Abstract
Excessive dietary phosphorus may increase cardiovascular risk in healthy individuals as well as in patients with chronic kidney disease, but the mechanisms underlying this risk are not completely understood. To determine whether postprandial hyperphosphatemia may promote endothelial dysfunction, we investigated the acute effect of phosphorus loading on endothelial function in vitro and in vivo. Exposing bovine aortic endothelial cells to a phosphorus load increased production of reactive oxygen species, which depended on phosphorus influx via sodium-dependent phosphate transporters, and decreased nitric oxide production via inhibitory phosphorylation of endothelial nitric oxide synthase. Phosphorus loading inhibited endothelium-dependent vasodilation of rat aortic rings. In 11 healthy men, we alternately served meals containing 400 mg or 1200 mg of phosphorus in a double-blind crossover study and measured flow-mediated dilation of the brachial artery before and 2 h after the meals. The high dietary phosphorus load increased serum phosphorus at 2 h and significantly decreased flow-mediated dilation. Flow-mediated dilation correlated inversely with serum phosphorus. Taken together, these findings suggest that endothelial dysfunction mediated by acute postprandial hyperphosphatemia may contribute to the relationship between serum phosphorus level and the risk for cardiovascular morbidity and mortality.
Cardiovascular disease (CVD) is the most important factor contributing to life expectancy in chronic kidney disease (CKD).1,2 Hyperphosphatemia has been recently recognized as an important factor in development of medial calcification by induction of differentiation of vascular smooth muscle cells to osteoblast-like cells.3–6 On the other hand, endothelial dysfunction is the principal cause of atherosclerosis resulting in CVD.7 However, the effect of hyperphosphatemia on endothelial cell function, and its influence on the development of CVD is still unclear.
Two recent papers reported that higher serum phosphorus (P) concentration, albeit within the normal range, was associated with development of atherosclerosis and mortality in the patients with normal kidney function8 and in the Framingham Offspring study participants.9 These studies suggested that long-term excessive P loading, even if it does not cause hyperphosphatemia, can be a risk factor for CVD. In addition, Onufrak et al. also demonstrated that serum phosphorus level was associated with carotid intima media thickness in the general population.10 Interestingly, continuous hyperphosphatemia in both klotho mutant mice and fibroblast growth factor 23 (FGF23)-deficient mice may also be a risk factor of a premature aging-like phenotype.11–13 This may be inferred because P restriction diet partially ameliorated the phenotype in both klotho and FGF23 deficient mice.14,15 In addition, hyperphosphatemia involved by chronic renal failure frequently causes hyperparathyroidism that affects the homeostasis of calcium and phosphate in plasma. Increased parathyroid hormone (PTH) has also been recognized as a risk factor of CVD.16 These factors complicate to understand the linking mechanism between serum P and CVD. Thus, a possible mechanism linking serum P and CVD risk should be clarified to gain insight into the potential impact of P on CVD and aging-related diseases. Here, we focused on the direct effect of P on endothelial function.
In this study, we reported that dietary P loading in human caused postprandial elevation of serum P and impaired endothelium-dependent vasodilation and also demonstrated that the impairment of vasodilation was mainly due to decreased NO production in endothelial cells, suggesting that postprandial elevation of serum P level deteriorates endothelial function.
RESULTS
High P Loading Increased Reactive Oxygen Species Production and Decreased Nitric Oxide Production in Bovine Aortic Endothelial Cells
We reported that elevation of extracellular P level can induce reactive oxygen species (ROS) production in endothelial cells.17 In this study, we also confirmed that elevation of P concentration from the control value (0.8 mM) to 2.8 mM increased ROS production by both nitro blue tetrazolium (NBT) assay and time-lapse confocal microscopy analysis with aminophenyl fluorescein (APF) in bovine aortic endothelial cells (BAECs) (Figure 1A and B). P loading significantly increased the ROS production in BAECs in a dose-dependent manner (Figure 1A). In the endothelial cells, ROS is produced mainly by the activation of NAD phosphate [NAD(P)H] oxidase, xanthine oxidase, and mitochondrial respiratory chain. NAD(P)H oxidase and xanthine oxidase pathways can mainly produce O2-, and mitochondrial pathway can produce O2- and OH-. In addition, produced O2- can be converted to H2O2 by superoxide dismutase and, consequently, be converted to OH- in the presence of Fe2+. O2- is also reacted with nitric oxide (NO) to form ONOO-.18 Thus, we examined the effect of specific inhibitors of each pathway on the ROS production mediated by P loading. Figure 1A also demonstrates that diphenyl iodonium (DPI), a specific inhibitor of NAD(P)H oxidase decreased the ROS production. On the other hand, rotenone did not inhibit the ROS production, but oxypurinol partially inhibited it (Figure 1A). Furthermore, ebselen, a potent antioxidant, completely inhibited the ROS production induced by P loading (Figure 1B). The ROS production was also inhibited by treatment of phosphonoformic acid (PFA), which is a specific inhibitor of sodium-dependent phosphate transporters (Figure 1B). Figure 1C demonstrates that BAECs express both PiT-1 and PiT-2, which are type III sodium-dependent phosphate transporters. Therefore, these transporters would contribute to P influx in BAECs.
Figure 1.
