Abstract
Laminin and type IV collagen composition of the glomerular basement membrane changes during glomerular development and maturation. Although it is known that both glomerular endothelial cells and podocytes produce different laminin isoforms at the appropriate stages of development, the cellular origins for the different type IV collagen heterotrimers that appear during development are unknown. Here, immunoelectron microscopy demonstrated that endothelial cells, mesangial cells, and podocytes of immature glomeruli synthesize collagen α1α2α1(IV). However, intracellular labeling revealed that podocytes, but not endothelial or mesangial cells, contain collagen α3α4α5(IV). To evaluate the origins of collagen IV further, we transplanted embryonic kidneys from Col4a3-null mutants (Alport mice) into kidneys of newborn, wildtype mice. Hybrid glomeruli within grafts containing numerous host-derived, wildtype endothelial cells never expressed collagen α3α4α5(IV). Finally, confocal microscopy of glomeruli from infant Alport mice that had been dually labeled with anti-collagen α5(IV) and the podocyte marker anti-GLEPP1 showed immunolabeling exclusively within podocytes. Together, these results indicate that collagen α3α4α5(IV) originates solely from podocytes; therefore, glomerular Alport disease is a genetic defect that manifests specifically within this cell type.
Basement membranes are thin sheets of extracellular matrix that underlie epithelial cells, including the vascular endothelium, and surround all muscle cells, Schwann cells, and adipocytes. They are composed of polymers of laminin and type IV collagen, and also contain nidogen/entactin, and proteoglycans. During glomerulogenesis, a basement membrane beneath developing endothelial cells fuses with a separate basement membrane layer beneath differentiating podocytes, to produce the glomerular basement membrane (GBM) shared on opposing surfaces by both cell types.1
Unlike most basement membranes in the body, the laminin and collagen IV composition of the GBM changes temporally as the glomerulus develops.2 The earliest GBMs of comma- and S-shaped nephrons contain laminin α1β1γ1 (laminin 111), whereas those at later developmental stages and in adulthood contain laminin α5β2γ1 (laminin 521).2,3 Previously, we showed by postfixation immunoelectron microscopy that both endothelial cells and podocytes synthesize laminin α1 and β1 initially, and both cells then undergo a laminin isoform switch and synthesize laminin α5 and β2 as glomeruli mature.4 The mechanism and reason why laminin replacement occurs are unknown, but this may be necessary for achievement and maintenance of the highly differentiated states assumed by glomerular endothelial cells and podocytes. For example, mice with genetic deletions of laminin α5 fail to develop vascularized glomeruli and die before birth with incomplete neural tube closure and placental vascular defects.5 By transplanting embryonic day 12 (E12) metanephroi into kidney cortices of newborn wildtype mice, we partially rescued the kidney phenotype and observed well vascularized hybrid glomeruli within grafts containing wildtype endothelial cells and laminin α5 mutant podocytes.6 However, the GBMs that form within these glomerular hybrids are stratified and contain laminin α5 on their inner, subendothelial surfaces and laminin α1 on their outer, podocyte halves. Intriguingly, the podocytes fail to form foot processes, suggesting that an absence of the laminin α1-α5 isoform switch stunts podocyte differentiation.6 Unlike laminin α5, laminin β2 expression is not required for normal glomerular development. However, mice with genetic delection of the Lamb2 gene become proteinuric by 8 d after birth, display effaced foot processes, and suffer renal failure by 4 wk of age.7 Recently, a human mutation mapped to the LAMB2 locus has been linked to Pierson syndrome, a disease of variable phenotype that entails congenital nephrosis, diffuse mesangial sclerosis, perinatal or childhood renal failure, ocular abnormalities, and neuromuscular deficits.8–10
Six genetically distinct collagen type IV α chains form three different triple helical heterotrimers (protomers) in separate compartments of the mature glomerulus.11,12 A network of collagen α1α2α1(IV) is found in the mesangial matrix, collagen α3α4α5(IV) is present in the GBM, and Bowman's capsule contains networks of both α1α2α1(IV) and α5α6α5(IV).3,13 The immature GBMs of comma, S-shaped, and early capillary loop stage glomeruli all contain collagen α1α2α1(IV).2 Beginning in capillary loop stages, this network is replaced by α3α4α5(IV),2 which is the only collagen IV network normally present in mature GBM. Like laminins, the mechanism for collagen IV isoform substitution in the GBM is unknown. Nevertheless, the mature α3α4α5(IV) isoform is more resistant to proteolytic degradation than α1α2α1(IV),14 which may be critical for establishment and maintenance of glomerular permselective barrier properties.
Importantly, the collagen α3(IV) and α4(IV) chains associate exclusively with α5(IV), to form the α3α4α5(IV) protomer.15,16 The carboxyl terminal noncollagenous 1 (NC1) domains of the α chain polypeptides interact specifically to select and register appropriate chains for triple helix assembly. Collagen networks then assemble through head to head interactions between NC1 domains of two protomers, to produce a NC1 hexamer structure.11,12 Similarly, the amino termini of four protomers associate in an anti-parallel fashion, to form a three-dimensional network of polymerized collagen IV.11,12 Mutations of the human COL4A3, COL4A4, and/or COL4A5 genes prevent the proper assembly of a stable α3α4α5(IV) network, resulting in Alport disease.11,12,15,16 This disorder is characterized in kidney by an absence of collagen α3α4α5(IV) and persistence of collagen α1α2α1(IV) in the GBM, thickening and multilamination of the GBM, proteinuria, and in most cases eventually, renal failure. Mice homozygous for a Col4a3 mutation possess a strikingly similar phenotype, and therefore, represent an attractive experimental model of human Alport disease.17–19
As indicated earlier, we have previously shown that both glomerular endothelial cells and podocytes synthesize the different laminin isoforms at appropriate stages of glomerular development.4 Here, we sought to define unambiguously the cellular origins of GBM collagen α1α2α1(IV) and α3α4α5(IV) during glomerular development. Additionally, we attempted to rescue the murine Alport phenotype by grafting E12 kidneys from Col4a3 knockout mice into renal cortices of wildtype newborn hosts to generate glomerular hybrids containing wildtype endothelial cells and Alport podocytes. Our findings show that collagen α3α4α5(IV) originates only from podocytes, which indicates that Alport disease in the glomerulus is a genetic disorder of this cell type specifically.
