.1) | Diabetic nephropathy |
| Glomerulonephritis | 48 (11.6) | 84 (27.8) | Chronic glomerulonephritis |
| Renal vascular disease | 76 (18.4) | 12 (4.0) | Nephrosclerosis |
| Other | 214 (51.8) | 35 (11.6) | Polycystic kidney disease |
| 83 (27.5) | Other | ||
| Cardiovascular disease | 144 (34.9) | 99 (32.8) | |
| Diabetes mellitus | 105 (25.4) | 88 (29.1) | |
| Smoking | |||
| Never | 120 (29.2) | ||
| Former | 194 (47.2) | ||
| Current | 97 (23.6) | ||
| DBP (mmHg) | 83 (12.8) | 88 (13.2) | |
| SBP (mmHg) | 150 (25.4) | 151 (23.8) | |
| Antihypertensive medication | 356 (86.2) | ||
| Lipid lowering medication | 121 (29.3) | ||
| hsCRP (mg/L) | 5.1 (1.9 to 13.7) | 4.9 (2.0 to 14.0) | |
| hsCRP > 10 (mg/L) | 133 (32.2) | 101 (33.4) | |
| Cholesterol (mmol/l) | 5.0 (1.3) | 5.3 (1.5) | |
| Albumin (g/L) | 32.5 (6.9) | 33.2 (6.1) | |
| Hemoglobin (g/dl) | 11.0 (1.4) | 10.4 (1.4) | |
| GFR (ml/min) | 4.2 (3.1) | 6.6 (2.3) | |
| Kt/V/wk | 2.3 (0.9) | — | |
| CCR5 | |||
| Ins/ins | 333 (80.6) | 246 (81.5) | |
| Ins/del32 | 73 (17.7) | 51 (16.9) | |
| Del32/del32 | 7 (1.7) | 5 (1.7) |
Data are presented as number (percentage), median (25, 75th percentile), mean (standard deviation; SD). DBP, diastolic blood pressure; SBP, systolic blood pressure.
Mortality and Systemic Inflammation
A total of 170 (87 [51%] cardiovascular) patients died during follow-up of 5 yr. The mortality rate in the group with hsCRP ≤ 10 mg/L was 91 per 1000 person years as opposed to 207 per 1000 person years in the group with hsCRP > 10 mg/L. Kaplan-Meier survival analysis showed a statistically significant difference in all-cause, cardiovascular and noncardiovascular mortality for the two hsCRP level groups; those with hsCRP > 10 mg/L had the worst survival (log rank: P < 0.01). We confirmed these univariate findings by Cox regression analysis (adjusted hazard ratio [HR] for patients with hsCRP ≤ 10 mg/L compared with hsCPR > 10 mg/L for all-cause mortality: 1.78 [95% confidence interval (CI): 1.31 to 2.42; P < 0.01], for cardiovascular mortality: 1.70 [95% CI: 1.10 to 2.63; P = 0.02], and for noncardiovascular mortality: 1.87 [95% CI: 1.20 to 2.91; P < 0.01]).
When analyzed as a continuous variable, the adjusted mortality risk per unit hsCRP increase was 1.31 (95% CI: 1.17 to 1.48; P < 0.01) for all-cause mortality, 1.24 (95% CI: 1.04 to 1.46; P = 0.01) for cardiovascular mortality, and 1.41 (95% CI: 1.18 to 1.67; P < 0.01) for noncardiovascular mortality.
Mortality and Systemic Inflammation and CCR5 Polymorphism Interaction
Of the 170 patients who died within 5 yr of follow-up, 140 patients were noncarriers of the CCR5Δ32 polymorphism (42% of all noncarriers), and 30 patients were carriers of the polymorphism (38% of carriers).
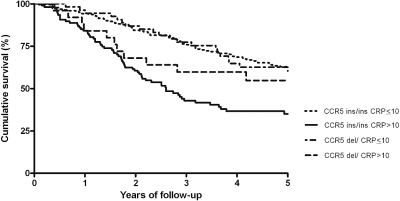
The Kaplan Meier survival curve (Figure 1) shows that patients with hsCRP > 10 mg/L and not carrying a deletion allele had the worst survival (log rank: P < 0.01). In Table 2, the HRs for all-cause mortality are presented. In the NECOSAD cohort, noncarriers of the deletion allele with hsCRP > 10 mg/L had an increased all-cause mortality risk compared with noncarriers with hsCRP ≤ 10 mg/L. Patients in the group with hsCRP > 10 mg/L, carrying the deletion allele showed a much less elevated HR for all-cause mortality. This effect was even more pronounced for cardiovascular mortality (Table 2). For noncardiovascular mortality, the protective effect of the deletion allele in patients with an elevated hsCRP was less pronounced (Table 2).
Figure 1.
Kaplan-Meier overall survival curve for patients in the NECOSAD cohort according to CCR5 genotype combined with or without elevated hsCRP (mg/L) levels (log rank: P < 0.001).
Table 2.