Effects of high P loading on ROS production. (A) P loading increased O2- generation in a dose-dependent manner, and the effects of various oxidase inhibitors on the O2- generation were tested by NBT assay. Data are expressed as mean ± SEM (n = 8 to 20). aP = 0.004 for column 3, <0.0001 for column 6, 0.005 for column 7, <0.0001 for column 8 versus column 1, bP = 0.019 for column 5 versus column 3. (B) Effect of ebselen or PFA on the P loading-mediated ROS production determined by time-lapse confocal microscopy analysis with APF. Data points shown are mean ± SEM for control (0.9 mM P, n = 3, closed circle), 2.8 mM P (n = 12, open circle), 2.8 mM P with 200 μM PFA (n = 6, square), or 2.8 mM P with 10 μM ebselen (n = 9, triangle). #P < 0.05 versus 2.8 mM P, and *P < 0.05 versus control. (C) Expression of type III sodium-dependent phosphate transporter in BAECs. RT-PCR analysis was performed with specific primer sets for four different types of sodium-dependent phosphate transporter (Type IIa, Type IIb, Type III [PiT-1 and PiT-2]) as described in Supplemental Appendix.
High P Loading Decreased Endothelium-Dependent Vasodilation of Rat Aorta Rings
To confirm the impairment of endothelium-dependent vasodilation by P loading, we investigated the effects of high P loading on acetylcholine-induced vasodilation using thoracic aorta rings from rats maintained under normal condition. When aorta rings were preincubated in the medium containing 2.4 mM P for 1 h, acetylcholine-induced vasodilation decreased by 70% compared with rings preincubated in the medium containing 1.2 mM P (Figure 2A). On the other hand, when aorta rings stripped of endothelium were treated similarly, no differences were observed between the rings incubating in the medium containing different P concentrations (Figure 2B). Preincubation of intact aortic rings in the medium containing 2.4 mM P slightly increased vasoconstriction mediated by 1 μM phenylephrine; however, the difference was not statistically significant (1.374 ± 0.17 versus 1.645 ± 0.239, P = 0.38). We attempted to confirm the role of ROS in the impairment of endothelium-dependent vasodilation caused by high P loading using rat aortic rings. However, ebselen did not significantly ameliorate the impaired vasodilation (Figure 2C).
Figure 2.
Effects of high P loading on aortic vasodilation. (A) Dose-response curves of vasodilation induced by acetylcholine in rat aortic rings pretreated with Krebs-Henseleit bicarbonate (KHB) buffer containing 1.2 mM P (n = 3, closed circle) or 2.4 mM P (n = 2, open circle). Data are expressed as mean ± SEM. There was significant difference in intercepts between the curves of 1.2 mM P and 2.4 mM P (P = 0.0067). (B) Dose-response curves of vasodilation induced by sodium nitroprusside (SNP) in rat aortic rings stripped of the endothelium, preincubated with KHB buffer containing 1.2 mM P (n = 4, closed circle) or 2.4 mM P (n = 4, open circle). Data are expressed as mean ± SEM. (C) Effect of ebselen on the inhibition of vasodilation by high P medium in rat aortic rings. Rat aortic rings were preincubated with KHB buffer containing with 1.2 mM P (n = 4), 2.4 mM P (n = 3), or 2.4 mM P with 10 μM ebselen (n = 3). Data are expressed as mean ± SEM, *P < 0.05 versus column 1.
High P Loading Decreased NO Production in BAECs
To elucidate the inhibitory mechanism on vasodilation by P loading, we investigated the effect of high P loading on NO production and intracellular Ca2+ concentration in BAECs. High P loading completely inhibited the NO production mediated by bradykinin (Figure 3A). However, ebselen did not affect the inhibitory effect of P loading on bradykinin-mediated NO production. In addition, since intracellular Ca2+ increase plays an important role in the activation of endothelial NO synthase (eNOS), we investigated whether P loading can affect the receptor-mediated intracellular Ca2+ increase in BAECs. High P loading did not affect the intracellular Ca2+ increase by adenosine triphosphate (ATP) (Figure 3B).
Figure 3.
Effect of high P loading on NO production. (A) Effect of bradykinin-induced NO production in BAECs measured by time-lapse confocal microscopy with DAF-2DA. The cells were pretreated with control (0.9 mM P, n = 8, closed circle), 1.8 mM P (n = 7, open circle), or 1.8 mM P with 10 μM ebselen (n = 6, closed triangle). *P < 0.05 versus control. (B) Effect of high P loading on intracellular Ca2+ increase induced by ATP in BAECs. BAECs were preincubated with control (0.9 mM P, solid line) or 2.8 mM P (dotted line) for 1 h before intracellular Ca2+ measurement. Intracellular Ca2+ increase induced by 0.5 μM and 2 μM ATP, and 10 μM calcium ionophore was sequentially estimated by time-lapse confocal microscopy analysis with Fluo-4 and Fura-Red. Change of intracellular Ca2+ concentration is expressed as change of fluorescence ratio (Fluo-4/Fura-Red). The curves are representative of three independent experiments. (C) Effects of high P loading on phosphorylation of eNOS at Thr497. Western blot analysis of phosphorylated eNOS at Thr497, total eNOS, and caveolin. BAECs were treated with control (0.9 mM P), 3 mM P, 3 mM P with 10 μM Gö6976, 3 mM P with 1 mM Tempol, or 3 mM Na2SO4 for 60 min. We subjected isolated caveolar membrane fractions from the cells to western blot analysis with anti-phospho-eNOS (Thr495) monoclonal antibody (mAb), anti-eNOS mAb, or anti-caveolin polyclonal antibody (pAb). Representative blots from four separate experiments and densitometric analysis data are shown. *P < 0.05 versus control, **P < 0.01 versus 3 mM P.