RESULTS
Ultrastructural Localization of Collagen α1α2α1(IV) and Collagen α3α4α5(IV) in Developing Glomeruli
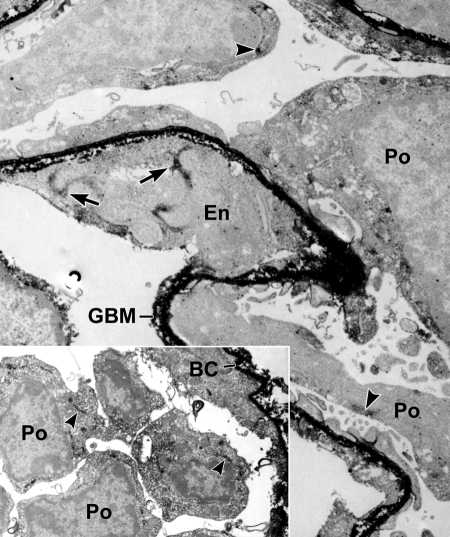
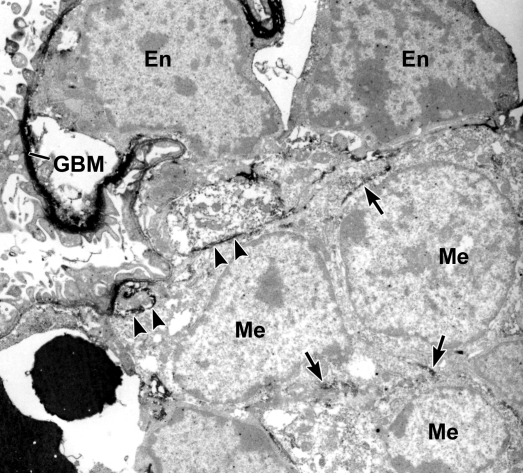
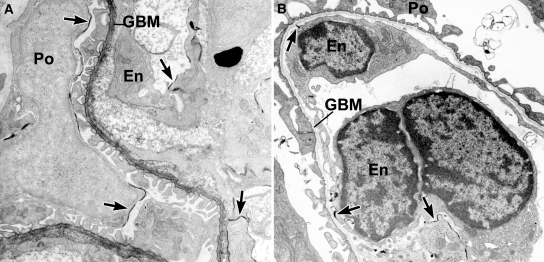
To identify cells engaged in collagen α1α2α1(IV) synthesis during glomerulogenesis, we lightly fixed developing mouse kidneys and incubated cryostat sections with anti-collagen α1α2α1(IV) and then secondary antibodies conjugated to horseradish peroxidase (HRP). After washing, sections were refixed with full-strength Karonovsky's fixative, developed for peroxidase histochemistry, postfixed with osmium, embedded, and ultrathin sectioned. As shown in Figure 1, electron dense peroxidase reaction product heavily labeled developing GBM of immature glomeruli, as well as the basement membrane of Bowman's capsule (inset). In addition, intracellular labeling was seen in both glomerular endothelial cells and podocytes (Figure 1) and developing mesangial cells (Figure 2), signifying that all three cell types were actively synthesizing collagen α1α2α1(IV).
Figure 1.
Postfixation immunoperoxidase labeling of developing glomerulus with anti-α1α2α1 collagen (IV). Both the GBM and Boman's capsule (BC in inset) basement membranes contain heavy, electron dense peroxidase reaction product. Note intracellular labeling of biosynthetic organelles in glomerular endothelial (En) cells (arrows) and podocytes (Po, arrowheads).
Figure 2.
Postfixation immunolabeling of developing glomerulus with anti-α1α2α1 collagen (IV). Immunoperoxidase labeling is observed intracellularly within mesangial cells (arrows, Me) and developing mesangial matrix (arrowheads), as well as in the GBM. En: Endothelium.
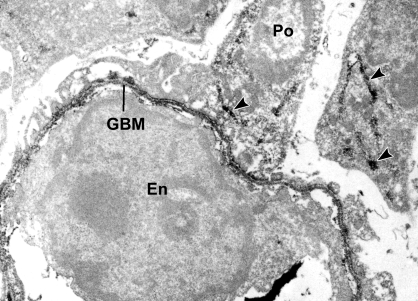
Similarly, when sections were sequentially incubated with mouse anti-α3α4α5(IV) IgG and anti-mouse IgG-HRP, the developing GBM was again labeled (Figure 2). Unlike what occurred with anti-collagen α1α2α1(IV), however, intracellular labeling with anti-α3α4α5(IV) could only be identified in podocytes (Figure 3). Moreover, the monoclonal antibody used in these immunoelectron microscopic localization experiments reacts specifically with the hexamer of NC1 domains of collagen α3α4α5(IV),20 indicating that collagen IV network polymerization was beginning to occur intracellularly. Examination of sections from several animals and multiple glomeruli at various stages of development failed to show anti-collagen α3α4α5(IV) labeling of biosynthetic organelles within glomerular endothelium or mesangial cells.
Figure 3.
