Abstract
In polycystic kidney disease (PKD), genetic mutations in polycystin 1 and 2 lead to defective intracellular trafficking of calcium, thereby decreasing intracellular calcium and altering cAMP signaling to favor proliferation. We hypothesized that calcimimetics, allosteric modulators of the calcium-sensing receptor, would reduce cyst growth by increasing intracellular calcium. We randomly assigned 20-wk-old male rats with a form of autosomal dominant PKD (heterozygote Cy/+) to one of four groups for 14 to 18 wk of treatment: (group 1) no treatment; (group 2) calcimimetic R-568 formulated in the diet; (group 3) R-568 plus calcium-supplemented drinking water (R-568 plus Ca); or (group 4) Ca-supplemented drinking water with a normal diet (Ca). Severity of PKD did not progress in any of the three treatment groups between 34 and 38 wk. Compared with no treatment, cyst growth was unaffected at 34 wk by all treatments, but cyst volume and fibrosis were lower at 38 wk, with both R-568-treated groups demonstrating a greater reduction than calcium alone. Between 34 and 38 wk, the total kidney weight increased by 78% in the control group (P < 0.001) and by 19% in the Ca group (P < 0.01), but did not increase in the R-568 or R-568 plus Ca groups, suggesting inhibition of disease progression despite equivalent suppression of parathyroid hormone. In summary, treatment of hyperparathyroidism halts late-stage progression of rodent cystic kidney disease. The benefit of R-568 alone suggests calcium-sensing receptor modulation may have additional inhibitory effects on late-stage cyst growth resulting from a direct modulation of intracellular calcium.
Hepatorenal fibrocystic diseases, including polycystic kidney disease (PKD), are a group of inherited conditions associated with the development of renal cysts. This renal pathology can induce renal demise requiring renal replacement therapy at an annual cost of approximately two billion dollars in the United States. In recent years, there have been significant gains in our understanding of the processes involved in the development of renal cysts, with new knowledge of the importance of cyclic AMP from G-protein receptors and intracellular calcium signaling in the pathogenesis of cyst growth.1 In PKD, genetic mutations in polycystin 1 and 2 lead to defects in the intracellular trafficking of calcium. This results in decreased intracellular calcium leading to alterations in cAMP signaling pathways that are pro-proliferative.1
Extracellular calcium is tightly regulated, principally by the action of parathyroid hormone (PTH). When ionized calcium levels decrease, PTH secretion is stimulated and restores serum levels by increasing renal calcium reabsorption, bone calcium resorption, and increased intestinal calcium absorption via calcitriol. The actions of PTH and calcitriol prevent even minor decreases in serum calcium in the setting of normal kidney function. In the setting of kidney disease, there is hyperphosphatemia, decreased calcitriol production, and hypocalcemia. Together, these contribute to the development of secondary hyperparathyroidism, a common cause of morbidity and mortality in dialysis patients.2 PTH acts via the parathyroid receptor-1 (PTH1R), which is found in proximal convoluted and straight tubules, the cortical thick ascending limb, the distal convoluted tubules, and the cortical collecting duct.3 The receptor is a Gs-protein linked receptor that increases calcium reabsorption, phosphate excretion, and increases urinary excretion of cAMP.3
The synthesis and secretion of PTH from the parathyroid gland is regulated by the calcium-sensing receptor (CaR), a seven membrane-spanning G-protein coupled receptor. When activated by increases in serum ionized calcium, the CaR acts through the Gi complex to inhibit cAMP generation and increase intracellular calcium leading to a decrease in PTH secretion.4 Calcimimetics, which are allosteric modulators of the CaR, effectively lower PTH secretion in secondary hyperparathyroidism.5 In addition to the parathyroid gland, the CaR is also localized in other tissues, including the kidney, where its role is not as clear. Renal expression of the CaR has been localized throughout the nephron in similar, but not identical, locations of the PTH1R.3,6 In the thick ascending limb, the CaR is located basolaterally and senses changes in extracellular calcium that modify cell signaling and reduce calcium reabsorption. The CaR is located apically in the inner medullary collecting duct and appears to sense increased luminal calcium and decreases arginine vasopressin (AVP)-dependent aquaporin-2 expression, perhaps to dilute the urine and prevent hypercalcuria.7 Activation of the CaR increases intracellular calcium, which may counteract the abnormally low intracellular calcium in cystic epithelia.