We postulated that high P loading may modulate eNOS activity through its activation/inactivation pathway through phosphorylation. To address that, we investigated whether high P loading can phosphorylate Thr497 (corresponding to Thr495 in the human19,20) of eNOS in BAECs, because the phosphorylation of this threonine causes eNOS inactivation. Loading with 3 mM P for 1 h significantly increased phosphorylation of eNOS at Thr497 on caveolar membranes of BAECs (Figure 3C). The phosphorylation was completely inhibited by conventional protein kinase C (PKC) inhibitor Gö6976 and partially by tempol (Figure 3C). However, protein kinase A inhibitor H-89 and PI3 kinase inhibitor wortmannin did not inhibit the phosphorylation (data not shown). Sulfate ion did not increase the phosphorylation of eNOS (Figure 3C).
Activation of Conventional PKC by High P Loading
To confirm that high P loading can activate conventional PKC in endothelial cells, we investigated if the activity of PKC can be stimulated by high P loading. P loading also increased PKC activity in BAECs as shown in Figure 4A. The activation of PKC activity was inhibited by PFA, suggesting that influx of P into the cells should be required for PKC activation in response to P loading. In addition, the translocation of PKCα from cytosol to caveolae membrane was also investigated, because previous reports have demonstrated that activated PKCα can be targeted to caveolae, where eNOS is enriched and regulated.11–23 P loading increased the translocation of PKCα in a dose-dependent manner in BAECs (Figure 4B). The increased level of translocation by 3 mM P loading was almost same as that by 25 mM glucose.
Figure 4.
Activation of conventional PKC by high P loading in BAECs. (A) Effect of high P loading on the PKC activity of BAECs. We treated BAECs with control (0.9 mM P), 1.5 mM P, 3 mM P, or 3 mM P with 200 μM PFA for 15 min, then pretreated whole cell lysates, and subjected lysates to measurement of conventional PKC activity using the TruLight PKCα Assay kit. *P < 0.05 versus column 1, †P < 0.05 versus 3 mM P (column 3). (B) Effect of high P loading on the subcellular localization of PKCα in BAECs. We treated BAECs with the indicated concentration of P or glucose in Medium 199 medium without serum for 1 h, and then fractionated the cells into cytosol and caveolar membrane fractions. We separated 20 μg of protein of each fraction by SDS-PAGE and performed western blot analysis with anti-PKCα mAb and anti-caveolin pAb. The data are representative from two separate experiments.
Effects of Dietary P Loading on Serum P, Glucose, and Intact-PTH Levels in Young Healthy Men
To confirm the effect of P loading on endothelial function in vivo, we examined the effect of dietary P loading on endothelial function in healthy men (Table 1). Mean values of all serum chemistry measurements were within the respective normal ranges in the preprandial status as shown in Table 2. Furthermore, there was no significant difference in the preprandial data between the experimental days. Serum P level increased significantly 2 h after the ingestion of a P1200 meal (containing 1200 mg P per meal) (Table 2). The serum P level exceeded the normal range (2.5 to 4.5 mg/dl [0.8 to 1.5 mM]) in 8 out of 11 subjects. On the other hand, postprandial serum P level after the ingestion of a P400 meal (containing 400 mg P per meal) did not change significantly. We did not measure urinary P excretion because a previous study demonstrated that urinary P excretion increased after the ingestion of the P1200 meal compared with the P400 meal.24 In addition, in vitro experiments suggest that increased serum P level would more directly affect on endothelial function rather than increased urinary P excretion. Serum glucose levels at 2 h after the meal were not significantly different between those receiving a P400 meal and a P1200 meal. Serum intact-PTH (iPTH) level tended to decrease after the breakfast due to circadian regulation. However, the postprandial iPTH level of subjects receiving a P1200 meal was significantly higher than those receiving a P400 meal (Table 2). Other laboratory data were not significantly different between the subjects receiving the two types of meal.
Table 1.
Baseline characteristics of 11 healthy male subjects
| Characteristic | n = 11 |
|---|---|
| Age | 24.6 ±3.4 |
| Height (cm) | 170.1 ±4.1 |
| Weight (kg) | 60.4 ±6.6 |
| BMI (kg/m2) | 20.9 ±2.0 |
| Percent of body fat (%) | 12.5 ±3.6 |
| Lean body mass (kg) | 52.7 ±4.7 |
Table 2.