Postfixation immunolabeling of developing glomerular capillary with anti-α3α4α5 collagen (IV). Note peroxidase labeling of GBM and biosynthetic organelles within podocytes (arrowheads, Po), and absence of label within the endothelium (En).
Localization of Collagen α1α2α1(IV) and Collagen α3α4α5(IV) in Hybrid Glomeruli
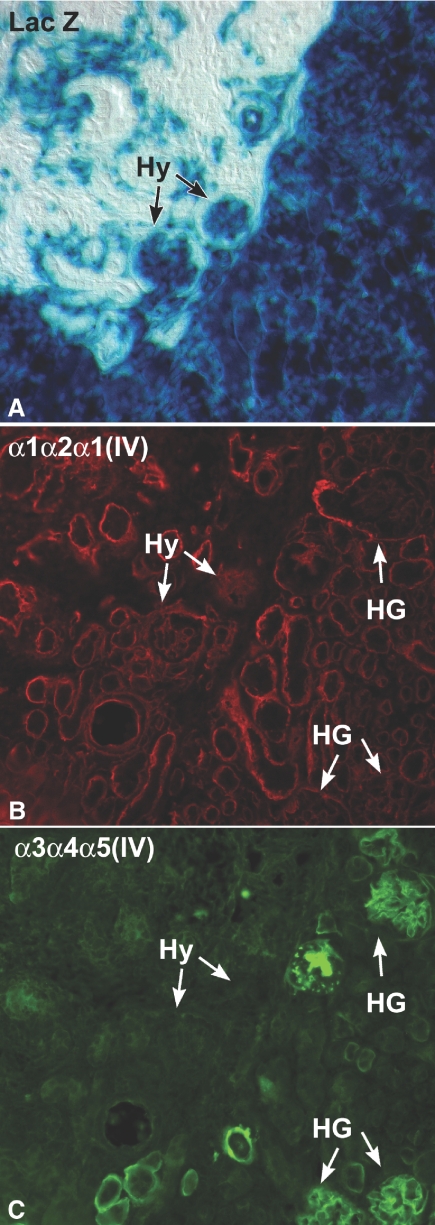
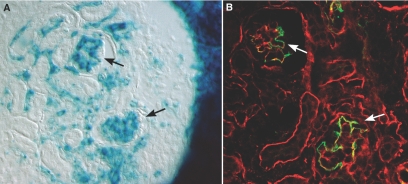
As a rule, negative immunohistochemical findings need to be interpreted cautiously. Therefore, as a further test for the exclusive, podocyte origin of collagen α3α4α5(IV), we created hybrid glomeruli by surgically grafting Col4a3 knockout metanephroi under the renal capsule of ROSA26 newborn hosts. These host mice ubiquitously express the bacterial transgene, LacZ, which therefore provides a cell lineage tracer, but they are normal otherwise and synthesize and transition GBM proteins like wildtype animals. Within 5 d after transplantation, sections of the engrafted Col4a3 knockout kidneys contained numerous LacZ-positive, host-derived endothelial cells (Figure 4A). As shown previously, this indicated the formation of hybrid glomeruli within grafts,6 which in this case contained wildtype endothelial cells and Col4a3 mutant podocytes. When serial sections were incubated with anti-collagen α1α2α1(IV), most basement membranes within the host kidney as well as within grafts labeled positively, including the immature GBMs found within the host kidney glomeruli and hybrid glomeruli within grafts (Figure 4B). In contrast, anti-collagen α3α4α5(IV) only labeled maturing GBMs in host tissue, along with basement membranes surrounding a few tubular segments (Figure 4C). All glomeruli within Alport grafts, including the glomerular hybrids containing host-derived, wildtype endothelium, were entirely negative for collagen α3α4α5(IV) (Figure 4C).
Figure 4.
ections showing grafts of Alport metanephroi into ROSA26 host kidneys. (A) Section developed for β-galactosidase activity to show LacZ transgene expression (blue). Note presence of hybrid glomeruli (Hy) containing host-derived endothelial cells. (B) Serial section immunolabeled with anti-collagen α1α2α1(IV). GBMs within hybrid glomeruli (Hy) and host glomeruli (HG) are positive, as are tubular basement membranes in host and graft tissue. (C) Same section as in (B), but immunolabeled with anti-collagen α3α4α5(IV). Only GBMs in host glomeruli (HG) and a few tubular basement membranes are labeled; hybrid glomeruli (Hy) and tubules within graft tissue are negative.
To examine the origins of collagen α3α4α5(IV) further, anti-collagen α3α4α5(IV) IgG conjugated to HRP was injected intraperitoneally into infant mice that had received intrarenal grafts of Col4a3 null metanephroi 5 d earlier. One day after injection, we removed host kidneys bearing grafts and processed tissues for the dual ultrastructural localization of the LacZ-encoded enzyme, β-galactosidase, and HRP.21 As shown in Figure 5A, host glomeruli were recognized by the expression of an electron dense, crystalline reaction product of β-galactosidase on plasma membranes of both endothelial cells and podocytes. Note that the GBMs of these glomeruli also reacted positively for injected anti-collagen α3α4α5(IV)-HRP (Figure 5A). By contrast, although many glomeruli within grafts contained β-galactosidase-positive endothelial cells, signifiying glomerular hybridity, β-galactosidase reaction product was absent on Col4a3 mutant podocytes (Figure 5B). As shown by indirect immunofluorescence microscopy earlier (Figure 3C), GBMs of these hybrid glomeruli failed to bind injected anti-collagen α3α4α5(IV)-HRP (Figure 5B).
Figure 5.