Progression of the autosomal dominant PKD (ADPKD) leads to chronic kidney disease (CKD) in association with hyperparathyroidism in humans and the male Cy/+ rat model with a form of ADPKD.8 The male Cy/+ rat develops a persistent azotemia starting at about 10 wk of age, with progressive secondary hyperthyroidism and uremia until demise about 40 wks. 8,9 In the late stage of this rodent form of ADPKD, a variable number of large cysts develop that are surrounded by a fibrotic interstitium,9 factors directly associated with renal demise.
We hypothesized that the use of the calcimimetic R-568 would slow or halt the progression of the cystic disease by directly increasing intracellular calcium and/or reducing PTH secretion in the secondary hyperparathyroidism of CKD. In the present study, R-568 was administered to male Cy/+ rats from 20 to 34 or from 20 to 38 wk of age. This treatment was associated with a reduction in the cystic pathology seen at 38 wk, but not 34 wk. R-568 appears to inhibit the development of the late, relatively large renal cysts observed with prolonged CKD and the fibrosis in rat ADPKD that is associated with renal demise.
RESULTS
The data from the present study are found in Table 1. As expected and previously reported,10 R-568 decreased PTH concentrations (P < 0.05) with reduction in serum calcium levels (38 wk control, 9.8 ± 0.8 mg/dl versus R-568, 7.7 ± 0.7 mg/dl, P ≤ 0.05). The addition of calcium to the drinking water further decreased the PTH (Table 1) and increased the serum calcium. Thus, all three interventions (R-568, R-568 plus Ca, and Ca) reduced PTH, but only R-568 alone reduced serum calcium. These treatments therefore allow us to isolate the effects of R-568 from those that lowered PTH, which was also observed in the group receiving calcium alone.
Table 1.
Effect of R-568 on the late stage of Rat ADPKD
| Age at Termination | 34 wkn = 8 | n = 7 | n = 8 | n = 8 | 38 wkn = 7 | n = 7 | n = 8 | n = 7 |
|---|---|---|---|---|---|---|---|---|
| Group | PKD-Ctl | R-568 | R-568 Plus Ca | Ca Alone | PKD-Ctl | R-568 | R-568 Plus Ca | Ca Alone |
| Body weighta (g) | 559 ±23 | 531 ±16b | 504 ±31b | 516 ±28b | 513 ±25 | 528 ±23c | 490 ±31 | 468 ±45 |
| Kidney weighte (g) | 7.6 ±1.1 | 7.8 ±2.0 | 7.0 ±0.5 | 8.2 ±1.5 | 13.5 ±1.3 | 9.2 ±1.3b | 8.4 ±1.0b,c | 10.5 ±1.0b |
| KWe (%BW) | 1.4 ±0.2 | 1.5 ±0.18 | 1.4 ±0.2 | 1.5 ±0.3 | 2.6 ±0.3 | 1.7 ±0.3b,c | 1.7 ±0.2b,c | 2.3 ±0.3b |
| Cyst vole (cc) | 3.6 ±1.0 | 3.4 ±0.75 | 2.9 ±0.5 | 3.4 ±1.1 | 6.2 ±1.2 | 3.1 ±0.5b | 3.4 ±0.8b | 4.0 ±0.9b |
| Cyst vole (%BW) | 0.64 ±0.17 | 0.64 ±0.16 | 0.58 ±0.14 | 0.66 ±0.22 | 1.2 ±0.2 | 0.58 ±0.12b,c | 0.71 ± 0.15b | 0.85 ±0.15b |
| Fibrosis scoree | 3.1 ±0.29 | 2.6 ±0.20b | 2.7 ±0.20b | 2.8 ±0.17 | ||||
| BUNe (mg/dl) | 60 ±8 | 67 ±12 | 62 ±13 | 64 ±9 | 102 ±19 | 66 ±11b | 71 ±9b | 68 ±25b |
| Δ BUNe (mg/dl) | 20.7 ±10.3 | 22.0 ±12.5 | 21.1 ±12.3 | 22.4 ±5.3 | 53.7 ±19.9 | 24.0 ±6.6b | 23.5 ±7.9b | 30.7 ±9.2b |
| PTHa (pg/ml) | 314 ±300 | 66 ±14b | 47 ±8b,d | 64 ±30b | 953 ±855 | 92 ±76b | 52 ±6b | 36 ±30b |
Mean ± standard deviation (SD), KW, kidney weight, BW, body weight, BUN, blood urea nitrogen, ΔBUN, change in BUN from 20 wk to end point (34 or 38 wk), PTH, parathyroid hormone.