Serum chemistry, blood pressure, and %FMD findings before and 2 h after test meal ingestion
| Normal Range | P400 Meal
|
P1200 Meal
|
P Value | |||
|---|---|---|---|---|---|---|
| Preprandial | Postprandial | Preprandial | Postprandial | |||
| %FMD | 8.81±0.3 | 8.42±0.5 | 9.26±0.4 | 5.02±0.3a,b | <0.0001 | |
| Blood pressure systolic | <130 | 113.3±3.8 | 114.5±2.5 | 112.8±2.9 | 117.5±3.8 | 0.27 |
| Diastolic | <85 | 67.1±2.1 | 64.7±2.3 | 68.0±2.7 | 62.9±3.1 | 0.28 |
| UA (mg/dl) | 3.8to7.0 | 5.42±0.3 | 5.43±0.3 | 5.37±0.2 | 5.35±0.2 | 0.53 |
| Glucose (mg/dl) | 70to109 | 81.7±2.7 | 100.8±5.9a | 82.3±2.4 | 94.7±4.6 | 0.28 |
| TG (mg/dl) | 30to149 | 56.8±5.6 | 57.0±6.8 | 54.5±4.8 | 57.1±6.6 | 0.53 |
| HDL-Cho (mg/dl) | 40to85 | 56.5±3.0 | 53.9±2.7 | 53.7±2.6 | 52.4±2.6 | 0.21 |
| LDL-Cho (mg/dl) | 65to139 | 85.7±5.2 | 82.8±5.5 | 82.7±5.4 | 80.2±5.4 | 0.78 |
| Na (mEq/L) | 137to147 | 140.6±0.3 | 141.4±0.2 | 140.8±0.4 | 141.8±0.4 | 0.68 |
| K (mEq/L) | 3.5to5.0 | 3.92±0.1 | 3.93±0.1 | 4.01±0.1 | 3.78±0.1 | 0.08 |
| Cl (mEq/L) | 98to108 | 103.2±0.6 | 103.6±0.4 | 103.5±0.5 | 103.5±0.5 | 0.44 |
| Ca (mg/dl) | 8.4to10.4 | 9.66±0.1 | 9.60±0.1 | 9.56±0.1 | 9.47±0.1 | 0.69 |
| Ionized-Ca (mEq/L) | 2.41to2.72 | 2.64±0.1 | 2.70±0.1 | 2.62±0.1 | 2.55±0.0 | 0.11 |
| P (mg/dl) | 2.5to4.5 | 3.94±0.1 | 3.66±0.2 | 3.79±0.1 | 4.56±0.1a,b | <0.0001 |
| Intact-PTH (pg/dl) | 10to65 | 31.6±2.4 | 24.0±1.0a | 32.4±2.2 | 29.6±1.4 | 0.07 |
Data are expressed as means ± SEM (n = 11).
P values (right end) are resulted from repeated measures analysis of variance between P400 meal and P1200 meal.
P < 0.05 preprandial versus postprandial by Tukey-Kramer test.
P < 0.05 postprandial in P400 diet versus postprandial in P1200 diet by Tukey-Kramer test.
Effect of Dietary P Loading on Endothelium-Dependent Vasodilation in Young Healthy Men
We evaluated the effect of dietary P loading on endothelium-dependent vasodilation in 11 young healthy men by postprandial changes of percent flow-mediated dilatation (%FMD). %FMD decreased significantly 2 h after the ingestion of the P1200 meal, whereas there was no significant change after the ingestion of the P400 meal. As shown in Figure 5, the ingested P1200 meal, there was significant negative correlation between serum P level and %FMD (Spearman's coefficient rs = −0.42, P = 0.006) (Figure 5B). With the ingested P400 meal, on the other hand, there was no significant correlation (Figure 5A). There was no statistically significant correlation between serum glucose and %FMD (rs = −0.26, P = 0.09). In addition, the decreased %FMD at 2 h after the ingestion of the P1200 meal was normalized at least 24 h later (%FMD at 24 h was 8.60 ± 0.2 [NS versus before the ingestion]).
Figure 5.
Univariate association between serum P or serum glucose and %FMD. Univariate association between serum P and %FMD in a P400 meal (A) and in a P1200 meal (B). Symbols denote the same group in each graph (preprandial, closed circle; postprandial, open circle). Spearman's correlation coefficient (rs) and its P value for r = 0 are represented in each association.