Mortality rate per 1000 person years, HR (95% CI), and P values for all-cause, cardiovascular, and noncardiovascular mortality in the NECOSAD cohort and HR (95% CI) and P values for all-cause mortality in the Swedish cohort
| CCR5 ins/ins and hsCRP < 10 mg/L (n = 225) | CCR5 ins/ins and hsCRP > 10 mg/L (n = 108) | CCR5Δ32 and hsCRP < 10 mg/L (n = 55) | CCR5Δ32 and hsCRP > 10 mg/L (n = 25) | |
|---|---|---|---|---|
| NECOSAD | ||||
| All-cause mortality | ||||
| Mortality rate | 91 | 228 | 90 | 133 |
| Crude HR | 1 | 2.55 (1.83 to 3.56) | 1.00 (0.60 to 1.65) | 1.48 (0.79 to 2.79) |
| P | <0.01 | 0.99 | 0.22 | |
| Adjusted HR | 1 | 1.82 (1.29 to 2.58) | 0.90 (0.54 to 1.50) | 1.39 (0.73 to 2.62) |
| P | <0.01 | 0.69 | 0.32 | |
| Cardiovascular mortality | ||||
| Mortality rate | 47 | 112 | 52 | 60 |
| Crude HR | 1 | 2.50 (1.56 to 4.00) | 1.11 (0.57 to 2.17) | 1.30 (0.51 to 3.31) |
| P | <0.01 | 0.76 | 0.58 | |
| Adjusted HR | 1 | 1.85 (1.13 to 3.01) | 1.05 (0.53 to 2.07) | 1.25 (0.49 to 3.17) |
| P | 0.01 | 0.89 | 0.64 | |
| Noncardiovascular mortality | ||||
| Mortality rate | 44 | 116 | 38 | 72 |
| Crude HR | 1 | 2.60 (1.62 to 4.19) | 0.88 (0.41 to 1.88) | 1.67 (0.71 to 3.97) |
| P | <0.01 | 0.73 | 0.24 | |
| Adjusted HR | 1 | 1.82 (1.12 to 2.97) | 0.76 (0.35 to 1.65) | 1.52 (0.64 to 3.63) |
| P | 0.02 | 0.49 | 0.34 | |
| Swedish | (n = 167) | (n = 79) | (n = 34) | (n = 22) |
| All-cause mortality | ||||
| Crude HR | 1 | 2.44 (1.50 to 3.99) | 1.50 (0.72 to 3.15) | 1.50 (0.66 to 3.40) |
| P | <0.01 | 0.28 | 0.33 | |
| Adjusted HR | 1 | 1.67 (1.01 to 2.77) | 1.95 (0.91 to 4.19) | 0.94 (0.41 to 2.17) |
| P | 0.05 | 0.09 | 0.89 |
Adjusted for gender, age at inclusion, cardiovascular disease, diabetes, and dialysis modality.
In Table 3, the HRs for all-cause, cardiovascular, and noncardiovascular mortality for hsCRP level as a continuous variable for the two genotype groups are presented; hsCRP levels are associated with mortality in patients with the CCR5 ins/ins genotype and not in patients carrying a deletion allele, for cardiovascular mortality the results are more pronounced. For noncardiovascular mortality in both CCR5 genotype groups, there was a significant increase in mortality risk per unit hsCRP increase.
Table 3.
HR (95% CI) and P values for all-cause, cardiovascular, and noncardiovascular mortality per unit hsCRP increase in the NECOSAD cohort and HR (95% CI) and P values for all-cause mortality per unit hsCRP increase in the Swedish cohort
| CCR5 ins/ins | CCR5Δ32 | |
|---|---|---|
| NECOSAD | ||
| All-cause mortality | ||
| Crude HR | 1.52 (1.34 to 1.71) | 1.31 (0.93 to 1.84) |
| P | <0.01 | 0.13 |
| Adjusted HR | 1.34 (1.18 to 1.52) | 1.32 (0.89 to 1.97) |
| P | <0.01 | 0.17 |
| Cardiovascular mortality | ||
| Crude HR | 1.47 (1.24 to 1.75) | 1.05 (0.68 to 1.61) |
| P | <0.01 | 0.83 |
| Adjusted HR | 1.30 (1.08 to 1.55) | 1.02 (0.61 to 1.68) |
| P | <0.01 | 0.95 |
| Noncardiovascular mortality | ||
| Crude HR | 1.56 (1.31 to 1.86) | 1.79 (1.02 to 3.14) |
| P | <0.01 | 0.04 |
| Adjusted HR | 1.39 (1.16 to 1.66) | 2.37 (1.16 to 4.86) |
| P | <0.01 | 0.02 |
| Swedish | ||
| All-cause mortality | ||
| Crude HR | 1.40 (1.17 to 1.66) | 1.18 (0.82 to 1.69) |
| P | <0.01 | 0.36 |
| Adjusted HR | 1.18 (0.98 to 1.43) | 0.73 (0.46 to 1.15) |
| P | 0.08 | 0.17 |
Adjusted for gender, age at inclusion, cardiovascular disease, diabetes, and dialysis modality.
Limiting the analysis to patients with hsCRP level <50 mg/L to exclude patients who could have had an acute infection showed the same HRs for overall and cardiovascular mortality. The HRs for noncardiovascular mortality for the group with hsCRP > 10 mg/L, carrying the deletion allele resulted in a lower value (adjusted HR: 1.08 [95% CI: 0.38 to 3.04; P = 0.89]).
Also, limiting the analysis to Caucasian patients resulted in comparable HRs (data not shown).