P < 0.05 at both 34 and 38 wk with treatment by analysis of variance (ANOVA) with all groups.
P < 0.05 for difference from age matched CKD control value.
P < 0.05 for difference from age matched calcium alone value.
P < 0.05 for difference from age matched R-568 alone value.
P < 0.05 at 38 wk only with treatment by ANOVA with all groups.
KW %BW, the kidney weight as a percent of the total body weight.
Cyst vol, the cyst volume in cubic centimeters (cc) determined from the cyst volume density times kidney weight.
Cyst vol %BW, the cyst volume (assuming 1 g/cc of cyst) expressed as a percent of the total body weight.
Fibrosis score, based on a qualitative, 1+ to 4+ scale.
Δ BUN, the difference in BUN value from the start of the experimental diet at 20 wk.
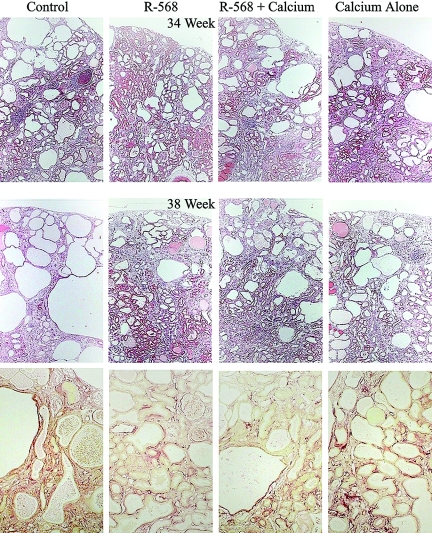
All three treatment groups showed a reduction in the severity of the CKD between 34 and 38 wk in the male Cy/+ rats, however, there were differences. At 34 wk, all three treatments were associated with a reduced total body weight compared with control. However, between 34 and 38 wk, the control group exhibited a reduction in body weight that appeared to be associated with their development of a moderately severe azotemia. Between the weeks 34 and 38, there was a 78% increase in total kidney weight in the control group (P < 0.001) and a 19% increase in those rats receiving calcium alone (P < 0.01), but no significant increase in the kidney weight in those receiving R-568 (alone or with Ca). These data demonstrate a significant late increase in cystic renal enlargement between 34 and 38 wk that was halted by the use of R-568. When renal size was expressed as a percent of total body weight, the increases in renal enlargement between 34 and 38 wk in both the control and Ca alone groups were even more pronounced (Table 1 and Figure 1). At 38 wk, the kidney weight as a percent of total body weight was reduced in the two groups receiving R-568 compared with either the CKD controls or the Ca alone group, which itself was reduced compared with the control group (Table 1 and Figure 1). This renal enlargement between 34 and 38 wk appears to be due to the expansion of the cystic space, which again was more pronounced in the control group and, to a lesser extent, the Ca alone group (Table 1 and Figure 1). At 38 wk, the change in cyst volume corresponded with the amount of fibrosis (Figure 1), suggesting a possible interrelationship between fibrosis and cyst expansion. Fibrosis generally correlated positively with reduction of renal function, with the R-568 group having a significantly reduced fibrosis and less functional loss compared with the control group at 38 wk (Table 1). Additionally, at 38 wk, the control group had the poorest renal function, as defined by increased blood urea nitrogen (BUN) levels (Table 1 and Figure 1). BUN concentrations increased significantly between when the treatments started at 20 wk (BUN levels in the low to mid 40 mg/dl range) and when the study terminated at 38 wk (Figure 1). The greatest increases in BUN levels were seen in the control and Ca alone groups (Table 1).