DISCUSSION
This study demonstrates that elevation of extracellular P level causes endothelial dysfunction in vitro and in vivo. In addition, in vitro experiments demonstrated that high P loading inhibited NO production through increased ROS production and eNOS inactivation via conventional PKC, resulting in impaired endothelium-dependent vasodilation. Furthermore, dietary P loading can deteriorate flow-mediated vasodilation in healthy men, suggesting that dietary P loading or elevation of serum P level may be a risk factor for CVD in healthy persons as well as CKD patients. Our data also provide a novel explanation why higher serum P levels, even although within normal range, are associated with an increased CVD risk as previously reported.8–10
Recently, we reported that P loading increased ROS production and decreased NO production in BAECs.17 In this study, we demonstrated that the ROS production was mainly mediated by NAD(P)H oxidase. Contribution of other ROS generators may be partial. Elevation of glucose can increase ROS production via NAD(P)H oxidase by activating conventional PKC in endothelial cells.25 We found that high P loading also can activate conventional PKC in BAECs depending on P influx. Although we cannot ascertain how P influx activates the PKC pathway, our data agreed with a recent report by Di Marco et al. that high P loading increased ROS production via P influx and induced apoptosis in endothelial cells.26
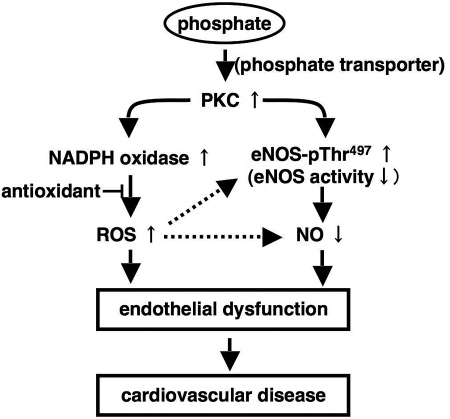
ROS is a scavenger of NO and depletes the available NO resulting in dysregulation of vascular tone.18,27,28 However, antioxidants were not enough to ameliorate the impaired vasodilation mediated by high P loading, suggesting that other factor(s) rather than ROS may be involved. The activity of eNOS can be regulated by Ca2+ and phosphorylation.19,20 Increased P influx did not influence intracellular Ca2+ increase in response to ATP, which can activate eNOS activity via Ca2+ signal. Therefore, we considered that P loading may regulate phosphorylation of eNOS. We demonstrated that high P loading enhances the phosphorylation at Thr497 of eNOS via PKC. Phosphorylation of eNOS at Thr497 can inhibit eNOS activity.19,20,29 Our data suggest that elevation of serum P by dietary P loading can primarily activate conventional PKC and then decrease NO availability via eNOS inactivation. In addition, increased ROS also reduce the available NO by converting NO to ONOO-. These steps eventually lead to endothelial dysfunction (Figure 6).
Figure 6.
Possible pathway of phosphate-mediated endothelial dysfunction. High P loading increases ROS production through PKC and NAD(P)H oxidase in endothelial cells. In addition, high P loading also decreases NO production through phosphorylation of eNOS. Increased ROS production and decreased NO production may cause endothelial dysfunction and CVD.
Until now, hyperphosphatemia was recognized as a risk factor for CVD through promotion of medial calcification.2,5,6 Jono et al. demonstrated that more than 1.4 mM P dose-dependently increased calcium deposition after a 6-d incubation in human smooth muscle cells.5 Additionally, dietary P restriction ameliorated vascular calcification in FGF23-deficient mice that show a premature aging phenotype with hyperphosphatemia and hypervitaminosis D.15 These results can explain the correlation between hyperphosphatemia in CKD patients and vascular calcification, but are not enough to explain the recent epidemiologic findings that higher serum P level within normal range (0.9 to 1.4 mM P) can be a risk factor for CVD in persons with normal kidney function.8–10 Both endothelial dysfunction and medial calcification are closely associated with development of CVD. It is well known that long-term exposure to P, generally observed in end-stage renal failure patients, can mediate vascular calcification.5,6 Our data demonstrated that dietary high P loading can be involved in the postprandial elevation of serum P level, and this short-term exposure to P was enough to decrease endothelium-dependent vasodilation.
In healthy persons, serum P level is maintained within normal range by various hormones, such as iPTH, and vitamin D. Nonetheless, higher serum P concentration, albeit within the normal range, was closely associated with development of atherosclerosis and carotid intima media thickness in the general population.9,10 Our previous data demonstrated that over intake of dietary P, even in a single meal challenge, in healthy young men was involved in postprandial elevation of serum P over normal range.24 In addition, over intake of P in a single meal also caused the impairment of endothelial mediated vasodilation as shown in this study. Portale et al. demonstrated that the range of circadian variation of serum P levels in humans was dependent on the amount of dietary P intake.30 For dietary prevention of CVD, therefore, we have to consider the contributions of both postprandial elevation of serum P and continuous elevation of serum P to the development of CVD. The former would be associated with endothelial dysfunction and progress of atherosclerosis, and the latter would be involved in medial calcification and progress of Mönkeberg-type arteriosclerosis.
Until now, the effect of dietary P intake on vascular function has been underestimated, although association of serum P and vascular dysfunction has been well investigated, because fasting serum P level could not increase in healthy persons, even if dietary P was overloaded.30,31 Generally, fasting blood in the morning was collected and analyzed in most of the epidemiologic studies to investigate the relationship between serum biomarkers and lifestyle-related diseases. However, our data demonstrate that postprandial P elevation was associated with %FMD in humans. Therefore, we propose that postprandial elevation of serum P should be evaluated to assess the relationship between dietary P intake and CVD, as postprandial blood glucose elevation has been well-established as a risk factor for CVD.
There are some limitations in this study. First, we investigated the effect of maximum dose of dietary P on the FMD in young healthy men. Studies with various doses of P in more elderly subjects and women should be performed in the future to better understand these effects in both sexes and different age groups to determine adequate dietary intake of phosphorus for CVD prevention. Second, because this human study is carried out with a limited number of subjects, the relationship between the amount of dietary P and endothelial function and risk of CVD should be verified in the general population epidemiologically. Third, our study strongly indicates that elevation of serum P level causes endothelial dysfunction, however we cannot exclude a coexistent defect in smooth muscle vasodilator responsiveness in this study.