By taking the median hsCRP level as a cut-off point instead of using a cut off hsCRP level of 10 mg/L to divide patients into two groups (with or without a systemic inflammation, respectively) yielded similar results. Also, extending the follow-up to more than 5 yr did not significantly change the results; neither did further adjusting for primary kidney disease, smoking, BP, and medication use (data not shown).
Independent Replication
The population used for confirmation consisted of 302 ESRD patients characterized for CCR5 genotype and hsCRP level. Baseline characteristics are given in the second column of Table 1. There were no differences in baseline characteristics between CCR5Δ32 carriers and noncarriers. The causes of primary kidney disease differed between the NECOSAD and the Swedish cohort. Also, compared with the NECOSAD study population, the Swedish cohort was, by inclusion criteria, significantly younger and a lower proportion of patients started on HD. Whereas mean diastolic BP, cholesterol levels, and GFR were higher, the hemoglobin level was lower. The median follow-up was 1457 d (3.99 yr; 712 to 1826 d). The CCR5Δ32 genotype distribution did not deviate significantly from Hardy-Weinberg equilibrium (P = 0.22), and the allele frequencies did not differ from the NECOSAD cohort (P = 0.96). During 5 yr of follow-up, 80 patients died (57 cardiovascular): 64 (26%) patients without the CCR5Δ32 polymorphism and 16 (29%) of the patients carrying the CCR5Δ32 polymorphism. In accordance with the NECOSAD cohort, in the Swedish cohort noncarriers of the deletion allele with an elevated hsCRP level had an increased mortality rate (Table 2). Similarly, carriers of the deletion allele without and with an elevated hsCRP showed a lower effect estimate in survival. For cardiovascular mortality, the HRs with CCR5 ins/ins and hsCRP ≤ 10 as reference were as follows: CCR5 ins/ins and hsCRP > 10: 2.55 (95% CI: 1.44 to 4.51; P < 0.01), adjusted 1.69 (95% CI: 0.94 to 3.05; P = 0.08); CCR5Δ32 and hsCRP ≤ 10: 1.17 (95% CI: 0.44 to 3.06; P = 0.76), adjusted 1.73 (95% CI: 0.64 to 4.68; P = 0.28); CCR5Δ32 and hsCRP > 10: 1.50 (95% CI: 0.57 to 3.95; P = 0.41), adjusted 0.98 (95% CI: 0.37 to 2.61; P = 0.96). For noncardiovascular mortality, there were no statistically significant differences between the four groups.
In Table 3, the HRs for all-cause mortality for hsCRP level as a continuous variable for the two genotype groups are presented. For cardiovascular mortality, the unadjusted and adjusted risks were respectively 1.43 (95% CI: 1.17 to 1.76; P < 0.01) and 1.24 (95% CI: 1.00 to 1.55; P = 0.05) in the CCR5 ins/ins genotype group and, respectively, 1.20 (95% CI: 0.76 to 1.89; P = 0.45) and 0.55 (95% CI: 0.27 to 1.11; P = 0.09) in the CCR5Δ32 genotype group.
Combining the Swedish cohort with the NECOSAD cohort gave comparable results (with smaller CIs) as initially found in the NECOSAD population (Table 4).
Table 4.
HR (95% CI) and P values for all-cause, cardiovascular, and noncardiovascular mortality in the combined cohort
| NECOSAD and Swedish | CCR5 ins/ins and hsCRP < 10 mg/L (n = 392) | CCR5 ins/ins and hsCRP > 10 mg/L (n = 187) | CCR5Δ32 and hsCRP < 10 mg/L (n = 89) | CCR5Δ32 and hsCRP > 10 mg/L (n = 47) |
|---|---|---|---|---|
| All-cause mortality | ||||
| Crude HR | 1 | 2.46 (1.87 to 3.25) | 1.16 (0.77 to 1.76) | 1.42 (0.86 to 2.34) |
| P | <0.01 | 0.49 | 0.17 | |
| Adjusted HR | 1 | 1.73 (1.31 to 2.30) | 1.09 (0.72 to 1.66) | 1.23 (0.74 to 2.02) |
| P | <0.01 | 0.69 | 0.42 | |
| Cardiovascular mortality | ||||
| Crude HR | 1 | 2.48 (1.72 to 3.56) | 1.14 (0.66 to 1.98) | 1.36 (0.70 to 2.65) |
| P | <0.01 | 0.63 | 0.37 | |
| Adjusted HR | 1 | 1.76 (1.21 to 2.54) | 1.16 (0.67 to 2.03) | 1.15 (0.59 to 2.25) |
| P | <0.01 | 0.59 | 0.68 | |
| Noncardiovascular mortality | ||||
| Crude HR | 1 | 2.45 (1.60 to 3.74) | 1.18 (0.63 to 2.23) | 1.50 (0.71 to 3.18) |
| P | <0.01 | 0.61 | 0.29 | |
| Adjusted HR | 1 | 1.70 (1.10 to 2.62) | 1.03 (0.54 to 1.95) | 1.31 (0.62 to 2.79) |
| P | 0.02 | 0.94 | 0.48 |
Adjusted for gender, age at inclusion, cardiovascular disease, diabetes, and dialysis modality.