Electron microscopy of kidneys developed for the dual localization of anti-collagen α3α4α5(IV)-HRP and LacZ expression. (A) Host glomerulus contains peroxidase reaction product in the GBM and β-galactosidase reaction product (arrows) on membranes of endothelial cells (En) and podocytes (Po). (B) Hybrid glomerulus within graft lacks peroxidase reaction product in GBM and shows β-galactosidase reaction product (arrows) only on endothelial cells; podocytes are negative.
To confirm that the absence of binding of anti-collagen α3α4α5(IV) to hybrid glomeruli within Alport grafts was not due to an artifact of the kidney grafting procedure, we set up grafts in which wildtype E12 metanephroi were transplanted into newborn kidneys of bigenic Col4a3 knockout/ROSA26 hosts. In these cases, grafts again developed well and numerous hybrid glomeruli were identified containing abundant Col4a3 null/LacZ-positive endothelial cells (Figure 6A). Unlike what was seen earlier when Col4a3 knockout kidneys were grafted into wildtype hosts, however, here we readily detected the expression of collagen α3α4α5(IV) within GBMs of glomerular hybrids containing wildtype podocytes and Col4a3 null/LacZ endothelial cells (Figure 6B).
Figure 6.
Sections from graft of wildtype metanephroi into Alport/ROSA26 host. (A) Section developed for LacZ expression showing hybrid glomeruli within graft (arrows) containing host-derived, Alport/LacZ endothelial cells. (B) Section dually immunolabeled with anti-collagen α1α2α1(IV) (red) and anti-collagen α3α4α5(green) showing expression of α3α4α5(IV) in hybrid GBM.
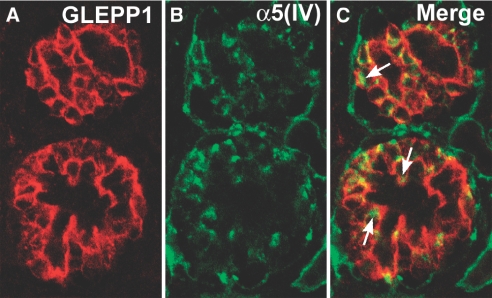
Finally, to verify further the strictly podocyte origin of collagen α3α4α5(IV) within developing GBM, cryostat sections of newborn Alport kidneys were doubly immunolabeled with anti-collagen α5(IV) and anti-GLEPP1, which binds to a podocyte membrane receptor tyrosine phosphatase. As shown in Figure 7A, anti-GLEPP1 labeled the apical and basolateral membranes of immature podocytes, as seen previously in developing kidneys.22 Although the GBMs were negative, anti-collagen α5(IV) appeared to label discrete spots within glomeruli (Figure 7B). When the two images were merged, the intraglomerular anti-collagen α5(IV) immunolabel was surrounded by the anti-GLEPP1 positive, podocyte membrane marker, signifying that the anti-collagen α5(IV) labeled podocyte cytoplasm exclusively.
Figure 7.
Section of developing Alport glomeruli immunolabeled with the podocyte plasma membrane marker, anti-GLEPP1 (A), and anti-collagen α5(IV) (B). Merged image shows intracellular labeling for anti-collagen α5(IV) within podocytes exclusively.
DISCUSSION
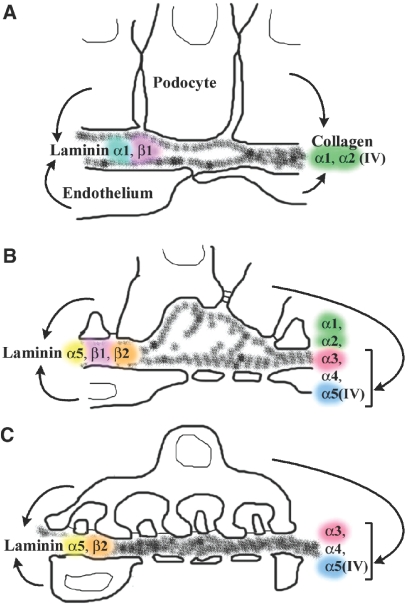
Our study reveals several new findings about the biosynthesis of collagen IV in developing glomeruli. First, postfixation immunoelectron microscopy of immature glomeruli with anti-collagen α1α2α1(IV) showed that biosynthetic organelles within endothelial cells, podocytes, and mesangial cells all labeled positively, demonstrating that all three cell types actively synthesize these collagen IV proteins during glomerular development. In contrast, the same immunolabeling technique with antibodies against collagen α3α4α5(IV) resulted only in podocyte immunolabeling; endothelial and mesangial cells were consistently negative. Second, when collagen α3(IV) null (Alport) metanephroi were grafted into wildtype newborn kidneys, hybrid glomeruli within grafts that contained host-derived, wildtype endothelial cells failed to express collagen α3α4α5(IV), providing further evidence that endothelial cells do not assemble this collagen IV heterotrimer. On the other hand, when wildtype metanephroi were grafted into newborn kidneys of collagen α3(IV) knockout hosts, collagen α3α4α5(IV) was found within GBMs of hybrid glomeruli comprised of mutant endothelial cells and wildtype podocytes. Finally, double immunolabeling of Alport glomeruli with anti-collagen α5(IV) and anti-GLEPP1 showed that anti-α5(IV) labeled only podocytes. Taken together, we conclude that both endothelial cells and podocytes synthesize collagen α1α2α1(IV) for the GBM, whereas collagen α3α4α5(IV) originates solely from podocytes (Figure 8). By extension, these results indicate that Alport disease in the glomerulus is a genetic disorder specifically of the podocyte.
Figure 8.
Diagram showing development of the glomerular capillary wall, stages of GBM assembly, and cellular origins of different laminin isoforms. Both endothelial cells and podocytes produce laminin α1 and β1, and then α5 and β2. Similarly, both cell types produce the collagen α1α2α1(IV) network. By contrast, the collagen α3α4α5(IV) network originates solely from podocytes. (Redrawn from ref. 4).