Figure 1.
Renal histopathology in Cy/+ rats treated with R-568. The treatments, which started at 20 wk of age, had little effect on the renal disease at 34 wk of age (top row of micrographs); however, the late-stage increase in renal pathology at 38 wk was attenuated (middle row). While the control Cy/+ kidneys progressed to develop more significant cystic pathology, including the generation of some very large cysts, treatment appeared to limit further progression of the cystic change as well as attenuate the renal fibrosis (picrosirius red-stained sections, bottom row). Renal functional deterioration was accompanied by an increase in cystic volume (i.e., larger and more numerous cysts) and the development of more significant interstitial fibrosis (red). Treating with R-568 limited the progression of cystic pathology (cysts and fibrosis) at 38 wk but not at 34 wk.
Histopathology
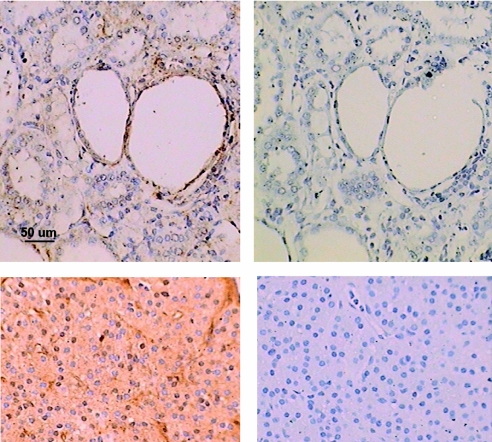
The histopathology of the cystic change and fibrosis are presented in Figure 1. At 34 wk, the relative amount of cystic change was similar across all groups (top row). However, at 38 wk, the control cystic kidneys had noticeably greater cystic change, with larger cysts (middle row) and more fibrosis (bottom row), compared with kidneys from the three treated animals groups. The CaR is described in the literature as being present in proximal tubules, distal tubules, and collecting ducts.3 In the Cy/+ rat, CaR staining was evident by immunostaining in the proximal tubules, distal tubules, and collecting ducts, and staining was prominently associated with cystic epithelial cells (Figure 2).
Figure 2.
Immunohistochemistry for the calcium-sensing receptor. While some CaR staining was found in several tubule regions, it was especially prominent in the cystic epithelium (top left). Staining was eliminated by omitting the primary antibody for both kidney (upper right) and parathyroid gland (lower right). Parathyroid gland served as a positive control and exhibited prominent staining for the CaR (lower left). All micrographs are at the same magnification.