DISCUSSION
Our prospective study of Dutch incident dialysis patients suggests that mortality in ESRD associated with elevated serum hsCRP concentrations is modulated by the CCR5Δ32 polymorphism. Elevated hsCRP was significantly associated with decreased survival in patients who were homozygous for the major allele and thus had a functional receptor. Interestingly, even although elevated hsCRP conferred an increased hazard for mortality in carriers of the deletion allele, this was one-third the magnitude observed in noncarriers and NS. For cardiovascular mortality, this suggested protective effect of a deletion allele was even more pronounced. For all-cause mortality, this finding was replicated in an independent Swedish cohort of ESRD patients. HRs for cardiovascular mortality showed the same trend, although not statistically significant. Also, when analyzed on a continuous scale, hsCRP levels were associated with (cardiovascular) mortality in patients with the CCR5 ins/ins genotype and not in patients carrying a deletion allele. These results suggest that CCR5 deficiency (implicated by one or two copies of a nonfunctional CCR5 gene) attenuates the adverse effects of a persistent inflammatory state that may lead to mortality in ESRD patients. Considering the importance of the inflammatory state for prognosis and the current developments in pharmacotherapy, this finding may have considerable potential clinical implications.
Blocking CCR5 has been proposed as a novel therapeutic approach for cardiovascular conditions by interfering with systemic inflammation. This concept is supported by an animal study by Veillard et al. 24, in which treatment of hypercholesterolemic mice with the CCR5 antagonist Met-RANTES reduced progression of atherosclerosis. Moreover, Schober et al.25 demonstrated that treatment of apoE-deficient mice with Met-RANTES reduced neointimal plaque area and macrophage infiltration. Finally, treatment with TAK-799, a CCR5 chemokine receptor antagonist, reduced lesion development in a collar-induced carotid artery atherosclerosis model.26
To our knowledge, this is the first study investigating the interaction between CCR5 genotype, elevated (hs)CRP levels, and (cardiovascular) mortality in dialysis patients. In line with our data, studies in other populations generally have shown an association of CCR5Δ32 with a favorable cardiovascular outcome, albeit not invariably so.27 In males, the presence of CCR5Δ32 is associated with reduced incidence of myocardial infarction at a younger age.19 Another study suggested that the CCR5Δ32 genotype protected against coronary heart disease.21 In the Nurses’ Health study, a possible association between the CCR5Δ32 polymorphism and a reduced incidence of early-onset coronary heart disease was found.20 Our data extend these findings in a population with a particularly high cardiovascular mortality, namely ESRD, showing that CCR5Δ32 attenuates the risk of mortality in patients with systemic inflammation as determined by a high hsCRP. Replication in an independent, somewhat different ESRD population supports the robustness of this finding. In patients without systemic inflammation, the CCR5Δ32 had no effect on mortality risk, strongly suggesting that the CCR5Δ32, possibly by receptor deficiency, ameliorates the downstream effects of systemic inflammation.
Our data elicit the hypothesis that CCR5Δ32 modulates inflammation-driven atherosclerosis. Whereas our study does not allow any conclusion on the underlying mechanisms of the impact of CCR5Δ32 on inflammation-driven mortality, several inferences can be made. Chemokines play an important role in the recruitment of inflammatory cells mediated through chemokine receptors. The presence of a dysfunctional CCR5 chemokine receptor can modulate inflammation. This was first described in HIV-infected persons. Homozygous carriers of the CCR5Δ32 polymorphism were protected against HIV infection, whereas heterozygotes showed a delayed progression to AIDS compared with noncarriers.28–30 As inflammation is involved in atherogenesis, it has been suggested that CCR5 ligands, CCR5, and genetic variation in CCR5 could play a role in the pathogenesis of vascular disease.10–14,24,31–34 Thus, it can be hypothesized that CCR5 deficiency in carriers of the CCR5Δ32 polymorphism could be beneficial during an enhanced state of vascular inflammation. We used elevated hsCRP levels as an indicator of the state of (vascular) inflammation in our population, a phenotype that was shown to be a predictor of CVD and mortality in dialysis patients.5,6,35 Little is known about the relationship between CCR5 or its ligands and CRP during ESRD or dialysis. However, in one study, it has been observed that acute transcription of anti-inflammatory cytokines following HD was significantly lower in patients with high serum CRP levels.36 In contrast, transcription of proinflammatory cytokines (including IL-1β and TNF-α) was induced in equal concentrations, regardless of baseline CRP levels. IL-1β and TNF-α are known to induce expression of the CCR5 ligands CCL4 and CCL5 in renal disease.37,38 Thus, the diminished up regulation of anti-inflammatory cytokines, together with enhanced expression of cytokines that stimulate CCR5-mediated inflammation, could underlie the observed hsCRP-dependent increased mortality in dialysis patients homozygous for a functional CCR5 gene in our study.
A potential limitation of our study is that we were not able to study all included patients from the NECOSAD cohort, because data on hsCRP and/or CCR5 were not available in a subset of the patients. In this subset, mortality rate was slightly higher than in the patients available for analysis. In a single-cohort study, selection bias and population stratification cannot be excluded, despite the presence of Hardy-Weinberg equilibrium and despite confirmation of results when only the Caucasian subjects were analyzed. However, independent replication on a Swedish cohort, which was in Hardy Weinberg equilibrium as well and in which data were available for all patients, confirmed our results, hereby showing the robustness of our findings. As, moreover, the number of patients in the CCR5Δ32 groups was small, we did an analysis on the two cohorts combined, leading to the same results.