Although this is among the very first reports showing the disparate cellular origin of GBM collagen α1α2α1(IV) and collagen α3α4α5(IV), others have pursued aspects of this topic previously. For example, postembedding colloidal gold immunolabeling of 4-d-old rat kidney localized collagen α1(IV) and α2(IV) chains to developing GBM and intracellularly in both endothelial cells and podocytes, but there were no comments regarding mesangial cells.23 The same study also showed a progressive loss of GBM labeling for the collagen α1(IV) and α2(IV) chains and increased labeling for collagen α3(IV) as glomeruli matured,23 but the cellular origin of α3(IV) was not investigated. In contrast to these immunolabeling results from infant rats, in situ reverse transcription experiments on sections of adult rat kidney showed expression of collagen type IV α1 and α2 mRNAs by a minority of glomerular endothelial cells and no apparent labeling of mature mesangial cells or podocytes.24 Whether this truly reflects restricted expression of collagen α1(IV) and α2(IV) only to select endothelial cells in fully mature glomeruli, therefore, needs to be confirmed immunohistochemically.
On the other hand, podocytes have been suspected of synthesizing collagen α3α4α5(IV) for some time. Incubation of rat kidney sections with 35S-labeled anti-sense riboprobe to α4(IV) collagen mRNA showed the densest radioactive label over what appeared to be podocytes based on their location in the periphery of the glomerular tuft.25 Similarly, in mice with chronic graft versus host disease, and in rats with chronic serum sickness, increased glomerular hybridization of 35S-riboprobes to collagen α3(IV) and α4(IV) was observed over peripheral cells of sclerosing glomeruli, probably representing podocytes.26 Others have used in situ hybridization of human kidney sections with 35S-labeled anti-sense riboprobes against collagen α3, α4, and α5(IV) mRNAs.27 Their findings also showed that the riboprobes appeared concentrated mainly over presumptive podocytes.27 However, the results from all three of these in situ hybridization papers were somewhat equivocal due to the limited resolution of isotopic localization techniques and light microscopy. Additionally, using an amplified immunofluorescence approach on sections from some human patients with X-linked Alport disease, cells probably corresponding to podocytes were found to be immunolabeled with anti-α3(IV) collagen (but not with anti-α4(IV) or anti-α5(IV)).27 Another study examined sections of Wilms tumor and showed that the avascular glomeruloid bodies were labeled with antibodies against Goodpasture determinants (previously shown to be structural motifs located specifically on the NC1 domain of the α3 chain of collagen IV),16,28 providing additional evidence that this protein is a product of podocytes.29 Although results from cultured immortalized podocytes need to be interpreted carefully, experiments have also shown that these cells synthesize α3(IV) collagen in vitro. For example, addition of exogenous vascular endothelial growth factor (VEGF) to immortalized mouse podocytes increases the production of collagen α3(IV), and this is reversed by treatment with the VEGF receptor inhibitor, SU5416.30 Additionally, cultured mouse podocytes treated with angiotensin II similarly upregulate collagen α3(IV), and this was also shown to be mediated by mechanisms involving TGF-β and VEGF signaling.31 Immortalized podocytes also synthesize more α5(IV) collagen when cultured on NC1 hexamers of collagen IV purified from normal canine GBM.32
Taking together all of the previous reports and our results presented here, there is now highly compelling evidence that podocytes synthesize GBM collagen α3α4α5(IV). However, the finding that collagen α1α2α1(IV) originates from both glomerular endothelial cells and podocytes, but collagen α3α4α5(IV) comes from podocytes alone, was unexpected. We previously showed that laminin α1 and β1, and then laminin α5 and β2, are all synthesized jointly by both glomerular endothelial cells and podocytes during glomerular development.4 Furthermore, when laminin α5 knockout metanephroi are grafted into renal cortices of wildtype newborn host mice, host-derived endothelial cells migrate into glomeruli developing within grafts and these cells express laminin α5.6 This partially rescues the glomerular Lama5 null phenotype, which ordinarily results in completely avascular glomeruli. Furthermore, quantitative confocal image analysis showed that GBMs that form in these wildtype/Lama5 mutant glomerular hybrids can contain up to approximately one-half the full complement of laminin α5 immunolabel, indicating that substantial amounts of GBM laminin can stem from the endothelium.6 By contrast, and as shown here, when Alport mouse metanephroi were grafted into wildtype hosts, there was no expression of collagen α3α4α5(IV) in hybrid glomeruli within grafts, despite the presence of abundant wildtype endothelial cells, indicating once again a strictly podocyte origin for this collagen IV heterotrimer.