DISCUSSION
The present study evaluated the efficacy of R-568 and/or calcium treatment from week 20 to week 34 or from week 20 to week 38 in male Cy/+ rats. Current treatments in development for PKD generally target the early phase of the disease. In contrast, our study specifically evaluated a treatment for the later stage of renal cystic pathology. Treatment with R-568 or R-568 plus Ca from 20 to 38 wk led to significantly smaller kidneys and cyst volume (as a percentage of body weight) compared with the Ca alone group (P < 0.05). The kidney cyst volumes at 38 wk in the R-568 treatment groups were not significantly different from values seen for animals similarly treated from 20 to 34 wk, suggesting that the cystic disease failed to progress after 34 wk. The R-568 treatment inhibited the development of large cysts that ordinarily only appear late in the natural history in this rat model.9 These large cysts in the Cy/+ model may parallel aspects of the human disease, including formation of the large cyst seen in humans with ADPKD and the development of acquired cysts found commonly in patients with prolonged azotemia. Thus, R-568, a calcimimetic similar to cinacalcet used in humans to treat secondary hyperparathyroidism, was effective in inhibiting further cyst growth in the late-stage cystic disease in this rat model.
The pathogenesis and progression of inherited forms of PKD appear to be significantly dependent on both intracellular cAMP and calcium.1 Lowering cAMP by the therapeutic use of vasopressin 2 receptor (V2R) antagonists inhibits disease progression,11 whereas the use of calcium channel blockers accelerates PKD progression.12 Renal epithelial cells from humans with ADPKD showed increased generation of cAMP in response to both vasopressin and PTH.13 In patients with CKD, both vasopressin and PTH are elevated. Hyperparathyroidism is also very common in CKD. A recent cohort study demonstrated that 60% of patients with CKD 3 had elevated PTH, with increasing prevalence of this abnormality as the CKD progressed.14
There are multiple possible mechanisms by which calcimimetics ameliorated cyst growth in the present study. First, by lowering PTH secretion, and therefore tubular activation of PTH1R, cAMP may be reduced, slowing the abnormal proliferation of cystic epithelial cells. Second, the elevation of cellular calcium through the activation of the CaR in cystic epithelia could correct the problems created by abnormally low cellular calcium concentrations in these cells. Last, because calcimimetics inhibit the effects of vasopressin and there is a prominent vasopressin V2 receptor abnormality in most forms of PKD, they may inhibit the PKD by modulating the activity of the vasopressin V2 receptor.
In the present study, we directly compared the effects of R-568, R-568 with calcium gluconate, and calcium gluconate alone. All three treatments were effective in reducing PTH at the 34 wk time point, but the calcium levels were significantly higher in both calcium-treated groups compared with R-568 alone. Treatment with R-568 was also associated with a smaller increase in renal cystic enlargement compared with calcium alone treatment, despite comparable PTH values between the two groups (mean ± standard deviation, 66 ± 14 versus 64 ± 30 pg/ml, respectively), suggesting that the beneficial effect of R-568 may be, at least in part, non-PTH-mediated. The exact mechanism by which R-568 inhibits the renal cystic disease will require further study. In addition, it is unclear if other treatments that alter PTH secretion in CKD would similarly impact on late-stage cyst growth.
In PKD, the current view holds that once renal function has significantly declined, no subsequent treatment will likely be beneficial. In the present study, utilizing the male Cy/+ rat model of autosomal dominant PKD at an age when renal cystic disease and azotemia was already well established, treatment was found to be efficacious in ameliorating the renal cystic disease. It is not yet clear whether this improvement in renal cystic disease would also occur in humans similarly treated. However, at present, there are no treatments for the later stages of PKD and thus, our results offer the possibility that later stages of PKD can be modified to slow or halt further deterioration of kidney function.