We used a single value of hsCRP in our analysis. It is possible that the hsCRP level was elevated because of acute infectious reasons and not due to a persistent inflammatory state. To exclude patients with an acute infection, we redid the analyses by excluding patients with a CRP ≥ 50 mg/L. This resulted in comparable HRs for all-cause and cardiovascular mortality. For noncardiovascular mortality, the HRs were comparable with those found for cardiovascular mortality, thereby underlining the importance of the CCR5 genotype in chronic, inflammation-driven mortality.
We used the previously used hsCRP cut-off point of 10 mg/L to divide patients in a group with or without a chronic inflammatory state. This cut-off point could potentially have influenced our results. However, analyzing the interaction between CCR5 genotype and CRP level with the median hsCRP level as cut-off did not alter the results. Moreover, when analyzing hsCRP as a continuous variable, the hsCRP level seemed to influence mortality and cardiovascular mortality in the CCR5 ins/ins genotype group and not in the CCR5Δ32 genotype group.
Adjustments in genetic association studies could potentially introduce interference in the causal pathway and thereby bias through overadjustment.39 For this reason, we reported unadjusted HRs in the manuscript. However, as we studied CCR5 as an effect modificator of the association between CRP levels and mortality, and CRP levels can be affected by confounding variables, such as CVD, diabetes, and dialysis modality, we also reported adjusted HRs in the manuscript.
Finally, we only studied a single polymorphism. The observed effect does not necessarily causally implicate this particular polymorphism, but could be due to another variant in linkage disequilibrium with the studied deletion. This variant does not necessarily have to be located within the CCR5 gene, since patterns of linkage disequilibrium do not follow the patterns of genes in the genome. This is a point that deserves further investigation. However, our efforts as reported in the present study were not toward in-depth characterization of the gene locus, but rather to investigate whether the impact of the polymorphism, reported in the literature to be associated with mortality, was modified by inflammatory status.
In conclusion, our results indicate that CCR5 genotype modifies the prognosis of mortality associated with inflammation in incident dialysis patients. Data from the literature suggest that this could be due to CCR5 deficiency (implicated by one or two copies of a nonfunctional CCR5 gene). This could lead to attenuation of the adverse effects of a persistent inflammatory state that is involved in increased all-cause and cardiovascular mortality in dialysis patients. Recently CCR5 blockade has become feasible in humans.40 Our data suggest that it may be worthwhile to study whether pharmacologic blockade of CCR5 could have therapeutic benefits in dialysis patients with persistent inflammation.
CONCISE METHODS
Patients
This study is part of the NECOSAD. This is a multicenter prospective follow-up study in which new ESRD patients from 38 Dutch dialysis centers are included at start of chronic dialysis treatment. All patients gave informed consent, and all local medical ethics committees gave their approval.
Eligibility criteria were: 18 yr and older and no previous renal replacement therapy. For the current analyses, we used data from patients included between July 1998 and December 2001 in 23 centers that approved of DNA analysis and had a follow-up of at least 3 mo. An additional criterium was that the response rate was more than 50% for DNA analyses of patients in these centers.
Demographic and Clinical Data
The collected data included: age, gender, smoking, primary kidney disease, systolic and diastolic BP, comorbidity, dialysis modality, and medication use. We obtained blood and 24-h urine samples at 3 mo after start of dialysis. We determined plasma hemoglobin, creatinine, urea, albumin, and cholesterol levels. We measured hsCRP by means of particle-enhanced immunonephelometry using a standard CardioPhase hsCRP for BNII (Dade Behring Holding GmbH, Liederbach, Germany). Detection limit was 0.1 mg/L, precision 0.1 mg/L.6 In addition, we collected blood for DNA analysis. We also analyzed urea and creatinine in urine samples. We calculated renal function, expressed as GFR, as the mean of creatinine and urea clearance, corrected for body surface area (ml/min/1.73 m2). We followed patients at 3 and 6 mo after start of dialysis and thereafter every 6 mo until date of death or date of censoring, i.e., transfer to a nonparticipating dialysis center, withdrawal from the study, or end of the follow-up period in June 2007. We did not censor patients receiving a kidney transplant.
Clinical Definitions
We classified primary kidney disease and causes of death according to the codes of the European Renal Association–European Dialysis and Transplantation Association (ERA-EDTA).41 The following codes were designated as cardiovascular mortality: myocardial ischemia and infarction; cardiac failure/fluid overload/pulmonary edema; cardiac arrest, cause unknown; cerebrovascular accident; hemorrhage from ruptured vascular aneurysm; mesenteric infarction; hyperkalemia; hypokalemia; cause of death uncertain/unknown. We grouped patients into four classes of primary kidney disease: glomerulonephritis, diabetes mellitus, renal vascular disease, and other kidney diseases. Other kidney diseases consisted of patients with interstitial nephritis, polycystic kidney diseases, other multisystem diseases, and unknown diseases.
Patients with a history of angina pectoris, myocardial infarction, heart failure, ischemic stroke, or claudication at time of inclusion were defined as having cardiovascular disease. Smoking habits are defined as never, current, or former smoker.