In conclusion, dietary P loading can cause endothelial dysfunction within a short time. Oxidative stress and decreased NO production in endothelial cells are possible mechanisms for the impaired endothelial function mediated by P loading. These findings suggest that elevation of serum P level by dietary P loading may be a novel risk factor for endothelial dysfunction and demonstrate a novel mechanism for explaining the relationship between higher serum P level and CVD risk or mortality. Our findings may also contribute to development of new pharmacologic (e.g., endothelium-specific inhibitor of P transporter) and nutritional approach to treatment and prevention of CVD.
CONCISE METHODS
Materials
We used the following inhibitors: DPI; a NAD(P)H oxidase inhibitor, oxypurinol; a xanthine oxidase inhibitor, rotenone; a mitochondrial respiratory chain system inhibitor, PFA; phosphonoformic acid; a sodium-dependent phosphate transporter inhibitor, Gö6976; a conventional PKC inhibitor, H-89; a PKA inhibitor, wortmannin; aPI3Kinase inhibitor. Other compounds were used in this study are listed in the Supplemental Appendix.
Examination of the Effects of High P Loading on Endothelial Cell Functions Using BAECs
We prepared BAECs as described previously.17,32 We maintained the cells in 100-mm plastic culture dishes in Medium 199 (Sigma-Aldrich, Tokyo, Japan) supplemented with 20% fetal bovine serum (FBS; Invitrogen, Carlsbad, California), penicillin 50 IU/ml, and streptomycin 50 μg/ml under humidified atmosphere of 5% CO2 at 37°C. We rountinely passaged the cells before they reached confluence.
P Loading Experiment in BAECs
We incubated BAECs overnight in FBS-free Medium 199. For inhibition experiments, the cells were incubated with either 10 μM Gö6976 for 10 min or one of the following for 30 min: 10 μM ebselen, 1 mM tempol, or 200 μM PFA before P loading. For P loading experiments, we added appropriate amounts of sodium phosphate buffer (0.1 M Na2HPO4/NaH2PO4, pH 7.4) to produce final P concentrations of 0.9 mM to 3 mM as indicated in figure legends.
NBT Assay
We incubated BAECs in serum-free Medium 199 under 5% CO2 at 37°C for 12 h and then in 0.2% NBT solution (0.2% weight per volume NBT in HBSS containing different concentrations of P [0.8 mM to 2.8 mM]) for 120 min. We washed the cells in HBSS, first by adding 2 M KOH to solubilize the cell membranes and then by adding dimethyl sulfoxide to dissolve blue formazan with gentle shaking for 10 min at room temperature. After solubilization, we determined the absorbance of the samples at 620 nm.
Time-Lapse Confocal Microscopy Analysis for the Measurement of ROS, NO Production, and Intracellular Ca2+ Change
We measured ROS and NO levels using the specific indicators APF and 4,5-diaminofluorescein-2 diacetate (DAF-2DA) (Daiichi Chemical, Tokyo, Japan), respectively. APF can specifically recognize OH-, ONOO-, and OCl- among many types of ROS.33 We performed the time-lapse experiments with confocal microscopy as previously reported. 17 For measurement of intracellular Ca2+ change, we used the combination of fluorescence indicators Fluo-4 and Fura-red (Molecular Probes, Eugene, Oregon). More detailed information is provided in the Supplemental Appendix.
Evaluation of Vasodilation and Vasoconstriction Using Rat Thoracic Aorta Rings
We measured vasoconstriction and vasodilation with rat aortic rings prepared from male 12-wk-old Sprague Dawley rats by Micro Easy Magnus (Kishimoto Medical, Kyoto, Japan) as described previously.34 The Animal Experimentation Committee of the University of Tokushima approved these experiments. More detailed information is provided in the Supplemental Appendix.
Reverse Transcription PCR (RT-PCR), Western Blot Analysis, and PKC Activity Assay
We performed RT-PCR and western blot analyses by standard method written elsewhere. We measured PKC activity by the TruLight PKCα Assay kit (Calbiochem, Tokyo, Japan). We provide detailed information for RT-PCR, western blot analysis, and PKC activity assay in the Supplemental Appendix.
Cell Fractionation
After the P loading as described above, we subjected BAECs to cell fractionation as described previously.32 Finally, we divided the cells into a postnuclear (PNS) soup, a cytosol (Cyt) fraction, a caveolar membrane (CM) fraction, and a noncaveolar membrane (NCM) fraction.
Dietary P Loading on Healthy Human Subjects
Eleven male volunteers (21 to 33 yrs of age) without apparent health problems were recruited for this study. The participants had no evidence of diabetes, abnormal glucose intolerance, obesity, hypertension, kidney diseases, cardiovascular disease, hyperlipidemia, and bone and mineral disorders. Demographic data for the participants are provided in Table 1. All participants were nonsmokers, had normal blood pressure (BP), consumed < 30 g/d alcohol, and took no medications or antioxidant supplements. Eligibility of participants for this study was determined as reported previously by us.24 The study used a double-blinded crossover design on two different days separated by more than 1 d. The subjects were alternately served either a P400 meal or a P1200 meal for breakfast at 8:30 a.m. The composition of the test meals and standard dinner is provided in the Supplemental Appendix. All of the meals were consumed over 7 to 14 min. On the day before each study day, the subjects were asked to abstain from foods and beverages other than water not containing P after 1:00 p.m. They were served a standard dinner at 8:00 p.m. We calculated the area under the curve (AUC) of serum P over 8 h after the ingestion of the P400 and P1200 meals in the previous study.24 The AUCs after the P400 and P1200 meals was 217 ± 56 mg•min/ml and 519 ± 38 mg•min/ml, respectively. In addition, the peak value of serum P level was 3.9 ± 0.12 mg/dl at 6 h after the ingestion of the P400 meal, while the peak value was 5.0 ± 0.11 mg/dl at 2 h after the ingestion of P1200 meal. These results suggest that enough of an amount of P can be absorbed from the intestine, and this dietary intervention is useful to control serum P level without changing other nutritional elements.