CONCISE METHODS
We performed these studies at the Indiana University School of Medicine with a colony of Cy rats (Han:SPRD-Cyiu) that are similar to those previously described.8,9 All animal procedures were approved by the Indiana University Institutional Animal Care and Use Committee. In this study, male and female Cy/+ heterozygotes were bred, but we used only the male Cy/+ offspring, since males have a more significant renal disease than females.9
Male Cy/+ rats were phenotyped by assessing BUN at 10 wk of age. At 20 wk of age, the animals were placed on one of the four following experimental diets: (group 1) Purina #5002 diet without any additive (this diet contains 0.862% Ca and 0.62% P); (group 2) Purina #5002 diet with 0.05% R-568 (to deliver a dosage of approximately 50 mg/kg per d); (group 3) Purina #5002 diet with 0.05% R-568 plus 2% calcium gluconate added to the drinking water; and (group 4) Purina #5002 diet without additive but 2% calcium gluconate added to the drinking water. The rats were provided food and water ad libitum and maintained on a 12 h:12 h, dark:light cycle. We evaluated the rats at either 34 or 38 wk of age (total treatment 14 or 18 wk), at which time they were weighed, anesthetized (sodium pentobarbital 100 mg/kg given intraperitoneally), and blood was collected via an intracardiac extraction. We performed a laparotomy and flushed the kidneys with saline. The left kidney was weighed and frozen, while the right kidney was weighed, and transverse sections were cut and fixed in 4% paraformaldehyde in 0.1 M phosphate buffer and then processed for paraffin embedment. We assayed sera for urea nitrogen (Sigma Urea Assay kit #640; Sigma-Aldrich, St. Louis, Missouri), PTH (enzyme-linked immunosorbent assay [ELISA] kit; ALPCO Diagnostics, Salem New Hampshire), calcium, and phosphate concentrations (Pointe Scientific, Canton, Michigan). We stained paraffin sections of the kidney with either H&E or picrosirius red (to assess fibrosis), and we photographed random regions of the cortex/outer medulla. We determined the amount of cystic change from random fields using point count stereology methods15 as described previously.16 The animals utilized for the present study represent a subset of animals in a separate publication in which the effect of R-568 on extraskeletal calcification and bone were examined.17
We performed immunohistochemistry on paraffin sections of kidney using a primary antibody to the CaR and a secondary antibody with a peroxidase chromagen product. We deparaffinized tissue sections (kidney and human parathyroid) in xylene and rehydrated them in descending alcohol concentrations. We incubated the sections for 10 min in hydrogen peroxide to block endogenous peroxidase and then with the primary antibody against the CaR (1:25 dilution; Abcam, Cambridge, Massachusetts). We then incubated the sections with the ABC staining system (Santa Cruz Biotechnology, Santa Cruz, California) followed by color development with diaminobenzidene and counterstaining with hematoxylin. We obtained negative controls by substituting the primary antibody with 1× blocking solution. We recorded images on a Nikon Coolpix 950 digital camera using a Nikon Eclipse E400 microscope (Nikon, Melville, New York).
We analyzed the data using analysis of variance for the group, and if significant, individual comparisons were made.
DISCLOSURES
D.M. and C.H. are employees of Amgen and had some input into the design, the preparation, and quality control of the R-568 and review of the manuscript. S.M. is a consultant and scientific advisor to Amgen and has received honoraria and other grant support. V.G., S.M., N.C., and M.S. have received royalties from Amgen.
Acknowledgments
This work was an investigator-initiated study supported by a grant from Amgen Inc. The design of the study, data collection, and analyses were done by V.G., S.M., N.C., and M.S.; the conduct of the study by V.G., S.M., N.C., M.S., R.S., and D.D. The authors wish to thank Faouzi Azzouz for his assistance in the statistical analyses and Michelle Murray for her secretarial assistance. In addition, the authors thank William W. Stark, Jr., PhD, and Jane Mannion, MS, (Amgen Inc) for editorial assistance in incorporating the multiple Amgen reviews into the final version of this manuscript.
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Type II Calcimimetics and Polycystic Kidney Disease: Unanswered Questions,” on pages 1421–1425.