Systemic inflammation was defined as hsCRP concentration above 10 mg/L. This cut-off point was previously used to divide patients in a group with or without a chronic inflammatory state.6 In ESRD patients, this cut-off point for CRP level has been shown to be the best with regard to the prediction of survival.35
CCR5 Polymorphism
The CCR5 gene is located on chromosome 3p21. We determined the genotypes with a PCR-based allelic discrimination assay using primers (Life Technologies, Foster City, CA) and allele-specific probes (PE Biosystems, Foster City, CA) as described previously.42
We divided patients into two groups based on their CCR5Δ32 genotype: those homozygous for the major allele (functional receptor), and those with one or two deletion alleles (dysfunctional receptor). Patients homo- or heterozygous for the deletion allele were clustered, since the presence of one minor allele has already been associated with reduced receptor function.43
Replication
For independent replication of the NECOSAD study, we analyzed results data from a Swedish cohort of ESRD patients. This is a prospective follow-up study in patients with ESRD close to the start of renal replacement therapy done in Sweden. We included only patients older than 18 yr and younger than 70 yr. We did not include patients who were hospitalized with clinical signs of infection and/or acute vasculitis at time of admission. We examined patients and collected blood close to start of dialysis treatment. A detailed description was published previously.44 We performed genotype analyses by PCR amplification followed by size separation of the resulting fragments on a 3% agarose gel and visualization by ethiduim bromide staining. The resulting fragments were 241 and 209 bp for the ins and the del allele, respectively. Patients were followed until date of death or date of censoring, i.e., end of the follow-up period (March 2007). Definitions for cardiovascular disease, cardiovascular death, and systemic inflammation were the same as described above for the NECOSAD cohort.
Statistical Analysis
We calculated Hardy-Weinberg equilibrium using the gene counting method. We tested differences between groups with the chi-square test for dichotomous and categorical variables and one-way ANOVA for continuous variables. The main outcome measure was all-cause and cardiovascular mortality within 5 yr of follow-up. We determined survival curves with the Kaplan-Meier method. We used the log rank test to determine differences between survival curves. We calculated unadjusted, adjusted (for gender, age at inclusion, cardiovascular disease, diabetes mellitus, and dialysis modality) HRs for all-cause, cardiovascular, and noncardiovascular mortality by Cox's proportional-hazard analysis.
To study the modification of the effect of hsCRP on mortality by the CCR5 genotype according to Rothmans approach of studying effect modification (i.e., additive interaction)45 between hsCRP and CCR5 genotype, we defined a new variable with four categories: CCR5 ins/ins with low hsCRP (≤10 mg/L), CCR5 ins/ins with high hsCRP (>10 mg/L), CCR5Δ32 with low hsCRP (≤10 mg/L), and CCR5Δ32 with high hsCRP level (>10 mg/L).
We also examined this association for hsCRP level as a continuous variable by studying its effect on mortality within the two separate CCR5 genotype groups. Because of the skewed distribution, we first log-transformed hsCRP.
Finally, to increase the number of patients in the different categories and hereby power, we combined the two cohorts. For the combined cohort, we performed Cox's proportional-hazard analysis also.
We used SPSS statistical software (version 14.0; SPSS, Chicago, Illinois) for all statistical analyses.
DISCLOSURES
None.
Acknowledgments
Parts of this work have previously been published in abstract form and presented at the annual meeting of the American Society of Nephrology 2006 (“CCR5 delta32 is associated with increased survival in dialysis patients with systemic inflammation.” FLH Muntinghe, MW Zuurman, DC Grootendorst, EW Boeschoten, RT Krediet, GJ Navis, FW Dekker. J Am Soc Nephrol 2006. Poster presentation at ASN 2006).
This work was supported by the applied Genomic Strategies for Treatment and Prevention of Cardiovascular Death in Uremia and End Stage Renal Disease (GENECURE) project (www.genecure.eu), a Specific Targeted Research or Innovation Project, funded by the European Commission under the Sixth Framework Programme as FP6–037696. GENECURE is led by Prof. Dr. G.J. Navis, University Medical Center Groningen in Groningen, The Netherlands; its goal is to elucidate the genomic basis of cardiovascular complications in renal disease. GENECURE is hosted by the Renal Genome Network (ReGeNet) project (www.regenet.eu), a pan European network of clinicians and scientists from academia and industry seeking to generate and facilitate genetic and genomic studies to the clinical benefit of the renal patient.
Juan Jesus Carrero is supported by the ERA-EDTA.
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Do Genes Allow Inflammation to Kill or Not to Kill?” on pages 1429–1431
Disclaimer: This publication has been produced with the assistance of the European Union. The content of this publication is the sole responsibility of the authors and can in no way be taken to reflect the views of the European Union.