We collected blood samples immediately before (0 h) and at 2 h after the test meal ingestion. Venous blood was taken from a median cubital vein for the measurement of serum P, Ca, ionized Ca, Na, K, Cl, iPTH, uric acid (UA), glucose, triacylglyceride (TG), LDL cholesterol (LDL-Cho), and HDL cholesterol (HDL-Cho) concentrations. All biochemical measurements and analyses were performed by MBC (Mitsubishi Kagaku Bio-Clinical Laboratories, Tokyo, Japan). We previously reported that serum P levels increased at 30 min and reached maximum level around 2 h after meal ingestion.24 Therefore, we measured serum chemistry, BP, and %FMD before and 2 h after the test meal ingestion.
The Ethics Committee of the Tokushima University Hospital approved the study protocols. All participants provided written informed consent. The investigators and sponsors in the University of Tokushima, University of Tokyo, and Hiroshima University all contributed to the design and execution of the study. All of the authors contributed to the review of drafts and revisions of the manuscript. Statistical analyses and data management were carried out according to the guideline of the Clinical Trial Center for Developmental Therapeutics and the Ethics Committee of Tokushima University Hospital. This study has been registered and opened on the University Hospital Medical Information Network-Clinical Trials Registry database in Japan according to the International Committee for Medical Journal Editors guidelines (UMIN000000803, Dietary phosphorus loading trial in humans).
Measurement of FMD
We evaluated endothelial function by measurement of FMD,35 according to previously published guidelines.36 We provide details in the Supplemental Appendix.
Statistical Analysis
We tested all data for normal distribution of variables of interests using Pearson's χ2 distribution test before further parametric or nonparametric statistical analysis. If the test judged the data as a normal distribution, we performed the following statistical analysis by parametric analysis. If not, we used nonparametric analysis.
In dietary P loading experiments, we performed comparisons between preprandial and postprandial values of serum chemistry measurements in a group and effects of meals on preprandial and postprandial values of these measurements by means of repeated measurements and Tukey-Kramer methods. We determined statistical significance of the difference between the P400 and P1200 meals by the Wilcoxon signed-rank test.
To examine associations between level of serum P and %FMD and the level of serum glucose and %FMD, we performed simple regression analysis and estimated Spearman's nonparametric correlation coefficients. We selected the nonparametric procedure to avert requirements of normal distributions and linear associations of variables of interests.
In the case of animal study, ex vivo and in vitro study, we determined statistical significance of the differences between the groups by ANOVA followed by post hoc testing using Fisher's protected least significant difference procedure for multiple comparisons.
For vasoconstriction and vasodilation studies, we performed linear regression analysis to compare between the dose-response curves. We estimated and compared slopes and intercepts and show a P value if there was a significant difference between the slopes or intercepts.
We performed all statistical analyses using Statview 5.0 (SAS Institute, Cary, North Carolina) or PRISM 5 (GraphPad Software, La Jolla, California), and considered a P value < 0.05 statistically significant.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
We thank Drs. Masanori Yoshizumi, Nara Medical College, and Toshiaki Tamaki, University of Tokushima, for their valuable suggestions and discussion. We also thank Support Center for Advance Medical Sciences in the University of Tokushima for their technical assistance. This work was supported by Grants-in-Aid for Scientific Research on Priority Areas (17790554), for Young Scientists (19680030, 18790567), for Scientific Research (B) (18300232), for Exploratory Research (17650225), and Knowledge Cluster Initiative from the Ministry of Education, Culture, Sports, Science, and Technology in Japan, and was also supported by the Uehara Memorial Foundation, the Kidney Foundation (JKFB08-22), the 21st Century COE Program “Human Nutritional Science and Stress Control,” and the Initiatives for Attractive Education in Graduate School, University of Tokushima, Japan.
Published online ahead of print. Publication date available at www.jasn.org.
E.S., Y.T., and R.T. contributed equally to this work.