REFERENCES
- 1.Torres VE, Harris, PC: Mechanisms of disease: Autosomal dominant and recessive polycystic kidney diseases. Nat Clin Pract Nephrol 2: 40–55; quiz 55, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Fadem SZ, Moe SM: Management of chronic kidney disease mineral-bone disorder. Adv Chronic Kidney Dis 14: 44–53, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Riccardi D, Lee WS, Lee K, Segre GV, Brown EM, Hebert SC: Localization of the extracellular Ca(2+)-sensing receptor and PTH/PTHrP receptor in rat kidney. Am J Physiol 271: F951–F956, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Brown EM: Physiology and pathophysiology of the extracellular calcium-sensing receptor. Am J Med 106: 238–253, 1999 [PubMed] [Google Scholar]
- 5.Moe SM, Chertow GM, Coburn JW, Quarles LD, Goodman WG, Block GA, Drueke TB, Cunningham J, Sherrard DJ, McCary LC, Olson KA, Turner SA, Martin KJ: Achieving NKF-K/DOQI bone metabolism and disease treatment goals with cinacalcet HCl. Kidney Int 67: 760–771, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Riccardi D, Hall AE, Chattopadhyay N, Xu JZ, Brown EM, Hebert SC: Localization of the extracellular Ca2+/polyvalent cation-sensing protein in rat kidney. Am J Physiol 274: F611–F622, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Sands JM, Naruse M, Baum M, Jo I, Hebert SC, Brown EM, Harris HW: Apical extracellular calcium/polyvalent cation-sensing receptor regulates vasopressin-elicited water permeability in rat kidney inner medullary collecting duct. J Clin Invest 99: 1399–1405, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaspareit-Rittinghausen J, Deerberg F, Wcislo A: Hereditary polycystic kidney disease. Adult polycystic kidney disease associated with renal hypertension, renal osteodystrophy, and uremic enteritis in SPRD rats. Am J Pathol 139: 693–696, 1991 [PMC free article] [PubMed] [Google Scholar]
- 9.Cowley BD, Jr., Gudapaty S, Kraybill AL, Barash BD, Harding MA, Calvet JP, Gattone 2nd, VH: Autosomal-dominant polycystic kidney disease in the rat. Kidney Int 43: 522–534, 1993 [DOI] [PubMed] [Google Scholar]
- 10.Levi R, Ben-Dov IZ, Lavi-Moshayoff V, Dinur M, Martin D, Naveh-Many T, Silver J: Increased parathyroid hormone gene expression in secondary hyperparathyroidism of experimental uremia is reversed by calcimimetics: Correlation with posttranslational modification of the trans acting factor AUF1. J Am Soc Nephrol 17: 107–112, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Gattone 2nd, VH, Wang X, Harris PC, Torres VE: Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med 9: 1323–1326, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Nagao S, Nishii K, Yoshihara D, Kurahashi H, Nagaoka K, Yamashita T, Takahashi H, Yamaguchi T, Calvet JP, Wallace DP: Calcium channel inhibition accelerates polycystic kidney disease progression in the Cy/+ rat. Kidney Int 73: 269–277, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Neufeld TK, Douglass D, Grant M, Ye M, Silva F, Nadasdy T, Grantham JJ: In vitro formation and expansion of cysts derived from human renal cortex epithelial cells. Kidney Int 41: 1222–1236, 1992 [DOI] [PubMed] [Google Scholar]
- 14.Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL: Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int 71: 31–38, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Nyengaard JR: Stereologic methods and their application in kidney research. J Am Soc Nephrol 10: 1100–1123, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Cowley BD, Jr., Rupp JC, Muessel MJ, Gattone 2nd, VH: Gender and the effect of gonadal hormones on the progression of inherited polycystic kidney disease in rats. Am J Kidney Dis 29: 265–272, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Moe SM, Seifert MF, Chen NX, Sinders RM, Chen X, Duan D, Henley C, Martin D, Gattone VH: R-568 reduces ectopic calcification in a rat model of chronic kidney disease-mineral bone disorder (CKD-MBD). Nephrol Dial Transplant March 3, 2009. [epub ahead of print] [DOI] [PubMed]