References
- 1.Foley RN, Parfrey PS, Sarnak MJ: Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol 9: S16–S23, 1998 [PubMed] [Google Scholar]
- 2.Lacson E, Jr., Levin NW: C-reactive protein and end-stage renal disease. Semin Dial 17: 438–448, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, III, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr., Taubert K, Tracy RP, Vinicor F: Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 107: 499–511, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Kovacs A, Tornvall P, Nilsson R, Tegner J, Hamsten A, Bjorkegren J: Human C-reactive protein slows atherosclerosis development in a mouse model with human-like hypercholesterolemia. Proc Natl Acad Sci U S A 104: 13768–13773, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.den Elzen WP, van Manen JG, Boeschoten EW, Krediet RT, Dekker FW: The effect of single and repeatedly high concentrations of C-reactive protein on cardiovascular and non-cardiovascular mortality in patients starting with dialysis. Nephrol Dial Transplant 21: 1588–1595, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Grootendorst DC, de Jager DJ, Brandenburg VM, Boeschoten EW, Krediet RT, Dekker FW: Excellent agreement between C-reactive protein measurement methods in end-stage renal disease patients—no additional power for mortality prediction with high-sensitivity CRP. Nephrol Dial Transplant 22: 3277–3284, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Racki S, Zaputovic L, Mavric Z, Vujicic B, Dvornik S: C-reactive protein is a strong predictor of mortality in hemodialysis patients. Ren Fail 28: 427–433, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Stenvinkel P: Inflammation in end-stage renal disease: The hidden enemy. Nephrology (Carlton) 11: 36–41, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Zimmermann J, Herrlinger S, Pruy A, Metzger T, Wanner C: Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int 55: 648–658, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Bursill CA, Channon KM, Greaves DR: The role of chemokines in atherosclerosis: Recent evidence from experimental models and population genetics. Curr Opin Lipidol 15: 145–149, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Charo IF, Taubman MB: Chemokines in the pathogenesis of vascular disease. Circ Res 95: 858–866, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Mach F: The role of chemokines in atherosclerosis. Curr Atheroscler Rep 3: 243–251, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Reape TJ, Groot PH: Chemokines and atherosclerosis. Atherosclerosis 147: 213–225, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Braunersreuther V, Mach F, Steffens S: The specific role of chemokines in atherosclerosis. Thromb Haemost 97: 714–721, 2007 [PubMed] [Google Scholar]
- 15.Raport CJ, Gosling J, Schweickart VL, Gray PW, Charo IF: Molecular cloning and functional characterization of a novel human CC chemokine receptor (CCR5) for RANTES, MIP-1beta, and MIP-1alpha. J Biol Chem 271: 17161–17166, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Schecter AD, Calderon TM, Berman AB, McManus CM, Fallon JT, Rossikhina M, Zhao W, Christ G, Berman JW, Taubman MB: Human vascular smooth muscle cells possess functional CCR5. J Biol Chem 275: 5466–5471, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Weber C, Schober A, Zernecke A: Chemokines: Key regulators of mononuclear cell recruitment in atherosclerotic vascular disease. Arterioscler Thromb Vasc Biol 24: 1997–2008, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Wilcox JN, Nelken NA, Coughlin SR, Gordon D, Schall TJ: Local expression of inflammatory cytokines in human atherosclerotic plaques. J Atheroscler Thromb 1 Suppl 1: S10–S13, 1994 [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez P, Alvarez R, Batalla A, Reguero JR, Alvarez V, Astudillo A, Cubero GI, Cortina A, Coto E: Genetic variation at the chemokine receptors CCR5/CCR2 in myocardial infarction. Genes Immun 2: 191–195, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Pai JK, Kraft P, Cannuscio CC, Manson JE, Rexrode KM, Albert CM, Hunter D, Rimm EB: Polymorphisms in the CC-chemokine receptor-2 (CCR2) and -5 (CCR5) genes and risk of coronary heart disease among US women. Atherosclerosis 186: 132–139, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Szalai C, Duba J, Prohaszka Z, Kalina A, Szabo T, Nagy B, Horvath L, Csaszar A: Involvement of polymorphisms in the chemokine system in the susceptibility for coronary artery disease (CAD). Coincidence of elevated Lp (a) and MCP-1–2518 G/G genotype in CAD patients. Atherosclerosis 158: 233–239, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Liu R, Paxton WA, Choe S, Ceradini D, Martin SR, Horuk R, MacDonald ME, Stuhlmann H, Koup RA, Landau NR: Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 86: 367–377, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Venkatesan S, Petrovic A, Van Ryk DI, Locati M, Weissman D, Murphy PM: Reduced cell surface expression of CCR5 in CCR5Delta 32 heterozygotes is mediated by gene dosage, rather than by receptor sequestration. J Biol Chem 277: 2287–2301, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Veillard NR, Kwak B, Pelli G, Mulhaupt F, James RW, Proudfoot AE, Mach F: Antagonism of RANTES receptors reduces atherosclerotic plaque formation in mice. Circ Res 94: 253–261, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Schober A, Manka D, von Hundelshausen P, Huo Y, Hanrath P, Sarembock IJ, Ley K, Weber C: Deposition of platelet RANTES triggering monocyte recruitment requires P-selectin and is involved in neointima formation after arterial injury. Circulation 106: 1523–1529, 2002 [DOI] [PubMed] [Google Scholar]