Finally, detailed knowledge about the glomerular sites of synthesis of the various collagen IV networks may inform new therapeutic approaches seeking to correct the underlying genetic defect in Alport syndrome. Specifically, we need to learn more about A) why an absence of the collagen α3α4α5(IV) network in the GBM leads to proteinuria, and B) whether there are any approaches that can rescue defective GBM assembly in Alport disease. There have been two reports recently in which Col4a3 knockout mice on the C57BL6 strain underwent irradiation and transplantation with wildtype bone marrow cells. In both cases, renal function improved, and there was partial restoration of immunostaining for collagen α3(IV) in glomeruli.33,34 Importantly, a small percentage of cells occupying the outer surfaces of glomerular capillary loops bore marrow donor markers, suggesting that the injected bone marrow stem cells traversed the Alport GBM and differentiated into podocytes.33,34 On the other hand, the possibility that the marrow cells might have fused with Alport podocytes could not be excluded. These studies raise many questions, including what factor(s) attract marrow-derived cells to Alport glomeruli, and what might incite a marrow cell, which ordinarily does not make collagen α3(IV), to undertake α3(IV) synthesis. In contrast, a third report regarding Col4a3 knockout mice on the 129 × 1/SvJ background that similarly underwent irradiation and bone marrow transplantation resulted in no differentiation of injected cells to renal phenotype and no delay in onset of renal failure or time of death.35 However, weekly injections of stem cells into Col4a3 knockouts did result in some histologic improvement but little change in renal function.35 To confuse matters further, another study with Col4a3 null mice in the 129 × 1/SvJ strain showed no differences in disease severity or longevity between knockouts transplanted with marrow from either wildtype or knockout animals.36 Moreover, there was no deposition of collagen α3(IV) in GBMs in either case.36 The authors provided evidence that irradiation itself, through an unknown mechanism, can contribute to the prolonged survival of Col4a3 null mice.36 As we learn more about GBM protein synthesis and secretion in general, and podocyte cell biology in particular, perhaps more effective therapies can be developed to repair collagen α3α4α5(IV) assembly by podocytes in Alport patients.
CONCISE METHODS
Animals
Embryonic day 15 (E15) CD-1 mice came from our colony originally purchased from Charles River (Wilmington, MA). We obtained both mice containing a targeted deletion of the NC1 domain of the α3(IV) collagen chain,18 and ROSA26 mice,37 which ubiquitously express the bacterial transgene, LacZ, from Jackson Laboratory (Bar Harbor, ME) (Jax mice strains 129-Col4a3tm1Dec/J and B6;129S-Gt(Rosa)26Sor/J, respectively). We crossed the Col4a3 null mice with ROSA26 transgenics to obtain mice bearing both the α3(IV) collagen deletion and the LacZ cell lineage marker. All animal experiments adhered to the NIH Guide for the Care and Use of Laboratory Animals.
Antibodies
We purchased goat anti-α1α2α1 collagen (IV) IgG from Southern Biotech (Birmingham, AL). We prepared and characterized mouse anti-α3α4α5 collagen (IV) IgG (mAb 26-20) and rat anti-α5 collagen (IV) IgG (mAb B14) as described previously.20,38 We directly conjugated the mAb 26-20 IgG to activated HRP as described previously for monoclonal anti-laminin IgGs.39 Rabbit anti-GLEPP1 antibodies were kindly provided by Dr. Roger Wiggins (University of Michigan, Ann Arbor, MI).22 We purchased chicken anti-goat IgG-AlexaFluor-594, goat anti-mouse IgG1 AlexaFluor-488, donkey anti-rabbit IgG-AlexaFluor-594, and chicken anti-rat IgG-AlexaFluor-488 from Invitrogen (Carlsbad, CA). We obtained rabbit anti-mouse IgG1-HRP from MP Biochemicals (Aurora, OH) and rabbit anti-goat IgG-HRP from Organon Teknika Corp. (West Chester, PA).
Immunohistochemistry
Postfixation immunoelectron microscopy was carried out on lightly fixed frozen sections of metanephroi from E15 CD-1 mice as described before.4 Briefly, 2-mm wedges of kidney cortices were fixed with 1% paraformaldehyde and 0.05% glutaraldehyde in 0.1 M sodium phosphate buffer, pH 7.3, for 1.5 h on ice. Tissues were washed, equilibrated with 30% sucrose in buffer, and snap frozen in tissue freezing medium (Triangle Biomedical Sciences, Durham, NC) using isopentane chilled in a dry ice-acetone bath. We collected 30-μm thick frozen sections on Thermanox coverslips (Miles Laboratories, Inc., Naperville, IL), and then air dried sections at room temperature. Sections were blocked for 30 min each in 0.5 M ammonium chloride in PBS and then with 5% goat serum-0.1% BSA in PBS. Sections were then immunolabeled with goat anti-α1α2α1 collagen IV (20 μg/ml in PBS) for 1 h, and washed with PBS. Sections were then treated with rabbit anti-goat IgG-HRP (50 μg/ml in PBS) for 1 h, washed, refixed in Karnovsky's fixative, developed for peroxidase histochemistry, and processed for electron microscopy as described previously.39 Similarly, frozen sections were immunolabeled with mouse anti-α3α4α5 collagen IV (mAb 26-20; 50 μg/ml in PBS), washed, incubated with rabbit anti-mouse IgG1-HRP (50 μg/ml), and processed for peroxidase histochemistry and electron microscopy as before.4 As controls for nonspecific peroxidase localization, sections were labeled with irrelevant primary IgGs and appropriate secondary IgG-HRP conjugates, and secondary IgG-HRP conjugates alone, and then processed for peroxidase histochemistry and electron microscopy.