Supplemental information for this article is available online at http://www.jasn.org
REFERENCES
- 1.Poduval RD, Wolgemuth C, Ferrell J, Hammes MS: Hyperphosphatemia in dialysis patients: Is there a role for focused counseling? J Renal Nutr 13: 219–223, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Moe SM, Chen NX: Pathophysiology of vascular calcification in chronic kidney disease. Circ Res 95: 560–567, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK: Association of elevated serum PO4, Ca×PO4 product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol 12: 2131–2138, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, Morii H, Giachelli CM: Phosphate regulation of vascular smooth muscle cell calcification. Circ Res 87: e10–e17, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Giachelli CM: Vascular calcification: In vitro evidence for the role of inorganic phosphate. J Am Soc Nephrol 14: S300–S304, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Ross R: Atherosclerosis—an inflammatory disease. N Engl J Med 340: 115–126, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G: Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 112: 2627–2633, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Dhingra R, Sullivan LM, Fox CS, Wang TJ, D’Agostino RB Sr, Gaziano JM, Vasan RS: Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 167: 879–885, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Onufrak SJ, Bellasi A, Shaw LJ, Herzog CA, Cardarelli F, Wilson PW, Vaccarino V, Raggi P: Phosphorus levels are associated with subclinical atherosclerosis in the general population. Atherosclerosis 199: 424–431, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI: Mutation of the mouse klotho gene leads to a syndrome resembling aging. Nature 390: 45–51, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T: Targeted ablation of FGF23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113: 561–568, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arking DE, Atzmon G, Arking A, Barzilai N, Dietz HC: Association between a functional variant of the KLOTHO gene and high-density lipoprotein cholesterol, blood pressure, stroke, and longevity. Circ Res 96: 412–418, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Morishita K, Shirai A, Kubota M, Katakura Y, Nabeshima Y, Takeshige K, Kamiya T: The progression of aging in klotho mutant mice can be modified by dietary phosphorus and zinc. J Nutr 131: 3182–3188, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Stubbs JR, Liu S, Tang W, Zhou J, Wang Y, Yao X, Quarles LD: Role of hyperphosphatemia and 1,25-dihydroxyvitamin D in vascular calcification and mortality in fibroblastic growth factor 23 null mice. J Am Soc Nephrol 18: 2116–2124, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Block GA, Port FK: Re-evaluation of risks associated with hyperphosphatemia and hyperparathyroidism in dialysis patients: Recommendations for a change in management. Am J Kidney Dis 35: 1226–1237, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Takeda E, Taketani Y, Nashiki K, Nomoto M, Shuto E, Sawada N, Yamamoto H, Isshiki M: A novel function of phosphate-mediated intracellular signal transduction pathways. Adv Enzyme Regul 46: 154–161, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Li JM, Shah AM: Endothelial cell superoxide generation: Regulation and relevance for cardiovascular pathology. Am J Physiol Regul Integr Comp Physiol 287: R1014–R1030, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Mount PF, Kemp BE, Power DA: Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol 42: 271–279, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Kukreja RC, Xi L: eNOS phosphorylation: A pivotal molecular switch in vasodilation and cardioprotection? J Mol Cell Cardiol 42: 280–282, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaul PW: Regulation of endothelial nitric oxide synthase: Location, location, location. Annu Rev Physiol 64: 749–774, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Shaul PW: Endothelial nitric oxide synthase, caveolae and the development of atherosclerosis. J Physiol 547: 21–33, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mineo C, Ying YS, Chapline C, Jaken S, Anderson RG: Targeting of protein kinase Cα to caveolae. J Cell Biol 141: 601–610, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishida Y, Taketani Y, Yamanaka-Okumura H, Imamura F, Taniguchi A, Sato T, Shuto E, Nashiki K, Arai H, Yamamoto H, Takeda E: Acute effect of oral phosphate loading on serum fibroblast growth factor 23 levels in healthy men. Kidney Int 70: 2141–2147, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H: High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 49: 1939–1945, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Di Marco GS, Hausberg M, Hillebrand U, Rustemeyer P, Wittkowski W, Lang D, Pavenstädt H: Increased inorganic phosphate induces human endothelial cell apoptosis in vitro. Am J Physiol Renal Physiol 294: F1381–1387, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Cai H, Harrison DG: Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ Res 87: 840–844, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Förstermann U, Münzel T: Endothelial nitric oxide synthase in vascular disease from marvel to menace. Circulation 113: 1708–1714, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Matsubara M, Hayashi N, Jing T, Titani K: Regulation of endothelial nitric oxide synthase by protein kinase C. J Biochem 133: 773–781, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Portale AA, Halloran BP, Morris C: Dietary intake of phosphorus modulates the circadian rhythm in serum concentration of phosphorus. J Clin Invest 80: 1147–1154, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Portale AA, Halloran BP, Morris C: Physiologic regulation of the serum concentration of 1,25-dihydroxyvitamin D by phosphorus in normal men. J Clin Invest 83: 1494–1499, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isshiki M, Ying Y, Fujita T, Anderson RGW: A molecular sensor detects signal transduction from caveolae in living cells. J Biol Chem 277: 43389–43398, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Setsukinai K, Urano Y, Kakinuma K, Majima HJ, Nagano T: Development of novel fluorescence probes that can reliably detect reactive oxygen species and distinguish specific species. J Biol Chem 278: 3170–3175, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Harada N, Sakamoto S, Niwa Y, Nakaya Y: Involvement of adenosine in vascular contractile preconditioning. Am J Physiol Heart Circ Physiol 280: H2911–H2919, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Deanfield JE, Halcox JP, Rabelink TJ: Endothelial function and dysfunction: Testing and clinical relevance. Circulation 115: 1285–1295, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R: Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the international brachial artery reactivity task force. J Am Coll Cardiol 39: 257–265, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.