- 26.van Wanrooij EJ, Happe H, Hauer AD, de Vos P, Imanishi T, Fujiwara H, van Berkel TJ, Kuiper J: HIV entry inhibitor TAK-779 attenuates atherogenesis in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol 25: 2642–2647, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Simeoni E, Winkelmann BR, Hoffmann MM, Fleury S, Ruiz J, Kappenberger L, Marz W, Vassalli G: Association of RANTES G-403A gene polymorphism with increased risk of coronary arteriosclerosis. Eur Heart J 25: 1438–1446, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Dean M, Carrington M, Winkler C, Huttley GA, Smith MW, Allikmets R, Goedert JJ, Buchbinder SP, Vittinghoff E, Gomperts E, Donfield S, Vlahov D, Kaslow R, Saah A, Rinaldo C, Detels R, O'Brien SJ: Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science 273: 1856–1862, 1996 [DOI] [PubMed] [Google Scholar]
- 29.Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, Paxton WA: HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381: 667–673, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Samson M, Libert F, Doranz BJ, Rucker J, Liesnard C, Farber CM, Saragosti S, Lapoumeroulie C, Cognaux J, Forceille C, Muyldermans G, Verhofstede C, Burtonboy G, Georges M, Imai T, Rana S, Yi Y, Smyth RJ, Collman RG, Doms RW, Vassart G, Parmentier M: Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 382: 722–725, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Braunersreuther V, Zernecke A, Arnaud C, Liehn EA, Steffens S, Shagdarsuren E, Bidzhekov K, Burger F, Pelli G, Luckow B, Mach F, Weber C: Ccr5 but not Ccr1 deficiency reduces development of diet-induced atherosclerosis in mice. Arterioscler Thromb Vasc Biol 27: 373–379, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Potteaux S, Combadiere C, Esposito B, Lecureuil C, it-Oufella H, Merval R, Ardouin P, Tedgui A, Mallat Z: Role of bone marrow-derived CC-chemokine receptor 5 in the development of atherosclerosis of low-density lipoprotein receptor knockout mice. Arterioscler Thromb Vasc Biol 26: 1858–1863, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Quinones MP, Martinez HG, Jimenez F, Estrada CA, Dudley M, Willmon O, Kulkarni H, Reddick RL, Fernandes G, Kuziel WA, Ahuja SK, Ahuja SS: CC chemokine receptor 5 influences late-stage atherosclerosis. Atherosclerosis 195: e92–e103, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Zernecke A, Liehn EA, Gao JL, Kuziel WA, Murphy PM, Weber C: Deficiency in CCR5 but not CCR1 protects against neointima formation in atherosclerosis-prone mice: Involvement of IL-10. Blood 107: 4240–4243, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Stenvinkel P, Wanner C, Metzger T, Heimburger O, Mallamaci F, Tripepi G, Malatino L, Zoccali C: Inflammation and outcome in end-stage renal failure: Does female gender constitute a survival advantage? Kidney Int 62: 1791–1798, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Friedrich B, Alexander D, Janessa A, Haring HU, Lang F, Risler T: Acute effects of hemodialysis on cytokine transcription profiles: Evidence for C-reactive protein-dependency of mediator induction. Kidney Int 70: 2124–2130, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Schwarz M, Radeke HH, Resch K, Uciechowski P: Lymphocyte-derived cytokines induce sequential expression of monocyte- and T cell-specific chemokines in human mesangial cells. Kidney Int 52: 1521–1531, 1997 [DOI] [PubMed] [Google Scholar]
- 38.van Kooten C, van der Linde X, Woltman AM, van Es LA, Daha MR: Synergistic effect of interleukin-1 and CD40L on the activation of human renal tubular epithelial cells. Kidney Int 56: 41–51, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Jager KJ, Zoccali C, Macleod A, Dekker FW: Confounding: What it is and how to deal with it. Kidney Int 73: 256–260, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Fatkenheuer G, Pozniak AL, Johnson MA, Plettenberg A, Staszewski S, Hoepelman AI, Saag MS, Goebel FD, Rockstroh JK, Dezube BJ, Jenkins TM, Medhurst C, Sullivan JF, Ridgway C, Abel S, James IT, Youle M, van der RE: Efficacy of short-term monotherapy with maraviroc, a new CCR5 antagonist, in patients infected with HIV-1. Nat Med 11: 1170–1172, 2005 [DOI] [PubMed] [Google Scholar]
- 41.van Dijk PC, Jager KJ, de Charro F, Collart F, Cornet R, Dekker FW, Gronhagen-Riska C, Kramar R, Leivestad T, Simpson K, Briggs JD: Renal replacement therapy in Europe: The results of a collaborative effort by the ERA-EDTA registry and six national or regional registries. Nephrol Dial Transplant 16: 1120–1129, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Clark VJ, Metheny N, Dean M, Peterson RJ: Statistical estimation and pedigree analysis of CCR2-CCR5 haplotypes. Hum Genet 108: 484–493, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Benkirane M, Jin DY, Chun RF, Koup RA, Jeang KT: Mechanism of transdominant inhibition of CCR5-mediated HIV-1 infection by ccr5delta32. J Biol Chem 272: 30603–30606, 1997 [DOI] [PubMed] [Google Scholar]
- 44.Stenvinkel P, Heimburger O, Paultre F, Diczfalusy U, Wang T, Berglund L, Jogestrand T: Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int 55: 1899–1911, 1999 [DOI] [PubMed] [Google Scholar]
- 45.Rothman KJ: Measuring interactions. In: Epidemiology, New York, Oxford University Press, 2002, pp 168–180