Intrarenal Grafts
To obtain hybrid glomeruli, we prepared intrarenal grafts as described before.6 For these experiments, we engrafted E12 Alport metanephroi into newborn ROSA26 host kidneys (1 d after birth). Five to 7 d after implantation, we killed host animals and removed kidneys bearing grafts. Tissues were fixed with 0.2% paraformaldehyde in PBS overnight, washed three times with PBS, and then equilibrated with 30% sucrose in PBS and frozen. Serial cryostat sections were alternately developed with X-gal for β-galactosidase localization or stored at −80°C. For immunofluorescence, slides were brought to room temperature, treated with 0.5% Triton X-100 for 5 min, washed in PBS, and then blocked with 0.5M ammonium chloride for 10 min. Slides were then sequentially immunolabeled with the anti-α1α2α1(IV) collagen and anti-α3α4α5(IV) collagen antibodies (50 μg/ml in PBS), and then with chicken anti-goat IgG-AlexaFluor-594 and goat anti-mouse IgG1 AlexaFluor-488 (both at 1:200 in PBS). After washing, slides were coverslipped with ProLong Gold (Invitrogen). In some cases, direct conjugates of mAb 26-20-HRP (1.3 mg/ml) were injected in a volume of 50 μl intraperitoneally into 8-d-old ROSA26 hosts that had received intrarenal grafts of E12 Alport metanphroi 1 d after birth. In these cases, kidneys were fixed and processed for dual immunoperoxidase and β-galactosidase ultrastructural localization as described in detail previously.21
We also transplanted wildtype E12 CD-1 metanephroi under the renal capsule of newborn Col4a3 knockout/ROSA26 hosts. Five to 7 d after implantation, host kidneys were removed and processed for β-galactosidase localization with X-gal and immunofluorescence as described in the previous paragraph. Finally, kidneys from nongrafted infant Alport mice were frozen and processed for routine confocal dual immunolabeling with rabbit anti-GLEPP1 (30 μg/ml), rat anti-α5(IV) collagen (1:1000), and donkey anti-rabbit AlexaFluor-594 (1:200) and chicken anti-rat AlexaFluor-488 (1:200) secondary antibodies.
DISCLOSURES
None.
Acknowledgments
Funds came from National Institutes of Health grants DK052483, DK065123, and RR024214. We thank Roger Wiggins for the gift of anti-GLEPP1 antibodies and Eileen Roach for technical help.
Parts of this article were previously published in abstract form.40
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Miner JH, Abrahamson DR: Molecular and cellular mechanisms of glomerular capillary development. In: Seldin and Giebish's The Kidney, 4th Ed, edited by Alpern RJ, Hebert SC, Burlington, Academic Press, 2008, pp 691–706
- 2.Miner JH: Developmental biology of glomerular basement membrane components. Curr Opin Nephrol Hypertens 7: 13–19, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Miner JH: Renal basement membrane components. Kidney Int 56: 2016–2024, 1999 [DOI] [PubMed] [Google Scholar]
- 4.St.John PL, Abrahamson DR: Glomerular endothelial cells and podocytes jointly synthesize laminin-1 and -11 chains. Kidney Int 60: 1037–1046, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Miner JH, Li C: Defective glomerulogenesis in the absence of laminin α5 demonstrates a developmental role for the kidney glomerular basement membrane. Dev Biol 217: 278–298, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Abrahamson DR, St. John PL, Isom K, Robert B, Miner JH: Partial rescue of glomerular laminin α5 mutation by wildtype endothelial cells produce hybrid glomeruli. J Am Soc Nephrol 18: 2285–2297, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Noakes PG, Miner JH, Gautam M, Cunningham JM, Sanes JR, Merlie JP: The renal glomerulus of mice lacking s-laminin/laminin β2: Nephrosis despite molecular compensation by laminin β1. Nat Genet 10: 400–406, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Zenker M, Aigner T, Wendler O, Tralau T, Muntefering H, Fenski R, Pitz S, Schumacher V, Royer-Pokora b, Wuhl E, Cochat P, Bouvier R, Kraus C: Human laminin beta2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet 13: 2625–2632, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Wuhl E, Kogan J, Zurowska A, Matejas V, Vandervoorde RG, Aigner T, Wendler O, Lesniewska I, Bouvier R, Reis A, Cochat P, Zenker M: Neurodevelopmental deficits in Pierson (microcoria-congenital nephrosis) syndrome. Am J Med Genet A 143: 311–319, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Choi HJ, Lee BH, Kang JH, Jeong HJ, Moon KC, Ha IS, Yu YS, Matejas V, Zenker M, Choi Y, cheong HI: Variable phenotype of Pierson syndrome. Pediatr Nephrol 23: 995–2000, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Hudson BG, Tryggvason K, Sundarmoorthy M, Neilson EG: Alport syndrome, Goodpasture syndrome, and type IV collagen. N Engl J Med 348: 2543–2556, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Hudson BG: The molecular basis of Goodpasture and Alport syndromes: Beacons for the discovery of the collagen IV family. J Am Soc Nephrol 15: 2514–2527, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Ninomiya Y, Kagawa M, Iyama K, Naito I, Kishiro Y, Seyer JM, Sugimoto M, Oohashi T, Sado Y: Differential expression of two basement membrane collagen genes, COL4A6 and COL4A5, demonstrated by immunofluorescence staining using peptide specific monoclonal antibodies. J Cell Biol 130: 1219–1229, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalluri R, Shield CF, Todd P, Hudson BG, Neilson EG: Isoform switching of type IV collagen is developmentally arrested in X-linked Alport syndrome leading to increased susceptibility of renal basement membranes to endoproteolysis. J Clin Invest 99: 2470–2478, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khoshnoodi J, Pedchenko V, Hudson BG: Mammalian collagen IV. Microscop Res Tech 71: 357–370, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borza DB, Bondar O, Todd P, Sundaramoorthy M, Sado Y, Ninomiya Y, Hudson BG: Quaternary organization of the Goodpasture autoantigen, the α3(IV) collagen chain. J Biol Chem 277: 40075–40083, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Miner JH, Sanes JR: Molecular and functional defects in kidneys of mice lacking alpha collagen 3(IV): Implications for Alport syndrome. J Cell Biol 135: 1403–1413, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cosgrove D, Meehan DT, Grunkmeyer JA, Kornak JM, Sayers R, Hunter WJ, Samuelson GC: Collagen COL4A3 knockout: A mouse model for autosomal Alport syndrome. Genes Dev 10: 2981–2992, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Lu W, Phillips CL, Killen PD, Hlaing T, Harrison WR, Elder FF, Miner JH, Overbeek PA, Meisler MH: Insertional mutation of the collagen genes Col4a3 and Col4a4 in a mouse model of Alport syndrome. Genomics 61: 113–124, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Heidet L, Borza DB, Jouin M, Sich M, Mattei MG, Sado Y, Hudson BG, Hastie N, Antignac C, Gubler MC: A human-mouse chimera of the α3α4α5(IV) collagen promoter rescues the renal phenotype in Col4a3-/- Alport mice. Am J Pathol 165: 1633–1644, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.St. John PL, Abrahamson DR: LacZ transgenic mice and immunoelectron microscopy: An ultrastructural method for dual localization of β-galactosidase and horseradish peroxidase. J Histochem Cytochem 55: 1207–1211, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Wang R, St. John PL, Kretzler M, Wiggins RC, Abrahamson DR: Molecular cloning, expression, and distribution of GLEPP1 in developing mouse kidney. Kidney Int 57: 1847–1859, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Desjardins M, Bendayan M: Ontogenesis of glomerular basement membrane: Structural and functional properties. J Cell Biol 113: 689–700, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee LK, Pollock AS, Lovett DH: Asymmetric origins of the mature glomerular basement membrane. J Cell Physiol 157: 169–177, 1993 [DOI] [PubMed] [Google Scholar]
- 25.Minto AW, Kalluri R, Togawa M, Bergijk EC, Killen PD, Salant DJ: Augmented expression of glomerular basement membrane specific type IV collagen isoforms (alpha3-alpha5) in experimental membranous nephropathy. Proc Assoc Am Physicians 110: 207–217, 1998 [PubMed] [Google Scholar]
- 26.Bergijk EC, Van Alderwegen IE, Baelde HJ, De Heer E, Funabiki K, Miyai H, Killen PD, Kalluri RK, Bruijn JA: Differential expression of collagen IV isoforms in experimental glomerulosclerosis. J Pathol 184: 307–315, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Heidet L, Cai Y, Guicharnaud L, Antignac C, Gubler MC: Glomerular expression of type IV collagen chains in normal and X-linked Alport syndrome kidneys. Am J Pathol 156: 1901–1910, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saus J, Wieslander J, Langeveld JP, Quinones S, Hudson BG: Identification of the Goodpasture antigen as the alpha 3(IV) chain of collagen IV. J Biol Chem 263: 13374–13380, 1988 [PubMed] [Google Scholar]
- 29.Droz D, Diebold N, Jan D, Jaubert F: Deduction from Wilms’ tumor that glomerular podocytes produce the basement membrane material bearing Goodpasture determinants. J Pathol 162: 323–327, 1990 [DOI] [PubMed] [Google Scholar]
- 30.Chen S, Kasama Y, Lee JS, Jim B, Marin M, Ziyadeh FN: Podocyte-derived vascular endothelial growth factor mediates the stimulation of α3(IV) collagen production by transforming growth factor-β1 in mouse podocytes. Diabetes 53: 2939–2949, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Chen S, Lee JS, Iglesias-de la Cruz MC, Wang A, Izquierdo-Lahuerta A, Gandhi NK, Danesh FR, Wolf G, Ziyadeh FN: Angiotensin II stimulates α3(IV) collagen production in mouse podocytes via TGF-β and VEGF signaling: implications for diabetic glomerulopathy. Nephrol Dial Transplant 20: 1320–1328, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Perry J, Tam S, Zheng K, Sado Y, Dobson H, Jefferson B, Jacobs R, Thorner PS: Type IV collagen induces podocytic features in bone marrow stromal stem cells in vitro. J Am Soc Nephrol 17: 66–76, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Sugimoto H, Mundel TM, Sund M, Xie L, Cosgrove D, Kalluri R: Bone marrow-derived stem cells repair basement membrane collagen defects and reverse genetic kidney disease. Proc Natl Acad Sci U S A 103: 7321–7326, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prodromidi EI, Poulsom R, Jeffrey R, Roufosse, Pollard PJ, Pusey CD, Cook HT: Bone marrow-derived cells contribute to podocyte regeneration and amelioration of renal disease in a mouse model of Alport syndrome. Stem Cells 24: 2448–2455, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Ninichuk V, Gross O, Segerer S, Hoffmann R, Radomska E, Buchstaller A, Huss R, Akis N, Schlondorff D, Anders HJ: Multipotent mesenchymal stem cells reduce interstitial fibrosis but do not delay progression of chronic kidney disease in collagen 4A3-deficient mice. Kidney Int 70: 121–129, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Katayama K, Kawano M, Naito I, Ishikawa H, Sado Y, Asakawa N, Murata T, Oosugi K, Kiyohara M, Ishikawa E, Ito M, Nomura S: Irradiation prolongs survival of Alport mice. J Am Soc Nephrol 19: 1692–1700, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friedrich G, Soriano P: Promoter traps in embryonic stem cells: A genetic screen to identify and mutate developmental genes in mice. Genes Dev 5: 1513–1523, 1991 [DOI] [PubMed] [Google Scholar]
- 38.Kohda T, Okada SI, Hayashi A, Kanzaki S, Ninomiya Y, Taki M, Sado Y: High nephritogenicity of monoclonal antibodies belonging to IgG2a and IgG2b subclasses in rat anti-GBM nephritis. Kidney Int 66: 177–186, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Abrahamson DR, St. John PL: Loss of laminin epitopes during glomerular basement membrane assembly in developing mouse kidneys. J Histochem 40: 1943–1953, 1992 [DOI] [PubMed] [Google Scholar]
- 40.Abrahamson DR, Stroganova L, St. John PL, Borza DB: Is Alport a podocyte disease? J Am Soc Nephrol 17: 97A, 2006 [Google Scholar]